Pregnane X Receptor Mediates Atherosclerosis Induced by Dicyclohexyl Phthalate in LDL Receptor-Deficient Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals and Treatment
2.2. Blood Analysis
2.3. Atherosclerotic Lesion Analysis
2.4. Isolation of Primary Macrophages and Analysis of Their Related Functions
2.5. RNA Isolation and Quantitative Real-Time PCR Analysis
2.6. Immunohistochemistry
2.7. Statistical Analysis
3. Results
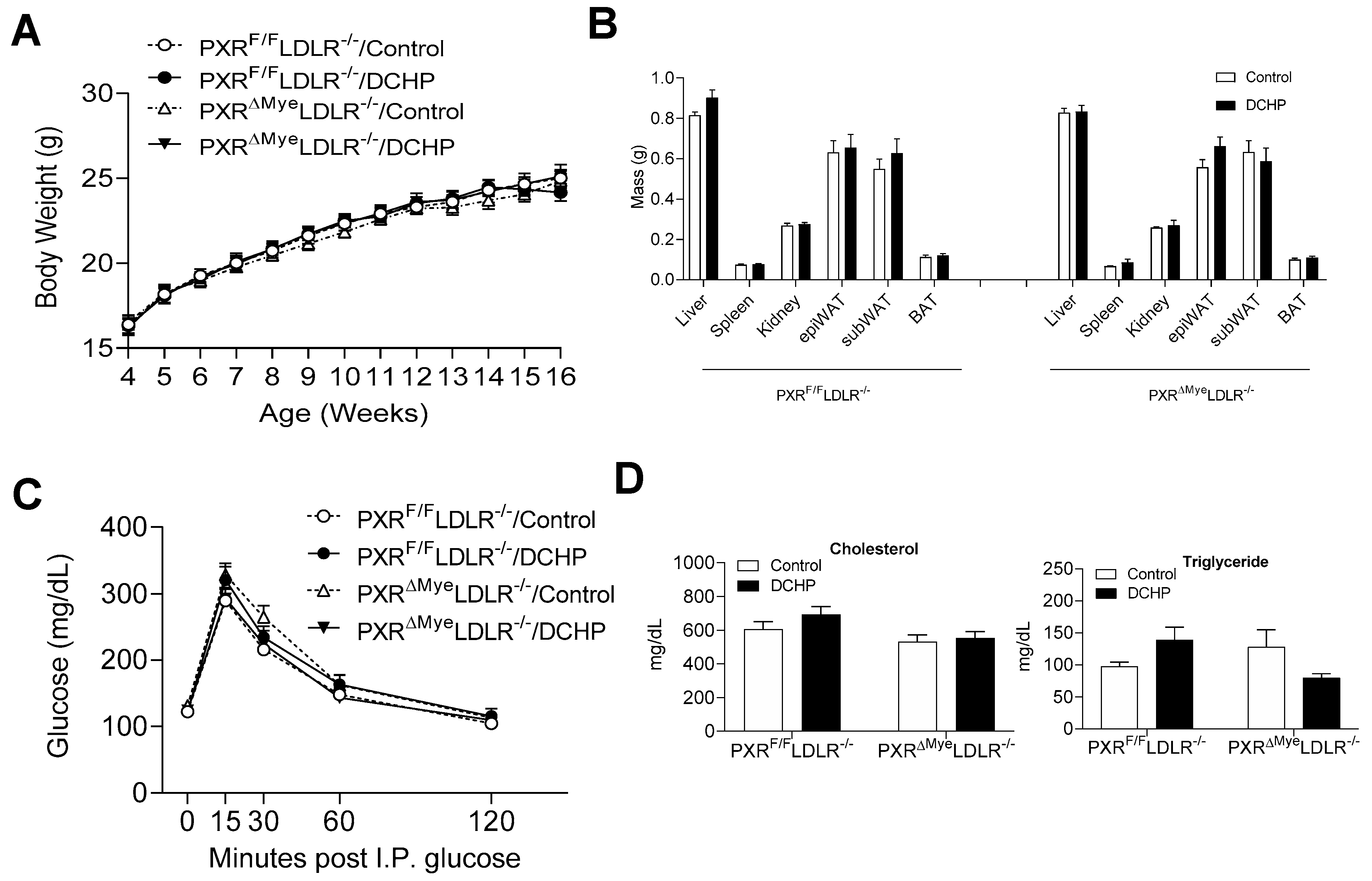
3.1. Exposure to DCHP Does Not Affect Metabolic Phenotypes and Lipid Profiles of PXRF/F LDLR−/− and PXRΔMyeLDLR−/− Mice
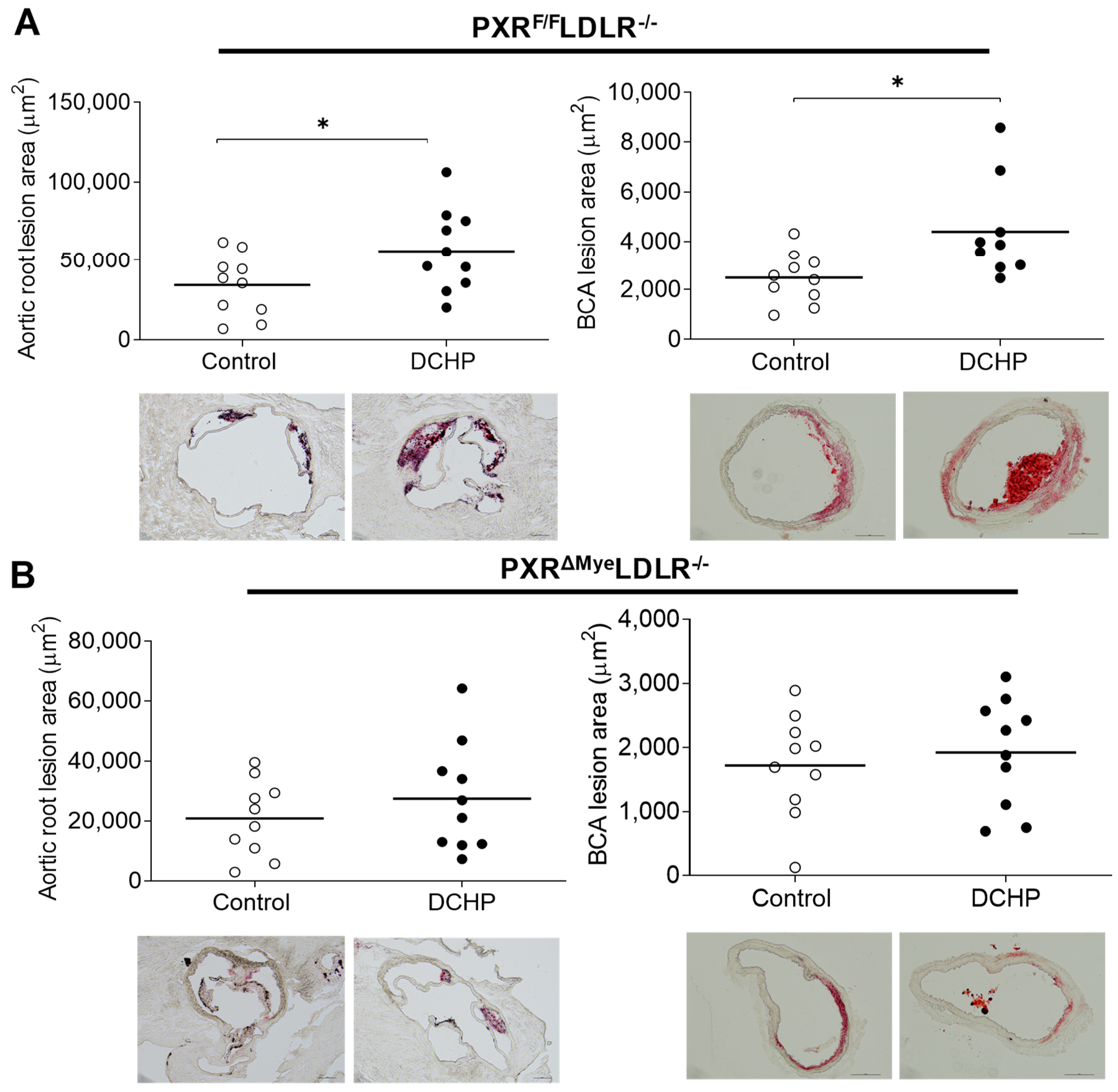
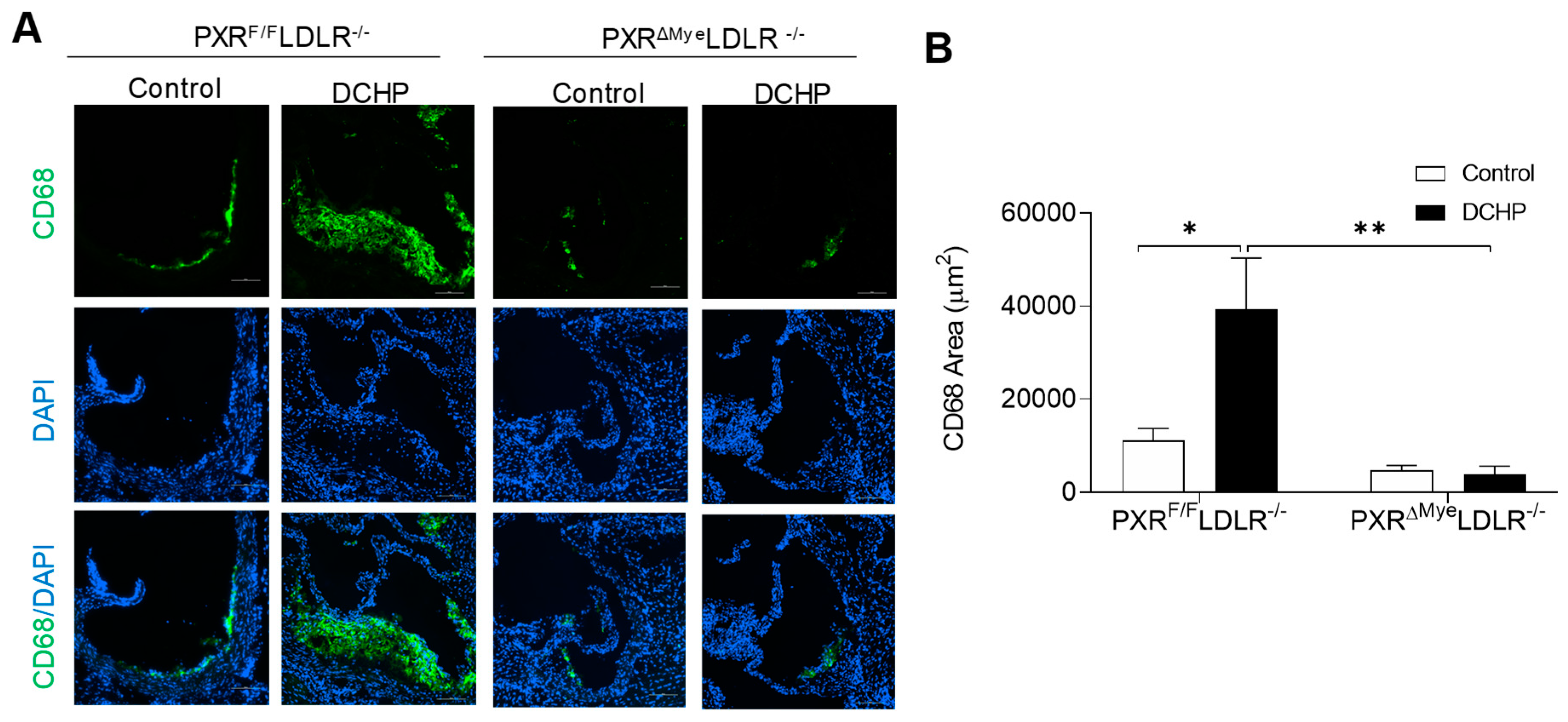
3.2. Chronic Exposure to DCHP Leads to Increased Atherosclerosis in PXRF/F LDLR−/− but Not PXRΔMyeLDLR−/− Mice
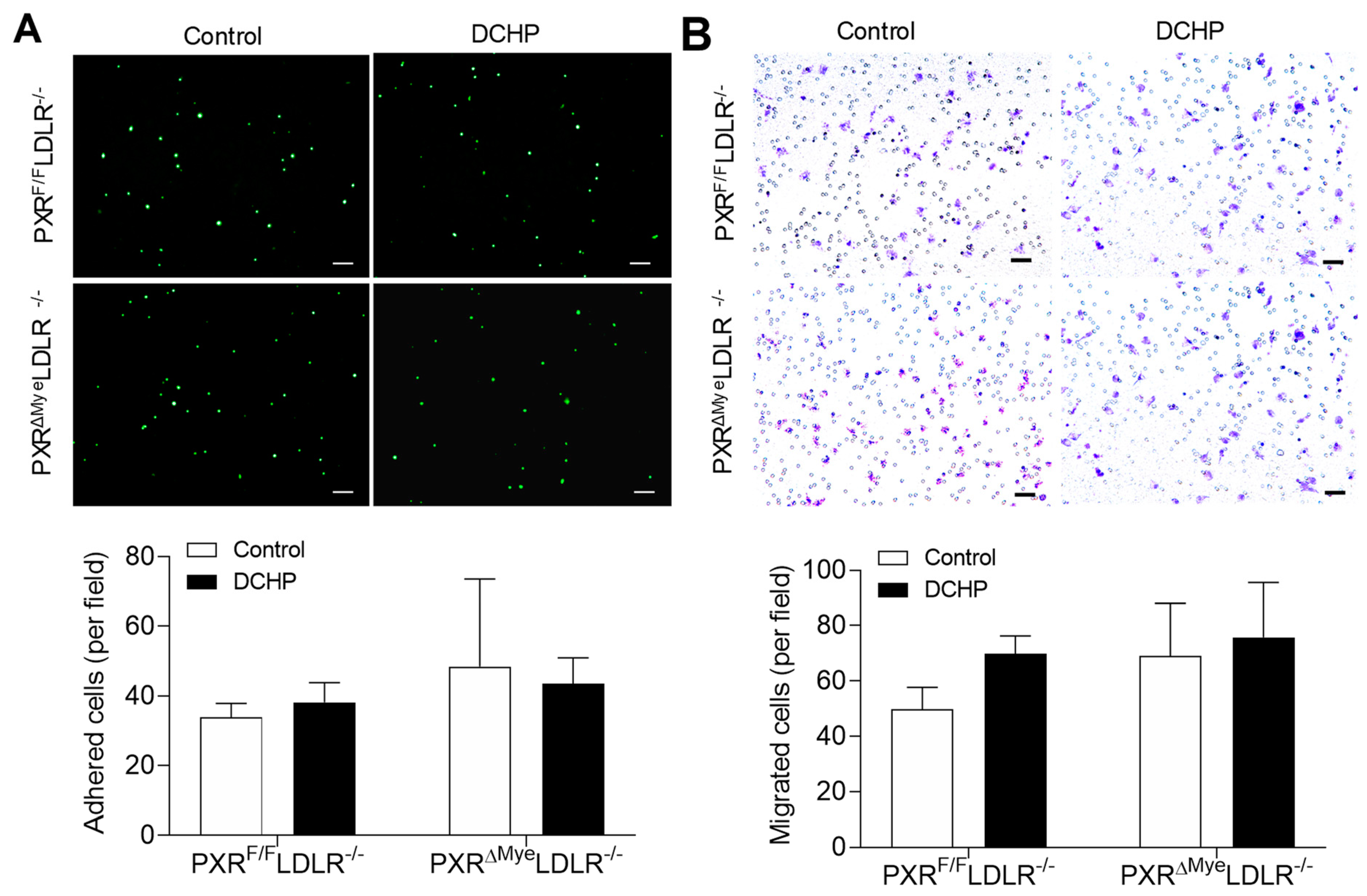
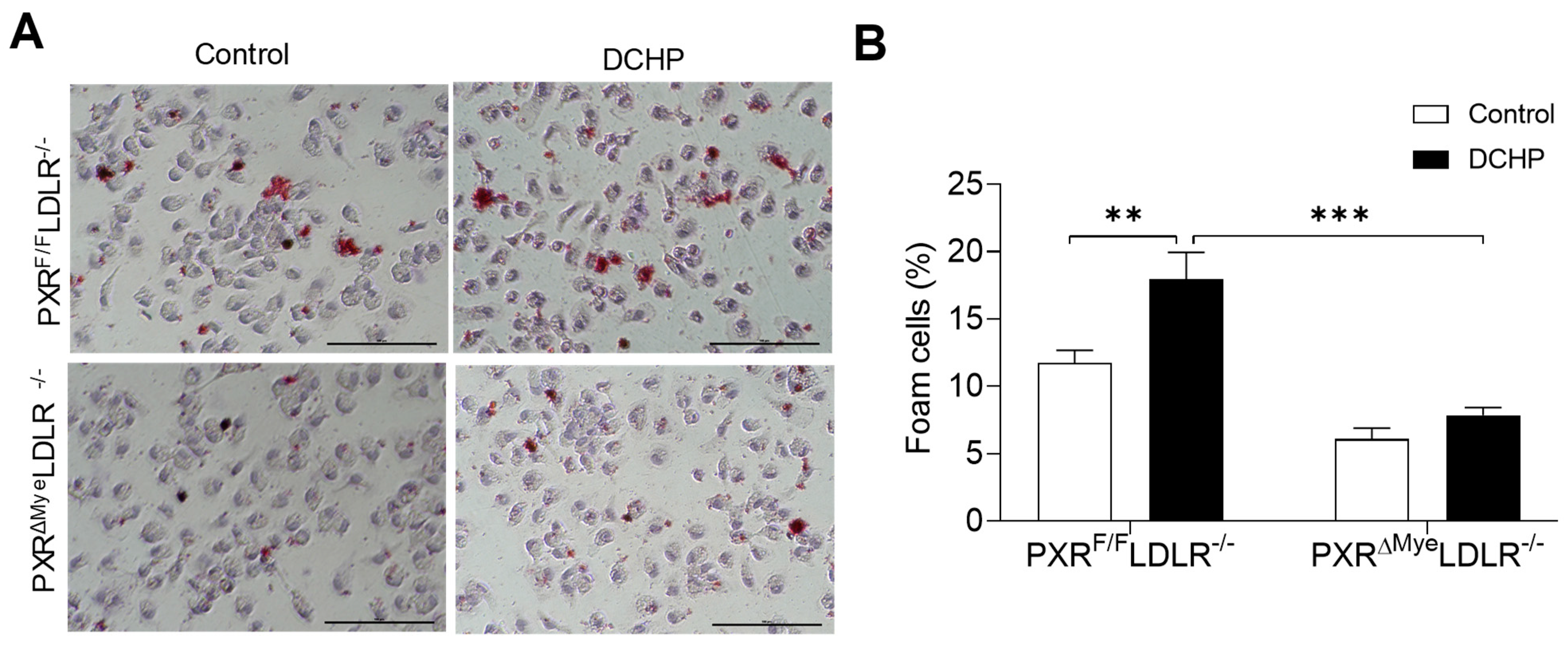
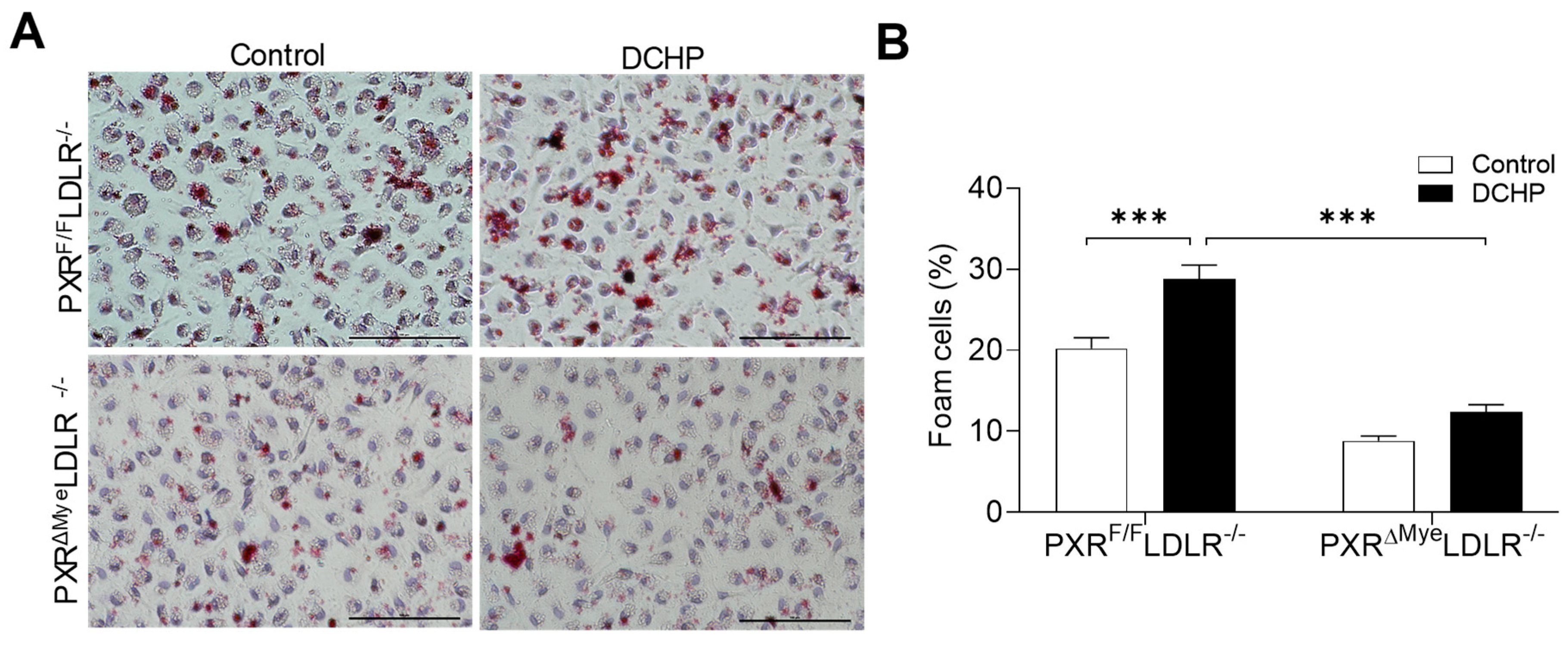
3.3. DCHP Exposure Increases Lipid Accumulation and Foam Cell Formation in Macrophages of PXRF/FLDLR−/− Mice
3.4. DCHP-Mediated PXR Activation Stimulates Macrophage CD36 Expression and Increases Lipid Uptake by Macrophages of PXRF/FLDLR−/− Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roth, G.A.; Forouzanfar, M.H.; Moran, A.E.; Barber, R.; Nguyen, G.; Feigin, V.L.; Naghavi, M.; Mensah, G.A.; Murray, C.J. Demographic and epidemiologic drivers of global cardiovascular mortality. N. Engl. J. Med. 2015, 372, 1333–1341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helsley, R.N.; Zhou, C. Epigenetic impact of endocrine disrupting chemicals on lipid homeostasis and atherosclerosis: A pregnane X receptor-centric view. Environ. Epigenet. 2017, 3, dvx017. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; de Ferranti, S.; Despres, J.P.; Fullerton, H.J.; Howard, V.J.; et al. Heart disease and stroke statistics--2015 update: A report from the American Heart Association. Circulation 2015, 131, e29–e322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dahlöf, B. Cardiovascular Disease Risk Factors: Epidemiology and Risk Assessment. Am. J. Cardiol. 2010, 105, 3A–9A. [Google Scholar] [CrossRef] [PubMed]
- Lind, L.; Araujo, J.A.; Barchowsky, A.; Belcher, S.; Berridge, B.R.; Chiamvimonvat, N.; Chiu, W.A.; Cogliano, V.J.; Elmore, S.; Farraj, A.K.; et al. Key Characteristics of Cardiovascular Toxicants. Environ. Health Perspect. 2021, 129, 95001. [Google Scholar] [CrossRef] [PubMed]
- Trasande, L.; Liu, B.; Bao, W. Phthalates and attributable mortality: A population-based longitudinal cohort study and cost analysis. Environ. Pollut. 2022, 292, 118021. [Google Scholar] [CrossRef] [PubMed]
- Lind, L.; Lind, P.M. Can persistent organic pollutants and plastic-associated chemicals cause cardiovascular disease? J. Intern. Med. 2012, 271, 537–553. [Google Scholar] [CrossRef] [Green Version]
- Melzer, D.; Rice, N.E.; Lewis, C.; Henley, W.E.; Galloway, T.S. Association of urinary bisphenol a concentration with heart disease: Evidence from NHANES 2003/06. PLoS ONE 2010, 5, e8673. [Google Scholar] [CrossRef]
- Melzer, D.; Osborne, N.J.; Henley, W.E.; Cipelli, R.; Young, A.; Money, C.; McCormack, P.; Luben, R.; Khaw, K.T.; Wareham, N.J.; et al. Urinary bisphenol a concentration and risk of future coronary artery disease in apparently healthy men and women. Circulation 2012, 125, 1482–1490. [Google Scholar] [CrossRef] [Green Version]
