L-Type Cav1.3 Calcium Channels Are Required for Beta-Adrenergic Triggered Automaticity in Dormant Mouse Sinoatrial Pacemaker Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Single Cell Isolation
2.2. Patch-Clamp Recordings of SAN Cells
2.2.1. ICaL Measurement
2.2.2. If Measurement
2.3. 2D Calcium Imaging of Single SAN Cells
2.4. Intact SAN Immunostaining
2.5. Cav1.3 and HCN4 Labeling in Isolated SANC
2.6. Dual Immunostaining of Phospholamban in Isolated SANC
2.7. Statistical Analysis
3. Results
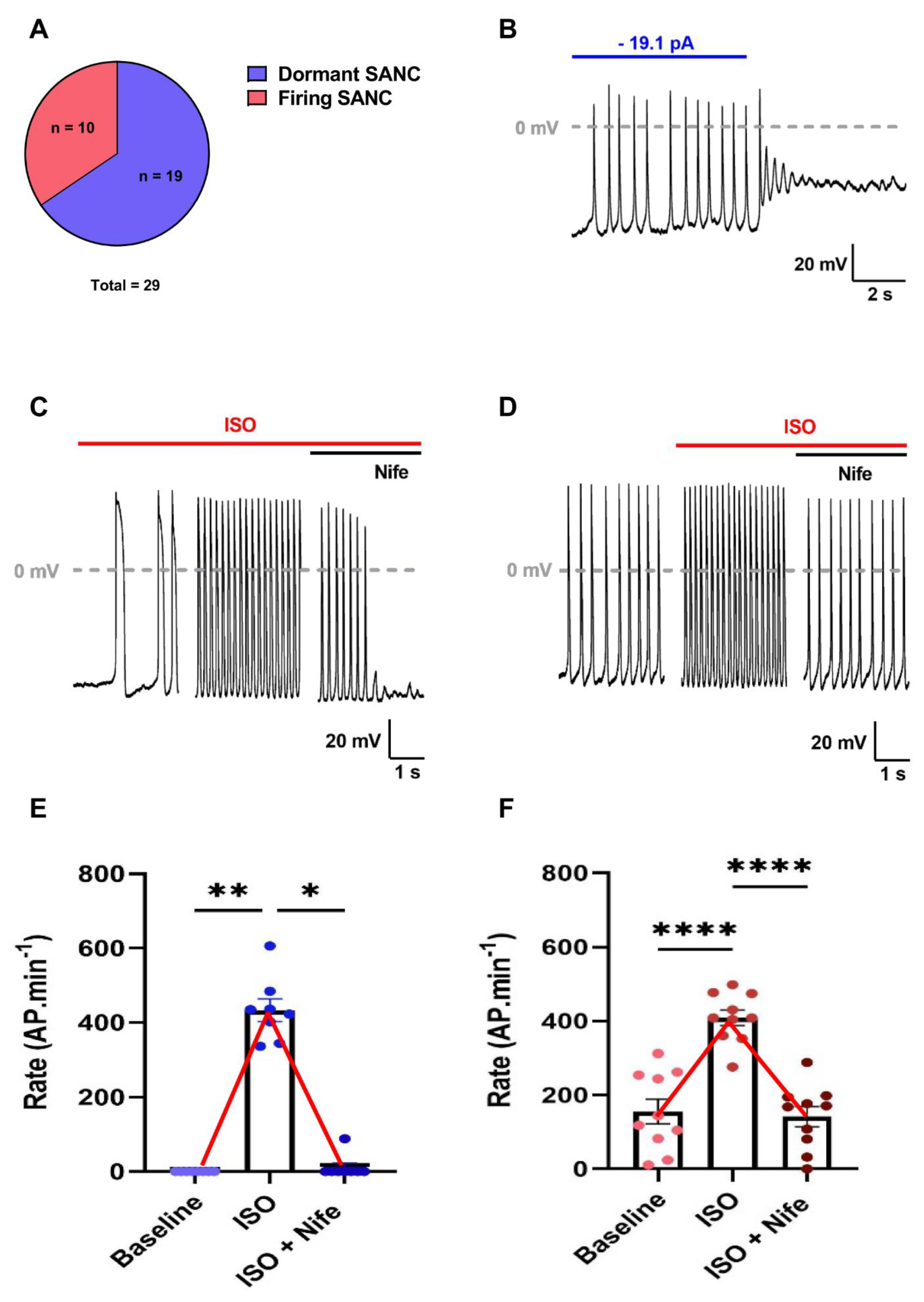
3.1. Inhibition of L-Type Ca2+ Channels Reversed Adrenergic-Induced Acceleration of AP Frequency in Isolated SANC from WT Mice
3.2. Selective Inhibition of L-Type Cav1.3 Ca2+ Channels Reversed Adrenergic-Induced Acceleration of AP Frequency in Isolated SANC of Cav1.2DHP−/− Mice
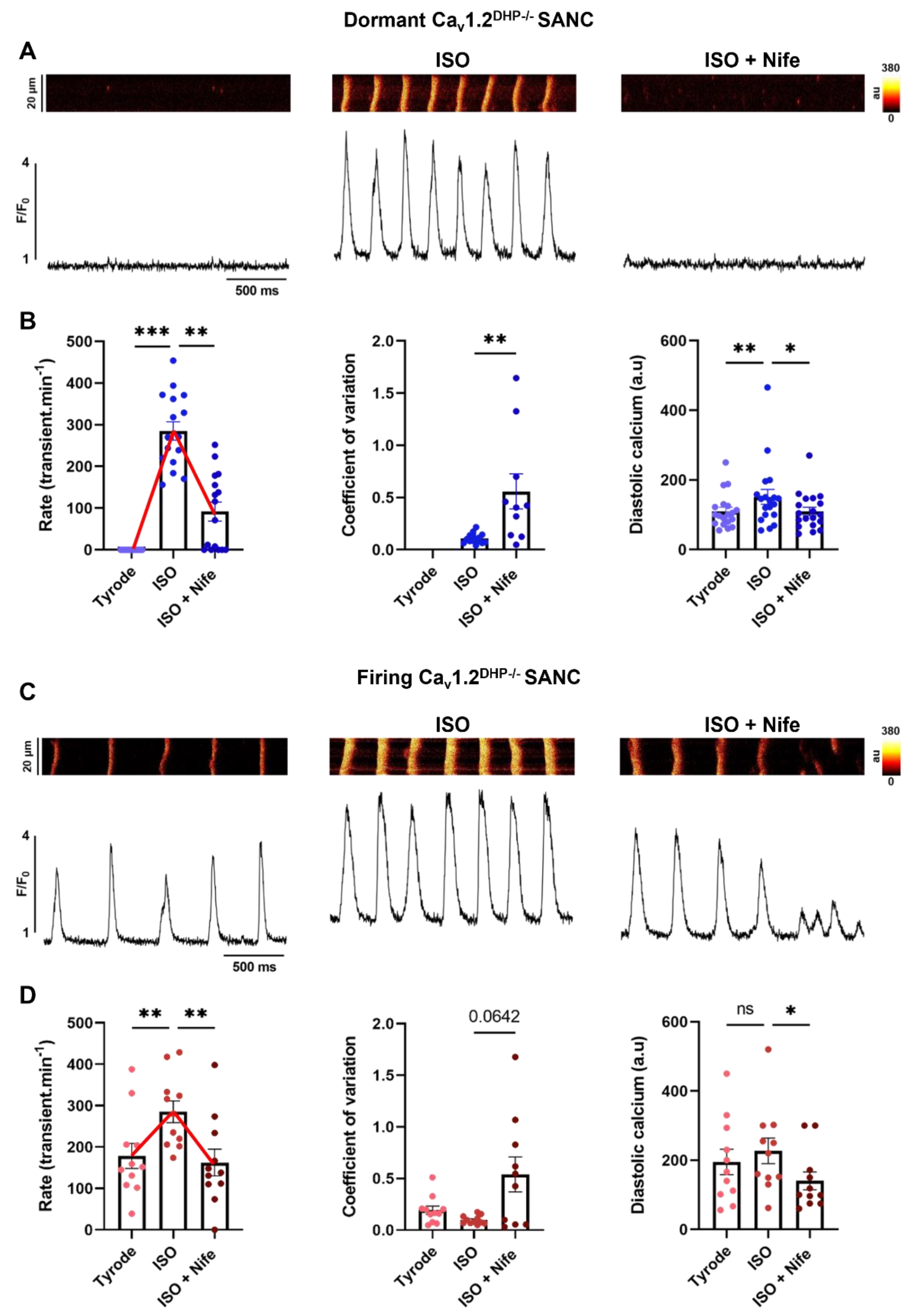
3.3. Selective Inhibition of L-Type Cav1.3 Ca2+ Channels Disrupts Ca2+ Release in Isolated Cav1.2DHP−/− SANC
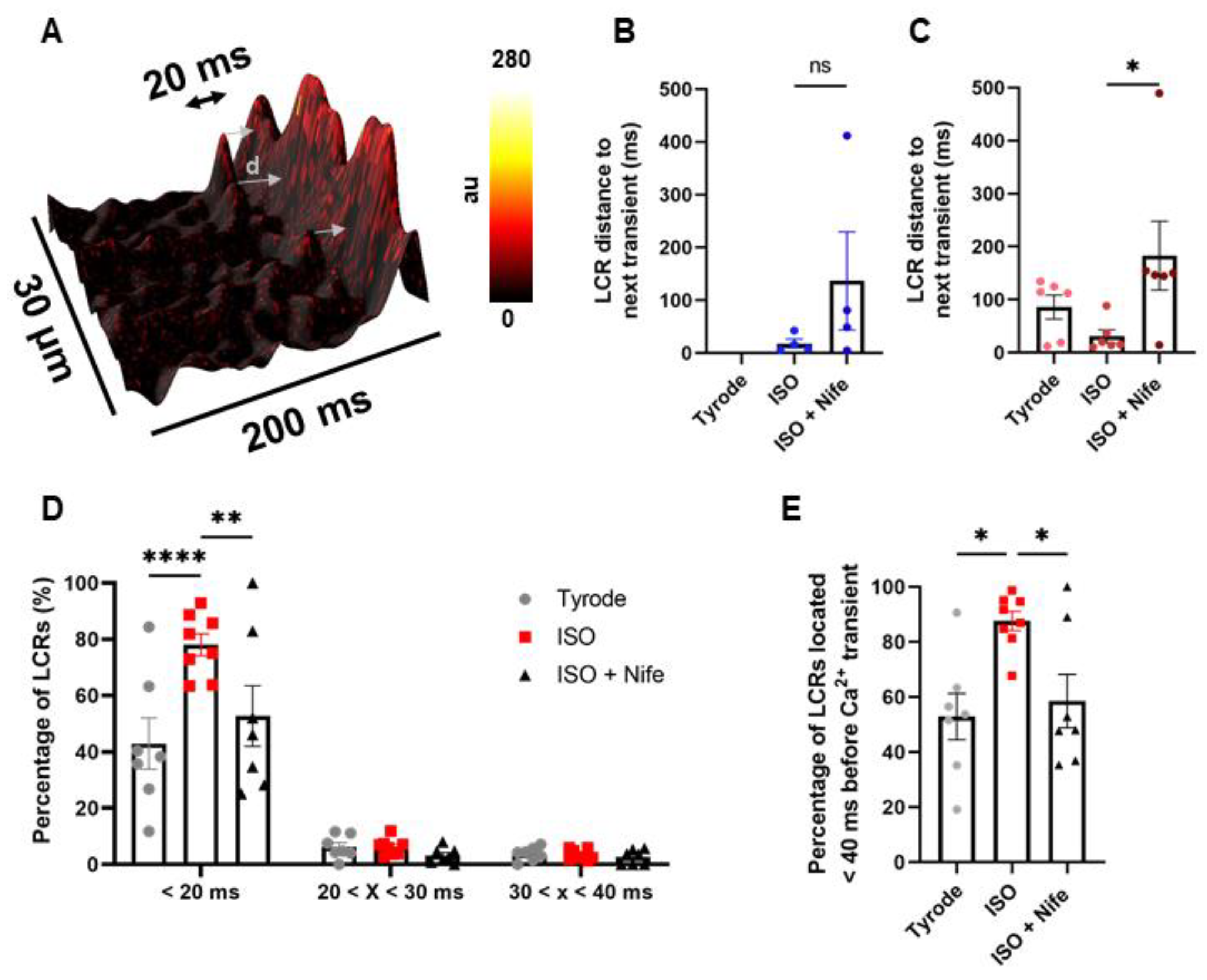
3.4. β-Adrenergic Activation of SANC Automaticity Synchronizes LCRs in Late Diastole and Is Dependent of L-Type Cav1.3 Ca2+ Channels
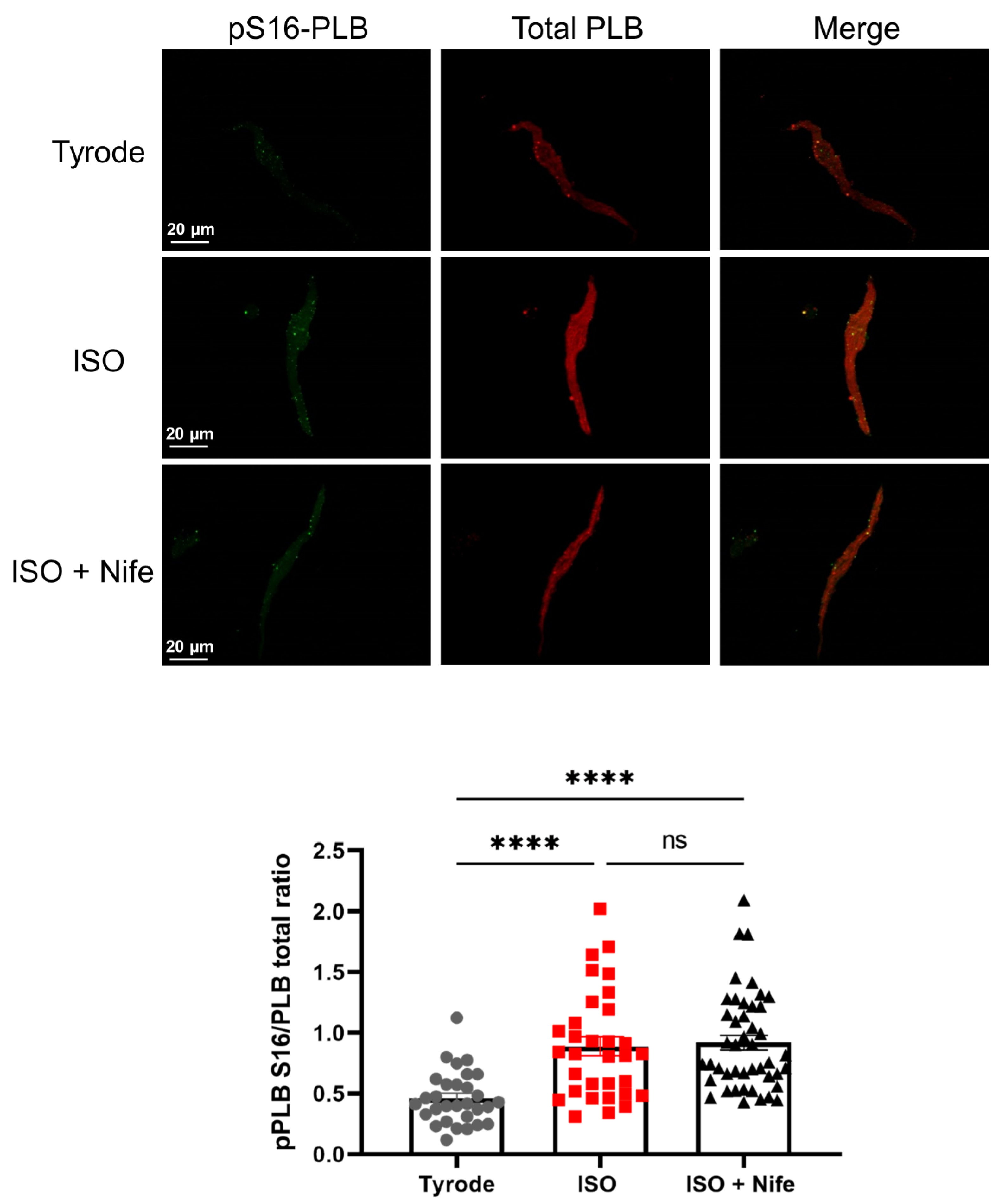
3.5. L-Type Cav1.3 Ca2+ Channels Are Not Necessary for PKA-Dependent Phosphorylation of PLB under β-Adrenergic Stimulation
3.6. Diminished Diastolic Ionic Currents in Dormant SANC
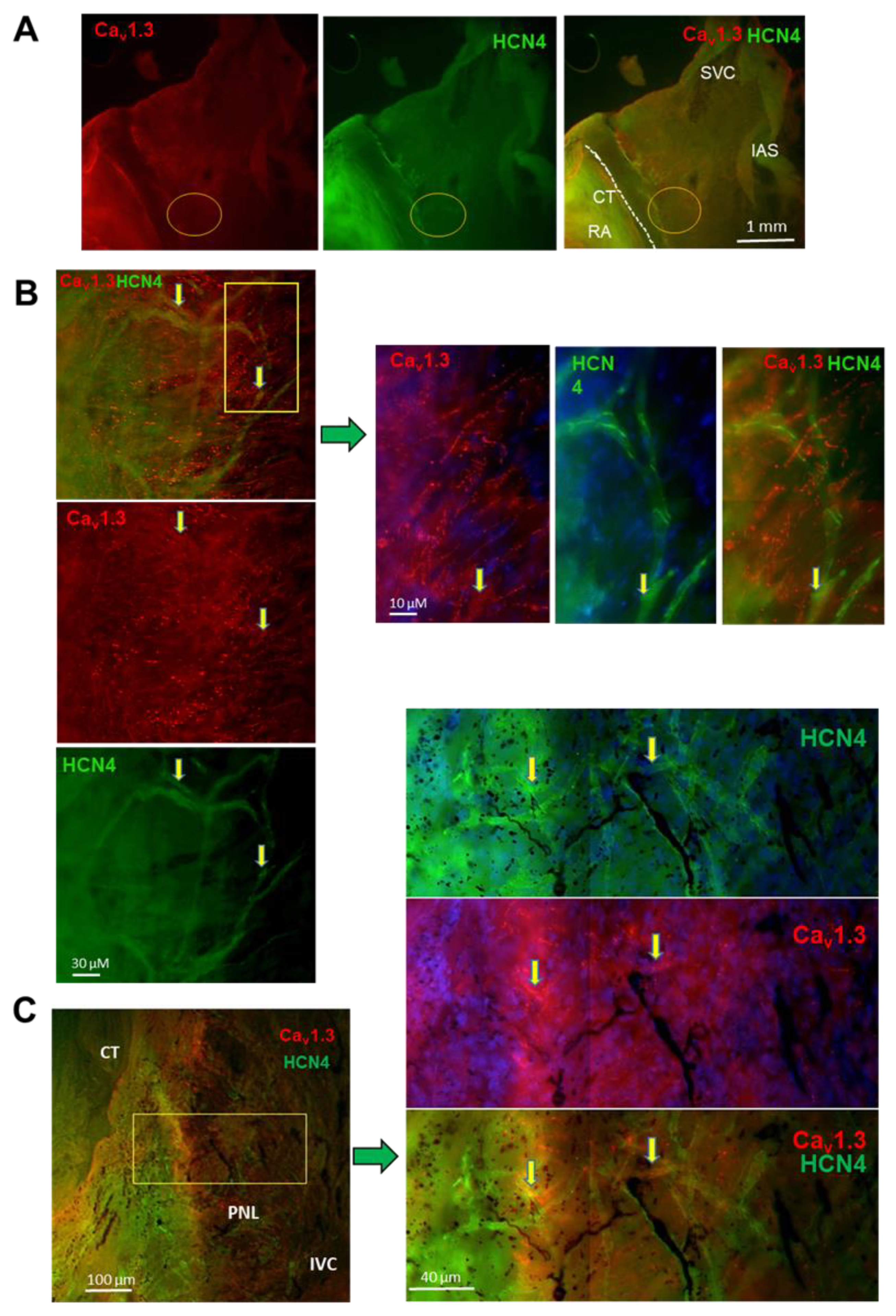
3.7. Expression of Cav1.3 and HCN4 in Intact SAN and Isolated SANC
4. Discussion
4.1. Cav1.3 Channels Are Critical β-Adrenergic Effectors in Maintaining Firing in Dormant SANC
4.2. Cav1.3 Channels Enhance Clock Coupling in Response to β-Adrenergic Stimulation
4.3. Cav1.3 Channels Link M-Clock and Ca2+-Clock by Regulating LCRs
4.4. Physiological Relevance of Dormant SANC In Vivo
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kennedy, A.; Finlay, D.D.; Guldenring, D.; Bond, R.; Moran, K.; McLaughlin, J. The Cardiac Conduction System: Generation and Conduction of the Cardiac Impulse. Crit. Care Nurs. Clin. N. Am. 2016, 28, 269–279. [Google Scholar] [CrossRef]
- Mangoni, M.E.; Nargeot, J. Genesis and Regulation of the Heart Automaticity. Physiol. Rev. 2008, 88, 919–982. [Google Scholar] [CrossRef]
- DiFrancesco, D. Pacemaker Mechanisms in Cardiac Tissue. Annu. Rev. Physiol. 1993, 55, 455–472. [Google Scholar] [CrossRef]
