The Non-Linear Path from Gene Dysfunction to Genetic Disease: Lessons from the MICPCH Mouse Model
Abstract
1. The Complexity of the Disease Process and Utility of Genetically Modified Animals
2. MICPCH and CASK-Linked Pathologies
3. Interpretation of Findings from CASK Null Animals
4. Further Revelations from CASK-Mutant Mice
5. The Molecular Function of CASK
6. Reconciling CASK’s Molecular Function with Its Loss-of-Function Pathology
7. Remaining Questions
8. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kumar, V.; Cotran, R.S.; Robbins, S.L.; Crain, B.J. Basic pathology. Arch. Pathol. Lab. Med. 1998, 122, 660. [Google Scholar]
- Gee, B.E. Biologic Complexity in Sickle Cell Disease: Implications for Developing Targeted Therapeutics. Sci. World J. 2013, 2013, 694146. [Google Scholar] [CrossRef] [PubMed]
- Piccin, A.; Murphy, C.; Eakins, E.; Rondinelli, M.B.; Daves, M.; Vecchiato, C.; Wolf, D.; Mc Mahon, C.; Smith, O.P. Insight into the complex pathophysiology of sickle cell anaemia and possible treatment. Eur. J. Haematol. 2019, 102, 319–330. [Google Scholar] [CrossRef] [PubMed]
- McColl, B.; Vadolas, J. Animal models of beta-hemoglobinopathies: Utility and limitations. J. Blood Med. 2016, 7, 263–274. [Google Scholar] [CrossRef] [PubMed]
- Pound, P.; Ritskes-Hoitinga, M. Is it possible to overcome issues of external validity in preclinical animal research? Why most animal models are bound to fail. J. Transl. Med. 2018, 16, 304. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.S.M. The trouble with animal models in brain research. In Neuroethics and Nonhuman Animals; Springer: Cham, Switzerland, 2020; pp. 271–286. [Google Scholar]
- Chadman, K.K.; Gong, S.; Scattoni, M.L.; Boltuck, S.E.; Gandhy, S.U.; Heintz, N.; Crawley, J.N. Minimal aberrant behavioral phenotypes of neuroligin-3 R451C knockin mice. Autism Res. 2008, 1, 147–158. [Google Scholar] [CrossRef]
- Tabuchi, K.; Blundell, J.; Etherton, M.R.; Hammer, R.E.; Liu, X.; Powell, C.M.; Südhof, T.C. A Neuroligin-3 Mutation Implicated in Autism Increases Inhibitory Synaptic Transmission in Mice. Science 2007, 318, 71–76. [Google Scholar] [CrossRef]
- Thomas, P.D. The Gene Ontology and the Meaning of Biological Function. Methods Mol. Biol. 2017, 1446, 14–24. [Google Scholar] [CrossRef]
- Otchy, T.M.; Wolff, S.B.E.; Rhee, J.; Pehlevan, C.; Kawai, R.; Kempf, A.; Gobes, S.; Ölveczky, B. Acute off-target effects of neural circuit manipulations. Nature 2015, 528, 358–363. [Google Scholar] [CrossRef]
- Südhof, T.C. Reproducibility: Experimental mismatch in neural circuits. Nature 2015, 528, 338–339. [Google Scholar] [CrossRef][Green Version]
- Etherton, M.; Földy, C.; Sharma, M.; Tabuchi, K.; Liu, X.; Shamloo, M.; Malenka, R.C.; Südhof, T.C. Autism-linked neuroligin-3 R451C mutation differentially alters hippocampal and cortical synaptic function. Proc. Natl. Acad. Sci. USA 2011, 108, 13764–13769. [Google Scholar] [CrossRef] [PubMed]
- Gratten, J.; Visscher, P. Genetic pleiotropy in complex traits and diseases: Implications for genomic medicine. Genome Med. 2016, 8, 78. [Google Scholar] [CrossRef] [PubMed]
- Zlotogora, J. Penetrance and expressivity in the molecular age. Genet. Med. 2003, 5, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Kammenga, J.E. The background puzzle: How identical mutations in the same gene lead to different disease symptoms. FEBS J. 2017, 284, 3362–3373. [Google Scholar] [CrossRef] [PubMed]
- Burga, A.; Lehner, B. Beyond genotype to phenotype: Why the phenotype of an individual cannot always be predicted from their genome sequence and the environment that they experience. FEBS J. 2012, 279, 3765–3775. [Google Scholar] [CrossRef]
- Gjuvsland, A.B.; Vik, J.O.; Beard, D.A.; Hunter, P.J.; Omholt, S.W. Bridging the genotype–phenotype gap: What does it take? J. Physiol. 2013, 591, 2055–2066. [Google Scholar] [CrossRef] [PubMed]
- Eisener-Dorman, A.F.; Lawrence, D.A.; Bolivar, V.J. Cautionary insights on knockout mouse studies: The gene or not the gene? Brain Behav. Immun. 2009, 23, 318–324. [Google Scholar] [CrossRef]
