Estrogens Play a Critical Role in Stress-Related Gastrointestinal Dysfunction in a Spontaneous Model of Disorders of Gut–Brain Interaction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experimental Design
2.2.1. Protocol 1: Effect of Maternal Separation on GI Phenotype of the Adult BBDP-N Rat
2.2.2. Protocol 2: Estrogen Involvement in Maternal-Separation-Induced GI Alterations in Adult BBDP-N Rats
2.3. Colonic Sensitivity
2.4. Surgery
2.5. Histology
2.6. Immunohistochemistry
2.7. Protein Extraction and β-Hexosaminidase Assay
2.8. Eosinophil Peroxidase
2.9. Real-Time PCR
2.10. Statistical Analysis
2.11. Ethical Approval
3. Results
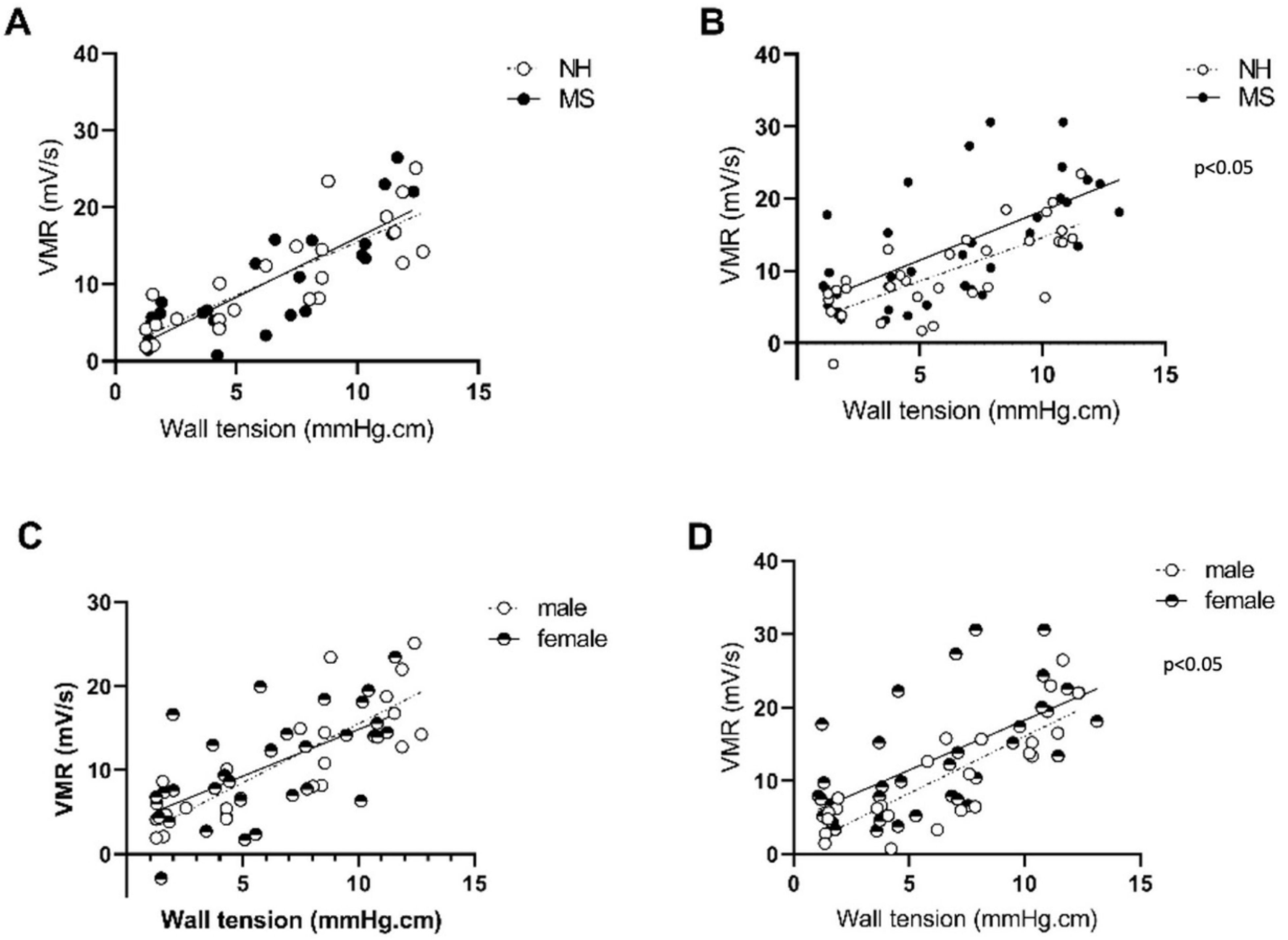
3.1. Protocol 1: Effect of Maternal Separation on Gastrointestinal Features of the Adult BBDP-N Rat
3.1.1. Stress Induces Visceral Sensitivity in Females Only
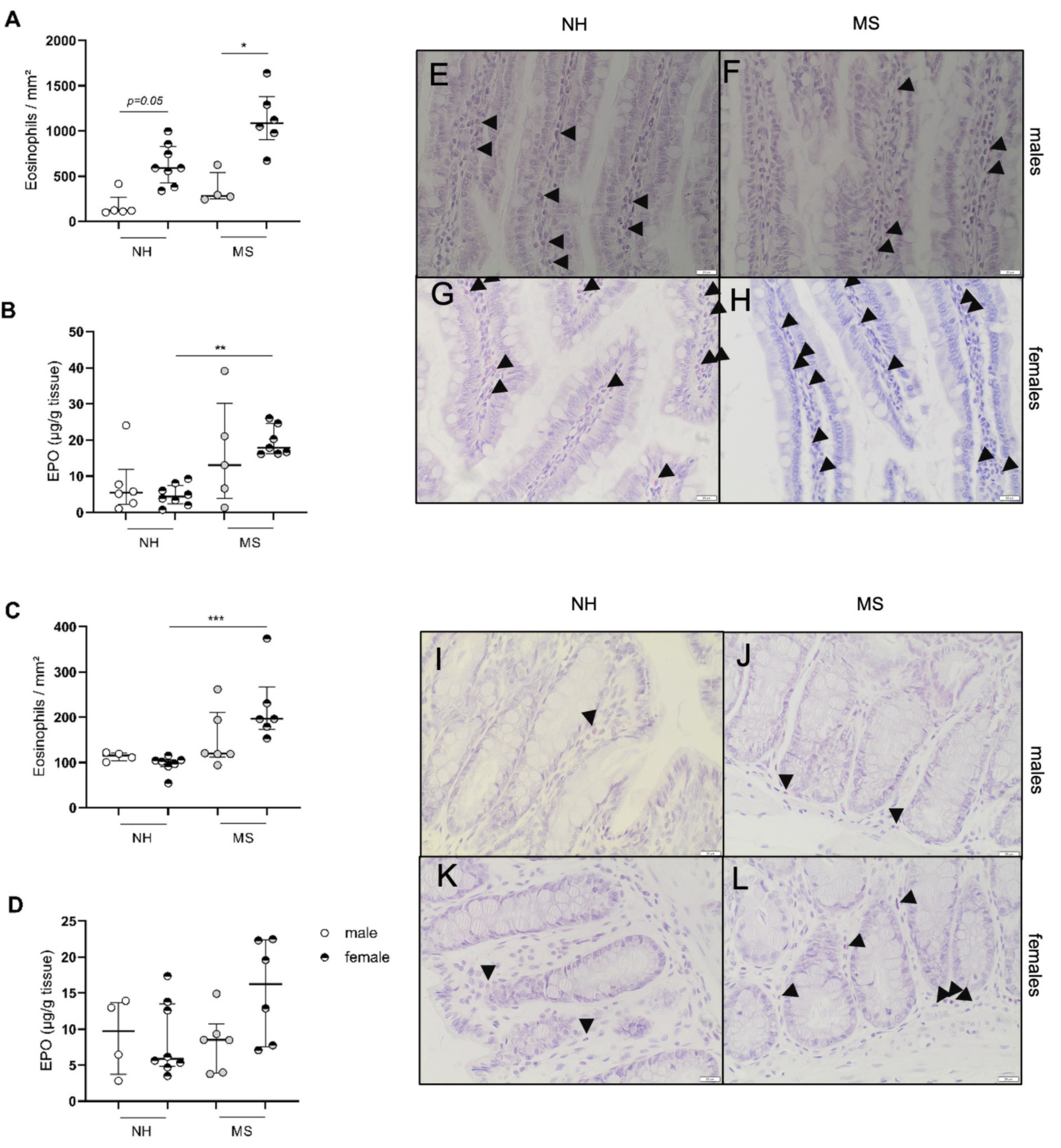
3.1.2. Stress Triggers Intestinal Inflammatory Changes in Females Only
3.2. Protocol 2: Estrogen Involvement in Maternal-Separation-Induced GI Alterations in Adult BBDP-N Rats
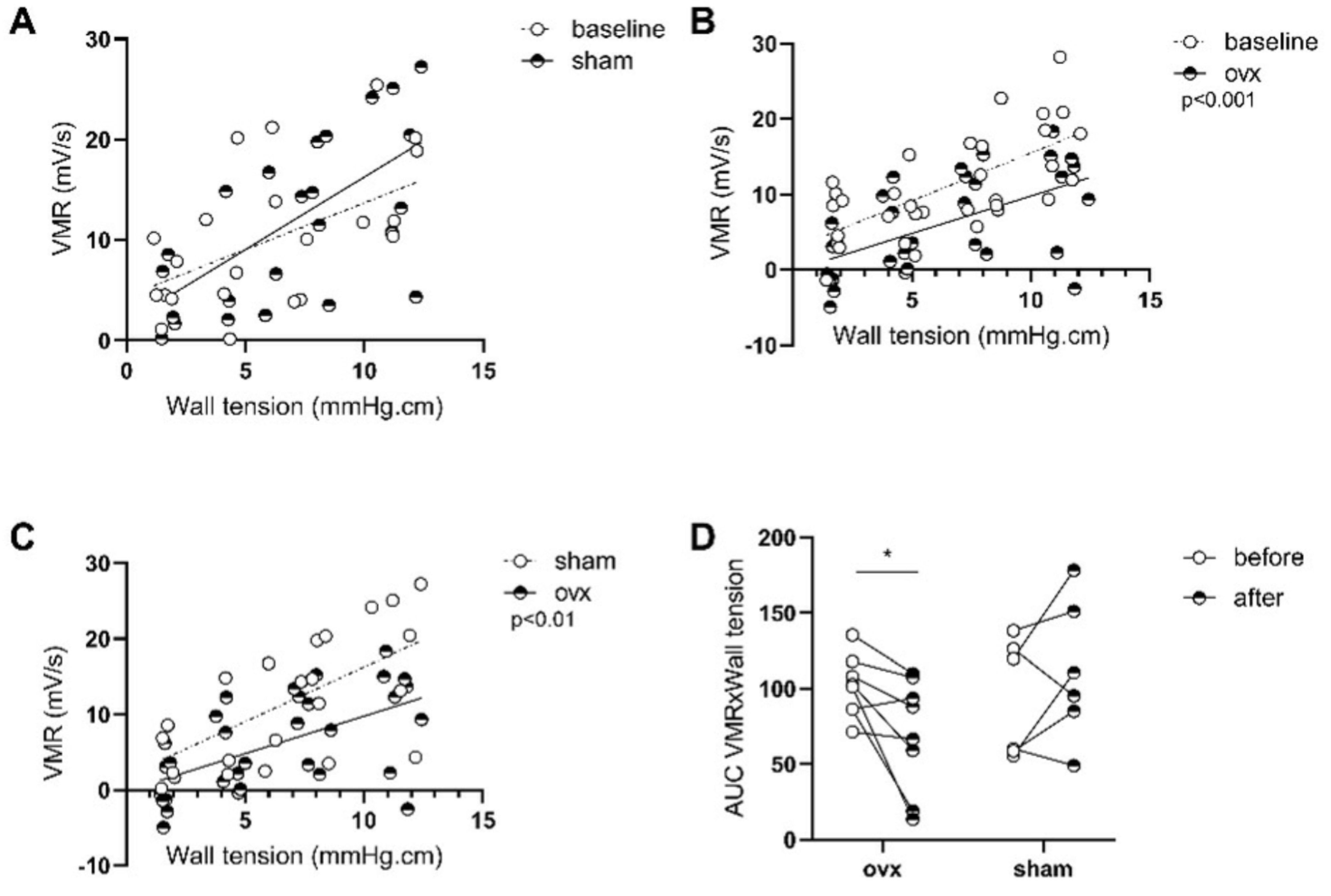
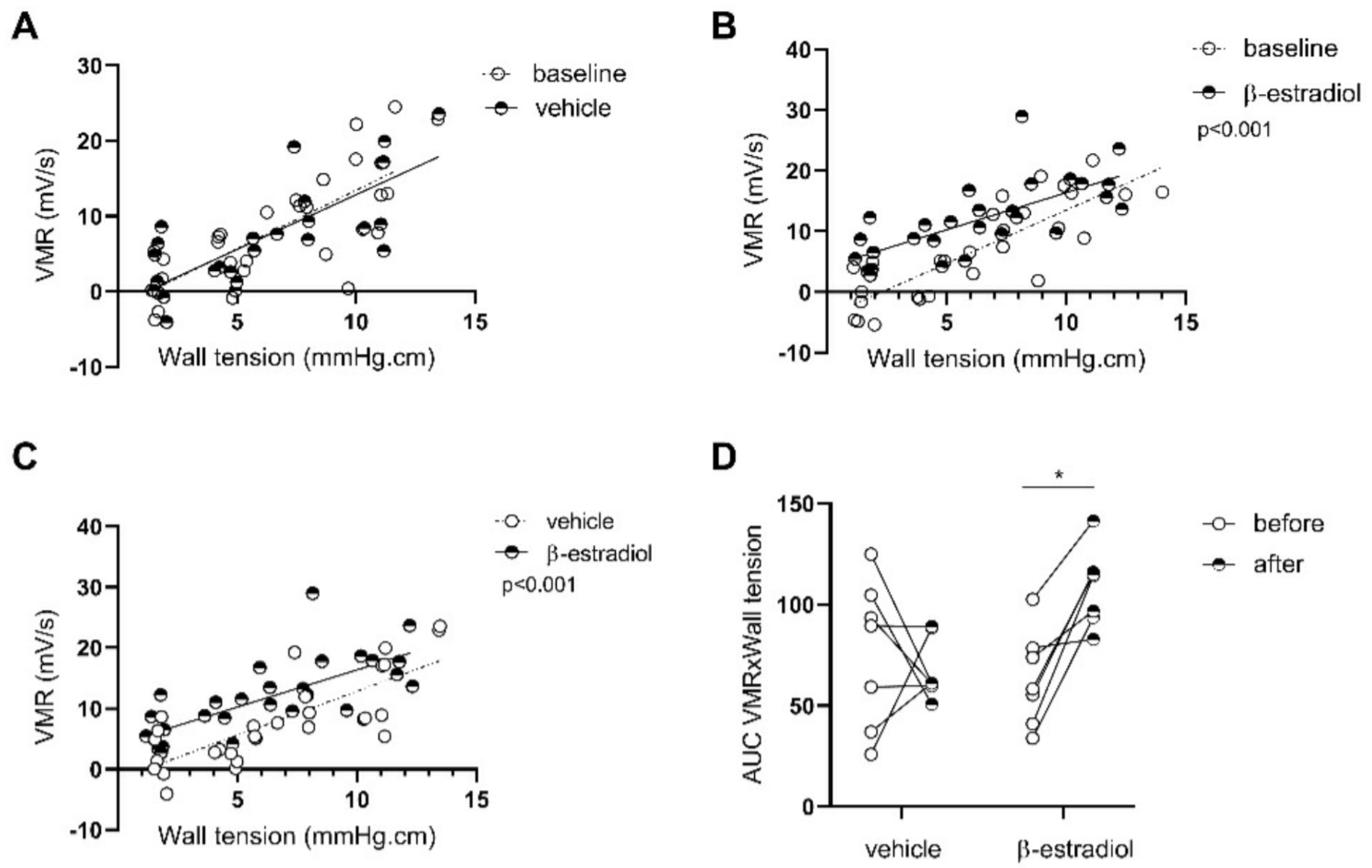
3.2.1. β-Estradiol Is Involved in Stress-Induced Visceral Hypersensitivity in Females
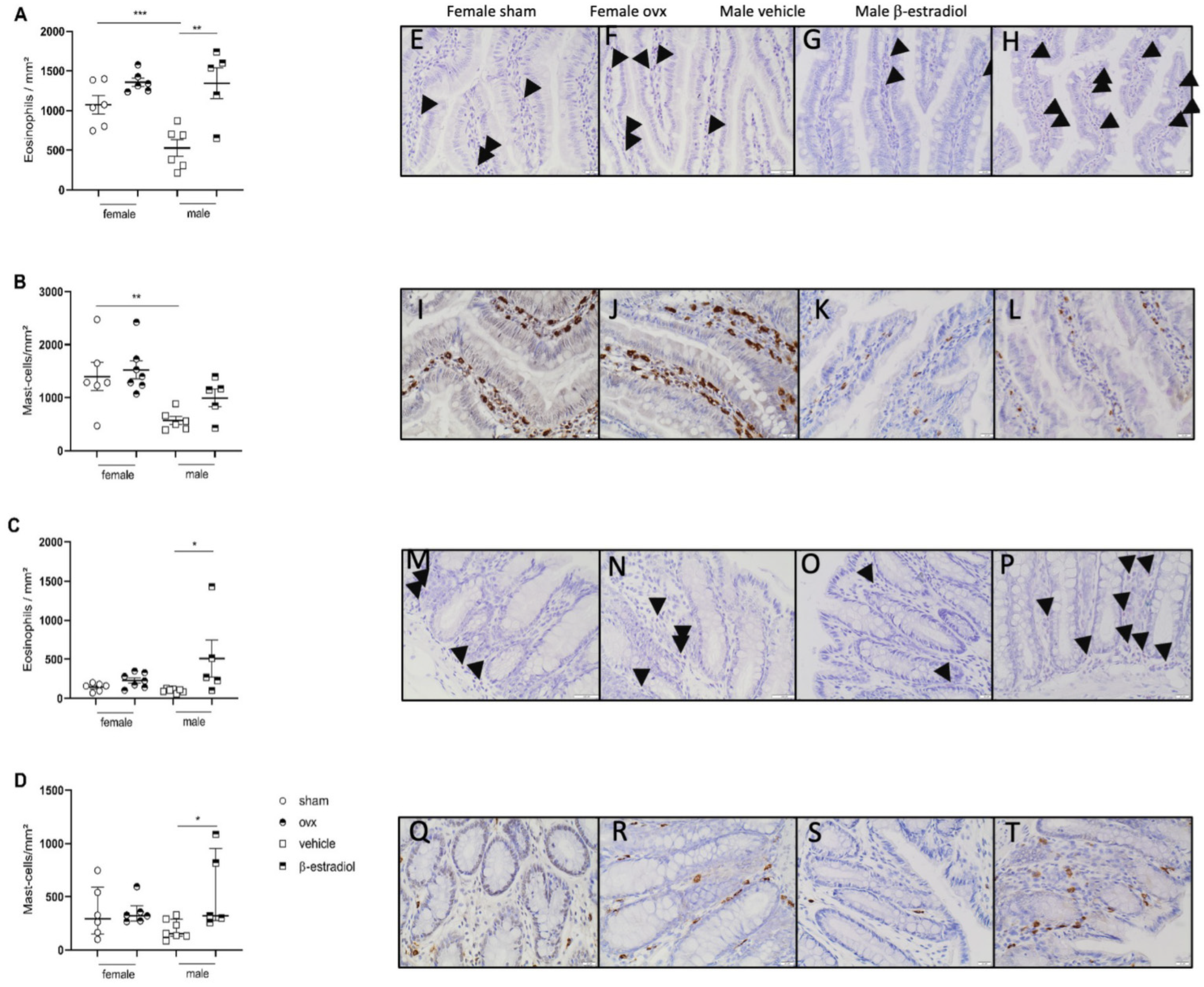
3.2.2. β-Estradiol Increases Colonic Sensitivity in Stressed Males
3.2.3. Stress-Induced Inflammatory Changes in Females Are Not Reversed by Ovariectomy While β-Estradiol Induces Inflammatory Changes in Stressed Males
3.2.4. Β-Estradiol Induces Gene Expression of Inflammatory-Related Proteins in the GI Tract
3.2.5. Gene Expression of the Estrogen Receptor GPER in the GI Tract
