Identification and Validation of Autophagy-Related Genes in Vitiligo
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tissue Specimens
2.2. RNA Isolation and Library Preparation
2.3. ARGs Dataset
2.4. Differentially Expressed Genes (DEGs) Analysis and Ingenuity Pathways Analysis (IPA)
2.5. qRT-PCR
2.6. Protein Extraction and Western Blotting
2.7. Statistical Analysis
3. Results
3.1. Identification of DEGs in Vitiligo Lesions
3.2. Identification of Differentially Expressed Autophagy-Related Genes (DEARGs) in Vitiligo Lesions
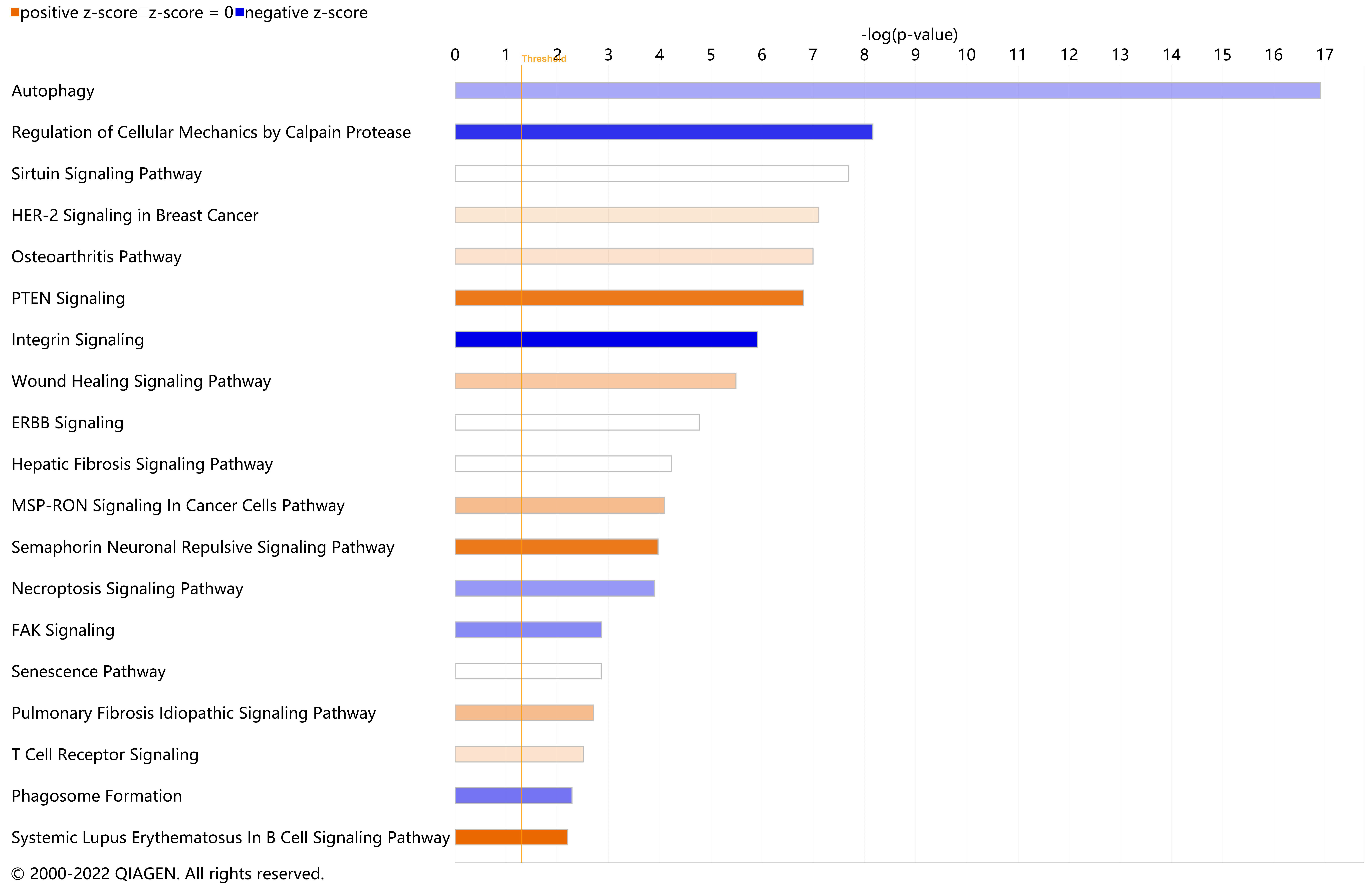
3.3. Canonical Pathway Analysis of DEARGs
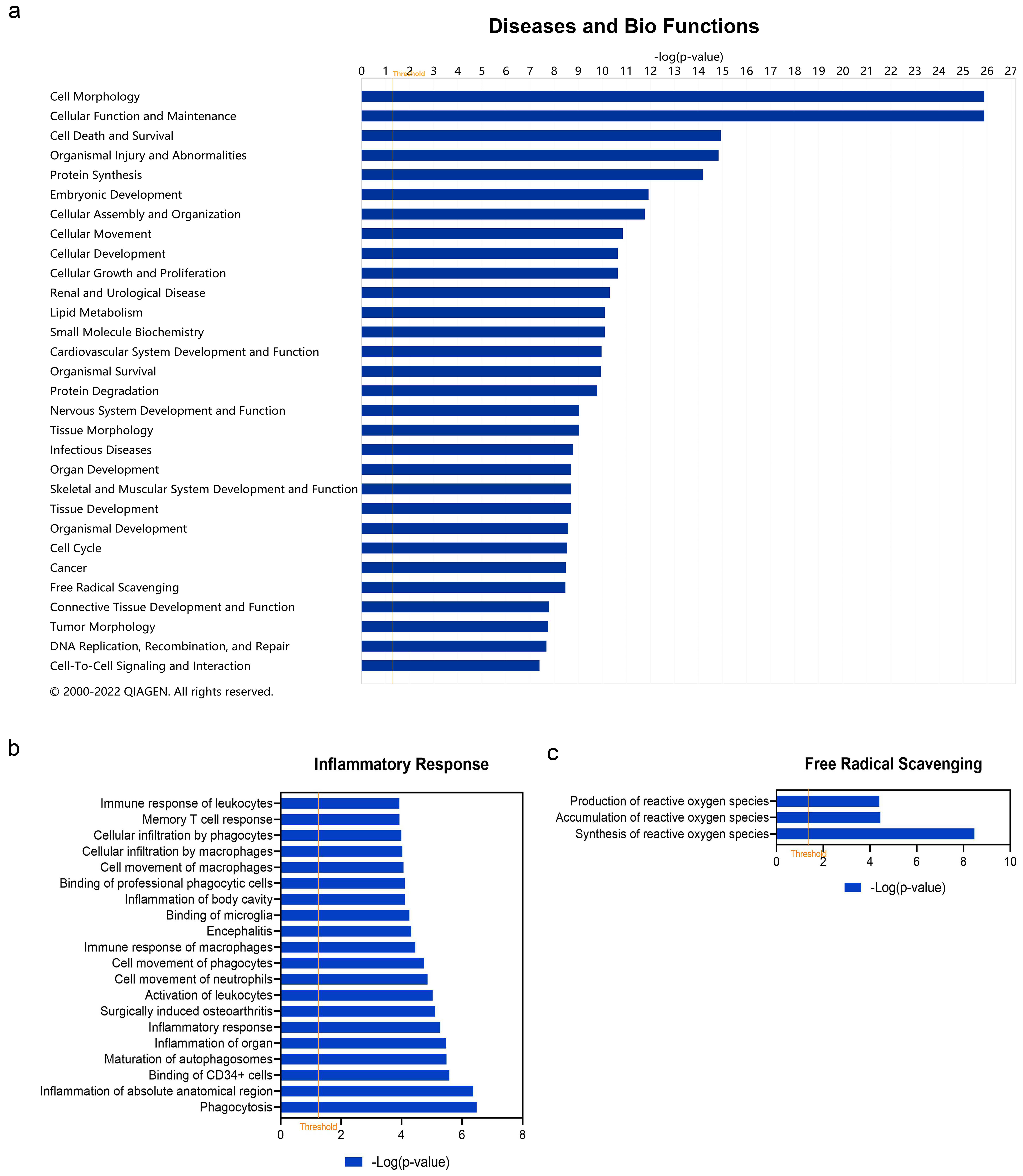
3.4. Diseases and Bio Function Analysis of DEARGs
3.5. Upstream Regulator Analysis of DEARGs
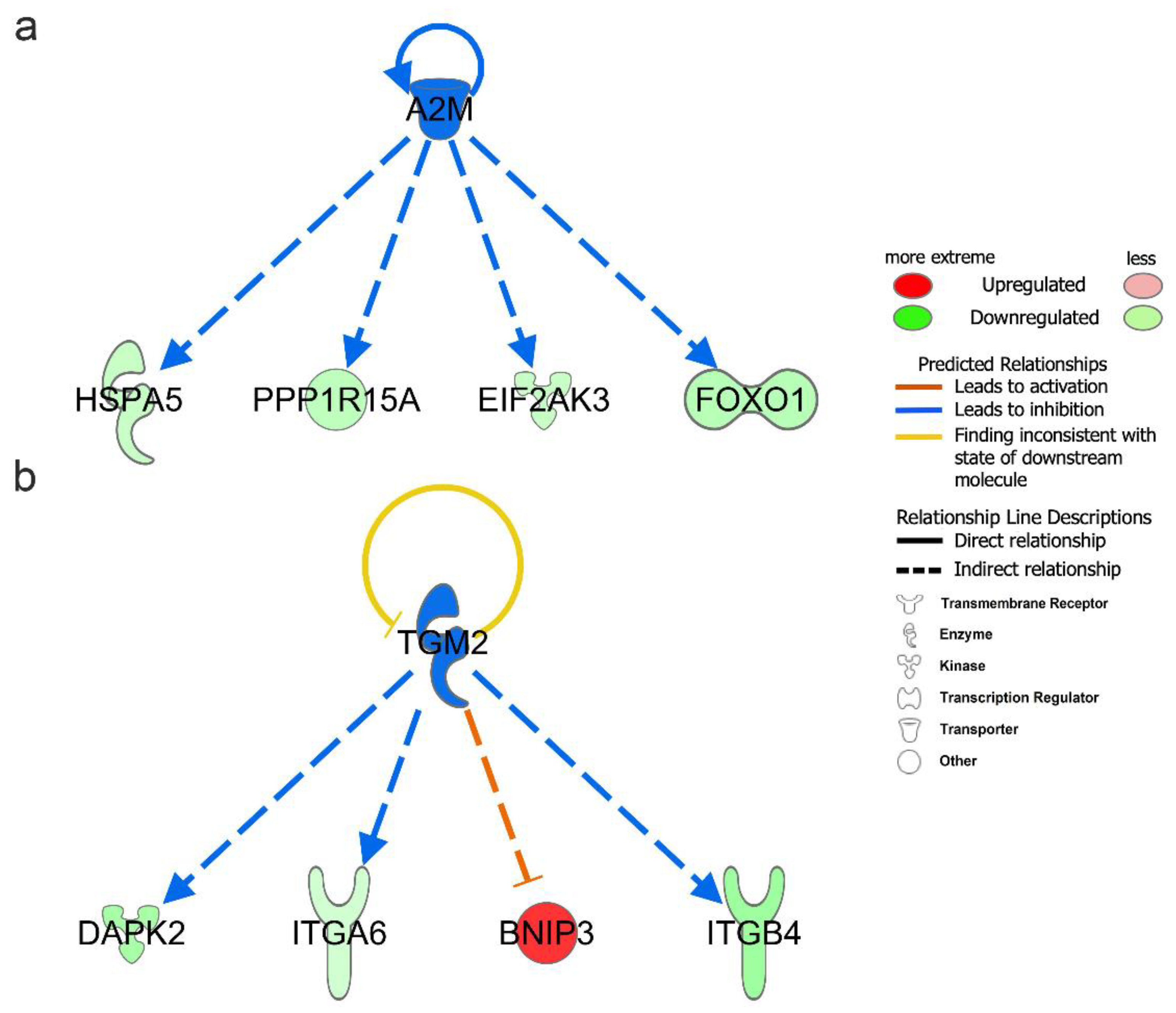
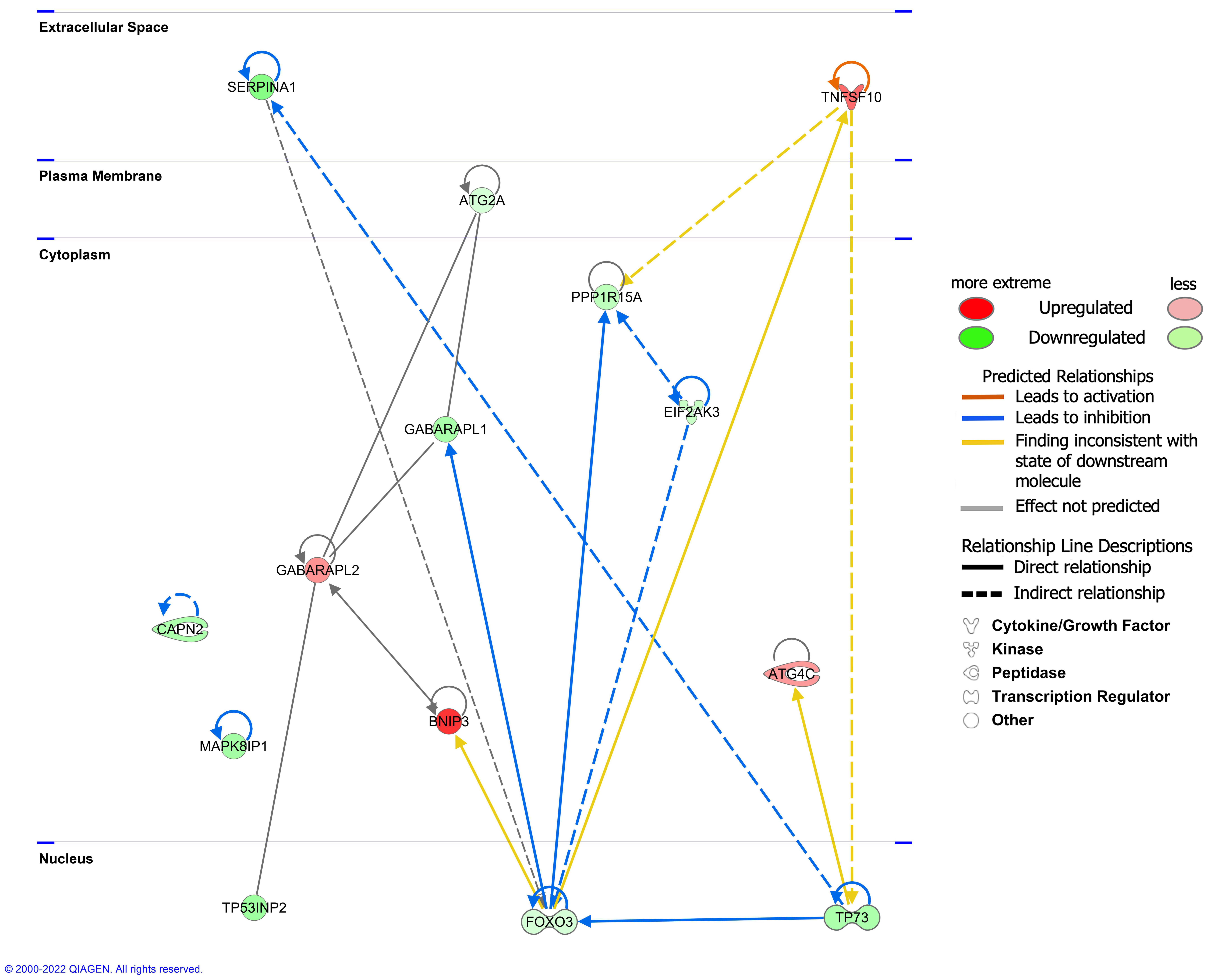
3.6. Molecular Network Analysis of DEARGs
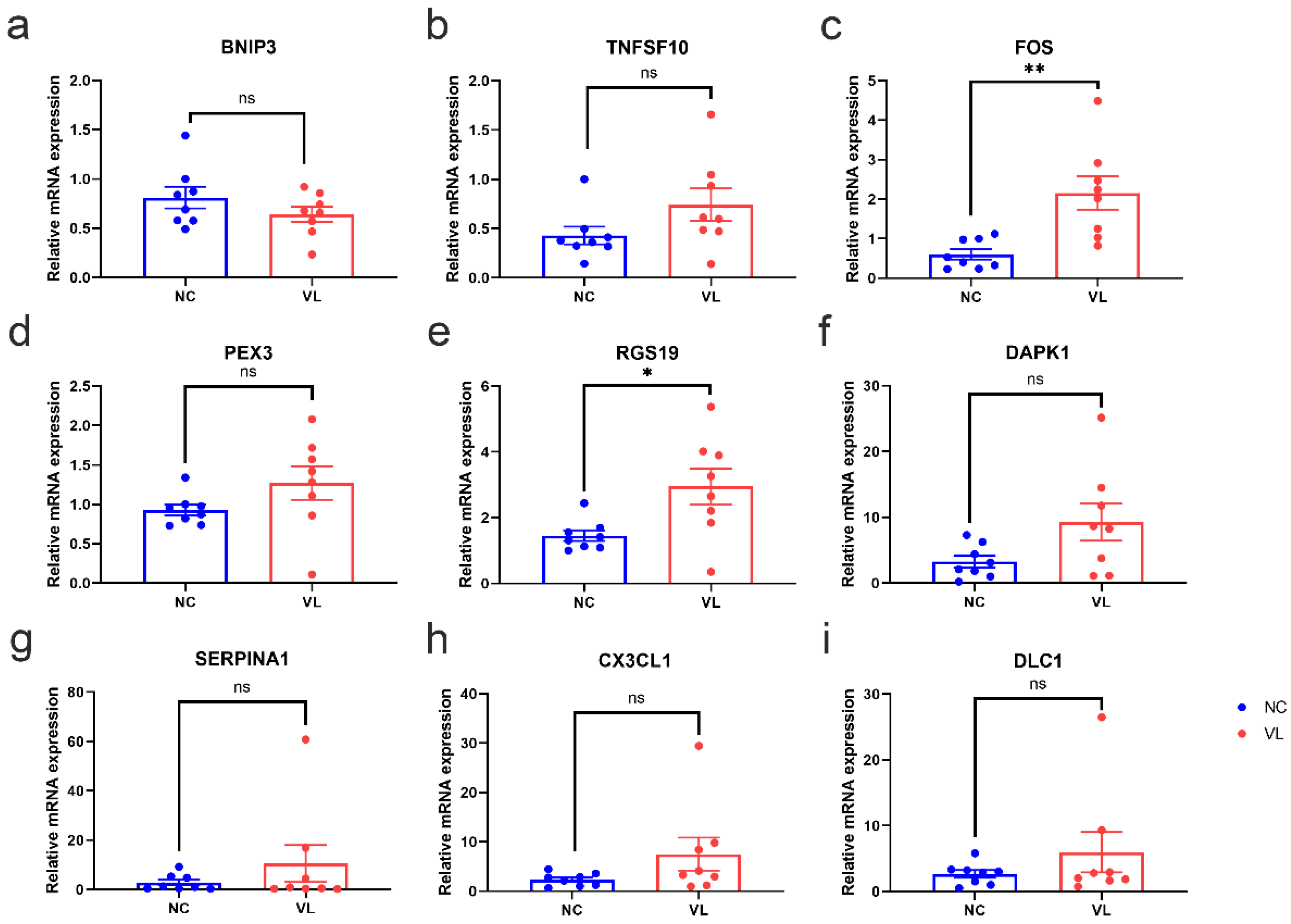
3.7. Validation of DEARGs’ Expression in Clinical Samples
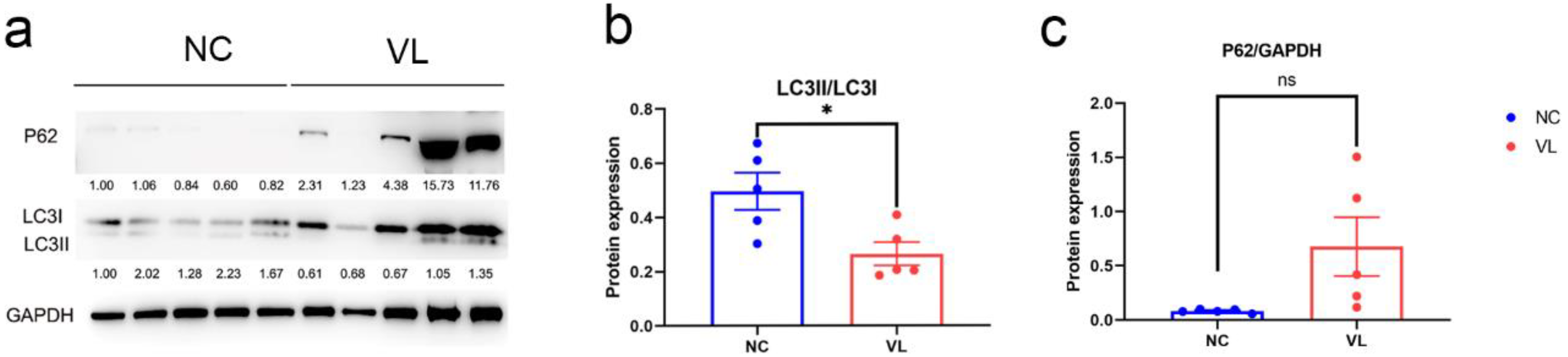
3.8. Autophagy Expression in Vitiligo Lesions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bergqvist, C.; Ezzedine, K. Vitiligo: A Review. Dermatology 2020, 236, 571–592. [Google Scholar] [CrossRef] [PubMed]
- Rashighi, M.; Harris, J.E. Vitiligo Pathogenesis and Emerging Treatments. Dermatol. Clin. 2017, 35, 257–265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Speeckaert, R.; Van Geel, N. Vitiligo: An Update on Pathophysiology and Treatment Options. Am. J. Clin. Dermatol. 2017, 18, 733–744. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Li, B.; Li, X.Y.; Wu, Z.B. The Role of Autophagy in Rheumatic Disease. Curr. Drug Targets 2018, 19, 1009–1017. [Google Scholar] [CrossRef]
- Boada-Romero, E.; Serramito-Gómez, I.; Sacristán, M.P.; Boone, D.L.; Xavier, R.J.; Pimentel-Muiños, F.X. The T300A Crohn’s disease risk polymorphism impairs function of the WD40 domain of ATG16L1. Nat. Commun. 2016, 7, 11821. [Google Scholar] [CrossRef] [Green Version]
- Qiao, Z.; Xu, Z.; Xiao, Q.; Yang, Y.; Ying, J.; Xiang, L.; Zhang, C. Dysfunction of ATG7-dependent autophagy dysregulates the antioxidant response and contributes to oxidative stress-induced biological impairments in human epidermal melanocytes. Cell Death Discov. 2020, 6, 31. [Google Scholar] [CrossRef]