- Wolfer, D.P.; Crusio, W.; Lipp, H.-P. Knockout mice: Simple solutions to the problems of genetic background and flanking genes. Trends Neurosci. 2002, 25, 336–340. [Google Scholar] [CrossRef]
- Gingrich, J.A.; Hen, R. The broken mouse: The role of development, plasticity and environment in the interpretation of phenotypic changes in knockout mice. Curr. Opin. Neurobiol. 2000, 10, 146–152. [Google Scholar] [CrossRef]
- Zhang, B.; Seigneur, E.; Wei, P.; Gokce, O.; Morgan, J.; Südhof, T.C. Developmental plasticity shapes synaptic phenotypes of autism-associated neuroligin-3 mutations in the calyx of Held. Mol. Psychiatry 2017, 22, 1483–1491. [Google Scholar] [CrossRef]
- Dimitratos, S.D.; Stathakis, D.G.; Nelson, C.A.; Woods, D.F.; Bryant, P.J. The Location of HumanCASKat Xp11.4 Identifies This Gene as a Candidate for X-Linked Optic Atrophy. Genomics 1998, 51, 308–309. [Google Scholar] [CrossRef]
- Froyen, G.; Van Esch, H.; Bauters, M.; Hollanders, K.; Frints, S.G.; Vermeesch, J.R.; Devriendt, K.; Fryns, J.P.; Marynen, P. Detection of genomic copy number changes in patients with idiopathic mental retardation by high-resolution X-array-CGH: Important role for increased gene dosage of XLMR genes. Hum. Mutat. 2007, 28, 1034–1042. [Google Scholar] [CrossRef]
- Hayashi, S.; Mizuno, S.; Migita, O.; Okuyama, T.; Makita, Y.; Hata, A.; Imoto, I.; Inazawa, J. The CASK gene harbored in a deletion detected by array-CGH as a potential candidate for a gene causative of X-linked dominant mental retardation. Am. J. Med. Genet. Part A 2008, 146A, 2145–2151. [Google Scholar] [CrossRef] [PubMed]
- Najm, J.; Horn, D.; Wimplinger, I.; Golden, J.A.; Chizhikov, V.V.; Sudi, J.; Christian, S.L.; Ullmann, R.; Kuechler, A.; Haas, C.A.; et al. Mutations of CASK cause an X-linked brain malformation phenotype with microcephaly and hypoplasia of the brainstem and cerebellum. Nat. Genet. 2008, 40, 1065–1067. [Google Scholar] [CrossRef]
- Tarpey, P.S.; Smith, R.; Pleasance, E.; Whibley, A.; Edkins, S.; Hardy, C.; O’Meara, S.; Latimer, C.; Dicks, E.; Menzies, A.; et al. A systematic, large-scale resequencing screen of X-chromosome coding exons in mental retardation. Nat. Genet. 2009, 41, 535–543. [Google Scholar] [CrossRef] [PubMed]
- Hackett, A.; Tarpey, P.S.; Licata, A.; Cox, J.; Whibley, A.; Boyle, J.; Rogers, C.; Grigg, J.; Partington, M.; Stevenson, R.E.; et al. CASK mutations are frequent in males and cause X-linked nystagmus and variable XLMR phenotypes. Eur. J. Hum. Genet. 2010, 18, 544–552. [Google Scholar] [CrossRef] [PubMed]
- Bailey, K.A.; Aldinger, K.A. An X-linked microcephaly syndrome caused by disruptions of CASK implicates the CASK-TBR1-RELN pathway in human brain development. Clin. Genet. 2009, 75, 424–425. [Google Scholar] [CrossRef] [PubMed]
- Piluso, G.; D’Amico, F.; Saccone, V.; Bismuto, E.; Rotundo, I.L.; Di Domenico, M.; Aurino, S.; Schwartz, C.E.; Neri, G.; Nigro, V. A Missense Mutation in CASK Causes FG Syndrome in an Italian Family. Am. J. Hum. Genet. 2009, 84, 162–177. [Google Scholar] [CrossRef] [PubMed][Green Version]
- LaConte, L.E.W.; Chavan, V.; Mukherjee, K. Identification and Glycerol-Induced Correction of Misfolding Mutations in the X-Linked Mental Retardation Gene CASK. PLoS ONE 2014, 9, e88276. [Google Scholar] [CrossRef] [PubMed]
- Burglen, L.; Chantot-Bastaraud, S.; Garel, C.; Milh, M.; Touraine, R.; Zanni, G.; Petit, F.; Afenjar, A.; Goizet, C.; Barresi, S.; et al. Spectrum of pontocerebellar hypoplasia in 13 girls and boys with CASK mutations: Confirmation of a recognizable phenotype and first description of a male mosaic patient. Orphanet J. Rare Dis. 2012, 7, 18. [Google Scholar] [CrossRef] [PubMed]
- Moog, U.; Kutsche, K.; Kortüm, F.; Chilian, B.; Bierhals, T.; Apeshiotis, N.; Balg, S.; Chassaing, N.; Coubes, C.; Das, S.; et al. Phenotypic spectrum associated with CASK loss-of-function mutations. J. Med Genet. 2011, 48, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, S.; Uehara, D.T.; Tanimoto, K.; Mizuno, S.; Chinen, Y.; Fukumura, S.; Takanashi, J.I.; Osaka, H.; Okamoto, N.; Inazawa, J. Comprehensive investigation of CASK mutations and other genetic etiologies in 41 patients with intellectual disability and microcephaly with pontine and cerebellar hypoplasia (MICPCH). PLoS ONE 2017, 12, e0181791. [Google Scholar] [CrossRef] [PubMed]