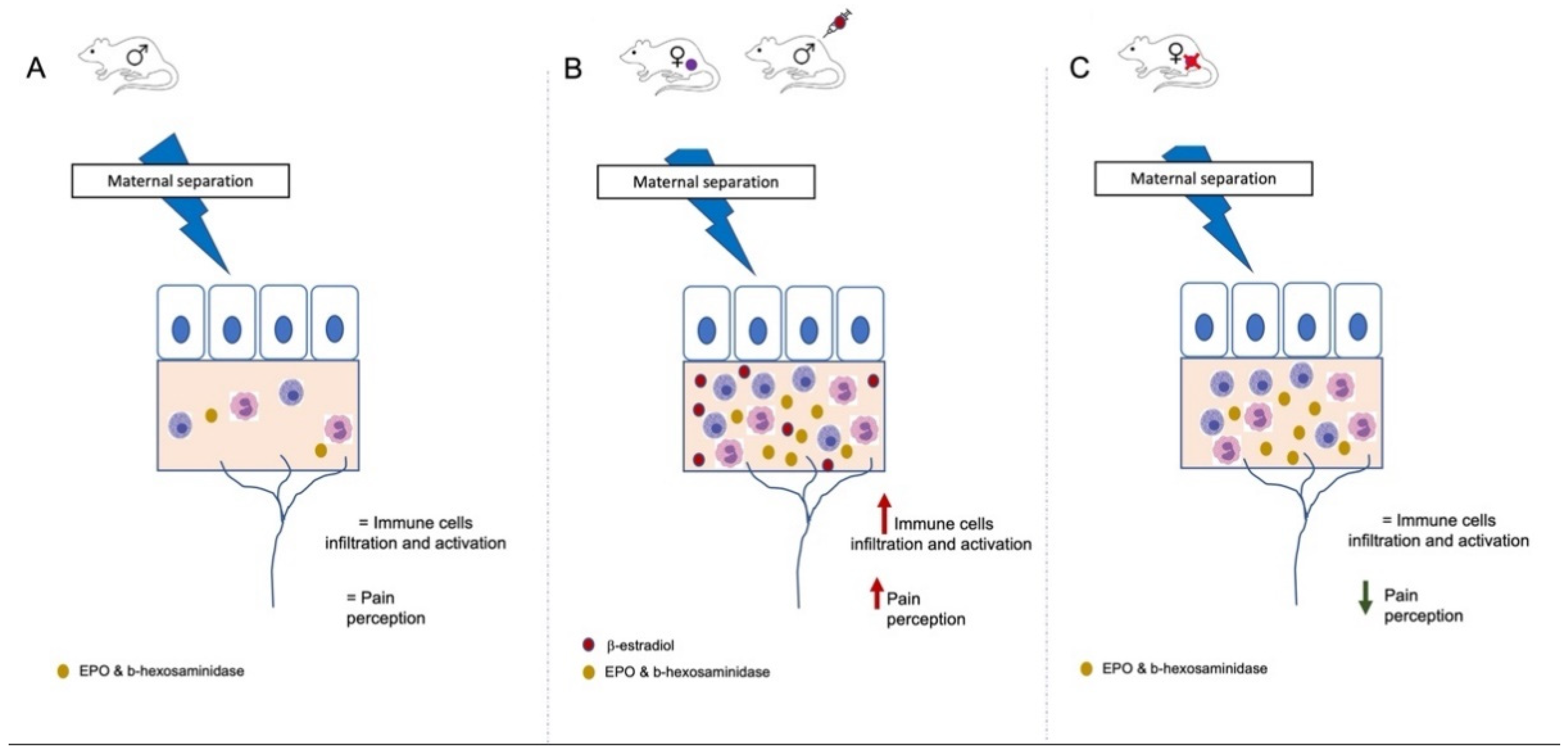
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nicholl, B.I.; Halder, S.L.; Macfarlane, G.J.; Thompson, D.G.; O’brien, S.; Musleh, M.; McBeth, J. Psychosocial risk markers for new onset irritable bowel syndrome--results of a large prospective population-based study. Pain 2008, 137, 147–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Creed, F. Review article: The incidence and risk factors for irritable bowel syndrome in population-based studies. Aliment. Pharmacol. Ther. 2019, 50, 507–516. [Google Scholar] [CrossRef] [PubMed]
- Moussa, L.; Bezirard, V.; Salvador-Cartier, C.; Bacquie, V.; Houdeau, E.; Theodorou, V. A new soy germ fermented ingredient displays estrogenic and protease inhibitor activities able to prevent irritable bowel syndrome-like symptoms in stressed female rats. Clin. Nutr. 2013, 32, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Wang, X.; Zhao, J.; Yang, S.; Dong, L.; Qin, B. GPER-mediated, oestrogen-dependent visceral hypersensitivity in stressed rats is associated with mast cell tryptase and histamine expression. Fundam. Clin. Pharmacol. 2020, 34, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Chaloner, A.; Greenwood-Van Meerveld, B. Sexually dimorphic effects of unpredictable early life adversity on visceral pain behavior in a rodent model. J. Pain 2013, 14, 270–280. [Google Scholar] [CrossRef]
- Whitehead, W.E.; Cheskin, L.J.; Heller, B.R.; Robinson, J.C.; Crowell, M.D.; Benjamin, C.; Schuster, M.M. Evidence for exacerbation of irritable bowel syndrome during menses. Gastroenterology 1990, 98, 1485–1489. [Google Scholar] [CrossRef]
