Abstract
The androgen receptor (AR) signalling pathway is the key driver in most prostate cancers (PCa), and is underpinned by several kinases both upstream and downstream of the AR. Many popular therapies for PCa that target the AR directly, however, have been circumvented by AR mutation, such as androgen receptor variants. Some upstream kinases promote AR signalling, including those which phosphorylate the AR and others that are AR-regulated, and androgen regulated kinase that can also form feed-forward activation circuits to promotes AR function. All of these kinases represent potentially druggable targets for PCa. There has generally been a divide in reviews reporting on pathways upstream of the AR and those reporting on AR-regulated genes despite the overlap that constitutes the promotion of AR signalling and PCa progression. In this review, we aim to elucidate which kinases—both upstream and AR-regulated—may be therapeutic targets and require future investigation and ongoing trials in developing kinase inhibitors for PCa.
1. Introduction
The biological effects of male sex hormones, androgens, on the target tissues are manifested by the androgen receptor (AR) to achieve a wide range of developmental as well as physiological responses. Thus, androgens play a crucial role in the differentiation and maturation of the male reproductive organs and androgens are the major regulators of cell proliferation and cell death of the epithelial cells within the prostate gland [1,2]. It is, therefore, not surprising that the majority of prostate cancers (PCas) remain reliant on the AR signalling axis throughout their life [3,4,5].
Therefore, to combat PCa, the AR and AR signalling pathways have been considered a key target, and androgen deprivation therapy (ADT) is the current standard of care for advanced PCa. Whilst some ADT reduce androgen synthesis, such as Abiraterone, other drugs, including enzalutamide, target the AR directly. However, in metastatic patients, ADT resistance due to transition to hormone-insensitive castration resistant PCa (CRPC) usually occurs within 18–36 months [6]. There are several resistance mechanisms, including AR amplification, increased AR promiscuity, AR variants (AR-V), and increased reliance on androgen-independent activation of the AR. CRPC can be treated with chemotherapeutic agents such as Docetaxel; however, CRPC is usually fatal within 13–30 months [7]. Notably, ADT-induced activation of the pro-survival poly (ADP-ribose) polymerase (PARP) pathway can also lead to therapy failure and, therefore, combination treatments to simultaneously block AR and PARP signalling hold promise for the treatment of CRPC [8]. Although there have been strives in developing new small molecules which can bind different AR domains reviewed in [9], the identification of other fundamental proteins in the AR signalling cascade to therapeutically target to reduce ADT resistance and treat CRPC is an active and ongoing area of research.
Owing to the fact that AR is a very dynamic phosphoprotein, it is unsurprising that its stability, activity, and cellular localisation are all differentially regulated by phosphorylation by specific signalling kinases [10,11,12]. As key post-translational modifiers in signal transduction, kinases can phosphorylate serine, threonine, and tyrosine amino acid residues within their target proteins. The AR belongs to the family of nuclear hormone receptors and functions as a ligand-activated transcription factor. While unliganded AR is cytosolic, androgen binding triggers the nuclear shuttling of the AR and binding to the androgen response elements (AREs) motifs that are often present in the promoters and enhancers of the AR target genes, including many kinases which mediate changes in cell proliferation and metabolism [13]. In several cases, downstream kinases also directly interact with the AR, resulting in feedback regulation of AR activity [14]. The following manuscript will review current knowledge on kinases around AR signalling and explore their role as potential therapeutic targets for the treatment of PCa.
1.1. AR as a Master Transcription Factor
The AR is overexpressed in PCa as a function of disease progression, with ADT providing a selection pressure to overcome androgen dependence and develop into advanced disease such as castration-resistant prostate cancer (CRPC) [3]. Thus, the AR is a major therapeutic target throughout PCa progression. The unstimulated AR resides in the cytosol where several chaperones interact with the AR to oppose its degradation by cytosolic proteases. However, once activated, the AR translocates from the cytoplasm to the nucleus, where it binds to a cis-regulatory consensus sequence with the DNA known as Androgen Response Elements (AREs) [15]. Not all androgen-regulated genes have canonical AREs in the proximal promoter; however, AREs may be found in the distal enhancer regions [16,17], or AR recruitment may otherwise be supported by transcription ancillary factors such as Sp1 and ETS [18,19,20]. While the major function of the AR is to act as a transcriptional activator (i.e., a transactivator), a chromatin-bound AR can also act as a repressor of the target gene expression. When acting as a transactivator, AR recruitment creates a platform for RNA Pol II machinery to bind, which increases the rate of transcription. TMPRSS2, PSA, and PSCA are all examples of genes whose expression is upregulated by AR in this manner [21,22,23]. Transrepression by the AR is less well understood. An example of AR-mediated suppression of the expression of Maspin gene transcript level. The AR and Maspin form a negative feedback loop where each bind to consensus sequences in the other’s promoter to reduce RNA Pol II binding and decrease transcription [24,25].
In addition to its primary role as a transcription factor, a few non-genomic (transcription-independent) functions of the AR have been revealed in the recent years, including the binding of AR with a scaffold protein filamin A in prostate fibroblasts to promote oncogenesis [26], and steroid-induced association of AR with oestrogen receptor beta and Src to promote cancer cell proliferation [27].
1.2. Functional Domains of the AR
The functionality of the AR is primarily split into three well-defined functionally critical domains; the amino terminus transactivation domain (NTD), the DNA binding domain (DBD), and the ligand-binding domain (LBD), with a hinge region connecting the LBD and DBD (Figure 1). All these domains can act as targets of small molecules designed to inhibit AR function in prostate cancer reviewed in [9]. The transactivation potential of the AR primarily involves the NTD, which contains activation function 1 (AF1) that harbours two transactivation units (TAU), TAU1 and TAU5 [28]. The AF1 is constitutively active on its own; however, its interaction with the LBD prevents the action of the receptor without an androgenic ligand [29]. These interactions are lost in clinically relevant AR variants (AR-Vs) that lack the LBD potentially making AR-Vs to become constitutively active. Androgen binding to the LBD results in a conformational change, which exposes the activation function 2 (AF2) within the LBD allowing it to bind to transcriptional cofactors to modulate gene transcription [30]. The conformational change also causes the chaperones to dissociate from the AR and the nuclear shuttling of the AR [30]. In the nucleus, the AR interacts with DNA through its DBD, exploiting the zinc finger motifs in the P-box and D-box, which recognise AREs [31]. The DBD is highly conserved between nuclear hormone receptors, and therefore, may not represent a promising therapeutic target due to potential off-target effects [32].
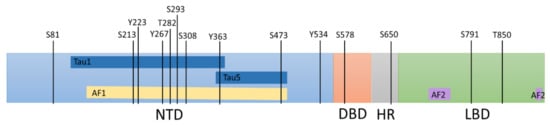
Figure 1.
A schematic summarising the phosphorylated sites within the androgen receptor which moderate its activity. The androgen receptor contains several serine (S), threonine (T), and tyrosine (Y) residues that can be phosphorylated to moderate localisation, stability, and transactivation activity. Amino terminus transactivation domain (NTD), the DNA binding domain (DBD), the Hinge region (HR), and the ligand-binding domain (LBD). The activation function 1 (AF1) region (yellow line) consist of the majority of Tau1 and Tau5 (dark blue lines). The activation function 2 (AF2) region (purple lines) in the LBD is primarily located in the 12th helix; however, parts of the 3rd and 4th helixes also make up the AF2 surface [33].
Another layer of intricacy to AR-dependent transcription is the involvement of the co-regulatory proteins, such as coactivators that can bind to the AR to enhance the rate of AR-mediated transcription, and transcriptional corepressors—which usually bind to the NTD—which can dampen AR transactivation function causing a reduction in AR target gene expression. Two main conformations of the AR have been proposed by which AR can regulate, which co-regulatory molecules bind: the ligand-bound conformation of the LBD and post-translational modifications, such as phosphorylation [11,34]. The binding of an agonist such as androgens can modulate AR conformation that allows preferential recruitment of transcriptional coactivators such as CBP/p300 and SRC [35]. In contrast, when an androgen antagonist such as cyproterone acetate (CPA) binds to the LBD of AR, the conformational change preferentially allows corepressors, such as Alien and Silencing-Mediator for Retinoid/Thyroid hormone (SMRT) to be preferentially recruited [36].
Phosphorylation of the AR by kinases is known to occur in at least 15 amino acid residues within the AR protein (Figure 1) and can potentially modulate which coregulators bind, as well as AR stability and location reviewed in [37,38]. In AR-Vs, such as AR-V7, which lack a functional LBD, ligand-dependent activation is lost whilst phosphorylation of the remains a key form of AR regulation [39]. Therefore, kinases that enhance AR-mediated transcription can represent surrogate therapeutic targets to stop constitutively active AR-Vs function. Given that the NTD is intrinsically disordered, anecdotal compound screening techniques will have to be used to find potential inhibitors rather than designed antagonists based on the structure [9], therefore targeting of kinases that modulate the NTD may prove more successful in tumours that are driven by such AR-Vs.
2. Upstream Kinases of the AR
Components of several key signal transduction pathways have been shown to interact with androgen signalling, including direct phosphorylation of the AR, through their kinase components. These include the IL-6 and EGF extracellular signal-mediated pathways and the PI3K/Akt and MAPK transduction networks, which are often found to be implicated in hormone-independent aggressive PCa such as CRPC [40]. Likewise, cell cycle-dependent kinases can also phosphorylate and regulate AR activity to allow cell proliferation. Within each of these pathways, there are key kinases that can act as potential therapeutic targets for cancer inhibition.
2.1. Interleukin 6 Pathway
Interleukin 6 (IL-6) is a pleiotropic cytokine that is involved in the regulation of the growth of the majority of malignant tumours [41], including PCa [42]. Exposure of PCa LNCaP cell line to Bicalutamide—an AR LBD antagonist—resulted in a 70% decrease in the IL-6 induced expression of AR target gene PSA, indicating the role of IL-6 signalling in promoting AR activity [43]. The role of IL-6 in regulating AR activity appears to be pleiotropic since several signal transduction cascades can be activated by the binding of IL-6 to its receptor, including the JAK/STAT, MAPK, PI3K/Akt, and PKA pathways, providing multiple possible mechanisms for the modulation of AR signalling. Using kinase inhibitors to disrupt these signalling pathways, IL-6 mediated activation of the NTD of AR was shown to occur primarily via JAK/STAT and MAPK pathways [43]. This IL-6 mediated activation of AR may occur via STAT3, which interacts with amino acids 234–558 of the AR NTD [43] and requires phosphorylation of STAT3 at Tyr705 and Ser727 for optimal activation [44]. JAK can phosphorylate STAT3 at Tyr705 [45] and kinases, such as p38MAPK and ERK in the MAPK pathway, can also phosphorylate STAT3 at Ser727, thus promoting STAT3-mediated AR activation [46,47]. Due to its role in IL-6 mediated signalling, the role of JAK has been explored as a potential therapeutic target to block PCa progression; however, this has proved a challenge. Ruxolitinib is a JAK1/2 inhibitor that reached Phase II trials for metastatic PCa, but the trial was terminated due to low efficiency (Trial identifier: NCT 00638378); however, this should be further investigated in the context of prolonged ADT with antiandrogens [48], where IL-6 signalling is more prevalent [49]. The Jak inhibitor AZD1480 initially also showed promise, suppressing metastasis in pre-clinical models [50]; however, three Phase I trials have been terminated due to unfavourable neurotoxicity profiles [48]. Another stalling factor in JAK inhibitor development may be a lack of clinically representative cell-based assays [43]. Src kinase is a non-receptor tyrosine kinase at the confluence of several signal transduction pathways, such as EGFR, IL6, MAPK, and PI3K [51]. Like JAK, Src can also phosphorylate STAT3 at Tyr705—albeit much weaker [52]—and promotes non-genomic AR signalling; however, beyond this, it also directly interacts with AR between residues 371 and 381 in the AF1 in the NTD domain [53]. Src is not to be confused with steroid hormone coactivators (SRC), which also interact with the AR and are required for ligand-independent activation by IL-6 [54]. Through the feed-forward regulation, the AR-Src interaction activates the Src [53], which results in Src-mediated phosphorylation of the AR at Tyr534. This increases AR stability by blocking E3 ligase CHIP and the subsequent proteasomal degradation [55], as well as causing recruitment p85α and the resultant activation of MAPK and PI3K/Akt pathways [37]. Src can also indirectly activate AR-mediated transcription by repressing the AR interaction of AR with AR corepressor LCoR [56]. Dasatinib inhibits Src and has shown promising results in Phase II trials of decreased metastasis; however, another Phase II trial did not improve on abiraterone, and in Phase III trials did not improve on docetaxel [51,57,58]. It has been suggested that identifying which subpopulation of patients have higher Src-driven PCa would be beneficial for future Dasatinib trials [58]. One such subpopulation may be AR-V7-driven CRPC [58,59].
2.2. Epidermal Growth Factor Pathway
There is also crosstalk between several growth factors signalling pathways and the AR signalling pathway, with the epidermal growth factor (EGF) receptor family amongst the most described in the literature. EGF pathways play a pivotal role in regulating AR signalling, e.g., ERBB1 (EGFR) expression is enhanced as a function of tumour severity, whilst the expression of ERBB2 (HER2) increases during and following androgen ablation, thus ensuring sustained survival of PCa cells [60,61].
There is functional redundancy in the activation of downstream signalling by IL-6 and EGF signal transduction pathways. Like IL-6, EGF signalling can also promote phosphorylation of STAT3 at Tyr705, although this occurs via the EGFR and Src [62,63]. EGFR signalling also cross talks with the AR synergistically during ligand-dependent AR activation via activation through the use of the ERK1/2 and p38 MAPK pathways [64], possibly via Ser515 phosphorylation which transactivates the AR and is involved in AR recycling [65]. The EGFR can also activate the protein kinase C (PKC) pathway, which phosphorylates Ser578 of the AR, which causes greater transactivation than Ser515 phosphorylation [65]. Notably, Ser578 is within the P box of the DBD, and its phosphorylation promotes AR-DNA interaction and specificity [65]. Intriguingly, phosphorylation of AR Ser515 and Ser578 are coregulated, with phosphorylation of P-Ser578 correlated with lower P-Ser515; this coregulation regulates AR nuclear-cytoplasmic shuttling through interactions with the Ku-70/80 [65].
