The Role of CD200–CD200 Receptor in Human Blood and Lymphatic Endothelial Cells in the Regulation of Skin Tissue Inflammation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Isolation and Culture
2.2. Flow Cytometry and Immunohistochemistry
2.3. Proliferation Assay
2.4. Cell Viability Assay
2.5. Immunoassay
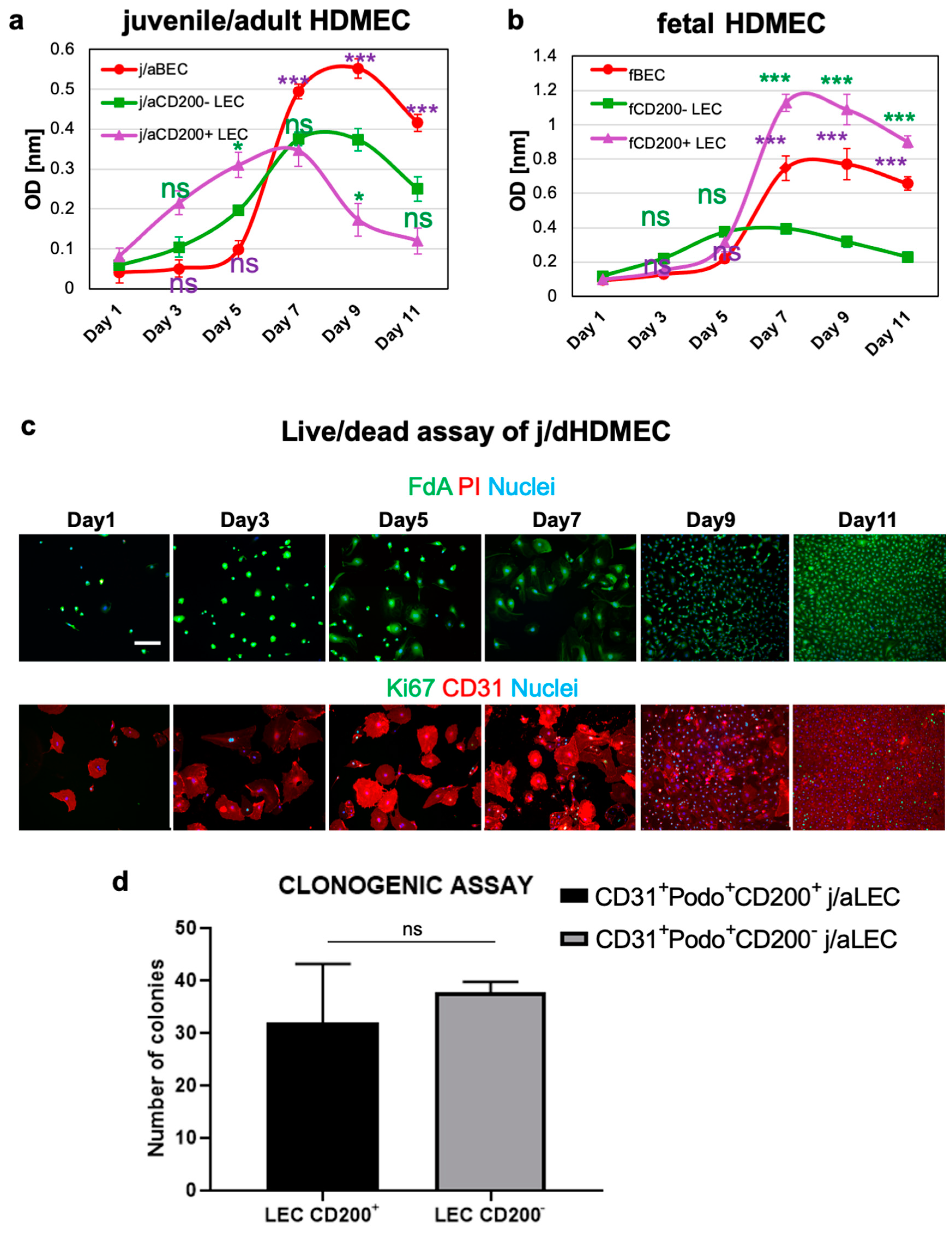
2.6. Clonogenic Assay
2.7. qRT-PCR
2.8. Preparation of vascDESS and Non-vascDESS
2.9. Transplantation of Tissue-Engineered Skin Substitutes
2.10. Quantification of CD200 and CD200R on Blood and Lymphatic Capillaries In Vivo
2.11. Statistical Analysis
3. Results
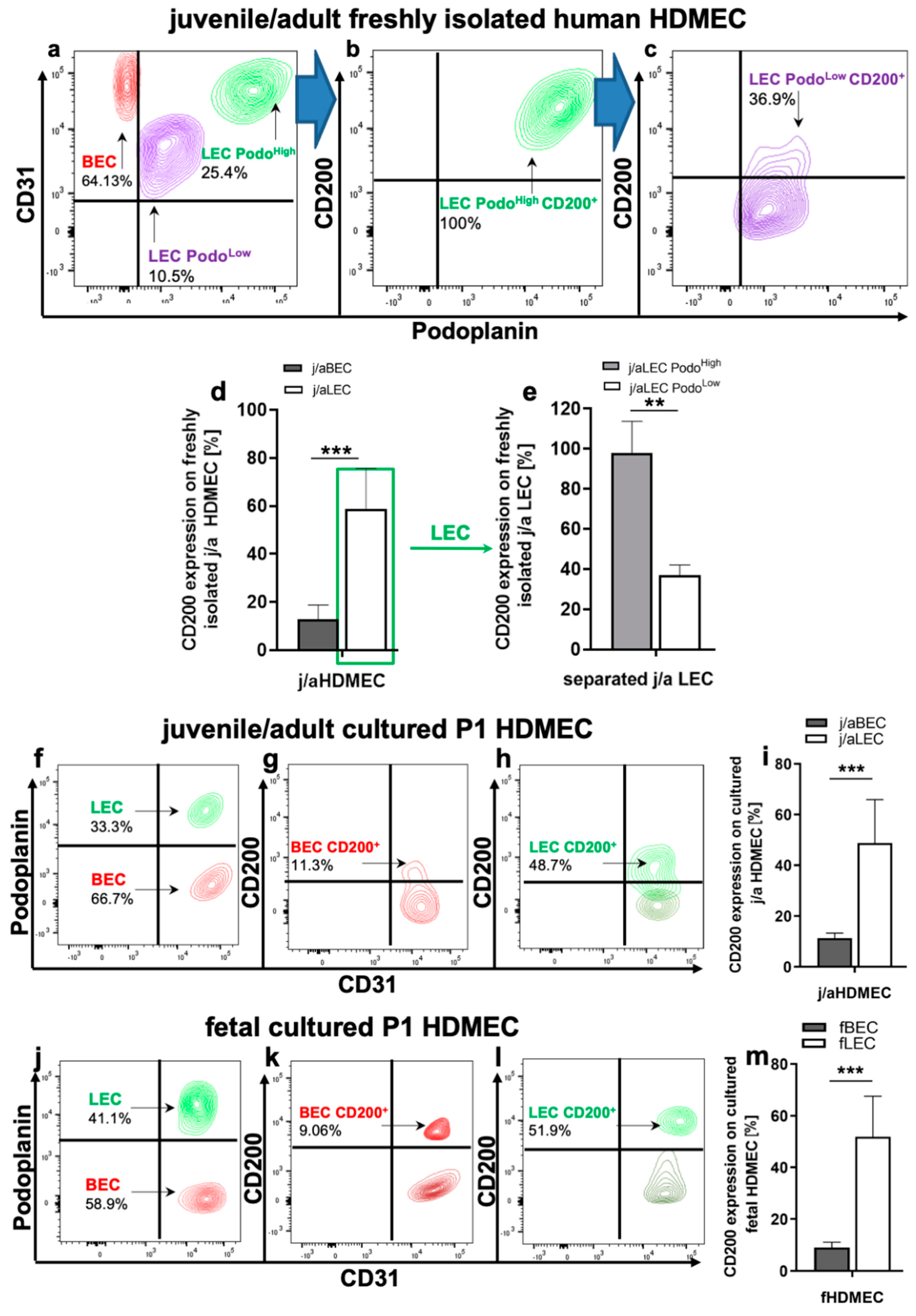
3.1. CD200 Is Expressed in Normal Human Juvenile/Adult and Fetal Skin
3.2. CD200 Is Differently Expressed on j/a and Fetal HDMEC Populations In Vitro
3.3. CD200+ LEC Demonstrate Higher Proliferation Rate Than CD200− LEC and BEC In Vitro
3.4. Specific Adhesion Molecules, Lymphatic Markers, and Chemokines Are Differently Expressed in Distinct HDMEC Populations In Vitro
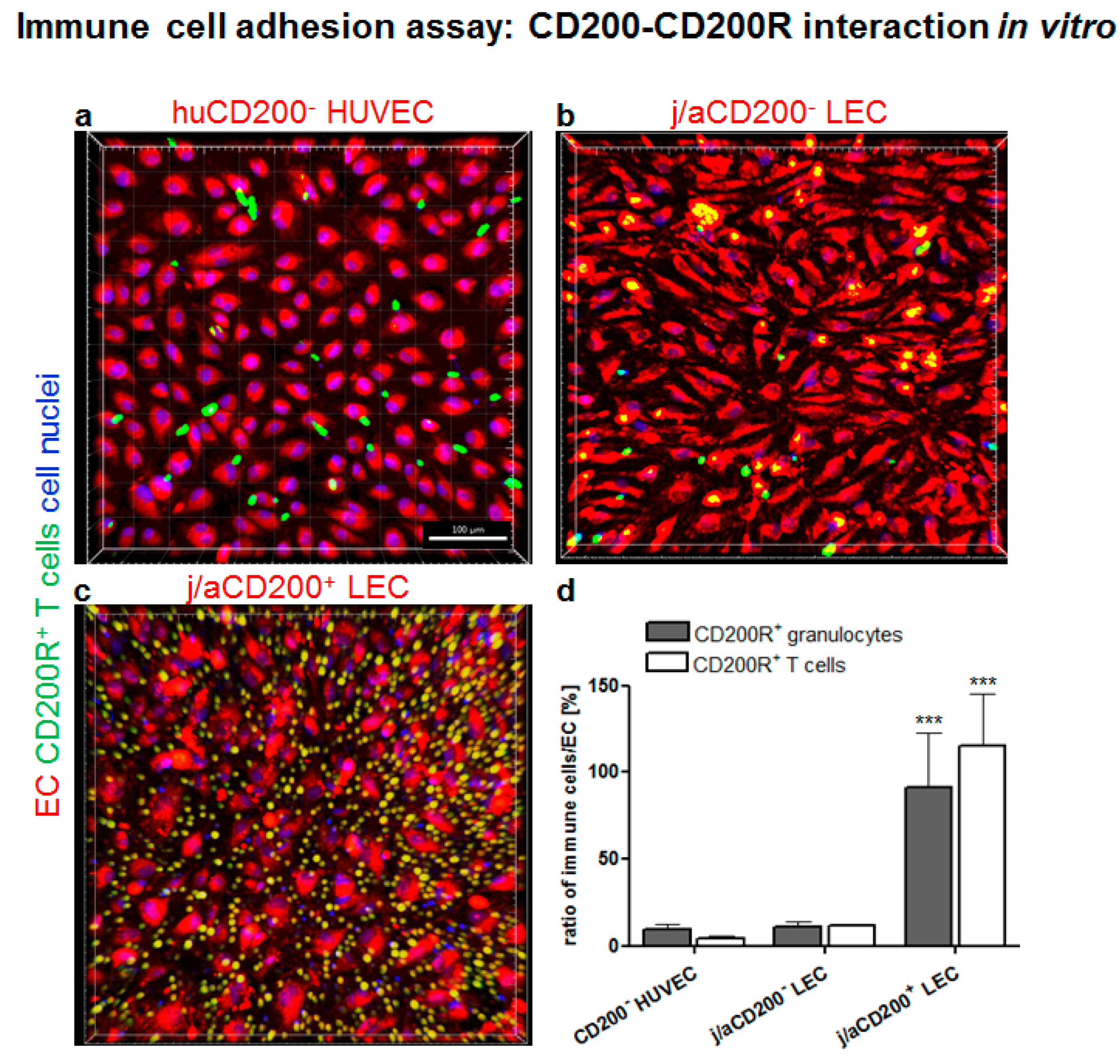
3.5. CD200R Is Expressed on Distinct Subsets of Human and Rat Immune Cells
3.6. Role of CD200 and CD200R in Endothelial-Immune Cell–Cell Interactions In Vitro
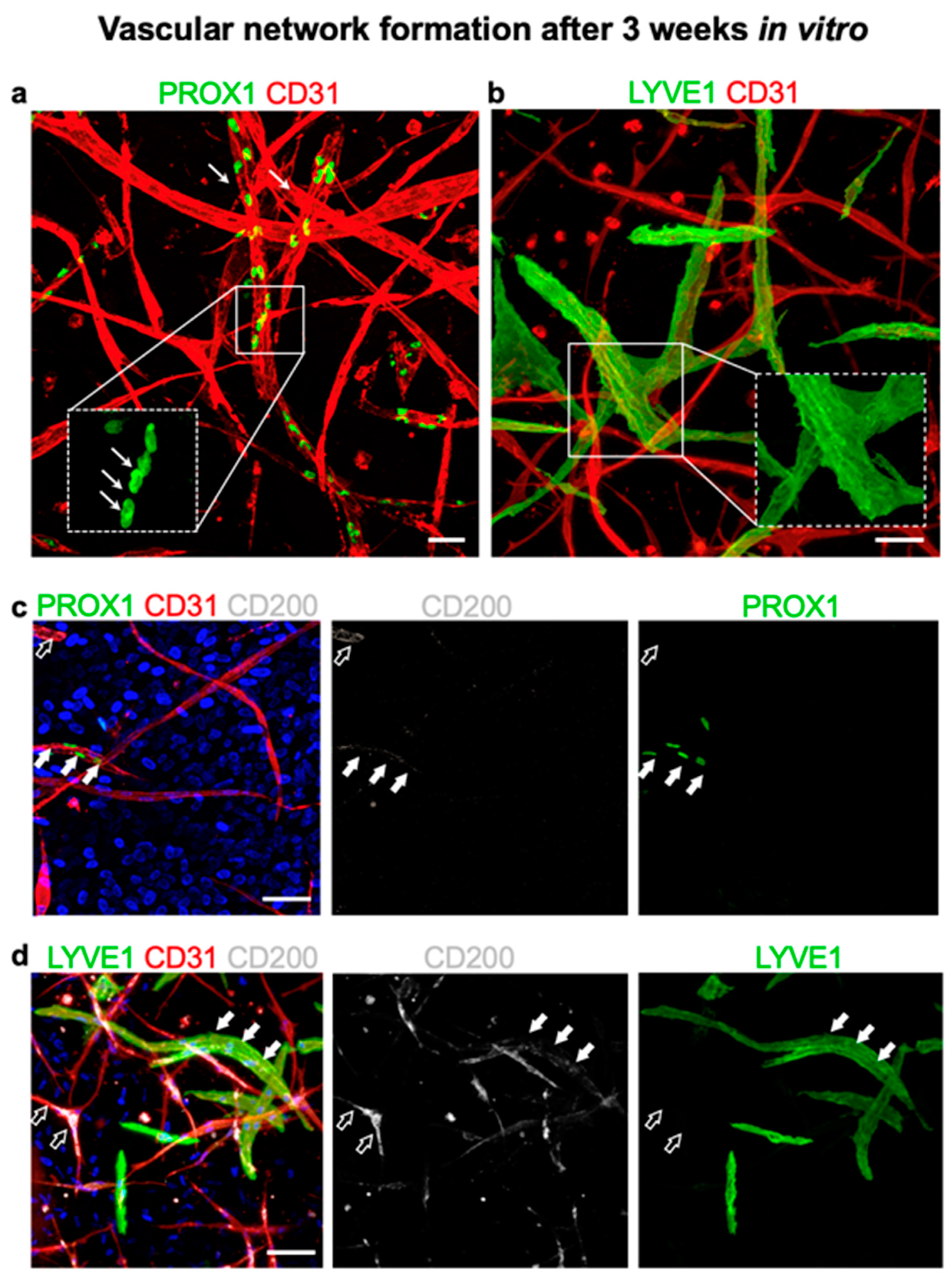
3.7. CD200 Is Expressed on Cultured Capillaries in 3D Hydrogels In Vitro
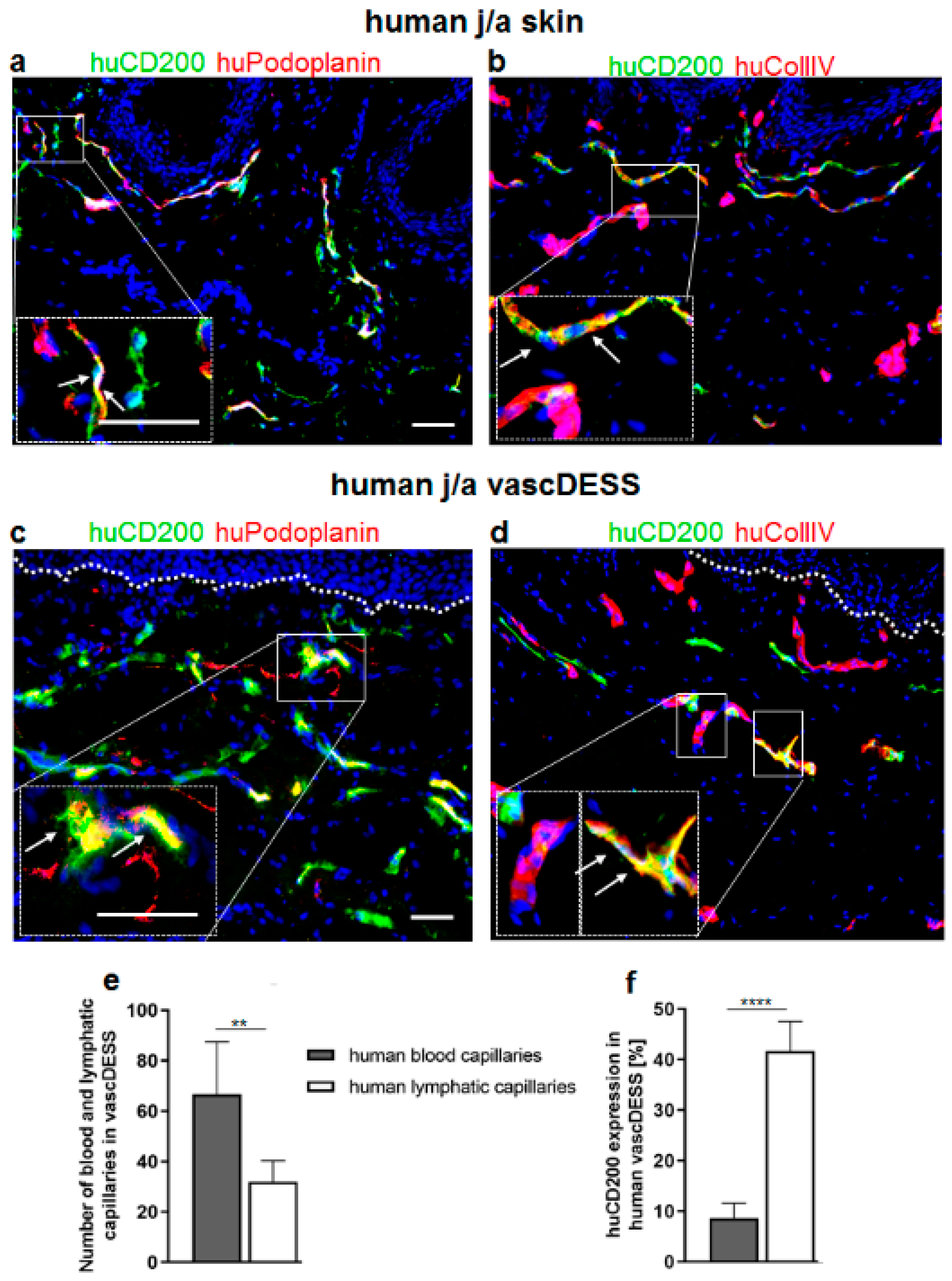
3.8. CD200 Is Expressed on Capillaries of Human Prevascularized DESS In Vivo
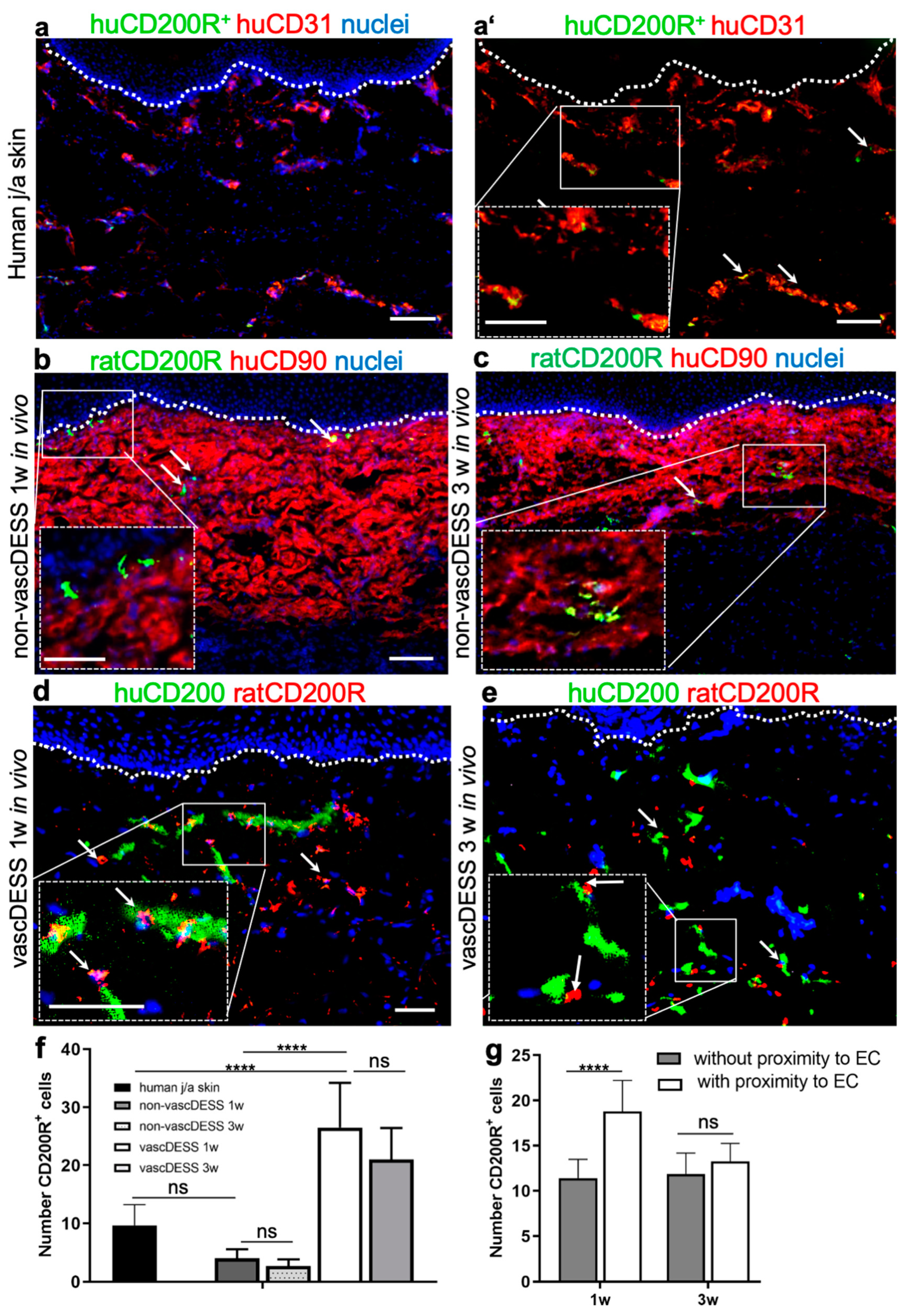
3.9. CD200 Binds to Its Cognitive Receptor, CD200R in Human Skin and Skin Substitutes In Vivo
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Biedermann, T.; Boettcher-Haberzeth, S.; Reichmann, E. Tissue Engineering of Skin for Wound Coverage. Eur. J. Pediatr. Surg. 2013, 23, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Biedermann, T.; Bottcher-Haberzeth, S.; Klar, A.S.; Widmer, D.S.; Pontiggia, L.; Weber, A.D.; Weber, D.M.; Schiestl, C.; Meuli, M.; Reichmann, E. The Influence of Stromal Cells on the Pigmentation of Tissue-Engineered Dermo-Epidermal Skin Grafts. Tissue Eng. Part A 2015, 21, 960–969. [Google Scholar] [CrossRef] [Green Version]
- Bottcher-Haberzeth, S.; Biedermann, T.; Klar, A.S.; Widmer, D.S.; Neuhaus, K.; Schiestl, C.; Meuli, M.; Reichmann, E. Characterization of pigmented dermo-epidermal skin substitutes in a long-term in vivo assay. Exp. Dermatol. 2015, 24, 16–21. [Google Scholar] [CrossRef]