- He, Y.; Li, S.; Zhang, W.; Dai, W.; Cui, T.; Wang, G.; Gao, T.; Li, C. Dysregulated autophagy increased melanocyte sensitivity to H2O2-induced oxidative stress in vitiligo. Sci. Rep. 2017, 7, srep42394. [Google Scholar] [CrossRef]
- Bastonini, E.; Kovacs, D.; Raffa, S.; Macchie, M.D.; Pacifico, A.; Iacovelli, P.; Torrisi, M.R.; Picardo, M. A protective role for autophagy in vitiligo. Cell Death Dis. 2021, 12, 318. [Google Scholar] [CrossRef]
- Yun, W.J.; Kim, E.-Y.; Park, J.-E.; Jo, S.Y.; Bang, S.H.; Chang, E.-J.; Chang, S.E. Microtubule-associated protein light chain 3 is involved in melanogenesis via regulation of MITF expression in melanocytes. Sci. Rep. 2016, 6, 19914. [Google Scholar] [CrossRef]
- Ganesan, A.K.; Ho, H.; Bodemann, B.; Petersen, S.; Aruri, J.; Koshy, S.; Richardson, Z.; Le, L.Q.; Krasieva, T.; Roth, M.G.; et al. Genome-Wide siRNA-Based Functional Genomics of Pigmentation Identifies Novel Genes and Pathways That Impact Melanogenesis in Human Cells. PLoS Genet. 2008, 4, e1000298. [Google Scholar] [CrossRef] [Green Version]
- Krämer, A.; Green, J.; Pollard, J., Jr.; Tugendreich, S. Causal analysis approaches in Ingenuity Pathway Analysis. Bioinformatics 2014, 30, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Hu, W.; Xu, W.; Zhou, M.; Xu, A. CXCL9 as a key biomarker of vitiligo activity and prediction of the success of cultured melanocyte transplantation. Sci. Rep. 2021, 11, 18298. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Kang, Y.; Chen, S.; Wang, L.; Jiang, M.; Xiang, L. Circulating CCL20: A potential biomarker for active vitiligo together with the number of Th1/17 cells. J. Dermatol. Sci. 2019, 93, 92–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaccaro, M.; Cicero, F.; Mannucci, C.; Calapai, G.; Spatari, G.; Barbuzza, O.; Cannavò, S.P.; Gangemi, S. IL-33 circulating serum levels are increased in patients with non-segmental generalized vitiligo. Arch. Dermatol. Res. 2016, 308, 527–530. [Google Scholar] [CrossRef]
- Zhu, L.; Lin, X.; Zhi, L.; Fang, Y.; Lin, K.; Li, K.; Wu, L. Mesenchymal stem cells promote human melanocytes proliferation and resistance to apoptosis through PTEN pathway in vitiligo. Stem Cell Res. Ther. 2020, 11, 26. [Google Scholar] [CrossRef] [Green Version]