EGF stimulation of the EGFR and HER2 receptors results in phosphorylation and activation of activated Cdc42-associated tyrosine kinase 1 (ACK1) [66,67], which can phosphorylate the AR and enhance transcription of the AR gene itself. ACK1 kinase phosphorylates the AR at Tyr267 and Tyr363, located in the AF1, to promote AR-mediated transactivation of AR-regulated genes, with Tyr267 being the functionally important site [67]. P-Tyr267 is required for the AR to bind to the Ataxia telangiectasia mutated (ATM) enhancer, and the consequential increase in ATM expression maintains genetic integrity from double-stranded DNA breaks. This correlates with PCa progression and affords greater resistance of CRPC to radiotherapy [68]. ACK1 can also phosphorylate Akt at Tyr176, which promotes Akt translocation to the plasma membrane and subsequent activation of Akt and the PI3K pathway [69]. ACK1 activation also results in an increase in AR expression via phosphorylation of histone 4 (H4) at Tyr88. P-Tyr88-H4 has been located at two sites upstream enhancer sites of the AR, creating an area of permissive chromatin to promote AR mRNA transcription in CRPC [70]. Therefore, ACK1 activation may be involved in AR transactivation and in the increase in AR expression in PCa to overcome androgen deprivation therapy (ADT) and the progression to lethal CRPC.
In normal prostate epithelia, the EGF pathway mediated phosphorylation of ACK1 results in the degradation of ACK1 and EGFR in a negative feedback mechanism, mediated via the interaction of E3 ligases with the ubiquitin association (UBA) domain of the ACK1. This appears to be overcome in CRPC, as both ACK1 and EGFR protein levels are increased, presumably due to changes in phosphorylation and protein interactions in the ACK1 UBA domain [71]. The reduced ACK1 degradation may allow for the previously mentioned oncogenic effects of ACK1 activation to be exacerbated.
Due to the role of ACK1 in AR transactivation and PCa genomic integrity, several inhibitors have been designed that show potential to overcome CRPC [66]. Notably, Dasatinib, previously mentioned for its role as an Src inhibitor, also inhibits ACK1 [72]. AIM-100 is more selective for ACK1 and inhibits LNCaP and LAPC-4 cell proliferation; however, poor pharmacokinetic properties have halted entry into clinical trials [73].
2.3. PI3K/Akt Signalling
The PI3K/Akt signalling network is involved in complex crosstalk between several cell signalling cascades, some of which have the potential to be oncogenic and have already been mentioned earlier. Two key points in the signalling network are mTOR1 and mTOR2 both of which are functionally distinct complexes downstream of PI3K/Akt, which regulate many cellular processes that promote tumour progression and drug resistance [74]. The intertwining branches in the PI3K/Akt signalling network contains many redundancies and feedback regulatory loops, which results in limited efficacy in clinical targeting. However, more promising results with the use of their inhibitors have been achieved in recent years, reviewed in [74].
PI3K/Akt/mTOR and AR signalling pathways have a complex relationship containing several redundancies, and downregulation of one can promote the other; this can occur during AR inhibitor therapy, where downregulation of AR-regulated genes can result in increased AKT-mTOR signalling and lead to therapeutic resistance [75]. Conditional activation of Akt in LNCaP cells promoted proliferation in absence of androgens, which was confirmed in transgenic mice, suggesting a role of the Akt pathway in the transition from androgen dependence to androgen independence [76]. These data suggest that Akt is an attractive therapeutic target for growth inhibition, in particular when PTEN—a tumour suppressor phosphatase that negatively regulates the PI3K/Akt pathway—is inactive or deleted, which occurs in up to 60% of CRPC [77]. Inhibition of both Akt and AR signalling pathways has been considered therapeutically in PTEN-deficient PCa in the hope of inducing synthetic lethality. Recently, the Akt inhibitor Ipatasertib was combined with the Abiraterone—an inhibitor of androgen biosynthesis—in Phase III trials. Encouragingly, this study showed an improved radiographical progression-free survival [78], indicating that PI3K/Akt blockade alongside ADT or AR inhibition is a viable option in the treatment of advanced PCa.
Growth factor and cytokine pathways mentioned previously activate kinases, such as Src and ACK1, which positively regulate the activity of both the AR and Akt, and therefore, activation of these membrane-associated receptors may be key in the development of therapeutic resistance and progression to CRPC. However, under low androgen conditions, the AR and Akt can also directly interact, with Akt phosphorylating the AR at Ser213 and Ser791 [79]. In vitro, the outcome of this interaction is varied and may depend on different cellular contexts, including the passage number of the PCa cells, with Akt enhancing AR activity in high passage cells but reducing AR activity in low passage cells [76].
2.4. Mitogen-Activated Protein Kinase Pathway
The activation of the MAPK signal transduction pathway is yet another mechanism that growth factors employ to activate PCa growth. The MAPK pathway is split into four parts, the extracellular signal-regulated ERK1/2 pathway, the EGF- and oxidative stress-associated ERK5 pathway, and the stress-activated JNK and p38 MAPK pathways. Androgen-mediated AR signalling can induce the ERK1/2 and p38 MAPK pathways [64], which can then mediate proliferation via phosphorylation of several transcription factors such as Myc [80,81]. Activation of the ERK1/2 pathway can also occur via IL-6 and growth factor signalling [64,82], and phosphorylation of ERK correlated with the severity of PCa [81,82,83]. The primary ERK1/2 kinases are MEK1/2, and MEK1/2 inhibition is being investigated as a potential avenue to dampen AR signalling [84,85]. Trametinib, a MEK1/2 inhibitor that is approved therapeutic for melanoma, is currently undergoing Phase II trials for CRPC (Trial identifier: NCT02881242).
Expression and activation of p38 MAPK and some of its associated upstream kinases occurs in PCa, making this pathway another possible therapeutic target [86]. Interestingly, androgen-driven activation of p38 MAPK results in the phosphorylation of AR-interacting heat shock protein 27 (hsp27), which subsequently replaces hsp90 as an AR chaperone for translocation into the nucleus and aids AR transactivation [87]. IL-6 can also activate p38 MAPK signalling independent of androgen, which by this mechanism would increase AR signalling [87]. Inhibition of p38 MAPK signalling decreased proliferation in AR-positive cell lines in normoxia, and growth was further compromised by hypoxia, which is another trigger for p38 MAPK activation [88].
2.5. Cell Cycle-Dependent Modulation of AR Activity
AR signalling results in the proliferation of PCa epithelial cells via regulating the transition between the G1 and S phases [89,90]. At the beginning of the G1 phase, AR activation induces mTOR-dependent translation of D1 cyclin, resulting in cyclin D1 accumulation and subsequent interaction with the Cyclin-dependent kinase (CDK)4/6 complex [91]. Activated D-cyclin/CDK4 or CDK6 complex phosphorylates and inactivates retinoblastoma tumour suppressor (Rb) in G1, causing it to release E2F transcription factors [92]. Activated E2F1 transcription factor not only enhances AR expression but also causes increased expression of cyclin A and activation of CDK2 for transition into S phase [93].
Many ADT rely on Rb [58,92], and thus, if Rb activity is lost due to deletion, mutation, or PTEN inactivity [26,27], the antiandrogen therapy can be compromised [92]. Indeed, Rb deletion is higher ADT resistant CRPC than in primary PCa [93]. Beyond the cell cycle, Rb loss changes AR transrepression activity and can alter the AR cistrome [94].
AR activity is also modulated through phosphorylation by other CDK throughout the cell cycle. Firstly, Ser81 of AR can be phosphorylated by CDK1, CDK2, CDK5, and CDK9 [11,95]. Phosphorylation of AR at Ser81 is associated with androgen-mediated activation of the AR [96]. Depending on the context of phosphorylation, P-Ser81 of AR results in resistance to AR degradation, increased nuclear localisation and chromatin binding, as well as interaction with cofactor CBP/p300 and expression of different subsets of AR-target genes [97,98,99]. CDK1 is active in the G2/M phase, and phosphorylation of Ser81 of AR in cytoplasm causes AR translocation to the nucleus whilst preventing degradation and may facilitate the transcription of AR target genes following mitosis [100]. As opposed to CDK1, the role of CDK9 is not cell cycle-dependent; however, once AR is recruited on its target genes, CDK9 can phosphorylate its Ser81 to couple the P-TEFb transcription elongation complex that activates RNA Pol II to synergise AR-dependent transcription [97,99,101]. CDK5, as well as promoting stability and transactivation of AR by phosphorylating Ser81, also promotes degradation of p21CIP1, an inhibitor of CDK2/3/4/6 [96,102]. CDK2 can also phosphorylate Ser81 of AR [103], and CDK6—beyond its role in Rb phosphorylation—can interact with the AR to promote transactivation [104]. The other main CDK in the cell cycle is CDK11, specifically the CDK11p58 isoform, which codes for a transiently active kinase in mitosis associated with spindle formation and cell cycle arrest [105,106]. The CDK11p58/cyclinD3 complex causes the phosphorylation of the AR on Ser308, in TAU1 within the NTD, which results in the exclusion of the AR from condensed chromatin and the repression of AR activity [107]. However, a therapy to promote CDK11p58 activation to reduce AR activity may not be a good strategy as CDK11—the isoform was not reported—has been reported to promote AR expression and activation in osteosarcoma [108]. This would be a concern, as the bone is the most common PCa metastasis site [109], and thus, increasing the activity of CDK11 may be harmful in metastatic CRPC. The majority of interest in CDK inhibitors for PCa currently lies with CDK4/6, rather than AR Ser81 kinases. Three CDK4/6 inhibitors—abemaciclib, palbociclib, and ribociclib—have been found safe and effective in hormone receptor-positive HER2-negative breast cancer and are now undergoing clinical trials for various stages of PCa reviewed in [110,111]. However, abemaciclib is also a CDK9 inhibitor [112], and several selective small-molecule CDK9 inhibitors have been designed. KB-0742 has been designed as ‘ultra-selective’ for CDK9 and showed promise by inhibiting AR-V7 dependent transactivation in CRPC xenografts [113], and has entered Phase I clinical trials (Trial identifier: NCT04718675).
3. Downstream Kinases of the AR
Normally, the AR acts as a transactivator for target genes leading to their increased expression. The AR can also repress gene expression by acting as a transrepressor. These target genes code for proteins with a variety of roles, including mediation of cell proliferation and metabolism. Some AR target genes code for downstream kinases which can reinforce AR signalling by interaction with the AR to change its stability, location, and transactivation by phosphorylation of key serine, threonine, and tyrosine residues. On the other hand, androgen-repressed kinases are also therapeutically important, as these may function as backup pathways that are activated in response to AR-targeting therapies and can mediate therapeutic resistance. We identified 49 androgen-regulated kinase genes in LNCaP cells, of which 25 were upregulated and 24 were downregulated [14], which are discussed further below (see Figure 2). Massie et al. partially defined AR target genes as having ARE within 25 kb of a gene, which, although allowing enough genes for pathway enrichment analysis, would have missed some genes regulated by more distal ARE [13], which may encode proteins that could present as potential therapeutic targets. Indeed, three AR-upregulated and sixteen AR-downregulated kinases identified by Asim et al. did not have ARE within 25 kb [14]. Protein interaction networks and clustering analysis of the 49 kinases highlighted clusters of functional enrichment, including growth factor signalling, MAPK signalling, and glycolysis [14]. To identify any other biological processes that increased or decreased after androgen signalling, Gene Ontology (GO) analysis was performed on the forty-nine kinases. GO analysis of upregulated kinases also indicated enrichment for regulation of the MAPK pathway, regulation of stress-activated MAPK pathway, and positive regulation of PI3K signalling. GO analysis of the downstream kinases implicated nuclear speck organisation, sodium and potassium iron transport across the membrane, and regulation of the ERK1/2 cascade.
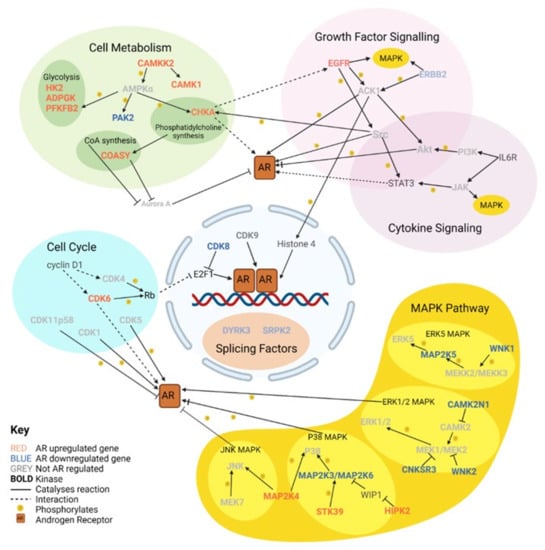
Figure 2.
Key cellular pathways involving upstream and downstream kinases of the androgen receptor. Different functional groups are contained within different coloured ovoids. Within Cell Metabolism and the MAPK Pathway are subgroups represented by further coloured ovals. There are 24 AR-regulated kinases that are discussed in the main text, with other key kinases and molecules these kinases interact with included.
3.1. Cell Metabolism
Cell metabolism is a broad term for biochemical reactions that are required to sustain a cell and consists of several overlapping groups of enzymes. A number of these enzymes are kinases, several of which are androgen regulated (Figure 2). Whilst some of the kinases are more specific to certain cellular processes, such as glycolysis, others are key regulators of metabolism.
3.1.1. Glycolysis
AR signalling has been shown to upregulate aerobic glycolysis and anabolic synthesis through upregulation of several enzymes including key rate-limiting kinases in the glycolytic pathway [13]. Hexokinase 2 (HK2) and ADP-Dependent Glucokinase (ADPGK) catalyse the first obligatory step of glucose metabolism in the cytosol and endoplasmic reticulum, respectively. Whilst HK2 utilises ATP, ADPGK utilises ADP and, thus, promotes glycolysis under nutrient-poor and anoxic conditions, such as in cancerous cells, and may support the Warburg effect [114]. AR signalling also increases 6-Phosphofructo-2-Kinase/Fructose-2,6-Biphosphatase 2 (PFKFB2) expression, although PFKFB2 activity can also be increased through phosphorylation of Ser446 and Ser483 by the PI3K/Akt signalling pathway [115]. Therefore, potentially in high passage cancer cells PI3K/Akt and AR signalling may synergistically [76] to increase glucose metabolism. By upregulating HK, ADPGK, and PFKFB2, AR signalling results in increased energy production via glycolysis and carbon is produced for macromolecule synthesis [13]. Although metabolic pathways are potential targets, these three glycolytic kinases are relatively ubiquitously expressed and may, therefore, be poor therapeutic targets for kinase inhibitors to achieve specificity. However, the increase in glucose uptake has been utilised clinically in fluorodeoxyglucose PET scans, used to identify if metastasis has occurred [116].
