Designing Personalized Antigen-Specific Immunotherapies for Autoimmune Diseases—The Case for Using Ignored Target Cell Antigen Determinants
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Antigens
2.3. T Cell Proliferation Assay
2.4. ELISPOT Analysis of the Numbers of Spontaneously Arising Splenic and Pancreatic Infiltrating IFNγ-Secreting Responses to Antigens
2.5. MHCII Tetramers and FACS Analysis
2.6. ELISPOT Analysis of Autoimmune Responses in Antigen-Immunized Mice
2.7. Treatments
2.8. Analysis of Treg and Tr-1-like Cell Frequencies
3. Results
3.1. Identification of an Immunogenic Ignored Determinant within Preproinsulin
3.2. A Large Pool of Cognate L4-20-Reactive Naïve T Cells Is Available for Priming during Late-Stage Pre-T1D
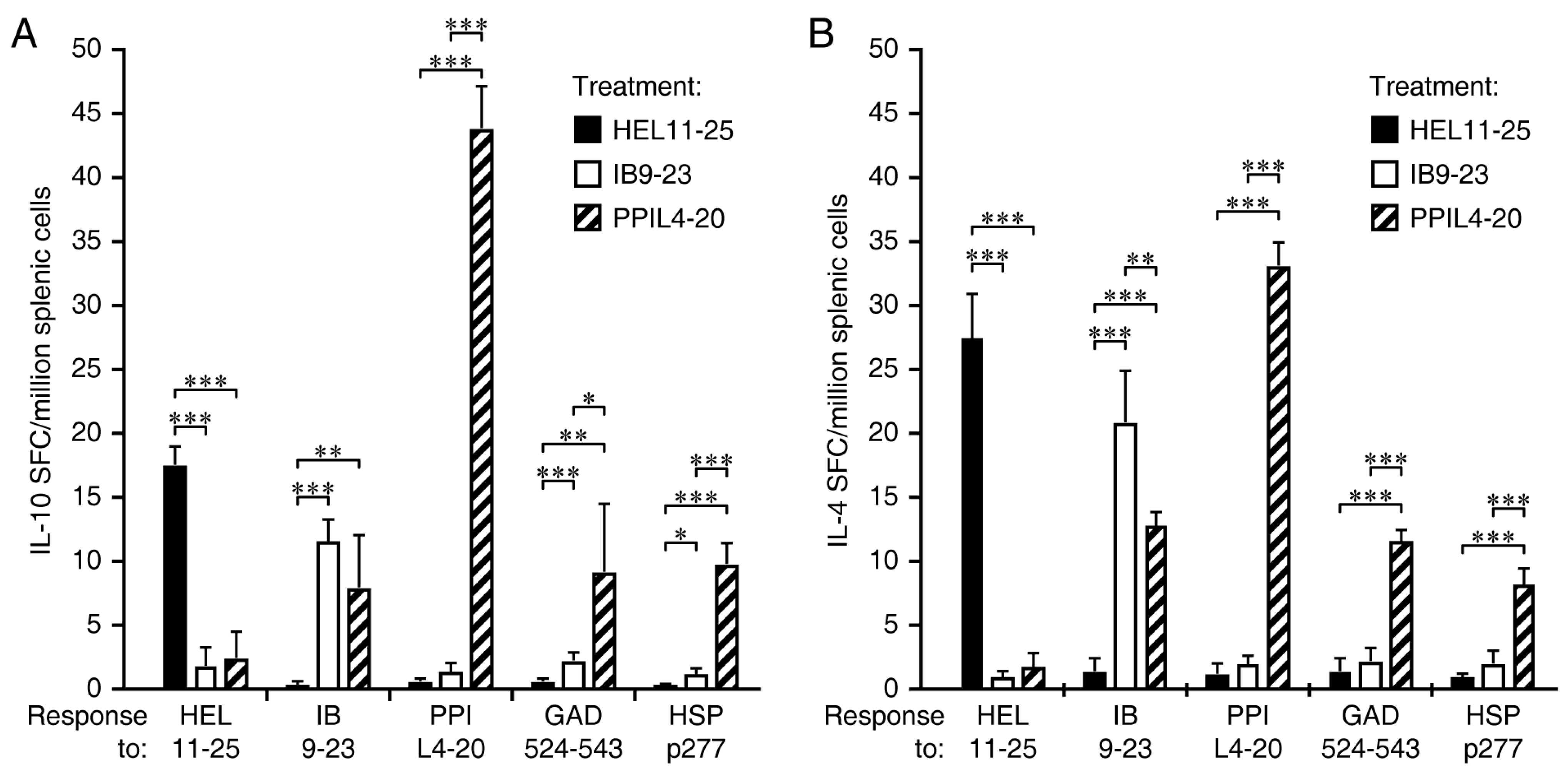
3.3. Immunization with PPIL4-20, but Not Ins B9-23, Induces Robust IL-10 and IL-4 Responses Which Spread to Other ß-Cell Antigens
3.4. Immunization Ins B9-23, but Not PPIL4-20, Primes IFNγ Responses and the Spreading of IFNγ Responses to Other ß-Cell Antigens
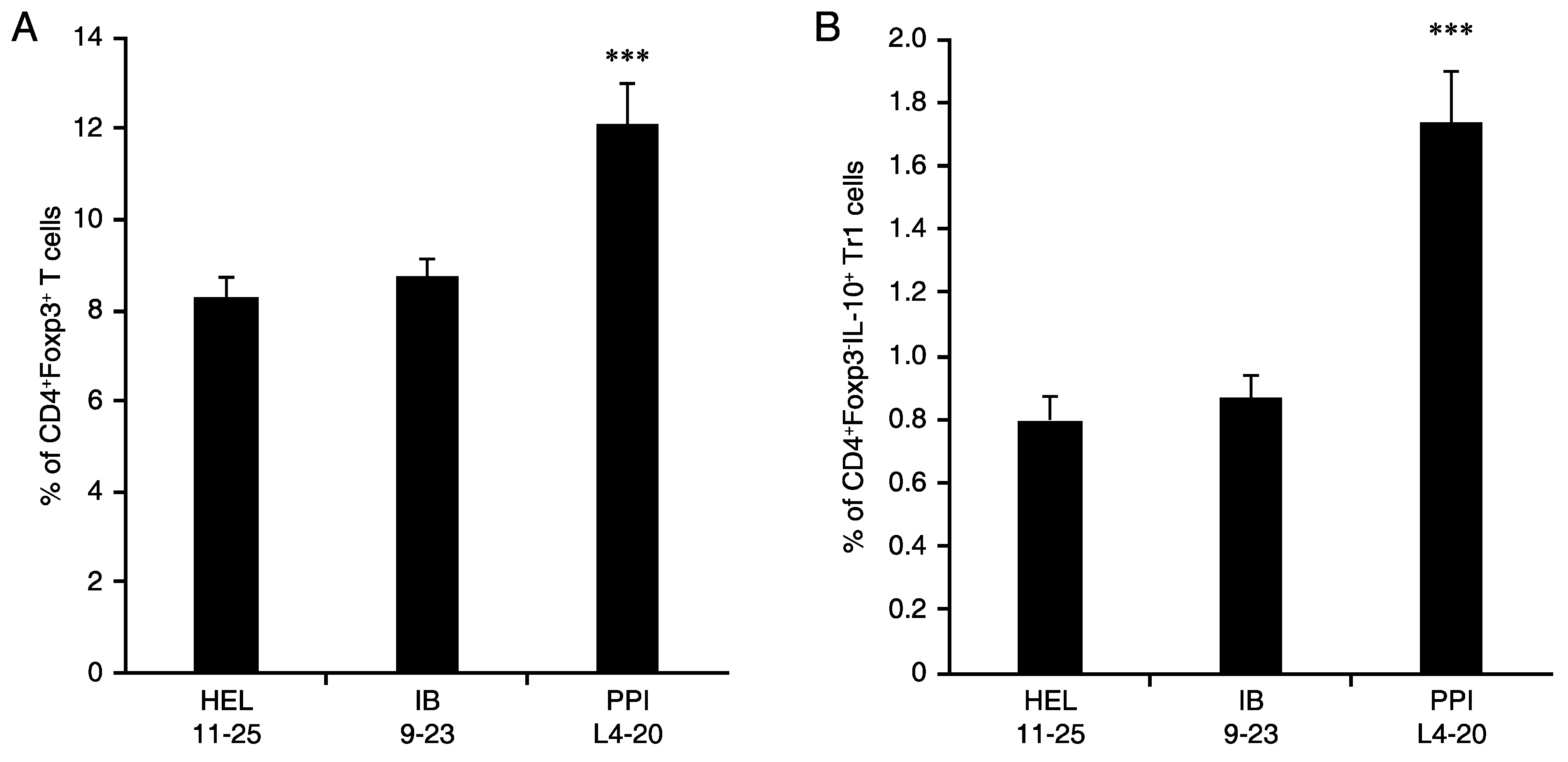
3.5. L4-20 Effectively Induces Antigen-Specific CD4+ Treg and Tr1 Responses
3.6. Studies of Disease Reversal Using Intraperitoneal and Intradermal Routes of ASI Administration
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tian, J.; Kaufman, D.L. Antigen-based therapy for the treatment of type 1 diabetes. Diabetes 2009, 58, 1939–1946. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Roep, B.O.; Wheeler, D.C.S.; Peakman, M. Antigen-based immune modulation therapy for type 1 diabetes: The era of precision medicine. Lancet Diabetes Endocrinol. 2019, 7, 65–74. [Google Scholar] [CrossRef]
- Rodriguez-Fernandez, S.; Almenara-Fuentes, L.; Perna-Barrull, D.; Barneda, B.; Vives-Pi, M. A century later, still fighting back: Antigen-specific immunotherapies for type 1 diabetes. Immunol. Cell Biol. 2021, 99, 461–474. [Google Scholar] [CrossRef]
