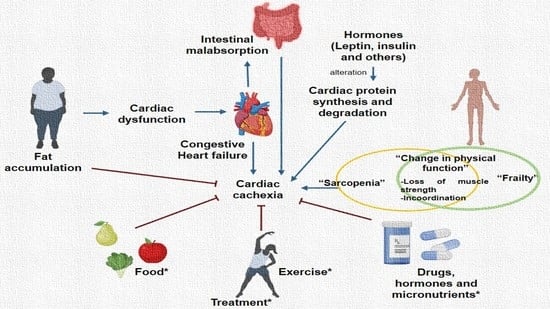
Interconnection between Cardiac Cachexia and Heart Failure—Protective Role of Cardiac Obesity
Abstract
1. Introduction
2. Cachexia in Heart Failure
Conditions Associated with Heart Failure and Cardiac Cachexia
3. Obesity, Cardiac Obesity and Cardiac Cachexia
4. Cardiac Cachexia, Heart Failure, Frailty and Aging
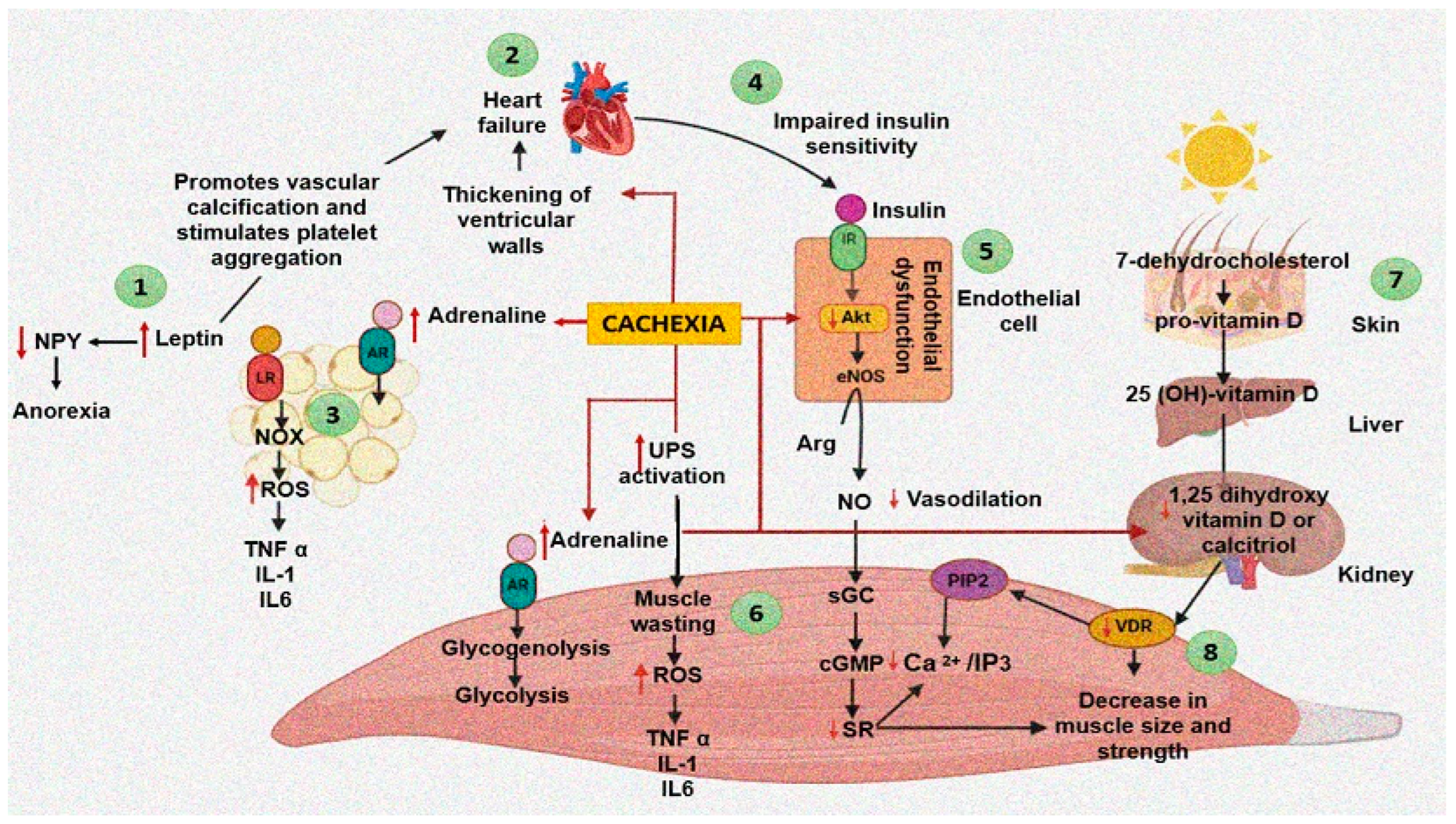
5. Pathogenesis of Cachexia
5.1. Cachexia and the Balance between Protein Synthesis and Breakdown in Heart Failure
5.2. Role of the Endocrine System in Heart Cachexia
5.3. Role of Micronutrients and Vitamin D in Cachexia
5.4. Inflammation in Cachexia
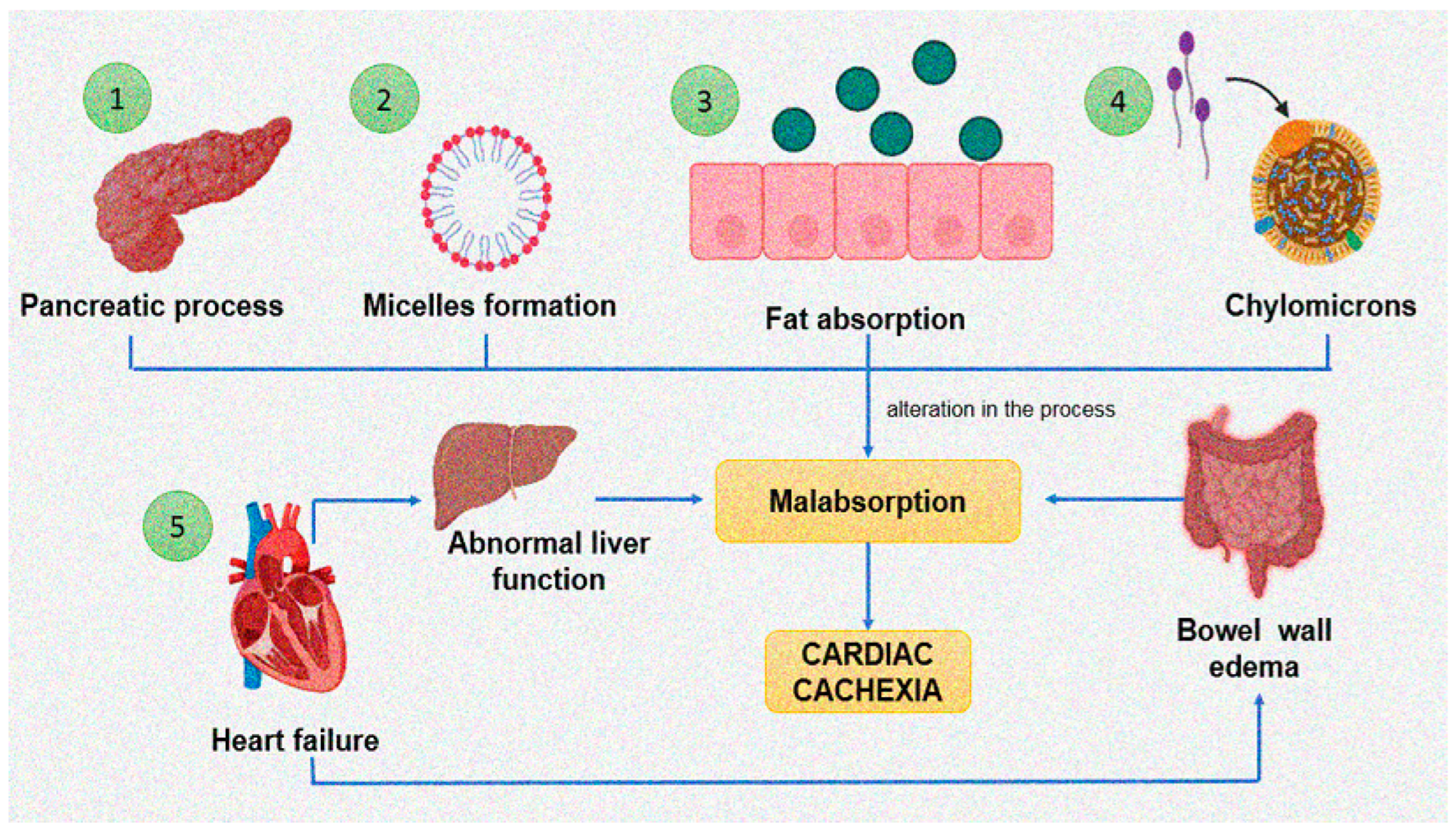
5.5. Intestinal Malabsorption in Cachexia
6. Timely Diagnosis of HF and Cachexia
7. Treatment of Cachexia
7.1. Nutritional Interventions
7.2. Micronutrients
7.3. Exercise
7.4. Treatment with Other Drugs
8. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Morley, J.E.; Thomas, D.R.; Wilson, M.M. Cachexia: Pathophysiology and clinical relevance. Am. J. Clin. Nutr. 2006, 83, 735–743. [Google Scholar] [CrossRef] [PubMed]
- von Haehling, S.; Anker, S.D. Prevalence, incidence and clinical impact of chaexia: Facts and numbers—Update 2014. J. Cachexia Sarcopenia Muscle 2014, 5, 261–263. [Google Scholar] [CrossRef] [PubMed]
- Morley, J.E.; Kalantar-Zadeh, K.; Anker, S.D. COVID-19: A major cause of cachexia and sarcopenia? J. Cachexia Sarcopenia Muscle 2020, 11, 863–865. [Google Scholar] [CrossRef] [PubMed]
- Anker, M.S.; Landmesser, U.; von Haehling, S.; Butler, J.; Coats, A.J.S.; Anker, S.D. Weight loss, malnutrition, and cachexia in COVID-19: Facts and numbers. J. Cachexia Sarcopenia Muscle 2021, 12, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Krysztofiak, H.; Wleklik, M.; Migaj, J.; Dudek, M.; Uchmanowicz, I.; Lisiak, M.; Kubielas, G.; Straburzyńska-Migaj, E.; Lesiak, M.; Kałużna-Oleksy, M. Cardiac Cachexia: A Well-known but challenging complication of heart failure. Clin. Interv. Aging 2020, 5, 2041–2051. [Google Scholar] [CrossRef] [PubMed]
- Selthofer-Relatić, K.; Kibel, A.; Delić-Brkljačić, D.; Bošnjak, I. Cardiac Obesity and Cardiac Cachexia: Is There a Pathophysiological Link? J. Obes. 2019, 2019, 9854085. [Google Scholar] [CrossRef]
- Mariotti, R.; Castrogiovanni, F.; Canale, M.L.; Borelli, G.; Rondinini, L. Weight loss and quality of life in chronic heart failure patients. J. Cardiovasc. Med. 2008, 9, 576–580. [Google Scholar] [CrossRef]
- Poirier, P.; Giles, T.D.; Bray, G.A.; Hong, Y.; Stern, J.S.; Pi-Sunyer, F.X.; Eckel, R.H. American Heart Association; Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Obesity and cardiovascular disease: Pathophysiology, evaluation, and effect of weight loss: An update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation 2006, 113, 898–918. [Google Scholar] [CrossRef]
- Flegal, K.M.; Ioannidis, J.P.A. The obesity paradox: A misleading term that should be abandoned. Obesity 2018, 26, 629–630. [Google Scholar] [CrossRef]
- Carbone, S.; Lavie, C.J.; Elagizi, A.; Arena, R.; Ventura, H.O. The impact of obesity in heart failure. Heart Fail. Clin. 2020, 16, 71–80. [Google Scholar] [CrossRef]
- Bays, H.; Dujovne, C.A. Adiposopathy is a more rational treatment target for metabolic disease than obesity alone. Curr. Atheroscler. Rep. 2006, 8, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Bowen, T.S.; Schuler, G.; Adams, V. Skeletal muscle wasting in cachexia and sarcopenia: Molecular pathophysiology and impact of exercise training. J. Cachexia Sarcopenia Muscle 2015, 6, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Evans, W.J.; Morley, J.E.; Argiles, J.; Bales, C.; Baracos, V.; Guttridge, D.; Jatoi, A.; Kalantar-Zadeh, K.; Lochs, H.; Mantovani, G.; et al. Cachexia: A new definition. Clin. Nutr. 2008, 27, 793–799. [Google Scholar] [CrossRef]
- Anker, S.D.; Negassa, A.; Coats, A.J.; Afzal, R.; Poole-Wilson, P.A.; Cohn, J.N.; Salim, Y. Prognostic importance of weight loss in chronic heart failure and the effect of treatment with angiotensin-converting-enzyme inhibitors: An observational study. Lancet 2003, 361, 1077–1083. [Google Scholar] [CrossRef]
