WRKY Gene Family Drives Dormancy Release in Onion Bulbs
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. OYDV Infection Evaluation Using DAS-ELISA and Real Time RT-PCR
2.3. Abscisic Acid (ABA) Quantification by Means of HPLC/MS
2.4. RNA Isolation and Sequencing
2.5. De Novo Assembly, Annotation, and Differential Gene Expression Analysis
2.6. WRKY Extraction and Validation in the Onion Genome Sequence
2.7. Phylogenetic Analysis and Structural Organization of AcWRKY
2.8. Validation of Differentially Expressed AcWRKY Genes by RT-PCR
2.9. Networking Analysis
3. Results
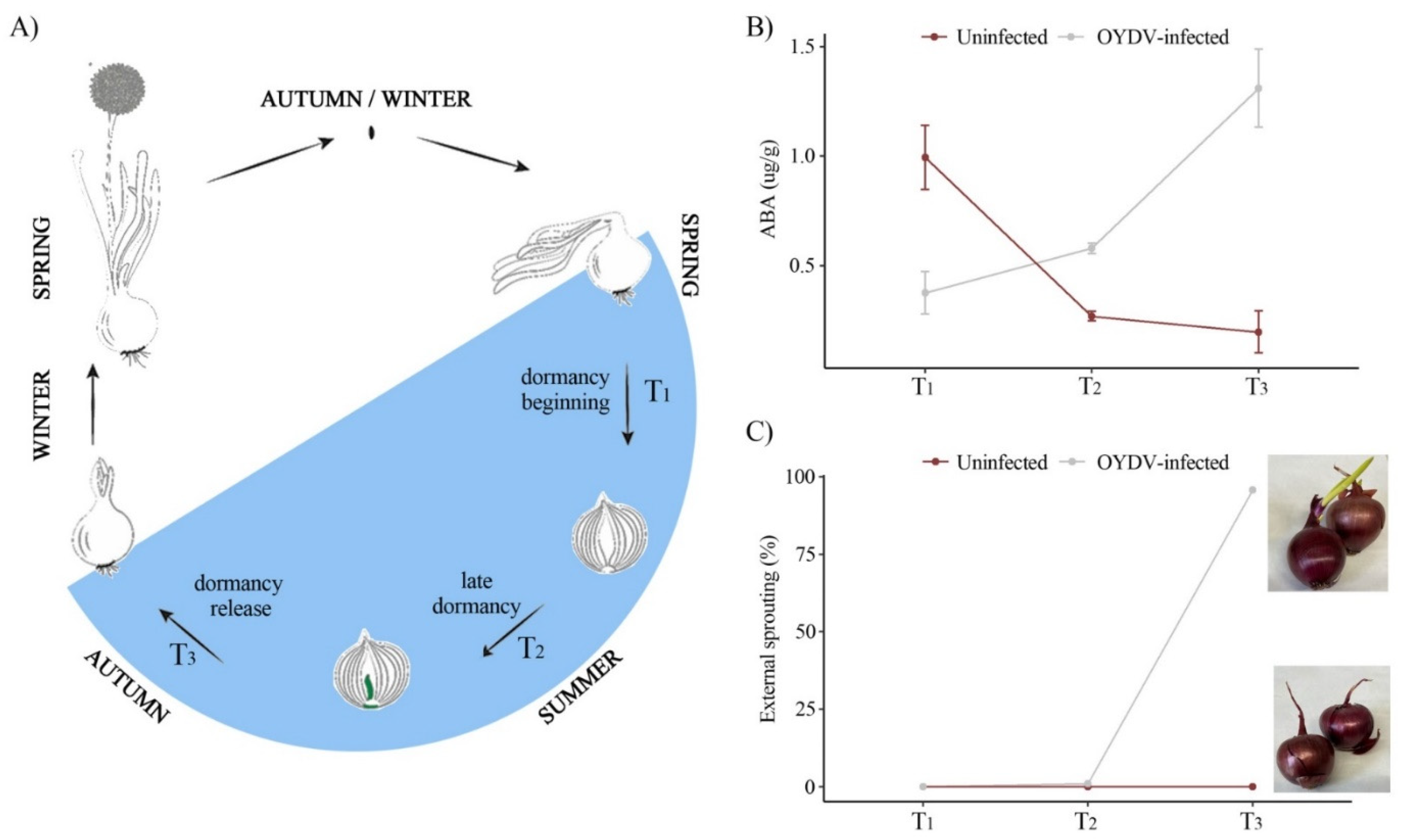
3.1. Infection of Onion with OYDV
3.2. ABA Concentration and Morphological Changes in Uninfected and OYDV-Infected Bulbs during Different Stages in Bulb Dormancy
3.3. Transcriptomic Profiles of Infected and Uninfected Onion Bulbs Comparing the Dormancy Stages at Three Time Points
3.4. Differentially Expressed Genes between OYDV-Infected and Uninfected Bulbs at Each Dormancy Stage
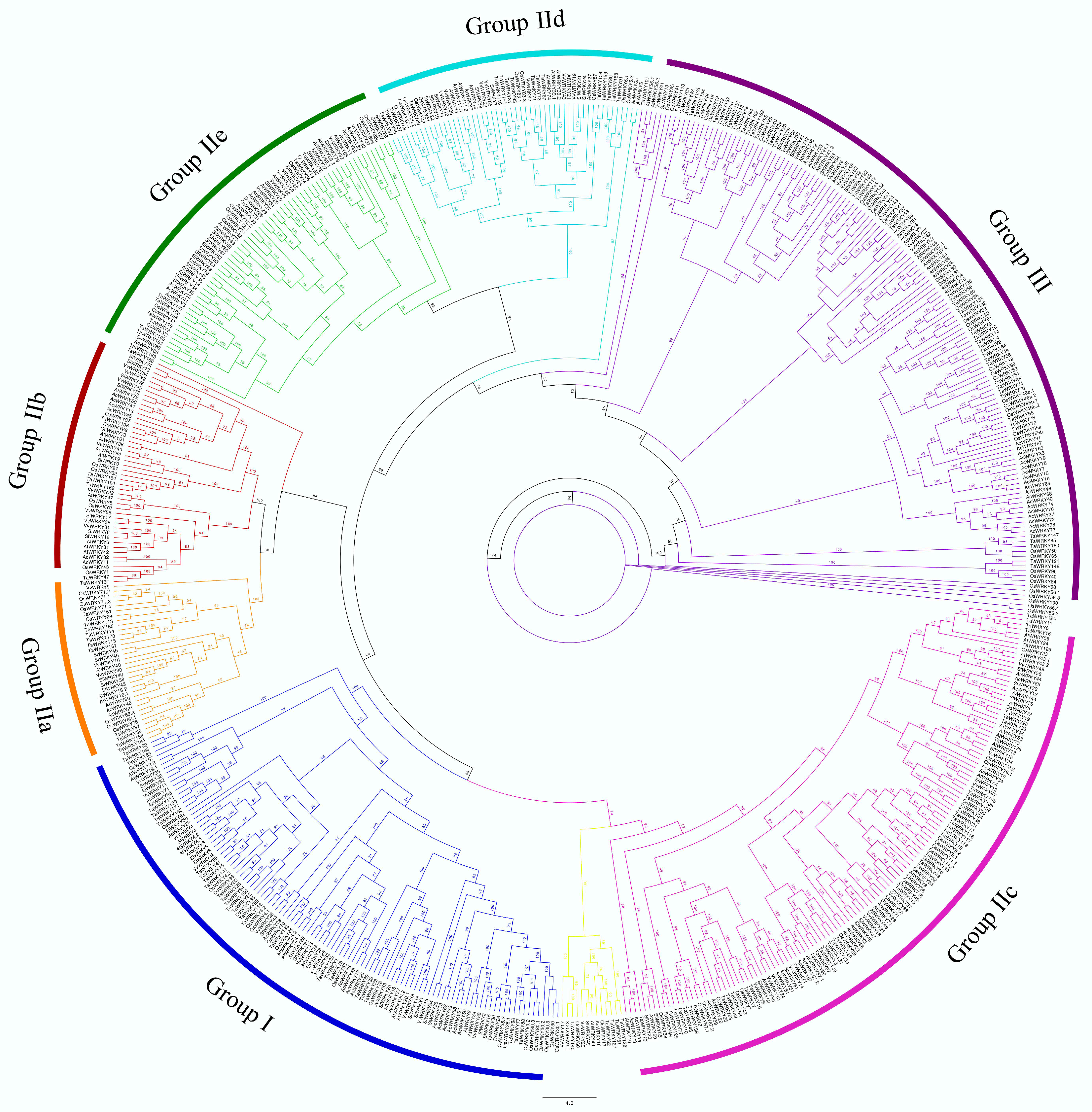
3.5. Characterization of WRKY Genes in the Onion Genome
3.6. Phylogenetic Analysis of Onion WRKY Genes
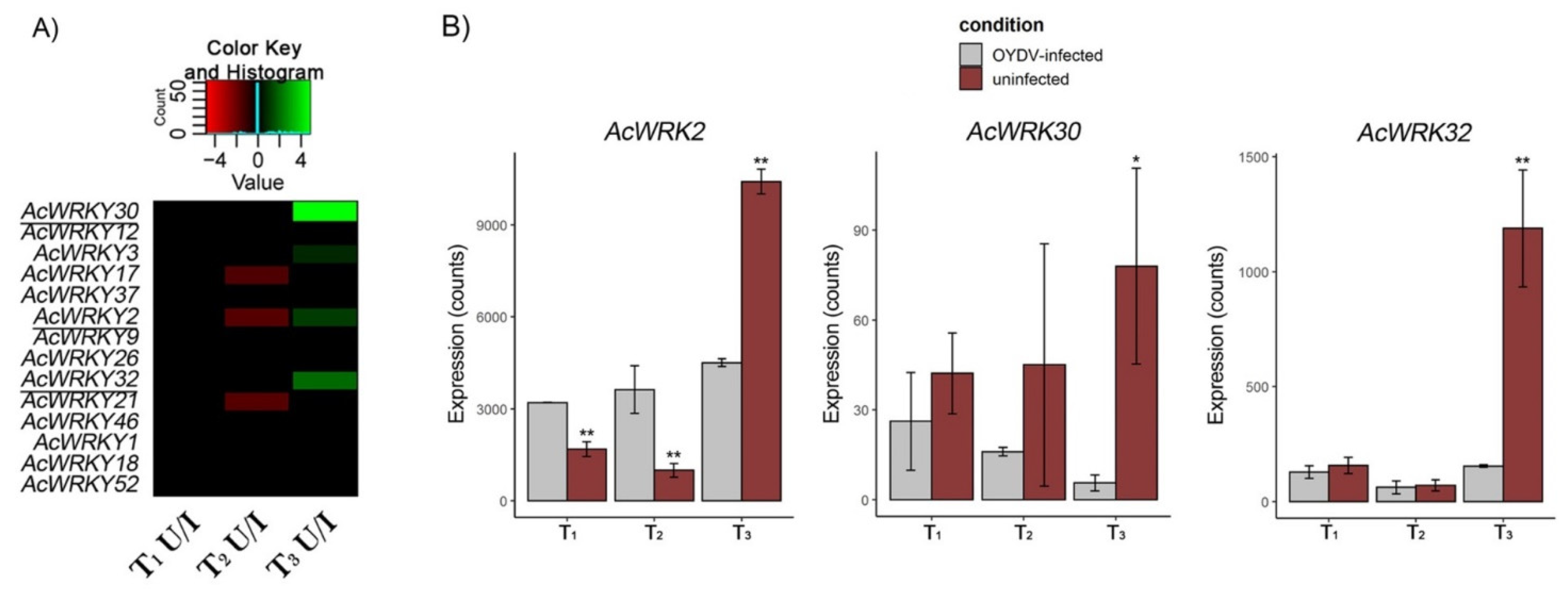
3.7. AcWRKYs Differentially Expressed during Dormancy Different Stages
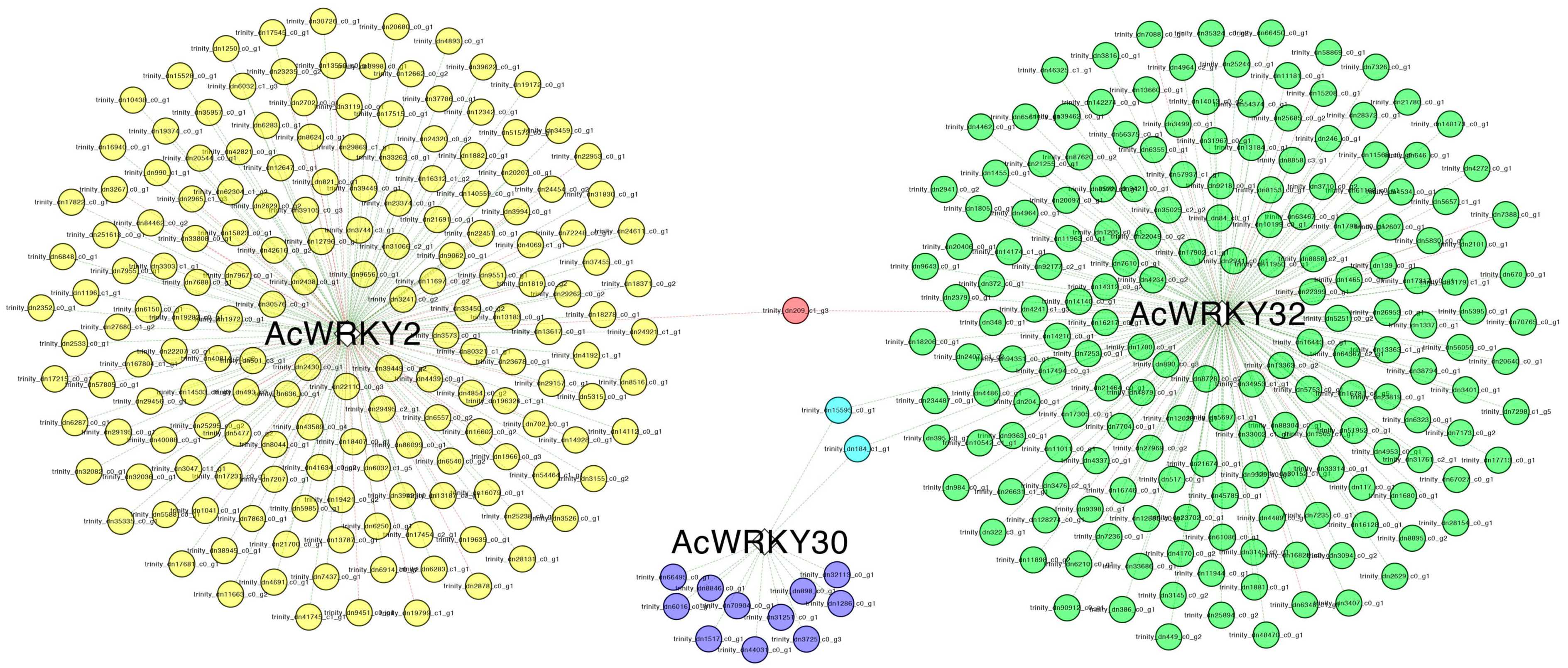
3.8. Gene Co-Expression Network Analysis
4. Discussion
4.1. Transcriptome and ABA Profiles during Onion Bulb Dormancy and Its Alteration Caused by OYDV Infection
4.2. Onion WRKY Gene Characterization
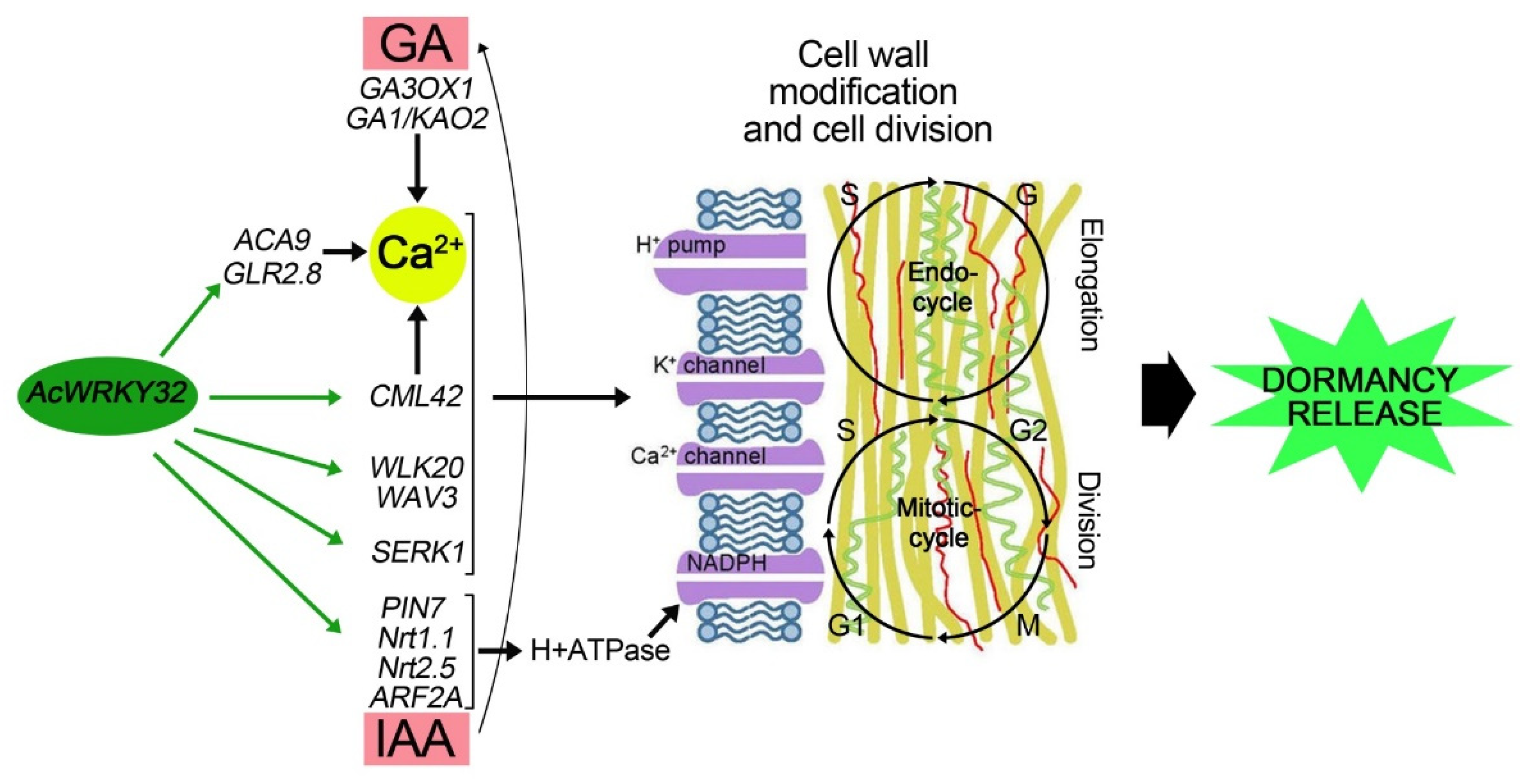
4.3. WRKY Potential Involvement in Onion Bulb Dormancy Release
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pareek, S.; Sagar, N.A.; Sharma, S.; Kumar, V. Onion (Allium cepa L.). In Fruit and Vegetable Phytochemicals: Chemistry and Human Health, 2nd ed.; Yahia, E.M., Ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2017; Volume 2, pp. 1145–1161. [Google Scholar]
- Hanci, F. A Comprehensive Overview of Onion Production: Worldwide and Turkey. J. Agric. Veter. Sci. 2018, 11, 17–27. [Google Scholar]
- Lang, G.A.; Early, J.D.; Martin, G.C.; Darnell, R.L. Endodormancy, Paradormancy, and Ecodormancy-Physiological Terminology and Classification for Dormancy Research. Hortscience 1987, 22, 371–377. [Google Scholar]
- Davis, F.; Terry, L.A.; Chope, G.A.; Faul, C.F.L. Effect of extraction procedure on measured sugar concentrations in onion (Allium cepa L.) bulbs. J. Agric. Food Chem. 2007, 55, 4299–4306. [Google Scholar]
- Cools, K.; Chope, G.A.; Hammond, J.P.; Thompson, A.J.; Terry, L.A. Ethylene and 1-methylcyclopropene differentially regulate gene expression during onion sprout suppression. Plant Physiol. 2011, 156, 1639–1652. [Google Scholar] [PubMed] [Green Version]