- Webb, A.E.; Brunet, A. FOXO transcription factors: Key regulators of cellular quality control. Trends Biochem. Sci. 2014, 39, 159–169. [Google Scholar] [CrossRef] [Green Version]
- Turkcu, U.O.; Tekin, N.S.; Edgunlu, T.G.; Çelik, S.K.; Oner, S. The association of FOXO3A gene polymorphisms with serum FOXO3A levels and oxidative stress markers in vitiligo patients. Gene 2014, 536, 129–134. [Google Scholar] [CrossRef]
- Egbeto, I.A.; Garelli, C.J.; Piedra-Mora, C.; Wong, N.B.; David, C.N.; Robinson, N.A.; Richmond, J.M. Case Series: Gene Expression Analysis in Canine Vogt-Koyanagi-Harada/Uveodermatologic Syndrome and Vitiligo Reveals Conserved Immunopathogenesis Pathways Between Dog and Human Autoimmune Pigmentary Disorders. Front. Immunol. 2020, 11, 590558. [Google Scholar] [CrossRef]
- Faria, A.R.; Jung, J.E.; de Castro, C.C.S.; de Noronha, L. Reduced immunohistochemical expression of adhesion molecules in vitiligo skin biopsies. Pathol. Res. Pract. 2017, 213, 199–204. [Google Scholar] [CrossRef]
- Goldstein, N.B.; Koster, M.I.; Jones, K.L.; Gao, B.; Hoaglin, L.G.; Robinson, S.E.; Wright, M.J.; Birlea, S.I.; Luman, A.; Lambert, K.A.; et al. Repigmentation of Human Vitiligo Skin by NBUVB Is Controlled by Transcription of GLI1 and Activation of the β-Catenin Pathway in the Hair Follicle Bulge Stem Cells. J. Investig. Dermatol. 2018, 138, 657–668. [Google Scholar] [CrossRef] [Green Version]
- Birlea, S.A.; Costin, G.-E.; Roop, D.R.; Norris, D.A. Trends in Regenerative Medicine: Repigmentation in Vitiligo Through Melanocyte Stem Cell Mobilization. Med. Res. Rev. 2017, 37, 907–935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, Y.; Zhou, X.; Zhang, H. Autophagy and immunological aberrations in systemic lupus erythematosus. Eur. J. Immunol. 2019, 49, 523–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haq, S.; Grondin, J.; Banskota, S.; Khan, W.I. Autophagy: Roles in intestinal mucosal homeostasis and inflammation. J. Biomed. Sci. 2019, 26, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Liu, D.; Jin, R.; Zhu, Y.; Xu, A. Differential Responses of Normal Human Melanocytes to Intra- and Extracellular dsRNA. DNA Cell Biol. 2015, 34, 391–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Yu, S.; Hu, W.; Wang, M.; Abudoureyimu, D.; Luo, D.; Li, T.; Long, L.; Zeng, H.; Cheng, C.; et al. Comprehensive Analysis of Cell Population Dynamics and Related Core Genes During Vitiligo Development. Front. Genet. 2021, 12, 627092. [Google Scholar] [CrossRef]