- Florea, V.G.; Henein, M.Y.; Rauchhaus, M.; Koloczek, V.; Sharma, R.; Doehner, W.; Poole-Wilson, P.A.; Coats, A.J.; Anker, S.D. The cardiac component of cardiac cachexia. Am. Heart J. 2002, 144, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Goyal, P.; Almarzooq, Z.I.; Horn, E.M.; Karas, M.G.; Sobol, I.; Swaminathan, R.V.; Feldman, D.N.; Minutello, R.M.; Singh, H.S.; Bergman, G.W.; et al. Characteristics of hospitalizations for heart failure with preserved ejection fraction. Am. J. Med. 2016, 129, 635.e15–635.e26. [Google Scholar] [CrossRef] [PubMed]
- Falk, K.; Swedberg, K.; Gaston-Johansson, F.; Ekman, I. Fatigue is a prevalent and severe symptom associated with uncertainty and sense of coherence in patients with chronic heart failure. Eur. J. Cardiovasc. Nurs. 2007, 6, 99–104. [Google Scholar] [CrossRef]
- Evangelista, L.S.; Moser, D.K.; Westlake, C.; Pike, N.; Ter-Galstanyan, A.; Dracup, K. Correlates of fatigue in patients with heart failure. Prog. Cardiovasc. Nurs. 2008, 23, 12–17. [Google Scholar] [CrossRef]
- Sandek, A.; Bauditz, J.; Swidsinski, A.; Buhner, S.; Weber-Eibel, J.; von Haehling, S.; Schroedl, W.; Karhausen, T.; Doehner, W.; Rauchhaus, M.; et al. Altered intestinal function in patients with chronic heart failure. J. Am. Coll. Cardiol. 2007, 50, 1561–1569. [Google Scholar] [CrossRef]
- Springer, J.; Haehling, S.V.; Anker, D.S. The need for a standardized definition for cachexia in chronic illness. Nat. Clin. Pract. Endocrinol. Metab. 2006, 2, 416–417. [Google Scholar] [CrossRef]
- Mustafa, I.; Leverve, X. Metabolic and nutritional disorders in cardiac cachexia. Nutrition 2001, 17, 756–760. [Google Scholar] [CrossRef]
- Araújo, J.P.; Lourenço, P.; Rocha-Gonçalves, F.; Ferreira, A.; Bettencourt, P. Nutritional markers and prognosis in cardiac cachexia. Int. J. Cardiol. 2011, 146, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Bays, H.E. “Sick fat,” metabolic disease, and atherosclerosis. Am. J. Med. 2009, 122, S26–S37. [Google Scholar] [CrossRef] [PubMed]
- Mazurek, T.; Zhang, L.; Zalewski, A.; Mannion, J.D.; Diehl, J.T.; Arafat, H.; Sarov-Blat, L.; O’Brien, S.; Keiper, E.A.; Johnson, A.G.; et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation 2003, 108, 2460–2466. [Google Scholar] [CrossRef]
- Baker, A.R.; Silva, N.F.; Quinn, D.W.; Harte, A.L.; Pagano, D.; Bonser, R.S.; Kumar, S.; McTernan, P.G. Human epicardial adipose tissue expresses a pathogenic profile of adipocytokines in patients with cardiovascular disease. Cardiovasc. Diabetol. 2006, 5, 1. [Google Scholar] [CrossRef]
- Ebong, I.A.; Goff, D.C., Jr.; Rodriguez, C.J.; Chen, H.; Bertoni, A.G. Mechanisms of heart failure in obesity. Obes. Res. Clin. Pract. 2014, 8, e540–e548. [Google Scholar] [CrossRef]
- Ibrahim, M.M. Subcutaneous and visceral adipose tissue: Structural and functional differences. Obes. Rev. 2010, 11, 11–18. [Google Scholar] [CrossRef]
- Savarese, G.; Lund, L.H. Global public health burden of heart failure. Card. Fail. Rev. 2017, 3, 7–11. [Google Scholar] [CrossRef]
- Oktay, A.A.; Aktürk, H.K.; Paul, T.K.; O’Keefe, J.H.; Ventura, H.O.; Koch, C.A.; Lavie, C.J. Diabetes, Cardiomyopathy, and Heart Failure. In Endotext; Feingold, K.R., Anawalt, B., Boyce, A., Eds.; MDText.com, Inc.: South Darmouth, MA, USA, 2020. [Google Scholar] [PubMed]
- Després, J.P. Cardiovascular disease under the influence of excess visceral fat. Crit. Pathw. Cardiol. 2007, 6, 51–59. [Google Scholar] [CrossRef]
- Stevenson-Hellerstein, H.K.; Santiago-Stevenson, D. Atrophy of the heart: A correlative study of eighty-five proved cases. Circulation 1950, 1, 93–126. [Google Scholar] [CrossRef]
- Bjørnstad, P.G.; Foerster, A.; Ihlen, H. Cardiac findings in generalized lipodystrophy. Acta Paediatr. Suppl. 1996, 413, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.D.; Victor, R.G.; Szczepaniak, E.W.; Simha, V.; Garg, A.; Szczepaniak, L.S. Cardiac steatosis and left ventricular hypertrophy in patients with generalized lipodystrophy as determined by magnetic resonance spectroscopy and imaging. Am. J. Cardiol. 2013, 112, 1019–1024. [Google Scholar] [CrossRef] [PubMed]
- McLean, D.S.; Stillman, A.E. Epicardial adipose tissue as a cardiovascular risk marker. Clin. Lipidol. 2009, 4, 55–62. [Google Scholar] [CrossRef]
- Fitzgibbons, T.P.; Czech, M.P. Epicardial and perivascular adipose tissues and their influence on cardiovascular disease: Basic mechanisms and clinical associations. J. Am. Heart Assoc. 2014, 3, e000582. [Google Scholar] [CrossRef]
