5-Azacytidine Inhibits the Activation of Senescence Program and Promotes Cytotoxic Autophagy during Trdmt1-Mediated Oxidative Stress Response in Insulinoma β-TC-6 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines and Culture Conditions
2.2. Cell Number
2.3. Cell Cycle
2.4. Senescence-Associated β-Galactosidase Activity (SA-β-Gal)
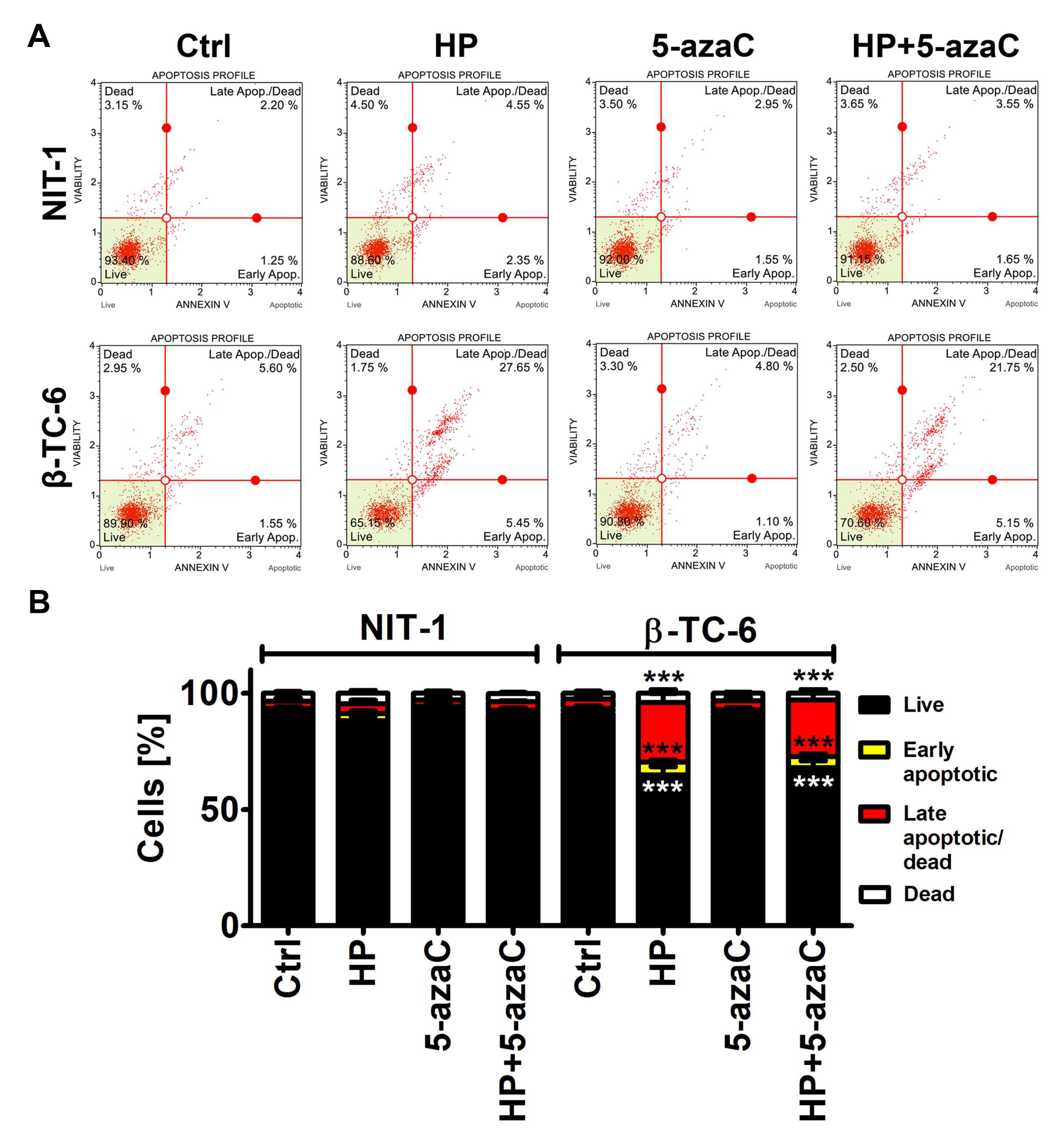
2.5. Apoptosis
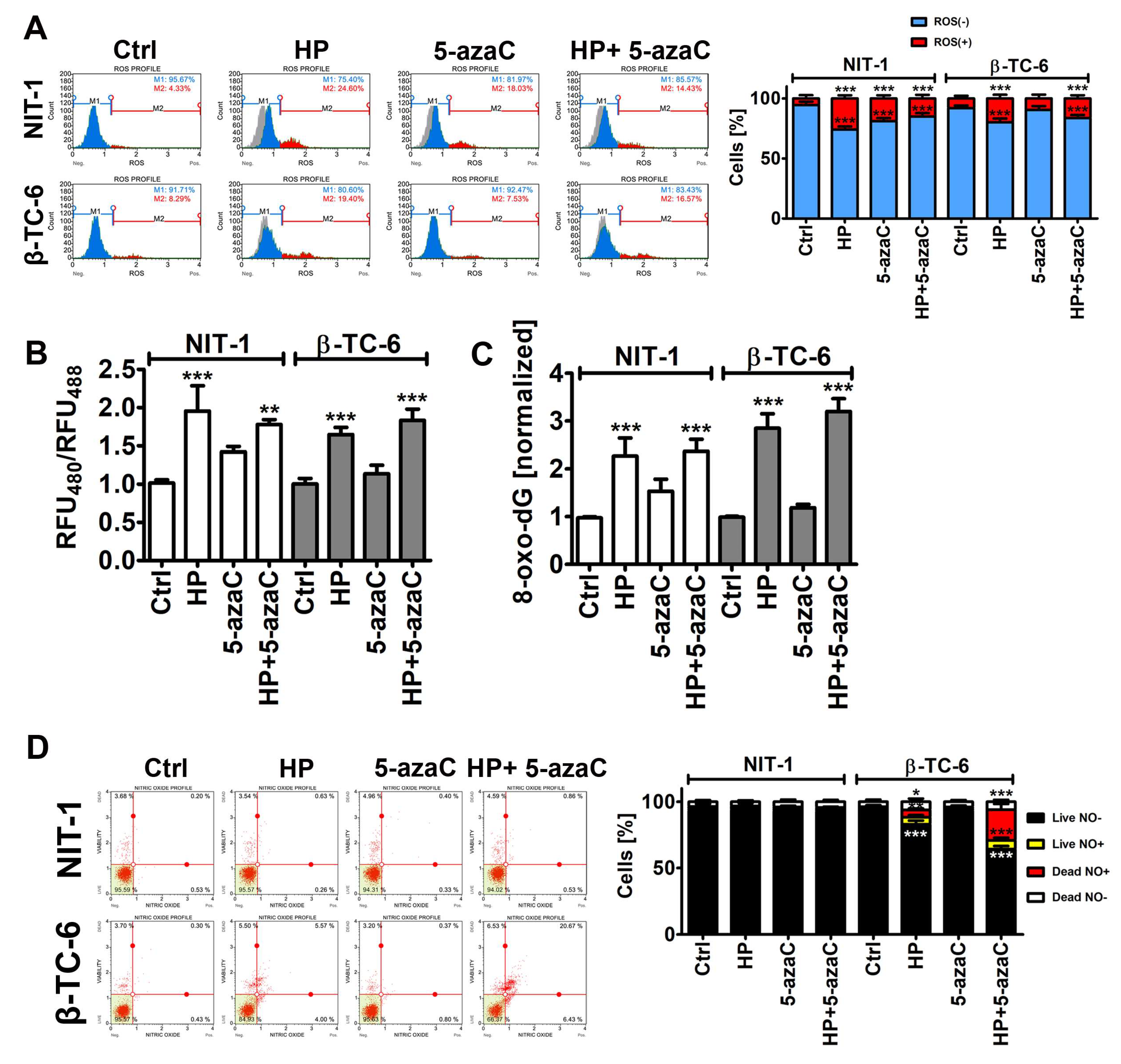
2.6. Oxidative Stress Parameters
2.7. Nitric Oxide Levels
2.8. RNA Methylation
2.9. Immunofluorescence Using Imaging Flow Cytometry
2.10. Statistical Analysis
3. Results and Discussion
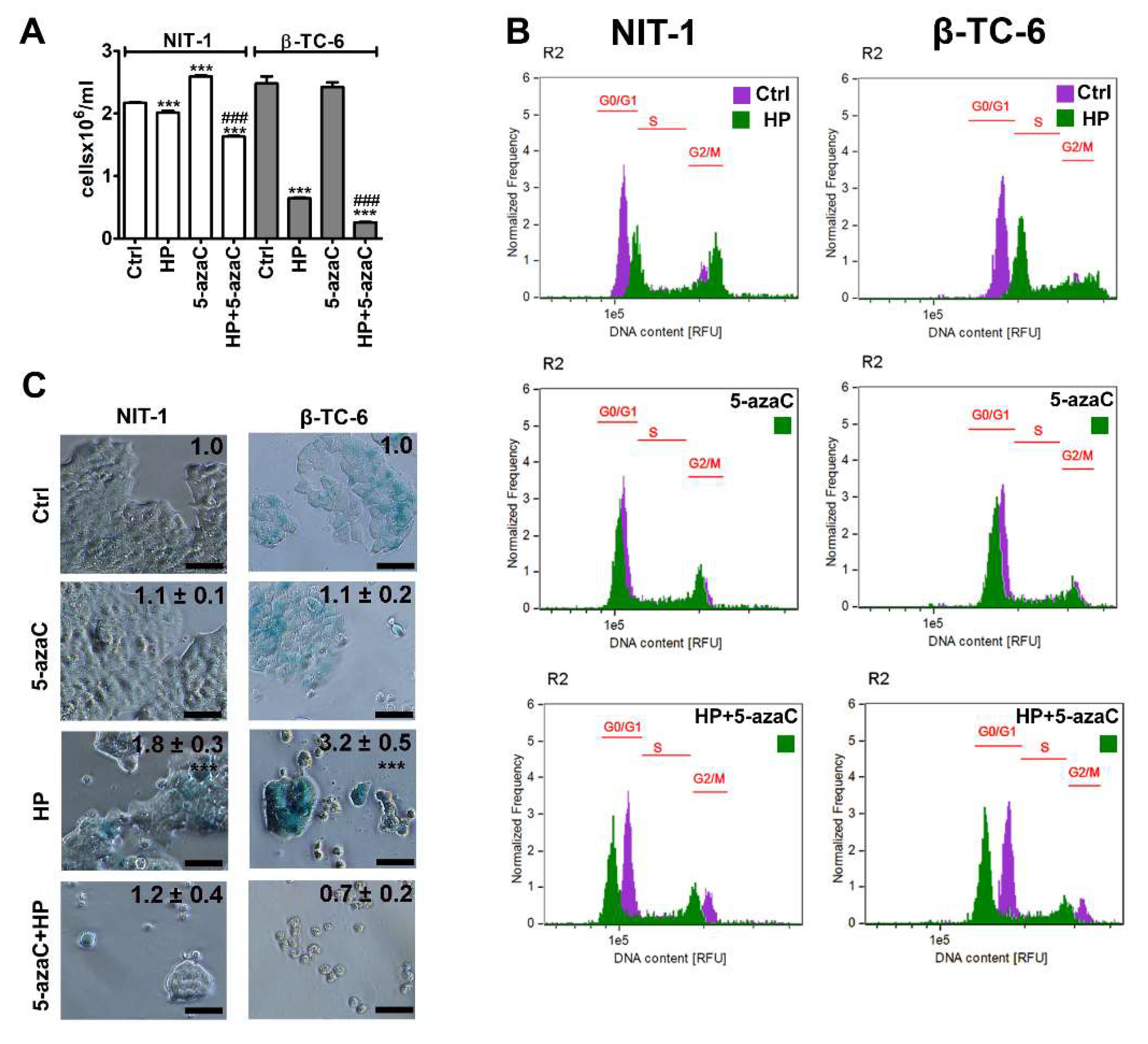
3.1. 5-AzaC Attenuates the Development of HP-Induced Senescent Phenotype in Insulinoma Cells
3.2. 5-AzaC Potentiates Nitric Oxide-Mediated Necrotic Cell Death in HP-Treated β-TC-6 Cells
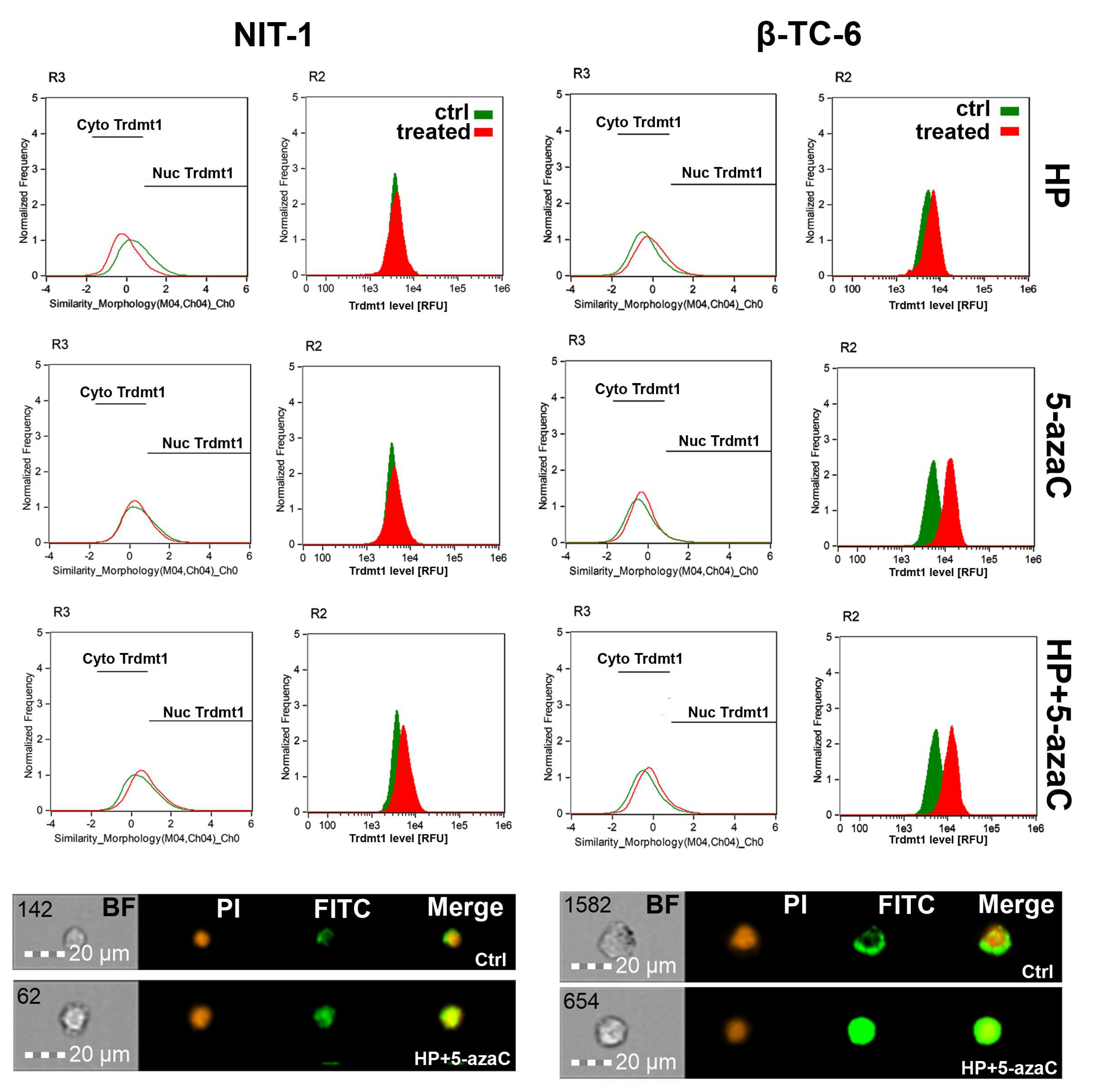
3.3. Trdmt1 Is Increased after HP and 5-azaC Post-Treatment in β-TC-6 Cells
3.4. 5-AzaC Stimulation Promotes Cytotoxic Autophagy in HP-Stressed β-TC-6 Cells
3.5. HP-Induced Oxidative Stress Promotes RNA Methylation in Insulinoma Cells
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Okabayashi, T. Diagnosis and Management of Insulinoma. World J. Gastroenterol. 2013, 19, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Eichelberger, G.S.; Carbono, J.; Field, Z.; Kainaur, K.; Montalvo, F. Case Report and Literature Review of Insulinoma in the Geriatric Population: An 86-Year-Old Female with Syncope of Unknown Origin. Case Rep. Endocrinol. 2020, 2020, 8879776. [Google Scholar] [CrossRef] [PubMed]
- Service, F.J.; McMahon, M.M.; O’Brien, P.C.; Ballard, D.J. Functioning Insulinoma—Incidence, Recurrence, and Long-Term Survival of Patients: A 60-Year Study. Mayo Clin. Proc. 1991, 66, 711–719. [Google Scholar] [CrossRef]