- Bottcher-Haberzeth, S.; Klar, A.S.; Biedermann, T.; Schiestl, C.; Meuli-Simmen, C.; Reichmann, E.; Meuli, M. “Trooping the color”: Restoring the original donor skin color by addition of melanocytes to bioengineered skin analogs. Pediatr. Surg. Int. 2013, 29, 239–247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klar, A.S.; Biedermann, T.; Michalak, K.; Michalczyk, T.; Meuli-Simmen, C.; Scherberich, A.; Meuli, M.; Reichmann, E. Human Adipose Mesenchymal Cells Inhibit Melanocyte Differentiation and the Pigmentation of Human Skin via Increased Expression of TGF-beta1. J. Invest. Dermatol. 2017, 137, 2560–2569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klar, A.S.; Guven, S.; Biedermann, T.; Luginbuhl, J.; Bottcher-Haberzeth, S.; Meuli-Simmen, C.; Meuli, M.; Martin, I.; Scherberich, A.; Reichmann, E. Tissue-engineered dermo-epidermal skin grafts prevascularized with adipose-derived cells. Biomaterials 2014, 35, 5065–5078. [Google Scholar] [CrossRef]
- Klar, A.S.; Biedermann, T.; Simmen-Meuli, C.; Reichmann, E.; Meuli, M. Comparison of in vivo immune responses following transplantation of vascularized and non-vascularized human dermo-epidermal skin substitutes. Pediatr. Surg. Int. 2017, 33, 377–382. [Google Scholar] [CrossRef]
- Klar, A.S.; Bottcher-Haberzeth, S.; Biedermann, T.; Michalak, K.; Kisiel, M.; Reichmann, E.; Meuli, M. Differential expression of granulocyte, macrophage, and hypoxia markers during early and late wound healing stages following transplantation of tissue-engineered skin substitutes of human origin. Pediatr. Surg. Int. 2014, 30, 1257–1264. [Google Scholar] [CrossRef]
- Tremblay, P.L.; Hudon, V.; Berthod, F.; Germain, L.; Auger, F.A. Inosculation of tissue-engineered capillaries with the host's vasculature in a reconstructed skin transplanted on mice. Am. J. Transplant. 2005, 5, 1002–1010. [Google Scholar] [CrossRef]
- MacNeil, S. Progress and opportunities for tissue-engineered skin. Nature 2007, 445, 874–880. [Google Scholar] [CrossRef]
- Marino, D.; Luginbuhl, J.; Scola, S.; Meuli, M.; Reichmann, E. Bioengineering dermo-epidermal skin grafts with blood and lymphatic capillaries. Sci. Transl. Med. 2014, 6, 221ra214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montano, I.; Schiestl, C.; Schneider, J.; Pontiggia, L.; Luginbuhl, J.; Biedermann, T.; Bottcher-Haberzeth, S.; Braziulis, E.; Meuli, M.; Reichmann, E. Formation of human capillaries in vitro: The engineering of prevascularized matrices. Tissue Eng. Part A 2010, 16, 269–282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Minas, K.; Liversidge, J. Is the CD200/CD200 receptor interaction more than just a myeloid cell lnhibitory signal? Crit. Rev. Immunol. 2006, 26, 213–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorczynski, R.; Chen, Z.; Kai, Y.; Lee, L.; Wong, S.; Marsden, P.A. CD200 is a ligand for all members of the CD200R family of immunoregulatory molecules. J. Immunol. 2004, 172, 7744–7749. [Google Scholar] [CrossRef]
- Hoek, R.M.; Ruuls, S.R.; Murphy, C.A.; Wright, G.J.; Goddard, R.; Zurawski, S.M.; Blom, B.; Homola, M.E.; Streit, W.J.; Brown, M.H.; et al. Down-regulation of the macrophage lineage through interaction with OX2 (CD200). Science 2000, 290, 1768–1771. [Google Scholar] [CrossRef] [PubMed]