- Ter Horst, G.J.; Wichmann, R.; Gerrits, M.; Westenbroek, C.; Lin, Y. Sex differences in stress responses: Focus on ovarian hormones. Physiol. Behav. 2009, 97, 239–249. [Google Scholar] [CrossRef]
- Bradesi, S.; Eutamene, H.; Garcia-Villar, R.; Fioramonti, J.; Bueno, L. Stress-induced visceral hypersensitivity in female rats is estrogen-dependent and involves tachykinin NK1 receptors. Pain 2003, 102, 227–234. [Google Scholar] [CrossRef]
- Bradesi, S.; Eutamene, H.; Fioramonti, J.; Bueno, L. Acute restraint stress activates functional NK1 receptor in the colon of female rats: Involvement of steroids. Gut 2002, 50, 349–354. [Google Scholar] [CrossRef]
- Knuesel, C.; Oulevey-Meier, M.; Flogerzi, B.; Krayer, M.; Gschossmann, I.; Miller, J.; Tovar, L.; Janko, S.; Gschossmann, J.M. Effect of estrogen on visceral sensory function in a non-inflammatory colonic hypersensitivity rat model. Neurogastroenterol. Motil. 2016, 28, 1570–1579. [Google Scholar] [CrossRef]
- Chen, J.; Li, Q.; Saliuk, G.; Bazhanov, S.; Winston, J.H. Estrogen and serotonin enhance stress-induced visceral hypersensitivity in female rats by up-regulating brain-derived neurotrophic factor in spinal cord. Neurogastroenterol. Motil. 2021, 33, e14117. [Google Scholar] [CrossRef] [PubMed]
- Qin, B.; Dong, L.; Guo, X.; Jiang, J.; He, Y.; Wang, X.; Li, L.; Zhao, J. Expression of G protein-coupled estrogen receptor in irritable bowel syndrome and its clinical significance. Int. J. Clin. Exp. Pathol. 2014, 7, 2238–2246. [Google Scholar] [PubMed]
- Li, Y.; Xu, J.; Jiang, F.; Jiang, Z.; Liu, C.; Li, L.; Luo, Y.; Lu, R.; Mu, Y.; Liu, Y.; et al. G protein-coupled estrogen receptor is involved in modulating colonic motor function via nitric oxide release in C57BL/6 female mice. Neurogastroenterol. Motil. 2016, 28, 432–442. [Google Scholar] [CrossRef] [PubMed]
