Functional Voltage-Gated Sodium Channels Are Present in the Human B Cell Membrane
Abstract
1. Introduction
2. Materials and Methods
2.1. B Cell Isolation
2.2. Electrophysiology
2.3. RT-qPCR
3. Results
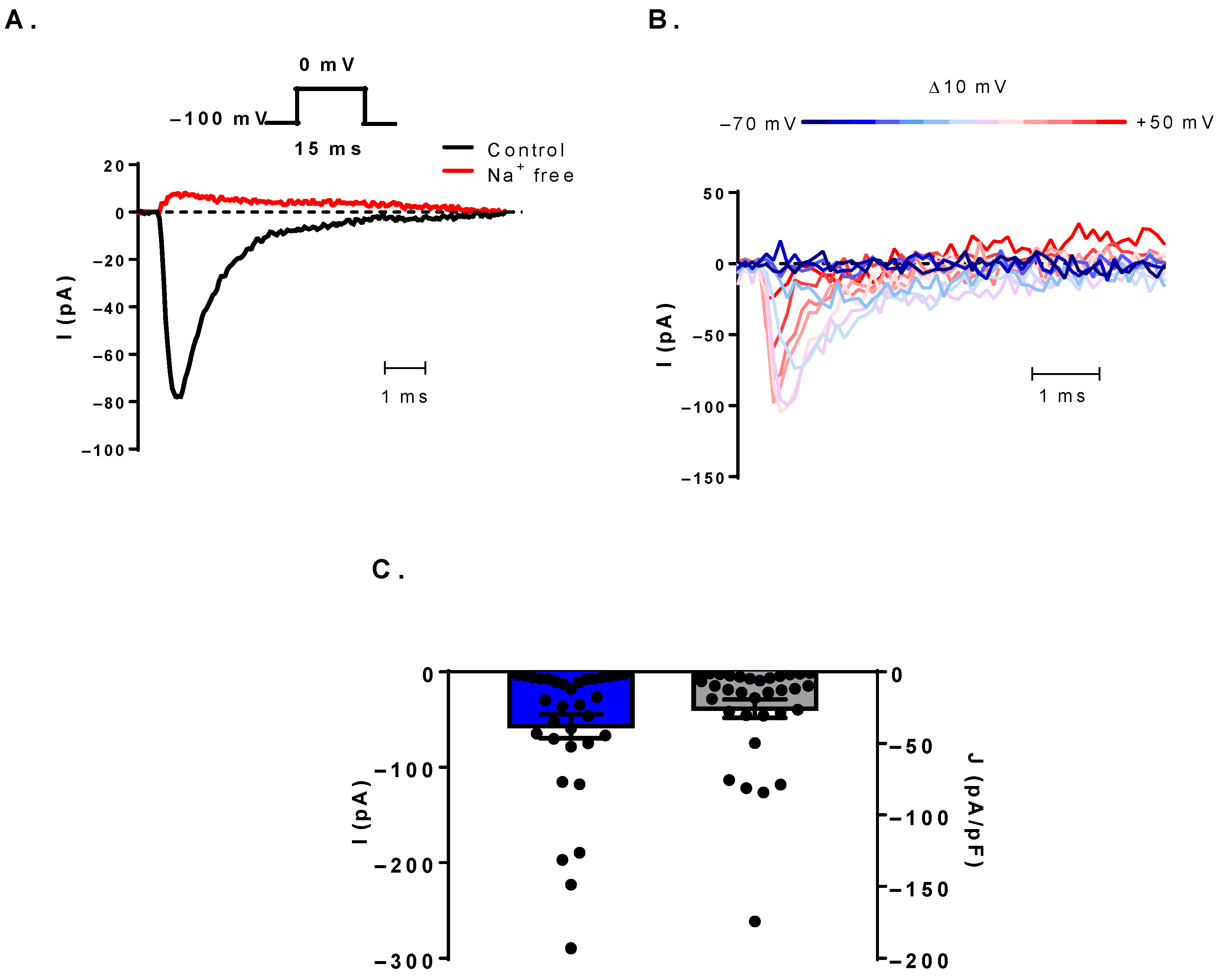
3.1. Voltage-Gated Sodium Currents Can Be Detected in Human B Cells
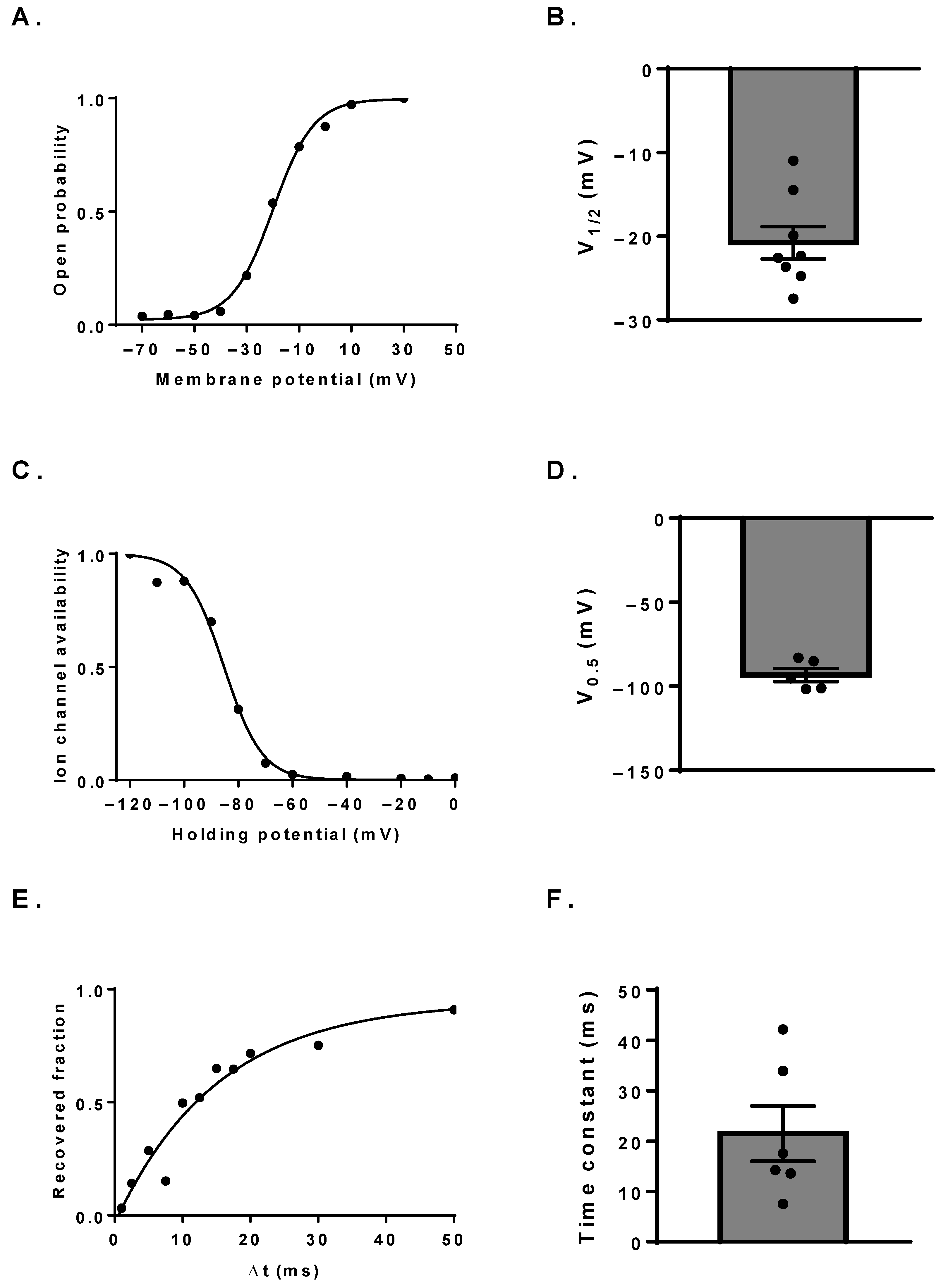
3.2. Biophysical Characterization of the NaV Channels
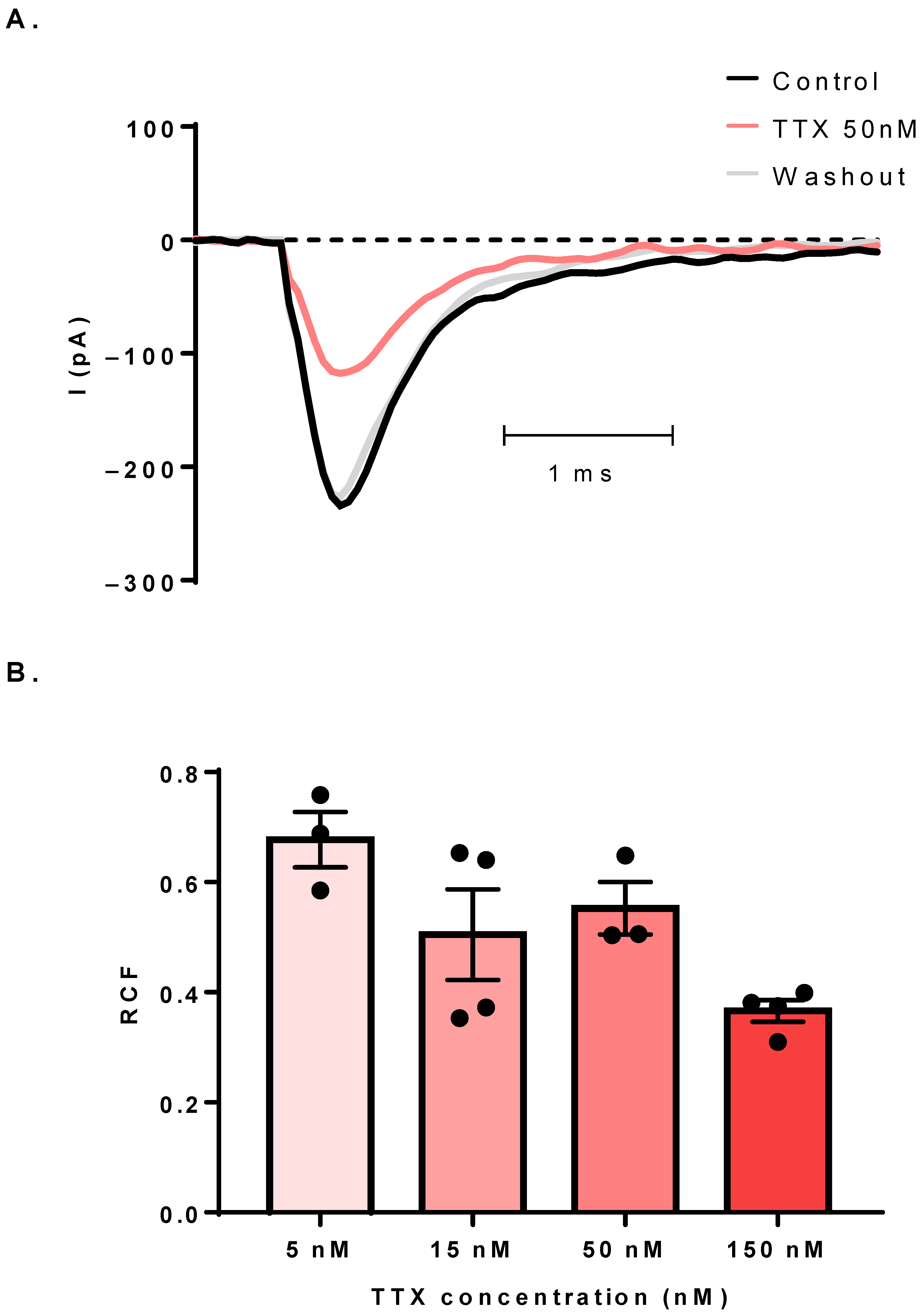
3.3. The Sodium Current Is TTX-Sensitive
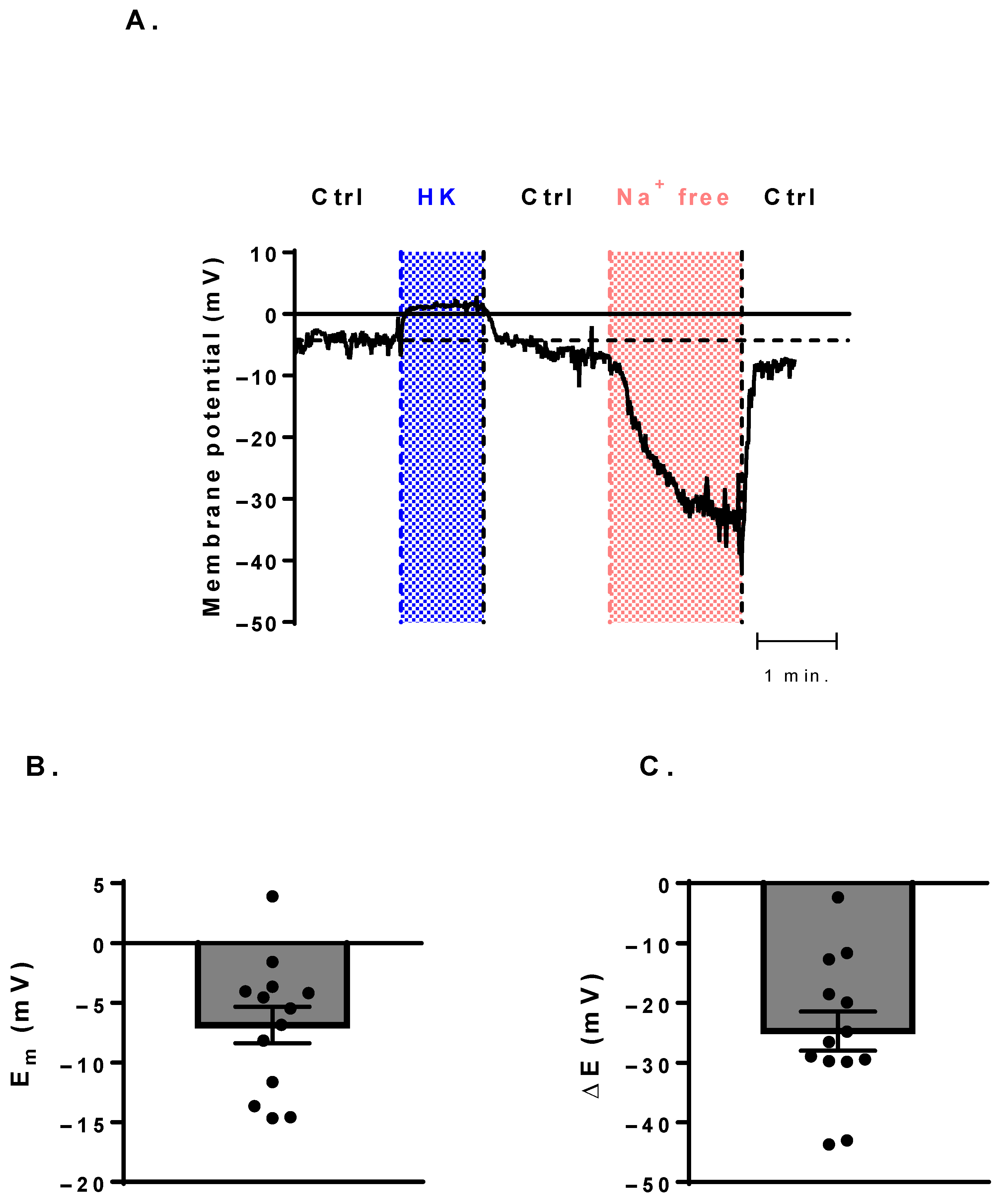
3.4. Membrane-Potential Measurements Show a Potential Canonical Function of NaV Channels
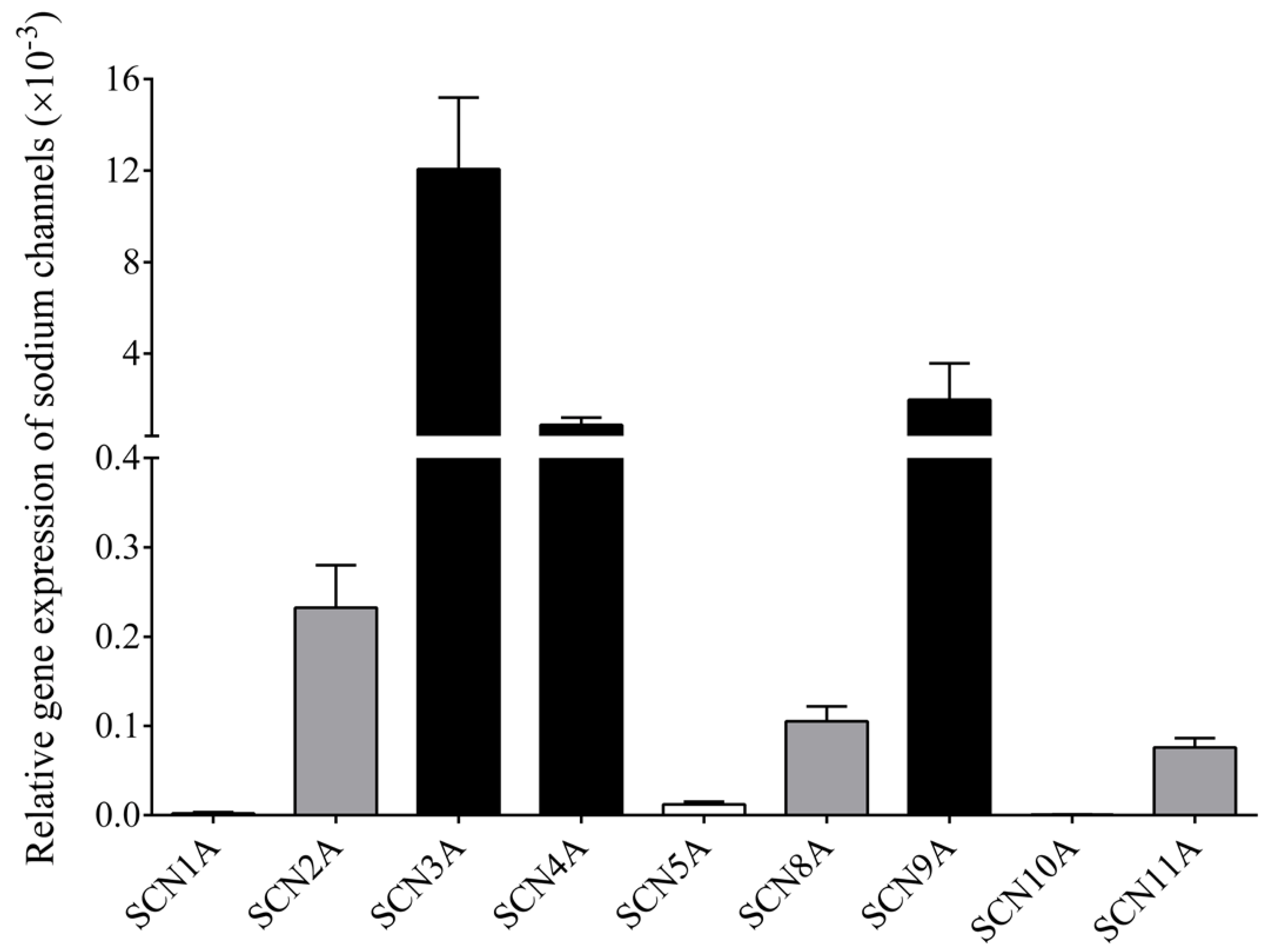
3.5. RT-qPCR Confirms Multiple NaV Channel Types in B Cells
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Catterall, W.A.; Goldin, A.L.; Waxman, S.G. International union of pharmacology. Xlvii. Nomenclature and structure-function relationships of voltage-gated sodium channels. Pharmacol. Rev. 2005, 57, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Goldin, A.L.; Barchi, R.L.; Caldwell, J.H.; Hofmann, F.; Howe, J.R.; Hunter, J.C.; Kallen, R.G.; Mandel, G.; Meisler, M.H.; Netter, Y.B.; et al. Nomenclature of voltage-gated sodium channels. Neuron 2000, 28, 365–368. [Google Scholar] [CrossRef]
- Zhang, Y.; Yip, G.W.; Chan, A.K.; Wang, M.; Lam, W.W.; Fung, J.W.; Chan, J.Y.; Sanderson, J.E.; Yu, C.M. Left ventricular systolic dyssynchrony is a predictor of cardiac remodeling after myocardial infarction. Am. Heart J. 2008, 156, 1124–1132. [Google Scholar] [CrossRef] [PubMed]
- Bane, V.; Lehane, M.; Dikshit, M.; O’Riordan, A.; Furey, A. Tetrodotoxin: Chemistry, toxicity, source, distribution and detection. Toxins 2014, 6, 693–755. [Google Scholar] [CrossRef]
- Narahashi, T.; Moore, J.W.; Scott, W.R. Tetrodotoxin blockage of sodium conductance increase in lobster giant axons. J. Gen. Physiol. 1964, 47, 965–974. [Google Scholar] [CrossRef]
- Alexander, S.P.; Kelly, E.; Marrion, N.; Peters, J.A.; Benson, H.E.; Faccenda, E.; Pawson, A.J.; Sharman, J.L.; Southan, C.; Buneman, O.P.; et al. The concise guide to pharmacology 2015/16: Overview. Br. J. Pharmacol. 2015, 172, 5729–5743. [Google Scholar] [CrossRef]
- Black, J.A.; Dib-Hajj, S.; Cohen, S.; Hinson, A.W.; Waxman, S.G. Glial cells have heart: Rh1 na+ channel mrna and protein in spinal cord astrocytes. Glia 1998, 23, 200–208. [Google Scholar] [CrossRef]