- Takanashi, J.; Okamoto, N.; Yamamoto, Y.; Hayashi, S.; Arai, H.; Takahashi, Y.; Maruyama, K.; Mizuno, S.; Shimakawa, S.; Ono, H.; et al. Clinical and radiological features of Japanese patients with a severe phenotype due to CASK mutations. Am. J. Med. Genet. Part A 2012, 158A, 3112–3118. [Google Scholar] [CrossRef] [PubMed]
- Moog, U.; Bierhals, T.; Brand, K.; Bautsch, J.; Biskup, S.; Brune, T.; Denecke, J.; de Die-Smulders, C.E.; Evers, C.; Hempel, M.; et al. Phenotypic and molecular insights into CASK-related disorders in males. Orphanet J. Rare Dis. 2015, 10, 44. [Google Scholar] [CrossRef][Green Version]
- Mukherjee, K.; Patel, P.A.; Rajan, D.S.; LaConte, L.E.W.; Srivastava, S. Survival of a male patient harboring CASK Arg27Ter mutation to adolescence. Mol. Genet. Genom. Med. 2020, 8, e1426. [Google Scholar] [CrossRef]
- Patel, P.A.; Hegert, J.; Cristian, I.; Kerr, A.; LaConte, L.E.; Fox, M.A.; Srivastava, S.; Mukherjee, K. Complete loss of CASK causes severe ataxia through cerebellar degeneration in human and mouse. bioRxiv 2021. [Google Scholar] [CrossRef]
- Saitsu, H.; Kato, M.; Osaka, H.; Moriyama, N.; Horita, H.; Nishiyama, K.; Yoneda, Y.; Kondo, Y.; Tsurusaki, Y.; Doi, H.; et al. CASK aberrations in male patients with Ohtahara syndrome and cerebellar hypoplasia. Epilepsia 2012, 53, 1441–1449. [Google Scholar] [CrossRef]
- Cristofoli, F.; Devriendt, K.; Davis, E.E.; Van Esch, H.; Vermeesch, J.R. Novel CASK mutations in cases with syndromic microcephaly. Hum. Mutat. 2018, 39, 993–1001. [Google Scholar] [CrossRef]
- Watkins, R.J.; Patil, R.; Goult, B.T.; Thomas, M.G.; Gottlob, I.; Shackleton, S. A novel interaction between FRMD7 and CASK: Evidence for a causal role in idiopathic infantile nystagmus. Hum. Mol. Genet. 2013, 22, 2105–2118. [Google Scholar] [CrossRef]
- LaConte, L.E.W.; Chavan, V.; DeLuca, S.; Rubin, K.; Malc, J.; Berry, S.; Summers, C.G.; Mukherjee, K. An N-terminal heterozygous missense CASK mutation is associated with microcephaly and bilateral retinal dystrophy plus optic nerve atrophy. Am. J. Med Genet. Part A 2018, 179, 94–103. [Google Scholar] [CrossRef]
- LaConte, L.E.W.; Chavan, V.; Elias, A.F.; Hudson, C.; Schwanke, C.; Styren, K.; Shoof, J.; Kok, F.; Srivastava, S.; Mukherjee, K. Two microcephaly-associated novel missense mutations in CASK specifically disrupt the CASK-neurexin interaction. Hum. Genet. 2018, 137, 231–246. [Google Scholar] [CrossRef] [PubMed]
- Horvitz, H.R.; Sulston, J.E. Isolation and genetic characterization of cell-lineage mutants of the nematode Caenorhabditis elegans. Genetics 1980, 96, 435–454. [Google Scholar] [CrossRef] [PubMed]
- Hoskins, R.; Hajnal, A.F.; Harp, S.A.; Kim, S.K. The C. elegans vulval induction gene lin-2 encodes a member of the MAGUK family of cell junction proteins. Development 1996, 122, 97–111. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.R.; Ollo, R. A new Drosophila Ca2+/calmodulin-dependent protein kinase (Caki) is localized in the central nervous system and implicated in walking speed. EMBO J. 1996, 15, 1865–1876. [Google Scholar] [CrossRef] [PubMed]
- Slawson, J.B.; Kuklin, E.A.; Ejima, A.; Mukherjee, K.; Ostrovsky, L.; Griffith, L.C. Central Regulation of Locomotor Behavior of Drosophila melanogaster Depends on a CASK Isoform Containing CaMK-Like and L27 Domains. Genetics 2011, 187, 171–184. [Google Scholar] [CrossRef] [PubMed]
- Atasoy, D.; Schoch, S.; Ho, A.; Nadasy, K.A.; Liu, X.; Zhang, W.; Mukherjee, K.; Nosyreva, E.D.; Fernandez-Chacon, R.; Missler, M.; et al. Deletion of CASK in mice is lethal and impairs synaptic function. Proc. Natl. Acad. Sci. USA 2007, 104, 2525–2530. [Google Scholar] [CrossRef]
