The Effects of the Combination of Mesenchymal Stromal Cells and Nanofiber-Hydrogel Composite on Repair of the Contused Spinal Cord
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. NHC Preparation
2.3. MSC Preparation
2.4. Spinal Cord Contusion and Injection
2.5. Assessment of Hind Limb Function
2.6. Histological Preparations
2.7. Immunohistochemistry
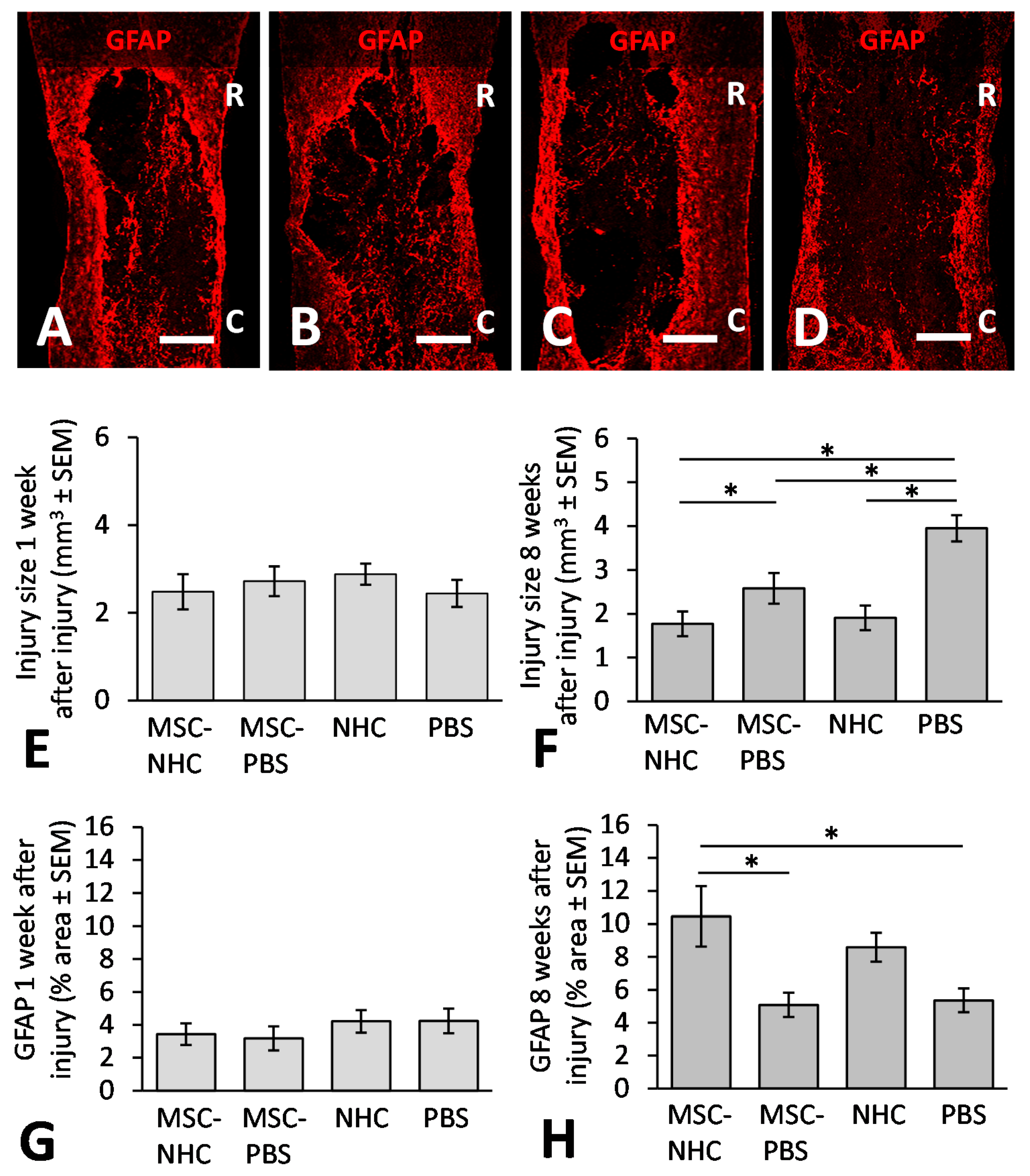
2.8. Injury Volume Assessment
2.9. Automated Quantification
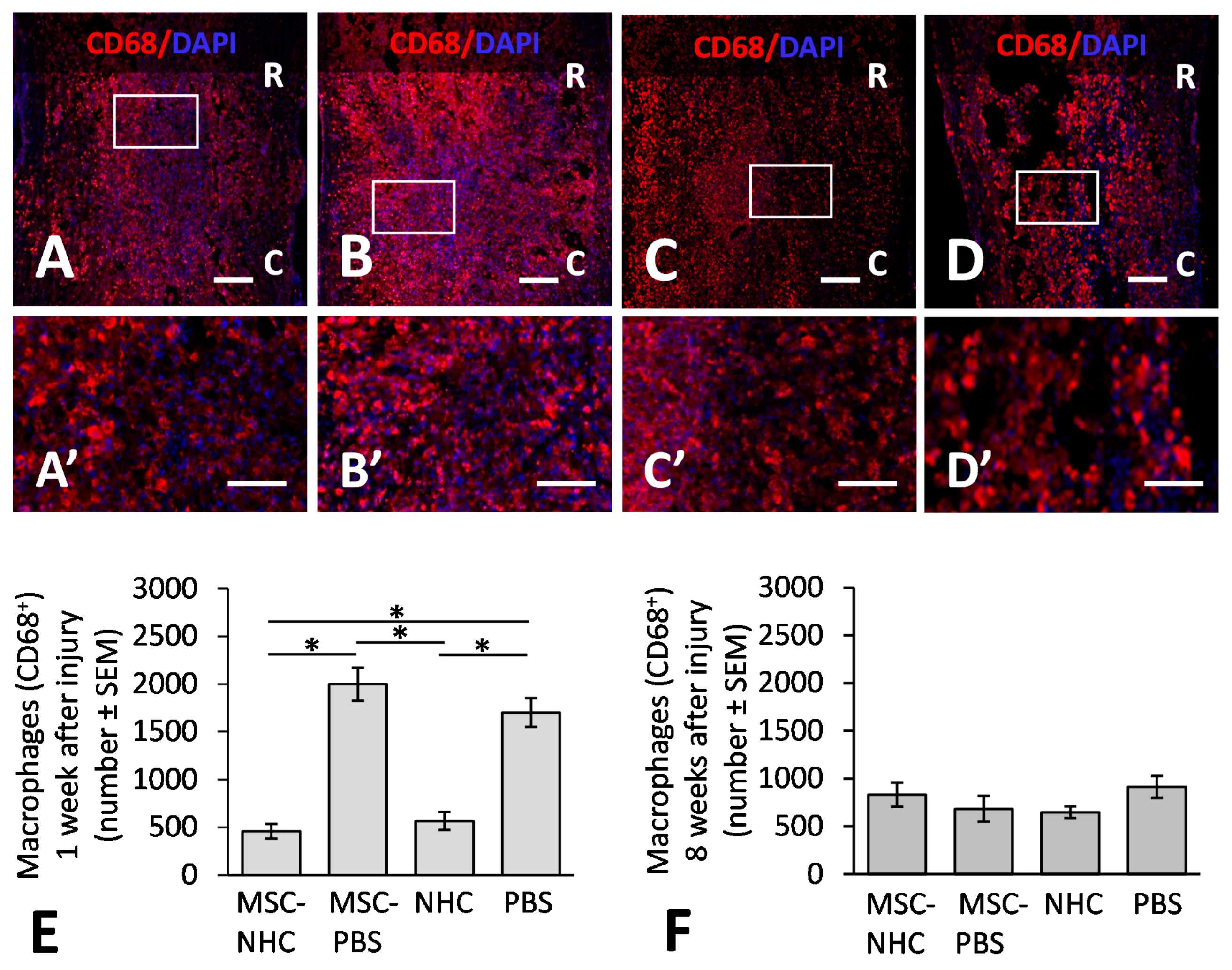
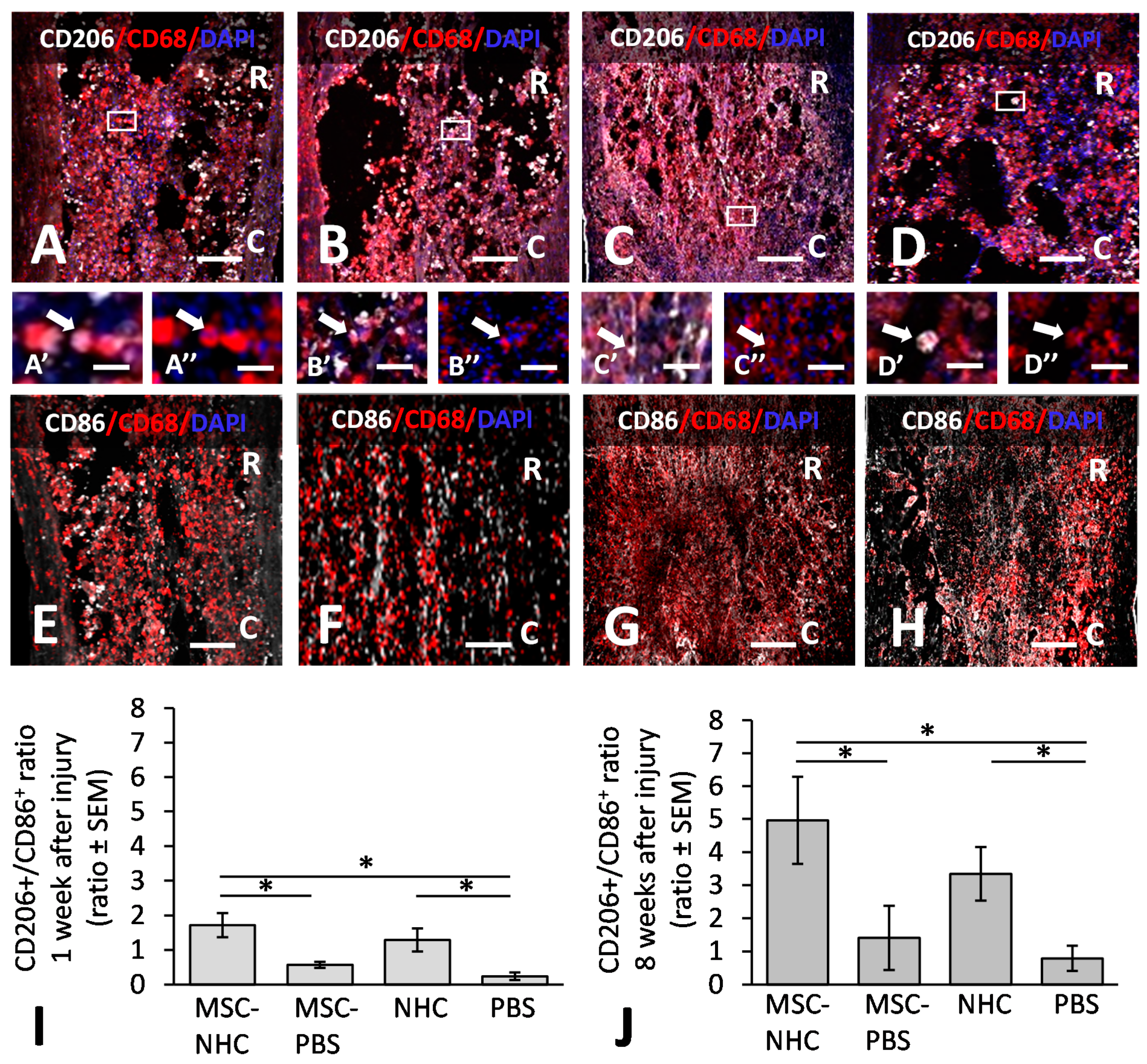
2.10. Macrophage Quantification
2.11. Astrocyte Presence Examination
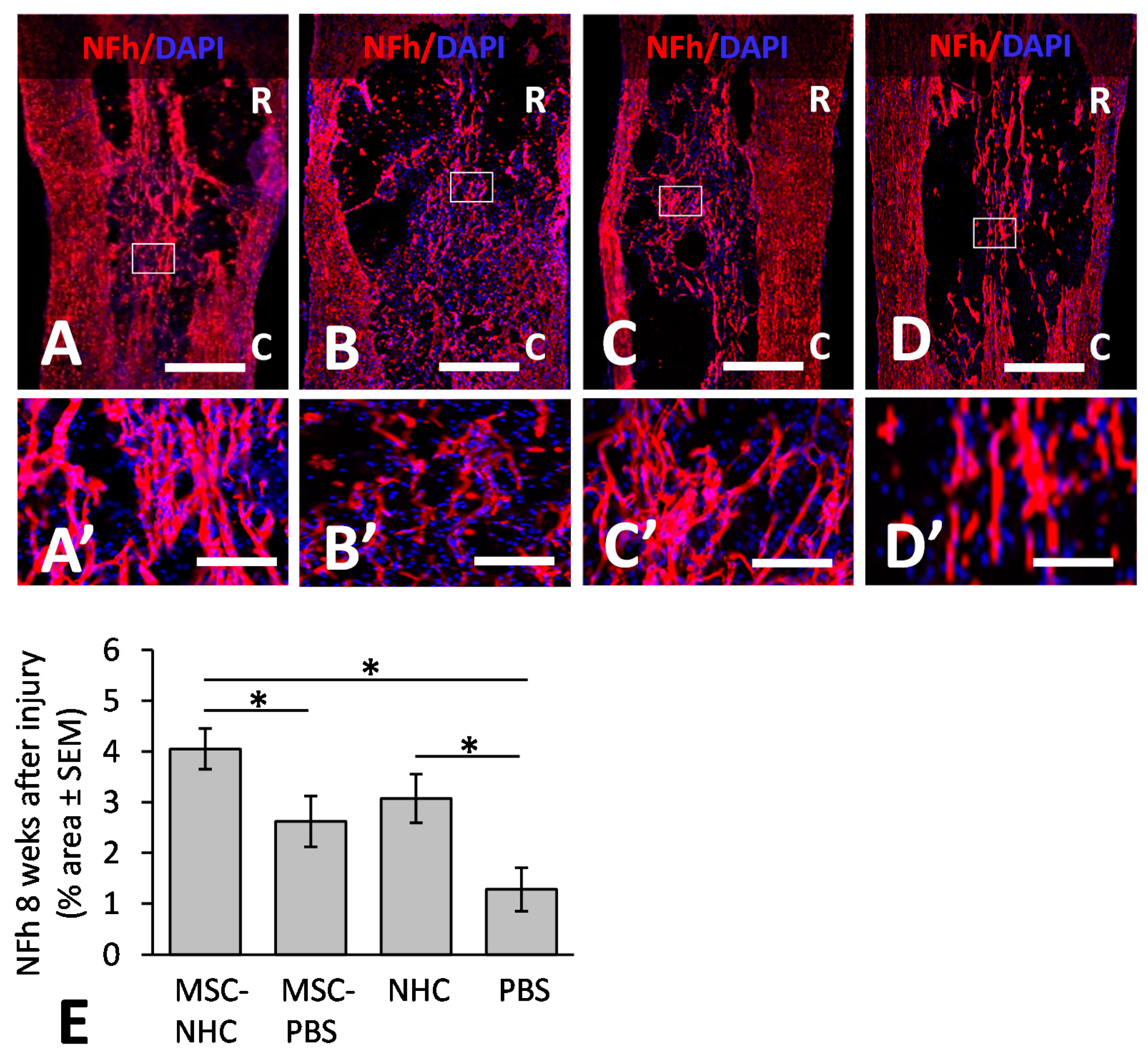
2.12. Axon Presence
2.13. Transplant Survival Assessment
2.14. Statistical Analyses
3. Results
3.1. Inflammatory Response in the Injury Site
3.2. Injury Site Volume
3.3. Astrocytes in the Injury Site
3.4. Axons in the Injury Site
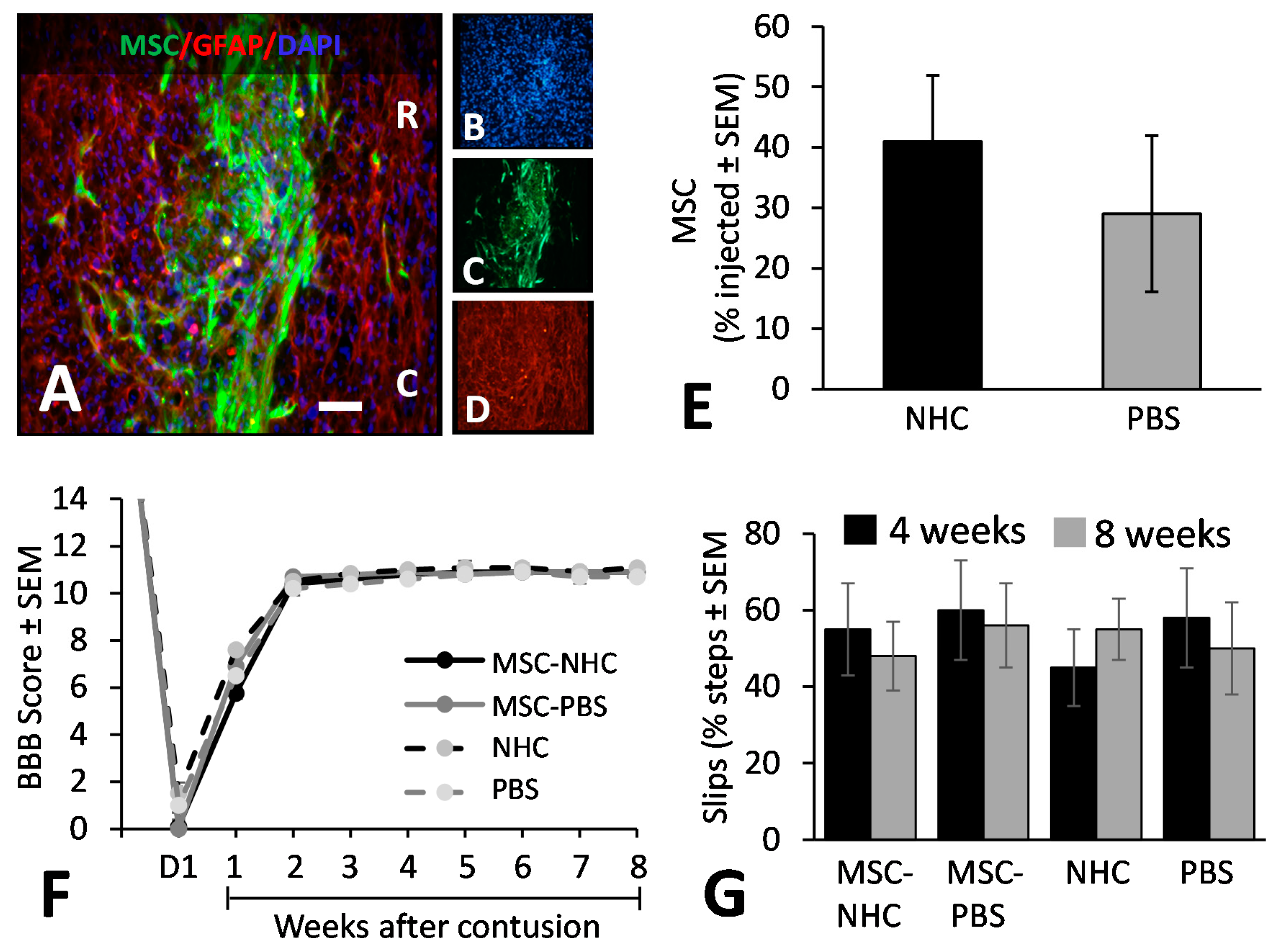
3.5. MSC Transplant Survival
3.6. Hindlimb Locomotor Function
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hachem, L.D.; Fehlings, M.G. Pathophysiology of Spinal Cord Injury. Neurosurg. Clin. N. Am. 2021, 32, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Hagg, T.; Oudega, M. Degenerative and spontaneous regenerative processes after spinal cord injury. J. Neurotrauma 2006, 23, 264–280. [Google Scholar] [CrossRef] [Green Version]
- van Niekerk, E.A.; Tuszynski, M.H.; Lu, P.; Dulin, J.N. Molecular and cellular mechanisms of axonal regeneration after spinal cord injury. Mol. Cell. Proteom. 2016, 15, 394–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walsh, C.M.; Wychowaniec, J.K.; Brougham, D.F.; Dooley, D. Functional hydrogels as therapeutic tools for spinal cord injury: New perspectives on immunopharmacological interventions. Pharmacol. Ther. 2021, 233, 108043. [Google Scholar] [CrossRef] [PubMed]