- Greenstein, A.S.; Khavandi, K.; Withers, S.B.; Sonoyama, K.; Clancy, O.; Jeziorska, M.; Laing, I.; Yates, A.P.; Pemberton, P.W.; Malik, R.A.; et al. Local inflammation and hypoxia abolish the protective anticontractile properties of perivascular fat in obese patients. Circulation 2009, 119, 1661–1670. [Google Scholar] [CrossRef]
- Budoff, M.J.; Achenbach, S.; Blumenthal, R.S.; Carr, J.J.; Goldin, J.G.; Greenland, P.; Guerci, A.D.; Lima, J.A.; Rader, D.J.; Rubin, G.D.; et al. Assessment of coronary artery disease by cardiac Computed tomography: A scientific statement from the American heart association committee on cardiovascular imaging and intervention, council on cardiovascular radiology and intervention, and committee on cardiac imaging, council on clinical cardiology. Circulation 2006, 114, 1761–1791. [Google Scholar]
- Pouliopoulos, J.; Chik, W.W.B.; Kanthan, A.; Sivagangabalan, G.; Barry, M.A.; Fahmy, P.N.; Midekin, C.; Lu, J.; Kizana, E.; Thomas, S.P.; et al. Intramyocardial adiposity after myocardial infarction: New implications of a substrate for ventricular tachycardia. Circulation 2013, 128, 2296–2308. [Google Scholar] [CrossRef]
- Samanta, R.; Kumar, S.; Chik, W.; Qian, P.; Barry, M.A.; Al Raisi, S.; Bhaskaran, A.; Farraha, M.; Nadri, F.; Kizana, E.; et al. Influence of intramyocardial adipose tissue on the accuracy of endocardial contact mapping of the chronic myocardial infarction substrate. Circulation 2017, 10, e004998. [Google Scholar] [CrossRef]
- Szczepaniak, L.S.; Dobbins, R.L.; Metzger, G.J.; Sartoni-D’Ambrosia, G.; Arbique, D.; Vongpatanasin, W.; Unger, R.; Victor, R.G. Myocardial triglycerides and systolic function in humans: In vivo evaluation by localized proton spectroscopy and cardiac imaging. Magn. Reason. Med. 2003, 49, 417–423. [Google Scholar] [CrossRef]
- Iozzo, P.; Lautamaki, R.; Borra, R.; Lehto, H.R.; Bucci, M.; Viljanen, A.; Parkka, J.; Lepomaki, V.; Maggio, R.; Parkkola, R.; et al. Contribution of glucose tolerance and gender to cardiac adiposity. J. Clin. Endocrinol. Metab. 2009, 94, 4472–4482. [Google Scholar] [CrossRef]
- Brindley, D.N.; Kok, B.P.C.; Kienesberger, P.C.; Lehner, R.; Dyck, J.R.B. Shedding light on the enigma of myocardial lipotoxicity: The involvement of known and putative regulators of fatty acid storage and mobilization. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E897–E908. [Google Scholar] [CrossRef] [PubMed]
- Van de Weijer, T.; Schrauwen-Hinderling, V.B.; Schrauwen, P. Lipotoxicity in type 2 diabetic Cardiomyopathy. Cardiovasc. Res. 2011, 92, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Sze, S.; Pellicori, P.; Zhang, J.; Weston, J.; Clark, A.L. Identification of frailty in chronic heart failure. JACC Heart Fail. 2019, 7, 291–302. [Google Scholar] [CrossRef] [PubMed]
- Haykowsky, M.J.; Brubaker, P.H.; Morgan, T.M.; Kritchevsky, S.; Eggebeen, J.; Kitzman, D.W. Impaired aerobic capacity and physical functional performance in older heart failure patients with preserved ejection fraction: Role of lean body mass. J. Gerontol. A Biol. Sci. Med. Sci. 2013, 68, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Fried, L.P.; Tangen, C.M.; Walston, J.; Newman, A.B.; Hirsch, C.; Gottdiener, J.; Seeman, T.; Tracy, R.; Kop, W.J.; Burke, G.; et al. Frailty in older adults: Evidence for a phenotype. J. Gerontol. A Biol. Sci. Med. Sci. 2001, 56, M146–M156. [Google Scholar] [CrossRef] [PubMed]
- Hoogendijk, E.O.; Afilalo, J.; Ensrud, K.E.; Kowal, P.; Onder, G.; Fried, L.P. Frailty: Implications for clinical practice and public health. Lancet 2019, 394, 1365–1375. [Google Scholar] [CrossRef]
- Soysal, P.; Veronese, N.; Thompson, T.; Kahl, K.G.; Fernandes, B.S.; Prina, A.M.; Solmi, M.; Schofield, P.; Koyanagi, A.; Tseng, P.T.; et al. Relationship between depression and frailty in older adults: A systematic review and meta-analysis. Ageing Res. Rev. 2017, 36, 78–87. [Google Scholar] [CrossRef]
- Gingrich, A.; Volkert, D.; Kiesswetter, E.; Thomanek, M.; Bach, S.; Sieber, C.C.; Zopf, Y. Prevalence and overlap of sarcopenia, frailty, cachexia and malnutrition in older medical inpatients. BMC Geriatr. 2019, 19, 120. [Google Scholar] [CrossRef]
- Wysokiński, A.; Sobów, T.; Kłoszewska, I.; Kostka, T. Mechanisms of the anorexia of aging—A review. Age 2015, 37, 9821. [Google Scholar] [CrossRef]
- Mahmoud, T.; Borgi, L. The interplay between nutrition, metabolic, and endocrine disorders in chronic kidney disease. Semin. Nephrol. 2021, 41, 180–188. [Google Scholar] [CrossRef]