- Straub, R.H. The complex role of estrogens in inflammation. Endocr. Rev. 2007, 28, 521–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanuytsel, T.; Vanormelingen, C.; Vanheel, H.; Masaoka, T.; Salim Rasoel, S.; Toth, J.; Houben, E.; Verbeke, K.; De Hertogh, G.; Vanden Berghe, P.; et al. From intestinal permeability to dysmotility: The biobreeding rat as a model for functional gastrointestinal disorders. PLoS ONE 2014, 9, e111132. [Google Scholar] [CrossRef]
- Meleine, M.; Accarie, A.; Wauters, L.; Toth, J.; Gourcerol, G.; Tack, J.; Farre, R.; Vanuytsel, T. Colonic hypersensitivity and low-grade inflammation in a spontaneous animal model for functional gastrointestinal disorders. Neurogastroenterol. Motil. 2019, 31, e13614. [Google Scholar] [CrossRef]
- Accarie, A.; Vanuytsel, T. Animal Models for Functional Gastrointestinal Disorders. Front. Psychiatry 2020, 11. [Google Scholar] [CrossRef]
- Cao, D.Y.; Bai, G.; Ji, Y.; Traub, R.J. Epigenetic upregulation of metabotropic glutamate receptor 2 in the spinal cord attenuates oestrogen-induced visceral hypersensitivity. Gut 2015, 64, 1913–1920. [Google Scholar] [CrossRef]
- Song, Y.; Yin, J.; Chang, H.; Zhou, Q.; Peng, H.; Ji, W.; Song, Q. Comparison of four staining methods for detecting eosinophils in nasal polyps. Sci. Rep. 2018, 8, 17718. [Google Scholar] [CrossRef]
- Zhu, T.H.; Ding, S.J.; Li, T.T.; Zhu, L.B.; Huang, X.F.; Zhang, X.M. Estrogen is an important mediator of mast cell activation in ovarian endometriomas. Reproduction 2018, 155, 73–83. [Google Scholar] [CrossRef]