- Black, J.A.; Westenbroek, R.; Minturn, J.E.; Ransom, B.R.; Catterall, W.A.; Waxman, S.G. Isoform-specific expression of sodium channels in astrocytes in vitro: Immunocytochemical observations. Glia 1995, 14, 133–144. [Google Scholar] [CrossRef]
- Reese, K.A.; Caldwell, J.H. Immunocytochemical localization of nach6 in cultured spinal cord astrocytes. Glia 1999, 26, 92–96. [Google Scholar] [CrossRef]
- Sontheimer, H.; Fernandez-Marques, E.; Ullrich, N.; Pappas, C.A.; Waxman, S.G. Astrocyte na+ channels are required for maintenance of na+/k(+)-atpase activity. J. Neurosci. 1994, 14, 2464–2475. [Google Scholar] [CrossRef]
- Donatsch, P.; Lowe, D.A.; Richardson, B.P.; Taylor, P. The functional significance of sodium channels in pancreatic beta-cell membranes. J. Physiol. 1977, 267, 357–376. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Barr, T.P.; Hou, Q.; Dib-Hajj, S.D.; Black, J.A.; Albrecht, P.J.; Petersen, K.; Eisenberg, E.; Wymer, J.P.; Rice, F.L.; et al. Voltage-gated sodium channel expression in rat and human epidermal keratinocytes: Evidence for a role in pain. Pain 2008, 139, 90–105. [Google Scholar] [CrossRef] [PubMed]
- Traub, O.; Ishida, T.; Ishida, M.; Tupper, J.C.; Berk, B.C. Shear stress-mediated extracellular signal-regulated kinase activation is regulated by sodium in endothelial cells. Potential role for a voltage-dependent sodium channel. J. Biol. Chem. 1999, 274, 20144–20150. [Google Scholar] [CrossRef] [PubMed]
- Kis-Toth, K.; Hajdu, P.; Bacskai, I.; Szilagyi, O.; Papp, F.; Szanto, A.; Posta, E.; Gogolak, P.; Panyi, G.; Rajnavolgyi, E. Voltage-gated sodium channel nav1.7 maintains the membrane potential and regulates the activation and chemokine-induced migration of a monocyte-derived dendritic cell subset. J. Immunol. 2011, 187, 1273–1280. [Google Scholar] [CrossRef] [PubMed]
- Black, J.A.; Liu, S.; Waxman, S.G. Sodium channel activity modulates multiple functions in microglia. Glia 2009, 57, 1072–1081. [Google Scholar] [CrossRef]
- Carrithers, M.D.; Chatterjee, G.; Carrithers, L.M.; Offoha, R.; Iheagwara, U.; Rahner, C.; Graham, M.; Waxman, S.G. Regulation of podosome formation in macrophages by a splice variant of the sodium channel scn8a. J. Biol. Chem. 2009, 284, 8114–8126. [Google Scholar] [CrossRef]
- de Lera Ruiz, M.; Kraus, R.L. Voltage-gated sodium channels: Structure, function, pharmacology, and clinical indications. J. Med. Chem. 2015, 58, 7093–7118. [Google Scholar] [CrossRef]