- Mori, T.; Kasem, E.A.; Suzuki-Kouyama, E.; Cao, X.S.; Li, X.; Kurihara, T.; Uemura, T.; Yanagawa, T.; Tabuchi, K. Deficiency of calcium/calmodulin-dependent serine protein kinase disrupts the excitatory-inhibitory balance of synapses by downregulating GluN2B. Mol. Psychiatry 2019, 24, 1079–1092. [Google Scholar] [CrossRef]
- Srivastava, S.; McMillan, R.; Willis, J.; Clark, H.; Chavan, V.; Liang, C.; Zhang, H.; Hulver, M.; Mukherjee, K. X-linked intellectual disability gene CASK regulates postnatal brain growth in a non-cell autonomous manner. Acta Neuropathol. Commun. 2016, 4, 30. [Google Scholar] [CrossRef]
- Patel, P.A.; Hegert, J.V.; Cristian, I.; Kerr, A.; LaConte, L.E.W.; Fox, M.A.; Srivastava, S.; Mukherjee, K. Complete loss of the X-linked gene CASK causes severe cerebellar degeneration. J. Med Genet. 2022. [Google Scholar] [CrossRef]
- Hata, Y.; Butz, S.; Südhof, T.C. CASK: A novel dlg/PSD95 homolog with an N-terminal calmodulin-dependent protein kinase domain identified by interaction with neurexins. J. Neurosci. 1996, 16, 2488–2494. [Google Scholar] [CrossRef]
- Biederer, T.; Südhof, T.C. CASK and Protein 4.1 Support F-actin Nucleation on Neurexins. J. Biol. Chem. 2001, 276, 47869–47876. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.R.; Wood, D.F.; Marfatia, S.M.; Walther, Z.; Chishti, A.H.; Anderson, J.M. Human CASK/LIN-2 Binds Syndecan-2 and Protein 4.1 and Localizes to the Basolateral Membrane of Epithelial Cells. J. Cell Biol. 1998, 142, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, Y.-P.; Yang, F.-C.; Kharazia, V.; Naisbitt, S.; Cohen, A.R.; Weinberg, R.; Sheng, M. Direct Interaction of CASK/LIN-2 and Syndecan Heparan Sulfate Proteoglycan and Their Overlapping Distribution in Neuronal Synapses. J. Cell Biol. 1998, 142, 139–151. [Google Scholar] [CrossRef] [PubMed]
- Butz, S.; Okamoto, M.; Südhof, T.C. A Tripartite Protein Complex with the Potential to Couple Synaptic Vesicle Exocytosis to Cell Adhesion in Brain. Cell 1998, 94, 773–782. [Google Scholar] [CrossRef]
- Tabuchi, K.; Biederer, T.; Butz, S.; Südhof, T.C. CASK Participates in Alternative Tripartite Complexes in which Mint 1 Competes for Binding with Caskin 1, a Novel CASK-Binding Protein. J. Neurosci. 2002, 22, 4264–4273. [Google Scholar] [CrossRef]
- Olsen, O.; Moore, K.A.; Fukata, M.; Kazuta, T.; Trinidad, J.C.; Kauer, F.W.; Streuli, M.; Misawa, H.; Burlingame, A.L.; Nicoll, R.A.; et al. Neurotransmitter release regulated by a MALS–liprin-α presynaptic complex. J. Cell Biol. 2005, 170, 1127–1134. [Google Scholar] [CrossRef]
- Hsueh, Y.-P.; Wang, T.-F.; Yang, F.-C.; Sheng, M. Nuclear translocation and transcription regulation by the membrane-associated guanylate kinase CASK/LIN-2. Nature 2000, 404, 298–302. [Google Scholar] [CrossRef]
- Caruana, G. Genetic studies define MAGUK proteins as regulators of epithelial cell polarity. Int. J. Dev. Biol. 2002, 46, 511–518. [Google Scholar]
- Lozovatsky, L.; Abayasekara, N.; Piawah, S.; Walther, Z. CASK Deletion in Intestinal Epithelia Causes Mislocalization of LIN7C and the DLG1/Scrib Polarity Complex without Affecting Cell Polarity. Mol. Biol. Cell 2009, 20, 4489–4499. [Google Scholar] [CrossRef]
- Qi, J.; Su, Y.; Sun, R.; Zhang, F.; Luo, X.; Yang, Z.; Luo, X. CASK inhibits ECV304 cell growth and interacts with Id1. Biochem. Biophys. Res. Commun. 2005, 328, 517–521. [Google Scholar] [CrossRef]
- Huang, T.-N.; Hsueh, Y.-P. Calcium/calmodulin-dependent serine protein kinase (CASK), a protein implicated in mental retardation and autism-spectrum disorders, interacts with T-Brain-1 (TBR1) to control extinction of associative memory in male mice. J. Psychiatry Neurosci. 2017, 42, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Milsom, W.; Kinkead, R.; Hedrick, M.; Gilmour, K.; Perry, S.; Gargaglioni, L.; Wang, T. Evolution of vertebrate respiratory central rhythm generators. Respir. Physiol. Neurobiol. 2021, 295, 103781. [Google Scholar] [CrossRef] [PubMed]
- LaConte, L.; Mukherjee, K. Structural constraints and functional divergences in CASK evolution. Biochem. Soc. Trans. 2013, 41, 1017–1022. [Google Scholar] [CrossRef]