- Cizkova, D.; Rosocha, J.; Vanicky, I.; Jergova, S.; Cizek, M. Transplants of human mesenchymal stem cells improve functional recovery after spinal cord injury in the rat. Cell. Mol. Neurobiol. 2006, 26, 1167–1180. [Google Scholar] [CrossRef] [PubMed]
- Nandoe Tewarie, R.D.; Hurtado, A.; Ritfeld, G.J.; Rahiem, S.T.; Wendell, D.F.; Barroso, M.M.; Grotenhuis, J.A.; Oudega, M. Bone marrow stromal cells elicit tissue sparing after acute but not delayed transplantation into the contused adult rat thoracic spinal cord. J. Neurotrauma 2009, 26, 2313–2322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakajima, H.; Uchida, K.; Guerrero, A.R.; Watanabe, S.; Sugita, D.; Takeura, N.; Yoshida, A.; Long, G.; Wright, K.T.; Johnson, W.E.; et al. Transplantation of mesenchymal stem cells promotes an alternative pathway of macrophage activation and functional recovery after spinal cord injury. J. Neurotrauma 2012, 29, 1614–1625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quertainmont, R.; Cantinieaux, D.; Botman, O.; Sid, S.; Schoenen, J.; Franzen, R. Mesenchymal stem cell graft improves recovery after spinal cord injury in adult rats through neurotrophic and pro-angiogenic actions. PLoS ONE 2012, 7, e39500. [Google Scholar] [CrossRef] [PubMed]
- Ritfeld, G.J.; Tewarie, R.N.; Vajn, K.; Rahiem, S.T.; Hurtado, A.; Wendell, D.F.; Roos, R.A.; Oudega, M. Bone marrow stromal cell-mediated tissue sparing enhances functional repair after spinal cord contusion in adult rats. Cell Transpl. 2012, 21, 1561–1575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ritfeld, G.J.; Rauck, B.M.; Novosat, T.L.; Park, D.; Patel, P.; Roos, R.A.; Wang, Y.D.; Oudega, M. The effect of a polyurethane-based reverse thermal gel on bone marrow stromal cell transplant survival and spinal cord repair. Biomaterials 2014, 35, 1924–1931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morita, T.; Sasaki, M.; Kataoka-Sasaki, Y.; Nakazaki, M.; Nagahama, H.; Oka, S.; Oshigiri, T.; Takebayashi, T.; Yamashita, T.; Kocsis, J.D.; et al. Intravenous infusion of mesenchymal stem cells promotes functional recovery in a model of chronic spinal cord injury. Neurosci 2016, 335, 221–231. [Google Scholar] [CrossRef]
