How Cargo Identity Alters the Uptake of Cell-Penetrating Peptide (CPP)/Cargo Complexes: A Study on the Effect of Net Cargo Charge and Length
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Peptide Synthesis and Purification
2.3. Circular Dichroism
2.4. Cell Culture and Lysate Generation
2.5. Quantification of CPP and CPP/Cargo Uptake
2.6. Cell Viability Assay
2.7. Fluorescent Microscopy
3. Results and Discussion
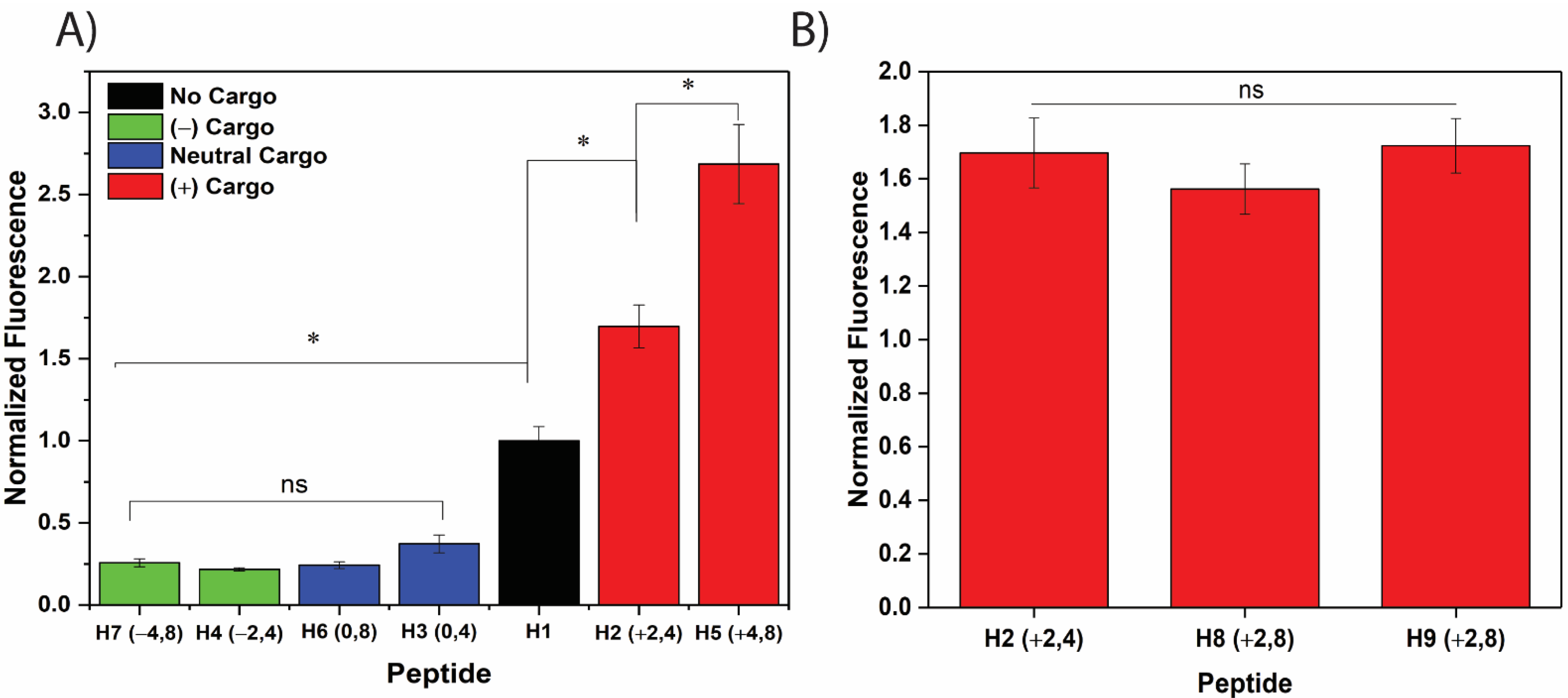
3.1. Net Positive Cargo Charge Enhances the Cellular Uptake of the CPP/Cargo Complex
3.2. Entry of the CPP/Cargo Complex into Intact Cells Does Not Significantly Diminish Cellular Viability
3.3. CPP/Cargo Complexes Exhibited a Non-Uniform Fluorescence Distribution in Intact HeLa Cells
3.4. Net Cargo Charge, but Not Net Cargo Length, Enhance CPP/Cargo Complex Uptake in Both Structured and Unstructured CPPs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- De Jong, H.; Bonger, K.M.; Lowik, D.W.P.M. Activatable Cell-Penetrating Peptides: 15 Years of Research. RSC Chem. Biol. 2020, 1, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Park, S.E.; Sajid, M.I.; Parang, K.; Tiwari, R.K. Cyclic Cell-Penetrating Peptides as Efficient Intracellular Drug Delivery Tools. Mol. Pharm. 2019, 16, 3727–3743. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Wang, Y.; Zhang, X.; Zhang, W.; Guo, S.; Jin, F. Recent progress of cell-penetrating peptides as new carriers for intracellular cargo delivery. J. Control. Release 2014, 174, 126–136. [Google Scholar] [CrossRef]
- Borrelli, A.; Tornesello, A.L.; Tornesello, M.L.; Buonaguro, F.M. Cell Penetrating Peptides as Molecular Carriers for Anti-Cancer Agents. Molecules 2018, 23, 295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurrikoff, K.; Vunk, B.; Langel, U. Status update in the use of cell-penetrating peptides for the delivery of macromolecular therapeutics. Expert Opin. Biol. Ther. 2021, 21, 361–370. [Google Scholar] [CrossRef]
- Dinca, A.; Chien, W.M.; Chin, M.T. Intracellular Delivery of Proteins with Cell-Penetrating Peptides for Therapeutic Uses in Human Disease. Int. J. Mol. Sci. 2016, 17, 263. [Google Scholar] [CrossRef]
- Liao, H.W.; Li, X.; Zhao, L.Z.; Wang, Y.L.; Wang, X.D.; Wu, Y.; Zhou, X.; Fu, W.; Liu, L.; Hu, H.G.; et al. A PROTAC peptide induces durable beta-catenin degradation and suppresses Wnt-dependent intestinal cancer. Cell Discov. 2020, 6, 1–12. [Google Scholar] [CrossRef]
- McCarthy, H.O.; McCaffrey, J.; McCrudden, C.M.; Zholobenko, A.; Ali, A.A.; McBride, J.W.; Massey, A.S.; Pentlavalli, S.; Chen, K.H.; Cole, G.; et al. Development and characterization of self-assembling nanoparticles using a bio-inspired amphipathic peptide for gene delivery. J. Control. Release 2014, 189, 141–149. [Google Scholar] [CrossRef]