- Liang, C.; Kerr, A.; Qiu, Y.; Cristofoli, F.; Van Esch, H.; Fox, M.A.; Mukherjee, K. Optic Nerve Hypoplasia Is a Pervasive Subcortical Pathology of Visual System in Neonates. Investig. Opthalmol. Vis. Sci. 2017, 58, 5485–5496. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Liang, C.; Arora, A.; Vijayan, S.; Ahuja, S.; Wagley, P.; Settlage, R.; LaConte, L.; Goodkin, H.; Lazar, I.; et al. Haploinsufficiency of X-linked intellectual disability gene CASK induces post-transcriptional changes in synaptic and cellular metabolic pathways. Exp. Neurol. 2020, 329, 113319. [Google Scholar] [CrossRef]
- Kerr, A.; Patel, P.A.; LaConte, L.E.W.; Liang, C.; Chen, C.-K.; Shah, V.; Fox, M.A.; Mukherjee, K. Non-Cell Autonomous Roles for CASK in Optic Nerve Hypoplasia. Investig. Opthalmol. Vis. Sci. 2019, 60, 3584–3594. [Google Scholar] [CrossRef] [PubMed]
- Marble, D.D.; Hegle, A.P.; Snyder, E.D., 2nd; Dimitratos, S.; Bryant, P.J.; Wilson, G.F. Camguk/CASK enhances Ether-a-go-go potassium current by a phosphorylation-dependent mechanism. J. Neurosci. 2005, 25, 4898–4907. [Google Scholar] [CrossRef]
- Kuo, T.-Y.; Hong, C.-J.; Chien, H.-L.; Hsueh, Y.-P. X-linked mental retardation gene CASK interacts with Bcl11A/CTIP1 and regulates axon branching and outgrowth. J. Neurosci. Res. 2010, 88, 2364–2373. [Google Scholar] [CrossRef]
- Jeyifous, O.; Waites, C.L.; Specht, C.G.; Fujisawa, S.; Schubert, M.; Lin, E.I.; Marshall, J.; Aoki, C.; de Silva, T.; Montgomery, J.M.; et al. SAP97 and CASK mediate sorting of NMDA receptors through a previously unknown secretory pathway. Nat. Neurosci. 2009, 12, 1011–1019. [Google Scholar] [CrossRef]
- Huang, T.-N.; Chang, H.-P.; Hsueh, Y.-P. CASK phosphorylation by PKA regulates the protein-protein interactions of CASK and expression of the NMDAR2b gene. J. Neurochem. 2010, 112, 1562–1573. [Google Scholar] [CrossRef]
- Hodge, J.; Mullasseril, P.; Griffith, L. Activity-Dependent Gating of CaMKII Autonomous Activity by Drosophila CASK. Neuron 2006, 51, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Chung, W.C.; Huang, T.N.; Hsueh, Y.P. Targeted deletion of CASK-interacting nucleosome assembly protein causes higher locomotor and exploratory activities. Neurosignals 2011, 19, 128–141. [Google Scholar] [CrossRef] [PubMed]
- Aravindan, R.G.; Fomin, V.P.; Naik, U.P.; Modelski, M.J.; Naik, M.U.; Galileo, D.S.; Duncan, R.L.; Martin-DeLeon, P.A. CASK interacts with PMCA4b and JAM-A on the mouse sperm flagellum to regulate Ca2+ homeostasis and motility. J. Cell. Physiol. 2011, 227, 3138–3150. [Google Scholar] [CrossRef]
- Ahn, S.Y.; Kim, Y.; Kim, S.T.; Swat, W.; Miner, J.H. Scaffolding Proteins DLG1 and CASK Cooperate to Maintain the Nephron Progenitor Population during Kidney Development. J. Am. Soc. Nephrol. 2013, 24, 1127–1138. [Google Scholar] [CrossRef] [PubMed]
- Márquez-Rosado, L.; Singh, D.; Rincón-Arano, H.; Solan, J.L.; Lampe, P.D. CASK (LIN2) interacts with Cx43 in wounded skin and their coexpression affects cell migration. J. Cell Sci. 2012, 125, 695–702. [Google Scholar] [CrossRef] [PubMed]
- Biederer, T.; Sara, Y.; Mozhayeva, M.; Atasoy, D.; Liu, X.; Kavalali, E.T.; Südhof, T.C. SynCAM, a Synaptic Adhesion Molecule That Drives Synapse Assembly. Science 2002, 297, 1525–1531. [Google Scholar] [CrossRef]
- Kaech, S.M.; Whitfield, C.W.; Kim, S.K. The LIN-2/LIN-7/LIN-10 complex mediates basolateral membrane localization of the C. elegans EGF receptor LET-23 in vulval epithelial cells. Cell 1998, 94, 761–771. [Google Scholar] [CrossRef]
- LaConte, L.E.; Chavan, V.; Liang, C.; Willis, J.; Schonhense, E.M.; Schoch, S.; Mukherjee, K. CASK stabilizes neurexin and links it to liprin-alpha in a neuronal activity-dependent manner. Cell. Mol. Life Sci. 2016, 73, 3599–3621. [Google Scholar] [CrossRef]
- Stafford, R.L.; Ear, J.; Knight, M.J.; Bowie, J.U. The Molecular Basis of the Caskin1 and Mint1 Interaction with CASK. J. Mol. Biol. 2011, 412, 3–13. [Google Scholar] [CrossRef]
- Wei, Z.; Zheng, S.; Spangler, S.A.; Yu, C.; Hoogenraad, C.C.; Zhang, M. Liprin-mediated large signaling complex organization revealed by the liprin-alpha/CASK and liprin-alpha/liprin-beta complex structures. Mol. Cell 2011, 43, 586–598. [Google Scholar] [CrossRef]