- Horstman, A.M.; Dillon, E.L.; Urban, R.J.; Sheffield-Moore, M. The role of androgens and estrogens on healthy aging and longevity. J. Gerontol. A Biol. Sci. Med. Sci. 2012, 67, 1140–1152. [Google Scholar] [CrossRef] [PubMed]
- McMillin, S.L.; Minchew, E.C.; Lowe, D.A.; Spangenburg, E.E. Skeletal muscle wasting: The estrogen side of sexual dimorphism. Am. J. Physiol. Cell Physiol. 2022, 322, C24–C37. [Google Scholar] [CrossRef] [PubMed]
- Nandam, L.S.; Brazel, M.; Zhou, M.; Jhaveri, D.J. Cortisol and Major Depressive Disorder-Translating Findings from Humans to Animal Models and Back. Front. Psychiatry 2020, 10, 974. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, M.; Tsutamoto, T.; Kawahara, C.; Nishiyama, K.; Yamamoto, T.; Fujii, M.; Horie, M. Serum cortisol as a useful predictor of cardiac events in patients with chronic heart failure: The impact of oxidative stress. Circ. Heart Fail. 2009, 2, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Koppe, L.; Fouque, D.; Kalantar-Zadeh, K.J. Kidney cachexia or protein-energy wasting in chronic kidney disease: Facts and numbers. Cachexia Sarcopenia Muscle 2019, 10, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Soria-Castro, E.; Guarner-Lans, V.; Soto, M.E.; Avila-Casado, C.; Manzano Pech, L.; Pérez-Torres, I. Alteration of the fatty acid metabolism in the rat kidney caused by the injection of serum from patients with collapsing glomerulopathy. Biomedicines 2020, 8, 388. [Google Scholar] [CrossRef]
- Cheung, W.W.; Paik, K.H.; Mak, R.H. Inflammation and cachexia in chronic kidney disease. Pediatr. Nephrol. 2010, 25, 711–724. [Google Scholar] [CrossRef]
- Mak, R.H.; Cheung, W. Energy homeostasis and cachexia in chronic kidney disease. Pediatr. Nephrol. 2006, 21, 1807–1814. [Google Scholar] [CrossRef]
- Schiaffino, S.; Dyar, K.A.; Ciciliot, S.; Blaauw, B.; Sandri, M. Mechanisms regulating skeletal muscle growth and atrophy. FEBS J. 2013, 280, 4294–4314. [Google Scholar] [CrossRef]
- Tham, Y.K.; Bernardo, B.C.; Ooi, J.Y.; Weeks, K.L.; McMullen, J.R. Pathophysiology of cardiac hypertrophy and heart failure: Signaling pathways and novel therapeutic targets. Arch. Toxicol. 2015, 89, 1401–1438. [Google Scholar] [CrossRef]
- Cheema, B.S.; Sabbah, H.N.; Greene, S.J.; Gheorghiade, M. Protein turnover in the failing heart: An ever-changing landscape. Eur. J. Heart Fail. 2017, 19, 1218–1221. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, S.S.; Evans, C.D.; Oratz, M.; Rothschild, M.A. Protein synthesis and degradation in cardiac stress. Circ. Res. 1981, 48, 601–611. [Google Scholar] [CrossRef] [PubMed]
- Volpi, E.; Mittendorfer, B.; Rasmussen, B.B.; Wolfe, R.R. The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the elderly. J. Clin. Endocrinol. Metab. 2000, 85, 4481–4490. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, B.B.; Fujita, S.; Wolfe, R.R.; Mittendorfer, B.; Roy, M.; Rowe, V.L.; Volpi, E. Insulin resistance of muscle protein metabolism in aging. FASEB J. 2006, 20, 768–769. [Google Scholar] [CrossRef] [PubMed]
- Lecker, S.H.; Solomon, V.; Mitch, W.E.; Goldberg, A.L. Muscle protein breakdown and the critical role of the ubiquitinproteasome pathway in normal and disease states. J. Nutr. 1999, 129, 227S–237S. [Google Scholar] [CrossRef] [PubMed]
- Wing, S.S. Deubiquitinases in skeletal muscle atrophy. Int. J. Biochem. Cell Biol. 2013, 45, 2130–2135. [Google Scholar] [CrossRef][Green Version]
- Wendt, A.; Thompson, V.F.; Goll, D.E. Interaction of calpastatin with calpain: A review. Biol. Chem. 2004, 385, 465–472. [Google Scholar] [CrossRef]
- Goll, D.E.; Thompson, V.F.; Li, H.; Wei, W.; Cong, J. The calpain system. Physiol. Rev. 2003, 83, 731–801. [Google Scholar] [CrossRef]
- Yin, Y.; Shen, H. Advances in Cardiotoxicity Induced by Altered Mitochondrial Dynamics and Mitophagy. Front. Cardiovasc. Med. 2021, 8, 739095. [Google Scholar] [CrossRef]
- de Castro, G.S.; Simoes, E.; Lima, J.D.C.C.; Ortiz-Silva, M.; Festuccia, W.T.; Tokeshi, F.; Alcântara, P.S.; Otoch, J.P.; Coletti, D.; Seelaender, M. Human Cachexia Induces Changes in Mitochondria, Autophagy and Apoptosis in the Skeletal Muscle. Cancers 2019, 11, 1264. [Google Scholar] [CrossRef]
