Abstract
Neuronal polarity established in developing neurons ensures proper function in the mature nervous system. As functionally distinct cellular compartments, axons and dendrites often require different subsets of proteins to maintain synaptic transmission and overall order. Although neurons in the mature CNS do not regenerate throughout life, their interactions with their extracellular environment are dynamic. The axon remains an overall protected area of the neuron where only certain proteins have access throughout the lifespan of the cell. This is in comparison to the somatodendritic compartment, where although it too has a specialised subset of proteins required for its maintenance, many proteins destined for the axonal compartment must first be trafficked through the former. Recent research has shown that axonal proteins contain specific axon-targeting motifs that permit access to the axonal compartment as well as downstream targeting to the axonal membrane. These motifs target proteins to the axonal compartment by a variety of mechanisms including: promoting segregation into axon-targeted secretory vesicles, increasing interaction with axonal kinesins and enhancing somatodendritic endocytosis. In this review, we will discuss axon-targeting motifs within the context of established neuron trafficking mechanisms. We will also include examples of how these motifs have been applied to target proteins to the axonal compartment to improve both tools for the study of axon biology, and for use as potential therapeutics for axonopathies.
1. Introduction
Mature central nervous system neurons display a unique morphology among mammalian cell types. Common multipolar neurons possess a highly polarised morphology consisting of a cell body (soma) with many branching dendrites and a single axon, which may end in a presynaptic terminal at distances ranging from microns to metres away from the cell body, such as in mammals [1]. This polarity of morphology is established de novo early in development, once migrating immature neurons reach their destination within the cerebrum. Immature neurons at first possess many processes but as they develop, one process is specified to become the axon and undergoes rapid outgrowth towards its anatomical target. Axon specification and outgrowth is driven by extracellular cues (e.g., laminin in the extracellular matrix) and intracellular changes such as cytoskeletal remodelling and trafficking of growth machinery into the nascent axon [2].
Having established polarity, mature neurons are considered to consist of two functionally distinct compartments, the somatodendritic and axonal compartments, which are responsible for the reception of post-synaptic potentials, and the initiation and transmission of action potentials, respectively. The distinct complement of proteins localised to either compartment underlies the normal function of mature neurons. Indeed, mislocalisation of axonal proteins may cause or contribute to neuropathology, such as in Alzheimer’s disease and amyotrophic lateral sclerosis [3,4]. Thus, much of the research has focused on characterising the mechanisms by which mature neurons may establish and maintain such polarity of protein localisation between the somatodendritic and axonal compartments, and how these mechanisms change during cellular maturation and go awry in pathology. This review discusses the mechanisms by which neurons maintain polarity of transmembrane protein localisation between the somatodendritic and axonal compartments through protein sorting, trafficking and exclusion mechanisms, and how short peptide axon-targeting motifs (ATMs) interact with these different pathways to promote axonal localisation of transmembrane proteins. Finally, the potential further methodological and therapeutic applications of ATMs are discussed.
2. Two Distinct Pathways Mediate Transmembrane Protein Trafficking in CNS Neurons
Transmembrane proteins are initially synthesised and adopt their native confirmation in the endoplasmic reticulum (ER) then undergo further maturation by post-translation modifications as they transit through the Golgi network. In the trans-Golgi network (TGN), transmembrane proteins are segregated into distinct populations of somatodendritic and axonal vesicles, a process that forms the basis of polarity maintenance in neurons. The mechanisms by which axonal cargoes are differentiated from somatodendritic cargoes in the neuronal TGN are not completely understood. However, recent evidence from Caenorhabditis elegans suggests that cargo recognition is dependent in part on competition between clathrin-associated adaptor protein (AP) complexes [5]. Binding of AP-3 complexes to axonal cargoes sorts these into vesicles destined for the axon whereas binding of AP-1 targets these to the somatodendritic domain [5,6]. Preferential binding of either AP complex is mediated by dileucine motifs ([D/E]xxxL[L/I]) present in the cytoplasmic tails of certain transmembrane proteins and those with higher affinity for AP-3 thus promoting axonal localisation of transmembrane proteins [5].
Having left the TGN, vesicles containing axonal proteins are trafficked towards both the somatodendritic and axonal compartments, whereas vesicles containing somatodendritic proteins, such as transferrin receptor (TfR), are excluded from the axon [7]. Some axonal transmembrane proteins are trafficked directly to the axon in secretory vesicles without interacting with the somatodendritic membrane, in a pathway resembling the canonical secretory pathway. Such trafficking is known to occur for tropomyosin receptor kinase B (TrkB) [8] and neuron–glia cell adhesion molecule (NgCAM) [9]. One might expect that vesicles carrying axonal proteins are efficiently routed directly to the axonal membrane without interacting with the somatodendritic membrane; however, a second level of transmembrane protein sorting occurs at the somatodendritic surface. Indeed, axonal transmembrane proteins may be exocytosed at the somatodendritic membrane then rapidly reinternalized into endosomes [9,10]. Endosomes containing axonal cargo are then either degraded [9,11] or recycled to the axon in a process resembling transcytosis in epithelial cells [9,12]. Interestingly, binding of ligands to TrkA and type-1 cannabinoid receptor (CB1R) at the axonal or somatodendritic surface, respectively, has been shown to stimulate transcytosis to the axonal compartment [13]. These findings could suggest that the local extracellular environment of both the axon and soma plays a role in regulating the contribution of transcytosis to axonal protein trafficking.
Since finding that these two pathways operate in neurons, their relative contribution to the sorting of axonal proteins has remained somewhat unclear. For example, seminal papers on this topic disagreed on the role of transcytosis in axonal expression of NgCAM [9,12]. With improvement of live-cell imaging of surface labelled proteins this dispute may soon be resolved. Indeed, a recent report using live-cell imaging of hippocampal neurons with surface labelled proteins found that the majority (84–90%) of vesicles containing somatodendritic surface-labelled VAMP2 and NgCAM were targeted to lysosome-like endosomes upon endocytosis and that direct axon-targeting in the secretory pathway accounted for the majority (85–94%) of anterograde vesicles carrying these proteins in the axon [11]. This is a remarkable finding, as it suggests that transcytotic trafficking of axonal proteins is quite inefficient in neurons as much of the axonal transmembrane protein retrieved from the somatodendritic surface is targeted for degradation.