- Boudeau, J.; Miranda-Saavedra, D.; Barton, G.; Alessi, D.R. Emerging roles of pseudokinases. Trends Cell Biol. 2006, 16, 443–452. [Google Scholar] [CrossRef] [PubMed]
- Olsen, O.; Bredt, D.S. Functional Analysis of the Nucleotide Binding Domain of Membrane-associated Guanylate Kinases. J. Biol. Chem. 2003, 278, 6873–6878. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, K.; Sharma, M.; Jahn, R.; Wahl, M.C.; Südhof, T.C. Evolution of CASK into a Mg2+ -Sensitive Kinase. Sci. Signal. 2010, 3, ra33. [Google Scholar] [CrossRef]
- Mukherjee, K.; Sharma, M.; Urlaub, H.; Bourenkov, G.P.; Jahn, R.; Südhof, T.C.; Wahl, M.C. CASK Functions as a Mg2+-Independent Neurexin Kinase. Cell 2008, 133, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wei, Z.; Yan, Y.; Wan, Q.; Du, Q.; Zhang, M. Structure of Crumbs tail in complex with the PALS1 PDZ–SH3–GK tandem reveals a highly specific assembly mechanism for the apical Crumbs complex. Proc. Natl. Acad. Sci. USA 2014, 111, 17444–17449. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.E.; Tibbe, D.; Harms, F.L.; Reissner, C.; Becker, K.; Dingmann, B.; Mirzaa, G.; Kattentidt-Mouravieva, A.A.; Shoukier, M.; Aggarwal, S.; et al. Missense mutations in CASK, coding for the calcium-/calmodulin-dependent serine protein kinase, interfere with neurexin binding and neurexin-induced oligomerization. J. Neurochem. 2021, 157, 1331–1350. [Google Scholar] [CrossRef] [PubMed]
- Bozarth, X.; Foss, K.; Mefford, H.C. A de novo in-frame deletion of CASK gene causes early onset infantile spasms and supratentorial cerebral malformation in a female patient. Am. J. Med. Genet. Part A 2018, 176, 2425–2429. [Google Scholar] [CrossRef] [PubMed]
- Studtmann, C.; LaConte, L.E.W.; Mukherjee, K. Comments on: A de novo in-frame deletion of CASK gene causes early onset infantile spasms and supratentorial cerebral malformation in a female patient. Am. J. Med. Genet. Part A 2019, 179, 2514–2516. [Google Scholar] [CrossRef] [PubMed]
- Spangler, S.A.; Schmitz, S.K.; Kevenaar, J.T.; de Graaff, E.; de Wit, H.; Demmers, J.; Toonen, R.F.; Hoogenraad, C.C. Liprin-alpha 2 promotes the presynaptic recruitment and turnover of RIM1/CASK to facilitate synaptic transmission. J. Cell. Biol. 2013, 201, 915–928. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.; Mastropasqua, F.; Reising, J.P.; Maier, S.; Ho, M.-L.; Rabkina, I.; Li, D.; Neufeld, J.; Ballenberger, L.; Myers, L.; et al. Presynaptic dysfunction in CASK-related neurodevelopmental disorders. Transl. Psychiatry 2020, 10, 312. [Google Scholar] [CrossRef]
- Lu, C.S.; Hodge, J.J.; Mehren, J.; Sun, X.X.; Griffith, L.C. Regulation of the Ca2+/CaM-responsive pool of CaMKII by scaffold-dependent autophosphorylation. Neuron 2003, 40, 1185–1197. [Google Scholar] [CrossRef]
- Samuels, B.A.; Hsueh, Y.-P.; Shu, T.; Liang, H.; Tseng, H.-C.; Hong, C.-J.; Su, S.C.; Volker, J.; Neve, R.L.; Yue, D.T.; et al. Cdk5 Promotes Synaptogenesis by Regulating the Subcellular Distribution of the MAGUK Family Member CASK. Neuron 2007, 56, 823–837. [Google Scholar] [CrossRef] [PubMed]
- Gao, R.; Piguel, N.; Melendez-Zaidi, A.; Martin-De-Saavedra, M.D.; Yoon, S.; Forrest, M.; Myczek, K.; Zhang, G.; Russell, T.A.; Csernansky, J.G.; et al. CNTNAP2 stabilizes interneuron dendritic arbors through CASK. Mol. Psychiatry 2018, 23, 1832–1850. [Google Scholar] [CrossRef] [PubMed]
- Penagarikano, O.; Abrahams, B.S.; Herman, E.I.; Winden, K.D.; Gdalyahu, A.; Dong, H.; Sonnenblick, L.I.; Gruver, R.; Almajano, J.; Bragin, A.; et al. Absence of CNTNAP2 leads to epilepsy, neuronal migration abnormalities, and core autism-related deficits. Cell 2011, 147, 235–246. [Google Scholar] [CrossRef]
- Somaiya, R.D.; Stebbins, K.; Xie, H.; Garcia, A.D.R.; Fox, M.A. Sonic hedgehog-dependent recruitment of GABAergic interneurons into the developing visual thalamus. bioRxiv 2022. [Google Scholar] [CrossRef]