- Perillo, E.; Herve-Aubert, K.; Allard-Vannier, E.; Falanga, A.; Galdiero, S.; Chourpa, I. Synthesis and in vitro evaluation of fluorescent and magnetic nanoparticles functionalized with a cell penetrating peptide for cancer theranosis. J. Colloid Interface Sci. 2017, 499, 209–217. [Google Scholar] [CrossRef]
- Green, M.; Loewenstein, P.M. Autonomous functional domains of chemically synthesized human immunodeficiency virus tat trans-activator protein. Cell 1988, 55, 1179–1188. [Google Scholar] [CrossRef]
- Viscidi, R.P.; Mayur, K.; Lederman, H.M.; Frankel, A.D. Inhibition of antigen-induced lymphocyte proliferation by Tat protein from HIV-1. Science 1989, 246, 1606–1608. [Google Scholar] [CrossRef]
- Joliot, A.; Pernelle, C.; Deagostinibazin, H.; Prochiantz, A. Antennapedia Homeobox Peptide Regulates Neural Morphogenesis. Proc. Natl. Acad. Sci. USA 1991, 88, 1864–1868. [Google Scholar] [CrossRef] [Green Version]
- Derossi, D.; Joliot, A.H.; Chassaing, G.; Prochiantz, A. The 3rd Helix of the Antennapedia Homeodomain Translocates through Biological-Membranes. J. Biol. Chem. 1994, 269, 10444–10450. [Google Scholar] [CrossRef]
- Vives, E.; Brodin, P.; Lebleu, B. A truncated HIV-1 Tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. J. Biol. Chem. 1997, 272, 16010–16017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bohmova, E.; Machova, D.; Pechar, M.; Pola, R.; Venclikova, K.; Janouskova, O.; Etrych, T. Cell-penetrating peptides: A useful tool for the delivery of various cargoes into cells. Physiol. Res. 2018, 67, S267–S279. [Google Scholar] [CrossRef]
- Elmquist, A.; Hansen, M.; Langel, Ü. Structure–activity relationship study of the cell-penetrating peptide pVEC. Biochim. Biophys. Acta (BBA) Biomembr. 2006, 1758, 721–729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, M.C.; Deshayes, S.; Heitz, F.; Divita, G. Cell-penetrating peptides: From molecular mechanisms to therapeutics. Biol. Cell 2008, 100, 201–217. [Google Scholar] [CrossRef] [Green Version]
- Vaithiyanathan, M.; Hymel, H.C.; Safa, N.; Sanchez, O.M.; Pettigrew, J.H.; Kirkpatrick, C.S.; Gauthier, T.J.; Melvin, A.T. Kinetic analysis of cellular internalization and expulsion of unstructured D-chirality cell penetrating peptides. Aiche J. 2021, 67, e17087. [Google Scholar] [CrossRef]
- Hanna, S.E.; Mozaffari, S.; Tiwari, R.K.; Parang, K. Comparative Molecular Transporter Efficiency of Cyclic Peptides Containing Tryptophan and Arginine Residues. ACS Omega 2018, 3, 16281–16291. [Google Scholar] [CrossRef]
- Qian, Z.; Martyna, A.; Hard, R.L.; Wang, J.; Appiah-Kubi, G.; Coss, C.; Phelps, M.A.; Rossman, J.S.; Pei, D. Discovery and Mechanism of Highly Efficient Cyclic Cell-Penetrating Peptides. Biochemistry 2016, 55, 2601–2612. [Google Scholar] [CrossRef]