- Beam, C.A.; MacCallum, C.; Herold, K.C.; Wherrett, D.K.; Palmer, J.; Ludvigsson, J. GAD vaccine reduces insulin loss in recently diagnosed type 1 diabetes: Findings from a Bayesian meta-analysis. Diabetologia 2017, 60, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Hannelius, U.; Beam, C.A.; Ludvigsson, J. Efficacy of GAD-alum immunotherapy associated with HLA-DR3-DQ2 in recently diagnosed type 1 diabetes. Diabetologia 2020, 63, 2177–2181. [Google Scholar] [CrossRef] [PubMed]
- Ludvigsson, J.; Sumnik, Z.; Pelikanova, T.; Chavez, L.N.; Lundberg, E.; Rica, I.; Martínez-Brocca, M.A.; de Adana, M.R.; Wahlberg, J.; Katsarou, A.; et al. Intralymphatic Glutamic Acid Decarboxylase With Vitamin D Supplementation in Recent-Onset Type 1 Diabetes: A Double-Blind, Randomized, Placebo-Controlled Phase IIb Trial. Diabetes Care 2021, 44, 1604–1612. [Google Scholar] [CrossRef]
- Jenkins, M.K.; Moon, J.J. The role of naive T cell precursor frequency and recruitment in dictating immune response magnitude. J. Immunol. 2012, 188, 4135–4140. [Google Scholar] [CrossRef]
- Moon, J.J.; Chu, H.H.; Pepper, M.; McSorley, S.J.; Jameson, S.C.; Kedl, R.M.; Jenkins, M.K. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity 2007, 27, 203–213. [Google Scholar] [CrossRef]
- Olcott, A.P.; Tian, J.; Walker, V.; Dang, H.; Middleton, B.; Adorini, L.; Washburn, L.; Kaufman, D.L. Antigen-based therapies using ignored determinants of beta cell antigens can more effectively inhibit late-stage autoimmune disease in diabetes-prone mice. J. Immunol. 2005, 175, 1991–1999. [Google Scholar] [CrossRef]
- Ott, P.A.; Hu, Z.; Keskin, D.B.; Shukla, S.A.; Sun, J.; Bozym, D.J.; Zhang, W.; Luoma, A.; Giobbie-Hurder, A.; Peter, L.; et al. An immunogenic personal neoantigen vaccine for patients with melanoma. Nature 2017, 547, 217–221. [Google Scholar] [CrossRef]
- Hu, Z.; Leet, D.E.; Allesøe, R.L.; Oliveira, G.; Li, S.; Luoma, A.M.; Liu, J.; Forman, J.; Huang, T.; Iorgulescu, J.B.; et al. Personal neoantigen vaccines induce persistent memory T cell responses and epitope spreading in patients with melanoma. Nat. Med. 2021, 27, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Kaufman, D.L. Attenuation of inducible Th2 immunity with autoimmune disease progression. J. Immunol. 1998, 161, 5399–5403. [Google Scholar] [PubMed]