- VanderVeen, B.N.; Fix, D.K.; Carson, J.A. Disrupted Skeletal muscle mitochondrial dynamics, mitophagy, and biogenesis during cancer cachexia: A role for inflammation. Oxid. Med. Cell. Longev. 2017, 2017, 3292087. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, Y.; Bi, X.; Hu, C.; Ding, F.; Ding, W. Therapeutic Approaches in mitochondrial dysfunction, inflammation, and autophagy in uremic cachexia: Role of aerobic exercise. Mediat. Inflamm. 2019, 2019, 2789014. [Google Scholar] [CrossRef] [PubMed]
- Von Haehling, S.; Doehner, W.; Anker, S.D. Nutrition, metabolism, and the complex pathophysiology of cachexia in chronic heart failure. Cardiovasc. Res. 2007, 73, 298–309. [Google Scholar] [CrossRef] [PubMed]
- Dei Cas, A.; Muoio, A.; Zavaroni, I. Fisiopatologia della cachessia nella insufficienza cardiaca: Ruolo del sistema neuroendocrino [Chronic heart failure and cachexia: Role of endocrine system]. Minerva Cardioangiol. 2011, 59, 601–612. [Google Scholar]
- Husmann, I.; Soulet, L.; Gautron, J.; Martelly, I.; Barritault, D. Growth factors in skeletal muscle regeneration. Cytokine Growth Factor Rev. 1996, 7, 249–258. [Google Scholar] [CrossRef]
- Cassano, M.; Quattrocelli, M.; Crippa, S.; Perini, I.; Ronzoni, F.; Sampaolesi, M. Cellular mechanisms and local progenitor activation to regulate skeletal muscle mass. J. Muscle Res. Cell Motil. 2009, 3, 243–253. [Google Scholar] [CrossRef]
- Sacheck, J.M.; Ohtsuka, A.; McLary, S.C.; Goldberg, A.L. IGF-1 stimulates muscle growth by suppressing protein breakdown and expression of atrophy-related ubiquitin ligases, atrogin-1 and MuRF1. Am. J. Physiol. Endocrinol. Metab. 2004, 287, E591–E601. [Google Scholar] [CrossRef]
- Chrysis, D.; Underwood, L.E. Regulation of components of the ubiquitin system by insulin-like growth factor I and growth hormone in skeletal muscle of rats made catabolic with dexamethasone. Endocrinology 1999, 140, 5635–5641. [Google Scholar] [CrossRef][Green Version]
- Hong, D.; Forsberg, N.E. Effects of serum and insulin-like growth factor I on protein degradation and protease gene expression in rat L8 myotubes. J. Anim. Sci. 1994, 72, 2279–2288. [Google Scholar] [CrossRef]
- Wenz, T.; Rossi, S.G.; Rotundo, R.L.; Spiegelman, B.M.; Moraes, C.T. Increased muscle PGC-1a expression protects from sarcopenia and metabolic disease during aging. Proc. Natl. Acad. Sci. USA 2009, 106, 20405–20410. [Google Scholar] [CrossRef]
- Anker, S.D.; Sharma, R. The syndrome of cardiac cachexia. Int. J. Cardiol. 2002, 85, 51–66. [Google Scholar] [CrossRef]
- Anker, S.D.; Rauchhaus, M. Insights into the pathogenesis of chronic heart failure: Immune activation and cachexia. Curr. Opin. Cardiol. 1999, 14, 211–216. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; McNeill, J.H. The emerging roles of leptin and ghrelin in cardiovascular physiology and pathophysiology. Curr. Vasc. Pharmacol. 2005, 3, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Raghay, K.; Akki, R.; Bensaid, D.; Errami, M. Ghrelin as an anti-inflammatory and protective agent in ischemia/reperfusion injury. Peptides 2020, 124, 170226. [Google Scholar] [CrossRef] [PubMed]
- Krim, S.R.; Campbell, P.; Lavie, C.J.; Ventura, H. Micronutrients in chronic heart failure. Curr. Heart Fail. Rep. 2013, 10, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Yang, A.; Lv, Q.; Chen, F.; Wang, Y.; Liu, Y.; Shi, W.; Liu, Y.; Wang, D. The effect of vitamin D on sarcopenia depends on the level of physical activity in older adults. J. Cachexia Sarcopenia Muscle 2020, 11, 678–689. [Google Scholar] [CrossRef]
- Vinke, P.; Wesselink, E.; van Orten-Luiten, W.; van Norren, K. The Use of proton pump inhibitors may increase symptoms of muscle function loss in patients with chronic illnesses. Int. J. Mol. Sci. 2020, 21, 323. [Google Scholar] [CrossRef]
- Polly, P.; Tan, T.C. The role of vitamin D in skeletal and cardiac muscle function. Front. Physiol. 2014, 5, 145. [Google Scholar] [CrossRef]
- Garcia, M.; Seelaender, M.; Sotiropoulos, A.; Coletti, D.; Lancha, A.H., Jr. Vitamin D, muscle recovery, sarcopenia, cachexia, and muscle atrophy. Nutrition 2019, 60, 66–69. [Google Scholar] [CrossRef]
- Girgis, C.M.; Cha, K.M.; So, B.; Tsang, M.; Chen, J.; Houweling, P.J.; Schindeler, A.; Stokes, R.; Swarbrick, M.M.; Evesson, F.J.; et al. Mice with myocyte deletion of vitamin D receptor have sarcopenia and impaired muscle function. J. Cachexia Sarcopenia Muscle 2019, 10, 1228–1240. [Google Scholar] [CrossRef]