- Chope, G.A.; Cools, K.; Terry, L.A.; Hammond, J.P.; Thompson, A.J. Association of gene expression data with dormancy and sprout suppression in onion bulbs using a newly developed onion microarray. Acta Hortic. 2012, 969, 169–174. [Google Scholar]
- Ohanenye, I.C.; Alamar, M.C.; Thompson, A.J.; Terry, L.A. Fructans redistribution prior to sprouting in stored onion bulbs is a potential marker for dormancy break. Postharvest Biol. Technol. 2019, 149, 221–234. [Google Scholar]
- Alamar, M.C.; Anastasiadi, M.; Lopez-Cobollo, R.; Bennett, M.H.; Thompson, A.J.; Turnbull, C.G.N.; Mohareb, F.; Terry, L.A. Transcriptome and phytohormone changes associated with ethylene induced onion bulb dormancy. Postharvest Biol. Technol. 2020, 168, 111267. [Google Scholar]
- Matsubara, S.; Kimura, I. Changes of ABA content during bulbing and dormancy and in vitro bulbing in onion plant. J. Jpn. Soc. Hort. Sci. 1991, 59, 757–762. [Google Scholar]
- Chope, G.A.; Terry, L.A.; White, P.J. Effect of controlled atmosphere storage on abscisic acid concentration and other biochemical attributes of onion bulbs. Postharvest Biol. Technol. 2006, 39, 233–246. [Google Scholar]
- Miedema, P.; Kamminga, G.C. Bulb dormancy in onion. II. The role of cytokinins in high-temperature imposed sprout inhibition. J. Hort. Sci. 1994, 69, 41–45. [Google Scholar]
- Pak, C.; Van der Plas, L.H.W.; de Boer, A.D. Importance of dormancy and sink strength in sprouting of onions (Allium cepa) during storage. Physiol. Plant. 1995, 94, 277–283. [Google Scholar]
- Brewster, J.L. Onion and Other Vegetable Alliums, 2nd ed.; Cab International: Wallingford, UK, 2008. [Google Scholar]
- Van Dijk, P. Survey and characterization of potyviruses and their strains of Allium species. Neth. J. Plant Pathol. 1993, 99, 1–48. [Google Scholar]
- Kamenetsky, R.; Rabinoswitch, H.D. The genus Allium: A developmental and horticultural analysis. Hort. Rev. 2006, 32, 329–337. [Google Scholar]
- Katis, N.I.; Maliogka, V.I.; Dovas, C.I. Viruses of the Genus Allium in the Mediterranean Region, Ed Gad Loebenstein, Hervé Lecoq. Adv. Virus Res. 2012, 84, 163–208. [Google Scholar] [PubMed]
- Sharma, K.; Lee, Y.R.; Park, S.W.; Nile, S.H. Importance of growth hormones and temperature for physiological regulation of dormancy and sprouting in onions. Food Rev. Int. 2016, 32, 233–255. [Google Scholar]
- Van Verk, M.C.; Pappaioannou, D.; Neeleman, L.; Bol, J.F.; Linthorst, H.J. A novel WRKY transcription factor is required for induction of PR-1a gene expression by salicylic acid and bacterial elicitors. Plant Physiol. 2008, 146, 1983–1995. [Google Scholar]
- Ülker, B.; Somssich, I.E. WRKY transcription factors: From DNA binding towards biological function. Curr. Opin. Plant Biol. 2004, 7, 491–498. [Google Scholar]
- Dou, L.; Zhang, X.; Pang, C.; Song, M.; Wei, H.; Fan, S.; Yu, S. Genome-wide analysis of the WRKY gene family in cotton. Mol. Genet. Genom. 2014, 289, 1103–1121. [Google Scholar]