3.1.2. CAMMK2 Signalling
Calcium/Calmodulin Dependent Protein Kinase Kinase 2 (CAMKK2) is a metabolic regulator, which is targeted by the AR in both androgen-dependent and androgen-independent PCa cell lines [13]. In PCa, CAMKK2 primarily acts through phosphorylation of AMP-activated protein kinase (AMPKα) [13], which in turn phosphorylates many kinases. Amongst AMPKα phosphorylated kinases are PFKFB2/3, which leads to a further increase in Glycolysis [117]. AMPKα also phosphorylates p21 Activated Kinase 2 (PAK2) at Ser20 to activate it [118]. PAK2 is repressed by AR signalling, but its level increases during ADT and has been identified as a ‘hub’ protein for metastasis development [119,120], indicating an oncogenic role for AMPKα in PCa. Knockdown of CAMKK2 reduces transitioning of cells from G1 to S phase of the cell cycle, as CAMKK2 is required for AR-mediated proliferation [121]. Expression of CAMKK2 increases with Gleason Score [121]. The CAMKK2 inhibitor STO-609 has been used in most CAMKK2 studies; however, STO-609 can also repress many other kinases [122]. Even so, treatment of AR-dependent cell lines with STO-609 has been shown to decrease their proliferation, migration, and invasion [13,121,123], indicating the viability of CAMKK2 as a potential target in PCa. The repression of the prostate tumour xenografts was also more pronounced in chemically castrated mice when they were administered with STO-609, despite its poor solubility in vivo [124]. However, this potentially could be the accumulated effect of several inhibited kinases [122]. The process to develop small-molecule inhibitors is ongoing, with the hinge region of CAMKK2 as the main target for achieving CAMKK2 inhibition [122,125,126].
Calcium/calmodulin-dependent protein kinase type 1 (CAMK1) is a transcriptional target of AR [13,14]. However, CAMKK2 inhibition did not change the phosphorylation status of CAMK1, and inhibition of CAMK1 had marginal effects on cell proliferation and aerobic glucose metabolism [13]. Therefore, whilst CAMKK2 has been investigated as a therapeutic target in PCa, CAMK1 has not and may be interrogated for its role as a therapeutic target in PCa.
3.1.3. Choline Kinase Alpha
Choline kinase alpha (CHKA) is an androgen-upregulated kinase that has roles in lipid metabolism and positive regulation of AR activity [14,127]. In lipid metabolism, CHKA catalyses the first step in the Kennedy pathway for the synthesis of phosphatidylcholine, a key component of cell membranes [127]. Furthermore, phosphorylation of CHKA at Ser279 by AMPKα in low glucose conditions increases lipolysis, which provides substrates for beta-oxidation, a key process for tumour growth [128]. As AMPKα is activated by CAMKK2, CHKA may play a role in CAMKK2-mediated alteration of metabolism. In addition to PCa, CHKA is oncogenic, and it is known to be upregulated in many cancer types and may, thus, represent an attractive therapeutic target [129,130,131].
However, CHKA is of particular therapeutic interest in PCa due to its interactions with AR both directly and indirectly. CHKA acts as an AR chaperone by binding to the LBD and maintaining AR stability and results in a feed-forward signalling loop that maintains AR activity [14]. However, although CHKA knockdown reduced full-length AR transcriptional activity, ARv567—a variant of AR—was not inhibited via CHKA knockdown [14]. CHKA may indirectly impact the AR by aiding in the activation of EGF-mediated signalling via interaction with the EGFR, which is Src-dependent [132]. Therefore, CHKA may be important for the progression of PCa to CRPC. Furthermore, the interaction between EGFR and CHKA is required for EGF-mediated DNA synthesis and increases proliferation, which may a mechanism by which CHKA promotes oncogenesis in many cancers [132]. CHKA inhibition may potentially reduce lipolysis, AR stability, and EGFR signalling, and therefore, presents as a key therapeutic target for investigation in PCa. Small-molecule inhibitors and small interfering RNA (siRNA) have been designed for CHKA inhibition reviewed in [131], one of which—TCD-717—has finished Phase I clinical trials for solid tumours (Trial identifier: NCT01215864), although no results have been posted. A suggested mechanism of TCD-717 action is inhibition of CHKA Tyr333 phosphorylation [133], an amino acid residue that is phosphorylated by Src to allow CHKA interaction with EGFR [132]. As yet, it is unknown if TCD-717 will affect the chaperone function of CHKA.
3.1.4. Coenzyme A Synthase
Coenzyme A synthase (COASY) was identified as upregulated by AR signalling [14]. COASY is a bifunctional enzyme with a synthase and a kinase domain, which catalyse the last two steps in the biosynthesis of CoA—a key metabolite in many biological processes reviewed in [133,134]—from pantothenic acid [135]. The lack of ARE within 25 kb may have excluded COASY from many studies of AR-regulated genes, and literature on the role of COASY within PCa is currently very limited. However, there is indirect evidence of COASY being an AR target gene, and that through the analysis of metabolites, CoA synthesis was identified as a key metabolic pathway changed in androgen ablation therapy [136]. Beyond synthesising CoA, COASY also has a role in the regulation of mitotic proteins, including Aurora A through association with CBP, and DNA repair [137]. COASY knockdown reduced activity of the PI3K pathway and DNA repair, which sensitised colorectal cancer to radiotherapy in vivo and in vitro [138], and thus, may also be a therapeutic target alongside radiotherapy and chemotherapy in CRPC. Although COASY inhibition did not alter proliferation in triple-negative breast cancer and colorectal cancer cell lines [138,139], the outcome in PCa may be different for several reasons. Firstly, COASY activity is strongly upregulated by phosphatidylcholine [135], in which CHKA—an AR-regulated gene [14]—is key for the synthesis of [127]. Thus, COASY may potentially be more active and depended upon in PCa. COASY inhibition may also indirectly impact the phosphorylation and stability of the AR. COASY inhibits Aurora A—a kinase that promotes AR degradation [140]—firstly in mitosis through reduction of CBP acetylation [137], and secondly through interaction with CoA during oxidative stress [141]. Furthermore, the COASY protein also interacts with PI3K-P85α, a regulatory subunit of PI3K, which leads to increased Akt phosphorylation [138] and in turn phosphorylation of the AR [79]. The impact of COASY inhibition of AR-driven proliferation and PCa is currently unknown and requires investigation in vivo and in vitro.
3.2. Growth Factor Signalling
As mentioned previously, growth factor signalling can crosstalk with AR signalling and can contribute to progression to androgen-independent and even AR-independent PCa. AR signalling increases EGFR and IGF1R transcription [142,143], and although in normal prostate epithelium EGFR is degraded by prolonged AR signalling [64], this can be overcome by various mechanisms including increased autocrine and paracrine EGF expression [144,145] and potentially changes in ACK1 UBD interaction [71]. Despite its increased expression with severe disease, EGFR is not a key signalling pathway that confers androgen independence [146]. On the other hand, HER2 is post-translationally repressed by the AR [143], and ADT removes this repression leading to its increased in situ expression in advancing disease and tumour recurrence [147]. Therefore, HER2 inhibition is potentially a better therapeutic target than EGFR for CRPC.
3.3. MAPK Signalling
One of the functional clusters identified in Asim et al. was MAPK signalling [14]. MAPK signalling is split into four branches, and each branch can be modulated by androgen signalling (Figure 2). Therefore, the level and activity of various MAPK proteins can be modulated not only by the AR inhibition but also as a function of tumour progression.
3.3.1. ERK1/2 MAPK
AR signalling potentiates ERK1/2 MAPK activity by inducing and enhancing the ERK1/2 MAPK pathway, partially through genomic transactivation and repression. Firstly, EGFR and IGF1R—which transduce signals via ERK1/2 signalling—are upregulated [142,143]. There are also several AR-repressed kinases we identified [14] which can negatively regulate the ERK1/2 MAPK cascade, such as Connector Enhancer Of Kinase Suppressor Of Ras 3 (CNKSR3) [148,149], WNK Lysine-Deficient Protein Kinase 2 (WNK2) [150], and Calcium/Calmodulin-Dependent Protein Kinase II Inhibitor 1 [151,152]. CNKSR3 codes for MAGI1, which is a Membrane-Associated Guanylate Kinase, and its depletion by RNAi can increase MEK1/2 phosphorylation and, thus, its activity [149,153]. WNK2 depletion also increases MEK1 activity and sensitivity to lower concentrations of EGF [154]. Repression of CAMK2N1 reduces inhibition of CAMK2 signalling, which could lead to increased MEK1 phosphorylation [155]. Therefore, repression of these three genes—which all encode proteins that reduce phosphorylation of MEK1/2—allows increased activation of the ERK1/2 MAPK pathway.
3.3.2. ERK5 MAPK
The ERK5 MAPK pathway is also activated by growth factors—as well by mitogenic signals and oxidative stress [156]—however, its primary activator Mitogen-Activated Protein Kinase Kinase 5 (MAP2K5) and a further upstream kinase WNK Lysine-Deficient Protein Kinase 1 (WNK1) [157] are downregulated by AR signalling [13]. During ADT, the ERK5 pathway is no longer repressed by AR signalling and has been implicated as a key mechanism underlying AR inhibitor resistance [158], as well as PCa proliferation and metastasis [159,160]. ERK5 inhibitors—such as XMD8-92—have been investigated in other cancers alongside other MAPK inhibitors to combat resistance mechanisms [158,159].
3.3.3. P38 MAPK
The AR-mediated genomic regulation of the pro-survival p38 MAPK appears to be varied. MAP2K3 and MAP2K6 are the primary activators of p38 and are downregulated. Conversely, the AR-mediated increase in Homeodomain Interacting Protein Kinase 2 (HIPK2) [13,14] and Serine/Threonine Kinase 39 (STK39) [14,161] would promote the p38 MAPK pathway. HIPK2 enhances the degradation of WIP1 [162], an inhibitor of p38 MAPK under oxidative stress conditions [163], and the STK39 encoded protein SPAK can activate p38 MAPK [161]. However, the increased expression of HIPK2 and STK39 may not be reflected in the activation level of the encoded protein. HIPK2 has a short half-life [164] and an autoinhibitory domain, but is stabilised and cleaved for activation during DNA damage [165]. HIPK2 can impact AR signalling, but only in low hormone conditions [166]. SPAK has lower phosphorylation of the Ser385 activation residue in hormone naive than metastatic PCa [167]. Whilst HIPK2 and STK39 can activate the p38 MAPK pathway, the reduced expression of MAP2K3 and MAP2K6—as primary activators of the p38 MAPK pathway—are likely to have a much greater impact on p38 MAPK signalling. Therefore, the overall result is likely to be androgen-mediated downregulation of the p38 MAPK pathway.
During ADT, the genomic repression of the p38 MAPK pathway would be ablated and reversed. One of the results would be increased hsp27, which would be predicted to increase stability and nuclear translocation of the AR [87,88], as well as enhance survival via the p38 MAPK pathway [88]. Activation of the p38 MAPK pathway has also been linked to epithelial-mesenchymal transition (EMT) and metastasis [88,168]. By identifying MAP2K3 and MAP2K6 repression by the AR, continuing to repress these alongside ADT could reduce androgen independence caused by the increase in the p38 MAPK pathway. However, only a few MEK3/6 inhibitors have thus far been designed [169,170].
3.3.4. JNK and MAP2K4
MAP2K4 is another key MAPK gene that we and others have found to be transcriptionally regulated by the AR [13,14,171]. MAP2K4 codes for MEK4, which can activate the JNK and p38 MAPK pathways. Both of these pathways result in the phosphorylation of AR on Ser650 in its hinge region, leading to AR translocation from the nucleus into the cytoplasm, therefore decreasing AR transactivation [172]. MEK4 is primarily involved with JNK-mediated Ser650 phosphorylation [173]. The AR-mediated MEK4 increase, thus, forms a negative feedback mechanism on AR signalling. Although this implies that the JNK pathway should be repressed during ADT, this has not been reported. Indeed, overall JNK has been reported as repressed by androgen signalling [173], and therefore, JNK levels would be predicted to increase following treatment with enzalutamide [174], genipin [174], and guggulsterone [175], and can, thus, cause apoptosis. Conversely, JNK activation is also implicated in enzalutamide-mediated metastasis [176]. This builds a contrary picture of the role of the JNK MAPK pathway in prostate cancer progression. It should be noted that whilst MEK4 is required for optimal JNK activation, MEK7—which phosphorylates a different JNK residue—is essential JNK activation [177]. To our knowledge, MEK7 is not an AR-responsive gene. Thus, whilst a decrease in MEK4 due to ADT may reduce, JNK activation is primarily activated by a non-AR target gene.
Although targeting MEK4 may have limited effects in modulating the JNK MAPK pathway, MEK4 is still targeted for the activation of the p38 MAPK pathway, which increases AR stability by hsp27 expression and metastasis via matrix metalloproteinase-2 (MMP-2) expression. However, chronic overexpression of MAP2K4 in the LNCaP cell line indicated MEK4 can also bypass p38 MAPK and increase the stability of hsp27 and MMP-2 expression [178]. Phase II trials of the MEK4 inhibitor Genistein decreased prostate cancer metastasis in the short term; however, compensatory mechanisms may reduce the efficiency of long-term MEK4 inhibition [179].
3.4. CDK8 and the Mediator Complex
CDK8 has been identified as an AR-repressed gene [180,181]. Whilst CDK8 expression remains unchanged between benign prostate tissue and primary PCa, it is elevated in ADT-treated metastatic and CRPC [182]. CDK8 is a component of the mediator complexes [183] and opposes the interaction of the mediator complex with the preinitiation complex at a gene promoter and RNA Pol II, inhibiting transcription [184]. However, during stressful conditions, potentially including ADT and CRPC which have higher oxidative stress than those without PCa [185,186], CDK8 becomes active and dissociates from the mediator complex to allow increased transcription [187,188].