- Lang, I.A.; Galloway, T.S.; Scarlett, A.; Henley, W.E.; Depledge, M.; Wallace, R.B.; Melzer, D. Association of urinary bisphenol a concentration with medical disorders and laboratory abnormalities in adults. JAMA 2008, 300, 1303–1310. [Google Scholar] [CrossRef]
- Lind, P.M.; Lind, L. Circulating levels of bisphenol A and phthalates are related to carotid atherosclerosis in the elderly. Atherosclerosis 2011, 218, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Vom Saal, F.S.; Myers, J.P. Bisphenol a and risk of metabolic disorders. JAMA 2008, 300, 1353–1355. [Google Scholar] [CrossRef] [PubMed]
- Olsen, L.; Lampa, E.; Birkholz, D.A.; Lind, L.; Lind, P.M. Circulating levels of bisphenol A (BPA) and phthalates in an elderly population in Sweden, based on the Prospective Investigation of the Vasculature in Uppsala Seniors (PIVUS). Ecotoxicol. Environ. Saf. 2012, 75, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Olsen, L.; Lind, L.; Lind, P.M. Associations between circulating levels of bisphenol A and phthalate metabolites and coronary risk in the elderly. Ecotoxicology and environmental safety 2012, 80, 179–183. [Google Scholar] [CrossRef]
- Halden, R.U. Plastics and health risks. Annu. Rev. Public Health 2010, 31, 179–194. [Google Scholar] [CrossRef] [Green Version]
- Casals-Casas, C.; Desvergne, B. Endocrine disruptors: From endocrine to metabolic disruption. Annu. Rev. Physiol. 2011, 73, 135–162. [Google Scholar] [CrossRef] [Green Version]
- Blumberg, B.; Sabbagh, W., Jr.; Juguilon, H.; Bolado, J., Jr.; van Meter, C.M.; Ong, E.S.; Evans, R.M. SXR, a novel steroid and xenobiotic-sensing nuclear receptor. Genes Dev. 1998, 12, 3195–3205. [Google Scholar] [CrossRef] [Green Version]
- Kliewer, S.A.; Goodwin, B.; Willson, T.M. The nuclear pregnane X receptor: A key regulator of xenobiotic metabolism. Endocr. Rev. 2002, 23, 687–702. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.; Verma, S.; Blumberg, B. The steroid and xenobiotic receptor (SXR), beyond xenobiotic metabolism. Nucl. Recept. Signal. 2009, 7, nrs-07001. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C. Novel functions of PXR in cardiometabolic disease. Biochim. Biophys. Acta 2016, 1859, 1112–1120. [Google Scholar] [CrossRef] [Green Version]
- Sui, Y.; Ai, N.; Park, S.H.; Rios-Pilier, J.; Perkins, J.T.; Welsh, W.J.; Zhou, C. Bisphenol a and its analogues activate human pregnane x receptor. Environ. Health Perspect. 2012, 120, 399–405. [Google Scholar] [CrossRef] [PubMed]
- DeKeyser, J.G.; Laurenzana, E.M.; Peterson, E.C.; Chen, T.; Omiecinski, C.J. Selective phthalate activation of naturally occurring human constitutive androstane receptor splice variants and the pregnane X receptor. Toxicol. Sci. 2011, 120, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.; Helsley, R.N.; Park, S.H.; Song, X.; Liu, Z.; Zhou, C. Intestinal pregnane x receptor links xenobiotic exposure and hypercholesterolemia. Mol. Endocrinol. 2015, 29, 765–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sui, Y.; Meng, Z.; Chen, J.; Liu, J.; Hernandez, R.; Gonzales, M.B.; Gwag, T.; Morris, A.J.; Zhou, C. Effects of Dicyclohexyl Phthalate Exposure on PXR Activation and Lipid Homeostasis in Mice. Environ. Health Perspect. 