- Cheng, W.; Jiang, Y.; Peng, J.; Guo, J.; Lin, M.; Jin, C.; Huang, J.; Tang, W.; Guan, D.; He, S. The transcriptional reprograming and functional identification of WRKY family members in pepper’s response to Phytophthora capsici infection. BMC Plant Biol. 2020, 20, 256. [Google Scholar]
- Wang, M.; Vannozzi, A.; Wang, G.; Liang, Y.H.; Tornielli, G.B.; Zenoni, S.; Cavallini, E.; Pezzotti, M.; Cheng, Z.M. Genome and transcriptome analysis of the grapevine (Vitis vinifera L.) WRKY gene family. Hort. Res. 2014, 1, 16. [Google Scholar]
- Chen, M.; Tan, Q.; Sun, M.; Li, D.; Fu, X.; Chen, X.; Xiao, W.; Li, L.; Gao, D. Genome-wide identification of WRKY family genes in peach and analysis of WRKY expression during bud dormancy. Mol. Gen. Genom. 2016, 291, 1319–1332. [Google Scholar]
- Jin, J.; Tian, F.; Yang, D.C.; Meng, Y.Q.; Kong, L.; Luo, J.; Gao, G. PlantTFDB 4.0: Toward a central hub for transcription factors and regulatory interactions in plants. Nucleic Acids Res. 2016, 45, 1040–1045. [Google Scholar]
- Tang, Y.; Guo, J.; Zhang, T.; Bai, S.; He, K.; Wang, Z. Genome-Wide Analysis of WRKY Gene Family and the Dynamic Responses of Key WRKY Genes Involved in Ostrinia furnacalis attack in Zea mays. Int. J. Mol. Sci. 2021, 22, 13045. [Google Scholar] [PubMed]
- Wang, P.; Yue, C.; Chen, D.; Zheng, Y.; Zhang, Q.; Yang, J.; Ye, N. Genome-wide identification of WRKY family genes and their response to abiotic stresses in tea plant (Camellia sinensis). Genes Genom. 2019, 41, 17–33. [Google Scholar]
- Zhou, Q.-Y.; Tian, A.-G.; Zou, H.-F.; Xie, Z.-M.; Lei, G.; Huang, J.; Wang, C.-M.; Wang, H.-W.; Zhang, J.-S.; Chen, S.-Y. Soybean WRKY-type transcription factor genes, GmWRKY13, GmWRKY21, and GmWRKY54, confer differential tolerance to abiotic stresses in transgenic Arabidopsis plants. Plant Biotechnol. J. 2008, 6, 486–503. [Google Scholar]
- Li, W.; Wang, H.; Yu, D. The Arabidopsis WRKY transcription factors WRKY12 and WRKY13 oppositely regulate flowering under short-day conditions. Mol. Plant 2016, 9, 1492–1503. [Google Scholar]
- Viana, V.E.; Busanello, C.; da Maia, L.C.; Pegoraro, C.; de Oliveira, A.C. Activation of rice WRKY transcription factors: An army of stress fighting soldiers? Curr. Opin. Plant Biol. 2018, 45, 268–275. [Google Scholar]
- Xie, Z.; Zhang, Z.L.; Hanzlik, S.; Cook, E.; Shen, Q.J. Salicylic acid inhibits gibberellin-induced alpha-amylase expression and seed germination via a pathway involving an abscisic-acid-inducible WRKY gene. Plant Mol. Biol. 2007, 64, 293–303. [Google Scholar]
- Ding, Z.J.; Yan, J.Y.; Li, G.X.; Wu, Z.C.; Zhang, S.Q.; Zheng, S.J. WRKY 41 controls Arabidopsis seed dormancy via direct regulation of ABI3 transcript levels not downstream of ABA. Plant J. 2014, 79, 810–823. [Google Scholar]