- Moiola, M.; Memeo, M.G.; Quadrelli, P. Stapled Peptides-A Useful Improvement for Peptide-Based Drugs. Molecules 2019, 24, 3654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cline, L.L.; Waters, M.L. The structure of well-folded beta-hairpin peptides promotes resistance to peptidase degradation. Biopolymers 2009, 92, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Safa, N.; Anderson, J.C.; Vaithiyanathan, M.; Pettigrew, J.H.; Pappas, G.A.; Liu, D.; Gauthier, T.J.; Melvin, A.T. CPProtectides: Rapid uptake of well-folded beta-hairpin peptides with enhanced resistance to intracellular degradation. Pept. Sci. 2019, 111, e24092. [Google Scholar] [CrossRef] [PubMed]
- Jin, J.M.; Wu, Y.; Chen, J.J.; Shen, Y.W.; Zhang, L.J.; Zhang, H.; Chen, L.L.; Yuan, H.B.; Chen, H.Z.; Zhang, W.D.; et al. The peptide PROTAC modality: A novel strategy for targeted protein ubiquitination. Theranostics 2020, 10, 10141–10153. [Google Scholar] [CrossRef] [PubMed]
- Ottis, P.; Crews, C.M. Proteolysis-Targeting Chimeras: Induced Protein Degradation as a Therapeutic Strategy. ACS Chem. Biol. 2017, 12, 892–898. [Google Scholar] [CrossRef]
- Pazos, E.; Vazquez, O.; Mascarenas, J.L.; Vazquez, M.E. Peptide-based fluorescent biosensors. Chem. Soc. Rev. 2009, 38, 3348–3359. [Google Scholar] [CrossRef]
- Safa, N.; Vaithiyanathan, M.; Sombolestani, S.; Charles, S.; Melvin, A.T. Population-based analysis of cell-penetrating peptide uptake using a microfluidic droplet trapping array. Anal. Bioanal. Chem. 2019, 411, 2729–2741. [Google Scholar] [CrossRef]
- Hallbrink, M.; Floren, A.; Elmquist, A.; Pooga, M.; Bartfai, T.; Langel, U. Cargo delivery kinetics of cell-penetrating peptides. Biochim. Biophys. Acta 2001, 1515, 101–109. [Google Scholar] [CrossRef] [Green Version]
- Jones, S.W.; Christison, R.; Bundell, K.; Voyce, C.J.; Brockbank, S.M.; Newham, P.; Lindsay, M.A. Characterisation of cell-penetrating peptide-mediated peptide delivery. Br. J. Pharmacol. 2005, 145, 1093–1102. [Google Scholar] [CrossRef]
- Patel, S.G.; Sayers, E.J.; He, L.; Narayan, R.; Williams, T.L.; Mills, E.M.; Allemann, R.K.; Luk, L.Y.P.; Jones, A.T.; Tsai, Y.H. Cell-penetrating peptide sequence and modification dependent uptake and subcellular distribution of green florescent protein in different cell lines. Sci. Rep. 2019, 9, 6298. [Google Scholar] [CrossRef]
- Khayyatnejad Shoushtari, S.; Zoghebi, K.; Sajid, M.I.; Tiwari, R.K.; Parang, K. Hybrid Cyclic-Linear Cell-Penetrating Peptides Containing Alternative Positively Charged and Hydrophobic Residues as Molecular Transporters. Mol. Pharm. 2021, 18, 3909–3919. [Google Scholar] [CrossRef] [PubMed]