2021, 129, 127001. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.; Xu, J.; Rios-Pilier, J.; Zhou, C. Deficiency of PXR decreases atherosclerosis in apoE-deficient mice. J. Lipid Res. 2011, 52, 1652–1659. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.; King, N.; Chen, K.Y.; Breslow, J.L. Activation of PXR induces hypercholesterolemia in wild-type and accelerates atherosclerosis in apoE deficient mice. J. Lipid Res. 2009, 50, 2004–2013. [Google Scholar] [CrossRef] [Green Version]
- de Haan, W.; de Vries-van der Weij, J.; Mol, I.M.; Hoekstra, M.; Romijn, J.A.; Jukema, J.W.; Havekes, L.M.; Princen, H.M.; Rensen, P.C. PXR agonism decreases plasma HDL levels in ApoE3-Leiden.CETP mice. Biochim. Biophys. Acta 2009, 1791, 191–197. [Google Scholar] [CrossRef]
- Cheng, J.; Krausz, K.W.; Tanaka, N.; Gonzalez, F.J. Chronic exposure to rifaximin causes hepatic steatosis in pregnane x receptor-humanized mice. Toxicol. Sci. 2012, 129, 456–468. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Zhai, Y.; Mu, Y.; Gong, H.; Uppal, H.; Toma, D.; Ren, S.; Evans, R.M.; Xie, W. A novel pregnane X receptor-mediated and sterol regulatory element-binding protein-independent lipogenic pathway. J. Biol. Chem. 2006, 281, 15013–15020. [Google Scholar] [CrossRef] [Green Version]
- He, J.; Gao, J.; Xu, M.; Ren, S.; Stefanovic-Racic, M.; O’Doherty, R.M.; Xie, W. PXR ablation alleviates diet-induced and genetic obesity and insulin resistance in mice. Diabetes 2013, 62, 1876–1887. [Google Scholar] [CrossRef] [Green Version]
- Helsley, R.N.; Sui, Y.; Ai, N.; Park, S.H.; Welsh, W.J.; Zhou, C. Pregnane X receptor mediates dyslipidemia induced by the HIV protease inhibitor amprenavir in mice. Mol. Pharmacol. 2013, 83, 1190–1199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sui, Y.; Park, S.H.; Helsley, R.N.; Sunkara, M.; Gonzalez, F.J.; Morris, A.J.; Zhou, C. Bisphenol A increases atherosclerosis in pregnane X receptor-humanized ApoE deficient mice. J. Am. Heart Assoc. 2014, 3, e000492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sui, Y.; Park, S.H.; Wang, F.; Zhou, C. Perinatal Bisphenol A Exposure Increases Atherosclerosis in Adult Male PXR-Humanized Mice. Endocrinology 2018, 159, 1595–1608. [Google Scholar] [CrossRef] [PubMed]
- Gwag, T.; Meng, Z.; Sui, Y.; Helsley, R.N.; Park, S.H.; Wang, S.; Greenberg, R.N.; Zhou, C. Non-nucleoside reverse transcriptase inhibitor efavirenz activates PXR to induce hypercholesterolemia and hepatic steatosis. J. Hepatol. 2019, 70, 930–940. [Google Scholar] [CrossRef]
- Meng, Z.; Gwag, T.; Sui, Y.; Park, S.H.; Zhou, X.; Zhou, C. The atypical antipsychotic quetiapine induces hyperlipidemia by activating intestinal PXR signaling. JCI Insight 2019, 4, e125657. [Google Scholar] [CrossRef] [Green Version]
- Karpale, M.; Hukkanen, J.; Hakkola, J. Nuclear Receptor PXR in Drug-Induced Hypercholesterolemia. Cells 2022, 11, 313. [Google Scholar] [CrossRef]
- Dubrac, S.; Elentner, A.; Ebner, S.; Horejs-Hoeck, J.; Schmuth, M. Modulation of T lymphocyte function by the pregnane X receptor. J. Immunol. 2010, 184, 2949–2957. [Google Scholar] [CrossRef] [Green Version]