In the VCaP cell line—which is androgen-dependent and represents primary PCa [16]—the inhibition of CDK8 increased transition from G1 to S phase [189], possibly due to the increase in transcription-promoting mediator complexes without CDK8. Thus, androgen-mediated repression of CDK8 in primary PCa may also promote G1 to S phase transition. Although CDK8 expression is increased in ADT and CRPC [182], this may not reduce transcription-promoting mediator complexes, as the increased oxidative stress in PCa cells [185,186] may result in CDK8 activation, which causes CDK8 to release from the mediator complex [187,188]. The increase of CDK8 expression in CRPC may also have a role in the increase in WNT signalling [182], a pathway which is almost always inactive in normal prostate cells but is often dysregulated in CRPC [190] and is linked to ADT [191] and chemotherapy resistance [192]. Furthermore, WNT signalling downregulates cadherin, a key molecule in cell-cell adhesion, and is implicated in metastasis and the epithelial-mesenchymal transition [193]. Many studies have looked at genetic alterations of the WNT pathway; however, aberrant WNT signalling can also occur through other mechanisms [193]. Β-catenin is a key effector molecule of canonical WNT signalling and is inhibited and degraded in multiple ways, including by E2F1, which CDK8 phosphorylates and inhibits [194]. Thus, the increased level of β-catenin in the cytoplasm of prostate cancer cells [193] may be partially mediated by increased CDK8 expression. Β-catenin can interact with AR in its active conformation to increase androgen-dependent AR transactional activity [195] and β-catenin expression [196] to create a positive feedback loop. WNT/β-catenin signalling increases AR expression [197], which may perpetuate the AR-β catenin-positive feedback loop in CRPC. Therefore, CDK8 may be a therapeutic target for CRPC, or to inhibit alongside ADT. CDK8 and its analogue CDK19 regulate distinct gene profiles, and both may need to be inhibited for the optimum therapeutic benefit [182]. Indeed, several CDK8/CDK19 inhibitors are in preclinical development, and one—BCD-115—has completed Phase I clinical trials (Trial identifier: NCT03065010), although the results have not yet been posted [198]. Recently, Ma et al. reviewed the binding profiles of 24 CDK8 inhibitors and highlighted four methods of identifying new inhibitors, which may be more selective for CDK8 [199].
3.5. Splicing Factors
GO analysis of the kinases we previously found to be downregulated [14] showed enrichment for nuclear speck organisation, due to Dual Specificity Tyrosine Phosphorylation Regulated Kinase 3 (DYRK3) and SRSF Protein Kinase 2 (SRPK2) repression. The function of nuclear specks is debated; however, they consist of high concentrations of Serine and arginine-rich (SR) proteins and other splicing factors [200]. DYRK3 and SRPK2 both cause the dissolution of nuclear speckles [201,202].
Indeed, SRPK2 overexpression in PCa is associated with high Gleason grade and metastasis in AR-dependent and AR-independent cell lines, indicating SRPK2 may have a role in the progression of PCa and initiation of metastasis [203]. In androgen ablation, PI3K/Akt/mTOR1 signalling increases [75], which results in SPRK2 phosphorylation and translocation to the nucleus [202]. In the nucleus SRPK2 promotes disassembly of nuclear speckles by phosphorylating certain splicing factors, including ASF/SF2, resulting in translocation out of the nuclear speckles, where they associate with nascent mRNA [202]. Lui et al. found that ASF/SF2 was recruited to 3′ splice sites on AR pre-mRNA for AR-V7 during ADT with enzalutamide [204]. Therefore, the increased expression of SRPK2 due to androgen ablation could be a mechanism by which AR-V7 transcripts increase and the subsequent issues with ADT resistance. SRPK2 inhibition could potentially re-sensitise CRPC to ADT through the reduction in AR-V7, however, the roles of SRPK2 and DYRK3 in PCa and splicing of the AR requires further investigation.
4. Clinical Implications and Future Perspectives
Several kinase inhibitors have been tested for in a large range of human malignancies, and a search on the clinicaltrial.gov database (accessed on 30 January 2022) with the terms “prostate cancer” and “kinase inhibitor” returned 106 clinical trials that were either recruiting, active, or completed. Looking forward, Table 1 lists 20 active or recruiting clinical trials, and which of the functional pathways or groups discussed in the review are targeted. The SMMART and MATCH clinical trials were excluded as kinase inhibitors were given were dependent on the genotype of the patient.

Table 1.
Overview of active or recruiting clinical trials of kinase inhibitors for prostate cancer.
5. Conclusions
Kinases are heavily involved in the regulation and biological effects of AR activation and are interrelated in a complex robust network, where AR-responsive genes also affect cascades upstream of the AR and the AR directly. Kinases upstream of the AR, such as ACK1 and Src, present as therapeutic targets to reduce androgen-independent activation of the AR due to extracellular signalling by EGF and IL-6. Androgen-responsive kinases, which are upregulated, present as targets to reduce the proliferation of AR-dependent prostate cancer, and downregulated kinases imply changes that can occur during ADT and, thus, may be therapeutic targets for reducing the progression of androgen independence of prostate cancer. Some of the androgen-responsive kinases are under-researched and could present as therapeutic targets, such as COASY and SRPK2.
Author Contributions
Writing—original draft preparation, K.J.M.; writing—review and editing, M.A. All authors have read and agreed to the published version of the manuscript.
Funding
Prostate Cancer Foundation (Young Investigator Award to M.A.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
Figure 2 was created using Biorender (Toronto, ON, Canada).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cooke, P.S.; Young, P.; Cunha, G.R. Androgen receptor expression in developing male reproductive organs. Endocrinology 1991, 128, 2867–2873. [Google Scholar] [CrossRef] [PubMed]
- Isaacs, J.T. Antagonistic effect of androgen on prostatic cell death. Prostate 1984, 5, 545–557. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.D.; Welsbie, D.S.; Tran, C.; Baek, S.H.; Chen, R.; Vessella, R.; Rosenfeld, M.G.; Sawyers, C.L. Molecular determinants of resistance to antiandrogen therapy. Nat. Med. 2004, 10, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Fujita, K.; Nonomura, N. Role of Androgen Receptor in Prostate Cancer: A Review. World J. Men’s Health 2019, 37, 288–295. [Google Scholar] [CrossRef]
- Heinlein, C.A.; Chang, C. Androgen receptor in prostate cancer. Endocr. Rev. 2004, 25, 276–308. [Google Scholar] [CrossRef]
- Pienta, K.J.; Bradley, D. Mechanisms underlying the development of androgen-independent prostate cancer. Clin. Cancer Res. 2006, 12, 1665–1671. [Google Scholar] [CrossRef]
- Moreira, D.M.; Howard, L.E.; Sourbeer, K.N.; Amarasekara, H.S.; Chow, L.C.; Cockrell, D.C.; Pratson, C.L.; Hanyok, B.T.; Aronson, W.J.; Kane, C.J.; et al. Predicting Time from Metastasis to Overall Survival in Castration-Resistant Prostate Cancer: Results from SEARCH. Clin. Genitourin. Cancer 2016, 15, 60–66. [Google Scholar] [CrossRef]
- Asim, M.; Tarish, F.; Zecchini, H.; Sanjiv, K.; Gelali, E.; Massie, C.; Baridi, A.; Warren, A.; Zhao, W.; Ogris, C.; et al. Synthetic lethality between androgen receptor signalling and the PARP pathway in prostate cancer. Nat. Commun. 2017, 8, 374. [Google Scholar] [CrossRef]
- Maylin, Z.R.; Nicolescu, R.C.; Pandha, H.; Asim, M. Breaking androgen receptor addiction of prostate cancer by targeting different functional domains in the treatment of advanced disease. Transl. Oncol. 2021, 14, 101115. [Google Scholar] [CrossRef]
- Gioeli, D.; Ficarro, S.B.; Kwiek, J.J.; Aaronson, D.; Hancock, M.; Catling, A.D.; White, F.M.; Christian, R.E.; Settlage, R.E.; Shabanowitz, J.; et al. Androgen receptor phosphorylation. Regulation and identification of the phosphorylation sites. J. Biol. Chem. 2002, 277, 29304–29314. [Google Scholar] [CrossRef]
- Wen, S.; Niu, Y.; Huang, H. Posttranslational regulation of androgen dependent and independent androgen receptor activities in prostate cancer. Asian J. Urol. 2020, 7, 203–218. [Google Scholar] [CrossRef] [PubMed]
- Shah, K.; Bradbury, N.A. Kinase modulation of androgen receptor signaling: Implications for prostate cancer. Cancer Cell Microenviron. 2015, 2, 123e. [Google Scholar] [CrossRef]
- Massie, C.E.; Lynch, A.; Ramos-Montoya, A.; Boren, J.; Stark, R.; Fazli, L.; Warren, A.; Scott, H.; Madhu, B.; Sharma, N.; et al. The androgen receptor fuels prostate cancer by regulating central metabolism and biosynthesis. EMBO J. 2011, 30, 2719–2733. [Google Scholar] [CrossRef] [PubMed]
- Asim, M.; Massie, C.E.; Orafidiya, F.; Pertega-Gomes, N.; Warren, A.Y.; Esmaeili, M.; Selth, L.A.; Zecchini, H.I.; Luko, K.; Qureshi, A.; et al. Choline Kinase Alpha as an Androgen Receptor Chaperone and Prostate Cancer Therapeutic Target. J. Natl. Cancer Inst. 2015, 108, djv371. [Google Scholar] [CrossRef] [PubMed]
- Claessens, F.; Verrijdt, G.; Schoenmakers, E.; Haelens, A.; Peeters, B.; Verhoeven, G.; Rombauts, W. Selective DNA binding by the androgen receptor as a mechanism for hormone-specific gene regulation. J. Steroid Biochem. Mol. Biol. 2001, 76, 23–30. [Google Scholar] [CrossRef]
- Pomerantz, M.M.; Li, F.; Takeda, D.Y.; Lenci, R.; Chonkar, A.; Chabot, M.; Cejas, P.; Vazquez, F.; Cook, J.; Shivdasani, R.A.; et al. The androgen receptor cistrome is extensively reprogrammed in human prostate tumorigenesis. Nat. Genet. 2015, 47, 1346–1351. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, C.; Shen, Y.; Nephew, K.P.; Wang, Q. Androgen receptor-driven chromatin looping in prostate cancer. Trends Endocrinol. Metab. 2011, 22, 474–480. [Google Scholar] [CrossRef]
- Vlahopoulos, S.; Zimmer, W.E.; Jenster, G.; Belaguli, N.S.; Balk, S.P.; Brinkmann, A.O.; Lanz, R.B.; Zoumpourlis, V.C.; Schwartz, R.J. Recruitment of the androgen receptor via serum response factor facilitates expression of a myogenic gene. J. Biol. Chem. 2005, 280, 7786–7792. [Google Scholar] [CrossRef]
- Eisermann, K.; Broderick, C.J.; Bazarov, A.; Moazam, M.M.; Fraizer, G.C. Androgen up-regulates vascular endothelial growth factor expression in prostate cancer cells via an Sp1 binding site. Mol. Cancer 2013, 12, 7. [Google Scholar] [CrossRef]
- Cai, C.; Wang, H.; He, H.H.; Chen, S.; He, L.; Ma, F.; Mucci, L.; Wang, Q.; Fiore, C.; Sowalsky, A.G.; et al. ERG induces androgen receptor-mediated regulation of SOX9 in prostate cancer. J. Clin. Investig. 2013, 123, 1109–1122. [Google Scholar] [CrossRef]
- Lin, B.; Ferguson, C.; White, J.T.; Wang, S.; Vessella, R.; True, L.D.; Hood, L.; Nelson, P.S. Prostate-localized and androgen-regulated expression of the membrane-bound serine protease TMPRSS2. Cancer Res. 1999, 59, 4180–4184. [Google Scholar] [PubMed]