- Sato, Y.; Iwamoto, J.; Kanoko, T.; Satoh, K. Low-dose vitamin D prevents muscular atrophy and reduces falls and hip fractures in women after stroke: A randomized controlled trial. Cerebrovasc. Dis. 2005, 20, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Endo, I.; Inoue, D.; Mitsui, T.; Umaki, Y.; Akaike, M.; Yoshizawa, T.; Kato, S.; Matsumoto, T. Deletion of vitamin D receptor gene in mice results in abnormal skeletal muscle development with deregulated expression of myoregulatory transcription factors. Endocrinology 2003, 144, 5138–5144. [Google Scholar] [CrossRef] [PubMed]
- Penna, F.; Camperi, A.; Muscaritoli, M.; Filigheddu, N.; Costelli, P. The role of vitamin D in cancer cachexia. Curr. Opin. Support. Palliat. Care 2017, 11, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Dev, R.; Del Fabbro, E.; Schwartz, G.G.; Hui, D.; Palla, S.L.; Gutierrez, N.; Bruera, E. Preliminary report: Vitamin D deficiency in advanced cancer patients with symptoms of fatigue or anorexia. Oncologist 2011, 16, 1637–1641. [Google Scholar] [CrossRef] [PubMed]
- Gröber, U.; Kisters, K.; Adamietz, I.A. Vitamin D in oncology: Update 2015. Med. Monatsschr. Pharm. 2015, 38, 512–516. [Google Scholar] [PubMed]
- Vest, A.R.; Chan, M.; Deswal, A.; Givertz, M.M.; Lekavich, C.; Lennie, T.; Litwin, S.E.; Parsly, L.; Rodgers, J.E.; Rich, M.W.; et al. Nutrition, obesity, and cachexia in patients with heart failure: A consensus statement from the Heart Failure Society of America scientific statements committee. J. Card. Fail. 2019, 25, 380–400. [Google Scholar] [CrossRef] [PubMed]
- Mochamat; Cuhls, H.; Marinova, M.; Kaasa, S.; Stieber, C.; Conrad, R.; Radbruch, L.; Mücke, M. A systematic review on the role of vitamins, minerals, proteins, and other supplements for the treatment of cachexia in cancer: A European Palliative Care Research Centre cachexia project. J. Cachexia Sarcopenia Muscle 2017, 8, 25–39. [Google Scholar] [CrossRef]
- Cleland Witte, K.K.; Clark, A.L.; Cleland, J.G. Chronic heart failure and micronutrients. J. Am. Coll. Cardiol. 2001, 37, 1765–1774. [Google Scholar] [CrossRef]
- Batista, M.L., Jr.; Peres, S.B.; McDonald, M.E.; Alcantara, P.S.; Olivan, M.; Otoch, J.P.; Farmer, S.R.; Seelaender, M. Adipose tissue inflammation and cancer cachexia: Possible role of nuclear transcription factors. Cytokine 2012, 57, 9–16. [Google Scholar] [CrossRef]
- Webster, J.M.; Kempen, L.J.A.P.; Hardy, R.S.; Langen, R.C.J. Inflammation and skeletal muscle wasting during cachexia. Front. Physiol. 2020, 11, 597675. [Google Scholar] [CrossRef]
- Wisniacki, N. Is anaemia a cause or a consequence of heart failure in the elderly? Heart 2001, 85 (Suppl. I), P4. [Google Scholar]
- Bindels, L.B.; Neyrinck, A.M.; Claus, S.P.; Le Roy, C.I.; Grangette, C.; Pot, B.; Martinez, I.; Walter, J.; Cani, P.D.; Delzenne, N.M. Synbiotic approach restores intestinal homeostasis and prolongs survival in leukaemic mice with cachexia. ISME J. 2016, 10, 1456–1470. [Google Scholar] [CrossRef] [PubMed]
- Vrieze, A.; Van Nood, E.; Holleman, F.; Salojärvi, J.; Kootte, R.S.; Bartelsman, J.F.W.M.; Dallinga-Thie, G.M.; Ackermans, M.T.; Serlie, M.J.; Oozeer, R.; et al. Transfer of intestinal microbiota from lean donors increases insulin sensitivity in individuals with metabolic syndrome. Gastroenterology 2012, 143, 913–916. [Google Scholar] [CrossRef] [PubMed]
- Kojima, M.; Hosoda, H.; Date, Y.; Nakazato, M.; Matsuo, H.; Kangawa, K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999, 402, 656–660. [Google Scholar] [CrossRef]
- King, D.; Smith, M.L.; Chapman, T.J.; Stockdale, H.R.; Lye, M. Fat malabsorption in elderly patients with cardiac cachexia. Age Ageing 1996, 25, 144–149. [Google Scholar] [CrossRef]
- Rohm, M.; Zeigerer, A.; Machado, J.; Herzig, S. Energy metabolism in cachexia. EMBO Rep. 2019, 20, e47258. [Google Scholar] [CrossRef]
- Marcondes, M.C.; Honma, H.N.; Areas, M.A.; Cury, L. Effect ofWalker256tumor growth on intestinal absorption of leucine, methion-ine and glucose in newly weaned and mature rats. Braz. J. Med. Biol. Res. 1998, 31, 1345–1348. [Google Scholar] [CrossRef]
- Sandek, A.; Rauchhaus, M.; Anker, S.D.; von Haehling, S. The emergingrole of the gut in chronic heart failure. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 632–639. [Google Scholar] [CrossRef]
- Julienne, C.M.; Tardieu, M.; Chevalier, S.; Pinault, M.; Bougnoux, P.; Labarthe, F.; Couet, C.; Servais, S.; Dumas, J.F. Cardiolipin content is involved in liver mitochondrial energy wasting associated with cancer-induced cachexia without the involvement of adenine nucleotide translocase. Biochim. Biophys. Acta 2014, 1842, 726–733. [Google Scholar] [CrossRef]