- Copland, D.A.; Calder, C.J.; Raveney, B.J.; Nicholson, L.B.; Phillips, J.; Cherwinski, H.; Jenmalm, M.; Sedgwick, J.D.; Dick, A.D. Monoclonal antibody-mediated CD200 receptor signaling suppresses macrophage activation and tissue damage in experimental autoimmune uveoretinitis. Am. J. Pathol. 2007, 171, 580–588. [Google Scholar] [CrossRef] [Green Version]
- Preston, S.; Wright, G.J.; Starr, K.; Barclay, A.N.; Brown, M.H. The leukocyte/neuron cell surface antigen OX2 binds to a ligand on macrophages. Eur. J. Immunol. 1997, 27, 1911–1918. [Google Scholar] [CrossRef]
- Meuth, S.G.; Simon, O.J.; Grimm, A.; Melzer, N.; Herrmann, A.M.; Spitzer, P.; Landgraf, P.; Wiendl, H. CNS inflammation and neuronal degeneration is aggravated by impaired CD200-CD200R-mediated macrophage silencing. J. Neuroimmunol. 2008, 194, 62–69. [Google Scholar] [CrossRef]
- Ko, Y.C.; Chien, H.F.; Jiang-Shieh, Y.F.; Chang, C.Y.; Pai, M.H.; Huang, J.P.; Chen, H.M.; Wu, C.H. Endothelial CD200 is heterogeneously distributed, regulated and involved in immune cell-endothelium interactions. J. Anat. 2009, 214, 183–195. [Google Scholar] [CrossRef]
- Rosenblum, M.D.; Olasz, E.B.; Yancey, K.B.; Woodliff, J.E.; Lazarova, Z.; Gerber, K.A.; Truitt, R.L. Expression of CD200 on epithelial cells of the murine hair follicle: A role in tissue-specific immune tolerance? J. Investig. Dermatol. 2004, 123, 880–887. [Google Scholar] [CrossRef] [Green Version]
- Wright, G.J.; Cherwinski, H.; Foster-Cuevas, M.; Brooke, G.; Puklavec, M.J.; Bigler, M.; Song, Y.; Jenmalm, M.; Gorman, D.; McClanahan, T.; et al. Characterization of the CD200 receptor family in mice and humans and their interactions with CD200. J. Immunol. 2003, 171, 3034–3046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Broderick, C.; Hoek, R.M.; Forrester, J.V.; Liversidge, J.; Sedgwick, J.D.; Dick, A.D. Constitutive retinal CD200 expression regulates resident microglia and activation state of inflammatory cells during experimental autoimmune uveoretinitis. Am. J. Pathol. 2002, 161, 1669–1677. [Google Scholar] [CrossRef] [Green Version]
- Christine, A.; Vaine, R.J.S. Chapter Five—The CD200–CD200R1 Inhibitory Signaling Pathway: Immune Regulation and Host–Pathogen Interactions. Adv. Immunol. 2014, 121, 191–211. [Google Scholar] [CrossRef]
- Belkin, D.A.; Mitsui, H.; Wang, C.Q.; Gonzalez, J.; Zhang, S.; Shah, K.R.; Coats, I.; Suarez-Farinas, M.; Krueger, J.G.; Felsen, D.; et al. CD200 upregulation in vascular endothelium surrounding cutaneous squamous cell carcinoma. JAMA Dermatol. 2013, 149, 178–186. [Google Scholar] [CrossRef] [Green Version]