- Kuehn, H.S.; Radinger, M.; Gilfillan, A.M. Measuring mast cell mediator release. Curr. Protoc. Immunol. 2010, 7, 381–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bao, A.M.; Hestiantoro, A.; Van Someren, E.J.; Swaab, D.F.; Zhou, J.N. Colocalization of corticotropin-releasing hormone and oestrogen receptor-alpha in the paraventricular nucleus of the hypothalamus in mood disorders. Brain 2005, 128 Pt 6, 1301–1313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camilleri, M. Sex as a biological variable in irritable bowel syndrome. Neurogastroenterol. Motil. 2020, 32, e13802. [Google Scholar] [CrossRef] [PubMed]
- Chaban, V. Estrogen modulation of visceral nociceptors. Curr. Trends Neurol. 2013, 7, 51–55. [Google Scholar] [PubMed]
- Zhang, L.; Song, J.; Bai, T.; Wang, R.; Hou, X. Sustained pain hypersensitivity in the stressed colon: Role of mast cell-derived nerve growth factor-mediated enteric synaptic plasticity. Neurogastroenterol. Motil. 2018, 30, e13430. [Google Scholar] [CrossRef]
- Vicario, M.; Guilarte, M.; Alonso, C.; Yang, P.; Martinez, C.; Ramos, L.; Lobo, B.; Gonzalez, A.; Guila, M.; Pigrau, M.; et al. Chronological assessment of mast cell-mediated gut dysfunction and mucosal inflammation in a rat model of chronic psychosocial stress. Brain Behav. Immun. 2010, 24, 1166–1175. [Google Scholar] [CrossRef]
- Wouters, M.M.; Vicario, M.; Santos, J. The role of mast cells in functional GI disorders. Gut 2016, 65, 155–168. [Google Scholar] [CrossRef] [Green Version]
- Oertelt-Prigione, S. The influence of sex and gender on the immune response. Autoimmun. Rev. 2012, 11, A479–A485. [Google Scholar] [CrossRef]