- Palesty, J.A.; Dudrick, S.J. Cachexia, malnutrition, the refeeding syndrome, and lessons from Goldilocks. Surg. Clin. N. Am. 2011, 91, 653–673. [Google Scholar] [CrossRef]
- Kondrup, J.; Rasmussen, H.H.; Hamberg, O.; Stanga, Z. Ad Hoc ESPEN Working Group. Nutritional risk screening (NRS 2002): A new method based on an analysis of controlled clinical trials. Clin. Nutr. 2003, 22, 321–336. [Google Scholar] [CrossRef]
- Detsky, A.S.; McLaughlin, J.R.; Baker, J.P.; Johnston, N.; Whittaker, S.; Mendelson, R.A.; Jeejeebhoy, K.N. What is subjective global assessment of nutritional status? J. Parenter. Enter. Nutr. 1987, 11, 8–13. [Google Scholar] [CrossRef]
- Vellas, B.; Guigoz, Y.; Garry, P.J.; Nourhashemi, F.; Bennahum, D.; Lauque, S.; Albarede, J.L. The mini nutritional assessment (MNA) and its use in grading the nutritional state of elderly patients. Nutrition 1999, 15, 116–122. [Google Scholar] [CrossRef]
- Stratton, R.J.; Hackston, A.; Longmore, D.; Dixon, R.; Price, S.; Stroud, M.; King, C.; Elia, M. Malnutrition in hospital outpatients and inpatients: Prevalence, concurrent validity and ease of use of the ‘malnutrition universal screening tool’ (‘MUST’) for adults. Br. J. Nutr. 2004, 92, 799–808. [Google Scholar] [CrossRef] [PubMed]
- Kruizenga, H.M.; Seidell, J.C.; de Vet, H.C.; Wierdsma, N.J.; van Bokhorst-de van der Schueren, M.A. Development and validation of a hospital screening tool for malnutrition: The short nutritional assessment questionnaire (SNAQ). Clin. Nutr. 2005, 24, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Kenchaiah, S.; Pocock, S.J.; Wang, D.; Finn, P.V.; Zornoff, L.A.; Skali, H.; Pfeffer, M.A.; Yusuf, S.; Swedberg, K.; Michelson, E.L.; et al. Body mass index and prognosis in patients with chronic heart failure: Insights from the candesartan in heart failure: Assessment of reduction in mortality and morbidity (CHARM) program. Circulation 2007, 116, 627–636. [Google Scholar] [CrossRef]
- McClave, S.A.; Taylor, B.E.; Martindale, R.G.; Warren, M.M.; Johnson, D.R.; Braunschweig, C.; McCarthy, M.S.; Davanos, E.; Rice, T.W.; Cresci, G.A.; et al. Guidelines for the provision and assessment of nutrition support therapy in the adult critically ill patient: Society of critical care medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). J. Parenter. Enter. Nutr. 2016, 40, 159–211. [Google Scholar] [CrossRef]
- Singer, P.; Blaser, A.R.; Berger, M.M.; Alhazzani, W.; Calder, P.C.; Casaer, M.P.; Hiesmayr, M.; Mayer, K.; Montejo, J.C.; Pichard, C.; et al. ESPEN guideline on clinical nutrition in the intensive care unit. Clin. Nutr. 2019, 38, 48–79. [Google Scholar] [CrossRef]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. GLIM Core Leadership Committee, GLIM Working Group. GLIM criteria for the diagnosis of malnutrition e a consensus report from the global clinical nutrition community. Clin. Nutr. 2019, 38, 1–9. [Google Scholar] [CrossRef]
- da Silva, J.S.V.; Seres, D.S.; Sabino, K.; Adams, S.C.; Berdahl, G.J.; Citty, S.W.; Cober, M.P.; Evans, D.C.; Greaves, J.R.; Gura, K.M.; et al. Parenteral Nutrition Safety and Clinical Practice Committees, American Society for Parenteral and Enteral Nutrition. ASPEN consensus recommendations for refeeding syndrome. Nutr. Clin. Pract. 2020, 35, 178–195. [Google Scholar] [CrossRef]
- Hepgul, A.E.; Kon, N.; Maddocks, M. Sarcopenia and frailty in chronic respiratory disease. Chron. Respir. Dis. 2017, 14, 85–99. [Google Scholar]
- Cawthon, P.M.; Manini, T.; Patel, S.M.; Newman, A.; Travison, T.; Kiel, D.P.; Santanasto, A.J.; Ensrud, K.E.; Xue, Q.L.; Shardell, M.; et al. Putative cut-points in sarcopenia components and incident adverse health outcomes: An SDOC analysis. J. Am. Geriatr. Soc. 2020, 68, 1429–1437. [Google Scholar] [CrossRef] [PubMed]
- Newman, A.B.; Kupelian, V.; Visser, M.; Simonsick, E.M.; Goodpaster, B.H.; Kritchevesky, S.B.; Tylavsky, F.A.; Rubin, S.M.; Harris, T.B. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 72–77. [Google Scholar] [CrossRef] [PubMed]
- Linge, J.; Heymsfield, S.B.; Dahlqvist, L.O. On the definition of sarcopenia in the presence of aging and obesity—Initial results from UK Biobank. J. Gerontol. A Biol. Med. Sci. 2020, 75, 1309–1316. [Google Scholar] [CrossRef]
