Spatial Manipulation of Particles and Cells at Micro- and Nanoscale via Magnetic Forces
Abstract
:1. Introduction
2. Gradient Magnetic Field and Forces
3. Magnetic Field Configurations from Cylindrical Magnets
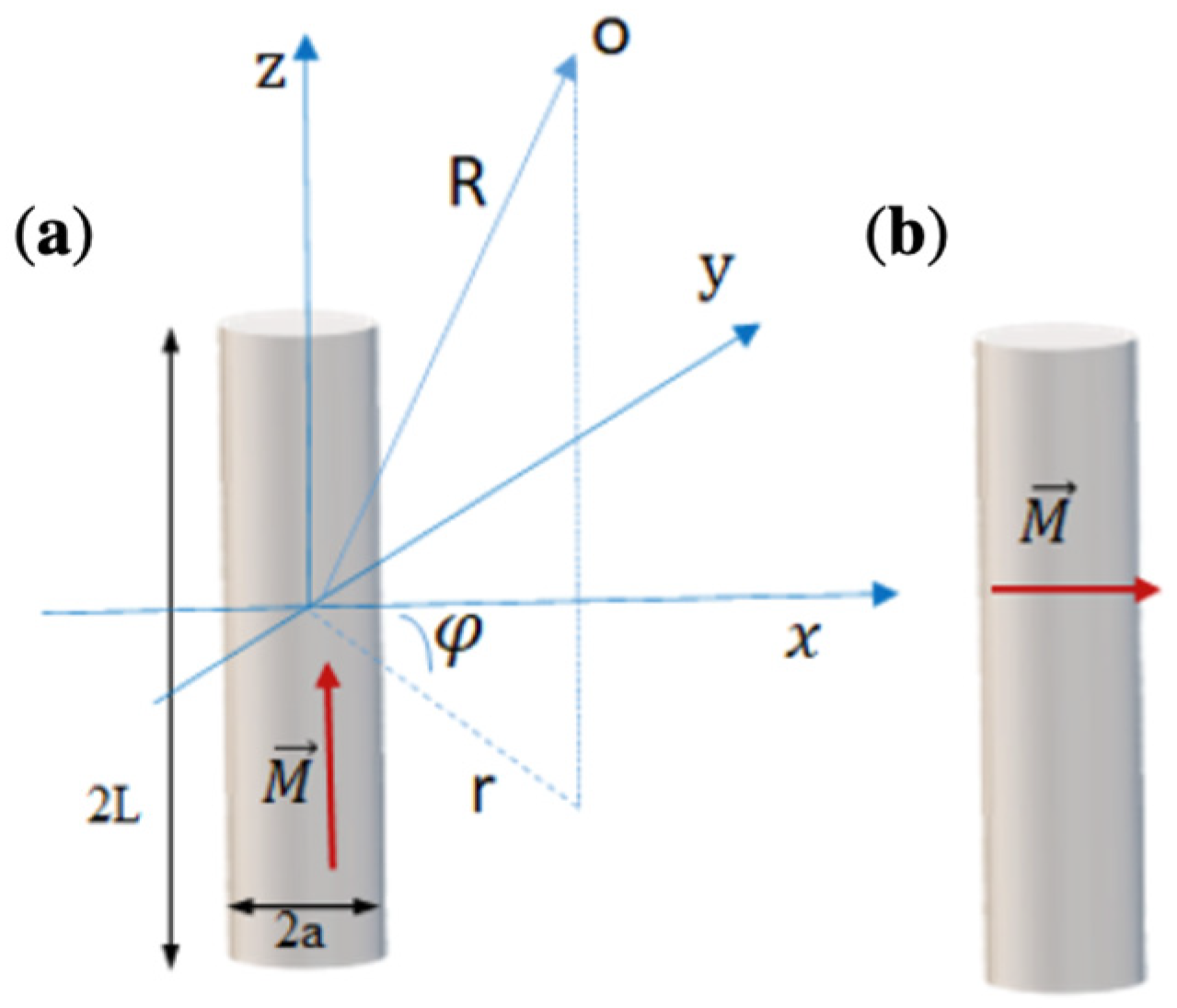
3.1. Cylinder with Axial Magnetisation
3.2. Diametrically Magnetised Cylinder
4. Basic Magnet Systems for Magnetic Particle Manipulation
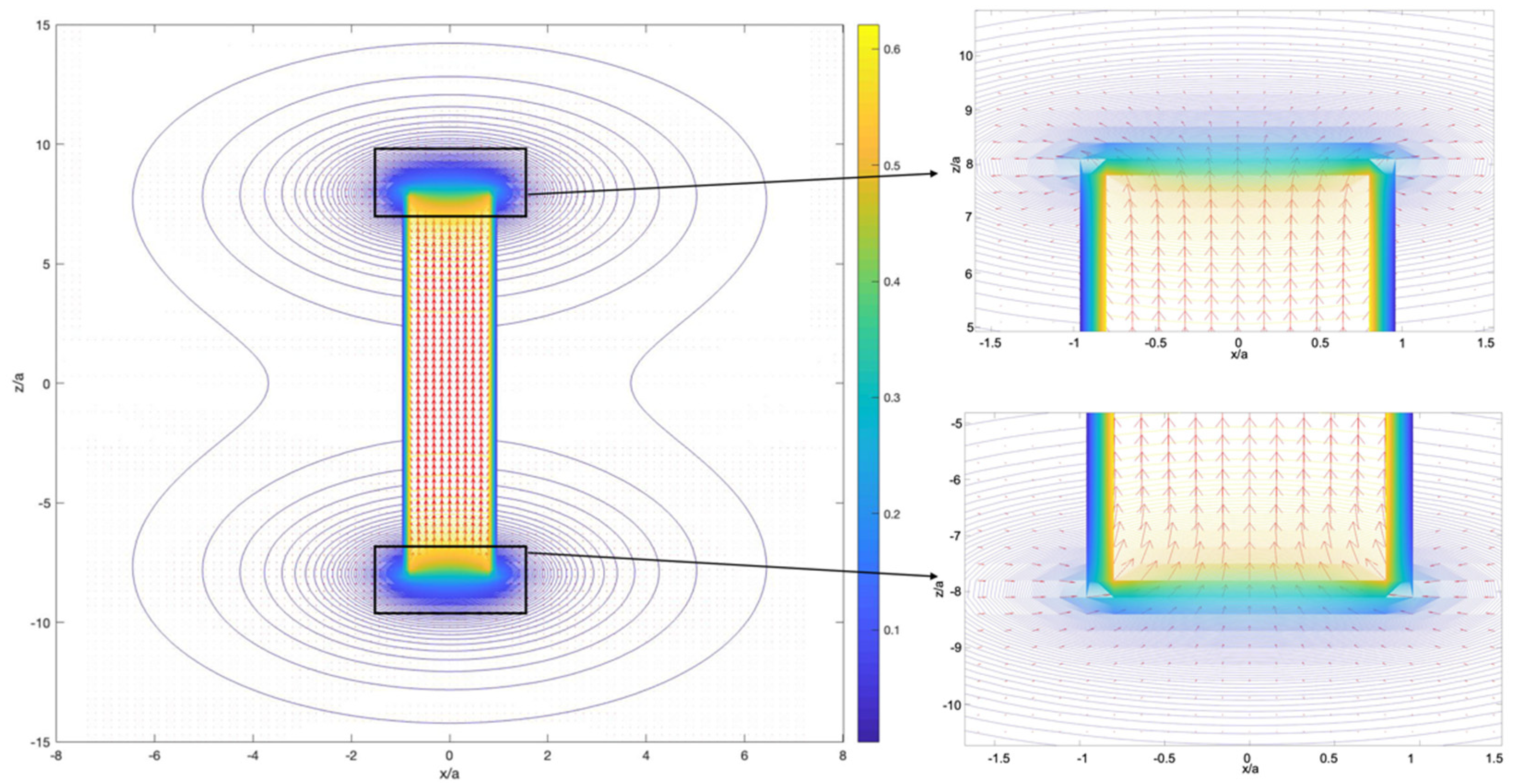
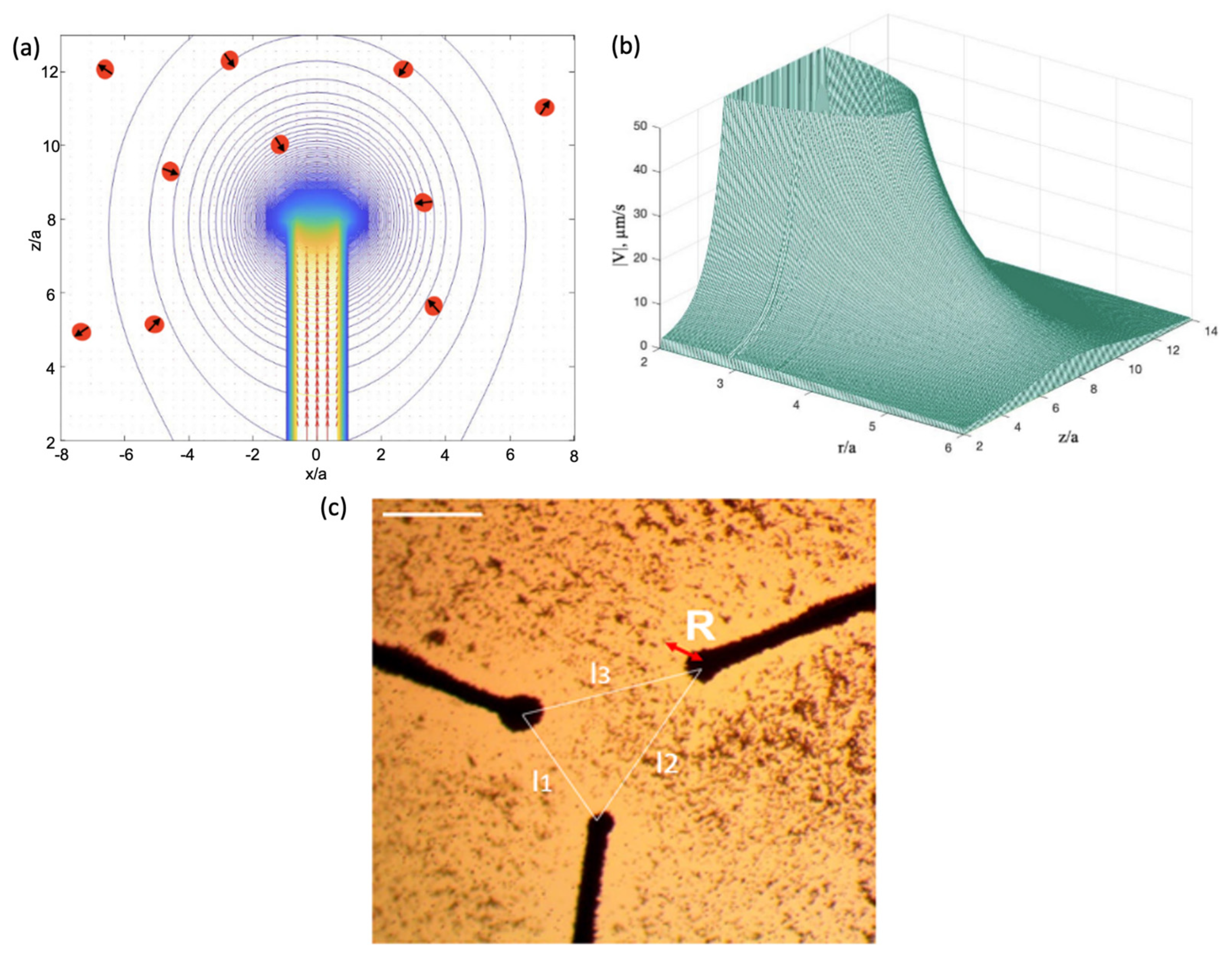
4.1. Magnetic Particle Manipulation with a Single Magnet
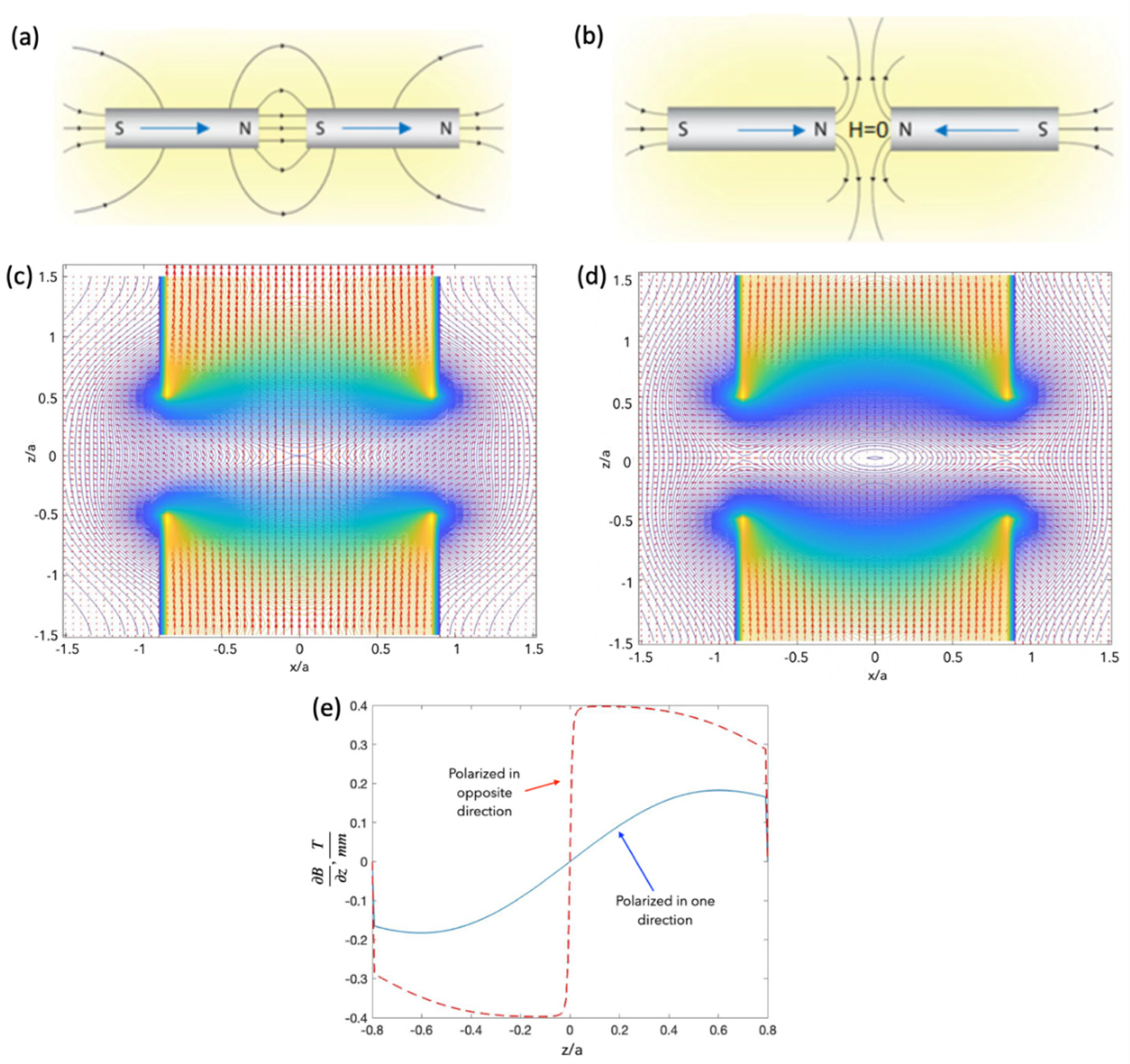
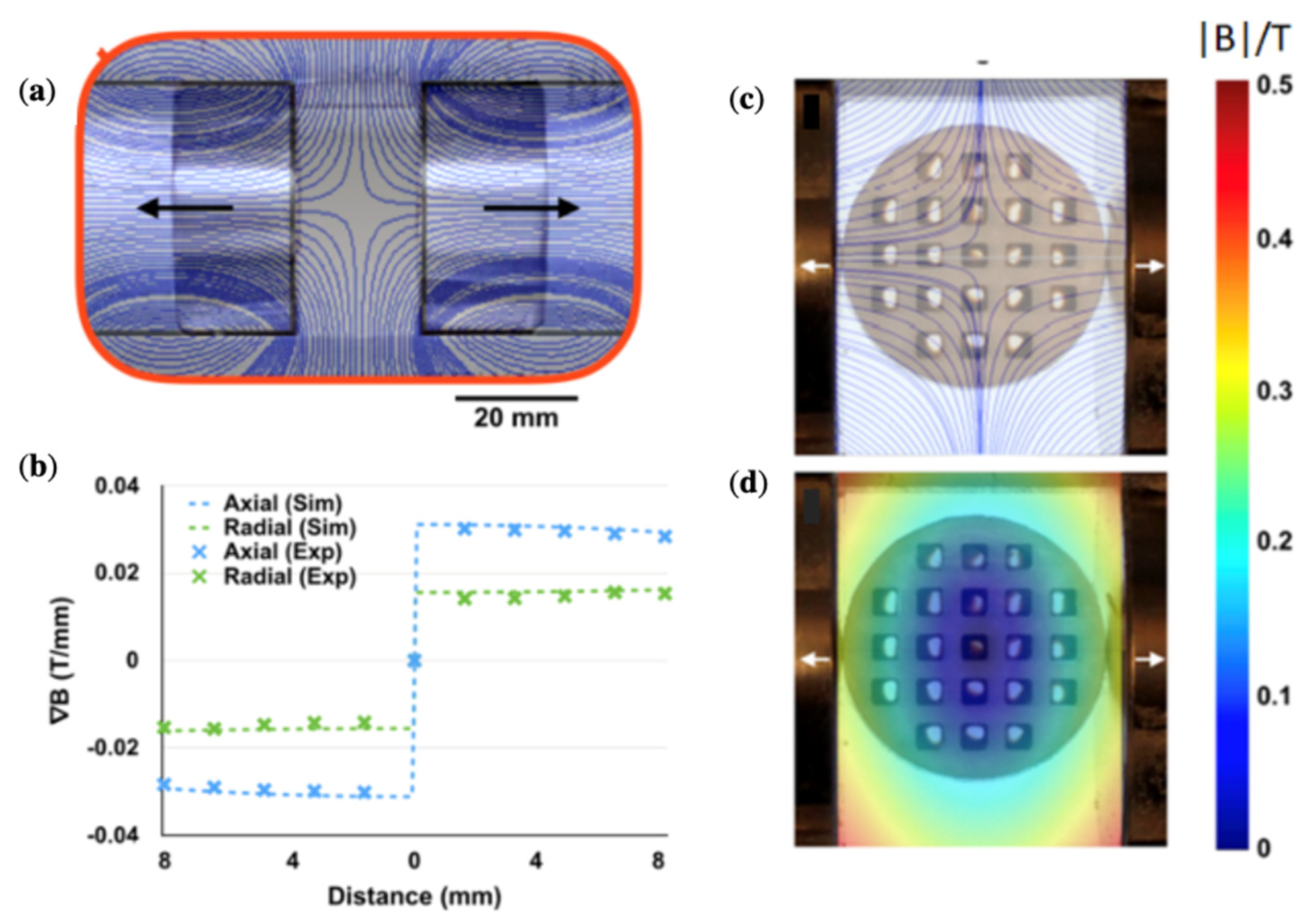
4.2. Magnetic Particle Manipulation with Two Axially Polarised Magnets
4.3. Magnetic Particle Manipulation with Two Cylindrical Magnets Magnetised along the Diameter
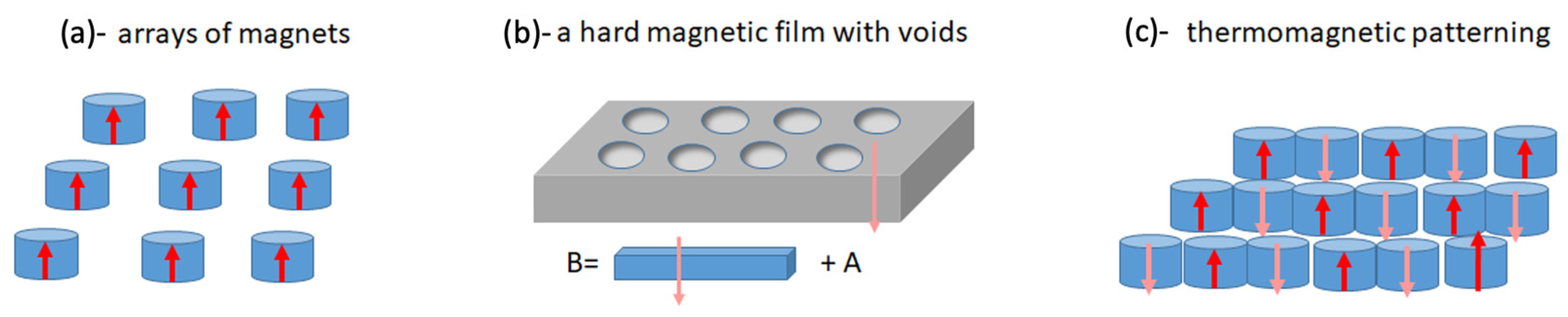
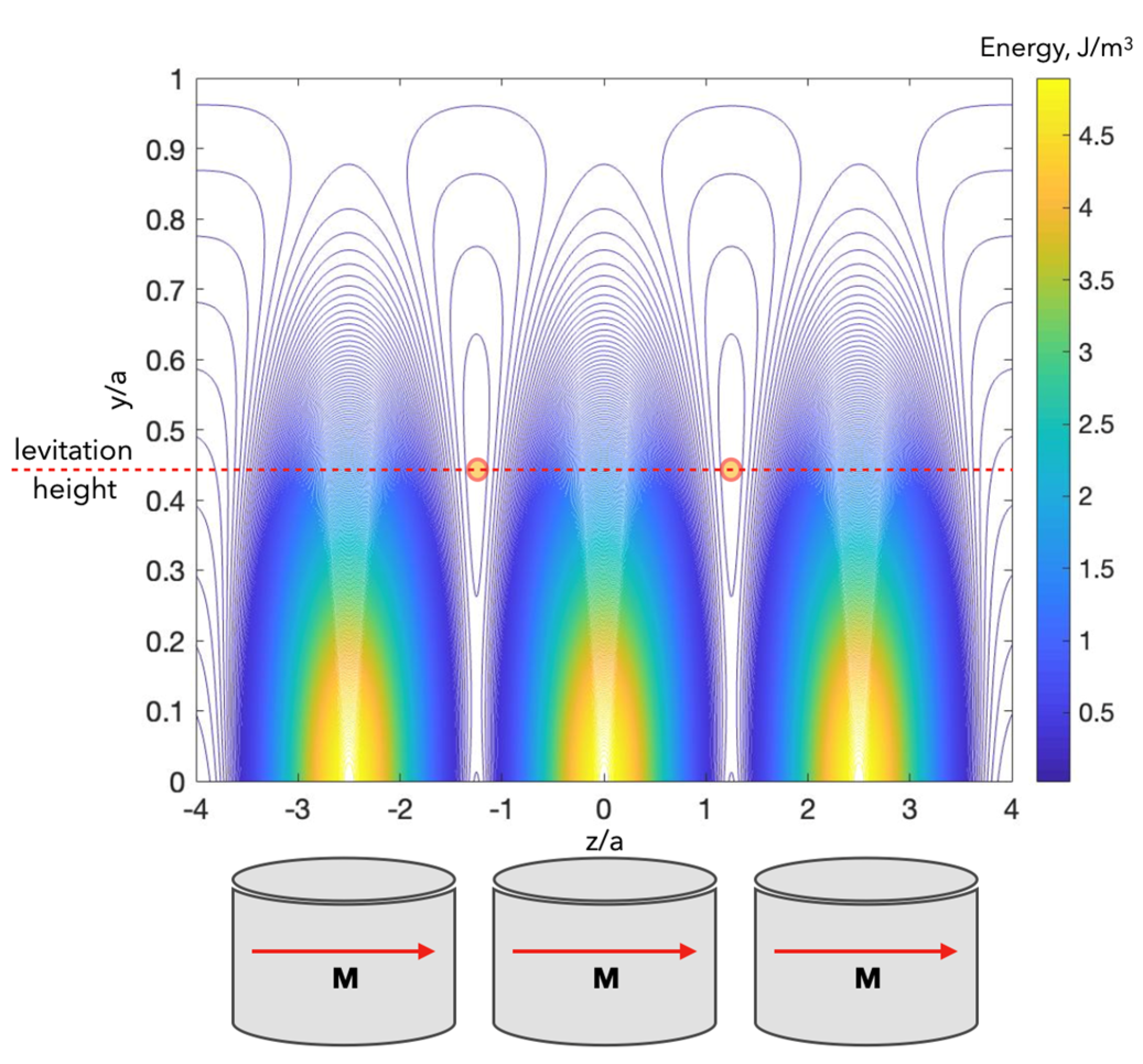
4.4. Arrays of Magnets
4.5. Methods of Computation of Magnetic Field Produced by Various Magnetic Grids
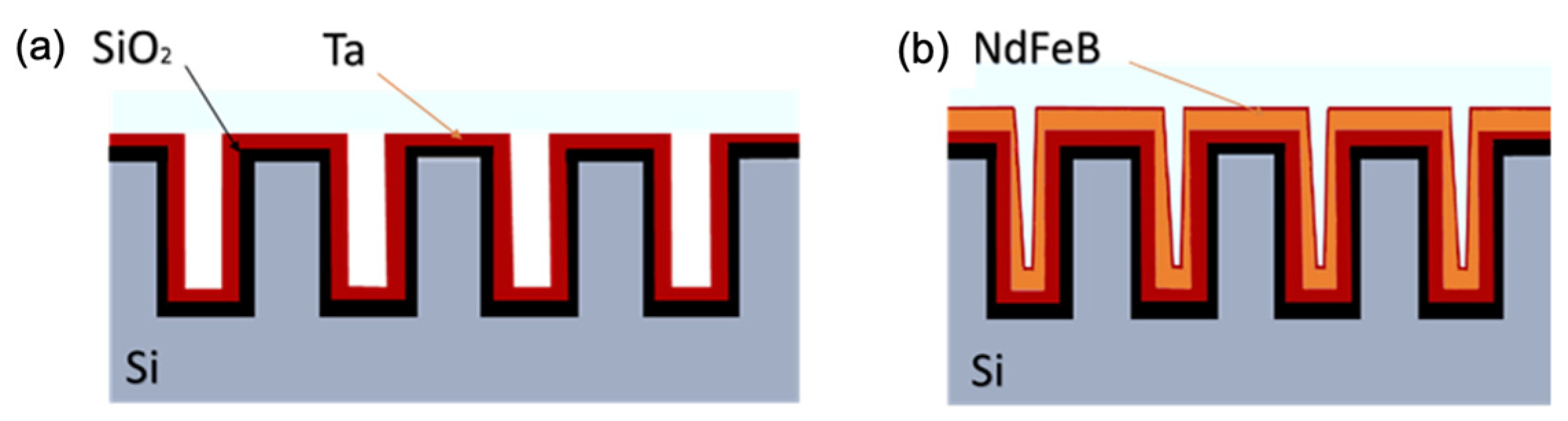
4.6. Microelectromagnetic Patterning
5. Diffusion of Magnetic Particles
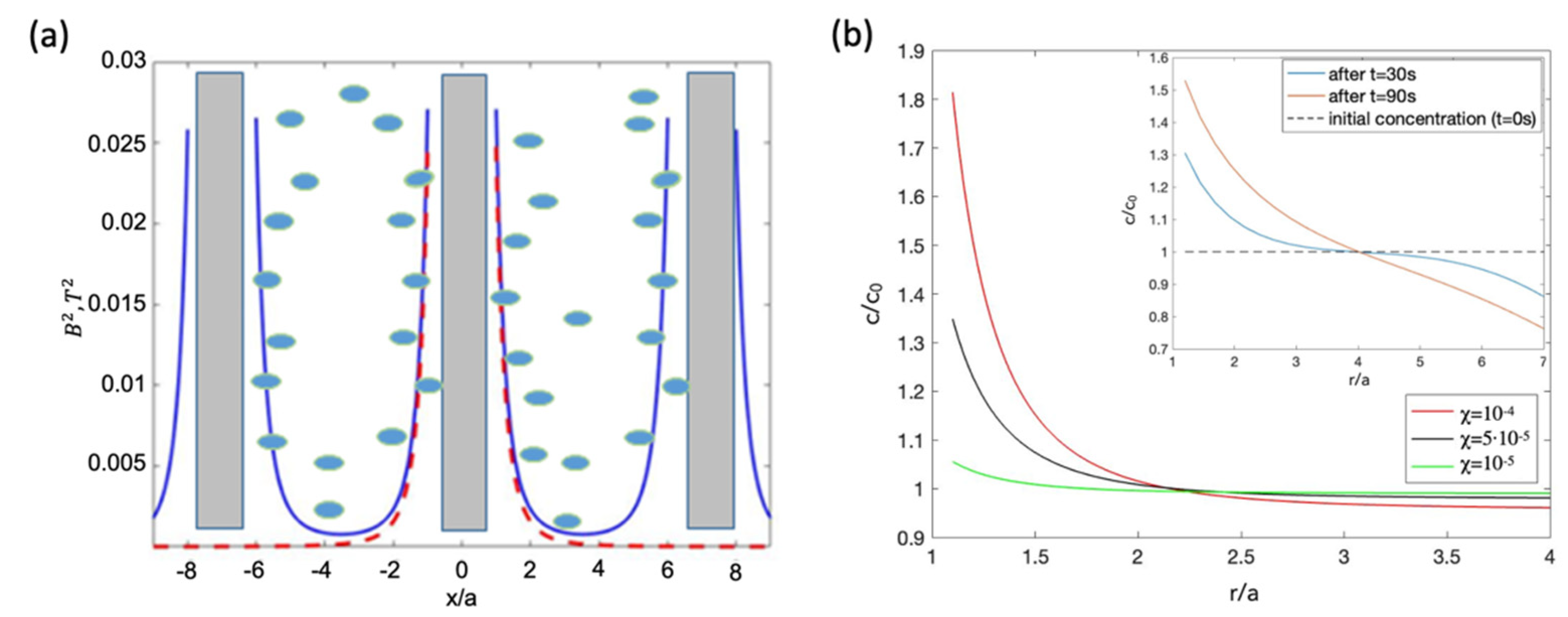
5.1. Diffusion of Paramagnetic NPs in a Magnetic Field Produced by Diametrically Magnetised Microwires
5.2. Regulated Submicron Magnetic Particle Diffusion using Tunable Domain Structure
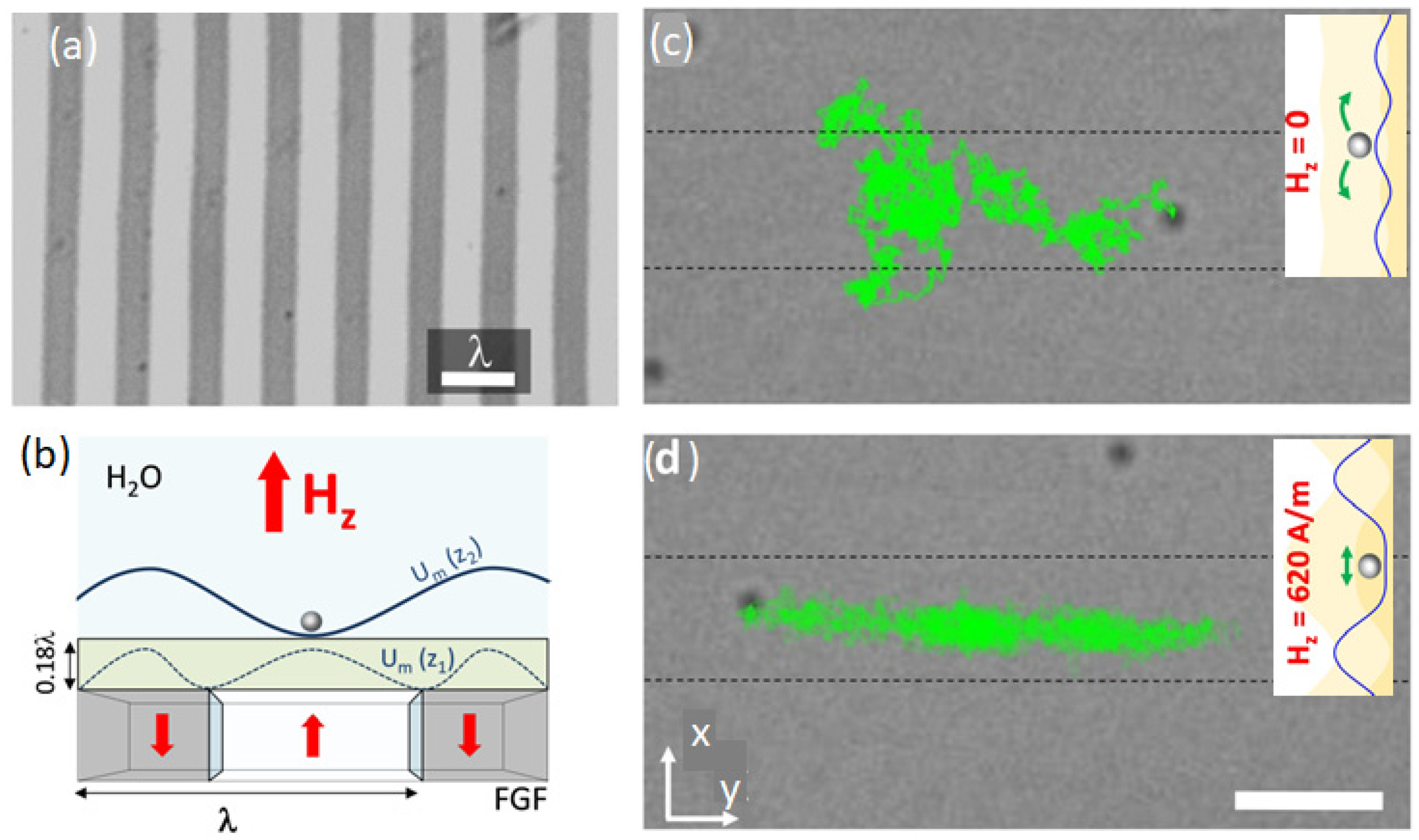
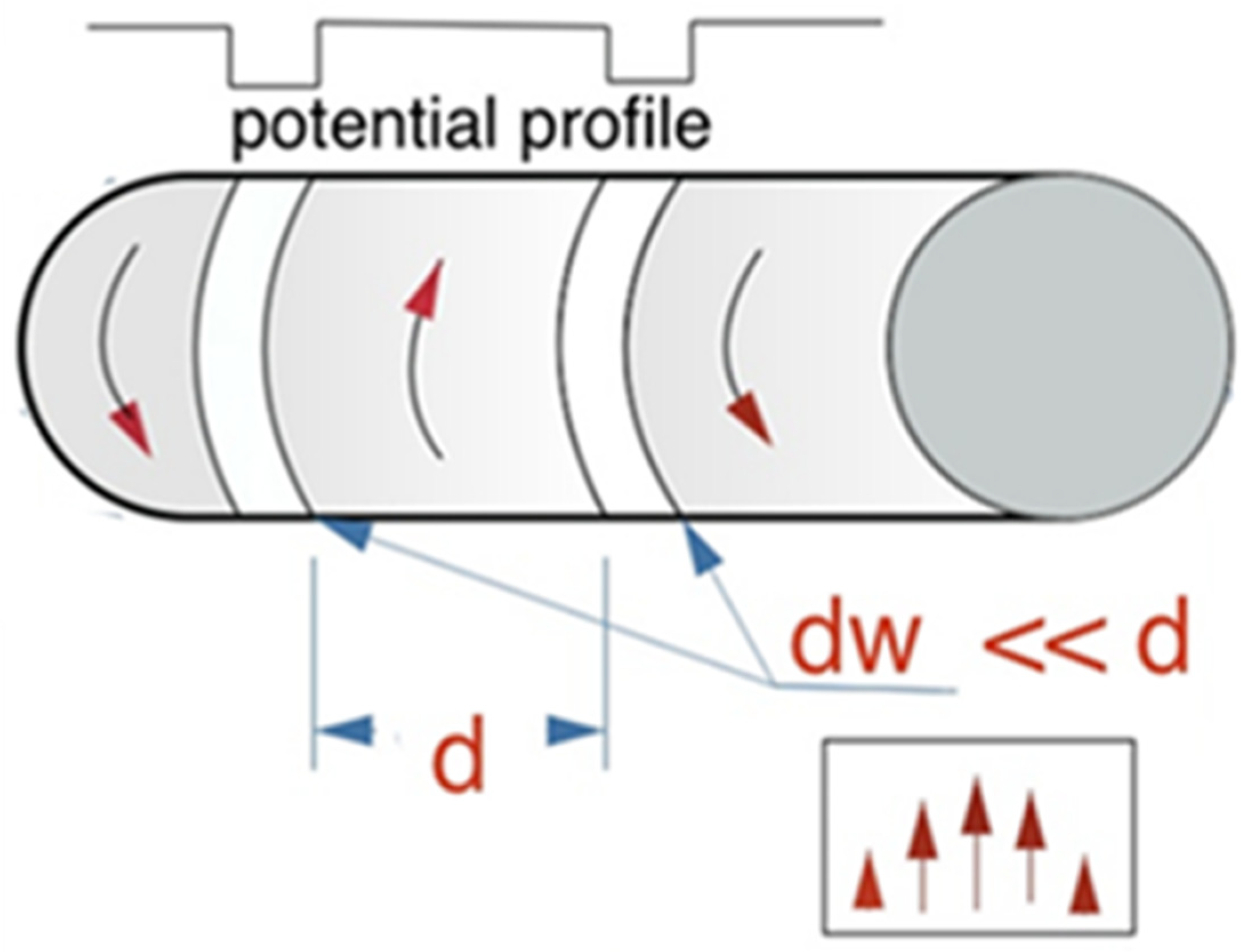
5.3. Enhanced Diffusion of Magnetic Particles Using Travelling Potential (Magnetic Ratchet)
6. Biocompatibility of Ferromagnetic Microwires with a Glass Coating
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cao, Q.L.; Fan, Q.; Chen, Q.; Liu, C.T.; Han, X.T.; Li, L. Recent advances in manipulation of micro- and nano-objects with magnetic fields at small scales. Mater. Horiz. 2020, 7, 638–666. [Google Scholar] [CrossRef]