- Salinas-Santander, M.; Trevino, V.; de la Rosa-Moreno, E.; Verduzco-Garza, B.; Sánchez-Domínguez, C.N.; Cantú-Salinas, C.; Ocampo-Garza, J.; Lagos-Rodríguez, A.; Ocampo-Candiani, J.; Ortiz-López, R. CAPN3, DCT, MLANA and TYRP1 are overexpressed in skin of vitiligo vulgaris Mexican patients. Exp. Ther. Med. 2018, 15, 2804–2811. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.-F.; Gruber, F.; Ni, C.; Mildner, M.; Koenig, U.; Karner, S.; Barresi, C.; Rossiter, H.; Narzt, M.-S.; Nagelreiter, I.M.; et al. Suppression of Autophagy Dysregulates the Antioxidant Response and Causes Premature Senescence of Melanocytes. J. Investig. Dermatol. 2015, 135, 1348–1357. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Chen, X.; Gu, H. The signaling involved in autophagy machinery in keratinocytes and therapeutic approaches for skin diseases. Oncotarget 2016, 7, 50682–50697. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Tan, J.; Miao, Y.; Lei, P.; Zhang, Q. ROS and Autophagy: Interactions and Molecular Regulatory Mechanisms. Cell. Mol. Neurobiol. 2015, 35, 615–621. [Google Scholar] [CrossRef]
- Fang, C.; Gu, L.; Smerin, D.; Mao, S.; Xiong, X. The Interrelation between Reactive Oxygen Species and Autophagy in Neurological Disorders. Oxidative Med. Cell. Longev. 2017, 2017, 8495160. [Google Scholar] [CrossRef]
- Borth, W. Alpha 2 ″Macroglobulin, a multifunctional binding protein with targeting characteristics. FASEB J. 1992, 6, 3345–3353. [Google Scholar] [CrossRef] [PubMed]
- Vandooren, J.; Itoh, Y. Alpha-2-Macroglobulin in Inflammation, Immunity and Infections. Front. Immunol. 2021, 12, 803244. [Google Scholar] [CrossRef] [PubMed]
- Östensson, M.; Montén, C.; Bacelis, J.; Gudjonsdottir, A.H.; Adamovic, S.; Ek, J.; Ascher, H.; Pollak, E.; Arnell, H.; Browaldh, L.; et al. A Possible Mechanism behind Autoimmune Disorders Discovered by Genome-Wide Linkage and Association Analysis in Celiac Disease. PLoS ONE 2013, 8, e70174. [Google Scholar] [CrossRef] [PubMed]