- Albermann, N.; Schmitz-Winnenthal, F.H.; Z’Graggen, K.; Volk, C.; Hoffmann, M.M.; Haefeli, W.E.; Weiss, J. Expression of the drug transporters MDR1/ABCB1, MRP1/ABCC1, MRP2/ABCC2, BCRP/ABCG2, and PXR in peripheral blood mononuclear cells and their relationship with the expression in intestine and liver. Biochem. Pharmacol. 2005, 70, 949–958. [Google Scholar] [CrossRef]
- Owen, A.; Chandler, B.; Back, D.J.; Khoo, S.H. Expression of pregnane-X-receptor transcript in peripheral blood mononuclear cells and correlation with MDR1 mRNA. Antivir. Ther. 2004, 9, 819–821. [Google Scholar]
- Siest, G.; Jeannesson, E.; Marteau, J.B.; Samara, A.; Marie, B.; Pfister, M.; Visvikis-Siest, S. Transcription factor and drug-metabolizing enzyme gene expression in lymphocytes from healthy human subjects. Drug Metab. Dispos. 2008, 36, 182–189. [Google Scholar] [CrossRef] [Green Version]
- Casey, S.C.; Blumberg, B. The steroid and xenobiotic receptor negatively regulates B-1 cell development in the fetal liver. Mol. Endocrinol. 2012, 26, 916–925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casey, S.C.; Nelson, E.L.; Turco, G.M.; Janes, M.R.; Fruman, D.A.; Blumberg, B. B-1 cell lymphoma in mice lacking the steroid and xenobiotic receptor, SXR. Mol. Endocrinol. 2011, 25, 933–943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lusis, A.J. Atherosclerosis. Nature 2000, 407, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Glass, C.K.; Witztum, J.L. Atherosclerosis. the road ahead. Cell 2001, 104, 503–516. [Google Scholar] [CrossRef] [Green Version]
- Sui, Y.; Meng, Z.; Park, S.H.; Lu, W.; Livelo, C.; Chen, Q.; Zhou, T.; Zhou, C. Myeloid-specific deficiency of pregnane X receptor decreases atherosclerosis in LDL receptor-deficient mice. J. Lipid Res. 2020, 61, 696–706. [Google Scholar] [CrossRef] [Green Version]
- Clausen, B.E.; Burkhardt, C.; Reith, W.; Renkawitz, R.; Forster, I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999, 8, 265–277. [Google Scholar] [CrossRef]
- Teupser, D.; Persky, A.D.; Breslow, J.L. Induction of atherosclerosis by low-fat, semisynthetic diets in LDL receptor-deficient C57BL/6J and FVB/NJ mice: Comparison of lesions of the aortic root, brachiocephalic artery, and whole aorta (en face measurement). Arterioscler. Thromb. Vasc. Biol. 2003, 23, 1907–1913. [Google Scholar] [CrossRef] [Green Version]
- Sui, Y.; Park, S.H.; Xu, J.; Monette, S.; Helsley, R.N.; Han, S.S.; Zhou, C. IKKbeta links vascular inflammation to obesity and atherosclerosis. J. Exp. Med. 2014, 211, 869–886. [Google Scholar] [CrossRef]
- Meng, Z.; Hernandez, R.; Liu, J.; Gwag, T.; Lu, W.; Hsiai, T.K.; Kaul, M.; Zhou, T.; Zhou, C. HIV Protein Tat Induces Macrophage Dysfunction and Atherosclerosis Development in Low-Density Lipoprotein Receptor-Deficient Mice. Cardiovasc. Drugs Ther. 2022, 36, 201–205. [Google Scholar] [CrossRef]
- Helsley, R.N.; Sui, Y.; Park, S.H.; Liu, Z.; Lee, R.G.; Zhu, B.; Kern, P.A.; Zhou, C. Targeting IkappaB kinase beta in Adipocyte Lineage Cells for Treatment of Obesity and Metabolic Dysfunctions. Stem. Cells 2016, 34, 1883–1895. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Liu, Z.; Park, S.H.; Gwag, T.; Lu, W.; Ma, M.; Sui, Y.; Zhou, C. Myeloid beta-Catenin Deficiency Exacerbates Atherosclerosis in Low-Density Lipoprotein Receptor-Deficient Mice. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1468–1478. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Meng, Z.; Hernandez, R.; Zhou, C. Fibroblast-specific IKK-beta deficiency ameliorates angiotensin II-induced adverse cardiac remodeling in mice. JCI Insight 2021, 6, e150161. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Park, S.H.; Meng, Z.; Wang, F.; Zhou, C. Deficiency of Adipocyte IKKbeta Affects Atherosclerotic Plaque Vulnerability in Obese LDLR Deficient Mice. J. Am. Heart Assoc. 2019, 8, e012009. [Google Scholar] [CrossRef] [PubMed]