- Hoffman, J.F.; Dodson, A.; Wickrema, A.; Dib-Hajj, S.D. Tetrodotoxin-sensitive na+ channels and muscarinic and purinergic receptors identified in human erythroid progenitor cells and red blood cell ghosts. Proc. Natl. Acad. Sci. USA 2004, 101, 12370–12374. [Google Scholar] [CrossRef]
- Cahalan, M.D.; Chandy, K.G.; DeCoursey, T.E.; Gupta, S. A voltage-gated potassium channel in human t lymphocytes. J. Physiol. 1985, 358, 197–237. [Google Scholar] [CrossRef]
- Lo, W.L.; Donermeyer, D.L.; Allen, P.M. A voltage-gated sodium channel is essential for the positive selection of cd4(+) t cells. Nat. Immunol. 2012, 13, 880–887. [Google Scholar] [CrossRef]
- Poffers, M.; Buhne, N.; Herzog, C.; Thorenz, A.; Chen, R.; Guler, F.; Hage, A.; Leffler, A.; Echtermeyer, F. Sodium channel nav1.3 is expressed by polymorphonuclear neutrophils during mouse heart and kidney ischemia in vivo and regulates adhesion, transmigration, and chemotaxis of human and mouse neutrophils in vitro. Anesthesiology 2018, 128, 1151–1166. [Google Scholar] [CrossRef] [PubMed]
- Angus, M.; Ruben, P. Voltage gated sodium channels in cancer and their potential mechanisms of action. Channels (Austin) 2019, 13, 400–409. [Google Scholar] [CrossRef] [PubMed]
- Paez, P.M.; Fulton, D.; Colwell, C.S.; Campagnoni, A.T. Voltage-operated ca(2+) and na(+) channels in the oligodendrocyte lineage. J. Neurosci. Res. 2009, 87, 3259–3266. [Google Scholar] [CrossRef] [PubMed]
- Varga, Z.; Juhasz, T.; Matta, C.; Fodor, J.; Katona, E.; Bartok, A.; Olah, T.; Sebe, A.; Csernoch, L.; Panyi, G.; et al. Switch of voltage-gated k+ channel expression in the plasma membrane of chondrogenic cells affects cytosolic ca2+-oscillations and cartilage formation. PLoS ONE 2011, 6, e27957. [Google Scholar] [CrossRef] [PubMed]
- Zsiros, E.; Kis-Toth, K.; Hajdu, P.; Gaspar, R.; Bielanska, J.; Felipe, A.; Rajnavolgyi, E.; Panyi, G. Developmental switch of the expression of ion channels in human dendritic cells. J. Immunol. 2009, 183, 4483–4492. [Google Scholar] [CrossRef]
- Lund, F.E. Cytokine-producing b lymphocytes-key regulators of immunity. Curr. Opin. Immunol. 2008, 20, 332–338. [Google Scholar] [CrossRef]
- Lund, F.E.; Garvy, B.A.; Randall, T.D.; Harris, D.P. Regulatory roles for cytokine-producing b cells in infection and autoimmune disease. Curr. Dir. Autoimmun. 2005, 8, 25–54. [Google Scholar]
- Shen, P.; Fillatreau, S. Antibody-independent functions of b cells: A focus on cytokines. Nat. Rev. Immunol. 2015, 15, 441–451. [Google Scholar] [CrossRef]