- Szondy, Z.; Korponay-Szabó, I.; Király, R.; Sarang, Z.; Tsay, G.J. Transglutaminase 2 in human diseases. BioMedicine 2017, 7, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ameyar, M.; Wisniewska, M.; Weitzman, J. A role for AP-1 in apoptosis: The case for and against. Biochimie 2003, 85, 747–752. [Google Scholar] [CrossRef]
- Sastry, K.S.; Naeem, H.; Mokrab, Y.; Chouchane, A.I. RNA-seq Reveals Dysregulation of Novel Melanocyte Genes upon Oxidative Stress: Implications in Vitiligo Pathogenesis. Oxidative Med. Cell. Longev. 2019, 2019, 2841814. [Google Scholar] [CrossRef] [Green Version]
- Wijngaard, R.V.D.; Wankowicz-Kalinska, A.; Le Poole, C.; Tigges, B.J.; Westerhof, W.; Das, P.K. Local Immune Response in Skin of Generalized Vitiligo Patients. Lab. Investig. 2000, 80, 1299–1309. [Google Scholar] [CrossRef] [Green Version]
- Le Poole, I.C.; van den Wijngaard, R.M.; Westerhof, W.; Das, P.K. Presence of T cells and macrophages in inflammatory vitiligo skin parallels melanocyte disappearance. Am. J. Pathol. 1996, 148, 1219–1228. [Google Scholar]
- Oiso, N.; Tanemura, A.; Kotobuki, Y.; Kimura, M.; Katayama, I.; Kawada, A. Role of macrophage infiltration in successful repigmentation in a new periphery-spreading vitiligo lesion in a male Japanese patient. J. Dermatol. 2013, 40, 915–918. [Google Scholar] [CrossRef]
- Kehrl, J.H. The impact of RGS and other G-protein regulatory proteins on Gαi-mediated signaling in immunity. Biochem. Pharmacol. 2016, 114, 40–52. [Google Scholar] [CrossRef] [Green Version]
- Pugsley, H.R. Assessing Autophagic Flux by Measuring LC3, p62, and LAMP1 Co-localization Using Multispectral Imaging Flow Cytometry. J. Vis. Exp. 2017, e55637. [Google Scholar] [CrossRef] [PubMed]
- Naguib, R.M.; Rashed, A.L. Role of autophagy in nonsegmental vitiligo Naguid and Rashed. Egypt. J. Dermatol. Venerol. 2021, 41, 22–25. [Google Scholar] [CrossRef]
- Yu, H.; Lin, X.; Huang, Y.; Cheng, H.; Seifert, O. The Difference in Expression of Autophagy-Related Proteins in Lesional and Perilesional Skin in Adult Patients with Active and Stable Generalized Vitiligo-A Cross-Sectional Pilot Study. Indian J. Dermatol. 2021, 66, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Cui, T.; Wang, Y.; Song, P.; Yi, X.; Chen, J.; Yang, Y.; Wang, H.; Kang, P.; Guo, S.; Liu, L.; et al. HSF1-Dependent Autophagy Activation Contributes to the Survival of Melanocytes Under Oxidative Stress in Vitiligo. J. Investig. Dermatol. 2021. [Google Scholar] [CrossRef]