- Lakatta, E.G.; Maltsev, V.A.; Vinogradova, T.M. A Coupled SYSTEM of Intracellular Ca2+ Clocks and Surface Membrane Voltage Clocks Controls the Timekeeping Mechanism of the Heart’s Pacemaker. Circ. Res. 2010, 106, 659–673. [Google Scholar] [CrossRef]
- DiFrancesco, D. The Role of the Funny Current in Pacemaker Activity. Circ. Res 2010, 106, 434–446. [Google Scholar] [CrossRef]
- Mangoni, M.E.; Couette, B.; Marger, L.; Bourinet, E.; Striessnig, J.; Nargeot, J. Voltage-Dependent Calcium Channels and Cardiac Pacemaker Activity: From Ionic Currents to Genes. Prog. Biophys. Mol. Biol. 2006, 90, 38–63. [Google Scholar] [CrossRef]
- Mesirca, P.; Torrente, A.G.; Mangoni, M.E. Functional Role of Voltage Gated Ca2+ Channels in Heart Automaticity. Front. Physiol. 2015, 6, 19. [Google Scholar] [CrossRef]
- Lei, M.; Jones, S.A.; Liu, J.; Lancaster, M.K.; Fung, S.S.-M.; Dobrzynski, H.; Camelliti, P.; Maier, S.K.G.; Noble, D.; Boyett, M.R. Requirement of Neuronal- and Cardiac-Type Sodium Channels for Murine Sinoatrial Node Pacemaking. J. Physiol. 2004, 559, 835–848. [Google Scholar] [CrossRef]
- Demion, M.; Bois, P.; Launay, P.; Guinamard, R. TRPM4, a Ca2+-Activated Nonselective Cation Channel in Mouse Sinoatrial Node Cells. Cardiovasc. Res 2007, 73, 531–538. [Google Scholar] [CrossRef]
- Hof, T.; Simard, C.; Rouet, R.; Sallé, L.; Guinamard, R. Implication of the TRPM4 Nonselective Cation Channel in Mammalian Sinus Rhythm. Heart Rhythm 2013, 10, 1683–1689. [Google Scholar] [CrossRef]
- Ju, Y.-K.; Chu, Y.; Chaulet, H.; Lai, D.; Gervasio, O.L.; Graham, R.M.; Cannell, M.B.; Allen, D.G. Store-Operated Ca2+ Influx and Expression of TRPC Genes in Mouse Sinoatrial Node. Circ. Res. 2007, 100, 1605–1614. [Google Scholar] [CrossRef]
- Vinogradova, T.M.; Zhou, Y.-Y.; Maltsev, V.; Lyashkov, A.; Stern, M.; Lakatta, E.G. Rhythmic Ryanodine Receptor Ca2+ Releases during Diastolic Depolarization of Sinoatrial Pacemaker Cells Do Not Require Membrane Depolarization. Circ. Res. 2004, 94, 802–809. [Google Scholar] [CrossRef]
- Yaniv, Y.; Lakatta, E.G.; Maltsev, V.A. From Two Competing Oscillators to One Coupled-Clock Pacemaker Cell System. Front. Physiol. 2015, 6, 28. [Google Scholar] [CrossRef]
- Lyashkov, A.E.; Behar, J.; Lakatta, E.G.; Yaniv, Y.; Maltsev, V.A. Positive Feedback Mechanisms among Local Ca Releases, NCX, and ICaL Ignite Pacemaker Action Potentials. Biophys. J. 2018, 114, 1176–1189. [Google Scholar] [CrossRef]