- Liu, Y.-L.; Chen, D.; Shang, P.; Yin, D.-C. A review of magnet systems for targeted drug delivery. J. Control. Release 2019, 302, 90–104. [Google Scholar] [CrossRef] [PubMed]
- Day, N.B.; Wixson, W.C.; Shields IV, C.W. Magnetic systems for cancer immunotherapy. Acta Pharm. Sin. B. 2021, 11, 2172–2196. [Google Scholar] [CrossRef] [PubMed]
- Rajan, A.; Sahu, N.K. Review on magnetic nanoparticle-mediated hyperthermia for cancer therapy. J. Nanopart. Res. 2020, 22, 319. [Google Scholar] [CrossRef]
- Parfenov, V.A.; Khesuani, Y.D.; Petrov, S.V.; Karalkin, P.A.; Koudan, E.V.; Nezhurina, E.K.; Pereira, F.; Krokhmal, A.A.; Gryadunova, A.A.; Bulanova, E.A. Magnetic levitational bioassembly of 3D tissue construct in space. Sci. Adv. 2020, 6, 4174. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.A.; Yim, H.; Heo, J. Application of magnetic nanoparticle for controlled tissue assembly and Tissue Eng. Arch. Pharm. Res. 2014, 37, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Fayol, D.; Frasca, G.; Visage, C.L.; Gazeau, F.; Luciani, N.; Wilhelm, C. Use of magnetic forces to promote stem cell aggregation during differentiation, and cartilage tissue modeling. Adv. Mater. 2013, 25, 2611–2616. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.H.; Lee, E.J.; Son, M.; Lee, J.-H.; Yoo, D.; Kim, J.-w.; Park, S.W.; Shin, J.-S.; Cheon, J. A Magnetic Switch for The Control of Cell Death Signalling in in Vitro and in Vivo Systems. Nat. Mater. 2012, 11, 1038. [Google Scholar] [CrossRef] [PubMed]