- Feske, S.; Skolnik, E.Y.; Prakriya, M. Ion channels and transporters in lymphocyte function and immunity. Nat. Rev. Immunol. 2012, 12, 532–547. [Google Scholar] [CrossRef]
- Mahtani, T.; Treanor, B. Beyond the crac: Diversification of ion signaling in b cells. Immunol. Rev. 2019, 291, 104–122. [Google Scholar] [CrossRef]
- Zheng, H.; Nam, J.H.; Pang, B.; Shin, D.H.; Kim, J.S.; Chun, Y.S.; Park, J.W.; Bang, H.; Kim, W.K.; Earm, Y.E.; et al. Identification of the large-conductance background k+ channel in mouse b cells as trek-2. Am. J. Physiol. Cell Physiol. 2009, 297, C188–C197. [Google Scholar] [CrossRef]
- Stokes, L.; MacKenzie, A.B.; Sluyter, R. Editorial: Roles of ion channels in immune cells. Front. Immunol. 2016, 7, 48. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wulff, H.; Knaus, H.G.; Pennington, M.; Chandy, K.G. K+ channel expression during b cell differentiation: Implications for immunomodulation and autoimmunity. J. Immunol. 2004, 173, 776–786. [Google Scholar] [CrossRef]
- MacDougall, S.L.; Grinstein, S.; Gelfand, E.W. Activation of ca2+-dependent k+ channels in human b lymphocytes by anti-immunoglobulin. J. Clin. Investig. 1988, 81, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Ransom, J.T.; Cambier, J.C. The dynamics and relationship of k+ efflux and ca++ influx in b lymphocytes after antigen-receptor cross-linking. Soc. Gen. Physiol. Ser. 1988, 43, 241–251. [Google Scholar] [PubMed]
- Zhang, S.; Wang, X.; Ju, C.; Zhu, L.; Du, Y.; Gao, C. Blockage of k(ca)3.1 and kv1.3 channels of the b lymphocyte decreases the inflammatory monocyte chemotaxis. Int. Immunopharmacol. 2016, 31, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Davenport, B.; Li, Y.; Heizer, J.W.; Schmitz, C.; Perraud, A.L. Signature channels of excitability no more: L-type channels in immune cells. Front. Immunol. 2015, 6, 375. [Google Scholar] [CrossRef]
- Grafton, G.; Stokes, L.; Toellner, K.M.; Gordon, J. A non-voltage-gated calcium channel with l-type characteristics activated by b cell receptor ligation. Biochem. Pharmacol. 2003, 66, 2001–2009. [Google Scholar] [CrossRef] [PubMed]
- Kotturi, M.F.; Jefferies, W.A. Molecular characterization of l-type calcium channel splice variants expressed in human t lymphocytes. Mol. Immunol. 2005, 42, 1461–1474. [Google Scholar] [CrossRef]