- Stumpfova, M.; Ratner, D.; Desciak, E.B.; Eliezri, Y.D.; Owens, D.M. The Immunosuppressive Surface Ligand CD200 Augments the Metastatic Capacity of Squamous Cell Carcinoma. Cancer Res. 2010, 70, 2962–2972. [Google Scholar] [CrossRef] [Green Version]
- Kretz-Rommel, A.; Qin, F.; Dakappagari, N.; Ravey, E.P.; McWhirter, J.; Oltean, D.; Frederickson, S.; Maruyama, T.; Wild, M.A.; Nolan, M.J.; et al. CD200 expression on tumor cells suppresses antitumor immunity: New approaches to cancer immunotherapy. J. Immunol. 2007, 178, 5595–5605. [Google Scholar] [CrossRef] [Green Version]
- Tonks, A.; Hills, R.; White, P.; Rosie, B.; Mills, K.I.; Burnett, A.K.; Darley, R.L. CD200 as a prognostic factor in acute myeloid leukaemia. Leukemia 2007, 21, 566–568. [Google Scholar] [CrossRef]
- Petermann, K.B.; Rozenberg, G.I.; Zedek, D.; Groben, P.; McKinnon, K.; Buehler, C.; Kim, W.Y.; Shields, J.M.; Penland, S.; Bear, J.E.; et al. CD200 is induced by ERK and is a potential therapeutic target in melanoma. J. Clin. Investig. 2007, 117, 3922–3929. [Google Scholar] [CrossRef] [Green Version]
- Moreaux, J.; Hose, D.; Reme, T.; Jourdan, E.; Hundemer, M.; Legouffe, E.; Moine, P.; Bourin, P.; Moos, M.; Corre, J.; et al. CD200 is a new prognostic factor in multiple myeloma. Blood 2006, 108, 4194–4197. [Google Scholar] [CrossRef]
- Schwartzfarb, E.; Kirsner, R.S. Understanding scarring: Scarless fetal wound healing as a model. J. Investig. Dermatol. 2012, 132, 260. [Google Scholar] [CrossRef] [Green Version]
- Larson, B.J.; Longaker, M.T.; Lorenz, H.P. Scarless fetal wound healing: A basic science review. Plast. Reconstr. Surg. 2010, 126, 1172–1180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Walraven, M.; Talhout, W.; Beelen, R.H.; van Egmond, M.; Ulrich, M.M. Healthy human second-trimester fetal skin is deficient in leukocytes and associated homing chemokines. Wound Repair Regen. 2016, 24, 533–541. [Google Scholar] [CrossRef] [PubMed]
- Pontiggia, L.; Biedermann, T.; Meuli, M.; Widmer, D.; Bottcher-Haberzeth, S.; Schiestl, C.; Schneider, J.; Braziulis, E.; Montano, I.; Meuli-Simmen, C.; et al. Markers to evaluate the quality and self-renewing potential of engineered human skin substitutes in vitro and after transplantation. J. Investig. Dermatol. 2009, 129, 480–490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nolan, T.; Hands, R.E.; Bustin, S.A. Quantification of mRNA using real-time RT-PCR. Nat. Protoc. 2006, 1, 1559–1582. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Rao, X.; Huang, X.; Zhou, Z.; Lin, X. An improvement of the 2^(-delta delta CT) method for quantitative real-time polymerase chain reaction data analysis. Biostat. Bioinform. Biomath. 2013, 3, 71–85. [Google Scholar] [CrossRef]