- Lo, J.; Xia, C.Q.; Peng, R.; Clare-Salzler, M.J. Immature Dendritic Cell Therapy Confers Durable Immune Modulation in an Antigen-Dependent and Antigen-Independent Manner in Nonobese Diabetic Mice. J. Immunol. Res. 2018, 2018, 5463879. [Google Scholar] [CrossRef]
- Reich, E.P.; von Grafenstein, H.; Barlow, A.; Swenson, K.E.; Williams, K.; Janeway, C.A., Jr. Self peptides isolated from MHC glycoproteins of non-obese diabetic mice. J. Immunol. 1994, 152, 2279–2288. [Google Scholar] [PubMed]
- Harrison, L.C.; Honeyman, M.C.; Trembleau, S.; Gregori, S.; Gallazzi, F.; Augstein, P.; Brusic, V.; Hammer, J.; Adorini, L. A peptide-binding motif for I-A(g7), the class II major histocompatibility complex (MHC) molecule of NOD and Biozzi AB/H mice. J. Exp. Med. 1997, 185, 1013–1021. [Google Scholar] [CrossRef] [PubMed]
- Corper, A.L.; Stratmann, T.; Apostolopoulos, V.; Scott, C.A.; Garcia, K.C.; Kang, A.S.; Wilson, I.A.; Teyton, L. A structural framework for deciphering the link between I-Ag7 and autoimmune diabetes. Science 2000, 288, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Daniel, D.; Gill, R.G.; Schloot, N.; Wegmann, D. Epitope specificity, cytokine production profile and diabetogenic activity of insulin-specific T cell clones isolated from NOD mice. Eur. J. Immunol. 1995, 25, 1056–1062. [Google Scholar] [CrossRef]
- Crawford, F.; Stadinski, B.; Jin, N.; Michels, A.; Nakayama, M.; Pratt, P.; Marrack, P.; Eisenbarth, G.; Kappler, J.W. Specificity and detection of insulin-reactive CD4+ T cells in type 1 diabetes in the nonobese diabetic (NOD) mouse. Proc. Natl. Acad. Sci. USA 2011, 108, 16729–16734. [Google Scholar] [CrossRef]
- Alleva, D.G.; Crowe, P.D.; Jin, L.; Kwok, W.W.; Ling, N.; Gottschalk, M.; Conlon, P.J.; Gottlieb, P.A.; Putnam, A.L.; Gaur, A. A disease-associated cellular immune response in type 1 diabetics to an immunodominant epitope of insulin. J. Clin. Investig. 2001, 107, 173–180. [Google Scholar] [CrossRef]
- Kaufman, D.L.; Clare-Salzler, M.; Tian, J.; Forsthuber, T.; Ting, G.S.P.; Robinson, P.; Atkinson, M.A.; Sercarz, E.E.; Tobin, A.J.; Lehmann, P.V. Spontaneous loss of T-cell tolerance to glutamic acid decarboxylase in murine insulin-dependent diabetes. Nature 1993, 366, 69–72. [Google Scholar] [CrossRef]