3. Maintenance of Transmembrane Protein Polarity by the Proximal Axon
The two previously discussed trafficking pathways for transmembrane proteins in neurons are further supplemented by a host of mechanisms which function to exclude somatodendritic transmembrane proteins from the axonal compartment. Entry of vesicular cargo to the axon is regulated by two distinct regions at the proximal axon, the pre-axonal exclusion zone (PAEZ) and the axon initial segment (AIS).
The PAEZ lies just proximal to the axon hillock within the soma (Figure 1). Within the PAEZ, a population of somatodendritic vesicles are returned to the cell soma without entering the axon [14]. Sorting at the PAEZ appears to be regulated primarily by interactions of cargo-bound kinesins with post-translationally modified microtubules within the PAEZ, since fusion to a kinesin light chain binding sequence or overexpression of an acetylation mimic of α-tubulin results in axonal entry of TfR [14]. Microtubule associated proteins also play a role in filtering cargo at the PAEZ. Microtubule associated protein 2 (MAP2), which localises to the somatodendritic compartment, was shown to inhibit the binding of kinesin-1 to microtubules to favour anterograde trafficking of kinesin-3 bound cargoes in hippocampal neurons and dorsal root ganglion (DRG) neurons [15]. Similarly, the ability of secretory vesicles carrying TrkB to cross the PAEZ was shown to rely on kinesin-3 whereas rapid anterograde trafficking in the axon required kinesin-1 [8]. Thus, even in neurons lacking an AIS, such as DRG neurons [15], entry to the axon and correct distribution of cargo within the axon requires vesicles carrying axonal cargoes to associate with the appropriate complement of kinesins which preferentially bind to and ‘walk’ along axonal microtubules.
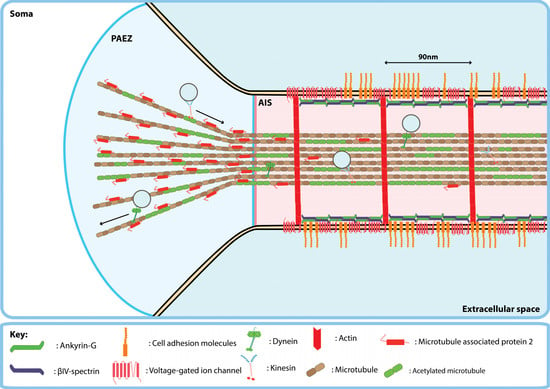
Figure 1.
Organisation of the pre-axonal exclusion zone (PAEZ) and axon initial segment (AIS) of mature central nervous system neurons. The PAEZ is located within the soma just proximal to the AIS. In the axon, the majority of microtubules are oriented with the plus-end pointing away from the soma. Plus-end directed kinesins drive anterograde transport and minus-end directed dyneins drive retrograde axonal transport of transmembrane proteins. The AIS is found in the axon hillock and is marked by a unique molecular architecture consisting of periodic rings of f-actin and a submembrane undercoat composed of ankyrin-G and βIV-spectrin. Furthermore, the AIS membrane is densely packed with transmembrane proteins such as voltage-gated ion channels and cell adhesion molecules.
Beyond the PAEZ lies the AIS, which makes up the first 20–60 µm of the axon in central nervous system neurons [16]. The AIS is marked by a unique molecular architecture that consists of the cytoskeletal proteins, filamentous actin (f-actin), ankyrin-G (AnkG) and βIV-spectrin that together form an annular cortex or ‘undercoat’ below the plasma membrane of the AIS [17] (Figure 1). Exclusion of somatodendritic transmembrane proteins at the AIS appears to be mediated in part via inhibition of lateral diffusion between the somatodendritic and axonal membranes and a cytoplasmic vesicle filter [18]. AnkG is necessary for the function of the AIS as a selective filter to maintain neuronal polarity since its knockdown allows somatodendritic proteins to cross the AIS and stimulates outgrowth of dendrite-like processes from the proximal axon [19,20]. Interaction with AnkG clusters many transmembrane proteins at high density on the AIS membrane, for example voltage-gated sodium channels (e.g., Nav1.2 and Nav1.6) and cell adhesion molecules (e.g., neurofascin-186 (NF-186) and L1) [21,22,23]. These clustered transmembrane proteins potentially inhibit lateral diffusion of membrane-associated proteins between neuronal compartments by greatly increasing the path length required to diffuse across the AIS due to molecular crowding [24,25].
As previously mentioned, the AIS also represents a cytoplasmic filtering point at which somatodendritic vesicles may be returned to the cell soma [16]. In contrast to somatodendritic vesicles, which pause before undergoing retrograde trafficking at the AIS [7,26], vesicles carrying axonal proteins appear unhindered as they traverse the AIS [22]. Indeed, as the AIS develops it recruits signaling proteins which interact with the cell’s trafficking machinery to exclude somatodendritic cargoes. For example, the ARF6 guanine-exchange factor EFA6 accumulates in the AIS during cortical neuron development where it activates ARF6, which induces retrograde trafficking of vesicles carrying integrins [20,27,28]. Similarly, nuclear distribution element-like 1 (NDEL1) localises to the AIS where it regulates dynein, potentially via lissencephaly 1 (Lis1), to enhance retrograde trafficking of vesicles carrying TfR that enter the AIS [29]. Besides regulation via the activity of microtubule-based motors, actin-based myosin motors also play a role in regulating vesicle trafficking at the AIS. Specifically, myosin Va, a plus-end directed motor, has been shown to be involved in excluding somatodendritic vesicles from the AIS and returning them to the somatodendritic domain, potentially by retrieving these vesicles from microtubules in the AIS where the plus-ends of actin filaments point toward the soma [30]. The accumulation of proteins that promote the activity of retrograde motors associated with somatodendritic cargoes following the initial assembly of the AIS may underlie further development of the AIS as a barrier to axonal entry of proteins. For example, in postnatal day 5 rat pups the AnkG is localized to the AIS however virally-expressed integrins are still permitted entry to the axon whereas in adult animals virally-expressed integrins are completely excluded from the axons of central neurons [31]. This suggests that the mechanical properties of the AIS scaffold alone may not be sufficient to exclude all somatodendritic cargoes and that maintenance of transmembrane protein polarity becomes more stringent with age.