- Waskaas, M. Short-Term Effects of Magnetic Fields on Diffusion in Stirred and Unstirred Paramagnetic Solutions. J. Phys. Chem. 1993, 97, 6470–6476. [Google Scholar] [CrossRef]
- Kinouchi, Y.; Tanimoto, S.; Ushita, T.; Sato, K.; Yamaguchi, H.; Miyamoto, H. Effects of Static Magnetic Fields on Diffusion in Solutions. Bioelectromagnetics 1988, 9, 159–166. [Google Scholar] [CrossRef]
- Chang, K.T.; Weng, C.I. An investigation into the structure of aqueous NaCl electrolyte solutions under magnetic fields. Comput. Mater. Sci. 2008, 43, 1048–1055. [Google Scholar] [CrossRef]
- Zablotskii, V.; Polyakova, T.; Dejneka, A. Effects of High Magnetic Fields on the Diffusion of Biologically Active Molecules. Cells 2022, 11, 81. [Google Scholar] [CrossRef] [PubMed]
- Sear Richard, P. Diffusiophoresis in Cells: A General Nonequilibrium, Nonmotor Mechanism for the Metabolism-Dependent Transport of Particles in Cells. Phys. Rev. Lett. 2019, 122, 128101. [Google Scholar] [CrossRef] [Green Version]
- Zablotskii, V.; Lunov, O.; Kubinova, S. Effects of high-gradient magnetic fields on living cell machinery. J. Phys. D Appl. Phys. 2016, 49, 493003. [Google Scholar] [CrossRef]
- Zablotskii, V.; Polyakova, T. How a High-Gradient Magnetic Field Could Affect Cell Life. Sci. Rep. 2016, 6, 37407. [Google Scholar] [CrossRef] [PubMed]
- Zablotskii, V.; Polyakova, T.; Dejneka, A. Cells in the Non-Uniform Magnetic World: How Cells Respond to High-Gradient Magnetic Fields. BioEssays 2018, 40, 1800017. [Google Scholar] [CrossRef] [PubMed]
- Shellock, F.G.; Kanal, E.J. Safety of magnetic resonance imaging contrast agents. Magn. Reson. Imaging 1999, 10, 477–484. [Google Scholar] [CrossRef]