- Galliano, E.; Gao, Z.; Schonewille, M.; Todorov, B.; Simons, E.; Pop, A.S.; D’Angelo, E.U.; Maagdenberg, A.M.V.D.; Hoebeek, F.E.; De Zeeuw, C.I. Silencing the Majority of Cerebellar Granule Cells Uncovers Their Essential Role in Motor Learning and Consolidation. Cell Rep. 2013, 3, 1239–1251. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, K.; Slawson, J.B.; Christmann, B.L.; Griffith, L.C. Neuron-specific protein interactions of Drosophila CASK-beta are revealed by mass spectrometry. Front. Mol. Neurosci. 2014, 7, 58. [Google Scholar] [CrossRef]
- Bonneh-Barkay, D.; Wiley, C.A. Brain Extracellular Matrix in Neurodegeneration. Brain Pathol. 2009, 19, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Fink, A.J.; Englund, C.; Daza, R.A.M.; Pham, D.; Lau, C.; Nivison, M.; Kowalczyk, T.; Hevner, R.F. Development of the Deep Cerebellar Nuclei: Transcription Factors and Cell Migration from the Rhombic Lip. J. Neurosci. 2006, 26, 3066–3076. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, K.D.; Rocheleau, C.E. Golgi localization of the LIN-2/7/10 complex points to a role in basolateral secretion of LET-23 EGFR in the Caenorhabditis elegans vulval precursor cells. Development 2021, 148, dev194167. [Google Scholar] [CrossRef] [PubMed]
- Thyrock, A.; Ossendorf, E.; Stehling, M.; Kail, M.; Kurtz, T.; Pohlentz, G.; Waschbüsch, D.; Eggert, S.; Formstecher, E.; Müthing, J.; et al. A New Mint1 Isoform, but Not the Conventional Mint1, Interacts with the Small GTPase Rab6. PLoS ONE 2013, 8, e64149. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, I.; Crespi, A.; Fornasari, D.; Pietrini, G. Novel localisation and possible function of LIN7 and IRSp53 in mitochondria of HeLa cells. Eur. J. Cell Biol. 2016, 95, 285–293. [Google Scholar] [CrossRef]
- Hung, V.; Lam, S.S.; Udeshi, N.D.; Svinkina, T.; Guzman, G.; Mootha, V.K.; Carr, S.A.; Ting, A.Y. Proteomic mapping of cytosol-facing outer mitochondrial and ER membranes in living human cells by proximity biotinylation. eLife 2017, 6, e24463. [Google Scholar] [CrossRef]
- Poston, C.N.; Krishnan, S.C.; Bazemore-Walker, C.R. In-depth proteomic analysis of mammalian mitochondria-associated membranes (MAM). J. Proteom. 2013, 79, 219–230. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, D.; Laverty, H.G.; Wenwieser, S.; Douglas, M.; Wilson, J.B. Mapping and expression analysis of the human CASK gene. Mamm. Genome 2000, 11, 934–937. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Bao, C.; Jin, L.; Xu, L.; Fan, W.; Lou, W. CASK Silence Overcomes Sorafenib Resistance of Hepatocellular Carcinoma Through Activating Apoptosis and Autophagic Cell Death. Front. Oncol. 2021, 11, 681683. [Google Scholar] [CrossRef]
- Burkin, H.R.; Zhao, L.; Miller, D.J. CASK is in the mammalian sperm head and is processed during epididymal maturation. Mol. Reprod. Dev. 2004, 68, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Mustroph, J.; Sag, C.M.; Bähr, F.; Schmidtmann, A.-L.; Gupta, S.N.; Dietz, A.; Islam, M.T.; Lücht, C.M.; Beuthner, B.E.; Pabel, S.; et al. Loss of CASK Accelerates Heart Failure Development. Circ. Res. 2021, 128, 1139–1155. [Google Scholar] [CrossRef]
- Liu, X.; Sun, P.; Yuan, Q.; Xie, J.; Xiao, T.; Zhang, K.; Chen, X.; Wang, Y.; Yuan, L.; Han, X. Specific Deletion of CASK in Pancreatic beta Cells Affects Glucose Homeostasis and Improves Insulin Sensitivity in Obese Mice by Reducing Hyperinsulinemia Running Title: Beta Cell CASK Deletion Reduces Hyperinsulinemia. Diabetes 2022, 71, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Gardeitchik, T.; Wyckmans, J.; Morava, E. Complex Phenotypes in Inborn Errors of Metabolism: Overlapping Presentations in Congenital Disorders of Glycosylation and Mitochondrial Disorders. Pediatric Clin. 2018, 65, 375–388. [Google Scholar] [CrossRef]
- Van Dijk, T.; Baas, F.; Barth, P.G. What’s new in pontocerebellar hypoplasia? An update on genes and subtypes. Orphanet J. Rare Dis. 2018, 13, 92. [Google Scholar] [CrossRef] [PubMed]
- Valayannopoulos, V.; Michot, C.; Rodriguez, D.; Hubert, L.; Saillour, Y.; Labrune, P.; De Laveaucoupet, J.; Brunelle, F.; Amiel, J.; Lyonnet, S.; et al. Mutations of TSEN and CASK genes are prevalent in pontocerebellar hypoplasias type 2 and 4. Brain 2011, 135, e199. [Google Scholar] [CrossRef] [PubMed]