- Peltola, E.; Hannula, P.; Huhtala, H.; Metso, S.; Kiviniemi, U.; Vornanen, M.; Sand, J.; Laukkarinen, J.; Tiikkainen, M.; Schalin-Jäntti, C.; et al. Characteristics and Outcomes of 79 Patients with an Insulinoma: A Nationwide Retrospective Study in Finland. Int. J. Endocrinol. 2018, 2018, 2059481. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikfarjam, M.; Warshaw, A.L.; Axelrod, L.; Deshpande, V.; Thayer, S.P.; Ferrone, C.R.; Fernández-del Castillo, C. Improved Contemporary Surgical Management of Insulinomas: A 25-Year Experience at the Massachusetts General Hospital. Ann. Surg. 2008, 247, 165–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagel, A.F.; Hagel, W.H.; Lindner, A.S.; Kammerer, F.J.; Neurath, M.F.; Konturek, P.C.; Harsch, I.A. Metastatic Insulinoma—Prolonged Survival after Multimodal Approach. Med. Sci. Monit. Int. Med. J. Exp. Clin. Res. 2011, 17, CS103–CS107. [Google Scholar] [CrossRef] [Green Version]
- Keen, F.; Iqbal, F.; Owen, P.; Christian, A.; Kumar, N.; Kalhan, A. Metastatic Insulinoma Presenting 14 Years after Benign Tumour Resection: A Rare Case and Management Dilemma. Endocrinol. Diabetes Metab. Case Rep. 2020, 2020, 20–0065. [Google Scholar] [CrossRef]
- AlJadir, S. Insulinoma: Literature’s Review (2). Endocrinol. Int. J. 2015, 2, 152–164. [Google Scholar] [CrossRef]
- Giannis, D.; Moris, D.; Karachaliou, G.S.; Tsilimigras, D.; Karaolanis, G.; Papalampros, A.; Felekouras, E. Insulinomas: From Diagnosis to Treatment. A Review of the Literature. J. BUON Off. J. Balk. Union Oncol. 2020, 25, 1302–1314. [Google Scholar]
- Bielak-Zmijewska, A.; Wnuk, M.; Przybylska, D.; Grabowska, W.; Lewinska, A.; Alster, O.; Korwek, Z.; Cmoch, A.; Myszka, A.; Pikula, S.; et al. A Comparison of Replicative Senescence and Doxorubicin-Induced Premature Senescence of Vascular Smooth Muscle Cells Isolated from Human Aorta. Biogerontology 2014, 15, 47–64. [Google Scholar] [CrossRef] [Green Version]