- Bakker, C.J.; de Roos, R. Concerning the preparation and use of substances with a magnetic susceptibility equal to the magnetic susceptibility of air. Magn. Reson. Med. 2006, 56, 1107–1113. [Google Scholar] [CrossRef] [PubMed]
- Press, D. Superparamagnetic iron oxide nanoparticles: Magnetic nanoplatforms as drug carriers. Int. J. Nanomed. 2012, 7, 3445–3471. [Google Scholar] [CrossRef] [Green Version]
- Lunov, O.; Uzhytchak, M.; Smolková, B.; Lunova, M.; Jirsa, M.; Dempsey, N.M.; Dias, A.L.; Bonfim, M.; Hof, M.; Jurkiewicz, P.; et al. Remote actuation of apoptosis in liver cancer cells via magneto-mechanical modulation of iron oxide nanoparticles. Cancers 2019, 11, 1873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Master, A.M.; Williams, P.N.; Pothayee, N.; Pothayee, N.; Zhang, R.; Vishwasrao, H.M.; Golovin, Y.I.; Riffle, J.S.; Sokolsky, M.; Kabanov, A.V. Remote actuation of magnetic nanoparticles for cancer cell selective treatment through cytoskeletal disruption. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Zablotskii, V.; Lunov, O.; Dejneka, A.; Jastrabk, L.; Polyakova, T.; Syrovets, T.; Simmet, T. Nanomechanics of magnetically driven cellular endocytosis. Appl. Phys. Lett. 2011, 99, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Pshenichnikov, S.; Omelyanchik, A.; Efremova, M.; Lunova, M.; Gazatova, N.; Malashchenko, V.; Khaziakhmatova, O.; Litvinova, L.; Perov, N.; Panina, L.; et al. Control of oxidative stress in Jurkat cells as a model of leukemia treatment. J. Magn. Magn. Mat. 2021, 523, 167623. [Google Scholar] [CrossRef]