- Svec, D.; Tichopad, A.; Novosadova, V.; Pfaffl, M.W.; Kubista, M. How good is a PCR efficiency estimate: Recommendations for precise and robust qPCR efficiency assessments. Biomol. Detect. Quantif. 2015, 3, 9–16. [Google Scholar] [CrossRef] [Green Version]
- Illescas-Montes, R.; Melguizo-Rodriguez, L.; Garcia-Martinez, O.; de Luna-Bertos, E.; Manzano-Moreno, F.J.; Ruiz, C.; Ramos-Torrecillas, J. Human Fibroblast Gene Expression Modulation Using 940 NM Diode Laser. Sci. Rep. 2019, 9, 12037. [Google Scholar] [CrossRef]
- Pontikoglou, C.; Langonne, A.; Ba, M.A.; Varin, A.; Rosset, P.; Charbord, P.; Sensebe, L.; Deschaseaux, F. CD200 expression in human cultured bone marrow mesenchymal stem cells is induced by pro-osteogenic and pro-inflammatory cues. J. Cell. Mol. Med. 2016, 20, 655–665. [Google Scholar] [CrossRef]
- Wakabayashi, T.; Naito, H.; Suehiro, J.; Lin, Y.; Kawaji, H.; Iba, T.; Kouno, T.; Ishikawa-Kato, S.; Furuno, M.; Takara, K.; et al. CD157 Marks Tissue-Resident Endothelial Stem Cells with Homeostatic and Regenerative Properties. Cell Stem Cell 2018, 22, 384–395. [Google Scholar] [CrossRef] [Green Version]
- Gorczynski, R.M.; Hadidi, S.; Yu, G.; Clark, D.A. The same immunoregulatory molecules contribute to successful pregnancy and transplantation. Am. J. Reprod. Immunol. 2002, 48, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, M.P.; Lin, Y.; Maas, M.L.; Van Keulen, V.P.; Johnston, P.B.; Peikert, T.; Gastineau, D.A.; Dietz, A.B. A Method for Identification and Analysis of Non-Overlapping Myeloid Immunophenotypes in Humans. PLoS ONE 2015, 10, e0121546. [Google Scholar] [CrossRef]
- Pitcher, L.A.; van Oers, N.S.C. T-cell receptor signal transmission: Who gives an ITAM? Trends Immunol. 2003, 24, 554–560. [Google Scholar] [CrossRef] [PubMed]
- Miyasaka, M.; Tanaka, T. Lymphocyte trafficking across high endothelial venules: Dogmas and enigmas. Nat. Rev. Immunol. 2004, 4, 360–370. [Google Scholar] [CrossRef] [PubMed]
- Luster, A.D.; Alon, R.; von Andrian, U.H. Immune cell migration in inflammation: Present and future therapeutic targets. Nat. Immunol. 2005, 6, 1182–1190. [Google Scholar] [CrossRef]
- Ruddle, N.H. High Endothelial Venules and Lymphatic Vessels in Tertiary Lymphoid Organs: Characteristics, Functions, and Regulation. Front. Immunol. 2016, 7, 491. [Google Scholar] [CrossRef] [Green Version]
- Acton, S.E.; Astarita, J.L.; Malhotra, D.; Lukacs-Kornek, V.; Franz, B.; Hess, P.R.; Jakus, Z.; Kuligowski, M.; Fletcher, A.L.; Elpek, K.G.; et al. Podoplanin-Rich Stromal Networks Induce Dendritic Cell Motility via Activation of the C-type Lectin Receptor CLEC-2. Immunity 2012, 37, 276–289. [Google Scholar] [CrossRef] [Green Version]
- Herzog, B.H.; Fu, J.X.; Wilson, S.J.; Hess, P.R.; Sen, A.; McDaniel, J.M.; Pan, Y.F.; Sheng, M.J.; Yago, T.; Silasi-Mansat, R.; et al. Podoplanin maintains high endothelial venule integrity by interacting with platelet CLEC-2. Nature 2013, 502, 105–109. [Google Scholar] [CrossRef] [Green Version]