- Fennell, A.Y.; Schlauch, K.A.; Gouthu, S.; Deluc, L.G.; Khadka, V.; Sreekantan, L.; Grimplet, J.; Cramer, G.R.; Mathiason, K.L. Short day transcriptomic programming during induction of dormancy in grapevine. Front. Plant Sci. 2015, 6, 834. [Google Scholar]
- Howe, G.T.; Horvath, D.P.; Dharmawardhana, P.; Priest, H.D.; Mockler, T.C.; Strauss, S.H. Extensive transcriptome changes during natural onset and release of vegetative bud dormancy in Populus. Front. Plant Sci. 2015, 6, 989. [Google Scholar] [PubMed] [Green Version]
- Zhou, C.; Lin, Q.; Lan, J.; Zhang, T.; Liu, X.; Miao, R.; Mou, C.; Nguyen, T.; Wang, J.; Zhang, X.; et al. WRKY Transcription Factor OsWRKY29 Represses Seed Dormancy in Rice by Weakening Abscisic Acid Response. Front. Plant Sci. 2020, 11, 691. [Google Scholar] [PubMed]
- Zhang, J.; Li, D.; Shi, X.; Zhang, D.; Qiu, S.; Wei, J.; Zhang, J.; Zhou, J.; Zhu, K.; Xia, Y. Mining and expression analysis of candidate genes involved in regulating the chilling requirement fulfillment of Paeonia lactiflora ‘Hang Baishao’. BMC Plant Biol. 2017, 17, 262. [Google Scholar]
- Finkers, R.; van Kaauwen, M.; Ament, K.; Burger-Meijer, K.; Egging, R.; Huits, H.; Kodde, L.; Kroon, L.; Shigyo, M.; Sato, S.; et al. Insights from the first genome assembly of Onion (Allium cepa). G3 2021, 11, jkab243. [Google Scholar] [PubMed]
- Taglienti, A.; Dell’Abate, M.T.; Ciampa, A.; Tomassoli, L.; Albanese, G.; Sironi, L.; Tiberini, A. Study on ultra-structural effects caused by Onion yellow dwarf virus infection in ‘Rossa di Tropea’ onion bulb by means of magnetic resonance imaging. Sci. Hort. 2020, 271, 109486. [Google Scholar]
- Tiberini, A.; Mangano, R.; Micali, G.; Leo, G.; Manglli, A.; Tomassoli, L.; Albanese, G. Onion yellow dwarf virus ∆∆Ct-based relative quantification obtained by using real-time polymerase chain reaction in ‘Rossa di Tropea’ onion. Eur. J. Plant Pathol. 2019, 153, 251–264. [Google Scholar]
- Khosa, J.S.; Lee, R.; Bräuning, S.; Lord, J.; Pither-Joyce, M.; McCallum, J.; Macknight, R.C. Doubled Haploid ‘CUDH2107′ as a Reference for Bulb Onion (Allium cepa L.) Research: Development of a Transcriptome Catalogue and Identification of Transcripts Associated with Male Fertility. PLoS ONE 2016, 11, e0166568. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar]
- Tsugama, D.; Liu, S.; Takano, T. A bZIP Protein, VIP1, is a Regulator of Osmosensory Signaling in Arabidopsis. Plant Physiol. 2012, 159, 144–155. [Google Scholar]
- Tellez-Robledo, B. The polyadenylation factor FIP1 is important for plant development and root responses to abiotic stresses. Plant J. 2019, 99, 1203–1219. [Google Scholar]
- Nishimura, N.; Tsuchiya, W.; Moresco, J.J.; Hayashi, Y.; Satoh, K.; Kaiwa, N.; Irisa, T.; Kinoshita, T.; Schroeder, J.I.; Yates, J.R., III; et al. Control of seed dormancy and germination by DOG1-AHG1 PP2C phosphatase complex via binding to heme. Nat. Commun. 2018, 9, 2132. [Google Scholar] [PubMed]