- Liu, Y.; Dulchavsky, D.S.; Gao, X.; Kwon, D.; Chopp, M.; Dulchavsky, S.; Gautam, S.C. Wound repair by bone marrow stromal cells through growth factor production. J. Surg. Res. 2006, 136, 336–341. [Google Scholar] [CrossRef] [PubMed]
- Nakano, N.; Nakai, Y.; Seo, T.B.; Yamada, Y.; Ohno, T.; Yamanaka, A.; Nagai, Y.; Fukushima, M.; Suzuki, Y.; Nakatani, T.; et al. Characterization of conditioned medium of cultured bone marrow stromal cells. Neurosci. Lett. 2010, 483, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Ritfeld, G.J.; Patel, A.; Chou, A.; Novosat, T.L.; Castillo, D.G.; Roos, R.A.; Oudega, M. The role of brain-derived neurotrophic factor in bone marrow stromal cell-mediated spinal cord repair. Cell Transpl. 2015, 24, 2209–2220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomchuck, S.L.; Zwezdaryk, K.J.; Coffelt, S.B.; Waterman, R.S.; Danka, E.S.; Scandurro, A.B. Toll-like receptors on human mesenchymal stem cells drive their migration and immunomodulating responses. Stem. Cells 2008, 26, 99–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, T.; Ahn, M.; Moon, C.; Kim, S.; Sim, K.B. Alternatively activated macrophages in spinal cord injury and remission: Another mechanism for repair? Mol. Neurobiol. 2013, 47, 1011–1019. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Wang, H.; Jin, T.; Xu, Y.; Mei, L.; Yang, J. TLR4 activation promotes bone marrow MSC proliferation and osteogenic differentiation via wnt3a and wnt5a signaling. PLoS ONE 2016, 11, e0149876. [Google Scholar] [CrossRef] [PubMed]