- Elias, D.; Reshef, T.; Birk, O.S.; van der Zee, R.; Walker, M.D.; Cohen, I.R. Vaccination against autoimmune mouse diabetes with a T-cell epitope of the human 65-kDa heat shock protein. Proc. Natl. Acad. Sci. USA 1991, 88, 3088–3091. [Google Scholar] [CrossRef] [PubMed]
- Yip, H.C.; Karulin, A.Y.; Tary-Lehmann, M.; Hesse, M.D.; Radeke, H.; Heeger, P.S.; Trezza, R.P.; Heinzel, F.P.; Forsthuber, T.; Lehmann, P.V. Adjuvant-guided type-1 and type-2 immunity: Infectious/noninfectious dichotomy defines the class of response. J. Immunol. 1999, 162, 3942–3949. [Google Scholar] [PubMed]
- Forsthuber, T.; Yip, H.C.; Lehmann, P.V. Induction of TH1 and TH2 immunity in neonatal mice. Science 1996, 271, 1728–1730. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Clere-Salzler, M.; Herschenfeld, A.; Middleton, B.; Newman, D.; Mueller, R.; Arita, S.; Evans, C.; Atkinson, M.A.; Mullen, Y.; et al. Modulating autoimmune responses to GAD inhibits disease progression and prolongs islet graft survival in diabetes-prone mice. Nat. Med. 1996, 2, 1348–1353. [Google Scholar] [CrossRef]
- Tian, J.; Dang, H.; Nguyen, A.V.; Chen, Z.; Kaufman, D.L. Combined therapy with GABA and proinsulin/alum acts synergistically to restore long-term normoglycemia by modulating T-cell autoimmunity and promoting beta-cell replication in newly diabetic NOD mice. Diabetes 2014, 63, 3128–3134. [Google Scholar] [CrossRef]
- Gibson, V.B.; Nikolic, T.; Pearce, V.Q.; Demengeot, J.; Roep, B.O.; Peakman, M. Proinsulin multi-peptide immunotherapy induces antigen-specific regulatory T cells and limits autoimmunity in a humanized model. Clin. Exp. Immunol. 2015, 182, 251–260. [Google Scholar] [CrossRef]
- Tian, J.; Yong, J.; Dang, H.; Kaufman, D.L. Oral GABA treatment downregulates inflammatory responses in a mouse model of rheumatoid arthritis. Autoimmunity 2011, 44, 465–470. [Google Scholar] [CrossRef]
- Wiles, T.A.; Delong, T.; Baker, R.L.; Bradley, B.; Barbour, G.; Powell, R.L.; Reisdorph, N.; Haskins, K. An insulin-IAPP hybrid peptide is an endogenous antigen for CD4 T cells in the non-obese diabetic mouse. J. Autoimmun. 2017, 78, 11–18. [Google Scholar] [CrossRef]