- Jeong, T.-J.; Shin, M.-K.; Uhm, Y.-K.; Kim, H.-J.; Chung, J.-H.; Lee, M.-H. Association of UVRAG polymorphisms with susceptibility to non-segmental vitiligo in a Korean sample. Exp. Dermatol. 2010, 19, e323–e325. [Google Scholar] [CrossRef]

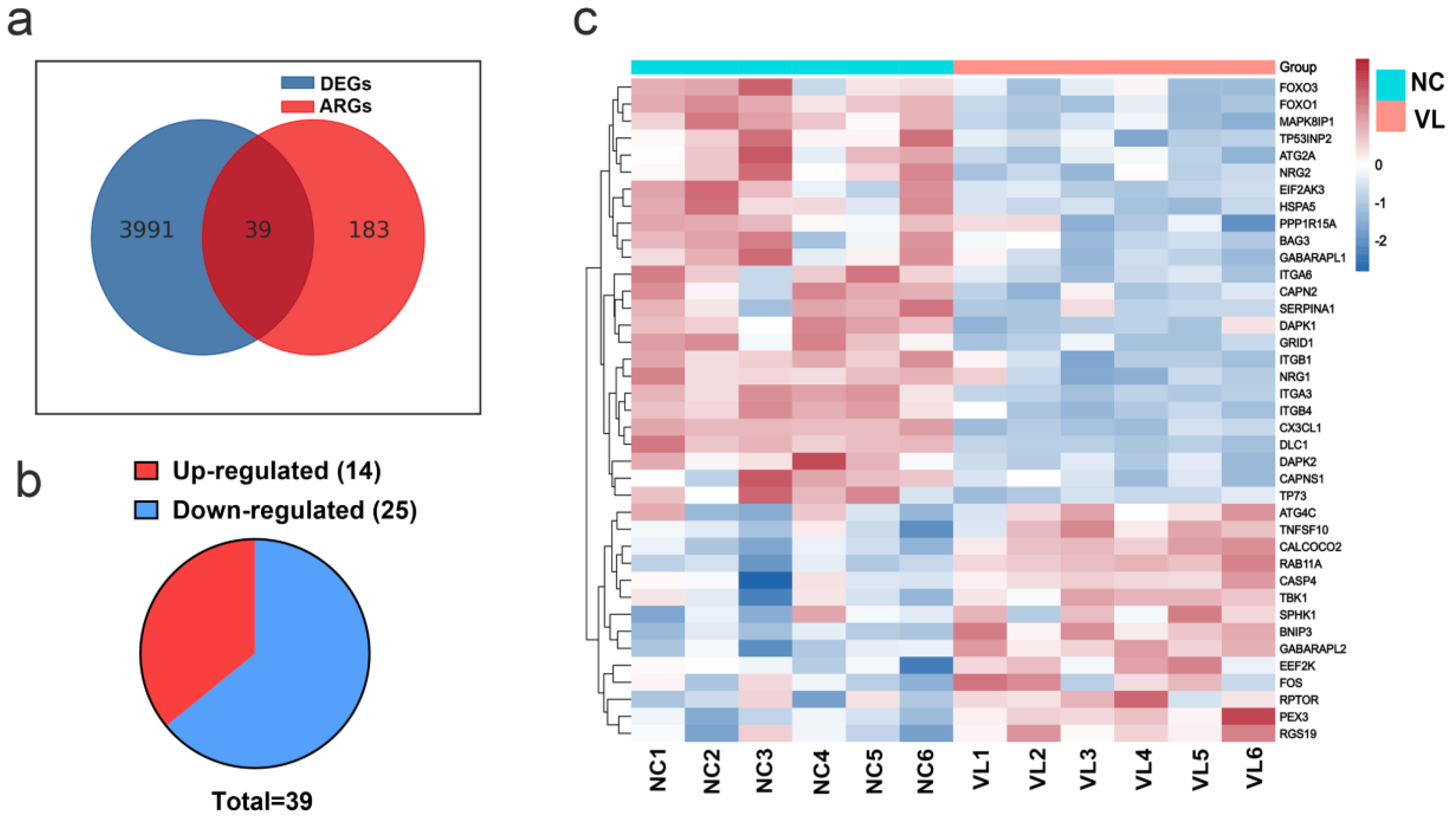
| Gene Symbol | FoldChange | log2FoldChange | p-Value | q-Value | Regulation |
|---|---|---|---|---|---|
| BNIP3 | 2.5595679 | 1.3559003 | 3.72 × 10−7 | 2.77 × 10−5 | Up |
| TNFSF10 | 1.9768091 | 0.9831735 | 0.0002049 | 0.0041653 | Up |
| FOS | 1.927316 | 0.9465932 | 0.0411468 | 0.1800061 | Up |
| PEX3 | 1.7269818 | 0.7882529 | 0.0024115 | 0.0264225 | Up |
| RGS19 | 1.6882659 | 0.7555421 | 0.0109968 | 0.0752503 | Up |
| GABARAPL2 | 1.6506423 | 0.7230275 | 0.0049887 | 0.0436477 | Up |
| EEF2K | 1.6256503 | 0.7010169 | 0.0066433 | 0.05304 | Up |
| CASP4 | 1.6253708 | 0.7007689 | 0.0074996 | 0.0576006 | Up |
| CALCOCO2 | 1.6038901 | 0.6815753 | 0.007666 | 0.0582566 | Up |
| ATG4C | 1.5917797 | 0.6706407 | 0.0318542 | 0.1519605 | Up |
| TBK1 | 1.5631494 | 0.6444557 | 0.0174337 | 0.1019376 | Up |
| RAB11A | 1.5397015 | 0.6226507 | 0.0129811 | 0.0843511 | Up |
| RPTOR | 1.524999 | 0.6088083 | 0.0185034 | 0.1058851 | Up |
| SPHK1 | 1.5135892 | 0.5979737 | 0.0352007 | 0.1624093 | Up |
| ATG2A | 0.6509159 | −0.619457 | 0.0220707 | 0.1191685 | Down |
| FOXO3 | 0.6484999 | −0.624822 | 0.0372126 | 0.1685346 | Down |
| ITGA6 | 0.6116547 | −0.709211 | 0.004182 | 0.0385211 | Down |
| CAPNS1 | 0.5507554 | −0.860516 | 0.0368733 | 0.1673774 | Down |
| EIF2AK3 | 0.5495273 | −0.863737 | 0.0190598 | 0.1082081 | Down |
| HSPA5 | 0.545728 | −0.873746 | 0.0036939 | 0.0351965 | Down |
| ITGB1 | 0.5452697 | −0.874958 | 0.0005796 | 0.0093808 | Down |
| BAG3 | 0.5318967 | −0.910782 | 0.0306816 | 0.1481352 | Down |
| PPP1R15A | 0.493106 | −1.02003 | 0.0158813 | 0.0965559 | Down |
| FOXO1 | 0.4646661 | −1.105734 | 2.24 × 10−5 | 0.0007508 | Down |
| NRG2 | 0.4609473 | −1.117326 | 0.0032613 | 0.032505 | Down |
| ITGA3 | 0.4119834 | −1.279342 | 7.33 × 10−7 | 4.81 × 10−5 | Down |
| CAPN2 | 0.410229 | −1.285498 | 0.0002022 | 0.0041223 | Down |
| TP73 | 0.4091502 | −1.289298 | 0.0020614 | 0.0235629 | Down |
| NRG1 | 0.4035998 | −1.309002 | 0.0002356 | 0.0046652 | Down |