- Dinesh, D.C.; Kovermann, M.; Gopalswamy, M.; Hellmuth, A.; Calderón Villalobos, L.I.; Lilie, H.; Balbach, J.; Abel, S. Solution structure of the PsIAA4 oligomerization domain reveals interaction modes for transcription factors in early auxin response. Proc. Natl. Acad. Sci. USA 2015, 112, 6230–6235. [Google Scholar] [PubMed] [Green Version]
- Eulgem, T.; Rushton, P.J.; Robatzek, S.; Somssich, I.E. The WRKY superfamily of plant transcription factors. Trends Plant Sci. 2000, 5, 199–206. [Google Scholar] [PubMed]
- Baillo, E.H.; Hanif, M.S.; Guo, Y.; Zhang, Z.; Xu, P.; Algam, S.A. Genome-wide Identification of WRKY transcription factor family members in sorghum (Sorghum bicolor (L.) Moench). PLoS ONE 2020, 15, e0236651. [Google Scholar]
- Grunewald, W.; De Smet, I.; De Rybel, B.; Robert, H.S.; van de Cotte, B.; Willemsen, V.; Gheysen, G.; Weijers, D.; Friml, J.; Beeckman, T. Tightly controlled WRKY23 expression mediates Arabidopsis embryo development. EMBO Rep. 2013, 14, 1136–1142. [Google Scholar]
- Jiang, Y.; Duan, Y.; Yin, J.; Ye, S.; Zhu, J.; Zhang, F.; Lu, W.; Fan, D.; Luo, K. Genome-wide identification and characterization of the Populus WRKY transcription factor family and analysis of their expression in response to biotic and abiotic stresses. J. Exp. Bot. 2014, 65, 6629–6644. [Google Scholar]
- Xie, T.; Chen, C.; Li, C.; Liu, J.; Liu, C.; He, Y. Genome-wide investigation of WRKY gene family in pineapple: Evolution and expression profiles during development and stress. BMC Genom. 2018, 19, 490. [Google Scholar]
- Wei, K.-F.; Chen, J.; Chen, Y.-F.; Wu, L.-J.; Xie, D.-X. Molecular Phylogenetic and Expression Analysis of the Complete WRKY Transcription Factor Family in Maize. DNA Res. 2012, 19, 153–164. [Google Scholar]
- Davidson, T.M.W. Dormancy in the potato tuber and the effects of storage conditions on initial sprouting and on subsequent sprout growth. Am. Potato J. 1958, 35, 451–465. [Google Scholar]
- Mandahar, C.L.; Gulati, A.; Nath, S. Factors involved in breaking bud dormancy in virus-infected stem cutting of Euphorbia pulcherrima. New Phytol. 1980, 84, 37–43. [Google Scholar]
- Gates, L. A virus causing axillary bud sprouting of tobacco in Rhodesia and Nyasaland. Ann. Appl. Biol. 1962, 50, 169–174. [Google Scholar]
- Alfenas-Zerbini, P.; Maia, I.G.; Fávaro, R.D.; Cascardo, J.C.; Brommonschenkel, S.H.; Zerbini, F.M. Genome-wide analysis of differentially expressed genes during the early stages of tomato infection by a potyvirus. Mol. Plant Microbe Interact. 2009, 22, 352–361. [Google Scholar] [PubMed]
- Ivanov, K.I.; Eskelin, K.; Lohmus, A.; Mäkinen, K. Molecular and cellular mechanisms underlying potyvirus infection. J. Gen. Virol. 2014, 95, 1415–1429. [Google Scholar] [PubMed] [Green Version]