- Jacenik, D.; Cygankiewicz, A.I.; Fichna, J.; Mokrowiecka, A.; Malecka-Panas, E.; Krajewska, W.M. Estrogen signaling deregulation related with local immune response modulation in irritable bowel syndrome. Mol. Cell Endocrinol. 2018, 471, 89–96. [Google Scholar] [CrossRef]
- Zielinska, M.; Fichna, J.; Bashashati, M.; Habibi, S.; Sibaev, A.; Timmermans, J.P.; Storr, M. G protein-coupled estrogen receptor and estrogen receptor ligands regulate colonic motility and visceral pain. Neurogastroenterol. Motil. 2017, 29. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, X.; Kong, Y.; Gou, L.; Lian, B.; Wang, Y.; Jiang, L.; Li, Q.; Sun, H.; Sun, L. Maternal Separation-Induced Histone Acetylation Correlates with BDNF-Programmed Synaptic Changes in an Animal Model of PTSD with Sex Differences. Mol. Neurobiol. 2021, 58, 1738–1754. [Google Scholar] [CrossRef] [PubMed]
- Kember, R.L.; Dempster, E.L.; Lee, T.H.A.; Schalkwyk, L.C.; Mill, J.; Fernandes, C. Maternal separation is associated with strain-specific responses to stress and epigenetic alterations to Nr3c1, Avp, and Nr4a1 in mouse. Brain Behav. 2012, 2, 455–467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Gong, X.; Yang, X.; Shang, X.; Du, Q.; Liao, Q.; Xie, R.; Chen, Y.; Xu, J. The roles of estrogen and estrogen receptors in gastrointestinal disease. Oncol. Lett. 2019, 18, 5673–5680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masuda, A.; Yoshikai, Y.; Aiba, K.; Matsuguchi, T. Th2 Cytokine Production from Mast Cells Is Directly Induced by Lipopolysaccharide and Distinctly Regulated by c-Jun N-Terminal Kinase and p38 Pathways. J. Immunol. 2002, 169, 3801. [Google Scholar] [CrossRef] [Green Version]