- Cofano, F.; Boido, M.; Monticelli, M.; Zenga, F.; Ducati, A.; Vercelli, A.; Garbossa, D. Mesenchymal stem cells for spinal cord injury: Current options, limitations, and future of cell therapy. Int. J. Mol. Sci. 2019, 20, 2698. [Google Scholar] [CrossRef] [Green Version]
- Okuda, A.; Horii-Hayashi, N.; Sasagawa, T.; Shimizu, T.; Shigematsu, H.; Iwata, E.; Morimoto, Y.; Masuda, K.; Koizumi, M.; Akahane, M.; et al. Bone marrow stromal cell sheets may promote axonal regeneration and functional recovery with suppression of glial scar formation after spinal cord transection injury in rats. J. Neurosurg. Spine 2017, 26, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Lin, H.; Bai, S.; Zheng, L.; Zhang, X. Bone marrow mesenchymal stem cells (BMSCs) improved functional recovery of spinal cord injury partly by promoting axonal regeneration. Neurochem. Int. 2018, 115, 80–84. [Google Scholar] [CrossRef]
- Nandoe Tewarie, R.D.; Hurtado, A.; Levi, A.D.; Grotenhuis, J.A.; Oudega, M. Bone marrow stromal cells for repair of the spinal cord: Towards clinical application. Cell Transplant 2006, 15, 563–577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Zhang, C.; Haggerty, A.E.; Yan, J.; Lan, M.; Seu, M.; Yang, M.; Marlow, M.M.; Maldonado-Lasunción, I.; Cho, B.; et al. The effect of a nanofiber-hydrogel composite on neural tissue repair and regeneration in the contused spinal cord. Biomaterials 2020, 245, 119978. [Google Scholar] [CrossRef]
- Ozawa, H.; Matsumoto, T.; Ohashi, T.; Sato, M.; Kokubun, S. Comparison of spinal cord gray matter and white matter softness: Measurement by pipette aspiration method. J. Neurosurg. 2001, 95 (Suppl. S2), 221–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ware, T.; Simon, D.; Arreaga-Salas, D.E.; Reeder, J.; Rennaker, R.; Keefer, E.W.; Voit, W. Fabrication of responsive, softening neural interfaces. Adv. Funct. Mater. 2012, 22, 3470–3479. [Google Scholar] [CrossRef]