- Cleutjens, K.B.; van der Korput, H.A.; Ehren-van Eekelen, C.C.; Sikes, R.A.; Fasciana, C.; Chung, L.W.; Trapman, J. A 6-kb promoter fragment mimics in transgenic mice the prostate-specific and androgen-regulated expression of the endogenous prostate-specific antigen gene in humans. Mol. Endocrinol. 1997, 11, 1256–1265. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.; Lam, A.; Vivanco, I.; Carey, M.F.; Reiter, R.E. Identification of an androgen-dependent enhancer within the prostate stem cell antigen gene. Mol. Endocrinol. 2002, 16, 2323–2337. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Magit, D.; Sager, R. Expression of maspin in prostate cells is regulated by a positive ets element and a negative hormonal responsive element site recognized by androgen receptor. Proc. Natl. Acad. Sci. USA 1997, 94, 5673–5678. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Lian, X.; Jiang, J.; Cheng, H.; Guo, J.; Huang, C.; Meng, H.; Li, X. Tumor Suppressive Maspin-Sensitized Prostate Cancer to Drug Treatment Through Negative Regulating Androgen Receptor Expression. Front. Cell Dev. Biol. 2020, 8, 573820. [Google Scholar] [CrossRef]
- Di Donato, M.; Zamagni, A.; Galasso, G.; Di Zazzo, E.; Giovannelli, P.; Barone, M.V.; Zanoni, M.; Gunelli, R.; Costantini, M.; Auricchio, F.; et al. The androgen receptor/filamin A complex as a target in prostate cancer microenvironment. Cell Death Dis. 2021, 12, 127. [Google Scholar] [CrossRef]
- Migliaccio, A.; Castoria, G.; Di Domenico, M.; de Falco, A.; Bilancio, A.; Lombardi, M.; Barone, M.V.; Ametrano, D.; Zannini, M.S.; Abbondanza, C.; et al. Steroid-induced androgen receptor-oestradiol receptor beta-Src complex triggers prostate cancer cell proliferation. EMBO J. 2000, 19, 5406–5417. [Google Scholar] [CrossRef]
- Tan, M.H.; Li, J.; Xu, H.E.; Melcher, K.; Yong, E.L. Androgen receptor: Structure, role in prostate cancer and drug discovery. Acta Pharmacol. Sin. 2015, 36, 3–23. [Google Scholar] [CrossRef]
- Callewaert, L.; Van Tilborgh, N.; Claessens, F. Interplay between two hormone-independent activation domains in the androgen receptor. Cancer Res. 2006, 66, 543–553. [Google Scholar] [CrossRef]
- Kuil, C.W.; Brinkmann, A.O. Androgens, antiandrogens and androgen receptor abnormalities. Eur. Urol. 1996, 29 (Suppl. S2), 78–82. [Google Scholar] [CrossRef]
- Shaffer, P.L.; Jivan, A.; Dollins, D.E.; Claessens, F.; Gewirth, D.T. Structural basis of androgen receptor binding to selective androgen response elements. Proc. Natl. Acad. Sci. USA 2004, 101, 4758–4763. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Ban, F.; Dalal, K.; Leblanc, E.; Frewin, K.; Ma, D.; Adomat, H.; Rennie, P.S.; Cherkasov, A. Discovery of small-molecule inhibitors selectively targeting the DNA-binding domain of the human androgen receptor. J. Med. Chem. 2014, 57, 6458–6467. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Kemppainen, J.A.; Voegel, J.J.; Gronemeyer, H.; Wilson, E.M. Activation function 2 in the human androgen receptor ligand binding domain mediates interdomain communication with the NH(2)-terminal domain. J. Biol. Chem. 1999, 274, 37219–37225. [Google Scholar] [CrossRef] [PubMed]
- Papaioannou, M.; Reeb, C.; Asim, M.; Dotzlaw, H.; Baniahmad, A. Co-activator and co-repressor interplay on the human androgen receptor. Andrologia 2005, 37, 211–212. [Google Scholar] [CrossRef]
- Powell, S.M.; Christiaens, V.; Voulgaraki, D.; Waxman, J.; Claessens, F.; Bevan, C.L. Mechanisms of androgen receptor signalling via steroid receptor coactivator-1 in prostate. Endocr. Relat. Cancer 2004, 11, 117–130. [Google Scholar] [CrossRef]
- Moehren, U.; Papaioannou, M.; Reeb, C.A.; Hong, W.; Baniahmad, A. Alien interacts with the human androgen receptor and inhibits prostate cancer cell growth. Mol. Endocrinol. 2007, 21, 1039–1048. [Google Scholar] [CrossRef]
- Koryakina, Y.; Ta, H.Q.; Gioeli, D. Androgen receptor phosphorylation: Biological context and functional consequences. Endocr. Relat. Cancer 2014, 21, 131. [Google Scholar] [CrossRef]
- Van der Steen, T.; Tindall, D.J.; Huang, H. Posttranslational modification of the androgen receptor in prostate cancer. Int. J. Mol. Sci. 2013, 14, 14833–14859. [Google Scholar] [CrossRef]
- Lu, C.; Brown, L.C.; Antonarakis, E.S.; Armstrong, A.J.; Luo, J. Androgen receptor variant-driven prostate cancer II: Advances in laboratory investigations. Prostate Cancer Prostatic Dis. 2020, 23, 381–397. [Google Scholar] [CrossRef]
- Lamont, K.R.; Tindall, D.J. Minireview: Alternative activation pathways for the androgen receptor in prostate cancer. Mol. Endocrinol. 2011, 25, 897–907. [Google Scholar] [CrossRef]
- Kumari, N.; Dwarakanath, B.S.; Das, A.; Bhatt, A.N. Role of interleukin-6 in cancer progression and therapeutic resistance. Tumour Biol. 2016, 37, 11553–11572. [Google Scholar] [CrossRef] [PubMed]
- Culig, Z.; Puhr, M. Interleukin-6: A multifunctional targetable cytokine in human prostate cancer. Mol. Cell. Endocrinol. 2012, 360, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Ueda, T.; Bruchovsky, N.; Sadar, M.D. Activation of the androgen receptor N-terminal domain by interleukin-6 via MAPK and STAT3 signal transduction pathways. J. Biol. Chem. 2002, 277, 7076–7085. [Google Scholar] [CrossRef] [PubMed]
- Yokogami, K.; Wakisaka, S.; Avruch, J.; Reeves, S.A. Serine phosphorylation and maximal activation of STAT3 during CNTF signaling is mediated by the rapamycin target mTOR. Curr. Biol. 2000, 10, 47–50. [Google Scholar] [CrossRef]
- Kaptein, A.; Paillard, V.; Saunders, M. Dominant negative stat3 mutant inhibits interleukin-6-induced Jak-STAT signal transduction. J. Biol. Chem. 1996, 271, 5961–5964. [Google Scholar] [CrossRef]
- Turkson, J.; Bowman, T.; Adnane, J.; Zhang, Y.; Djeu, J.Y.; Sekharam, M.; Frank, D.A.; Holzman, L.B.; Wu, J.; Sebti, S.; et al. Requirement for Ras/Rac1-mediated p38 and c-Jun N-terminal kinase signaling in Stat3 transcriptional activity induced by the Src oncoprotein. Mol. Cell. Biol. 1999, 19, 7519–7528. [Google Scholar] [CrossRef]
- Chung, J.; Uchida, E.; Grammer, T.C.; Blenis, J. STAT3 serine phosphorylation by ERK-dependent and -independent pathways negatively modulates its tyrosine phosphorylation. Mol. Cell. Biol. 1997, 17, 6508–6516. [Google Scholar] [CrossRef]
- Beinhoff, P.; Sabharwal, L.; Udhane, V.; Maranto, C.; LaViolette, P.S.; Jacobsohn, K.M.; Tsai, S.; Iczkowski, K.A.; Wang, L.; Hall, W.A.; et al. Second-Generation Jak2 Inhibitors for Advanced Prostate Cancer: Are We Ready for Clinical Development? Cancers 2021, 13, 5204. [Google Scholar] [CrossRef]
- Azevedo, A.; Cunha, V.; Teixeira, A.L.; Medeiros, R. IL-6/IL-6R as a potential key signaling pathway in prostate cancer development. World J. Clin. Oncol. 2011, 2, 384–396. [Google Scholar] [CrossRef]
- Gu, L.; Talati, P.; Vogiatzi, P.; Romero-Weaver, A.L.; Abdulghani, J.; Liao, Z.; Leiby, B.; Hoang, D.T.; Mirtti, T.; Alanen, K.; et al. Pharmacologic suppression of JAK1/2 by JAK1/2 inhibitor AZD1480 potently inhibits IL-6-induced experimental prostate cancer metastases formation. Mol. Cancer Ther. 2014, 13, 1246–1258. [Google Scholar] [CrossRef]
- Varkaris, A.; Katsiampoura, A.D.; Araujo, J.C.; Gallick, G.E.; Corn, P.G. Src signaling pathways in prostate cancer. Cancer Metastasis Rev. 2014, 33, 595–606. [Google Scholar] [CrossRef] [PubMed]
- Schreiner, S.J.; Schiavone, A.P.; Smithgall, T.E. Activation of STAT3 by the Src family kinase Hck requires a functional SH3 domain. J. Biol. Chem. 2002, 277, 45680–45687. [Google Scholar] [CrossRef] [PubMed]
- Zarif, J.C.; Lamb, L.E.; Schulz, V.V.; Nollet, E.A.; Miranti, C.K. Androgen receptor non-nuclear regulation of prostate cancer cell invasion mediated by Src and matriptase. Oncotarget 2015, 6, 6862–6876. [Google Scholar] [CrossRef] [PubMed]
- Ueda, T.; Mawji, N.R.; Bruchovsky, N.; Sadar, M.D. Ligand-independent activation of the androgen receptor by interleukin-6 and the role of steroid receptor coactivator-1 in prostate cancer cells. J. Biol. Chem. 2002, 277, 38087–38094. [Google Scholar] [CrossRef] [PubMed]
- DaSilva, J.; Gioeli, D.; Weber, M.J.; Parsons, S.J. The neuroendocrine-derived peptide parathyroid hormone-related protein promotes prostate cancer cell growth by stabilizing the androgen receptor. Cancer Res. 2009, 69, 7402–7411. [Google Scholar] [CrossRef] [PubMed]
- Asim, M.; Hafeez, B.B.; Siddiqui, I.A.; Gerlach, C.; Patz, M.; Mukhtar, H.; Baniahmad, A. Ligand-dependent corepressor acts as a novel androgen receptor corepressor, inhibits prostate cancer growth, and is functionally inactivated by the Src protein kinase. J. Biol. Chem. 2011, 286, 37108–37117. [Google Scholar] [CrossRef] [PubMed]
- Araujo, J.C.; Trudel, G.C.; Saad, F.; Armstrong, A.J.; Yu, E.Y.; Bellmunt, J.; Wilding, G.; McCaffrey, J.; Serrano, S.V.; Matveev, V.B.; et al. Docetaxel and dasatinib or placebo in men with metastatic castration-resistant prostate cancer (READY): A randomised, double-blind phase 3 trial. Lancet Oncol. 2013, 14, 1307–1316. [Google Scholar] [CrossRef]
- Szafran, A.T.; Stephan, C.; Bolt, M.; Mancini, M.G.; Marcelli, M.; Mancini, M.A. High-Content Screening Identifies Src Family Kinases as Potential Regulators of AR-V7 Expression and Androgen-Independent Cell Growth. Prostate 2017, 77, 82–93. [Google Scholar] [CrossRef]
- Dorff, T.B.; Quinn, D.I.; Pinski, J.K.; Goldkorn, A.; Sadeghi, S.; Tsao-Wei, D.; Groshen, S.; Kuhn, P.; Gross, M.E. Randomized Phase II Trial of Abiraterone Alone or With Dasatinib in Men With Metastatic Castration-resistant Prostate Cancer (mCRPC). Clin. Genitourin. Cancer 2019, 17, 241–247.e1. [Google Scholar] [CrossRef]
- Di Lorenzo, G.; Tortora, G.; D’Armiento, F.P.; De Rosa, G.; Staibano, S.; Autorino, R.; D’Armiento, M.; De Laurentiis, M.; De Placido, S.; Catalano, G.; et al. Expression of epidermal growth factor receptor correlates with disease relapse and progression to androgen-independence in human prostate cancer. Clin. Cancer Res. 2002, 8, 3438–3444. [Google Scholar]
- Lorenzo, G.D.; Bianco, R.; Tortora, G.; Ciardiello, F. Involvement of growth factor receptors of the epidermal growth factor receptor family in prostate cancer development and progression to androgen independence. Clin. Prostate Cancer 2003, 2, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Quesnelle, K.M.; Boehm, A.L.; Grandis, J.R. STAT-mediated EGFR signaling in cancer. J. Cell. Biochem. 2007, 102, 311–319. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.S.; Kehrl, J.H. Pyk2 amplifies epidermal growth factor and c-Src-induced Stat3 activation. J. Biol. Chem. 2004, 279, 17224–17231. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, B.; Mayer, D. Dihydrotestosterone interacts with EGFR/MAPK signalling and modulates EGFR levels in androgen receptor-positive LNCaP prostate cancer cells. Int. J. Oncol. 2008, 33, 623–629. [Google Scholar] [CrossRef]
- Ponguta, L.A.; Gregory, C.W.; French, F.S.; Wilson, E.M. Site-specific androgen receptor serine phosphorylation linked to epidermal growth factor-dependent growth of castration-recurrent prostate cancer. J. Biol. Chem. 2008, 283, 20989–21001. [Google Scholar] [CrossRef]
- Shen, F.; Lin, Q.; Gu, Y.; Childress, C.; Yang, W. Activated Cdc42-associated kinase 1 is a component of EGF receptor signaling complex and regulates EGF receptor degradation. Mol. Biol. Cell 2007, 18, 732–742. [Google Scholar] [CrossRef]
- Mahajan, N.P.; Liu, Y.; Majumder, S.; Warren, M.R.; Parker, C.E.; Mohler, J.L.; Earp, H.S.; Whang, Y.E. Activated Cdc42-associated kinase Ack1 promotes prostate cancer progression via androgen receptor tyrosine phosphorylation. Proc. Natl. Acad. Sci. USA 2007, 104, 8438–8443. [Google Scholar] [CrossRef]
- Mahajan, K.; Coppola, D.; Rawal, B.; Chen, Y.A.; Lawrence, H.R.; Engelman, R.W.; Lawrence, N.J.; Mahajan, N.P. Ack1-mediated androgen receptor phosphorylation modulates radiation resistance in castration-resistant prostate cancer. J. Biol. Chem. 2012, 287, 22112–22122. [Google Scholar] [CrossRef]
- Mahajan, K.; Coppola, D.; Challa, S.; Fang, B.; Chen, Y.A.; Zhu, W.; Lopez, A.S.; Koomen, J.; Engelman, R.W.; Rivera, C.; et al. Ack1 mediated AKT/PKB tyrosine 176 phosphorylation regulates its activation. PLoS ONE 2010, 5, e9646. [Google Scholar] [CrossRef]