- Satta, S.; Meng, Z.; Hernandez, R.; Cavallero, S.; Zhou, T.; Hsiai, T.K.; Zhou, C. An engineered nano-liposome-human ACE2 decoy neutralizes SARS-CoV-2 Spike protein-induced inflammation in both murine and human macrophages. Theranostics 2022, 12, 2639–2657. [Google Scholar] [CrossRef]
- Park, S.H.; Sui, Y.; Gizard, F.; Xu, J.; Rios-Pilier, J.; Helsley, R.N.; Han, S.S.; Zhou, C. Myeloid-specific IkappaB kinase beta deficiency decreases atherosclerosis in low-density lipoprotein receptor-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 2869–2876. [Google Scholar] [CrossRef] [Green Version]
- Zhou, C.; Pridgen, B.; King, N.; Xu, J.; Breslow, J.L. Hyperglycemic Ins2AkitaLdlr-/- mice show severely elevated lipid levels and increased atherosclerosis: A model of type 1 diabetic macrovascular disease. J. Lipid Res. 2011, 52, 1483–1493. [Google Scholar] [CrossRef] [Green Version]
- Rahaman, S.O.; Lennon, D.J.; Febbraio, M.; Podrez, E.A.; Hazen, S.L.; Silverstein, R.L. A CD36-dependent signaling cascade is necessary for macrophage foam cell formation. Cell Metab. 2006, 4, 211–221. [Google Scholar] [CrossRef] [Green Version]
- Kennedy, D.J.; Kuchibhotla, S.D.; Guy, E.; Park, Y.M.; Nimako, G.; Vanegas, D.; Morton, R.E.; Febbraio, M. Dietary cholesterol plays a role in CD36-mediated atherogenesis in LDLR-knockout mice. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1481–1487. [Google Scholar] [CrossRef] [Green Version]
- Geyer, R. Production, use, and fate of synthetic polymers. In Plastic Waste and Recycling; Elsevier: Amsterdam, The Netherlands, 2020; pp. 13–32. [Google Scholar]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [Green Version]
- Ramadan, M.; Cooper, B.; Posnack, N.G. Bisphenols and phthalates: Plastic chemical exposures can contribute to adverse cardiovascular health outcomes. Birth Defects Res. 2020, 112, 1362–1385. [Google Scholar] [CrossRef]
- Posnack, N.G. Plastics and cardiovascular disease. Nat. Rev. Cardiol. 2021, 18, 69–70. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.C.; Moore, C.J.; Vom Saal, F.S.; Swan, S.H. Plastics, the environment and human health: Current consensus and future trends. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2153–2166. [Google Scholar] [CrossRef] [PubMed]
- Pflieger-Bruss, S.; Schuppe, H.C.; Schill, W.B. The male reproductive system and its susceptibility to endocrine disrupting chemicals. Andrologia 2004, 36, 337–345. [Google Scholar] [CrossRef] [PubMed]
- Latini, G.; Del Vecchio, A.; Massaro, M.; Verrotti, A.; De Felice, C. Phthalate exposure and male infertility. Toxicology 2006, 226, 90–98. [Google Scholar] [CrossRef]
- Fisher, J.S. Environmental anti-androgens and male reproductive health: Focus on phthalates and testicular dysgenesis syndrome. Reproduction 2004, 127, 305–315. [Google Scholar] [CrossRef] [Green Version]