- Sadighi Akha, A.A.; Willmott, N.J.; Brickley, K.; Dolphin, A.C.; Galione, A.; Hunt, S.V. Anti-ig-induced calcium influx in rat b lymphocytes mediated by cgmp through a dihydropyridine-sensitive channel. J. Biol. Chem. 1996, 271, 7297–7300. [Google Scholar] [CrossRef]
- Stokes, L.; Gordon, J.; Grafton, G. Non-voltage-gated l-type ca2+ channels in human t cells: Pharmacology and molecular characterization of the major alpha pore-forming and auxiliary beta-subunits. J. Biol. Chem. 2004, 279, 19566–19573. [Google Scholar] [CrossRef] [PubMed]
- Feske, S.; Giltnane, J.; Dolmetsch, R.; Staudt, L.M.; Rao, A. Gene regulation mediated by calcium signals in t lymphocytes. Nat. Immunol. 2001, 2, 316–324. [Google Scholar] [CrossRef] [PubMed]
- Hogan, P.G.; Lewis, R.S.; Rao, A. Molecular basis of calcium signaling in lymphocytes: Stim and orai. Annu. Rev. Immunol. 2010, 28, 491–533. [Google Scholar] [CrossRef] [PubMed]
- Hoth, M.; Penner, R. Depletion of intracellular calcium stores activates a calcium current in mast cells. Nature 1992, 355, 353–356. [Google Scholar] [CrossRef] [PubMed]
- Limnander, A.; Depeille, P.; Freedman, T.S.; Liou, J.; Leitges, M.; Kurosaki, T.; Roose, J.P.; Weiss, A. Stim1, pkc-delta and rasgrp set a threshold for proapoptotic erk signaling during b cell development. Nat. Immunol. 2011, 12, 425–433. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Fujii, Y.; Baba, A.; Hikida, M.; Kurosaki, T.; Baba, Y. The calcium sensors stim1 and stim2 control b cell regulatory function through interleukin-10 production. Immunity 2011, 34, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Zweifach, A.; Lewis, R.S. Mitogen-regulated ca2+ current of t lymphocytes is activated by depletion of intracellular Ca2+ stores. Proc. Natl. Acad. Sci. USA 1993, 90, 6295–6299. [Google Scholar] [CrossRef]
- Pippel, A.; Bessler, B.; Klapperstuck, M.; Markwardt, F. Inhibition of antigen receptor-dependent ca(2+) signals and nf-at activation by p2x7 receptors in human b lymphocytes. Cell Calcium 2015, 57, 275–289. [Google Scholar] [CrossRef]
- Bradford, A.L.; Ismailov, I.I.; Achard, J.M.; Warnock, D.G.; Bubien, J.K.; Benos, D.J. Immunopurification and functional reconstitution of a na+ channel complex from rat lymphocytes. Am. J. Physiol. 1995, 269, C601–C611. [Google Scholar] [CrossRef]
- Launay, P.; Cheng, H.; Srivatsan, S.; Penner, R.; Fleig, A.; Kinet, J.P. Trpm4 regulates calcium oscillations after t cell activation. Science 2004, 306, 1374–1377. [Google Scholar] [CrossRef]