- Tian, J.; Lehmann, P.V.; Kaufman, D.L. Determinant spreading of T helper cell 2 (Th2) responses to pancreatic islet autoantigens. J. Exp. Med. 1997, 186, 2039–2043. [Google Scholar] [CrossRef]
- Tian, J.; Olcott, A.P.; Kaufman, D.L. Antigen-based immunotherapy drives the precocious development of autoimmunity. J Immunol. 2002, 169, 6564–6569. [Google Scholar] [CrossRef]
- Jain, R.; Tartar, D.M.; Gregg, R.K.; Divekar, R.D.; Bell, J.J.; Lee, H.-H.; Yu, P.; Ellis, J.S.; Hoeman, C.M.; Franklin, C.L.; et al. Innocuous IFNgamma induced by adjuvant-free antigen restores normoglycemia in NOD mice through inhibition of IL-17 production. J. Exp. Med. 2008, 205, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Bowman, M.; Atkinson, M.A. Heat shock protein therapy fails to prevent diabetes in NOD mice. Diabetologia 2002, 45, 1350–1351. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Lu, Y.; Zhang, H.; Chau, C.H.; Dang, H.N.; Kaufman, D.L. Gamma-aminobutyric acid inhibits T cell autoimmunity and the development of inflammatory responses in a mouse type 1 diabetes model. J. Immunol. 2004, 173, 5298–5304. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Dang, H.; Chen, Z.; Guan, A.; Jin, Y.; Atkinson, M.A.; Kaufman, D.L. gamma-Aminobutyric acid regulates both the survival and replication of human beta-cells. Diabetes 2013, 62, 3760–3765. [Google Scholar] [CrossRef] [PubMed]
- Soltani, N.; Qiu, H.; Aleksic, M.; Glinka, Y.; Zhao, F.; Liu, R.; Li, Y.; Zhang, N.; Chakrabarti, R.; Ng, T.; et al. GABA exerts protective and regenerative effects on islet beta cells and reverses diabetes. Proc. Natl. Acad. Sci. USA 2011, 108, 11692–11697. [Google Scholar] [CrossRef] [PubMed]
- Purwana, I.; Zheng, J.; Li, X.; Deurloo, M.; Son, D.O.; Zhang, Z.; Liang, C.; Shen, E.; Tadkase, A.; Feng, Z.-P.; et al. GABA promotes human beta-cell proliferation and modulates glucose homeostasis. Diabetes 2014, 63, 4197–4205. [Google Scholar] [CrossRef] [PubMed]
- Prud’homme, G.J.; Glinka, Y.; Hasilo, C.; Paraskevas, S.; Li, X.; Wang, Q. GABA protects human islet cells against the deleterious effects of immunosuppressive drugs and exerts immunoinhibitory effects alone. Transplantation 2013, 96, 616–623. [Google Scholar] [CrossRef]