- Bloniarz, D.; Adamczyk-Grochala, J.; Lewinska, A.; Wnuk, M. The Lack of Functional DNMT2/TRDMT1 Gene Modulates Cancer Cell Responses during Drug-Induced Senescence. Aging 2021, 13, 15833–15874. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Kohli, J.; Demaria, M. Senescent Cells in Cancer Therapy: Friends or Foes? Trends Cancer 2020, 6, 838–857. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-W.; Zhang, D.; Yu, B.-P. Senescent Cells in Cancer Therapy: Why and How to Remove Them. Cancer Lett. 2021, 520, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Coppé, J.-P.; Desprez, P.-Y.; Krtolica, A.; Campisi, J. The Senescence-Associated Secretory Phenotype: The Dark Side of Tumor Suppression. Annu. Rev. Pathol. Mech. Dis. 2010, 5, 99–118. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Pitcher, L.E.; Prahalad, V.; Niedernhofer, L.J.; Robbins, P.D. Recent Advances in the Discovery of Senolytics. Mech. Ageing Dev. 2021, 200, 111587. [Google Scholar] [CrossRef]
- Li, N.; Liu, F.; Yang, P.; Xiong, F.; Yu, Q.; Li, J.; Zhou, Z.; Zhang, S.; Wang, C.-Y. Aging and Stress Induced β Cell Senescence and Its Implication in Diabetes Development. Aging 2019, 11, 9947–9959. [Google Scholar] [CrossRef] [PubMed]
- Lewinska, A.; Adamczyk-Grochala, J.; Kwasniewicz, E.; Deregowska, A.; Semik, E.; Zabek, T.; Wnuk, M. Reduced Levels of Methyltransferase DNMT2 Sensitize Human Fibroblasts to Oxidative Stress and DNA Damage That Is Accompanied by Changes in Proliferation-Related MiRNA Expression. Redox Biol. 2018, 14, 20–34. [Google Scholar] [CrossRef]
- Lewinska, A.; Adamczyk-Grochala, J.; Bloniarz, D.; Olszowka, J.; Kulpa-Greszta, M.; Litwinienko, G.; Tomaszewska, A.; Wnuk, M.; Pazik, R. AMPK-Mediated Senolytic and Senostatic Activity of Quercetin Surface Functionalized Fe3O4 Nanoparticles during Oxidant-Induced Senescence in Human Fibroblasts. Redox Biol. 2020, 28, 101337. [Google Scholar] [CrossRef]
- Antoniak, M.A.; Pązik, R.; Bazylińska, U.; Wiwatowski, K.; Tomaszewska, A.; Kulpa-Greszta, M.; Adamczyk-Grochala, J.; Wnuk, M.; Maćkowski, S.; Lewińska, A.; et al. Multimodal Polymer Encapsulated CdSe/Fe3O4 Nanoplatform with Improved Biocompatibility for Two-Photon and Temperature Stimulated Bioapplications. Mater. Sci. Eng. C 2021, 127, 112224. [Google Scholar] [CrossRef]