- Mazzitelli, L.; Hancock, R.D.; Haupt, S.; Walker, P.G.; Pont, S.D.A.; McNicol, J.; Cardle, L.; Morris, J.; Viola, R.; Brennan, R.; et al. Coordinated gene expression during phases of dormancy release in raspberry (Rubus idaeus L.) buds. J. Expt. Bot. 2007, 58, 1035–1045. [Google Scholar]
- González, L.M.G.; Kayal, W.L.; Morris, J.S.; Cooke, J.E.K. Diverse chitinases are invoked during the activity-dormancy transition in spruce. Tree Genet. Genomes 2015, 11, 41. [Google Scholar]
- Wang, S.Y.; Faust, M. Changes of Membrane Lipids in Apple Buds During Dormancy and Budbreak. J. Am. Soc. Hort. Sci. 1990, 115, 803–808. [Google Scholar]
- Wojtyla, Ł.; Lechowska, K.; Kubala, S.; Garnczarska, M. Different Modes of Hydrogen Peroxide Action during Seed Germination. Front. Plant Sci. 2016, 7, 66. [Google Scholar]
- Mauri, N.; Fernández-Marcos, M.; Costas, C.; Desvoyes, B.; Pichel, A.; Caro, E.; Gutierrez, C. GEM, a member of the GRAM domain family of proteins, is part of the ABA signaling pathway. Sci. Rep. 2016, 6, 22660. [Google Scholar]
- Née, G.; Kramer, K.; Nakabayashi, K.; Yuan, B.; Xiang, Y.; Miatton, E.; Finkemeier, I.; Soppe, W.J.J. Delay of germination1 requires PP2C phosphatases of the ABA signalling pathway to control seed dormancy. Nat. Commun. 2017, 8, 72. [Google Scholar]
- Khokhar, K.M. A short review on onion bulb dormancy metabolism. Adv. Biotechnol. Microbiol. 2020, 15, 555915. [Google Scholar]
- Ma, Y.; Cao, J.; He, J.; Chen, Q.; Li, X.; Yang, Y. Molecular Mechanism for the Regulation of ABA Homeostasis During Plant Development and Stress Responses. Int. J. Mol. Sci. 2018, 19, 3643. [Google Scholar]
- Shu, K.; Zhou, W.; Chen, F.; Luo, X.; Yang, W. Abscisic Acid and Gibberellins Antagonistically Mediate Plant Development and Abiotic Stress Responses. Front. Plant Sci. 2018, 9, 416. [Google Scholar] [PubMed] [Green Version]
- Rinerson, C.I.; Rabara, R.C.; Tripathi, P.; Shen, Q.J.; Rushton, P.J. The evolution of WRKY transcription factors. BMC Plant Biol. 2015, 15, 66. [Google Scholar]
- Zhou, X.; Jiang, Y.; Yu, D. WRKY22 transcription factor mediates dark-induced leaf senescence in Arabidopsis. Mol. Cells 2011, 31, 303–313. [Google Scholar] [PubMed] [Green Version]
- Huang, Y.; Feng, C.-Z.; Ye, Q.; Wu, W.-H.; Chen, Y.-F. Arabidopsis WRKY6 Transcription Factor Acts as a Positive Regulator of Abscisic Acid Signaling during Seed Germination and Early Seedling Development. PLoS Genet. 2016, 12, e1005833. [Google Scholar]
- Khalil-Ur-Rehman, M.; Wang, W.; Dong, Y.; Faheem, M.; Xu, Y.; Gao, Z.; Guo Shen, Z.; Tao, J. Comparative Transcriptomic and Proteomic Analysis to Deeply Investigate the Role of Hydrogen Cyanamide in Grape Bud Dormancy. Int. J. Mol. Sci. 2019, 20, 3528. [Google Scholar]