| GABARAPL1 | 0.392561 | −1.349011 | 0.0102162 | 0.0713961 | Down |
| DAPK2 | 0.3887637 | −1.363035 | 0.0001956 | 0.0040326 | Down |
| MAPK8IP1 | 0.3750976 | −1.414662 | 0.0001184 | 0.0027454 | Down |
| ITGB4 | 0.3544888 | −1.496188 | 9.37 × 10−8 | 9.50 × 10−6 | Down |
| TP53INP2 | 0.3456572 | −1.532586 | 0.0128949 | 0.0840035 | Down |
| DAPK1 | 0.2971078 | −1.750942 | 6.87 × 10−8 | 7.48 × 10−6 | Down |
| SERPINA1 | 0.2734992 | −1.870392 | 0.0052844 | 0.0453088 | Down |
| GRID1 | 0.1295931 | −2.94794 | 0.0035153 | 0.0340916 | Down |
| CX3CL1 | 0.1253904 | −2.995501 | 4.33 × 10−23 | 1.56 × 10−19 | Down |
| DLC1 | 0.0883675 | −3.500341 | 2.79 × 10−21 | 8.37 × 10−18 | Down |
| Upstream Regulator | Molecule Type | Predicted Activation State | Activation z-Score | p-Value of Overlap | Target Molecules in Dataset |
|---|---|---|---|---|---|
| ERK1/2 | group | −1.708 | 5.14 × 10−8 | CAPN2, DAPK1, EIF2AK3, FOS, FOXO1, HSPA5, ITGA3, MAPK8IP1 | |
| TP63 | transcription regulator | −1.471 | 1.13 × 10−7 | ATG4C, FOS, FOXO3, ITGA3, ITGA6, ITGB1, ITGB4, TNFSF10, TP73 | |
| TGFB1 | growth factor | −1.203 | 4.52 × 10−7 | CALCOCO2, CAPNS1, CASP4, CX3CL1, DAPK1, FOS, FOXO1, FOXO3, HSPA5, ITGA3 | |
| PGR | ligand-dependent nuclear receptor | −1.633 | 8.11 × 10−7 | CAPN2, FOS, FOXO1, ITGA6, ITGB1, ITGB4, SERPINA1 | |
| NFKBIA | transcription regulator | 1.768 | 1.02 × 10−6 | BNIP3, CASP4, CX3CL1, FOS, HSPA5, ITGA3, ITGB1, TNFSF10 | |
| A2M | transporter | Inhibited | −2 | 1.09 × 10−6 | EIF2AK3, FOXO1, HSPA5, PPP1R15A |
| ATF3 | transcription regulator | −1.565 | 3.92 × 10−6 | CX3CL1, HSPA5, PPP1R15A, RAB11A, TP73 | |
| TP73 | transcription regulator | −1.689 | 6.06 × 10−6 | ATG4C, CASP4, CX3CL1, FOXO3, ITGB4, SERPINA1, TP73 | |
| SIRT1 | transcription regulator | Activated | 2.236 | 0.00001 | BNIP3, EIF2AK3, FOXO1, FOXO3, GABARAPL1, HSPA5, TP73 |
| AKT1 | kinase | 1.181 | 0.000012 | FOS, FOXO1, ITGB1, RAB11A, TNFSF10, TP73 | |
| HRAS | enzyme | −1.342 | 2.13 × 10−5 | BNIP3, CAPN2, FOS, FOXO1, ITGA6, ITGB1, ITGB4, TP73 | |
| TCF4 | transcription regulator | −1.633 | 3.35 × 10−5 | BNIP3, CASP4, FOXO1, ITGA3, TBK1, TNFSF10 | |
| Pkc(s) | group | 1.215 | 3.82 × 10−5 | FOS, HSPA5, ITGB1, PPP1R15A, TP73 | |
| CREB1 | transcription regulator | −1.151 | 5.28 × 10−5 | BAG3, BNIP3, FOS, FOXO3, HSPA5, PPP1R15A, TP53INP2 | |