- Hu, G.X.; Lian, Q.Q.; Ge, R.S.; Hardy, D.O.; Li, X.K. Phthalate-induced testicular dysgenesis syndrome: Leydig cell influence. Trends Endocrinol. Metab. 2009, 20, 139–145. [Google Scholar] [CrossRef] [Green Version]
- López-Carrillo, L.; Hernández-Ramírez, R.U.; Calafat, A.M.; Torres-Sánchez, L.; Galván-Portillo, M.; Needham, L.L.; Ruiz-Ramos, R.; Cebrián, M.E. Exposure to phthalates and breast cancer risk in northern Mexico. Environ. Health Perspect. 2010, 118, 539–544. [Google Scholar] [CrossRef]
- Sun, Q.; Cornelis, M.C.; Townsend, M.K.; Tobias, D.K.; Eliassen, A.H.; Franke, A.A.; Hauser, R.; Hu, F.B. Association of urinary concentrations of bisphenol A and phthalate metabolites with risk of type 2 diabetes: A prospective investigation in the Nurses’ Health Study (NHS) and NHSII cohorts. Environ. Health Perspect. 2014, 122, 616–623. [Google Scholar] [CrossRef]
- Zhang, H.; Ben, Y.; Han, Y.; Zhang, Y.; Li, Y.; Chen, X. Phthalate exposure and risk of diabetes mellitus: Implications from a systematic review and meta-analysis. Environ. Res. 2022, 204, 112109. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, T.; Hu, W.; Wang, X.; Xu, B.; Lin, Z.; Hofer, T.; Stefanoff, P.; Chen, Y.; Wang, X. Association between exposure to a mixture of phenols, pesticides, and phthalates and obesity: Comparison of three statistical models. Environ. Int. 2019, 123, 325–336. [Google Scholar] [CrossRef]
- Stojanoska, M.M.; Milosevic, N.; Milic, N.; Abenavoli, L. The influence of phthalates and bisphenol A on the obesity development and glucose metabolism. Disorders Endocr. 2017, 55, 666–681. [Google Scholar] [CrossRef] [PubMed]
- Mariana, M.; Cairrao, E. Phthalates implications in the cardiovascular system. J. Cardiovasc. Dev. Dis. 2020, 7, 26. [Google Scholar] [CrossRef] [PubMed]
- Golestanzadeh, M.; Riahi, R.; Kelishadi, R. Association of exposure to phthalates with cardiometabolic risk factors in children and adolescents: A systematic review and meta-analysis. Environ. Sci. Pollut. Res. 2019, 26, 35670–35686. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Xu, X.; Lin, Y.; Zhang, Y.; Huo, X. Phthalate exposure as a risk factor for hypertension. Environ. Sci. Pollut. Res. 2018, 25, 20550–20561. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Xu, J.; Zhang, R.; Yu, J. The association between environmental endocrine disruptors and cardiovascular diseases: A systematic review and meta-analysis. Environ. Res. 2020, 187, 109464. [Google Scholar] [CrossRef] [PubMed]
- Fierens, T.; Servaes, K.; Van Holderbeke, M.; Geerts, L.; De Henauw, S.; Sioen, I.; Vanermen, G. Analysis of phthalates in food products and packaging materials sold on the Belgian market. Food Chem. Toxicol. 2012, 50, 2575–2583. [Google Scholar] [CrossRef]
- Clara, M.; Windhofer, G.; Hartl, W.; Braun, K.; Simon, M.; Gans, O.; Scheffknecht, C.; Chovanec, A. Occurrence of phthalates in surface runoff, untreated and treated wastewater and fate during wastewater treatment. Chemosphere 2010, 78, 1078–1084. [Google Scholar] [CrossRef]
- EPA. Proposed Designation of Dicyclohexyl Phthalate (CASRN 84-61-7) as a High-Priority Substance for Risk Evaluation. 2019. Available online: https://nepis.epa.gov/Exe/ZyPURL.cgi?Dockey=P100XPOZ.txt (accessed on 10 January 2022).