- Cole, A.J.; Yang, V.C.; David, A.E. Cancer Theranostics: The Rise of Targeted Magnetic Nanoparticles. Trends Biotechnol. 2011, 29, 323–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, C.; Lee, J.S.H.; Zhang, M. Magnetic Nanoparticles in MR Imaging and Drug Delivery. Adv. Drug Deliv. Rev. 2008, 60, 1252–1265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dempsey, N.M.; Walther, A.; May, F.; Givord, D.; Khlopkov, K.; Gutfleisch, O. High performance hard magnetic NdFeB thick films for integration into micro-electro-mechanical systems. Appl. Phys. Lett. 2007, 90, 092509. [Google Scholar] [CrossRef] [Green Version]
- Walter, A.; Marcoux, C.; Desloges, B.; Grechishkin, R.; Givord, D.; Dempsey, N. Micro-patterning of NdFeB and SmCo magnet films for integration into micro-electro-mechanical-systems. J. Magn. Magn. Mater. 2009, 321, 590–594. [Google Scholar] [CrossRef] [Green Version]
- Omelyanchik, A.; Gurevich, A.; Pshenichnikov, S.; Kolesnikova, V.; Smolkova, B.; Uzhytchak, M.; Baraban, I.; Lunov, O.; Levada, K.; Panina, L.; et al. Ferromagnetic glass-coated microwires for cell manipulation. J. Magn. Magn. Mater. 2020, 512, 166991. [Google Scholar] [CrossRef]
- Gurevich, A.; Beklemisheva, A.V.; Levada, E.; Rodionova, V.; Panina, L.V. Ferromagnetic Microwire Systems as a High-Gradient Magnetic Field Source for Magnetophoresis. IEEE Magn. Lett. 2020, 11, 1–5. [Google Scholar] [CrossRef]
- Blümler, P. Magnetic Guiding with Permanent Magnets: Concept, Realization and Applications to Nanoparticles and Cells. Cells 2021, 10, 2708. [Google Scholar] [CrossRef]
- Baun, O.; Blümler, P. Permanent magnet system to guide superparamagnetic particles. J. Magn. Magn. Mater. 2017, 439, 294–304. [Google Scholar] [CrossRef] [Green Version]
- Tay, Z.W.; Chandrasekharan, P.; Zhou, X.Y.; Yu, E.; Zheng, B.; Conolly, S. In Vivo Tracking and Quantification of Inhaled Aerosol Using Magnetic Particle Imaging Towards Inhaled Therapeutic Monitoring. Theranostics 2018, 8, 3676–3687. [Google Scholar] [CrossRef] [PubMed]