4. Axon-Targeting Motifs Exploit Diverse Trafficking Pathways to Promote Axonal Localisation
The aforementioned mechanisms of protein sorting, trafficking and exclusion are dependent on the recognition of cargoes by the neuronal protein sorting machinery. Interactions between cargo proteins and protein sorting machinery are mediated in part by axon-targeting motifs. The term ‘axon-targeting motifs’ is generally used to describe relatively short peptide motifs, usually found in axonal proteins, that when tagged to a non-polarised or somatodendritic protein are sufficient to target it to the axon. Similarly, there also exist mRNA axon-targeting ‘zip-codes’, cis-acting elements that drive axonal localisation of mRNA in the 3′ UTR of axon-enriched mRNAs, for example, in β-actin [32] and the axonal microtubule associated protein tau [33] but these are outside the remit of this review (see review by Turner-Bridge and colleagues, 2020 [34]). ATMs display a variety of targeting mechanisms, reflecting the diversity of trafficking routes that proteins take to reach the axon. This section aims to discuss mechanisms by which ATMs target transmembrane proteins to the axonal compartment (see Table 1 for overview of ATMs discussed in this section).

Table 1.
Summary of previously identified and characterised axon-targeting motifs (ATMs). Examples of several ATMs characterised in the literature. The protein of origin, reported location and amino acid sequence of each ATM is given. Where possible, the putative targeting mechanism is also summarised alongside the model used to study the targeting mechanism.
Several ATMs have been identified that appear to target proteins to secretory vesicles destined for the axon at the TGN. For example, the C-terminus of neurexin-1α (Nxn1α) contains a PDZ-binding motif that targets Nxn1α to axon-targeted vesicles without the protein appearing on the somatodendritic membrane suggesting direct targeting [44]. Beyond interaction with the proteinaceous sorting machinery at the TGN, ATMs from both growth associated protein-43 (GAP-43) and paralemmin contain dicysteine palmitoylation motifs with adjacent dibasic amino acids that anchor these proteins into detergent-insoluble glycolipid-enriched complexes present in the plasma membrane (Figure 2A) [35,38]. These palmitoylation motifs may aid proteins in associating with vesicles in the secretory pathway during TGN sorting, as has been shown for GAP-43 [47]. Indeed, another cytosolic axonal protein, MAP6, also contains a similar N-terminal dicysteine palmitoylation motif with adjacent basic residues (RACCIAR) which promotes association with membranes of Rab6 positive secretory vesicles in the cell soma [48]. These motifs originate in lipid-anchored peripheral membrane proteins and studies using these motifs have tagged them to cytosolic proteins [35,36,49], thus whether they may successfully target transmembrane proteins remains unclear.
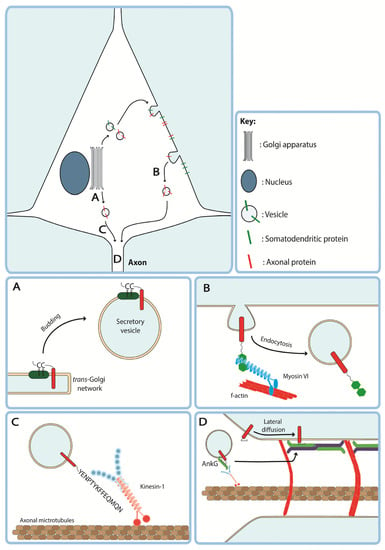
Figure 2.
Axon-targeting motifs influence diverse steps in the secretory and transcytotic trafficking pathways of transmembrane proteins to the axonal compartment. (A) A dicysteine palmitoylation motif found in Paralemmin and GAP-43 promotes association with lipid rafts in the trans-Golgi network, sorting transmembrane proteins into axon-targeted secretory vesicles. (B) Fused myosin VI binding domains of optineurin and DAB2 promote endocytosis of transmembrane proteins from the somatodendritic membrane for transcytotic delivery to the axon. (C) The C-terminus of APP drives association with light chains of kinesin-1, promoting axonal entry and anterograde trafficking. (D) Ankyrin-G (AnkG) binding domains found in Nav1.6 and Kv3.1 promote localisation to the axon initial segment potentially via association with AnkG on kinesin-3 driven vesicles or by anchoring transmembrane proteins in place following lateral diffusion into the AIS.
As previously discussed, many transmembrane proteins undergo transcytosis to reach the axonal membrane [9,12]. Therefore, increasing endocytosis of a protein from the somatodendritic membrane may increase the pool of endosomes available for transcytosis. Indeed, an early paper by Garrido and colleagues found that fusion of a dileucine-based endocytosis signal from the cytoplasmic domain of Nav1.2 to the non-polarised type 1 transmembrane protein CD4 increased its axonal localisation via clathrin-dependent endocytosis [10]. Another protein which may play a role in selective somatodendritic endocytosis of axonal proteins is the plus-end directed actin-based motor protein myosin VI. By fusing the myosin VI binding domains (MVIBD) of two proteins, optineurin (OPTN) and disabled homologue 2 (DAB2), to the C-terminus of non-polarised CD8 it, was shown that axonal enrichment of this transmembrane protein was dependent in part on endocytosis (Figure 2B) [43]. Further endocytosis-based motifs are also present in mGluR7 and Caspr2 which rely on the interaction with AP-2 and protein kinase C to induce endocytosis, respectively [45,46]. Some endocytosis-based ATMs have also been shown to drive axonal localization by both the transcytotic and secretory pathways, suggesting that sorting mechanisms at the somatodendritic membrane and the TGN may overlap mechanistically. For example, dynasore-mediated inhibition of endocytosis did not completely abrogate axon enrichment of channelrhodopsin tagged with the fused OPTN and DAB2 MVIBDs or the C-terminus Nav1.2 suggesting that these motifs may play a yet uncharacterised role in directing vesicles into the secretory pathway at the TGN [43].