- Futaki, S.; Suzuki, T.; Ohashi, W.; Yagami, T.; Tanaka, S.; Ueda, K.; Sugiura, Y. Arginine-rich peptides. An abundant source of membrane-permeable peptides having potential as carriers for intracellular protein delivery. J. Biol. Chem. 2001, 276, 5836–5840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tunnemann, G.; Ter-Avetisyan, G.; Martin, R.M.; Stockl, M.; Herrmann, A.; Cardoso, M.C. Live-cell analysis of cell penetration ability and toxicity of oligo-arginines. J. Pept. Sci. 2008, 14, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Zachowski, A. Phospholipids in animal eukaryotic membranes: Transverse asymmetry and movement. Biochem. J. 1993, 294 Pt 1, 1. [Google Scholar] [CrossRef]
- Utsugi, T.; Schroit, A.J.; Connor, J.; Bucana, C.D.; Fidler, I.J. Elevated expression of phosphatidylserine in the outer membrane leaflet of human tumor cells and recognition by activated human blood monocytes. Cancer Res. 1991, 51, 3062–3066. [Google Scholar] [PubMed]
- Amand, H.L.; Fant, K.; Nordén, B.; Esbjörner, E.K. Stimulated endocytosis in penetratin uptake: Effect of arginine and lysine. Biochem. Biophys. Res. Commun. 2008, 371, 621–625. [Google Scholar] [CrossRef]
- El-Sayed, N.S.; Miyake, T.; Shirazi, A.N.; Park, S.E.; Clark, J.; Buchholz, S.; Parang, K.; Tiwari, R. Design, Synthesis, and Evaluation of Homochiral Peptides Containing Arginine and Histidine as Molecular Transporters. Molecules 2018, 23, 1590. [Google Scholar] [CrossRef] [Green Version]
- Su, Y.; Doherty, T.; Waring, A.J.; Ruchala, P.; Hong, M. Roles of arginine and lysine residues in the translocation of a cell-penetrating peptide from (13)C, (31)P, and (19)F solid-state NMR. Biochemistry 2009, 48, 4587–4595. [Google Scholar] [CrossRef] [Green Version]
- Fei, L.; Ren, L.; Zaro, J.L.; Shen, W.C. The influence of net charge and charge distribution on cellular uptake and cytosolic localization of arginine-rich peptides. J. Drug Target. 2011, 19, 675–680. [Google Scholar] [CrossRef]
- Sun, C.; Shen, W.-C.; Tu, J.; Zaro, J.L. Interaction between Cell-Penetrating Peptides and Acid-Sensitive Anionic Oligopeptides as a Model for the Design of Targeted Drug Carriers. Mol. Pharm. 2014, 11, 1583–1590. [Google Scholar] [CrossRef]
- Fujita, H.; Yamagishi, M.; Kida, Y.; Sakaguchi, M. Positive charges on the translocating polypeptide chain arrest movement through the translocon. J. Cell Sci. 2011, 124, 4184–4193. [Google Scholar] [CrossRef] [Green Version]
- El-Andaloussi, S.; Jarver, P.; Johansson, H.J.; Langel, U. Cargo-dependent cytotoxicity and delivery efficacy of cell-penetrating peptides: A comparative study. Biochem. J. 2007, 407, 285–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardozo, A.K.; Buchillier, V.; Mathieu, M.; Chen, J.; Ortis, F.; Ladriere, L.; Allaman-Pillet, N.; Poirot, O.; Kellenberger, S.; Beckmann, J.S.; et al. Cell-permeable peptides induce dose- and length-dependent cytotoxic effects. Biochim. Biophys. Acta 2007, 1768, 2222–2234. [Google Scholar] [CrossRef]