- Van Dijk, T.; Barth, P.; Baas, F.; Reneman, L. Postnatal Brain Growth Patterns in Pontocerebellar Hypoplasia. Neuropediatrics 2021, 52, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Nuovo, S.; Micalizzi, A.; Romaniello, R.; Arrigoni, F.; Ginevrino, M.; Casella, A.; Serpieri, V.; D’Arrigo, S.; Briguglio, M.; Salerno, G.G.; et al. Refining the mutational spectrum and gene–phenotype correlates in pontocerebellar hypoplasia: Results of a multicentric study. J. Med. Genet. 2021. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Nishiyama, K.; Kodera, H.; Nakashima, M.; Tsurusaki, Y.; Miyake, N.; Matsumoto, N.; Saitsu, H.; Jinnou, H.; Ohki, S.; et al. A de novo CASK mutation in pontocerebellar hypoplasia type 3 with early myoclonic epilepsy and tetralogy of Fallot. Brain Dev. 2014, 36, 272–273. [Google Scholar] [CrossRef] [PubMed]
- Ilieva, H.; Polymenidou, M.; Cleveland, D.W. Non–cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. J. Cell Biol. 2009, 187, 761–772. [Google Scholar] [CrossRef]
- Pacheva, I.H.; Todorov, T.; Ivanov, I.; Tartova, D.; Gaberova, K.; Todorova, A.; Dimitrova, D. TSEN54 Gene-Related Pontocerebellar Hypoplasia Type 2 Could Mimic Dyskinetic Cerebral Palsy with Severe Psychomotor Retardation. Front. Pediatr. 2018, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Nishio, Y.; Kidokoro, H.; Takeo, T.; Narita, H.; Sawamura, F.; Narita, K.; Kawano, Y.; Nakata, T.; Muramatsu, H.; Hara, S.; et al. The eldest case of MICPCH with CASK mutation exhibiting gross motor regression. Brain Dev. 2021, 43, 459–463. [Google Scholar] [CrossRef] [PubMed]
- DeLuca, S.C.; Wallace, D.A.; Trucks, M.R.; Mukherjee, K. A clinical series using intensive neurorehabilitation to promote functional motor and cognitive skills in three girls with CASK mutation. BMC Res. Notes 2017, 10, 743. [Google Scholar] [CrossRef]
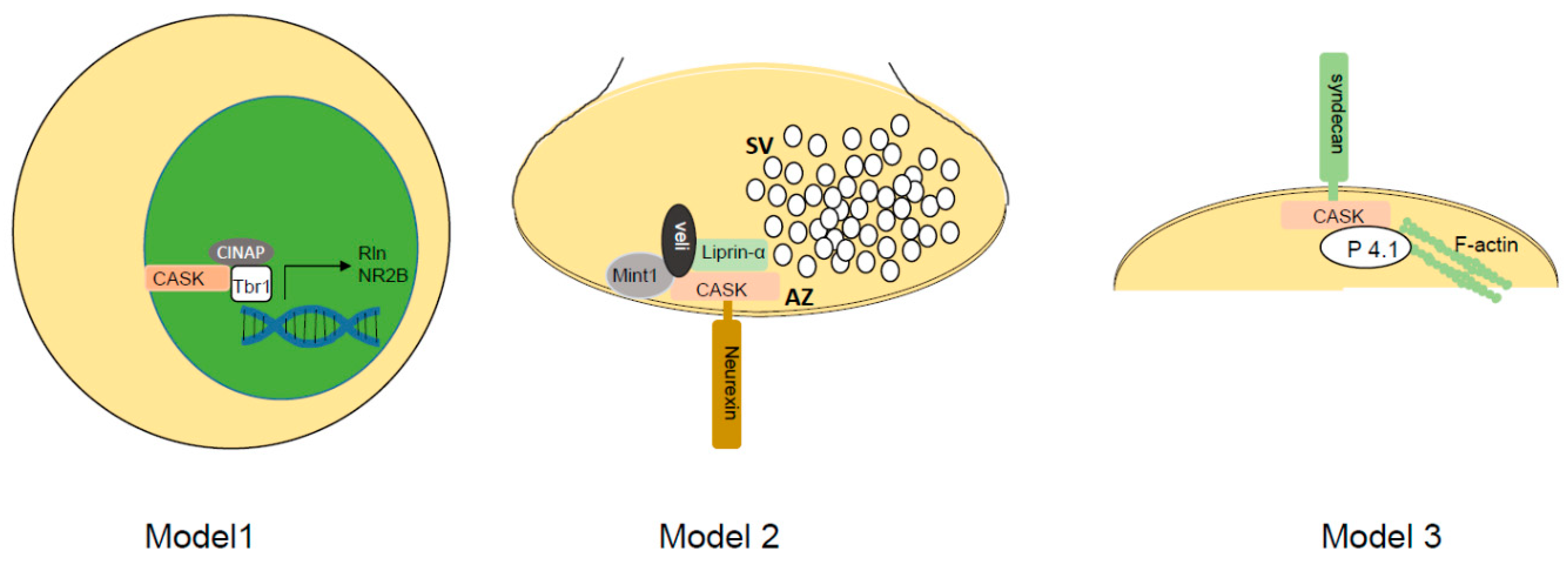

| Animal Model | Major Findings | Citation |
|---|---|---|
| C. elegans | Vulvaless phenotype. No other developmental or neuronal defect. | [43,44] |
| D. melanogaster | Reduced locomotion. No change in neuromuscular junction. | [45,46] |
| Constitutive Cask knockout mice | Death within hours of birth; cleft palate; normal-sized, well-laminated brains; increased neuronal death in thalamus. Increased excitatory synaptic miniature current frequency; decreased inhibitory synaptic miniature current frequency. | [47] |
| Constitutive heterozygous Cask knockout mice | Postnatal microencephaly; cerebellar hypoplasia; optic nerve hypoplasia; locomotor incoordination; scoliosis; occasional seizures. Increased excitatory synaptic miniature current frequency; decreased inhibitory synaptic miniature current frequency only in CASK-null cells. | [48,49] |
| Cask deletion in subset of cerebellar granule cells | No obvious phenotype. | [49] |
| Cask deletion in Purkinje cells | No obvious phenotype. | [49] |
| Cask deletion in all cerebellar granule cells | Cerebellar atrophy. | [50] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mukherjee, K.; LaConte, L.E.W.; Srivastava, S. The Non-Linear Path from Gene Dysfunction to Genetic Disease: Lessons from the MICPCH Mouse Model. Cells 2022, 11, 1131. https://doi.org/10.3390/cells11071131
Mukherjee K, LaConte LEW, Srivastava S. The Non-Linear Path from Gene Dysfunction to Genetic Disease: Lessons from the MICPCH Mouse Model. Cells. 2022; 11(7):1131. https://doi.org/10.3390/cells11071131
Chicago/Turabian StyleMukherjee, Konark, Leslie E. W. LaConte, and Sarika Srivastava. 2022. "The Non-Linear Path from Gene Dysfunction to Genetic Disease: Lessons from the MICPCH Mouse Model" Cells 11, no. 7: 1131. https://doi.org/10.3390/cells11071131
APA StyleMukherjee, K., LaConte, L. E. W., & Srivastava, S. (2022). The Non-Linear Path from Gene Dysfunction to Genetic Disease: Lessons from the MICPCH Mouse Model. Cells, 11(7), 1131. https://doi.org/10.3390/cells11071131