- Inoue, S.; Takahashi, K.; Okumura-Noda, H.; Kinoshita, T. Auxin Influx Carrier AUX1 Confers Acid Resistance for Arabidopsis Root Elongation Through the Regulation of Plasma Membrane H+-ATPase. Plant Cell Physiol. 2016, 57, 2194–2201. [Google Scholar]
- Lachaud, S. Participation of auxin and abscisic acid in the regulation of seasonal variations in cambial activity and xylogenesis. Trees 1989, 3, 125–137. [Google Scholar]
- Pang, X.; Halaly, T.; Crane, O.; Keilin, T.; Keren-Keiserman, A.; Ogrodovitch, A.; Galbraith, D.; Or, E. Involvement of calcium signalling in dormancy release of grape buds. J. Exp. Bot. 2007, 58, 3249–3262. [Google Scholar]
- Zhou, Y.P.; Wu, J.H.; Xiao, W.H.; Chen, W.; Chen, Q.H.; Fan, T.; Xie, C.P.; Tian, C.-E. Arabidopsis IQM4, a novel calmodulin-binding protein, is involved with seed dormancy and germination in Arabidopsis. Front. Plant Sci. 2018, 9, 721. [Google Scholar]
- Verma, G.; Khan, S.; Agarwal, S.K.; Sharma, S. Role of apoplastic calcium during germination and initial stages of seedling establishment in Vigna radiata seeds. J. Plant Physiol. 2019, 236, 66–73. [Google Scholar] [PubMed]
- Lovegrove, A.; Hooley, R. Gibberellin and abscisic acid signalling in aleurone. Trends Plant Sci. 2000, 5, 102–110. [Google Scholar] [PubMed]
- Zheng, C.; Acheampong, A.K.; Shi, Z.; Halaly, T.; Kamiya, Y.; Ophir, R.; Galbraith, D.W.; Or, E. Distinct gibberellin functions during and after grapevine bud dormancy release. J. Exp. Bot. 2018, 69, 1635–1648. [Google Scholar] [PubMed] [Green Version]
- Kim, S.Y.; Warpeha, K.M.; Huber, S.C. The brassinosteroid receptor kinase, BRI1, plays a role in seed germination and the release of dormancy by cold stratification. J. Plant Physiol. 2019, 241, 153031. [Google Scholar]
- Wang, Y.; Liu, X.; Su, H.; Yin, S.; Han, C.; Hao, D.; Dong, X. The regulatory mechanism of chilling-induced dormancy transition from endo-dormancy to non-dormancy in Polygonatum kingianum Coll.et Hemsl rhizome bud. Plant Mol. Biol. 2019, 99, 205–217. [Google Scholar]
- Albertos, P.; Romero-Puertas, M.; Tatematsu, K.; Mateos, I.; Sánchez-Vicente, I.; Nambara, E.; Lorenzo, O. S-nitrosylation triggers ABI5 degradation to promote seed germination and seedling growth. Nat. Commun. 2015, 6, 8669. [Google Scholar]
- Brocard, I.M.; Lynch, T.J.; Finkelstein, R.R. Regulation and role of the Arabidopsis abscisic acid-insensitive 5 gene in abscisic acid, sugar, and stress response. Plant Physiol. 2002, 129, 1533–1543. [Google Scholar]
- Clouse, S.D. Brassinosteroid Signal Transduction: From Receptor Kinase Activation to Transcriptional Networks Regulating Plant Development. Plant Cell 2011, 23, 1219–1230. [Google Scholar]
- Espinosa-Ruiz, A.; Martínez, C.; de Lucas, M.; Fàbregas, N.; Bosch, N.; Caño-Delgado, A.I.; Prat, S. TOPLESS mediates brassinosteroid control of shoot boundaries and root meristem development in Arabidopsis thaliana. Development 2017, 144, 1619–1628. [Google Scholar]