- Sodagam, L.; Lewinska, A.; Kwasniewicz, E.; Kokhanovska, S.; Wnuk, M.; Siems, K.; Rattan, S.I.S. Phytochemicals Rosmarinic Acid, Ampelopsin, and Amorfrutin-A Can Modulate Age-Related Phenotype of Serially Passaged Human Skin Fibroblasts In Vitro. Front. Genet. 2019, 10. [Google Scholar] [CrossRef]
- Lewinska, A.; Adamczyk-Grochala, J.; Deregowska, A.; Wnuk, M. Sulforaphane-Induced Cell Cycle Arrest and Senescence Are Accompanied by DNA Hypomethylation and Changes in MicroRNA Profile in Breast Cancer Cells. Theranostics 2017, 7, 3461–3477. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, M.; Hagemann, S.; Hanna, K.; Lyko, F. Azacytidine Inhibits RNA Methylation at DNMT2 Target Sites in Human Cancer Cell Lines. Cancer Res. 2009, 69, 8127–8132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venturelli, S.; Berger, A.; Weiland, T.; Essmann, F.; Waibel, M.; Nuebling, T.; Häcker, S.; Schenk, M.; Schulze-Osthoff, K.; Salih, H.R.; et al. Differential Induction of Apoptosis and Senescence by the DNA Methyltransferase Inhibitors 5-Azacytidine and 5-Aza-2′-Deoxycytidine in Solid Tumor Cells. Mol. Cancer Ther. 2013, 12, 2226–2236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsieh, Y.-Y.; Huang, T.-C.; Lo, H.-L.; Jhan, J.-Y.; Chen, S.-T.; Yang, P.-M. Systematic Discovery of Drug Action Mechanisms by an Integrated Chemical Genomics Approach: Identification of Functional Disparities between Azacytidine and Decitabine. Oncotarget 2016, 7, 27363–27378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schnekenburger, M.; Grandjenette, C.; Ghelfi, J.; Karius, T.; Foliguet, B.; Dicato, M.; Diederich, M. Sustained Exposure to the DNA Demethylating Agent, 2′-Deoxy-5-Azacytidine, Leads to Apoptotic Cell Death in Chronic Myeloid Leukemia by Promoting Differentiation, Senescence, and Autophagy. Biochem. Pharmacol. 2011, 81, 364–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandra, J. Oxidative Stress by Targeted Agents Promotes Cytotoxicity in Hematologic Malignancies. Antioxid. Redox Signal. 2009, 11, 1123–1137. [Google Scholar] [CrossRef] [Green Version]