- Zheng, B.; von See, M.P.; Yu, E.; Gunel, B.; Lu, K.; Vazin, T.; Schaffer, D.V.; Goodwill, P.W.; Conolly, S.M. Quantitative Magnetic Particle Imaging Monitors the Transplantation, Biodistribution, and Clearance of Stem Cells in Vivo. Theranostics 2016, 6, 291–301. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.F.; Lan, Z.; Ferrari, C.; Stein, J.M.; Higbee-Dempsey, E.; Yan, L.; Amirshaghaghi, A.; Cheng, Z.; Issadore, D.; Tsourkas, A. Use of Oppositely Polarised External Magnets to Improve the Accumulation and Penetration of Magnetic Nanocarriers into Solid Tumors. ACS Nano 2020, 14, 142–152. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Takizawa, Y.; Honda, H.; Hata, K.; Kagami, H.; Ueda, M.; Kobayashi, T. Tissue Eng. using magnetite nanoparticles and magnetic force: Heterotypic layers of cocultured hepatocytes and endothelial cells. Tissue Eng. 2004, 10, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Ino, K.; Hayashida, M.; Kobayashi, T.; Matsunuma, H.; Kagami, H.; Ueda, M.; Honda, H. Novel methodology for fabrication of tissue-engineered tubular constructs using magnetite nanoparticles and magnetic force. Tissue Eng. 2005, 11, 1553–1561. [Google Scholar] [CrossRef] [PubMed]
- Demirörs, A.; Pillai, P.; Kowalczyk, B.; Grzybowski, B.A. Colloidal assembly directed by virtual magnetic moulds. Nature 2013, 503, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, S. Programmed magnetic manipulation of vesicles into spatially coded prototissue architectures arrays. Nat. Commun. 2020, 11, 232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosuke, I.; Ito, A.; Honda, H. Cell patterning using magnetite nanoparticles and magnetic force. Biotechnol. Bioeng. 2007, 97, 1309–1317. [Google Scholar] [CrossRef]