- Tian, J.; Olcott, A.P.; Hanssen, L.R.; Zekzer, D.; Middleton, B.; Kaufman, D.L. Infectious Th1 and Th2 autoimmunity in diabetes-prone mice. Immunol. Rev. 1998, 164, 119–127. [Google Scholar] [CrossRef]
- Alpan, O.; Bachelder, E.; Isil, E.; Arnheiter, H.; Matzinger, P. ‘Educated’ dendritic cells act as messengers from memory to naive T helper cells. Nat. Immunol. 2004, 5, 615–622. [Google Scholar] [CrossRef]
- Tian, J.; Lu, Y.; Hanssen, L.; Dang, H.; Kaufman, D.L. Memory and effector T cells modulate subsequently primed immune responses to unrelated antigens. Cell. Immunol. 2003, 224, 74–85. [Google Scholar] [CrossRef][Green Version]
- Tian, J.; Dang, H.; Kaufman, D.L. Combining antigen-based therapy with GABA treatment synergistically prolongs survival of transplanted ss-cells in diabetic NOD mice. PLoS ONE 2011, 6, e25337. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Dang, H.; O’Laco, K.A.; Song, M.; Tiu, B.-C.; Gilles, S.; Zakarian, C.; Kaufman, D.L. Homotaurine treatment enhances CD4+ and CD8+ Treg responses and synergizes with low-dose anti-CD3 to enhance diabetes remission in type 1 diabetic mice. ImmunoHorizons 2019, 3, 498–510. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Dang, H.N.; Yong, J.; Chui, W.-S.; Dizon, M.P.G.; Yaw, C.K.Y.; Kaufman, D.L. Oral treatment with gamma-aminobutyric acid improves glucose tolerance and insulin sensitivity by inhibiting inflammation in high fat diet-fed mice. PLoS ONE 2011, 6, e25338. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Chau, C.; Hales, T.G.; Kaufman, D.L. GABA(A) receptors mediate inhibition of T cell responses. J. Neuroimmunol. 1999, 96, 21–28. [Google Scholar] [CrossRef]
- Davis, M.M.; Altman, J.D.; Newell, E.W. Interrogating the repertoire: Broadening the scope of peptide-MHC multimer analysis. Nat. Rev. Immunol. 2011, 11, 551–558. [Google Scholar] [CrossRef]
- Campion, S.L.; Brodie, T.M.; Fischer, W.; Korber, B.T.; Rossetti, A.; Goonetilleke, N.; McMichael, A.J.; Sallusto, F. Proteome-wide analysis of HIV-specific naive and memory CD4(+) T cells in unexposed blood donors. J. Exp. Med. 2014, 211, 1273–1280. [Google Scholar] [CrossRef]