- Mahajan, K.; Malla, P.; Lawrence, H.R.; Chen, Z.; Kumar-Sinha, C.; Malik, R.; Shukla, S.; Kim, J.; Coppola, D.; Lawrence, N.J.; et al. ACK1/TNK2 Regulates Histone H4 Tyr88-phosphorylation and AR Gene Expression in Castration-Resistant Prostate Cancer. Cancer Cell 2017, 31, 790–803.e8. [Google Scholar] [CrossRef]
- Lin, Q.; Wang, J.; Childress, C.; Sudol, M.; Carey, D.J.; Yang, W. HECT E3 ubiquitin ligase Nedd4-1 ubiquitinates ACK and regulates epidermal growth factor (EGF)-induced degradation of EGF receptor and ACK. Mol. Cell. Biol. 2010, 30, 1541–1554. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Karaca, M.; Zhang, Z.; Gioeli, D.; Earp, H.S.; Whang, Y.E. Dasatinib inhibits site-specific tyrosine phosphorylation of androgen receptor by Ack1 and Src kinases. Oncogene 2010, 29, 3208–3216. [Google Scholar] [CrossRef] [PubMed]
- Mahajan, K.; Mahajan, N.P. ACK1/TNK2 tyrosine kinase: Molecular signaling and evolving role in cancers. Oncogene 2015, 34, 4162–4167. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.; Tao, T.; Li, H.; Zhu, X. mTOR signaling pathway and mTOR inhibitors in cancer: Progress and challenges. Cell Biosci. 2020, 10, 31. [Google Scholar] [CrossRef]
- Shorning, B.Y.; Dass, M.S.; Smalley, M.J.; Pearson, H.B. The PI3K-AKT-mTOR Pathway and Prostate Cancer: At the Crossroads of AR, MAPK, and WNT Signaling. Int. J. Mol. Sci. 2020, 21, 4507. [Google Scholar] [CrossRef]
- Li, B.; Sun, A.; Youn, H.; Hong, Y.; Terranova, P.F.; Thrasher, J.B.; Xu, P.; Spencer, D. Conditional Akt activation promotes androgen-independent progression of prostate cancer. Carcinogenesis 2007, 28, 572–583. [Google Scholar] [CrossRef]
- Jamaspishvili, T.; Berman, D.M.; Ross, A.E.; Scher, H.I.; De Marzo, A.M.; Squire, J.A.; Lotan, T.L. Clinical implications of PTEN loss in prostate cancer. Nat. Rev. Urol. 2018, 15, 222–234. [Google Scholar] [CrossRef]
- Sweeney, C.; Bracarda, S.; Sternberg, C.N.; Chi, K.N.; Olmos, D.; Sandhu, S.; Massard, C.; Matsubara, N.; Alekseev, B.; Parnis, F.; et al. Ipatasertib plus abiraterone and prednisolone in metastatic castration-resistant prostate cancer (IPATential150): A multicentre, randomised, double-blind, phase 3 trial. Lancet 2021, 398, 131–142. [Google Scholar] [CrossRef]
- Wen, Y.; Hu, M.C.; Makino, K.; Spohn, B.; Bartholomeusz, G.; Yan, D.H.; Hung, M.C. HER-2/neu promotes androgen-independent survival and growth of prostate cancer cells through the Akt pathway. Cancer Res. 2000, 60, 6841–6845. [Google Scholar]
- Marampon, F.; Ciccarelli, C.; Zani, B.M. Down-regulation of c-Myc following MEK/ERK inhibition halts the expression of malignant phenotype in rhabdomyosarcoma and in non muscle-derived human tumors. Mol. Cancer 2006, 5, 31. [Google Scholar] [CrossRef]
- Bi, C.; Zhang, X.; Chen, Y.; Dong, Y.; Shi, Y.; Lei, Y.; Lv, D.; Cao, X.; Li, W.; Shi, H. MAGT1 is required for HeLa cell proliferation through regulating p21 expression, S-phase progress, and ERK/p38 MAPK MYC axis. Cell Cycle 2021, 20, 2233–2247. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Berriguete, G.; Prieto, A.; Fraile, B.; Bouraoui, Y.; de Bethencourt, F.R.; Martinez-Onsurbe, P.; Olmedilla, G.; Paniagua, R.; Royuela, M. Relationship between IL-6/ERK and NF-kappaB: A study in normal and pathological human prostate gland. Eur. Cytokine Netw. 2010, 21, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.L.; Whitney, M.C.; Yao, Z.; Keller, E.T. Interleukin-6 induces androgen responsiveness in prostate cancer cells through up-regulation of androgen receptor expression. Clin. Cancer Res. 2001, 7, 1773–1781. [Google Scholar]
- Nickols, N.G.; Nazarian, R.; Zhao, S.G.; Tan, V.; Uzunangelov, V.; Xia, Z.; Baertsch, R.; Neeman, E.; Gao, A.C.; Thomas, G.V.; et al. MEK-ERK signaling is a therapeutic target in metastatic castration resistant prostate cancer. Prostate Cancer Prostatic Dis. 2019, 22, 531–538. [Google Scholar] [CrossRef]
- Corno, C.; Arrighetti, N.; Ciusani, E.; Corna, E.; Carenini, N.; Zaffaroni, N.; Gatti, L.; Perego, P. Synergistic Interaction of Histone Deacetylase 6- and MEK-Inhibitors in Castration-Resistant Prostate Cancer Cells. Front. Cell Dev. Biol. 2020, 8, 610. [Google Scholar] [CrossRef]
- Ricote, M.; Garcia-Tunon, I.; Fraile, B.; Fernandez, C.; Aller, P.; Paniagua, R.; Royuela, M. P38 MAPK protects against TNF-alpha-provoked apoptosis in LNCaP prostatic cancer cells. Apoptosis 2006, 11, 1969–1975. [Google Scholar] [CrossRef] [PubMed]
- Zoubeidi, A.; Zardan, A.; Beraldi, E.; Fazli, L.; Sowery, R.; Rennie, P.; Nelson, C.; Gleave, M. Cooperative interactions between androgen receptor (AR) and heat-shock protein 27 facilitate AR transcriptional activity. Cancer Res. 2007, 67, 10455–10465. [Google Scholar] [CrossRef] [PubMed]
- Cheung, S.; Jain, P.; So, J.; Shahidi, S.; Chung, S.; Koritzinsky, M. p38 MAPK Inhibition Mitigates Hypoxia--Induced AR Signaling in Castration-Resistant Prostate Cancer. Cancers 2021, 13, 831. [Google Scholar] [CrossRef]
- Balk, S.P.; Knudsen, K.E. AR, the cell cycle, and prostate cancer. Nucl. Recept. Signal. 2008, 6, e001. [Google Scholar] [CrossRef]
- Murthy, S.; Wu, M.; Bai, V.U.; Hou, Z.; Menon, M.; Barrack, E.R.; Kim, S.H.; Reddy, G.P. Role of androgen receptor in progression of LNCaP prostate cancer cells from G1 to S phase. PLoS ONE 2013, 8, e56692. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, S.Y.; Ross, K.N.; Balk, S.P. Androgens induce prostate cancer cell proliferation through mammalian target of rapamycin activation and post-transcriptional increases in cyclin D proteins. Cancer Res. 2006, 66, 7783–7792. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, E.S.; Knudsen, K.E. Tailoring to RB: Tumour suppressor status and therapeutic response. Nat. Rev. Cancer 2008, 8, 714–724. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, K.E.; Arden, K.C.; Cavenee, W.K. Multiple G1 regulatory elements control the androgen-dependent proliferation of prostatic carcinoma cells. J. Biol. Chem. 1998, 273, 20213–20222. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Yeow, W.S.; Ertel, A.; Coleman, I.; Clegg, N.; Thangavel, C.; Morrissey, C.; Zhang, X.; Comstock, C.E.; Witkiewicz, A.K.; et al. The retinoblastoma tumor suppressor controls androgen signaling and human prostate cancer progression. J. Clin. Investig. 2010, 120, 4478–4492. [Google Scholar] [CrossRef] [PubMed]
- Oner, M.; Lin, E.; Chen, M.C.; Hsu, F.N.; Shazzad Hossain Prince, G.M.; Chiu, K.Y.; Teng, C.J.; Yang, T.Y.; Wang, H.Y.; Yue, C.H.; et al. Future Aspects of CDK5 in Prostate Cancer: From Pathogenesis to Therapeutic Implications. Int. J. Mol. Sci. 2019, 20, 3881. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Xin, H.W.; Kelley, J.B.; Spencer, A.; Brautigan, D.L.; Paschal, B.M. Ligand binding to the androgen receptor induces conformational changes that regulate phosphatase interactions. Mol. Cell. Biol. 2007, 27, 3390–3404. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Liang, J.; Wang, L.; Zhang, Z.; Yuan, P.; Wang, J.; Gao, Y.; Ma, F.; Calagua, C.; Ye, H.; et al. Phosphorylation of the androgen receptor at Ser81 is co-sustained by CDK1 and CDK9 and leads to AR-mediated transactivation in prostate cancer. Mol. Oncol. 2021, 15, 1901–1920. [Google Scholar] [CrossRef]
- Zhong, J.; Ding, L.; Bohrer, L.R.; Pan, Y.; Liu, P.; Zhang, J.; Sebo, T.J.; Karnes, R.J.; Tindall, D.J.; van Deursen, J.; et al. p300 acetyltransferase regulates androgen receptor degradation and PTEN-deficient prostate tumorigenesis. Cancer Res. 2014, 74, 1870–1880. [Google Scholar] [CrossRef]
- Liu, X.; Gao, Y.; Ye, H.; Gerrin, S.; Ma, F.; Wu, Y.; Zhang, T.; Russo, J.; Cai, C.; Yuan, X.; et al. Positive feedback loop mediated by protein phosphatase 1alpha mobilization of P-TEFb and basal CDK1 drives androgen receptor in prostate cancer. Nucleic Acids Res. 2017, 45, 3738–3751. [Google Scholar] [CrossRef][Green Version]
- Chen, S.; Xu, Y.; Yuan, X.; Bubley, G.J.; Balk, S.P. Androgen receptor phosphorylation and stabilization in prostate cancer by cyclin-dependent kinase 1. Proc. Natl. Acad. Sci. USA 2006, 103, 15969–15974. [Google Scholar] [CrossRef]
- Gordon, V.; Bhadel, S.; Wunderlich, W.; Zhang, J.; Ficarro, S.B.; Mollah, S.A.; Shabanowitz, J.; Hunt, D.F.; Xenarios, I.; Hahn, W.C.; et al. CDK9 regulates AR promoter selectivity and cell growth through serine 81 phosphorylation. Mol. Endocrinol. 2010, 24, 2267–2280. [Google Scholar] [CrossRef] [PubMed]
- Harper, J.W.; Elledge, S.J.; Keyomarsi, K.; Dynlacht, B.; Tsai, L.H.; Zhang, P.; Dobrowolski, S.; Bai, C.; Connell-Crowley, L.; Swindell, E. Inhibition of cyclin-dependent kinases by p21. Mol. Biol. Cell 1995, 6, 387–400. [Google Scholar] [CrossRef] [PubMed]
- Jorda, R.; Buckova, Z.; Reznickova, E.; Bouchal, J.; Krystof, V. Selective inhibition reveals cyclin-dependent kinase 2 as another kinase that phosphorylates the androgen receptor at serine 81. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.T.; Mansukhani, M.; Weinstein, I.B. Cyclin-dependent kinase 6 associates with the androgen receptor and enhances its transcriptional activity in prostate cancer cells. Proc. Natl. Acad. Sci. USA 2005, 102, 5156–5161. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Inoue, A.; Lahti, J.M.; Kidd, V.J. Failure to proliferate and mitotic arrest of CDK11(p110/p58)-null mutant mice at the blastocyst stage of embryonic cell development. Mol. Cell. Biol. 2004, 24, 3188–3197. [Google Scholar] [CrossRef] [PubMed]
- Petretti, C.; Savoian, M.; Montembault, E.; Glover, D.M.; Prigent, C.; Giet, R. The PITSLRE/CDK11p58 protein kinase promotes centrosome maturation and bipolar spindle formation. EMBO Rep. 2006, 7, 418–424. [Google Scholar] [CrossRef]
- Zong, H.; Chi, Y.; Wang, Y.; Yang, Y.; Zhang, L.; Chen, H.; Jiang, J.; Li, Z.; Hong, Y.; Wang, H.; et al. Cyclin D3/CDK11p58 complex is involved in the repression of androgen receptor. Mol. Cell. Biol. 2007, 27, 7125–7142. [Google Scholar] [CrossRef]
- Liao, Y.; Sassi, S.; Halvorsen, S.; Feng, Y.; Shen, J.; Gao, Y.; Cote, G.; Choy, E.; Harmon, D.; Mankin, H.; et al. Androgen receptor is a potential novel prognostic marker and oncogenic target in osteosarcoma with dependence on CDK11. Sci. Rep. 2017, 7, 43941. [Google Scholar] [CrossRef]
- Gandaglia, G.; Abdollah, F.; Schiffmann, J.; Trudeau, V.; Shariat, S.F.; Kim, S.P.; Perrotte, P.; Montorsi, F.; Briganti, A.; Trinh, Q.D.; et al. Distribution of metastatic sites in patients with prostate cancer: A population-based analysis. Prostate 2014, 74, 210–216. [Google Scholar] [CrossRef]
- Batra, A.; Winquist, E. Emerging cell cycle inhibitors for treating metastatic castration-resistant prostate cancer. Expert Opin. Emerg. Drugs 2018, 23, 271–282. [Google Scholar] [CrossRef]
- Brighi, N.; Conteduca, V.; Lolli, C.; Gurioli, G.; Schepisi, G.; Palleschi, M.; Mariotti, M.; Casadei, C.; De Giorgi, U. The cyclin-dependent kinases pathway as a target for prostate cancer treatment: Rationale and future perspectives. Crit. Rev. Oncol. Hematol. 2021, 157, 103199. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Lee, N.V.; Hu, W.; Xu, M.; Ferre, R.A.; Lam, H.; Bergqvist, S.; Solowiej, J.; Diehl, W.; He, Y.A.; et al. Spectrum and Degree of CDK Drug Interactions Predicts Clinical Performance. Mol. Cancer Ther. 2016, 15, 2273–2281. [Google Scholar] [CrossRef] [PubMed]
- Richters, A.; Doyle, S.K.; Freeman, D.B.; Lee, C.; Leifer, B.S.; Jagannathan, S.; Kabinger, F.; Koren, J.V.; Struntz, N.B.; Urgiles, J.; et al. Modulating Androgen Receptor-Driven Transcription in Prostate Cancer with Selective CDK9 Inhibitors. Cell Chem. Biol. 2021, 28, 134–147.e14. [Google Scholar] [CrossRef] [PubMed]