- Gorczynski, R.M.; Cattral, M.S.; Chen, Z.G.; Hu, J.A.; Lei, J.; Min, W.P.; Yu, G.; Ni, J. An immunoadhesin incorporating the molecule OX-2 is a potent immunosuppressant that prolongs allo- and xenograft survival. J. Immunol. 1999, 163, 1654–1660. [Google Scholar] [CrossRef]
- Koning, N.; van Eijk, M.; Pouwels, W.; Brouwer, M.S.M.; Voehringer, D.; Huitinga, I.; Hoek, R.M.; Raes, G.; Hamann, J. Expression of the Inhibitory CD200 Receptor Is Associated with Alternative Macrophage Activation. J. Innate Immun. 2010, 2, 195–200. [Google Scholar] [CrossRef] [Green Version]
- Taylor, N.; McConnachie, K.; Calder, C.; Dawson, R.; Dick, A.; Sedgwick, J.D.; Liversidge, J. Enhanced tolerance to autoimmune uveitis in CD200-deficient mice correlates with a pronounced Th2 switch in response to antigen challenge. J. Immunol. 2005, 174, 3818. [Google Scholar] [CrossRef]
- Zhang, S.L.; Cherwinski, H.; Sedgwick, J.D.; Phillips, J.H. Molecular mechanisms of CD200 inhibition of mast cell activation. J. Immunol. 2004, 173, 6786–6793. [Google Scholar] [CrossRef] [Green Version]
- Fallarino, F.; Asselin-Paturel, C.; Vacca, C.; Bianchi, R.; Gizzi, S.; Fioretti, M.C.; Trinchieri, G.; Grohmann, U.; Puccetti, P. Murine plasmacytoid dendritic cells initiate the immunosuppressive pathway of tryptophan catabolism in response to CD200 receptor engagement. J. Immunol. 2004, 173, 3748–3754. [Google Scholar] [CrossRef] [Green Version]
- Ngwa, C.; Liu, F. CD200-CD200R signaling and diseases: A potential therapeutic target? Int. J. Physiol. Pathophysiol. Pharmacol. 2019, 11, 297–309. [Google Scholar]
- Gorczynski, R.M.; Chen, Z.Q.; Yu, K.; Hu, J. CD200 immunoadhesin suppresses collagen-induced arthritis in mice. Clin. Immunol. 2001, 101, 328–334. [Google Scholar] [CrossRef]
- Chitnis, T.; Imitola, J.; Wang, Y.; Elyaman, W.; Chawla, P.; Sharuk, M.; Raddassi, K.; Bronson, R.T.; Khoury, S.J. Elevated neuronal expression of CD200 protects Wld(s) mice from inflammation-mediated neurodegeneration. Am. J. Pathol. 2007, 170, 1695–1712. [Google Scholar] [CrossRef] [Green Version]
- Wright, G.J.; Puklavec, M.J.; Willis, A.C.; Hoek, R.M.; Sedgwick, J.D.; Brown, M.H.; Barclay, A.N. Lymphoid/neuronal cell surface OX2 glycoprotein recognizes a novel receptor on macrophages implicated in the control of their function. Immunity 2000, 13, 233–242. [Google Scholar] [CrossRef] [Green Version]
- Kotwica-Mojzych, K.; Jodlowska-Jedrych, B.; Mojzych, M. CD200:CD200R Interactions and Their Importance in Immunoregulation. Int. J. Mol. Sci. 2021, 22, 1602. [Google Scholar] [CrossRef]