- Vinogradova, T.M.; Brochet, D.; Sirenko, S.; Li, Y.; Spurgeon, H.; Lakatta, E.G. Sarcoplasmic Reticulum Ca2+ Pumping Kinetics Regulates Timing of Local Ca2+ Releases and Spontaneous Beating Rate of Rabbit Sinoatrial Node Pacemaker Cells. Circ. Res 2010, 107, 767–775. [Google Scholar] [CrossRef]
- DiFrancesco, D.; Tortora, P. Direct Activation of Cardiac Pacemaker Channels by Intracellular Cyclic AMP. Nature 1991, 351, 145–147. [Google Scholar] [CrossRef]
- Vinogradova, T.M.; Lakatta, E.G. Dual Activation of Phosphodiesterase 3 and 4 Regulates Basal Cardiac Pacemaker Function and Beyond. Int. J. Mol. Sci. 2021, 22, 8414. [Google Scholar] [CrossRef]
- Bychkov, R.; Juhaszova, M.; Tsutsui, K.; Coletta, C.; Stern, M.D.; Maltsev, V.A.; Lakatta, E.G. Synchronized Cardiac Impulses Emerge From Heterogeneous Local Calcium Signals within and Among Cells of Pacemaker Tissue. JACC Clin. Electrophysiol. 2020, 6, 907–931. [Google Scholar] [CrossRef]
- Fenske, S.; Hennis, K.; Rötzer, R.D.; Brox, V.F.; Becirovic, E.; Scharr, A.; Gruner, C.; Ziegler, T.; Mehlfeld, V.; Brennan, J.; et al. CAMP-Dependent Regulation of HCN4 Controls the Tonic Entrainment Process in Sinoatrial Node Pacemaker Cells. Nat. Commun. 2020, 11, 5555. [Google Scholar] [CrossRef]
- Kim, M.S.; Maltsev, A.V.; Monfredi, O.; Maltseva, L.A.; Wirth, A.; Florio, M.C.; Tsutsui, K.; Riordon, D.R.; Parsons, S.P.; Tagirova, S.; et al. Heterogeneity of Calcium Clock Functions in Dormant, Dysrhythmically and Rhythmically Firing Single Pacemaker Cells Isolated from SA Node. Cell Calcium 2018, 74, 168–179. [Google Scholar] [CrossRef]
- Tsutsui, K.; Florio, M.C.; Yang, A.; Wirth, A.N.; Yang, D.; Kim, M.S.; Ziman, B.D.; Bychkov, R.; Monfredi, O.J.; Maltsev, V.A.; et al. CAMP-Dependent Signaling Restores AP Firing in Dormant SA Node Cells via Enhancement of Surface Membrane Currents and Calcium Coupling. Front. Physiol. 2021, 12, 596832. [Google Scholar] [CrossRef] [PubMed]
- Tsutsui, K.; Monfredi, O.; Sirenko-Tagirova, S.; Maltseva, L.; Bychkov, R.; Kim, M.; Ziman, B.; Tarasov, K.; Tarasova, Y.; Zhang, J.; et al. A Coupled-Clock System Drives the Automaticity of Human Sinoatrial Nodal Pacemaker Cells. Sci. Signal. 2018, 11, eaap7608. [Google Scholar] [CrossRef]
- Kim, M.S.; Monfredi, O.; Maltseva, L.A.; Lakatta, E.G.; Maltsev, V.A. β-Adrenergic Stimulation Synchronizes a Broad Spectrum of Action Potential Firing Rates of Cardiac Pacemaker Cells toward a Higher Population Average. Cells 2021, 10, 2124. [Google Scholar] [CrossRef]