Besides the previously discussed mechanisms of sorting proteins into axonal vesicles and removing them from the somatodendritic surface, ATMs may also interact with sorting machinery at the PAEZ and AIS to allow normally somatodendritic cargoes entry to the axon. As previously discussed, vesicles may be permitted entry to the axon and further trafficking to the distal axon by association with kinesin-3 and -1. Indeed, tagging three copies of the kinesin light chain binding sequence TNLEWDDSAI from cargo adaptor protein SifA and kinesin-interacting protein (SKIP) to somatodendritic TfR led to redirection of this protein to the distal axon in hippocampal neurons in vitro [14]. Interaction with axonal kinesin-1 may also underlie the axon-targeting effect of the C-terminal 15 amino acid ATM of amyloid precursor protein (APP) which contains a highly conserved GYENPTY motif [37]. The axon-targeting function of the C-terminus of APP as an ATM was first identified in experiments where fluorescent beads coated with the C-terminus of APP were shown to undergo fast anterograde transport when injected into the giant squid axon suggesting interaction with kinesin-1 [37].
Furthermore, the ATM of APP may interact directly with kinesin-1 or indirectly by the binding of jun N-terminal kinase (JNK) interacting protein JIP-1b which in turn interacts with kinesin light chain [50]. Thus, ATMs which drive interaction with axonal kinesins may function by first allowing tagged cargoes to cross the PAEZ and then facilitating fast anterograde transport within the axon itself (Figure 2C).
A subset of axon-targeting motifs containing AnkG binding motifs are found in voltage-gated sodium channels Nav1.2 and Nav1.6 [10,41] and potassium channel Kv3.1 [40] that cluster at the AIS. Nav1.2, for example, contains two putative axon-targeting sequences, an AnkG binding motif present in the second intracellular loop (II-III) and a 9 AA dileucine motif containing ATM in the intracellular C-terminal domain [10]. AnkG binding could increase axonal localisation of transmembrane proteins via two distinct mechanisms. First, lateral diffusion of transmembrane proteins into the AIS from the somatodendritic membrane could allow them to become anchored on the AIS membrane via interaction with AnkG. Secondly, it has previously been shown that Nav1.2 and AnkG are trafficked to the AIS in pre-assembled complexes driven by the interaction of AnkG with kinesin-1 [51] suggesting that AnkG binding motifs may also promote indirect association of transmembrane proteins with kinesin-1 via AnkG (Figure 2D). Whilst these may both be termed ATMs, the pattern of axon-targeting by these motifs appears to differ. By expressing CD4 that was tagged with either loop II-III or the C-terminus of Nav1.2 in hippocampal neurons, Garrido and colleagues showed that loop II-III targets CD4 to the AIS whereas CD4 tagged with the C-terminus was distributed throughout the axon suggesting that extra signals within the C-terminus are required for onward anterograde trafficking [10]. Thus, it appears that AnkG binding is sufficient to increase localisation at the AIS but further ATMs present in these proteins contribute to transport into the distal axon.
5. Further Applications of ATMs
5.1. Improving Characterisation of Mammalian Neuronal Circuity
Characterising the connectivity of neuronal circuitry is a pre-requisite to fully understanding its function; however, this is made difficult by the complexity of projections within the mammalian brain which may extend over long distances. ATMs could aid such characterisation by enhancing the ability of proteins to enter the axon and accumulate at the distal axon where otherwise they may not readily localise. Indeed, Padmanabhan and colleagues showed that by tagging the red fluorescent protein Tomato with the ATM of APP it was possible to efficiently label axons of the medial forebrain bundle and nigro-striatal tract, improving the ability to count axons within these tracts over non-targeted Tomato [35]. Thus, by simply targeting fluorescent proteins to the axon it could be possible to improve morphological characterisation of neuronal circuitry to study the number, size or complexity of axons.
Another class of fluorescent proteins are the GCaMPs, a family of genetically encoded calcium indicators (GECIs) that increase in fluorescence when Ca2+ binds to the protein as a result of conformational changes which exclude water from the fluorophore [52]. Thus, fluorescence can be used as a measurement of transient changes of intracellular Ca2+ concentration, such as those that occur during action potential firing [53]. Previous GCaMPs were limited by poor long-distance diffusion of cytosolic GCaMPs to the distal axon, which interferes with precise measurement of Ca2+ in the axonal compartment of neurons in vivo [49]. A synapse-targeted GCaMP was produced by fusing GCaMP2 to the cytoplasmic tail of full-length synaptophysin, a synaptic vesicle protein, to monitor spike activity in pre-synaptic boutons of the optic tectum in Zebrafish [54]. Improving on this application, a later study used the dicysteine palmitoylation motif of GAP-43, to develop an axon-targeted version of GCaMP6 for use in mice. This version of GCaMP6 offered lower background signal from somatodendritic domains, improved signal-to-noise ratio and photostability compared to non-targeted GCaMP [49].
5.2. Improving Genetic Therapies for Axonopathies
Following axotomy, the distal axon displays a stereotypical degeneration process known as “Wallerian degeneration” [55]. The Wallerian degeneration slow (Wlds) mouse however displays delayed onset of Wallerian degeneration following axotomy [56]. The Wlds gene encodes a chimeric fusion protein of ubiquitination factor Ube4b and the nuclear NAD+ synthesising enzyme nicotinamide nucleotide adenylyltransferase 1 (NMNAT1) [57]. The axoprotective effect of Wlds is dependent on its mislocalisation into the cytoplasm, with a portion of the mutant protein ending up in axons [58]. When NMNAT1 was tagged with the 15AA ATM of APP and its nuclear localisation signal was removed, the axonal localisation and axoprotective properties of NMNAT1 were increased compared to non-targeted NMNAT1 both in vivo and in vitro [36].
Besides potentiating the axoprotective effect of proteins, ATMs may also enhance the pro-regenerative effects of proteins by targeting them to the axon or growth cone. For example, chondroitinase ABC (ChABC), is a bacterial enzyme that degrades the glycosaminoglycan side chains of inhibitory extracellular matrix proteins chondroitin sulfate proteoglycans [59] deposited by reactive astrocytes following spinal cord injury [60]. Infusion of ChABC into spinal cord lesion sites following injury has been shown to promote regeneration of sensory and corticospinal tract axons beyond the lesion site [61]. Several modifications have been made to increase thermostability of ChABC at body temperature [62], allow controllable expression [63], and secretion from mammalian cells [64]. In line with these improvements, a recent in vitro study used the YENPTY ATM from APP to assess the effect of axon-targeting on the pro-regenerative effect of ChABC. Whilst the study did not provide evidence of ChABC being targeted to the neurites or axons of the cells used, potentially due to the lack of antibodies against ChABC, expression of axon-targeted ChABC did increase neurite outgrowth and branching from SH-SY5Y cells and dissociated primary DRG neurons on chondroitin-4-sulfate compared to non-targeted ChABC [65].