- Moloney, R.D.; Johnson, A.C.; O’Mahony, S.M.; Dinan, T.G.; Greenwood-Van Meerveld, B.; Cryan, J.F. Stress and the Microbiota-Gut-Brain Axis in Visceral Pain: Relevance to Irritable Bowel Syndrome. CNS Neurosci. Ther. 2016, 22, 102–117. [Google Scholar] [CrossRef]
- Sun, M.; He, C.; Cong, Y.; Liu, Z. Regulatory immune cells in regulation of intestinal inflammatory response to microbiota. Mucosal Immunol. 2015, 8, 969–978. [Google Scholar] [CrossRef]


| Gene | Males Vehicle | Males β-Estradiol | p-Value | Females Sham | Females Ovx | p-Value |
|---|---|---|---|---|---|---|
| F2rl1 | 1.3 ± 0.4 n = 6 | 2.4 ± 0.3 n = 6 | 0.05 | 0.7 (0.4–4.1) n = 5 | 0.9 (0.2–5.3) n = 7 | 0.87 |
| mcpt2 | 1.3 ± 0.5 n = 5 | 2.4 ± 0.5 n = 7 | 0.17 | 2 ± 0.99 n = 6 | 10.8 ± 4 n = 7 | 0.24 |
| ccl2 | 2.3 (0.1–6.1) n = 5 | 1 (0.1–1.8) n = 6 | 0.32 | 1.4 (0.1–9.9) n = 4 | 2 (0.9–4.5) n = 6 | 0.76 |
| ccl11 | 2.3 (0.1–4.3) n = 6 | 5.1 (4–6.1) n = 7 | 0.02 | 1.4 ± 0.6 n = 5 | 3.5 ± 1.2 n = 8 | 0.15 |
| il4 | 0.43 (0.05–2.4) n = 4 | 6.8 (1.3–8.8) n = 3 | 0.1 | 0.2 (0.1–0.3) n = 5 | 0.06 (0–4.6) n = 6 | 0.83 |
| Gene | Males Vehicle n = 4 | Males β-Estradiol n = 7 | p-Value | Females Sham n = 6–7 | Females Ovx n = 7 | p-Value |
|---|---|---|---|---|---|---|
| F2rl1 | 1 (0.7–1.3) | 1.4 (0.5–2) | 0.31 | 1.1 ± 0.2 | 0.8 ± 0.2 | 0.28 |
| mcpt2 | 1 (0.8–1.1) | 4.1 (3.1–18.9) | 0.07 | 1.7 ± 0.6 | 0.7 ± 0.2 | 0.15 |
| ccl2 | 0.8 (0.7–1.6) | 3.6 (0.3–10.4) | 0.31 | 1.6 ± 0.6 | 4.8 ± 2.6 | 0.28 |
| ccl11 | 1.1 (0.6–1.4) | 1 (0.7–2.4) | 0.99 | 1.7 ± 0.7 | 0.6 ± 0.2 | 0.18 |
| Females | Males | |||||
|---|---|---|---|---|---|---|