- Deprey, K.; Becker, L.; Kritzer, J.; Pluckthun, A. Trapped! A Critical Evaluation of Methods for Measuring Total Cellular Uptake versus Cytosolic Localization. Bioconjug. Chem. 2019, 30, 1006–1027. [Google Scholar] [CrossRef]
- Kovarik, M.L.; Allbritton, N.L. Measuring enzyme activity in single cells. Trends Biotechnol. 2011, 29, 222–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Proctor, A.; Wang, Q.; Lawrence, D.S.; Allbritton, N.L. Metabolism of peptide reporters in cell lysates and single cells. Analyst 2012, 137, 3028–3038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kristensen, M.; Birch, D.; Mørck Nielsen, H. Applications and Challenges afor Use of Cell-Penetrating Peptides as Delivery Vectors for Peptide and Protein Cargos. Int. J. Mol. Sci. 2016, 17, 185. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Name | Sequence | Cargo Charge | Cargo Length |
|---|---|---|---|
| H1 | RWVRVpGO(FAM)WIRQ | ||
| H2 | RWVRVpGO(FAM)WIRQ-GRGR | +2 | 4-mer |
| H3 | RWVRVpGO(FAM)WIRQ-GGGG | 0 | 4-mer |
| H4 | RWVRVpGO(FAM)WIRQ-GEGE | −2 | 4-mer |
| H5 | RWVRVpGO(FAM)WIRQ-GRGRGRGR | +4 | 8-mer |
| H6 | RWVRVpGO(FAM)WIRQ-GGGGGGGG | 0 | 8-mer |
| H7 | RWVRVpGO(FAM)WIRQ-GEGEGEGE | −4 | 8-mer |
| H8 | RWVRVpGO(FAM)WIRQ-GGGGGRGR | +2 | 8-mer |
| H9 | RWVRVpGO(FAM)WIRQ-GRGRGGGG | +2 | 8-mer |
| R1 | FAM-RRRRRRRRR | ||
| R2 | FAM-RRRRRRRRR-GRGR | +2 | 4-mer |
| R3 | FAM-RRRRRRRRR-GRGRGRGR | +4 | 8-mer |
| R4 | FAM-RRRRRRRRR-GGGGGGGG | 0 | 8-mer |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hymel, H.C.; Rahnama, A.; Sanchez, O.M.; Liu, D.; Gauthier, T.J.; Melvin, A.T. How Cargo Identity Alters the Uptake of Cell-Penetrating Peptide (CPP)/Cargo Complexes: A Study on the Effect of Net Cargo Charge and Length. Cells 2022, 11, 1195. https://doi.org/10.3390/cells11071195
Hymel HC, Rahnama A, Sanchez OM, Liu D, Gauthier TJ, Melvin AT. How Cargo Identity Alters the Uptake of Cell-Penetrating Peptide (CPP)/Cargo Complexes: A Study on the Effect of Net Cargo Charge and Length. Cells. 2022; 11(7):1195. https://doi.org/10.3390/cells11071195
Chicago/Turabian StyleHymel, Hannah C., Alireza Rahnama, Olivia M. Sanchez, Dong Liu, Ted J. Gauthier, and Adam T. Melvin. 2022. "How Cargo Identity Alters the Uptake of Cell-Penetrating Peptide (CPP)/Cargo Complexes: A Study on the Effect of Net Cargo Charge and Length" Cells 11, no. 7: 1195. https://doi.org/10.3390/cells11071195
APA StyleHymel, H. C., Rahnama, A., Sanchez, O. M., Liu, D., Gauthier, T. J., & Melvin, A. T. (2022). How Cargo Identity Alters the Uptake of Cell-Penetrating Peptide (CPP)/Cargo Complexes: A Study on the Effect of Net Cargo Charge and Length. Cells, 11(7), 1195. https://doi.org/10.3390/cells11071195