- Smolková, B.; Uzhytchak, M.; Lynnyk, A.; Kubinová, Š.; Dejneka, A.; Lunov, O. A critical review on selected external physical cues and modulation of cell behavior: Magnetic Nanoparticles, Non-thermal Plasma and Lasers. J. Funct. Biomater. 2019, 10, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lim, B.; Vavassori, P.; Sooryakumar, R.; Kim, C. Nano/micro-scale magnetophoretic devices for biomedical applications. J. Phys. D Appl. Phys. 2016, 50, 033002. [Google Scholar] [CrossRef]
- Halbach, K. Design of permanent multipole magnets with oriented rare earth cobalt material. Nucl. Instrum. Methods 1980, 169, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Van Oene, M.M.; Dickinson, L.E.; Pedaci, F.; Köber, M.; Dulin, D.; Lipfert, J.; Dekker, N. Biological Magnetometry: Torque on superparamagnetic beads in magnetic fields. Phys. Rev. Lett. 2015, 114, 218301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moerland, C.; van Ijzendoorn, L.; Prins, M. Rotating magnetic particles for lab-on-chip applications—A comprehensive review. Lab Chip 2019, 19, 919–933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bae, J.E.; Huh, M.I.; Ryu, B.K.; Do, J.Y.; Jin, S.U.; Moon, M.J.; Jung, J.C.; Chang, Y.; Kim, E.; Chi, S.G.; et al. The effect of static magnetic fields on the aggregation and cytotoxicity of magnetic nanoparticles. Biomaterials 2011, 32, 9401–9414. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, E.E.; Maslen, S.H. The Magnetic Field of a Finite Solenoid; National Aeronautics and Space Administration: Washington, DC, USA, 1960.