- Gaspar, R., Jr.; Krasznai, Z.; Marian, T.; Tron, L.; Recchioni, R.; Falasca, M.; Moroni, F.; Pieri, C.; Damjanovich, S. Bretylium-induced voltage-gated sodium current in human lymphocytes. Biochim. Biophys. Acta 1992, 1137, 143–147. [Google Scholar] [CrossRef]
- Pieri, C.; Recchioni, R.; Moroni, F.; Balkay, L.; Marian, T.; Tron, L.; Damjanovich, S. Ligand and voltage gated sodium channels may regulate electrogenic pump activity in human, mouse and rat lymphocytes. Biochem. Biophys. Res. Commun. 1989, 160, 999–1002. [Google Scholar] [CrossRef]
- Roselli, F.; Livrea, P.; Jirillo, E. Voltage-gated sodium channel blockers as immunomodulators. Recent Pat. CNS Drug Discov. 2006, 1, 83–91. [Google Scholar] [CrossRef]
- Fraser, S.P.; Diss, J.K.; Lloyd, L.J.; Pani, F.; Chioni, A.M.; George, A.J.; Djamgoz, M.B. T-lymphocyte invasiveness: Control by voltage-gated na+ channel activity. FEBS Lett. 2004, 569, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Lai, Z.F.; Chen, Y.Z.; Nishimura, Y.; Nishi, K. An amiloride-sensitive and voltage-dependent na+ channel in an hla-dr-restricted human t cell clone. J. Immunol. 2000, 165, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Fejes, Z.; Pocsi, M.; Takai, J.; Erdei, J.; Toth, A.; Balogh, E.; Rusznyak, A.; Fenyvesi, F.; Nagy, A.; Kappelmayer, J.; et al. Preterm intraventricular hemorrhage-induced inflammatory response in human choroid plexus epithelial cells. Int. J. Mol. Sci. 2021, 22, 8648. [Google Scholar] [CrossRef] [PubMed]
- Tsukamoto, T.; Chiba, Y.; Wakamori, M.; Yamada, T.; Tsunogae, S.; Cho, Y.; Sakakibara, R.; Imazu, T.; Tokoro, S.; Satake, Y.; et al. Differential binding of tetrodotoxin and its derivatives to voltage-sensitive sodium channel subtypes (nav 1.1 to nav 1.7). Br. J. Pharmacol. 2017, 174, 3881–3892. [Google Scholar] [CrossRef] [PubMed]
- Vanoye, C.G.; Kunic, J.D.; Ehring, G.R.; George, A.L., Jr. Mechanism of sodium channel nav1.9 potentiation by g-protein signaling. J. Gen. Physiol. 2013, 141, 193–202. [Google Scholar] [CrossRef]
- Beeton, C.; Wulff, H.; Standifer, N.E.; Azam, P.; Mullen, K.M.; Pennington, M.W.; Kolski-Andreaco, A.; Wei, E.; Grino, A.; Counts, D.R.; et al. Kv1.3 channels are a therapeutic target for t cell-mediated autoimmune diseases. Proc. Natl. Acad. Sci. USA 2006, 103, 17414–17419. [Google Scholar] [CrossRef]
- Macfarlane, S.N.; Sontheimer, H. Spinal cord astrocytes display a switch from ttx-sensitive to ttx-resistant sodium currents after injury-induced gliosis in vitro. J. Neurophysiol. 1998, 79, 2222–2226. [Google Scholar] [CrossRef]