- Klar, A.S.; Guven, S.; Zimoch, J.; Zapiorkowska, N.A.; Biedermann, T.; Bottcher-Haberzeth, S.; Meuli-Simmen, C.; Martin, I.; Scherberich, A.; Reichmann, E.; et al. Characterization of vasculogenic potential of human adipose-derived endothelial cells in a three-dimensional vascularized skin substitute. Pediatr. Surg. Int. 2016, 32, 17–27. [Google Scholar] [CrossRef]
- Klar, A.S.; Michalak-Micka, K.; Biedermann, T.; Simmen-Meuli, C.; Reichmann, E.; Meuli, M. Characterization of M1 and M2 polarization of macrophages in vascularized human dermo-epidermal skin substitutes in vivo. Pediatr. Surg. Int. 2018, 34, 129–135. [Google Scholar] [CrossRef]


| Antibody Conjugate | Clone | Product Number | Manufacturer | Dilution |
|---|---|---|---|---|
| CD15-PE (SSEA-1) | W6D3 | 323006 | BioLegend, Switzerland | 1:20 |
| PE Mouse IgG1, κ Isotype Ctrl | MOPC-21 | 400114 | BioLegend, Switzerland | 1:20 |
| CD56-APC | MEM-188 | 304610 | BioLegend, Switzerland | 1:20 |
| APC Mouse IgG2a, κ Isotype Ctrl | MOPC-173 | 400220 | BioLegend, Switzerland | 1:20 |
| CD3-FITC | UCHT1 | 300452 | BioLegend, Switzerland | 1:50 |
| FITC Mouse IgG1, κ Isotype Ctrl | MOPC-21 | 400108 | BioLegend, Switzerland | 1:50 |
| CD14-FITC | M5E2 | 555397 | BD Biosciences, Switzerland | 1:50 |
| CD200-AF647 | OX-104 | 329214 | BioLegend, Switzerland | 1:50 |
| Alexa Fluor 647 Mouse IgG1, κ Isotype Ctrl | MOPC-21 | 400130 | BioLegend, Switzerland | 1:50 |
| CD200R-APC | OX-108 | 329308 | BioLegend, Switzerland | 1:20 |
| APC Mouse IgG1, κ Isotype Ctrl | MOPC-21 | 400122 | BioLegend, Switzerland | 1:20 |
| CD31-PE | WM59 | 555446 | BD Biosciences, Switzerland | 1:20 |
| PE Mouse IgG1, κ Isotype Control | MOPC-21 | 555749 | BD Biosciences, Switzerland | 1:50 |
| Podoplanin-AF488 | NC-08 | 337006 | BioLegend, Switzerland | 1:50 |
| Alexa Fluor® 488 Rat IgG2a, κ Isotype Ctrl | RTK2758 | 400525 | BioLegend, Switzerland | 1:50 |
| ZombieAqua Fixable Viability Kit | - | 423102 | BioLegend, Switzerland | 1:500 |
| Antibody | Clone | Product Number | Manufacturer | Dilution |
|---|---|---|---|---|
| Anti-human | ||||
| CD31 | JC70A | M082301-2 | Dako, USA | 1:50 |
| CD31-PE | WM59 | 555446 | BD Biosciences, Switzerland | 1:50 |
| CD90 | AS02 | CP28-200UG | Merck Millipore, Germany | 1:100 |
| CD200 | OX-104 | 329202 | BioLegend, Switzerland | 1:50 |
| CD200-AF647 | OX-104 | 329214 | BioLegend, Switzerland | 1:50 |
| CD200R | OX-108 | 329302 | BioLegend, Switzerland | 1:50 |
| CD200R-APC | OX-108 | 329308 | BioLegend, Switzerland | 1:20 |
| Collagen IV | COL-94 | ab6311 | Abcam, UK | 1:200 |
| Ki67 | B56 | 550609 | BD Biosciences, Switzerland | 1:100 |
| LYVE1 | polyclonal | Ab10278 | Abcam, UK | 1:100 |
| Podoplanin | 18H5 | sc-59347 | Santa Cruz, USA | 1:100 |
| Podoplanin-AF488 | NC-08 | 337006 | BioLegend, Switzerland | 1:50 |
| Podoplanin-AF647 | NC-08 | 337008 | BioLegend, Switzerland | 1:20 |