Although limited in number these studies suggest that axon-targeting of either axoprotective or pro-regenerative proteins may potentiate their effects by delivering them to the axonal compartment. Several studies have already identified pro-regenerative proteins that are excluded from axons when virally expressed in mature neurons of the sensorimotor cortex and red nucleus, which supply axons to descending motor tracts. These include insulin-like growth factor type 1 receptor (IGF-IR), Trks and integrins [31,66,67]. It could therefore be possible to identify ATMs which could target these proteins to lesioned axons and assess whether this would potentiate the pro-regenerative effects of these proteins in the descending motor tracts.
6. Conclusions
Here we have discussed some of the mechanisms by which neurons establish and maintain a highly polarised distribution of transmembrane proteins between the somatodendritic and axonal compartments. Two main pathways via which transmembrane proteins leave the TGN to reach the axonal membrane have emerged, the indirect transcytosis pathway and the direct secretory pathway. Even for well-defined proteins, the relative contribution of each pathway to axonal localisation has been a point of contention. Recent evidence suggests that the majority of axonal proteins endocytosed from the somatodendritic membrane are trafficked to endolysosomes. Further study of the downstream fate of selectively eliminated axonal proteins will further aid in addressing this problem.
Polarised sorting of transmembrane proteins at the TGN and at the somatodendritic membrane is further coupled to selective filtration at both the PAEZ and the AIS of mature neurons. Evidence from in vitro studies suggests a model of proximal axon vesicle sorting whereby vesicles carrying axonal transmembrane proteins must associate with a specific complement of kinesin motors to traverse both the PAEZ and to gain entry to the AIS where somatodendritic cargoes are physically excluded by the AIS scaffold or by the action of proteins that regulated the motility of somatodendritic cargo-associated retrograde motor proteins.
Understanding the mechanisms of transmembrane protein trafficking in neurons is necessary to fully characterise how neurons establish and maintain polarity. Further mechanistic characterisation of these pathways will aid in manipulating transmembrane protein trafficking in adult animals. Polarised trafficking may be manipulated both in vitro and in vivo by the addition of ATMs to specific transmembrane proteins. Thus far, many ATMs have been identified and effort has been made to understand the mechanisms by which they are sufficient to induce axonal localisation of normally excluded proteins. It appears clear that ATMs target almost all identified steps within the trafficking and exclusion pathways described here. However, the research on ATMs beyond mechanistic characterisation is relatively sparse. We have described some applications of ATMs but to our knowledge in vivo studies have only been performed on targeting of non-polarised proteins used to study axon biology and potential genetic therapies. Therefore, studies assessing the impact of ATMs on transmembrane receptors localisation in the mature CNS in vivo where mature trafficking and exclusion mechanisms are active are needed.
Author Contributions
Conceptualisation, L.J.S.-N. and M.R.A.; writing-original draft preparation, L.J.S.-N. and M.R.A.; writing- review and editing, L.J.S.-N. and M.R.A. All authors have read and agreed to the published version of the manuscript.
Funding
L.J.S.-N. is supported by a studentship from the Gerald Kerkut Charitable Trust. M.R.A. is supported by a research grant from the Biotechnology and Biological Sciences Research Council (BBSRC) (Grant: BBN008189/1) and the International Foundation for Research in Paraplegia (IRP) (Grant: P182).
Acknowledgments
We acknowledge David A. Tumbarello (University of Southampton) for critical reading of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Smith, D.H. Stretch growth of integrated axon tracts: Extremes and exploitations. Prog. Neurobiol. 2009, 89, 231–239. [Google Scholar] [CrossRef] [PubMed]
- Esakakibara, A.; Ehatanaka, Y. Neuronal polarization in the developing cerebral cortex. Front. Neurosci. 2015, 9, 116. [Google Scholar] [CrossRef]
- Zempel, H.; Thies, E.; Mandelkow, E.-M. A Oligomers Cause Localized Ca2+ Elevation, Missorting of Endogenous Tau into Dendrites, Tau Phosphorylation, and Destruction of Microtubules and Spines. J. Neurosci. 2010, 30, 11938–11950. [Google Scholar] [CrossRef] [PubMed]
- Bilsland, L.G.; Sahai, E.; Kelly, G.; Golding, M.; Greensmith, L.; Schiavo, G. Deficits in axonal transport precede ALS symptoms in vivo. Proc. Natl. Acad. Sci. USA 2010, 107, 20523–20528. [Google Scholar] [CrossRef]