- Lottrup, G.; Andersson, A.M.; Leffers, H.; Mortensen, G.; Toppari, J.; Skakkebaek, N.; Main, K. Possible impact of phthalates on infant reproductive health. Int. J. Androl. 2006, 29, 172–180. [Google Scholar] [CrossRef]
- Swan, S.H. Environmental phthalate exposure in relation to reproductive outcomes and other health endpoints in humans. Environ. Res. 2008, 108, 177–184. [Google Scholar] [CrossRef] [Green Version]
- Sharman, M.; Read, W.A.; Castle, L.; Gilbert, J. Levels of di-(2-ethylhexyl)phthalate and total phthalate esters in milk, cream, butter and cheese. Food Addit. Contam. 1994, 11, 375–385. [Google Scholar] [CrossRef]
- Wittassek, M.; Wiesmuller, G.A.; Koch, H.M.; Eckard, R.; Dobler, L.; Muller, J.; Angerer, J.; Schluter, C. Internal phthalate exposure over the last two decades—A retrospective human biomonitoring study. Int. J. Hyg. Environ. Health 2007, 210, 319–333. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.J.; Barr, D.B.; Reidy, J.A.; Malek, N.A.; Hodge, C.C.; Caudill, S.P.; Brock, J.W.; Needham, L.L.; Calafat, A.M. Urinary levels of seven phthalate metabolites in the U.S. population from the National Health and Nutrition Examination Survey (NHANES) 1999–2000. Env. Health Perspect 2004, 112, 331–338. [Google Scholar] [CrossRef] [PubMed]
- Blount, B.C.; Silva, M.J.; Caudill, S.P.; Needham, L.L.; Pirkle, J.L.; Sampson, E.J.; Lucier, G.W.; Jackson, R.J.; Brock, J.W. Levels of seven urinary phthalate metabolites in a human reference population. Environ. Health Perspect. 2000, 108, 979–982. [Google Scholar] [CrossRef] [PubMed]
- Sakhi, A.K.; Lillegaard, I.T.; Voorspoels, S.; Carlsen, M.H.; Loken, E.B.; Brantsaeter, A.L.; Haugen, M.; Meltzer, H.M.; Thomsen, C. Concentrations of phthalates and bisphenol A in Norwegian foods and beverages and estimated dietary exposure in adults. Environ. Int. 2014, 73, 259–269. [Google Scholar] [CrossRef]
- Schecter, A.; Lorber, M.; Guo, Y.; Wu, Q.; Yun, S.H.; Kannan, K.; Hommel, M.; Imran, N.; Hynan, L.S.; Cheng, D.; et al. Phthalate concentrations and dietary exposure from food purchased in New York State. Environ. Health Perspect. 2013, 121, 473–494. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Z.; Li, H.H.; Wang, H.S.; Zhu, X.M.; Sthiannopkao, S.; Kim, K.W.; Yasin, M.S.M.; Hashim, J.H.; Wong, M.H. Dietary exposure and human risk assessment of phthalate esters based on total diet study in Cambodia. Environ. Res. 2016, 150, 423–430. [Google Scholar] [CrossRef]
- Cao, X.L.; Zhao, W.; Dabeka, R. Di-(2-ethylhexyl) adipate and 20 phthalates in composite food samples from the 2013 Canadian Total Diet Study. Food Addit. Contam. Part A Chem. Anal. Control Expo. Risk Assess 2015, 32, 1893–1901. [Google Scholar] [CrossRef]
- Huang, Y.; Li, J.; Garcia, J.M.; Lin, H.; Wang, Y.; Yan, P.; Wang, L.; Tan, Y.; Luo, J.; Qiu, Z.; et al. Phthalate levels in cord blood are associated with preterm delivery and fetal growth parameters in Chinese women. PLoS ONE 2014, 9, e87430. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Zhou, Y.; Tang, C.; He, Y.; Wu, J.; Chen, Y.; Jiang, Q. Urinary phthalate metabolites are associated with body mass index and waist circumference in Chinese school children. PLoS ONE 2013, 8, e56800. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, C.; Uhl, M.; Weiss, S.; Scharf, S.; Konig, J. Austrian reference values for phthalate metabolite exposure in children/adolescents and adults. Int. J. Hyg. Environ. Health 2018, 221, 985–989. [Google Scholar] [CrossRef]
- Saravanabhavan, G.; Guay, M.; Langlois, E.; Giroux, S.; Murray, J.; Haines, D. Biomonitoring of phthalate metabolites in the Canadian population through the Canadian Health Measures Survey (2007–2009). Int. J. Hyg. Environ. Health 2013, 216, 652–661. [Google Scholar] [CrossRef] [PubMed]
- Tabb, M.M.; Zhou, C.; Blumberg, B. SXR and the Xenobiotic Response; Springer Tokyo Inc.: Tokyo, Japan, 2003; pp. 115–125. [Google Scholar]
- Lake, B.G.; Foster, J.R.; Collins, M.A.; Stubberfield, C.R.; Gangolli, S.D.; Srivastava, S.P. Studies on the effects of orally administered dicyclohexyl phthalate in the rat. Acta Pharmacol. Toxicol. 1982, 51, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Fang, Y.; Chen, P.; Duan, Y.; Huang, T.; Ma, L.; Xie, L.; Chen, X.; Chen, X.; Gao, J.; et al. Dicyclohexyl phthalate blocks Leydig cell regeneration in adult rat testis. Toxicology 2019, 411, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, X.; Hu, G.; Li, L.; Su, H.; Wang, Y.; Chen, D.; Zhu, Q.; Li, C.; Li, J.; et al. Effects of in Utero Exposure to Dicyclohexyl Phthalate on Rat Fetal Leydig Cells. Int. J. Environ. Res. Public Health 2016, 13, 246. [Google Scholar] [CrossRef] [Green Version]
- Ahbab, M.A.; Guven, C.; Kockaya, E.A.; Barlas, N. Comparative developmental toxicity evaluation of di- n-hexyl phthalate and dicyclohexyl phthalate in rats. Toxicol. Ind. Health 2017, 33, 696–716. [Google Scholar] [CrossRef]
- Lapinskas, P.J.; Brown, S.; Leesnitzer, L.M.; Blanchard, S.; Swanson, C.; Cattley, R.C.; Corton, J.C. Role of PPARalpha in mediating the effects of phthalates and metabolites in the liver. Toxicology 2005, 207, 149–163. [Google Scholar] [CrossRef]