| Group | NH | MS | MS Sham | MS Ovx | MS Vehicle | MS β-Estradiol |
| jejunum | 1 ± 0.3 n = 6 | 2.4 ± 0.4 n = 7 | 1.3 ± 0.4 n = 7 | 1.6 ± 0.6 n = 7 | 0.9(0.4–3.7) n = 6 | 0.7(0.3–2.2) n = 7 |
| p-value | 0.03 | 0.66 | 0.73 | |||
| colon | 0.7(0.3–0.7) n = 5 | 0.5(0.1–2.9) n = 7 | 1.1 ± 0.2 n = 7 | 0.4 ± 0.2 n = 6 | 0.9 ± 0.3 n = 5 | 2.2 ± 0.6 n = 6 |
| p-value | 0.99 | 0.05 | 0.24 | |||
| Correlation with VMR AUC | r = −0.06 p = 0.82 | r = 0.58 p = 0.01 | r = 0.26 p = 0.42 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Accarie, A.; Toth, J.; Wauters, L.; Farré, R.; Tack, J.; Vanuytsel, T. Estrogens Play a Critical Role in Stress-Related Gastrointestinal Dysfunction in a Spontaneous Model of Disorders of Gut–Brain Interaction. Cells 2022, 11, 1214. https://doi.org/10.3390/cells11071214
Accarie A, Toth J, Wauters L, Farré R, Tack J, Vanuytsel T. Estrogens Play a Critical Role in Stress-Related Gastrointestinal Dysfunction in a Spontaneous Model of Disorders of Gut–Brain Interaction. Cells. 2022; 11(7):1214. https://doi.org/10.3390/cells11071214
Chicago/Turabian StyleAccarie, Alison, Joran Toth, Lucas Wauters, Ricard Farré, Jan Tack, and Tim Vanuytsel. 2022. "Estrogens Play a Critical Role in Stress-Related Gastrointestinal Dysfunction in a Spontaneous Model of Disorders of Gut–Brain Interaction" Cells 11, no. 7: 1214. https://doi.org/10.3390/cells11071214
APA StyleAccarie, A., Toth, J., Wauters, L., Farré, R., Tack, J., & Vanuytsel, T. (2022). Estrogens Play a Critical Role in Stress-Related Gastrointestinal Dysfunction in a Spontaneous Model of Disorders of Gut–Brain Interaction. Cells, 11(7), 1214. https://doi.org/10.3390/cells11071214