- Marionneau, C.; Couette, B.; Liu, J.; Li, H.; Mangoni, M.E.; Nargeot, J.; Lei, M.; Escande, D.; Demolombe, S. Specific Pattern of Ionic Channel Gene Expression Associated with Pacemaker Activity in the Mouse Heart. J. Physiol. 2005, 562, 223–234. [Google Scholar] [CrossRef]
- Mangoni, M.E.; Couette, B.; Bourinet, E.; Platzer, J.; Reimer, D.; Striessnig, J.; Nargeot, J. Functional Role of L-Type Cav1.3 Ca2+ Channels in Cardiac Pacemaker Activity. Proc. Natl. Acad. Sci. USA 2003, 100, 5543–5548. [Google Scholar] [CrossRef]
- Toyoda, F.; Mesirca, P.; Dubel, S.; Ding, W.-G.; Striessnig, J.; Mangoni, M.E.; Matsuura, H. CaV1.3 L-Type Ca2+ Channel Contributes to the Heartbeat by Generating a Dihydropyridine-Sensitive Persistent Na+ Current. Sci. Rep. 2017, 7, 7869. [Google Scholar] [CrossRef]
- Baudot, M.; Torre, E.; Bidaud, I.; Louradour, J.; Torrente, A.G.; Fossier, L.; Talssi, L.; Nargeot, J.; Barrère-Lemaire, S.; Mesirca, P.; et al. Concomitant Genetic Ablation of L-Type Cav1.3 (A1D) and T-Type Cav3.1 (A1G) Ca2+ Channels Disrupts Heart Automaticity. Sci. Rep. 2020, 10, 18906. [Google Scholar] [CrossRef]
- Christel, C.J.; Cardona, N.; Mesirca, P.; Herrmann, S.; Hofmann, F.; Striessnig, J.; Ludwig, A.; Mangoni, M.E.; Lee, A. Distinct Localization and Modulation of Cav1.2 and Cav1.3 L-Type Ca2+ Channels in Mouse Sinoatrial Node. J. Physiol. 2012, 590, 6327–6341. [Google Scholar] [CrossRef]
- Torrente, A.G.; Mesirca, P.; Neco, P.; Rizzetto, R.; Dubel, S.; Barrere, C.; Sinegger-Brauns, M.; Striessnig, J.; Richard, S.; Nargeot, J.; et al. L-Type Cav1.3 Channels Regulate Ryanodine Receptor-Dependent Ca2+ Release during Sinoatrial Node Pacemaker Activity. Cardiovasc. Res. 2016, 109, 451–461. [Google Scholar] [CrossRef]
- Sinnegger-Brauns, M.J.; Hetzenauer, A.; Huber, I.G.; Renström, E.; Wietzorrek, G.; Berjukov, S.; Cavalli, M.; Walter, D.; Koschak, A.; Waldschütz, R.; et al. Isoform-Specific Regulation of Mood Behavior and Pancreatic Beta Cell and Cardiovascular Function by L-Type Ca2+ Channels. J. Clin. Investig. 2004, 113, 1430–1439. [Google Scholar] [CrossRef]
- Mangoni, M.E.; Nargeot, J. Properties of the Hyperpolarization-Activated Current (I(f)) in Isolated Mouse Sinoatrial Cells. Cardiovasc. Res. 2001, 52, 51–64. [Google Scholar] [CrossRef]
- Laasmaa, M.; Karro, N.; Birkedal, R.; Vendelin, M. IOCBIO Sparks Detection and Analysis Software. PeerJ 2019, 7, e6652. [Google Scholar] [CrossRef]