- Pittet, M.J.; Valmori, D.; Dunbar, P.R.; Speiser, D.E.; Liénard, D.; Lejeune, F.; Fleischhauer, K.; Cerundolo, V.; Cerottini, J.C.; Romero, P. High frequencies of naive Melan-A/MART-1-specific CD8(+) T cells in a large proportion of human histocompatibility leukocyte antigen (HLA)-A2 individuals. J. Exp. Med. 1999, 190, 705–715. [Google Scholar] [CrossRef]
- Ma, K.-Y.; Schonnesen, A.A.; He, C.; Xia, A.Y.; Sun, E.; Chen, E.; Sebastian, K.R.; Guo, Y.-W.; Balderas, R.; Kulkarni-Date, M.; et al. High-throughput and high-dimensional single-cell analysis of antigen-specific CD8(+) T cells. Nat. Immunol. 2021, 22, 1590–1598. [Google Scholar] [CrossRef]
- Zhang, W.; Hawkins, P.G.; He, J.; Gupta, N.T.; Liu, J.; Choonoo, G.; Jeong, S.W.; Chen, C.R.; Dhanik, A.; Dillon, M.; et al. A framework for highly multiplexed dextramer mapping and prediction of T cell receptor sequences to antigen specificity. Sci. Adv. 2021, 7, eabf5835. [Google Scholar] [CrossRef]
- Massilamany, C.; Krishnan, B.; Reddy, J. Major Histocompatibility Complex Class II Dextramers: New Tools for the Detection of antigen-Specific, CD4 T Cells in Basic and Clinical Research. Scand. J. Immunol. 2015, 82, 399–408. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tian, J.; Song, M.; Kaufman, D.L. Designing Personalized Antigen-Specific Immunotherapies for Autoimmune Diseases—The Case for Using Ignored Target Cell Antigen Determinants. Cells 2022, 11, 1081. https://doi.org/10.3390/cells11071081
Tian J, Song M, Kaufman DL. Designing Personalized Antigen-Specific Immunotherapies for Autoimmune Diseases—The Case for Using Ignored Target Cell Antigen Determinants. Cells. 2022; 11(7):1081. https://doi.org/10.3390/cells11071081
Chicago/Turabian StyleTian, Jide, Min Song, and Daniel L. Kaufman. 2022. "Designing Personalized Antigen-Specific Immunotherapies for Autoimmune Diseases—The Case for Using Ignored Target Cell Antigen Determinants" Cells 11, no. 7: 1081. https://doi.org/10.3390/cells11071081
APA StyleTian, J., Song, M., & Kaufman, D. L. (2022). Designing Personalized Antigen-Specific Immunotherapies for Autoimmune Diseases—The Case for Using Ignored Target Cell Antigen Determinants. Cells, 11(7), 1081. https://doi.org/10.3390/cells11071081