- Bendahhou, S.; Cummins, T.R.; Tawil, R.; Waxman, S.G.; Ptacek, L.J. Activation and inactivation of the voltage-gated sodium channel: Role of segment s5 revealed by a novel hyperkalaemic periodic paralysis mutation. J. Neurosci. 1999, 19, 4762–4771. [Google Scholar] [CrossRef] [PubMed]
- Cummins, T.R.; Aglieco, F.; Renganathan, M.; Herzog, R.I.; Dib-Hajj, S.D.; Waxman, S.G. Nav1.3 sodium channels: Rapid repriming and slow closed-state inactivation display quantitative differences after expression in a mammalian cell line and in spinal sensory neurons. J. Neurosci. 2001, 21, 5952–5961. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Voelker, T.L.; Varga, Z.; Schubert, A.R.; Nerbonne, J.M.; Silva, J.R. Mechanisms of noncovalent beta subunit regulation of nav channel gating. J. Gen. Physiol. 2017, 149, 813–831. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Xiao, Z.; Xu, Y.; Zhang, Y.; Tang, D.; Wu, X.; Tang, C.; Chen, M.; Shi, X.; Chen, P.; et al. Electrophysiological and pharmacological analyses of nav1.9 voltage-gated sodium channel by establishing a heterologous expression system. Front. Pharmacol. 2017, 8, 852. [Google Scholar] [CrossRef] [PubMed]
- Ohtubo, Y. Slow recovery from the inactivation of voltage-gated sodium channel nav1.3 in mouse taste receptor cells. Pflügers Arch.-Eur. J. Physiol. 2021, 473, 953–968. [Google Scholar] [CrossRef]
- Herzog, R.I.; Cummins, T.R.; Ghassemi, F.; Dib-Hajj, S.D.; Waxman, S.G. Distinct repriming and closed-state inactivation kinetics of nav1.6 and nav1.7 sodium channels in mouse spinal sensory neurons. J. Physiol. 2003, 551, 741–750. [Google Scholar] [CrossRef]
- Rink, T.J.; Montecucco, C.; Hesketh, T.R.; Tsien, R.Y. Lymphocyte membrane potential assessed with fluorescent probes. Biochim. Biophys. Acta 1980, 595, 15–30. [Google Scholar] [CrossRef]
- Wilson, H.A.; Chused, T.M. Lymphocyte membrane potential and ca2+-sensitive potassium channels described by oxonol dye fluorescence measurements. J. Cell Physiol. 1985, 125, 72–81. [Google Scholar] [CrossRef]
- Malofiejew, M.; Kostrzewska, A.; Kowal, E. electrical potential of blood lymphocytes. Acta Haematol. Pol. 1975, 6, 97–104. [Google Scholar]
- Taki, M. Studies on blastogenesis of human lymphocytes by phytohemagglutinin, with special reference to changes of membrane potential during blastoid transformation. Mie Med. J. 1970, 19, 245–262. [Google Scholar]