- Tian, E.; Tang, H.; Xu, R.; Liu, C.; Deng, H.; Wang, Q. Azacytidine Induces Necrosis of Multiple Myeloma Cells through Oxidative Stress. Proteome Sci. 2013, 11, 24. [Google Scholar] [CrossRef] [Green Version]
- Gao, S.; Mobley, A.; Miller, C.; Boklan, J.; Chandra, J. Potentiation of Reactive Oxygen Species Is a Marker for Synergistic Cytotoxicity of MS-275 and 5-Azacytidine in Leukemic Cells. Leuk. Res. 2008, 32, 771–780. [Google Scholar] [CrossRef] [Green Version]
- Gleneadie, H.J.; Baker, A.H.; Batis, N.; Bryant, J.; Jiang, Y.; Clokie, S.J.H.; Mehanna, H.; Garcia, P.; Gendoo, D.M.A.; Roberts, S.; et al. The Anti-Tumour Activity of DNA Methylation Inhibitor 5-Aza-2′-Deoxycytidine Is Enhanced by the Common Analgesic Paracetamol through Induction of Oxidative Stress. Cancer Lett. 2021, 501, 172–186. [Google Scholar] [CrossRef]
- Cvetkovic, I.; Popadic, D.; Vuckovic, O.; Harhaji, L.; Miljkovic, D.; Trajkovic, V. 5-Aza-2′-Deoxycytidine Stimulates Inducible Nitric Oxide Synthase Induction in C6 Astrocytoma Cells. Brain Res. 2004, 998, 83–90. [Google Scholar] [CrossRef]
- Miljkovic, D.; Cvetkovic, I.; Sajic, M.; Vuckovic, O.; Harhaji, L.; Markovic, M.; Trajkovic, V. 5-Aza-2′-Deoxycytidine and Paclitaxel Inhibit Inducible Nitric Oxide Synthase Activation in Fibrosarcoma Cells. Eur. J. Pharmacol. 2004, 485, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Goll, M.G.; Kirpekar, F.; Maggert, K.A.; Yoder, J.A.; Hsieh, C.-L.; Zhang, X.; Golic, K.G.; Jacobsen, S.E.; Bestor, T.H. Methylation of tRNAAsp by the DNA Methyltransferase Homolog Dnmt2. Science 2006, 311, 395–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaefer, M.; Lyko, F. Solving the Dnmt2 Enigma. Chromosoma 2010, 119, 35–40. [Google Scholar] [CrossRef]
- Tuorto, F.; Liebers, R.; Musch, T.; Schaefer, M.; Hofmann, S.; Kellner, S.; Frye, M.; Helm, M.; Stoecklin, G.; Lyko, F. RNA Cytosine Methylation by Dnmt2 and NSun2 Promotes TRNA Stability and Protein Synthesis. Nat. Struct. Mol. Biol. 2012, 19, 900–905. [Google Scholar] [CrossRef] [PubMed]