- Derby, N.; Olbert, S. Cylindrical magnets and ideal solenoids. Am. J. Phys. 2010, 78, 229–235. [Google Scholar] [CrossRef] [Green Version]
- Taniguchi, T. An analytical computation of magnetic field generated from a cylinder ferromagnet. J. Magn. Magn. Mater. 2017, 11, 464–472. [Google Scholar] [CrossRef] [Green Version]
- Uzhytchak, M.; Lynnyk, A.; Zablotskii, V.; Dempsey, N.M.; Dias, A.L.; Bonfim, M.; Dejneka, A. The use of pulsed magnetic fields to increase the uptake of iron oxide nanoparticles by living cells. Appl. Phys. Lett. 2017, 111, 243703. [Google Scholar] [CrossRef]
- Vazquez, M.; Chiriac, H.; Zhukov, A.; Panina, L.; Uchiyama, T. On the state-of-the-art in magnetic microwires and expected trends for scientific and technological studies. Phys. Status Solidi A 2011, 208, 493. [Google Scholar] [CrossRef]
- Chiriac, H.; Corodeanu, S.; Lostun, M.; Stoian, G.; Ababei, G.; Ovari, T.A. Rapidly solidified amorphous nanowires. J. Appl. Phys. 2011, 109, 063902. [Google Scholar] [CrossRef]
- Baranov, S.A.; Larin, V.S.; Torcunov, A.V. Technology, preparation and properties of the cast glass-coated magnetic microwires. Crystals 2017, 7, 136. [Google Scholar] [CrossRef]
- Evstigneeva, S.A.; Nematov, M.G.; Omelyanchik, A.; Yudanov, N.A.; Rodionova, V.V.; Panina, L.V. Hard Magnetic Properties of Co-Rich Microwires Crystallized by Current Annealing. IEEE Magn. Lett. 2020, 11, 1–5. [Google Scholar] [CrossRef]
- Gunawan, O.; Virgus, Y.; Fai, K. A parallel dipole line system. Appl. Phys. Lett. 2015, 106, 062407. [Google Scholar] [CrossRef] [Green Version]
- Beklemisheva, A.V.; Yudanov, N.A.; Gurevich, A.A.; Panina, L.V.; Zablotskiy, V.A.; Deyneka, A. Matrices of Ferromagnetic Microwires for the Control of Cellular Dynamics and Localized Delivery of Medicines. Phys. Met. Metallogr. 2019, 120, 556–562. [Google Scholar] [CrossRef]
- Runge, V.M. Safety of Magnetic Resonance Contrast Media. Top. Magn. Reson. Imaging 2001, 12, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Kauffmann, P.; Dempsey, M.; Ith, A.; O’Brien, D.; Gaude, V.; Boué, F.; Combe, S.; Bruckert, F.; Schaack, B.; Haguet, V.; et al. Diamagnetically Trapped arrays of living cells above micromagnets. Lab Chip 2011, 11, 3153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosuke, I.; Okochi, M.; Konishi, N.; Nakatochi, M.; Imai, R.; Shikida, M.; Ito, A.; Honda, H. Cell culture arrays using magnetic force-based cell patterning for dynamic single cell analysis. Lab Chip 2008, 8, 134–142. [Google Scholar] [CrossRef]
- Okochi, M.; Matsumura, T.; Honda, H. Magnetic force-based cell patterning for evaluation of the effect of stromal fibroblasts on invasive capacity in 3D cultures. Biosens. Bioelectron. 2013, 42, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Okochi, M.; Matsumura, T.; Yamamoto, S.; Nakayama, E.; Jimbow, K.; Honda, H. Cell behavior observation and gene expression analysis of melanoma associated with stromal fibroblasts in a three-dimensional magnetic cell culture array. Biotechnol. Prog. 2013, 29, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, N.M.; Roy, D.L.; Marelli-Mathevon, H.; Shaw, G.; Dias, A.; Kramer, R.B.G.; Cuong, L.V.; Kustov, M.; Zanini, L.F.; Villard, C.; et al. Micro-magnetic imprinting of high field gradient magnetic flux sources. Appl. Phys. Lett. 2014, 104, 262401. [Google Scholar] [CrossRef]
- Dumas-Bouchiat, F.; Zanini, L.F.; Kustov, M.; Dempsey, N.M.; Grechishkin, R.; Hasselbach, K.; Orlianges, J.C.; Champeaux, C.; Catherinot, A.; Givord, D. Thermomagnetically patterned micromagnets. Appl. Phys. Lett. 2010, 96, 102511. [Google Scholar] [CrossRef]
- Huang, C.Y.; Chen, P.J.; Tsai, K.L.; Chen, J.Y. Cell Trapping by Local Magnetic Force Using Sinewave Magnetic Structure. IEEE Trans. Magn. 2015, 51, 1–4. [Google Scholar] [CrossRef]
- Omelyanchik, A.; Levada, E.; Ding, J.; Lendinez, S.; Pearson, J.; Efremova, M.; Rodionova, V. Design of conductive microwire systems for manipulation of biological cells. IEEE Trans. Magn. 2018, 54, 1–5. [Google Scholar] [CrossRef]
- Lee, C.S.; Lee, H.; Westervelt, R.M. Microelectromagnets for the control of magnetic nanoparticles. Appl. Phys. Lett. 2001, 79, 3308–3310. [Google Scholar] [CrossRef]
- Lee, H.; Purdon, A.M.; Westervelt, R.M. Manipulation of biological cells using a microelectromagnet matrix. Appl. Phys. Lett. 2004, 85, 1063. [Google Scholar] [CrossRef]
- Lee, H.; Liu, Y.; Hamb, D.; Westervelt, R.M. Integrated cell manipulation system—CMOS/microfluidic hybrid. Lab Chip 2007, 7, 331–337. [Google Scholar] [CrossRef] [PubMed]
- Gertz, F.; Azimov, R.; Khitun, A. Biological cell positioning and spatially selective destruction via magnetic nanoparticles. Appl. Phys. Lett. 2012, 101, 013701. [Google Scholar] [CrossRef]
- Reimann, P. Brownian motors: Noisy transport far from equilibrium. Phys. Rep. 2002, 361, 57–265. [Google Scholar] [CrossRef] [Green Version]
- Helseth, L.Е.; Fischer, T.M. Paramagnetic beads surfing on domain walls. Phys. Rev. E 2003, 67, 042401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pietro, T.; Johansen, T.H.; Sancho, J.M. A Tunable Magnetic Domain Wall Conduit Regulating Nanoparticle Diffusion. Nano Lett. 2016, 16, 5169–5175. [Google Scholar]
- Ralph, L.S.; Arthur, V.S.; Pietro, T. Enhancing Nanoparticle Diffusion on a Unidirectional Domain Wall Magnetic Ratchet. Nano Lett. 2019, 19, 433–440. [Google Scholar]
- Beklemisheva, A.V.; Gurevich, A.A.; Panina, L.V.; Zolotoreva, M.G.; Beklemishev, V.N.; Suetina, I.A. Micromagnetic Manipulators—Ferromagnetic Microwire Systems for Diffusion and Separation of Para and Dia- magnetic Particles in Gradient Magnetic Field. J. Nanomed. Nanotech. 2020, 11, 554. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Panina, L.V.; Gurevich, A.; Beklemisheva, A.; Omelyanchik, A.; Levada, K.; Rodionova, V. Spatial Manipulation of Particles and Cells at Micro- and Nanoscale via Magnetic Forces. Cells 2022, 11, 950. https://doi.org/10.3390/cells11060950
Panina LV, Gurevich A, Beklemisheva A, Omelyanchik A, Levada K, Rodionova V. Spatial Manipulation of Particles and Cells at Micro- and Nanoscale via Magnetic Forces. Cells. 2022; 11(6):950. https://doi.org/10.3390/cells11060950
Chicago/Turabian StylePanina, Larissa V., Anastasiya Gurevich, Anna Beklemisheva, Alexander Omelyanchik, Kateryna Levada, and Valeria Rodionova. 2022. "Spatial Manipulation of Particles and Cells at Micro- and Nanoscale via Magnetic Forces" Cells 11, no. 6: 950. https://doi.org/10.3390/cells11060950
APA StylePanina, L. V., Gurevich, A., Beklemisheva, A., Omelyanchik, A., Levada, K., & Rodionova, V. (2022). Spatial Manipulation of Particles and Cells at Micro- and Nanoscale via Magnetic Forces. Cells, 11(6), 950. https://doi.org/10.3390/cells11060950