- Richter, S.; Richter, J.P.; Mehta, S.Y.; Gribble, A.M.; Sutherland-Smith, A.J.; Stowell, K.M.; Print, C.G.; Ronimus, R.S.; Wilson, W.R. Expression and role in glycolysis of human ADP-dependent glucokinase. Mol. Cell. Biochem. 2012, 364, 131–145. [Google Scholar] [CrossRef]
- Moon, J.S.; Jin, W.J.; Kwak, J.H.; Kim, H.J.; Yun, M.J.; Kim, J.W.; Park, S.W.; Kim, K.S. Androgen stimulates glycolysis for de novo lipid synthesis by increasing the activities of hexokinase 2 and 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 2 in prostate cancer cells. Biochem. J. 2011, 433, 225–233. [Google Scholar] [CrossRef]
- Jadvar, H. PET of Glucose Metabolism and Cellular Proliferation in Prostate Cancer. J. Nucl. Med. 2016, 57, 25S–29S. [Google Scholar] [CrossRef][Green Version]
- Marsin, A.S.; Bouzin, C.; Bertrand, L.; Hue, L. The stimulation of glycolysis by hypoxia in activated monocytes is mediated by AMP-activated protein kinase and inducible 6-phosphofructo-2-kinase. J. Biol. Chem. 2002, 277, 30778–30783. [Google Scholar] [CrossRef]
- Banko, M.R.; Allen, J.J.; Schaffer, B.E.; Wilker, E.W.; Tsou, P.; White, J.L.; Villen, J.; Wang, B.; Kim, S.R.; Sakamoto, K.; et al. Chemical genetic screen for AMPKalpha2 substrates uncovers a network of proteins involved in mitosis. Mol. Cell 2011, 44, 878–892. [Google Scholar] [CrossRef]
- Gao, Y.; Ha, Y.S.; Kwon, T.G.; Cho, Y.C.; Lee, S.; Lee, J.N. Characterization of Kinase Expression Related to Increased Migration of PC-3M Cells Using Global Comparative Phosphoproteome Analysis. Cancer Genom. Proteom. 2020, 17, 543–553. [Google Scholar] [CrossRef]
- Jiang, N.; Hjorth-Jensen, K.; Hekmat, O.; Iglesias-Gato, D.; Kruse, T.; Wang, C.; Wei, W.; Ke, B.; Yan, B.; Niu, Y.; et al. In vivo quantitative phosphoproteomic profiling identifies novel regulators of castration-resistant prostate cancer growth. Oncogene 2015, 34, 2764–2776. [Google Scholar] [CrossRef]
- Karacosta, L.G.; Foster, B.A.; Azabdaftari, G.; Feliciano, D.M.; Edelman, A.M. A regulatory feedback loop between Ca2+/calmodulin-dependent protein kinase kinase 2 (CaMKK2) and the androgen receptor in prostate cancer progression. J. Biol. Chem. 2012, 287, 24832–24843. [Google Scholar] [CrossRef] [PubMed]
- O’Byrne, S.N.; Scott, J.W.; Pilotte, J.R.; Santiago, A.D.S.; Langendorf, C.G.; Oakhill, J.S.; Eduful, B.J.; Counago, R.M.; Wells, C.I.; Zuercher, W.J.; et al. In Depth Analysis of Kinase Cross Screening Data to Identify CAMKK2 Inhibitory Scaffolds. Molecules 2020, 25, 325. [Google Scholar] [CrossRef] [PubMed]
- Frigo, D.E.; Howe, M.K.; Wittmann, B.M.; Brunner, A.M.; Cushman, I.; Wang, Q.; Brown, M.; Means, A.R.; McDonnell, D.P. CaM kinase kinase beta-mediated activation of the growth regulatory kinase AMPK is required for androgen-dependent migration of prostate cancer cells. Cancer Res. 2011, 71, 528–537. [Google Scholar] [CrossRef] [PubMed]
- York, B.; Li, F.; Lin, F.; Marcelo, K.L.; Mao, J.; Dean, A.; Gonzales, N.; Gooden, D.; Maity, S.; Coarfa, C.; et al. Pharmacological inhibition of CaMKK2 with the selective antagonist STO-609 regresses NAFLD. Sci. Rep. 2017, 7, 11793. [Google Scholar] [CrossRef]
- Profeta, G.S.; Dos Reis, C.V.; Santiago, A.D.S.; Godoi, P.H.C.; Fala, A.M.; Wells, C.I.; Sartori, R.; Salmazo, A.P.T.; Ramos, P.Z.; Massirer, K.B.; et al. Binding and structural analyses of potent inhibitors of the human Ca2+/calmodulin dependent protein kinase kinase 2 (CAMKK2) identified from a collection of commercially-available kinase inhibitors. Sci. Rep. 2019, 9, 16452. [Google Scholar] [CrossRef]
- Eduful, B.J.; O’Byrne, S.N.; Temme, L.; Asquith, C.R.M.; Liang, Y.; Picado, A.; Pilotte, J.R.; Hossain, M.A.; Wells, C.I.; Zuercher, W.J.; et al. Hinge Binder Scaffold Hopping Identifies Potent Calcium/Calmodulin-Dependent Protein Kinase Kinase 2 (CAMKK2) Inhibitor Chemotypes. J. Med. Chem. 2021, 64, 10849–10877. [Google Scholar] [CrossRef]
- Aoyama, C.; Liao, H.; Ishidate, K. Structure and function of choline kinase isoforms in mammalian cells. Prog. Lipid Res. 2004, 43, 266–281. [Google Scholar] [CrossRef]
- Liu, R.; Lee, J.H.; Li, J.; Yu, R.; Tan, L.; Xia, Y.; Zheng, Y.; Bian, X.L.; Lorenzi, P.L.; Chen, Q.; et al. Choline kinase alpha 2 acts as a protein kinase to promote lipolysis of lipid droplets. Mol. Cell 2021, 81, 2722–2735.e9. [Google Scholar] [CrossRef]
- Glunde, K.; Bhujwalla, Z.M.; Ronen, S.M. Choline metabolism in malignant transformation. Nat. Rev. Cancer 2011, 11, 835–848. [Google Scholar] [CrossRef]
- Arlauckas, S.P.; Popov, A.V.; Delikatny, E.J. Choline kinase alpha-Putting the ChoK-hold on tumor metabolism. Prog. Lipid Res. 2016, 63, 28–40. [Google Scholar] [CrossRef]
- Lacal, J.C.; Zimmerman, T.; Campos, J.M. Choline Kinase: An Unexpected Journey for a Precision Medicine Strategy in Human Diseases. Pharmaceutics 2021, 13, 788. [Google Scholar] [CrossRef] [PubMed]
- Miyake, T.; Parsons, S.J. Functional interactions between Choline kinase alpha, epidermal growth factor receptor and c-Src in breast cancer cell proliferation. Oncogene 2012, 31, 1431–1441. [Google Scholar] [CrossRef] [PubMed]
- Kall, S.L.; Delikatny, E.J.; Lavie, A. Identification of a Unique Inhibitor-Binding Site on Choline Kinase alpha. Biochemistry 2018, 57, 1316–1325. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, B.; Sibon, O.C. Coenzyme A, more than ‘just’ a metabolic cofactor. Biochem. Soc. Trans. 2014, 42, 1075–1079. [Google Scholar] [CrossRef]
- Zhyvoloup, A.; Nemazanyy, I.; Panasyuk, G.; Valovka, T.; Fenton, T.; Rebholz, H.; Wang, M.L.; Foxon, R.; Lyzogubov, V.; Usenko, V.; et al. Subcellular localization and regulation of coenzyme A synthase. J. Biol. Chem. 2003, 278, 50316–50321. [Google Scholar] [CrossRef]
- Chi, J.T.; Lin, P.H.; Tolstikov, V.; Oyekunle, T.; Chen, E.Y.; Bussberg, V.; Greenwood, B.; Sarangarajan, R.; Narain, N.R.; Kiebish, M.A.; et al. Metabolomic effects of androgen deprivation therapy treatment for prostate cancer. Cancer Med. 2020, 9, 3691–3702. [Google Scholar] [CrossRef]
- Lin, C.C.; Kitagawa, M.; Tang, X.; Hou, M.H.; Wu, J.; Qu, D.C.; Srinivas, V.; Liu, X.; Thompson, J.W.; Mathey-Prevot, B.; et al. CoA synthase regulates mitotic fidelity via CBP-mediated acetylation. Nat. Commun. 2018, 9, 1039. [Google Scholar] [CrossRef]
- Ferrandon, S.; DeVecchio, J.; Duraes, L.; Chouhan, H.; Karagkounis, G.; Davenport, J.; Orloff, M.; Liska, D.; Kalady, M.F. CoA Synthase (COASY) Mediates Radiation Resistance via PI3K Signaling in Rectal Cancer. Cancer Res. 2020, 80, 334–346. [Google Scholar] [CrossRef]
- Kharabsheh, H.A.; Scott, J.E. CoAsy knockdown in TNBC cell lines resulted in no overt effect on cell proliferation in vitro. Biochem. Biophys. Res. Commun. 2020, 530, 136–141. [Google Scholar] [CrossRef]
- Sarkar, S.; Brautigan, D.L.; Larner, J.M. Aurora Kinase A Promotes AR Degradation via the E3 Ligase CHIP. Mol. Cancer Res. 2017, 15, 1063–1072. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Byrne, D.P.; Burgess, S.G.; Bormann, J.; Bakovic, J.; Huang, Y.; Zhyvoloup, A.; Yu, B.Y.K.; Peak-Chew, S.; Tran, T.; et al. Covalent Aurora A regulation by the metabolic integrator coenzyme A. Redox Biol. 2020, 28, 101318. [Google Scholar] [CrossRef] [PubMed]
- Hellawell, G.O.; Turner, G.D.; Davies, D.R.; Poulsom, R.; Brewster, S.F.; Macaulay, V.M. Expression of the type 1 insulin-like growth factor receptor is up-regulated in primary prostate cancer and commonly persists in metastatic disease. Cancer Res. 2002, 62, 2942–2950. [Google Scholar] [PubMed]
- Pignon, J.C.; Koopmansch, B.; Nolens, G.; Delacroix, L.; Waltregny, D.; Winkler, R. Androgen receptor controls EGFR and ERBB2 gene expression at different levels in prostate cancer cell lines. Cancer Res. 2009, 69, 2941–2949. [Google Scholar] [CrossRef] [PubMed]
- Traish, A.M.; Morgentaler, A. Epidermal growth factor receptor expression escapes androgen regulation in prostate cancer: A potential molecular switch for tumour growth. Br. J. Cancer 2009, 101, 1949–1956. [Google Scholar] [CrossRef] [PubMed]
- Montano, X.; Djamgoz, M.B. Epidermal growth factor, neurotrophins and the metastatic cascade in prostate cancer. FEBS Lett. 2004, 571, 1–8. [Google Scholar] [CrossRef]
- Shah, R.B.; Ghosh, D.; Elder, J.T. Epidermal growth factor receptor (ErbB1) expression in prostate cancer progression: Correlation with androgen independence. Prostate 2006, 66, 1437–1444. [Google Scholar] [CrossRef]
- Minner, S.; Jessen, B.; Stiedenroth, L.; Burandt, E.; Kollermann, J.; Mirlacher, M.; Erbersdobler, A.; Eichelberg, C.; Fisch, M.; Brummendorf, T.H.; et al. Low level HER2 overexpression is associated with rapid tumor cell proliferation and poor prognosis in prostate cancer. Clin. Cancer Res. 2010, 16, 1553–1560. [Google Scholar] [CrossRef]
- Bresson, E.; Seaborn, T.; Cote, M.; Cormier, G.; Provost, P.R.; Piedboeuf, B.; Tremblay, Y. Gene expression profile of androgen modulated genes in the murine fetal developing lung. Reprod. Biol. Endocrinol. 2010, 8, 2. [Google Scholar] [CrossRef]
- Jia, S.; Lu, J.; Qu, T.; Feng, Y.; Wang, X.; Liu, C.; Ji, J. MAGI1 inhibits migration and invasion via blocking MAPK/ERK signaling pathway in gastric cancer. Chin. J. Cancer Res. 2017, 29, 25–35. [Google Scholar] [CrossRef]
- Moniz, S.; Matos, P.; Jordan, P. WNK2 modulates MEK1 activity through the Rho GTPase pathway. Cell. Signal. 2008, 20, 1762–1768. [Google Scholar] [CrossRef]
- Wang, T.; Guo, S.; Liu, Z.; Wu, L.; Li, M.; Yang, J.; Chen, R.; Liu, X.; Xu, H.; Cai, S.; et al. CAMK2N1 inhibits prostate cancer progression through androgen receptor-dependent signaling. Oncotarget 2014, 5, 10293–10306. [Google Scholar] [CrossRef]
- Zhang, J.; Li, N.; Yu, J.; Zhang, W.; Cao, X. Molecular cloning and characterization of a novel calcium/calmodulin-dependent protein kinase II inhibitor from human dendritic cells. Biochem. Biophys. Res. Commun. 2001, 285, 229–234. [Google Scholar] [CrossRef]
- Ziera, T.; Irlbacher, H.; Fromm, A.; Latouche, C.; Krug, S.M.; Fromm, M.; Jaisser, F.; Borden, S.A. Cnksr3 is a direct mineralocorticoid receptor target gene and plays a key role in the regulation of the epithelial sodium channel. FASEB J. 2009, 23, 3936–3946. [Google Scholar] [CrossRef]
- Moniz, S.; Verissimo, F.; Matos, P.; Brazao, R.; Silva, E.; Kotelevets, L.; Chastre, E.; Gespach, C.; Jordan, P. Protein kinase WNK2 inhibits cell proliferation by negatively modulating the activation of MEK1/ERK1/2. Oncogene 2007, 26, 6071–6081. [Google Scholar] [CrossRef]
- Li, N.; Wang, C.; Wu, Y.; Liu, X.; Cao, X. Ca(2+)/calmodulin-dependent protein kinase II promotes cell cycle progression by directly activating MEK1 and subsequently modulating p27 phosphorylation. J. Biol. Chem. 2009, 284, 3021–3027. [Google Scholar] [CrossRef]
- Mody, N.; Campbell, D.G.; Morrice, N.; Peggie, M.; Cohen, P. An analysis of the phosphorylation and activation of extracellular-signal-regulated protein kinase 5 (ERK5) by mitogen-activated protein kinase kinase 5 (MKK5) in vitro. Biochem. J. 2003, 372, 567–575. [Google Scholar] [CrossRef] [PubMed]
- Adams, N.R.; Vasquez, Y.M.; Mo, Q.; Gibbons, W.; Kovanci, E.; DeMayo, F.J. WNK lysine deficient protein kinase 1 regulates human endometrial stromal cell decidualization, proliferation, and migration in part through mitogen-activated protein kinase 7. Biol. Reprod. 2017, 97, 400–412. [Google Scholar] [CrossRef]
- Paudel, R.; Fusi, L.; Schmidt, M. The MEK5/ERK5 Pathway in Health and Disease. Int. J. Mol. Sci. 2021, 22, 7594. [Google Scholar] [CrossRef] [PubMed]