- Jeltsch, A.; Ehrenhofer-Murray, A.; Jurkowski, T.P.; Lyko, F.; Reuter, G.; Ankri, S.; Nellen, W.; Schaefer, M.; Helm, M. Mechanism and Biological Role of Dnmt2 in Nucleic Acid Methylation. RNA Biol. 2017, 14, 1108–1123. [Google Scholar] [CrossRef] [Green Version]
- Lewinska, A.; Adamczyk-Grochala, J.; Kwasniewicz, E.; Wnuk, M. Downregulation of Methyltransferase Dnmt2 Results in Condition-Dependent Telomere Shortening and Senescence or Apoptosis in Mouse Fibroblasts. J. Cell. Physiol. 2017, 232, 3714–3726. [Google Scholar] [CrossRef]
- Xue, S.; Xu, H.; Sun, Z.; Shen, H.; Chen, S.; Ouyang, J.; Zhou, Q.; Hu, X.; Cui, H. Depletion of TRDMT1 Affects 5-Methylcytosine Modification of MRNA and Inhibits HEK293 Cell Proliferation and Migration. Biochem. Biophys. Res. Commun. 2019, 520, 60–66. [Google Scholar] [CrossRef]
- Chen, H.; Yang, H.; Zhu, X.; Yadav, T.; Ouyang, J.; Truesdell, S.S.; Tan, J.; Wang, Y.; Duan, M.; Wei, L.; et al. M5C Modification of MRNA Serves a DNA Damage Code to Promote Homologous Recombination. Nat. Commun. 2020, 11, 2834. [Google Scholar] [CrossRef]
- Betlej, G.; Lewińska, A.; Adamczyk-Grochala, J.; Błoniarz, D.; Rzeszutek, I.; Wnuk, M. Deficiency of TRDMT1 Impairs Exogenous RNA-Based Response and Promotes Retrotransposon Activity during Long-Term Culture of Osteosarcoma Cells. Toxicol. In Vitro 2022, 80, 105323. [Google Scholar] [CrossRef]
- Thiagarajan, D.; Dev, R.R.; Khosla, S. The DNA Methyltranferase Dnmt2 Participates in RNA Processing during Cellular Stress. Epigenetics 2011, 6, 103–113. [Google Scholar] [CrossRef]
- Gewirtz, D.A. The Four Faces of Autophagy: Implications for Cancer Therapy. Cancer Res. 2014, 74, 647–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gewirtz, D.A. An Autophagic Switch in the Response of Tumor Cells to Radiation and Chemotherapy. Biochem. Pharmacol. 2014, 90, 208–211. [Google Scholar] [CrossRef] [PubMed]