- Li, P.; Merrill, S.A.; Jorgensen, E.M.; Shen, K. Two Clathrin Adaptor Protein Complexes Instruct Axon-Dendrite Polarity. Neuron 2016, 90, 564–580. [Google Scholar] [CrossRef]
- Margeta, M.A.; Wang, G.J.; Shen, K. Clathrin adaptor AP-1 complex excludes multiple postsynaptic receptors from axons in C. elegans. Proc. Natl. Acad. Sci. USA 2009, 106, 1632–1637. [Google Scholar] [CrossRef]
- Burack, M.A.; Silverman, M.A.; Banker, G. The Role of Selective Transport in Neuronal Protein Sorting. Neuron 2000, 26, 465–472. [Google Scholar] [CrossRef]
- Zahavi, E.E.; Hummel, J.J.; Han, Y.; Bar, C.; Stucchi, R.; Altelaar, M.; Hoogenraad, C.C. Combined kinesin-1 and kinesin-3 activity drives axonal trafficking of TrkB receptors in Rab6 carriers. Dev. Cell 2021, 56, 494–508. [Google Scholar] [CrossRef]
- Sampo, B.; Kaech, S.; Kunz, S.; Banker, G. Two Distinct Mechanisms Target Membrane Proteins to the Axonal Surface. Neuron 2003, 37, 611–624. [Google Scholar] [CrossRef]
- Garrido, J.J.; Fernandes, F.; Giraud, P.; Mouret, I.; Pasqualini, E.; Fache, M.; Jullien, F.; Dargent, B. Identification of an axonal determinant in the C-terminus of the sodium channel Nav1.2. EMBO J. 2001, 20, 5950–5961. [Google Scholar] [CrossRef]
- Nabb, A.T.; Bentley, M. NgCAM and VAMP2 Reveal that Direct Delivery and Dendritic Degradation Maintain Axonal Polarity. Mol. Biol. Cell 2022, 33, ar3. [Google Scholar] [CrossRef] [PubMed]
- Wisco, D.; Anderson, E.D.; Chang, M.C.; Norden, C.; Boiko, T.; Folsch, H.; Winckler, B. Uncovering multiple axonal targeting pathways in hippocampal neurons. J. Cell Biol. 2003, 162, 1317–1328. [Google Scholar] [CrossRef] [PubMed]
- Ascaño, M.; Richmond, A.; Borden, P.; Kuruvilla, R. Axonal Targeting of Trk Receptors via Transcytosis Regulates Sensitivity to Neurotrophin Responses. J. Neurosci. 2009, 29, 11674–11685. [Google Scholar] [CrossRef] [PubMed]
- Farías, G.G.; Guardia, C.M.; Britt, D.J.; Guo, X.; Bonifacino, J.S. Sorting of Dendritic and Axonal Vesicles at the Pre-axonal Exclusion Zone. Cell Rep. 2015, 13, 1221–1232. [Google Scholar] [CrossRef]
- Gumy, L.F.; Katrukha, E.A.; Grigoriev, I.; Jaarsma, D.; Kapitein, L.C.; Akhmanova, A.; Hoogenraad, C.C. MAP2 Defines a Pre-axonal Filtering Zone to Regulate KIF1- versus KIF5-Dependent Cargo Transport in Sensory Neurons. Neuron 2017, 94, 347–362.e7. [Google Scholar] [CrossRef]
- Song, A.-H.; Wang, D.; Chen, G.; Li, Y.; Luo, J.; Duan, S.; Poo, M.-M. A Selective Filter for Cytoplasmic Transport at the Axon Initial Segment. Cell 2009, 136, 1148–1160. [Google Scholar] [CrossRef]
- Quistgaard, E.M.; Nissen, J.D.; Hansen, S.; Nissen, P. Mind the Gap: Molecular Architecture of the Axon Initial Segment—From Fold Prediction to a Mechanistic Model of Function? J. Mol. Biol. 2021, 433, 167176. [Google Scholar] [CrossRef]
- Leterrier, C.; Dargent, B. No Pasaran! Role of the axon initial segment in the regulation of protein transport and the maintenance of axonal identity. Semin. Cell Dev. Biol. 2013, 27, 44–51. [Google Scholar] [CrossRef]
- Hedstrom, K.L.; Ogawa, Y.; Rasband, M.N. AnkyrinG is required for maintenance of the axon initial segment and neuronal polarity. J. Cell Biol. 2008, 183, 635–640. [Google Scholar] [CrossRef]
- Franssen, E.H.P.; Zhao, R.-R.; Koseki, H.; Kanamarlapudi, V.; Hoogenraad, C.; Eva, R.; Fawcett, J. Exclusion of Integrins from CNS Axons Is Regulated by Arf6 Activation and the AIS. J. Neurosci. 2015, 35, 8359–8375. [Google Scholar] [CrossRef]
- Zhou, D.; Lambert, S.; Malen, P.L.; Carpenter, S.; Boland, L.; Bennett, V. AnkyrinG Is Required for Clustering of Voltage-gated Na Channels at Axon Initial Segments and for Normal Action Potential Firing. J. Cell Biol. 1998, 143, 1295–1304. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, S.M.; Bennett, V. Ankyrin-G coordinates assembly of the spectrin-based membrane skeleton, voltage-gated sodium channels, and L1 CAMs at Purkinje neuron initial segments. J. Cell Biol. 2001, 155, 739–746. [Google Scholar] [CrossRef] [PubMed]
- Hedstrom, K.L.; Xu, X.; Ogawa, Y.; Frischknecht, R.; Seidenbecher, C.I.; Shrager, P.; Rasband, M.N. Neurofascin assembles a specialized extracellular matrix at the axon initial segment. J. Cell Biol. 2007, 178, 875–886. [Google Scholar] [CrossRef] [PubMed]
- Nakada, C.; Ritchie, K.; Oba, Y.; Nakamura, M.; Hotta, Y.; Iino, R.; Kasai, R.; Yamaguchi, K.; Fujiwara, T.; Kusumi, A. Accumulation of anchored proteins forms membrane diffusion barriers during neuronal polarization. Nat. Cell Biol. 2003, 5, 626–632. [Google Scholar] [CrossRef]
- Albrecht, D.; Winterflood, C.M.; Sadeghi, M.; Tschager, T.; Noé, F.; Ewers, H. Nanoscopic compartmentalization of membrane protein motion at the axon initial segment. J. Cell Biol. 2016, 215, 37–46. [Google Scholar] [CrossRef]
- Al-Bassam, S.; Xu, M.; Wandless, T.J.; Arnold, D.B. Differential Trafficking of Transport Vesicles Contributes to the Localization of Dendritic Proteins. Cell Rep. 2012, 2, 89–100. [Google Scholar] [CrossRef]