- Fordyce, C.B.; Jagasia, R.; Zhu, X.; Schlichter, L.C. Microglia kv1.3 channels contribute to their ability to kill neurons. J. Neurosci. 2005, 25, 7139–7149. [Google Scholar] [CrossRef] [PubMed]
- Mullen, K.M.; Rozycka, M.; Rus, H.; Hu, L.; Cudrici, C.; Zafranskaia, E.; Pennington, M.W.; Johns, D.C.; Judge, S.I.; Calabresi, P.A. Potassium channels kv1.3 and kv1.5 are expressed on blood-derived dendritic cells in the central nervous system. Ann. Neurol. 2006, 60, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.; Leek, A.N.; Sole, L.; Maverick, E.E.; Levine, T.P.; Tamkun, M.M. Kv2 potassium channels form endoplasmic reticulum/plasma membrane junctions via interaction with vapa and vapb. Proc. Natl. Acad. Sci. USA 2018, 115, E7331–E7340. [Google Scholar] [CrossRef] [PubMed]
- King, A.N.; Manning, C.F.; Trimmer, J.S. A unique ion channel clustering domain on the axon initial segment of mammalian neurons. J. Comp. Neurol 2014, 522, 2594–2608. [Google Scholar] [CrossRef] [PubMed]
- Cidad, P.; Jimenez-Perez, L.; Garcia-Arribas, D.; Miguel-Velado, E.; Tajada, S.; Ruiz-McDavitt, C.; Lopez-Lopez, J.R.; Perez-Garcia, M.T. Kv1.3 channels can modulate cell proliferation during phenotypic switch by an ion-flux independent mechanism. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 1299–1307. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feher, A.; Pócsi, M.; Papp, F.; Szanto, T.G.; Csoti, A.; Fejes, Z.; Nagy, B., Jr.; Nemes, B.; Varga, Z. Functional Voltage-Gated Sodium Channels Are Present in the Human B Cell Membrane. Cells 2022, 11, 1225. https://doi.org/10.3390/cells11071225
Feher A, Pócsi M, Papp F, Szanto TG, Csoti A, Fejes Z, Nagy B Jr., Nemes B, Varga Z. Functional Voltage-Gated Sodium Channels Are Present in the Human B Cell Membrane. Cells. 2022; 11(7):1225. https://doi.org/10.3390/cells11071225
Chicago/Turabian StyleFeher, Adam, Marianna Pócsi, Ferenc Papp, Tibor G. Szanto, Agota Csoti, Zsolt Fejes, Béla Nagy, Jr., Balázs Nemes, and Zoltan Varga. 2022. "Functional Voltage-Gated Sodium Channels Are Present in the Human B Cell Membrane" Cells 11, no. 7: 1225. https://doi.org/10.3390/cells11071225
APA StyleFeher, A., Pócsi, M., Papp, F., Szanto, T. G., Csoti, A., Fejes, Z., Nagy, B., Jr., Nemes, B., & Varga, Z. (2022). Functional Voltage-Gated Sodium Channels Are Present in the Human B Cell Membrane. Cells, 11(7), 1225. https://doi.org/10.3390/cells11071225