- Cluzeau, T.; Robert, G.; Puissant, A.; Jean-Michel, K.; Cassuto, J.-P.; Raynaud, S.; Auberger, P. Azacitidine-Resistant SKM1 Myeloid Cells Are Defective for AZA-Induced Mitochondrial Apoptosis and Autophagy. Cell Cycle 2011, 10, 2339–2343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Humeau, J.; Leduc, M.; Cerrato, G.; Loos, F.; Kepp, O.; Kroemer, G. Phosphorylation of Eukaryotic Initiation Factor-2α (EIF2α) in Autophagy. Cell Death Dis. 2020, 11, 433. [Google Scholar] [CrossRef]
- He, L.; Li, H.; Wu, A.; Peng, Y.; Shu, G.; Yin, G. Functions of N6-Methyladenosine and Its Role in Cancer. Mol. Cancer 2019, 18, 176. [Google Scholar] [CrossRef] [Green Version]
- Liang, Z.; Kidwell, R.L.; Deng, H.; Xie, Q. Epigenetic N6-Methyladenosine Modification of RNA and DNA Regulates Cancer. Cancer Biol. Med. 2020, 17, 9–19. [Google Scholar] [CrossRef]
- Liu, L.; Li, H.; Hu, D.; Wang, Y.; Shao, W.; Zhong, J.; Yang, S.; Liu, J.; Zhang, J. Insights into N6-Methyladenosine and Programmed Cell Death in Cancer. Mol. Cancer 2022, 21, 32. [Google Scholar] [CrossRef]
- Jin, S.; Zhang, X.; Miao, Y.; Liang, P.; Zhu, K.; She, Y.; Wu, Y.; Liu, D.-A.; Huang, J.; Ren, J.; et al. M6A RNA Modification Controls Autophagy through Upregulating ULK1 Protein Abundance. Cell Res. 2018, 28, 955–957. [Google Scholar] [CrossRef]
- Wang, X.; Wu, R.; Liu, Y.; Zhao, Y.; Bi, Z.; Yao, Y.; Liu, Q.; Shi, H.; Wang, F.; Wang, Y. M6A MRNA Methylation Controls Autophagy and Adipogenesis by Targeting Atg5 and Atg7. Autophagy 2020, 16, 1221–1235. [Google Scholar] [CrossRef]
- Chen, X.; Wang, J.; Tahir, M.; Zhang, F.; Ran, Y.; Liu, Z.; Wang, J. Current Insights into the Implications of M6A RNA Methylation and Autophagy Interaction in Human Diseases. Cell Biosci. 2021, 11, 147. [Google Scholar] [CrossRef]