| PROX1 | polyclonal | 102-PA32S | ReliaTech, Germany | 1:100 |
| Anti-rat | ||||
| Anti-rat Granulocytes | HIS48 | sc-19613 | Santa Cruz, USA | 1:100 |
| Anti-rat Myeloid Lineage Antibody-FITC | OX-82 | 205103 | BioLegend, Switzerland | 1:50 |
| CD68 | PG-M1 | Ab783 | Abcam, UK | 1:100 |
| CD200R-FITC | OX-102 | 204905 | BioLegend, Switzerland | 1:20 |
| Secondary antibodies | ||||
| Donkey anti-mouse IgG H&L AF488 | ab150105 | Abcam, UK | 1:400 | |
| Donkey anti-rabbit IgG H&L AF488 | ab150073 | Abcam, UK | 1:200 | |
| Donkey anti-mouse IgG H&L AF568 | ab175472 | Abcam, UK | 1:400 | |
| Anti-rabbit IgG H&L AF568 | ab175470 | Abcam, UK | 1:200 | |
| Anti-mouse IgG H&L AF647 | ab150107 | Abcam, UK | 1:200 | |
| Gene Name | Forward | Reverse |
|---|---|---|
| CCL21 | CCATCCCAGCTATCCTGTTCTT | TTCTGTGGGGATGGTGTCTTG |
| CCL27 | CAGACCCTACAGCAGCATTCC | CACGAAAGCCTGGAGGTGAC |
| CD62P | CCCGAGTCCTTAAGGTTTCCAT | GGAAACAGGGTTGGTCCAGA |
| CD54 | CAGTGACCATCTACAGCTTTCC | CATTCAGCGTCACCTTGGCT |
| VCAM1 | GGAAATGACCTTCATCCCTACCA | ATCTCTGGGGGCAACATTGA |
| ICAM2 | GATTTTGGCAGTGTCGAGGTCT | GGAGCCTGAGGTGTTTCACTTT |
| F11R | CGAGGCCACTTTGACAGAACA | CCTTCACTTCGGGCACTAGG |
| TGFBeta1 | TGAACCGGCCTTTCCTGCTTCTCATG | GCGGAAGTCAATGTACAGCTGCCGC |
| CD200/OX2 | CCTAAGAATCAGGTGGGGAAGGA | GACGAGAAGAATTACCAGGGAAACA |
| CD157/BST1 | CGCACACTTGCGGGACATC | AGTTCTTGTTCCGCTGCTCG |
| Podoplanin | AAGAGCTGAAGGGTTACGCC | CACGGGTCATCTTCTCCCAC |
| Prox1 | AAGCAAATGACTTTGAGGTTCC | CAGCTTGCAGATGACCTTGT |
| GAPDH | AGTCAGCCGCATCTTCTTTT | CCAATACGACCAAATCCGTTG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rütsche, D.; Michalak-Micka, K.; Zielinska, D.; Moll, H.; Moehrlen, U.; Biedermann, T.; Klar, A.S. The Role of CD200–CD200 Receptor in Human Blood and Lymphatic Endothelial Cells in the Regulation of Skin Tissue Inflammation. Cells 2022, 11, 1055. https://doi.org/10.3390/cells11061055
Rütsche D, Michalak-Micka K, Zielinska D, Moll H, Moehrlen U, Biedermann T, Klar AS. The Role of CD200–CD200 Receptor in Human Blood and Lymphatic Endothelial Cells in the Regulation of Skin Tissue Inflammation. Cells. 2022; 11(6):1055. https://doi.org/10.3390/cells11061055
Chicago/Turabian StyleRütsche, Dominic, Katarzyna Michalak-Micka, Dominika Zielinska, Hannah Moll, Ueli Moehrlen, Thomas Biedermann, and Agnes S. Klar. 2022. "The Role of CD200–CD200 Receptor in Human Blood and Lymphatic Endothelial Cells in the Regulation of Skin Tissue Inflammation" Cells 11, no. 6: 1055. https://doi.org/10.3390/cells11061055
APA StyleRütsche, D., Michalak-Micka, K., Zielinska, D., Moll, H., Moehrlen, U., Biedermann, T., & Klar, A. S. (2022). The Role of CD200–CD200 Receptor in Human Blood and Lymphatic Endothelial Cells in the Regulation of Skin Tissue Inflammation. Cells, 11(6), 1055. https://doi.org/10.3390/cells11061055