- Li, X.; Cho, B.; Martin, R.; Seu, M.; Zhang, C.; Zhou, Z.; Choi, J.S.; Jiang, X.; Chen, L.; Walia, G.; et al. Nanofiber-hydrogel composite mediated angiogenesis for soft tissue reconstruction. Sci. Transl. Med. 2019, 11, eaau6210. [Google Scholar] [CrossRef]
- Azizi, S.A.; Stokes, D.; Augelli, B.J.; DiGirolamo, C.; Prockop, D.J. Engraftment and migration of human bone marrow stromal cells implanted in the brains of albino rats—Similarities to astrocyte grafts. Proc. Natl. Acad. Sci. USA 1998, 95, 908–913. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheff, S.W.; Rabchevsky, A.G.; Fugaccia, I.; Main, J.A.; Lumpp, J.E., Jr. Experimental modeling of spinal cord injury: Characterization of a force-defined injury device. J. Neurotrauma 2003, 20, 179–193. [Google Scholar] [CrossRef]
- Rauck, B.M.; Novosat, T.L.; Oudega, M.; Wang, Y. Biocompatibility of a coacervate-based controlled release system for protein delivery to the injured spinal cord. Acta Biomater. 2015, 11, 204–211. [Google Scholar] [CrossRef] [Green Version]
- Basso, M.; Beattie, M.S.; Bresnahan, J.C. A sensitive and reliable locomotor rating scale for open field testing in rats. J. Neurotrauma 1995, 12, 1–21. [Google Scholar] [CrossRef]
- Kunkel-Bagden, E.; Dai, H.N.; Bregman, B.S. Methods to assess the development and recovery of locomotor function after spinal cord injury in rats. Exp. Neurol. 1993, 119, 153–164. [Google Scholar] [CrossRef]
- Metz, G.A.; Whishaw, I.Q. Drug-induced rotation intensity in unilateral dopamine-depleted rats is not correlated with end point or qualitative measures of forelimb or hindlimb motor performance. Neuroscience 2002, 111, 325–336. [Google Scholar] [CrossRef]
- Noller, C.M.; Boulina, M.; McNamara, G.; Szeto, A.; McCabe, P.M.; Mendez, A.J. A practical approach to quantitative processing and analysis of small biological structures by fluorescent imaging. J. Biomol. Tech. 2016, 27, 90–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gensel, J.C.; Zhang, B. Macrophage activation and its role in repair and pathology after spinal cord injury. Brain Res. 2015, 1619, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kigerl, K.A.; Gensel, J.C.; Ankeny, D.P.; Alexander, J.K.; Donnelly, D.J.; Popovich, P.G. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J. Neurosci. 2009, 29, 13435–13444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kroner, A.; Greenhalgh, A.D.; Zarruk, J.G.; Passos Dos Santos, R.; Gaestel, M.; David, S. TNF and increased intracellular iron alter macrophage polarization to a detrimental M1 phenotype in the injured spinal cord. Neuron 2014, 83, 1098–1116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maldonado-Lasunción, I.; Verhaagen, J.; Oudega, M. Mesenchymal stem cell-macrophage choreography supporting spinal cord repair. Neurotherapeutics 2018, 15, 578–587. [Google Scholar] [CrossRef] [Green Version]
- Ge, W.; Jiang, J.; Arp, J.; Liu, W.; Garcia, B.; Wang, H. Regulatory T-cell generation and kidney allograft tolerance induced by mesenchymal stem cells associated with indoleamine 2,3-dioxygenase expression. Transplantation 2010, 90, 1312–1320. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, K.C.; Jena, M.K.; Pradhan, B.S.; Nayak, N.; Das, S.; Hsu, C.D.; Wheeler, D.S.; Chen, K.; Nayak, N.R. VEGF may contribute to macrophage recruitment and M2 polarization in the decidua. PLoS ONE 2018, 13, e0191040. [Google Scholar] [CrossRef] [PubMed]
- Garg, K.; Pullen, N.A.; Oskeritzian, C.A.; Ryan, J.J.; Bowlin, G.L. Macrophage functional polarization (M1/M2) in response to varying fiber and pore dimensions of electrospun scaffolds. Biomaterials 2013, 34, 4439–4451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McWhorter, F.Y.; Wang, T.; Nguyen, P.; Chung, T.; Liu, W.F. Modulation of macrophage phenotype by cell shape. Proc. Natl. Acad. Sci. USA 2013, 110, 17253–17258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popovich, P.G.; Guan, Z.; McGaughy, V.; Fisher, L.; Hickey, W.F.; Basso, D.M. The neuropathological and behavioral consequences of intraspinal microglial/macrophage activation. J. Neuropathol. Exp. Neurol. 2002, 61, 623–633. [Google Scholar] [CrossRef] [Green Version]