- Gregory, F.D.; Bryan, K.E.; Pangršič, T.; Calin-Jageman, I.E.; Moser, T.; Lee, A. Harmonin Inhibits Presynaptic Cav1.3 Ca2+ Channels in Mouse Inner Hair Cells. Nat. Neurosci. 2011, 14, 1109–1111. [Google Scholar] [CrossRef]
- Clark, R.B.; Mangoni, M.E.; Lueger, A.; Couette, B.; Nargeot, J.; Giles, W.R. A Rapidly Activating Delayed Rectifier K+ Current Regulates Pacemaker Activity in Adult Mouse Sinoatrial Node Cells. Am. J. Physiol. Heart Circ. Physiol. 2004, 286, H1757–H1766. [Google Scholar] [CrossRef][Green Version]
- Verheijck, E.E.; van Ginneken, A.C.; Bourier, J.; Bouman, L.N. Effects of Delayed Rectifier Current Blockade by E-4031 on Impulse Generation in Single Sinoatrial Nodal Myocytes of the Rabbit. Circ. Res. 1995, 76, 607–615. [Google Scholar] [CrossRef]
- Verheijck, E.E.; van Ginneken, A.C.; Wilders, R.; Bouman, L.N. Contribution of L-Type Ca2+ Current to Electrical Activity in Sinoatrial Nodal Myocytes of Rabbits. Am. J. Physiol. 1999, 276, H1064–H1077. [Google Scholar] [CrossRef]
- Vinogradova, T.M.; Lyashkov, A.E.; Zhu, W.; Ruknudin, A.M.; Sirenko, S.; Yang, D.; Deo, S.; Barlow, M.; Johnson, S.; Caffrey, J.L.; et al. High Basal Protein Kinase A-Dependent Phosphorylation Drives Rhythmic Internal Ca2+ Store Oscillations and Spontaneous Beating of Cardiac Pacemaker Cells. Circ. Res. 2006, 98, 505–514. [Google Scholar] [CrossRef]
- Vinogradova, T.M.; Zhou, Y.Y.; Bogdanov, K.Y.; Yang, D.; Kuschel, M.; Cheng, H.; Xiao, R.P. Sinoatrial Node Pacemaker Activity Requires Ca2+/Calmodulin-Dependent Protein Kinase II Activation. Circ. Res. 2000, 87, 760–767. [Google Scholar] [CrossRef]
- Mesirca, P.; Marger, L.; Toyoda, F.; Rizzetto, R.; Audoubert, M.; Dubel, S.; Torrente, A.G.; Difrancesco, M.L.; Muller, J.C.; Leoni, A.-L.; et al. The G-Protein-Gated K+ Channel, IKACh, Is Required for Regulation of Pacemaker Activity and Recovery of Resting Heart Rate after Sympathetic Stimulation. J. Gen. Physiol. 2013, 142, 113–126. [Google Scholar] [CrossRef]
- Van Borren, M.M.G.J.; Verkerk, A.O.; Wilders, R.; Hajji, N.; Zegers, J.G.; Bourier, J.; Tan, H.L.; Verheijck, E.E.; Peters, S.L.M.; Alewijnse, A.E.; et al. Effects of Muscarinic Receptor Stimulation on Ca2+ Transient, CAMP Production and Pacemaker Frequency of Rabbit Sinoatrial Node Cells. Basic Res. Cardiol. 2010, 105, 73–87. [Google Scholar] [CrossRef]