- Huang, X.; Zheng, G.; Dai, S.; Gai, S.-P. Identification of differentially expressed genes associated with bud dormancy release in tree peony (Paeonia suffruticosa) by suppression subtractive hybridization. For. Stud. China 2008, 10, 88–94. [Google Scholar]
- Huang, X.; Xue, T.; Dai, S.; Gai, S.; Zheng, C.; Zheng, G. Genes associated with the release of dormant buds in tree peonies (Paeonia suffruticosa). Acta Physiol. Plant. 2008, 30, 797. [Google Scholar]
- Talapatra, S.; Goswami, P.; Das, S.; Raychaudhuri, S.S. Role of SERK during Somatic Embryogenesis and Its Interaction with Brassinosteroids. In Somatic Embryogenesis in Ornamentals and Its Applications; Mujib, A., Ed.; Springer: New Delhi, India, 2016. [Google Scholar]
- Fan, X.; Yang, Y.; Li, M.; Fu, L.; Zang, Y.; Wang, C.; Hao, T.; Sun, H. Transcriptomics and targeted metabolomics reveal the regulatory network of Lilium davidii var. unicolor during bulb dormancy release. Planta 2021, 254, 59. [Google Scholar] [PubMed]

| Species | Group I | Group IIa | Group IIb | Group IIc | Group IId | Group IIe | Group III | Unclassified | Total |
|---|---|---|---|---|---|---|---|---|---|
| AcWRKY | 16 | 2 | 8 | 9 | 2 | 11 | 25 | 1 | 74 |
| AtWRKY | 14 | 4 | 7 | 19 | 9 | 9 | 16 | 3 | 81 |
| OsWRKY | 24 | 8 | 5 | 18 | 10 | 14 | 40 | 2 | 121 |
| VvWRKY | 16 | 3 | 7 | 13 | 7 | 5 | 6 | 2 | 59 |
| TaWRKY | 45 | 12 | 7 | 34 | 17 | 10 | 40 | 6 | 171 |
| SlWRKY | 15 | 5 | 7 | 16 | 5 | 21 | 11 | 1 | 81 |
| Total | 130 | 34 | 41 | 109 | 50 | 69 | 138 | 15 | 587 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puccio, G.; Crucitti, A.; Tiberini, A.; Mauceri, A.; Taglienti, A.; Palumbo Piccionello, A.; Carimi, F.; van Kaauwen, M.; Scholten, O.; Sunseri, F.; et al. WRKY Gene Family Drives Dormancy Release in Onion Bulbs. Cells 2022, 11, 1100. https://doi.org/10.3390/cells11071100
Puccio G, Crucitti A, Tiberini A, Mauceri A, Taglienti A, Palumbo Piccionello A, Carimi F, van Kaauwen M, Scholten O, Sunseri F, et al. WRKY Gene Family Drives Dormancy Release in Onion Bulbs. Cells. 2022; 11(7):1100. https://doi.org/10.3390/cells11071100
Chicago/Turabian StylePuccio, Guglielmo, Antonino Crucitti, Antonio Tiberini, Antonio Mauceri, Anna Taglienti, Antonio Palumbo Piccionello, Francesco Carimi, Martijn van Kaauwen, Olga Scholten, Francesco Sunseri, and et al. 2022. "WRKY Gene Family Drives Dormancy Release in Onion Bulbs" Cells 11, no. 7: 1100. https://doi.org/10.3390/cells11071100
APA StylePuccio, G., Crucitti, A., Tiberini, A., Mauceri, A., Taglienti, A., Palumbo Piccionello, A., Carimi, F., van Kaauwen, M., Scholten, O., Sunseri, F., Vosman, B., & Mercati, F. (2022). WRKY Gene Family Drives Dormancy Release in Onion Bulbs. Cells, 11(7), 1100. https://doi.org/10.3390/cells11071100