- Fiani, B.; Kondilis, A.; Soula, M.; Tao, A.; Alvi, M.A. Novel methods of necroptosis inhibition for spinal cord injury using translational research to limit secondary injury and enhance endogenous repair and regeneration. Neurospine 2021, 18, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Labrador-Velandia, S.; Alonso-Alonso, M.L.; Di Lauro, S.; García-Gutierrez, M.T.; Srivastava, G.K.; Pastor, J.C.; Fernandez-Bueno, I. Mesenchymal stem cells provide paracrine neuroprotective resources that delay degeneration of co-cultured organotypic neuroretinal cultures. Exp. Eye Res. 2019, 185, 107671. [Google Scholar] [CrossRef] [PubMed]
- Krupa, P.; Vackova, I.; Ruzicka, J.; Zaviskova, K.; Dubisova, J.; Koci, Z.; Turnovcova, K.; Urdzikova, L.M.; Kubinova, S.; Rehak, S.; et al. The effect of human mesenchymal stem cells derived from Wharton’s Jelly in spinal cord injury: Treatment is dose-dependent and can be facilitated by repeated application. Int. J. Mol. Sci. 2018, 19, 1503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Z.; Chen, Y.; Zhang, H.; Min, S.; Yu, B.; He, B.; Jin, A. Comparison of mesenchymal stromal cells from human bone marrow and adipose tissue for the treatment of spinal cord injury. Cytotherapy 2013, 15, 434–448. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, A.; Nakajima, H.; Uchida, K.; Takeura, N.; Honjoh, K.; Watanabe, S.; Kitade, M.; Kokubo, Y.; Johnson, W.E.B.; Matsumine, A. Comparison of Mesenchymal Stromal Cells Isolated from Murine Adipose Tissue and Bone Marrow in the Treatment of Spinal Cord Injury. Cell Transpl. 2018, 27, 1126–1139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Primary Antibody * | Source | Catalog Number | Dilution ** |