| FSH | complex | 1.969 | 6.53 × 10−5 | CASP4, CX3CL1, DAPK1, FOS, FOXO1, ITGA3 | |
| SYVN1 | transporter | Inhibited | −2 | 0.000114 | ITGA3, ITGA6, ITGB1, ITGB4 |
| ESR2 | ligand-dependent nuclear receptor | −1.109 | 0.000233 | CAPN2, FOS, FOXO1, FOXO3, ITGB1, NRG1, SPHK1 | |
| GLI1 | transcription regulator | 1.633 | 0.000299 | FOS, FOXO1, ITGA3, ITGB4, NRG1, SERPINA1 | |
| IL6 | cytokine | −1.174 | 0.000412 | CASP4, FOS, HSPA5, ITGB1, PPP1R15A, SERPINA1, TNFSF10 | |
| P38 MAPK | group | 1.071 | 0.000462 | BNIP3, FOS, HSPA5, ITGB4, TNFSF10 | |
| TGM2 | enzyme | Inhibited | −2 | 0.000648 | BNIP3, DAPK2, ITGA6, ITGB4 |
| STAT1 | transcription regulator | 1.501 | 0.000778 | CASP4, CX3CL1, FOS, FOXO1, TNFSF10 | |
| Growth hormone | group | 1.9 | 0.000822 | CX3CL1, FOS, PPP1R15A, TNFSF10 | |
| AHR | ligand-dependent nuclear receptor | 1.97 | 0.00109 | FOS, HSPA5, ITGA6, PPP1R15A, TP73 | |
| Tgf beta | group | −1.154 | 0.00194 | FOS, ITGA6, ITGB1, TNFSF10 | |
| IL1 | group | 1.236 | 0.0032 | FOS, NRG1, SPHK1, TNFSF10 | |
| CEBPB | transcription regulator | −1.239 | 0.00394 | DAPK1, FOS, FOXO3, PPP1R15A, SERPINA1 | |
| IL13 | cytokine | 1.199 | 0.00853 | CAPN2, CX3CL1, SERPINA1, SPHK1 | |
| CG | complex | 1.067 | 0.00891 | CX3CL1, FOS, ITGA3, ITGB1 | |
| LEP | growth factor | 1.184 | 0.0105 | FOS, FOXO3, HSPA5, TNFSF10 | |
| IGF1 | growth factor | 1.191 | 0.0199 | FOS, FOXO1, HSPA5, ITGA3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Wu, X.; Lu, X.; Wang, C.; Xiang, L.; Zhang, C. Identification and Validation of Autophagy-Related Genes in Vitiligo. Cells 2022, 11, 1116. https://doi.org/10.3390/cells11071116
Yang Y, Wu X, Lu X, Wang C, Xiang L, Zhang C. Identification and Validation of Autophagy-Related Genes in Vitiligo. Cells. 2022; 11(7):1116. https://doi.org/10.3390/cells11071116
Chicago/Turabian StyleYang, Yiwen, Xiuyi Wu, Xiaoli Lu, Chen Wang, Leihong Xiang, and Chengfeng Zhang. 2022. "Identification and Validation of Autophagy-Related Genes in Vitiligo" Cells 11, no. 7: 1116. https://doi.org/10.3390/cells11071116
APA StyleYang, Y., Wu, X., Lu, X., Wang, C., Xiang, L., & Zhang, C. (2022). Identification and Validation of Autophagy-Related Genes in Vitiligo. Cells, 11(7), 1116. https://doi.org/10.3390/cells11071116