- Nair, A.B.; Jacob, S. A simple practice guide for dose conversion between animals and human. J. Basic. Clin. Pharm. 2016, 7, 27–31. [Google Scholar] [CrossRef] [Green Version]
- Sharma, V.; McNeill, J.H. To scale or not to scale: The principles of dose extrapolation. Br. J. Pharmacol. 2009, 157, 907–921. [Google Scholar] [CrossRef] [Green Version]
- Stoddart, C.A.; Bales, C.A.; Bare, J.C.; Chkhenkeli, G.; Galkina, S.A.; Kinkade, A.N.; Moreno, M.E.; Rivera, J.M.; Ronquillo, R.E.; Sloan, B.; et al. Validation of the SCID-hu Thy/Liv mouse model with four classes of licensed antiretrovirals. PLoS ONE 2007, 2, e655. [Google Scholar] [CrossRef] [Green Version]
- Du, Z.H.; Xia, J.; Sun, X.C.; Li, X.N.; Zhang, C.; Zhao, H.S.; Zhu, S.Y.; Li, J.L. A novel nuclear xenobiotic receptors (AhR/PXR/CAR)-mediated mechanism of DEHP-induced cerebellar toxicity in quails (Coturnix japonica) via disrupting CYP enzyme system homeostasis. Environ. Pollut. 2017, 226, 435–443. [Google Scholar] [CrossRef]
- Dvorak, Z.; Sokol, H.; Mani, S. Drug Mimicry: Promiscuous Receptors PXR and AhR, and Microbial Metabolite Interactions in the Intestine. Trends Pharmacol. Sci. 2020, 41, 900–908. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, Z.; Kopp, F.; Costello, C.M.; Kemp, J.S.; Li, H.; Vrzalova, A.; Stepankova, M.; Bartonkova, I.; Jiskrova, E.; Poulikova, K.; et al. Targeting the pregnane X receptor using microbial metabolite mimicry. EMBO Mol. Med. 2020, 12, e11621. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, M.; Mukherjee, S.; Wang, H.; Li, H.; Sun, K.; Benechet, A.P.; Qiu, Z.; Maher, L.; Redinbo, M.R.; Phillips, R.S.; et al. Symbiotic bacterial metabolites regulate gastrointestinal barrier function via the xenobiotic sensor PXR and Toll-like receptor 4. Immunity 2014, 41, 296–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansson, G.K.; Hermansson, A. The immune system in atherosclerosis. Nat. Immunol. 2011, 12, 204–212. [Google Scholar] [CrossRef]
- Hamers, A.A.; Vos, M.; Rassam, F.; Marinkovic, G.; Kurakula, K.; van Gorp, P.J.; de Winther, M.P.; Gijbels, M.J.; de Waard, V.; de Vries, C.J. Bone marrow-specific deficiency of nuclear receptor Nur77 enhances atherosclerosis. Circ. Res. 2012, 110, 428–438. [Google Scholar] [CrossRef] [Green Version]
- Moore, K.J.; Tabas, I. Macrophages in the pathogenesis of atherosclerosis. Cell 2011, 145, 341–355. [Google Scholar] [CrossRef] [Green Version]
- de Villiers, W.J.; Smart, E.J. Macrophage scavenger receptors and foam cell formation. J. Leukoc. Biol. 1999, 66, 740–746. [Google Scholar] [CrossRef]
- Kunjathoor, V.V.; Febbraio, M.; Podrez, E.A.; Moore, K.J.; Andersson, L.; Koehn, S.; Rhee, J.S.; Silverstein, R.; Hoff, H.F.; Freeman, M.W. Scavenger receptors class A-I/II and CD36 are the principal receptors responsible for the uptake of modified low density lipoprotein leading to lipid loading in macrophages. J. Biol. Chem. 2002, 277, 49982–49988. [Google Scholar] [CrossRef] [Green Version]
- Collot-Teixeira, S.; Martin, J.; McDermott-Roe, C.; Poston, R.; McGregor, J.L. CD36 and macrophages in atherosclerosis. Cardiovasc. Res. 2007, 75, 468–477. [Google Scholar] [CrossRef]
- Hukkanen, J.; Hakkola, J. PXR and 4beta-Hydroxycholesterol Axis and the Components of Metabolic Syndrome. Cells 2020, 9, 2445. [Google Scholar] [CrossRef]
- Moreau, A.; Teruel, C.; Beylot, M.; Albalea, V.; Tamasi, V.; Umbdenstock, T.; Parmentier, Y.; Sa-Cunha, A.; Suc, B.; Fabre, J.M.; et al. A novel pregnane X receptor and S14-mediated lipogenic pathway in human hepatocyte. Hepatology 2009, 49, 2068–2079. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Tabb, M.M.; Sadatrafiei, A.; Grun, F.; Blumberg, B. Tocotrienols activate the steroid and xenobiotic receptor, SXR, and selectively regulate expression of its target genes. Drug Metab. Dispos. 2004, 32, 1075–1082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, J.; Shah, Y.M.; Ma, X.; Pang, X.; Tanaka, T.; Kodama, T.; Krausz, K.W.; Gonzalez, F.J. Therapeutic role of rifaximin in inflammatory bowel disease: Clinical implication of human pregnane X receptor activation. J. Pharmacol. Exp. Ther. 2010, 335, 32–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Hernandez, R.; Li, X.; Meng, Z.; Chen, H.; Zhou, C. Pregnane X Receptor Mediates Atherosclerosis Induced by Dicyclohexyl Phthalate in LDL Receptor-Deficient Mice. Cells 2022, 11, 1125. https://doi.org/10.3390/cells11071125
Liu J, Hernandez R, Li X, Meng Z, Chen H, Zhou C. Pregnane X Receptor Mediates Atherosclerosis Induced by Dicyclohexyl Phthalate in LDL Receptor-Deficient Mice. Cells. 2022; 11(7):1125. https://doi.org/10.3390/cells11071125
Chicago/Turabian StyleLiu, Jingwei, Rebecca Hernandez, Xiuchun Li, Zhaojie Meng, Hong Chen, and Changcheng Zhou. 2022. "Pregnane X Receptor Mediates Atherosclerosis Induced by Dicyclohexyl Phthalate in LDL Receptor-Deficient Mice" Cells 11, no. 7: 1125. https://doi.org/10.3390/cells11071125
APA StyleLiu, J., Hernandez, R., Li, X., Meng, Z., Chen, H., & Zhou, C. (2022). Pregnane X Receptor Mediates Atherosclerosis Induced by Dicyclohexyl Phthalate in LDL Receptor-Deficient Mice. Cells, 11(7), 1125. https://doi.org/10.3390/cells11071125