- Eva, R.; Crisp, S.; Marland, J.; Norman, J.C.; Kanamarlapudi, V.; Ffrench-Constant, C.; Fawcett, J. ARF6 Directs Axon Transport and Traffic of Integrins and Regulates Axon Growth in Adult DRG Neurons. J. Neurosci. 2012, 32, 10352–10364. [Google Scholar] [CrossRef]
- Eva, R.; Koseki, H.; Kanamarlapudi, V.; Fawcett, J.W. EFA6 regulates selective polarised transport and axon regeneration from the axon initial segment. J. Cell Sci. 2017, 130, 3663–3675. [Google Scholar] [CrossRef]
- Kuijpers, M.; van de Willige, D.; Freal, A.; Chazeau, A.; Franker, M.A.; Hofenk, J.; Rodrigues, R.J.C.; Kapitein, L.C.; Akhmanova, A.; Jaarsma, D.; et al. Dynein Regulator NDEL1 Controls Polarized Cargo Transport at the Axon Initial Segment. Neuron 2016, 89, 461–471. [Google Scholar] [CrossRef]
- Lewis, T.L.; Mao, T.; Svoboda, K.; Arnold, D.B. Myosin-dependent targeting of transmembrane proteins to neuronal dendrites. Nat. Neurosci. 2009, 12, 568–576. [Google Scholar] [CrossRef]
- Andrews, M.R.; Soleman, S.; Cheah, M.; Tumbarello, D.A.; Mason, M.R.J.; Moloney, E.; Verhaagen, J.; Bensadoun, J.-C.; Schneider, B.; Aebischer, P.; et al. Axonal Localization of Integrins in the CNS Is Neuronal Type and Age Dependent. eNeuro 2016, 3, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Eom, T.; Oleynikov, Y.; Shenoy, S.; Liebelt, D.; Dictenberg, J.; Singer, R.; Bassell, G. Neurotrophin-Induced Transport of a β-Actin mRNP Complex Increases β-Actin Levels and Stimulates Growth Cone Motility. Neuron 2001, 31, 261–275. [Google Scholar] [CrossRef]
- Aronov, S.; Aranda, G.; Behar, L.; Ginzburg, I. Axonal Tau mRNA Localization Coincides with Tau Protein in Living Neuronal Cells and Depends on Axonal Targeting Signal. J. Neurosci. 2001, 21, 6577–6587. [Google Scholar] [CrossRef] [PubMed]
- Turner-Bridger, B.; Caterino, C.; Cioni, J.-M. Molecular mechanisms behind mRNA localization in axons. Open Biol. 2020, 10, 200177. [Google Scholar] [CrossRef]
- Padmanabhan, S.; Kareva, T.; Kholodilov, N.; E Burke, R. Quantitative morphological comparison of axon-targeting strategies for gene therapies directed to the nigro-striatal projection. Gene Ther. 2013, 21, 115–122. [Google Scholar] [CrossRef]
- Babetto, E.; Beirowski, B.; Janeckova, L.; Brown, R.; Gilley, J.; Thomson, D.; Ribchester, R.R.; Coleman, M.P. Targeting NMNAT1 to Axons and Synapses Transforms Its Neuroprotective Potency In Vivo. J. Neurosci. 2010, 30, 13291–13304. [Google Scholar] [CrossRef]
- Satpute-Krishnan, P.; DeGiorgis, J.A.; Conley, M.P.; Jang, M.; Bearer, E.L. A peptide zipcode sufficient for anterograde transport within amyloid precursor protein. Proc. Natl. Acad. Sci. USA 2006, 103, 16532–16537. [Google Scholar] [CrossRef]
- El-Husseini, A.E.-D.; Craven, S.E.; Brock, S.C.; Bredt, D.S. Polarized Targeting of Peripheral Membrane Proteins in Neurons. J. Biol. Chem. 2001, 276, 44984–44992. [Google Scholar] [CrossRef]
- Zuber, M.X.; Strittmatter, S.M.; Fishman, M.C. A membrane-targeting signal in the amino terminus of the neuronal protein GAP-43. Nature 1989, 341, 345–348. [Google Scholar] [CrossRef]
- Xu, M.; Cao, R.; Xiao, R.; Zhu, M.X.; Gu, C. The Axon Dendrite Targeting of Kv3 (Shaw) Channels Is Determined by a Targeting Motif That Associates with the T1 Domain and Ankyrin G. J. Neurosci. 2007, 27, 14158–14170. [Google Scholar] [CrossRef]
- Gasser, A.; Ho, T.S.-Y.; Cheng, X.; Chang, K.-J.; Waxman, S.G.; Rasband, M.N.; Dib-Hajj, S.D. An AnkyrinG-Binding Motif Is Necessary and Sufficient for Targeting Nav1.6 Sodium Channels to Axon Initial Segments and Nodes of Ranvier. J. Neurosci. 2012, 32, 7232–7243. [Google Scholar] [CrossRef] [PubMed]
- Lemaillet, G.; Walker, B.; Lambert, S. Identification of a Conserved Ankyrin-binding Motif in the Family of Sodium Channel α Subunits. J. Biol. Chem. 2003, 278, 27333–27339. [Google Scholar] [CrossRef] [PubMed]
- Lewis, T.L., Jr.; Mao, T.; Arnold, D.B. A Role for Myosin VI in the Localization of Axonal Proteins. PLoS Biol. 2011, 9, e1001021. [Google Scholar] [CrossRef]
- Fairless, R.; Masius, H.; Rohlmann, A.; Heupel, K.; Ahmad, M.; Reissner, C.; Dresbach, T.; Missler, M. Polarized Targeting of Neurexins to Synapses Is Regulated by their C-Terminal Sequences. J. Neurosci. 2008, 28, 12969–12981. [Google Scholar] [CrossRef]
- Xu, J.; Zhu, Y.; Heinemann, S.F. Identification of Sequence Motifs That Target Neuronal Nicotinic Receptors to Dendrites and Axons. J. Neurosci. 2006, 26, 9780–9793. [Google Scholar] [CrossRef]
- Bel, C.; Oguievetskaia, K.; Pitaval, C.; Goutebroze, L.; Faivre-Sarrailh, C. Axonal targeting of Caspr2 in hippocampal neurons via selective somatodendritic endocytosis. J. Cell Sci. 2009, 122, 3403–3413. [Google Scholar] [CrossRef]
- Gauthier-Kemper, A.; Igaev, M.; Sündermann, F.; Janning, D.; Brühmann, J.; Moschner, K.; Reyher, H.-J.; Junge, W.; Glebov, K.; Walter, J.; et al. Interplay between phosphorylation and palmitoylation mediates plasma membrane targeting and sorting of GAP43. Mol. Biol. Cell 2014, 25, 3284–3299. [Google Scholar] [CrossRef]