- Koschak, A.; Reimer, D.; Huber, I.; Grabner, M.; Glossmann, H.; Engel, J.; Striessnig, J. Alpha 1D (Cav1.3) Subunits Can Form l-Type Ca2+ Channels Activating at Negative Voltages. J. Biol. Chem. 2001, 276, 22100–22106. [Google Scholar] [CrossRef] [PubMed]
- Weisbrod, D.; Khun, S.H.; Bueno, H.; Peretz, A.; Attali, B. Mechanisms Underlying the Cardiac Pacemaker: The Role of SK4 Calcium-Activated Potassium Channels. Acta Pharmacol. Sin. 2016, 37, 82–97. [Google Scholar] [CrossRef] [PubMed]
- Alig, J.; Marger, L.; Mesirca, P.; Ehmke, H.; Mangoni, M.E.; Isbrandt, D. Control of Heart Rate by CAMP Sensitivity of HCN Channels. Proc. Natl. Acad. Sci. USA 2009, 106, 12189–12194. [Google Scholar] [CrossRef] [PubMed]
- Saponaro, A.; Cantini, F.; Porro, A.; Bucchi, A.; DiFrancesco, D.; Maione, V.; Donadoni, C.; Introini, B.; Mesirca, P.; Mangoni, M.E.; et al. A Synthetic Peptide That Prevents CAMP Regulation in Mammalian Hyperpolarization-Activated Cyclic Nucleotide-Gated (HCN) Channels. eLife 2018, 7, e35753. [Google Scholar] [CrossRef]
- Vinogradova, T.M.; Sirenko, S.; Lyashkov, A.E.; Younes, A.; Li, Y.; Zhu, W.; Yang, D.; Ruknudin, A.M.; Spurgeon, H.; Lakatta, E.G. Constitutive Phosphodiesterase Activity Restricts Spontaneous Beating Rate of Cardiac Pacemaker Cells by Suppressing Local Ca2+ Releases. Circ. Res. 2008, 102, 761–769. [Google Scholar] [CrossRef]
- Bidaud, I.; Chong, A.C.Y.; Carcouet, A.; Waard, S.D.; Charpentier, F.; Ronjat, M.; Waard, M.D.; Isbrandt, D.; Wickman, K.; Vincent, A.; et al. Inhibition of G Protein-Gated K+ Channels by Tertiapin-Q Rescues Sinus Node Dysfunction and Atrioventricular Conduction in Mouse Models of Primary Bradycardia. Sci. Rep. 2020, 10, 9835. [Google Scholar] [CrossRef]
- Schweizer, P.A.; Duhme, N.; Thomas, D.; Becker, R.; Zehelein, J.; Draguhn, A.; Bruehl, C.; Katus, H.A.; Koenen, M. CAMP Sensitivity of HCN Pacemaker Channels Determines Basal Heart Rate but Is Not Critical for Autonomic Rate Control. Circ. Arrhythmia Electrophysiol. 2010, 3, 542–552. [Google Scholar] [CrossRef]
- Baruscotti, M.; Bucchi, A.; Viscomi, C.; Mandelli, G.; Consalez, G.; Gnecchi-Rusconi, T.; Montano, N.; Casali, K.R.; Micheloni, S.; Barbuti, A.; et al. Deep Bradycardia and Heart Block Caused by Inducible Cardiac-Specific Knockout of the Pacemaker Channel Gene Hcn4. Proc. Natl. Acad. Sci. USA 2011, 108, 1705–1710. [Google Scholar] [CrossRef]