- Stecca, B.; Rovida, E. Impact of ERK5 on the Hallmarks of Cancer. Int. J. Mol. Sci. 2019, 20, 1426. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.B.; Jenkins, B.L.; McCarthy, L.; Thilak, L.; Robson, C.N.; Neal, D.E.; Leung, H.Y. MEK5 overexpression is associated with metastatic prostate cancer, and stimulates proliferation, MMP-9 expression and invasion. Oncogene 2003, 22, 1381–1389. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Labrie, Y.; Grenier, J.; Fournier, A.; Fillion, C.; Labrie, C. Androgens induce expression of SPAK, a STE20/SPS1-related kinase, in LNCaP human prostate cancer cells. Mol. Cell. Endocrinol. 2001, 182, 181–192. [Google Scholar] [CrossRef]
- Choi, D.W.; Na, W.; Kabir, M.H.; Yi, E.; Kwon, S.; Yeom, J.; Ahn, J.W.; Choi, H.H.; Lee, Y.; Seo, K.W.; et al. WIP1, a homeostatic regulator of the DNA damage response, is targeted by HIPK2 for phosphorylation and degradation. Mol. Cell 2013, 51, 374–385. [Google Scholar] [CrossRef] [PubMed]
- Takekawa, M.; Adachi, M.; Nakahata, A.; Nakayama, I.; Itoh, F.; Tsukuda, H.; Taya, Y.; Imai, K. p53-inducible wip1 phosphatase mediates a negative feedback regulation of p38 MAPK-p53 signaling in response to UV radiation. EMBO J. 2000, 19, 6517–6526. [Google Scholar] [CrossRef] [PubMed]
- Winter, M.; Sombroek, D.; Dauth, I.; Moehlenbrink, J.; Scheuermann, K.; Crone, J.; Hofmann, T.G. Control of HIPK2 stability by ubiquitin ligase Siah-1 and checkpoint kinases ATM and ATR. Nat. Cell Biol. 2008, 10, 812–824. [Google Scholar] [CrossRef] [PubMed]
- Gresko, E.; Roscic, A.; Ritterhoff, S.; Vichalkovski, A.; del Sal, G.; Schmitz, M.L. Autoregulatory control of the p53 response by caspase-mediated processing of HIPK2. EMBO J. 2006, 25, 1883–1894. [Google Scholar] [CrossRef]
- Imberg-Kazdan, K.; Ha, S.; Greenfield, A.; Poultney, C.S.; Bonneau, R.; Logan, S.K.; Garabedian, M.J. A genome-wide RNA interference screen identifies new regulators of androgen receptor function in prostate cancer cells. Genome Res. 2013, 23, 581–591. [Google Scholar] [CrossRef]
- Drake, J.M.; Paull, E.O.; Graham, N.A.; Lee, J.K.; Smith, B.A.; Titz, B.; Stoyanova, T.; Faltermeier, C.M.; Uzunangelov, V.; Carlin, D.E.; et al. Phosphoproteome Integration Reveals Patient-Specific Networks in Prostate Cancer. Cell 2016, 166, 1041–1054. [Google Scholar] [CrossRef]
- Khandrika, L.; Lieberman, R.; Koul, S.; Kumar, B.; Maroni, P.; Chandhoke, R.; Meacham, R.B.; Koul, H.K. Hypoxia-associated p38 mitogen-activated protein kinase-mediated androgen receptor activation and increased HIF-1alpha levels contribute to emergence of an aggressive phenotype in prostate cancer. Oncogene 2009, 28, 1248–1260. [Google Scholar] [CrossRef]
- Kwong, A.J.; Scheidt, K.A. Non-‘classical’ MEKs: A review of MEK3-7 inhibitors. Bioorg. Med. Chem. Lett. 2020, 30, 127203. [Google Scholar] [CrossRef]
- Adams, M.; Kobayashi, T.; Lawson, J.D.; Saitoh, M.; Shimokawa, K.; Bigi, S.V.; Hixon, M.S.; Smith, C.R.; Tatamiya, T.; Goto, M.; et al. Fragment-based drug discovery of potent and selective MKK3/6 inhibitors. Bioorg. Med. Chem. Lett. 2016, 26, 1086–1089. [Google Scholar] [CrossRef]
- Venkadakrishnan, V.B.; Ben-Salem, S.; Heemers, H.V. AR-dependent phosphorylation and phospho-proteome targets in prostate cancer. Endocr. Relat. Cancer 2020, 27, R193–R210. [Google Scholar] [CrossRef] [PubMed]
- Gioeli, D.; Black, B.E.; Gordon, V.; Spencer, A.; Kesler, C.T.; Eblen, S.T.; Paschal, B.M.; Weber, M.J. Stress kinase signaling regulates androgen receptor phosphorylation, transcription, and localization. Mol. Endocrinol. 2006, 20, 503–515. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, P.I.; Saatcioglu, F. Inhibition of apoptosis in prostate cancer cells by androgens is mediated through downregulation of c-Jun N-terminal kinase activation. Neoplasia 2008, 10, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Hong, H.Y.; Kim, B.C. Mixed lineage kinase 3 connects reactive oxygen species to c-Jun NH2-terminal kinase-induced mitochondrial apoptosis in genipin-treated PC3 human prostate cancer cells. Biochem. Biophys. Res. Commun. 2007, 362, 307–312. [Google Scholar] [CrossRef]
- Singh, S.V.; Choi, S.; Zeng, Y.; Hahm, E.R.; Xiao, D. Guggulsterone-induced apoptosis in human prostate cancer cells is caused by reactive oxygen intermediate dependent activation of c-Jun NH2-terminal kinase. Cancer Res. 2007, 67, 7439–7449. [Google Scholar] [CrossRef]
- Chen, J.; Li, L.; Yang, Z.; Luo, J.; Yeh, S.; Chang, C. Androgen-deprivation therapy with enzalutamide enhances prostate cancer metastasis via decreasing the EPHB6 suppressor expression. Cancer Lett. 2017, 408, 155–163. [Google Scholar] [CrossRef]
- Park, J.G.; Aziz, N.; Cho, J.Y. MKK7, the essential regulator of JNK signaling involved in cancer cell survival: A newly emerging anticancer therapeutic target. Ther. Adv. Med. Oncol. 2019, 11, 1758835919875574. [Google Scholar] [CrossRef]
- Pavese, J.M.; Ogden, I.M.; Voll, E.A.; Huang, X.; Xu, L.; Jovanovic, B.; Bergan, R.C. Mitogen-activated protein kinase kinase 4 (MAP2K4) promotes human prostate cancer metastasis. PLoS ONE 2014, 9, e102289. [Google Scholar] [CrossRef]
- Zhang, H.; Gordon, R.; Li, W.; Yang, X.; Pattanayak, A.; Fowler, G.; Zhang, L.; Catalona, W.J.; Ding, Y.; Xu, L.; et al. Genistein treatment duration effects biomarkers of cell motility in human prostate. PLoS ONE 2019, 14, e0214078. [Google Scholar] [CrossRef]
- Liu, S.; Kumari, S.; Hu, Q.; Senapati, D.; Venkadakrishnan, V.B.; Wang, D.; DePriest, A.D.; Schlanger, S.E.; Ben-Salem, S.; Valenzuela, M.M.; et al. A comprehensive analysis of coregulator recruitment, androgen receptor function and gene expression in prostate cancer. Elife 2017, 6, e28482. [Google Scholar] [CrossRef]
- Schmidt, L.J.; Murillo, H.; Tindall, D.J. Gene expression in prostate cancer cells treated with the dual 5 alpha-reductase inhibitor dutasteride. J. Androl. 2004, 25, 944–953. [Google Scholar] [CrossRef] [PubMed]
- Bragelmann, J.; Klumper, N.; Offermann, A.; von Massenhausen, A.; Bohm, D.; Deng, M.; Queisser, A.; Sanders, C.; Syring, I.; Merseburger, A.S.; et al. Pan-Cancer Analysis of the Mediator Complex Transcriptome Identifies CDK19 and CDK8 as Therapeutic Targets in Advanced Prostate Cancer. Clin. Cancer Res. 2017, 23, 1829–1840. [Google Scholar] [CrossRef] [PubMed]
- Andrau, J.C.; van de Pasch, L.; Lijnzaad, P.; Bijma, T.; Koerkamp, M.G.; van de Peppel, J.; Werner, M.; Holstege, F.C. Genome-wide location of the coactivator mediator: Binding without activation and transient Cdk8 interaction on DNA. Mol. Cell 2006, 22, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Elmlund, H.; Baraznenok, V.; Lindahl, M.; Samuelsen, C.O.; Koeck, P.J.; Holmberg, S.; Hebert, H.; Gustafsson, C.M. The cyclin-dependent kinase 8 module sterically blocks Mediator interactions with RNA polymerase II. Proc. Natl. Acad. Sci. USA 2006, 103, 15788–15793. [Google Scholar] [CrossRef] [PubMed]
- Bassey, I.E.; Emodi, B.A.; Akpan, U.O.; Iyakndue, I.F.A.; Anakebe, E.A.; Icha, B.E.; Efobi, H.A.; Ntinya, A.J.; Udoh, A.E. Impact of Androgen Deprivation on Oxidative Stress and Antioxidant Status in Nigerian Patients With Prostate Cancer Undergoing Androgen Deprivation Therapy. JCO Glob. Oncol. 2020, 6, 1481–1489. [Google Scholar] [CrossRef]
- Shiota, M.; Yokomizo, A.; Naito, S. Oxidative stress and androgen receptor signaling in the development and progression of castration-resistant prostate cancer. Free Radic. Biol. Med. 2011, 51, 1320–1328. [Google Scholar] [CrossRef]
- Osman, S.; Mohammad, E.; Lidschreiber, M.; Stuetzer, A.; Bazso, F.L.; Maier, K.C.; Urlaub, H.; Cramer, P. The Cdk8 kinase module regulates interaction of the mediator complex with RNA polymerase II. J. Biol. Chem. 2021, 296, 100734. [Google Scholar] [CrossRef]
- Friedson, B.; Cooper, K.F. Cdk8 Kinase Module: A Mediator of Life and Death Decisions in Times of Stress. Microorganisms 2021, 9, 2152. [Google Scholar] [CrossRef]
- Nakamura, A.; Nakata, D.; Kakoi, Y.; Kunitomo, M.; Murai, S.; Ebara, S.; Hata, A.; Hara, T. CDK8/19 inhibition induces premature G1/S transition and ATR-dependent cell death in prostate cancer cells. Oncotarget 2018, 9, 13474–13487. [Google Scholar] [CrossRef]
- Yeh, Y.; Guo, Q.; Connelly, Z.; Cheng, S.; Yang, S.; Prieto-Dominguez, N.; Yu, X. Wnt/Beta-Catenin Signaling and Prostate Cancer Therapy Resistance. In Prostate Cancer; Advances in Experimental Medicine and Biology; Springer: Cham, Switzerland, 2019; Volume 1210, pp. 351–378. [Google Scholar] [CrossRef]
- Zhang, Z.; Cheng, L.; Li, J.; Farah, E.; Atallah, N.M.; Pascuzzi, P.E.; Gupta, S.; Liu, X. Inhibition of the Wnt/beta-Catenin Pathway Overcomes Resistance to Enzalutamide in Castration-Resistant Prostate Cancer. Cancer Res. 2018, 78, 3147–3162. [Google Scholar] [CrossRef]
- Johnsen, J.I.; Wickstrom, M.; Baryawno, N. Wingless/beta-catenin signaling as a modulator of chemoresistance in cancer. Mol. Cell. Oncol. 2016, 3, e1131356. [Google Scholar] [CrossRef] [PubMed]
- Kypta, R.M.; Waxman, J. Wnt/beta-catenin signalling in prostate cancer. Nat. Rev. Urol. 2012, 9, 418–428. [Google Scholar] [CrossRef] [PubMed]
- Morris, E.J.; Ji, J.Y.; Yang, F.; Di Stefano, L.; Herr, A.; Moon, N.S.; Kwon, E.J.; Haigis, K.M.; Naar, A.M.; Dyson, N.J. E2F1 represses beta-catenin transcription and is antagonized by both pRB and CDK8. Nature 2008, 455, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Song, L.N.; Herrell, R.; Byers, S.; Shah, S.; Wilson, E.M.; Gelmann, E.P. Beta-catenin binds to the activation function 2 region of the androgen receptor and modulates the effects of the N-terminal domain and TIF2 on ligand-dependent transcription. Mol. Cell. Biol. 2003, 23, 1674–1687. [Google Scholar] [CrossRef]
- Urbanucci, A.; Waltering, K.K.; Suikki, H.E.; Helenius, M.A.; Visakorpi, T. Androgen regulation of the androgen receptor coregulators. BMC Cancer 2008, 8, 219. [Google Scholar] [CrossRef]
- Truica, C.I.; Byers, S.; Gelmann, E.P. Beta-catenin affects androgen receptor transcriptional activity and ligand specificity. Cancer Res. 2000, 60, 4709–4713. [Google Scholar]
- Bhurta, D.; Bharate, S.B. Analyzing the scaffold diversity of cyclin-dependent kinase inhibitors and revisiting the clinical and preclinical pipeline. Med. Res. Rev. 2021, 42, 654–709. [Google Scholar] [CrossRef]
- Ma, D.; Chen, X.; Shen, X.B.; Sheng, L.Q.; Liu, X.H. Binding patterns and structure-activity relationship of CDK8 inhibitors. Bioorg. Chem. 2020, 96, 103624. [Google Scholar] [CrossRef]
- Galganski, L.; Urbanek, M.O.; Krzyzosiak, W.J. Nuclear speckles: Molecular organization, biological function and role in disease. Nucleic Acids Res. 2017, 45, 10350–10368. [Google Scholar] [CrossRef]
- Lee, G.; Zheng, Y.; Cho, S.; Jang, C.; England, C.; Dempsey, J.M.; Yu, Y.; Liu, X.; He, L.; Cavaliere, P.M.; et al. Post-transcriptional Regulation of De Novo Lipogenesis by mTORC1-S6K1-SRPK2 Signaling. Cell 2017, 171, 1545–1558.e18. [Google Scholar] [CrossRef] [PubMed]
- Rai, A.K.; Chen, J.X.; Selbach, M.; Pelkmans, L. Kinase-controlled phase transition of membraneless organelles in mitosis. Nature 2018, 559, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, Y.J.; Liu, Z.Z.; Wan, S.; Cai, Z.D.; Xie, J.J.; Cai, Z.D.; Song, S.D.; Wan, Y.P.; Hua, W.; Zhong, W.; et al. Enhanced expression of SRPK2 contributes to aggressive progression and metastasis in prostate cancer. Biomed. Pharmacother. 2018, 102, 531–538. [Google Scholar] [CrossRef]
- Liu, L.L.; Xie, N.; Sun, S.; Plymate, S.; Mostaghel, E.; Dong, X. Mechanisms of the androgen receptor splicing in prostate cancer cells. Oncogene 2014, 33, 3140–3150. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).