- Tortosa, E.; Adolfs, Y.; Fukata, M.; Pasterkamp, R.J.; Kapitein, L.C.; Hoogenraad, C.C. Dynamic Palmitoylation Targets MAP6 to the Axon to Promote Microtubule Stabilization during Neuronal Polarization. Neuron 2017, 94, 809–825.e7. [Google Scholar] [CrossRef]
- Broussard, G.; Liang, Y.; Fridman, M.; Unger, E.; Meng, G.; Xiao, X.; Ji, N.; Petreanu, L.; Tian, L. In vivo measurement of afferent activity with axon-specific calcium imaging. Nat. Neurosci. 2018, 21, 1272–1280. [Google Scholar] [CrossRef]
- Inomata, H.; Nakamura, Y.; Hayakawa, A.; Takata, H.; Suzuki, T.; Miyazawa, K.; Kitamura, N. A Scaffold Protein JIP-1b Enhances Amyloid Precursor Protein Phosphorylation by JNK and Its Association with Kinesin Light Chain 1. J. Biol. Chem. 2003, 278, 22946–22955. [Google Scholar] [CrossRef]
- Barry, J.; Gu, Y.; Jukkola, P.; O’Neill, B.; Gu, H.; Mohler, P.J.; Rajamani, K.T.; Gu, C. Ankyrin-G Directly Binds to Kinesin-1 to Transport Voltage-Gated Na+ Channels into Axons. Dev. Cell 2014, 28, 117–131. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Shui, B.; Kotlikoff, M.I.; Sondermann, H. Structural Basis for Calcium Sensing by GCaMP2. Structure 2008, 16, 1817–1827. [Google Scholar] [CrossRef] [PubMed]
- Broussard, G.; Liang, R.; Etian, L. Monitoring activity in neural circuits with genetically encoded indicators. Front. Mol. Neurosci. 2014, 7, 97. [Google Scholar] [CrossRef] [PubMed]
- Dreosti, E.; Odermatt, B.; Dorostkar, M.; Lagnado, L. A genetically encoded reporter of synaptic activity in vivo. Nat. Methods 2009, 6, 883–889. [Google Scholar] [CrossRef]
- Waller, A. Experiments on the Section of the Glosso-Pharyngeal and Hypoglossal Nerves of the Frog, and Observations of the Alterations Produced Thereby in the Structure of Their Primitive Fibres. Edinb. Med Surg. J. 1851, 76, 369–376. [Google Scholar] [PubMed]
- Perry, V.H.; Brown, M.C.; Lunn, E.R. Very Slow Retrograde and Wallerian Degeneration in the CNS of C57BL/Ola Mice. Eur. J. Neurosci. 1991, 3, 102–105. [Google Scholar] [CrossRef]
- Mack, T.G.A.; Reiner, M.; Beirowski, B.; Mi, W.; Emanuelli, M.; Wagner, D.; Thomson, D.; Gillingwater, T.; Court, F.; Conforti, L.; et al. Wallerian degeneration of injured axons and synapses is delayed by a Ube4b/Nmnat chimeric gene. Nat. Neurosci. 2001, 4, 1199–1206. [Google Scholar] [CrossRef]
- Beirowski, B.; Babetto, E.; Gilley, J.; Mazzola, F.; Conforti, L.; Janeckova, L.; Magni, G.; Ribchester, R.R.; Coleman, M.P. Non-Nuclear WldS Determines Its Neuroprotective Efficacy for Axons and Synapses In Vivo. J. Neurosci. 2009, 29, 653–668. [Google Scholar] [CrossRef]
- Yamagata, T.; Saito, H.; Habuchi, O.; Suzuki, S. Purification and Properties of Bacterial Chondroitinases and Chondrosulfatases. J. Biol. Chem. 1968, 243, 1523–1535. [Google Scholar] [CrossRef]
- Hussein, R.K.; Mencio, C.P.; Katagiri, Y.; Brake, A.M.; Geller, H.M. Role of Chondroitin Sulfation Following Spinal Cord Injury. Front. Cell. Neurosci. 2020, 14, 208. [Google Scholar] [CrossRef]
- Bradbury, E.J.; Moon, L.; Popat, R.J.; King, V.R.; Bennett, G.S.; Patel, P.N.; Fawcett, J.; McMahon, S. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 2002, 416, 636–640. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; McKeon, R.J.; Bellamkonda, R.V. Sustained delivery of thermostabilized chABC enhances axonal sprouting and functional recovery after spinal cord injury. Proc. Natl. Acad. Sci. USA 2009, 107, 3340–3345. [Google Scholar] [CrossRef] [PubMed]
- Burnside, E.R.; De Winter, F.; Didangelos, A.; James, N.D.; Andreica, E.-C.; Horsfall, H.L.; Muir, E.M.; Verhaagen, J.; Bradbury, E. Immune-evasive gene switch enables regulated delivery of chondroitinase after spinal cord injury. Brain 2018, 141, 2362–2381. [Google Scholar] [CrossRef] [PubMed]
- Muir, E.M.; Fyfe, I.; Gardiner, S.; Li, L.; Warren, P.; Fawcett, J.W.; Keynes, R.J.; Rogers, J.H. Modification of N-glycosylation sites allows secretion of bacterial chondroitinase ABC from mammalian cells. J. Biotechnol. 2010, 145, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Day, P.; Alves, N.; Daniell, E.; Dasgupta, D.; Ogborne, R.; Steeper, A.; Raza, M.; Ellis, C.; Fawcett, J.; Keynes, R.; et al. Targeting chondroitinase ABC to axons enhances the ability of chondroitinase to promote neurite outgrowth and sprouting. PLoS ONE 2020, 15, e0221851. [Google Scholar] [CrossRef] [PubMed]
- Hollis, E.R.; Lu, P.; Blesch, A.; Tuszynski, M.H. IGF-I gene delivery promotes corticospinal neuronal survival but not regeneration after adult CNS injury. Exp. Neurol. 2009, 215, 53–59. [Google Scholar] [CrossRef][Green Version]
- Hollis, E.; Jamshidi, P.; Löw, K.; Blesch, A.; Tuszynski, M.H. Induction of corticospinal regeneration by lentiviral trkB-induced Erk activation. Proc. Natl. Acad. Sci. USA 2009, 106, 7215–7220. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).