- Herrmann, S.; Stieber, J.; Stöckl, G.; Hofmann, F.; Ludwig, A. HCN4 Provides a “depolarization Reserve” and Is Not Required for Heart Rate Acceleration in Mice. EMBO J. 2007, 26, 4423–4432. [Google Scholar] [CrossRef]
- Mesirca, P.; Alig, J.; Torrente, A.G.; Müller, J.C.; Marger, L.; Rollin, A.; Marquilly, C.; Vincent, A.; Dubel, S.; Bidaud, I.; et al. Cardiac Arrhythmia Induced by Genetic Silencing of “funny” (f) Channels Is Rescued by GIRK4 Inactivation. Nat. Commun. 2014, 5, 4664. [Google Scholar] [CrossRef]
- Maltsev, V.A.; Lakatta, E.G. A Novel Quantitative Explanation for the Autonomic Modulation of Cardiac Pacemaker Cell Automaticity via a Dynamic System of Sarcolemmal and Intracellular Proteins. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H2010–H2023. [Google Scholar] [CrossRef] [PubMed]
- Hennis, K.; Rötzer, R.D.; Piantoni, C.; Biel, M.; Wahl-Schott, C.; Fenske, S. Speeding Up the Heart? Traditional and New Perspectives on HCN4 Function. Front. Physiol. 2021, 12, 669029. [Google Scholar] [CrossRef] [PubMed]
- Boyett, M.R.; Honjo, H.; Kodama, I. The Sinoatrial Node, a Heterogeneous Pacemaker Structure. Cardiovasc. Res. 2000, 47, 658–687. [Google Scholar] [CrossRef]
- Verheijck, E.E.; van Kempen, M.J.; Veereschild, M.; Lurvink, J.; Jongsma, H.J.; Bouman, L.N. Electrophysiological Features of the Mouse Sinoatrial Node in Relation to Connexin Distribution. Cardiovasc. Res. 2001, 52, 40–50. [Google Scholar] [CrossRef]
- Lang, D.; Petrov, V.; Lou, Q.; Osipov, G.; Efimov, I.R. Spatiotemporal Control of Heart Rate in a Rabbit Heart. J. Electrocardiol. 2011, 44, 626–634. [Google Scholar] [CrossRef]
- Opthof, T.; de Jonge, B.; Masson-Pevet, M.; Jongsma, H.J.; Bouman, L.N. Functional and Morphological Organization of the Cat Sinoatrial Node. J. Mol. Cell. Cardiol. 1986, 18, 1015–1031. [Google Scholar] [CrossRef]



| Firing Cav1.2DHP−/− SANC (n = 21) | Responder Dormant Cav1.2DHP−/− SANC (n = 12) | |||||
|---|---|---|---|---|---|---|
| Baseline | ISO | ISO + Nife | Baseline | ISO | ISO + Nife | |
| Frequency (AP·min−1) | 197 ± 22 | 401 ± 15 **** | 176 ± 30 $$$$ | NA | 320 ± 46 | 49 ± 31 |
| MDP (mV) | −59.8 ± 1.5 | −60.3 ± 1.4 | −61.7 ± 1.6 | NA | −53.4 ± 2 | −59.9 ± 3.3 |
| Linear slope (mV·ms−1) | 0.042 ± 0.005 | 0.092 ± 0.009 *** | 0.053 ± 0.004 $$$ | NA | 0.080 ± 0.008 | 0.079 ± 0.005 |
| Expo slope (mV·ms−1) | 0.317 ± 0.019 | 0.596 ± 0.042 **** | 0.340 ± 0.018 $$$$ | NA | 0.452 ± 0.046 | 0.411 ± 0.012 |
| AP threshold (mV) | −43.7 ± 1.5 | −42.2 ± 1.5 * | −41.7 ± 1.7 | NA | −30.1 ± 2.5 | −34.9 ± 2.8 |