- Paramasivam, A.; Priyadharsini, J.V. RNA N6-Methyladenosine: A New Player in Autophagy-Mediated Anti-Cancer Drug Resistance. Br. J. Cancer 2021, 124, 1621–1622. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Niu, Y.; Wan, A.; Chen, D.; Liang, H.; Chen, X.; Sun, L.; Zhan, S.; Chen, L.; Cheng, C.; et al. RNA m6A Methylation Regulates Sorafenib Resistance in Liver Cancer through FOXO 3-mediated Autophagy. EMBO J. 2020, 39, e103181. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, Q.; Li, G.; Zhang, Q.; Zhuo, L.; Han, X.; Zhang, M.; Chen, X.; Pan, T.; Yan, L.; et al. The Mechanism of M6A Methyltransferase METTL3-Mediated Autophagy in Reversing Gefitinib Resistance in NSCLC Cells by β-Elemene. Cell Death Dis. 2020, 11, 969. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filip, K.; Lewińska, A.; Adamczyk-Grochala, J.; Marino Gammazza, A.; Cappello, F.; Lauricella, M.; Wnuk, M. 5-Azacytidine Inhibits the Activation of Senescence Program and Promotes Cytotoxic Autophagy during Trdmt1-Mediated Oxidative Stress Response in Insulinoma β-TC-6 Cells. Cells 2022, 11, 1213. https://doi.org/10.3390/cells11071213
Filip K, Lewińska A, Adamczyk-Grochala J, Marino Gammazza A, Cappello F, Lauricella M, Wnuk M. 5-Azacytidine Inhibits the Activation of Senescence Program and Promotes Cytotoxic Autophagy during Trdmt1-Mediated Oxidative Stress Response in Insulinoma β-TC-6 Cells. Cells. 2022; 11(7):1213. https://doi.org/10.3390/cells11071213
Chicago/Turabian StyleFilip, Kamila, Anna Lewińska, Jagoda Adamczyk-Grochala, Antonella Marino Gammazza, Francesco Cappello, Marianna Lauricella, and Maciej Wnuk. 2022. "5-Azacytidine Inhibits the Activation of Senescence Program and Promotes Cytotoxic Autophagy during Trdmt1-Mediated Oxidative Stress Response in Insulinoma β-TC-6 Cells" Cells 11, no. 7: 1213. https://doi.org/10.3390/cells11071213
APA StyleFilip, K., Lewińska, A., Adamczyk-Grochala, J., Marino Gammazza, A., Cappello, F., Lauricella, M., & Wnuk, M. (2022). 5-Azacytidine Inhibits the Activation of Senescence Program and Promotes Cytotoxic Autophagy during Trdmt1-Mediated Oxidative Stress Response in Insulinoma β-TC-6 Cells. Cells, 11(7), 1213. https://doi.org/10.3390/cells11071213