|---|---|---|---|
| Rabbit anti-GFP | Millipore | AB3080P | 1:1000 |
| Mouse anti-GFAP | Sigma | G3893 | 1:500 |
| Rabbit anti-GFAP | PhosphoSolutions | 620-GFAP | 1:1000 |
| Mouse anti-ED1 (CD68) | Millipore | MAB1435 | 1:200 |
| Rabbit anti-NeuN | Millipore | ABN78 | 1:500 |
| Rabbit anti-CD206 | Abcam | Ab64693 | 1:500 |
| Rabbit anti-CD86 | Abcam | Ab53004 | 1:500 |
| Rabbit anti-NFh | Millipore | AB1991 | 1:500 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haggerty, A.E.; Maldonado-Lasunción, I.; Nitobe, Y.; Yamane, K.; Marlow, M.M.; You, H.; Zhang, C.; Cho, B.; Li, X.; Reddy, S.; et al. The Effects of the Combination of Mesenchymal Stromal Cells and Nanofiber-Hydrogel Composite on Repair of the Contused Spinal Cord. Cells 2022, 11, 1137. https://doi.org/10.3390/cells11071137
Haggerty AE, Maldonado-Lasunción I, Nitobe Y, Yamane K, Marlow MM, You H, Zhang C, Cho B, Li X, Reddy S, et al. The Effects of the Combination of Mesenchymal Stromal Cells and Nanofiber-Hydrogel Composite on Repair of the Contused Spinal Cord. Cells. 2022; 11(7):1137. https://doi.org/10.3390/cells11071137
Chicago/Turabian StyleHaggerty, Agnes E., Ines Maldonado-Lasunción, Yohshiro Nitobe, Kentaro Yamane, Megan M. Marlow, Hua You, Chi Zhang, Brian Cho, Xiaowei Li, Sashank Reddy, and et al. 2022. "The Effects of the Combination of Mesenchymal Stromal Cells and Nanofiber-Hydrogel Composite on Repair of the Contused Spinal Cord" Cells 11, no. 7: 1137. https://doi.org/10.3390/cells11071137
APA StyleHaggerty, A. E., Maldonado-Lasunción, I., Nitobe, Y., Yamane, K., Marlow, M. M., You, H., Zhang, C., Cho, B., Li, X., Reddy, S., Mao, H.-Q., & Oudega, M. (2022). The Effects of the Combination of Mesenchymal Stromal Cells and Nanofiber-Hydrogel Composite on Repair of the Contused Spinal Cord. Cells, 11(7), 1137. https://doi.org/10.3390/cells11071137