| Upstroke (mV·ms−1) | 13.9 ± 2.8 | 13.3 ± 2.6 | 13.4 ± 2.8 | NA | 6.1 ± 2.9 | 5.4 ± 0.3 |
| AP amplitude (mV) | 82.3 ± 2.9 | 85.9 ± 3.3 | 89.1 ± 4 | NA | 68.3 ± 4 | 84.1 ± 7.1 |
| AP duration (ms) | 133.6 ± 4.4 | 108.9 ± 3.5 **** | 111.4 ± 4.3 | NA | 117.1 ± 9.1 | 105.2 ± 15.1 |
| Coefficient of variation | 0.42 ± 0.12 | 0.06 ± 0.01 **** | 0.47 ± 0.15 $$$ | NA | 0.43 ± 0.22 | 1.11 ± 0.67 |
| Firing Cav1.2DHP−/− SANC (n = 8) | Responder Dormant Cav1.2DHP−/− SANC (n = 10) | |||||
|---|---|---|---|---|---|---|
| Baseline | ISO | ISO + Nife | Baseline | ISO | ISO + Nife | |
| LCR number (LCRs/s/20 µm) | 1.25 ± 0.31 | 1.08 ± 0.18 | 0.78 ± 0.2 | 0.23 ± 0.1 | 1.01 ± 0.24 * | 0.41 ± 0.15 |
| LCR size (µm) | 4.71 ± 0.39 | 5.76 ± 0.59 | 4.28 ± 0.21 $ | 3.61 ± 0.24 | 5.98 ± 0.67 * | 4.42 ± 0.39 |
| LCR duration (ms) | 22.76 ± 3.14 | 18.83 ± 0.98 | 19.42 ± 3.01 | 18.38 ± 2.17 | 19.35 ± 1.66 | 17.74 ± 2.26 |
| LCR amplitude (F/F0) | 2.32 ± 0.1 | 2.28 ± 0.12 | 2.2 ± 0.07 | 2.43 ± 0.11 | 2.49 ± 0.12 | 2.46 ± 0.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Louradour, J.; Bortolotti, O.; Torre, E.; Bidaud, I.; Lamb, N.; Fernandez, A.; Le Guennec, J.-Y.; Mangoni, M.E.; Mesirca, P. L-Type Cav1.3 Calcium Channels Are Required for Beta-Adrenergic Triggered Automaticity in Dormant Mouse Sinoatrial Pacemaker Cells. Cells 2022, 11, 1114. https://doi.org/10.3390/cells11071114
Louradour J, Bortolotti O, Torre E, Bidaud I, Lamb N, Fernandez A, Le Guennec J-Y, Mangoni ME, Mesirca P. L-Type Cav1.3 Calcium Channels Are Required for Beta-Adrenergic Triggered Automaticity in Dormant Mouse Sinoatrial Pacemaker Cells. Cells. 2022; 11(7):1114. https://doi.org/10.3390/cells11071114
Chicago/Turabian StyleLouradour, Julien, Olivier Bortolotti, Eleonora Torre, Isabelle Bidaud, Ned Lamb, Anne Fernandez, Jean-Yves Le Guennec, Matteo E. Mangoni, and Pietro Mesirca. 2022. "L-Type Cav1.3 Calcium Channels Are Required for Beta-Adrenergic Triggered Automaticity in Dormant Mouse Sinoatrial Pacemaker Cells" Cells 11, no. 7: 1114. https://doi.org/10.3390/cells11071114
APA StyleLouradour, J., Bortolotti, O., Torre, E., Bidaud, I., Lamb, N., Fernandez, A., Le Guennec, J.-Y., Mangoni, M. E., & Mesirca, P. (2022). L-Type Cav1.3 Calcium Channels Are Required for Beta-Adrenergic Triggered Automaticity in Dormant Mouse Sinoatrial Pacemaker Cells. Cells, 11(7), 1114. https://doi.org/10.3390/cells11071114