- Cawthon, P.M.; Blackwell, T.; Cummings, S.R.; Orwoll, E.S.; Duchowny, K.A.; Kado, D.M.; Stone, K.L.; Ensrud, K.E.; Cauley, J.A.; Evans, W.J. Muscle mass assessed by the D3-creatine dilution method and incident self-reported disability and mortality in a prospective observational study of community-dwelling older men. J. Gerontol. A Biol. Sci. Med. Sci. 2021, 76, 123–130. [Google Scholar] [CrossRef]
- Cruz-Jentoft, A.J.; Bahat, G.; Bauer, J.; Boirie, Y.; Bruyere, O.; Cederholm, T.; Cooper, C.; Landi, F.; Rolland, Y.; Sayer, A.A.; et al. Writing group for the European Working group on sarcopenia in older people 2 (EWGSOP2), and the extended group for EWGSOP2. Sarcopenia: Revised European consensus on definition and diagnosis. Age Ageing 2019, 48, 16–31. [Google Scholar] [CrossRef]
- Castillo-Martínez, L.; Colín-Ramírez, E.; Orea-Tejeda, A.; Gonzalez-Islas, D.G.; Rodríguez-García, W.D.; Santillan-Díaz, C.; Gutiérrez Rodríguez, A.E.; Vázquez Durán, M.; Keirns Davies, C. Cachexia assessed by bio-impedance vector analysis as a prognostic indicator in chronic stable heart failure patients. Nutrition 2012, 28, 886–891. [Google Scholar] [CrossRef]
- Gonzalez, M.C.; Heymsfield, S.B. Bioelectrical impedance analysis for diagnosing sarcopenia and cachexia: What are we really estimating? J. Cachexia Sarcopenia Muscle 2017, 8, 187–189. [Google Scholar] [CrossRef]
- Cunha, G.J.L.; Rocha, B.M.L.; Freitas, P.; Sousa, J.A.; Paiva, M.; Santos, A.C.; Guerreiro, S.; Tralhão, A.; Ventosa, A.; Aguiar, C.M.; et al. Pectoralis major muscle quantification by cardiac MRI is a strong predictor of major events in HF. Heart Vessel. 2021, 1–10. [Google Scholar] [CrossRef]
- Kumar, A.; Ansari, B.A.; Kim, J.; Suri, A.; Gaddam, S.; Yenigalla, S.; Vanjarapu, J.M.; Selvaraj, S.; Tamvada, D.; Lee, J.; et al. Axial muscle size as a strong predictor of death in subjects with and without heart failure. J. Am. Heart Assoc. 2019, 8, e010554. [Google Scholar] [CrossRef]
- Vangelov, B.; Bauer, J.; Kotevski, D.; Smee, R.I. The use of alternate vertebral levels to L3 in computed tomography scans for skeletal muscle mass evaluation and sarcopenia assessment in patients with cancer: A systematic review. Br. J. Nutr. 2021, 127, 1–49. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.J.; Wu, C.; Jones, M.; Kato, T.S.; Dam, T.T.; Givens, R.C.; Templeton, D.L.; Maurer, M.S.; Naka, Y.; Takayama, H.; et al. Reduced handgrip strength as a marker of frailty predicts clinical outcomes in patients with heart failure undergoing ventricular assist device placement. J. Card. Fail. 2014, 20, 310–315. [Google Scholar] [CrossRef] [PubMed]
- Budzynski, J.; Tojek, K.; Czerniak, B.; Banaszkiewicz, Z. Scores of nutritional risk and parameters of nutritional status assessment as predictors of in-hospital mortality and readmissions in the general hospital population. Clin. Nutr. 2016, 35, 1464–1471. [Google Scholar] [CrossRef]
- Murphy, L.; Gray, A.; Joyce, E. Anabolism to catabolism: Serologic clues to nutritional status in heart failure. Curr. Heart Fail. Rep. 2019, 16, 189–200. [Google Scholar] [CrossRef] [PubMed]
- Nagaya, N.; Uematsu, M.; Kojima, M.; Date, Y.; Nakazato, M.; Okumura, H.; Hosoda, H.; Shimizu, W.; Yamagishi, M.; Oya, H.; et al. Elevated circulating level of ghrelin in cachexia associated with chronic heart failure: Relationships between ghrelin and anabolic/catabolic factors. Circulation 2001, 104, 2034–2038. [Google Scholar] [CrossRef] [PubMed]
- Szabo, T.; Scherbakov, N.; Sandek, A.; Kung, T.; von Haehling, S.; Lainscak, M.; Jankowska, E.A.; Rudovich, N.; Anker, S.D.; Frystyk, J.; et al. Plasma adiponectin in heart failure with and without cachexia: Catabolic signal linking catabolism, symptomatic status, and prognosis. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 50–56. [Google Scholar] [CrossRef]
- Breitbart, A.; Auger-Messier, M.; Molkentin, J.D.; Heineke, J. Myostatin from the heart: Local and systemic actions in cardiac failure and muscle wasting. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H1973–H1982. [Google Scholar] [CrossRef]
- Shyu, K.G.; Lu, M.J.; Wang, B.W.; Sun, H.Y.; Chang, H. Myostatin expression in ventricular myocardium in a rat model of volume-overload heart failure. Eur. J. Clin. Investig. 2006, 36, 713–719. [Google Scholar] [CrossRef]
- Okoshi, M.P.; Capalbo, R.V.; Romeiro, F.G.; Okoshi, K. Cardiac cachexia: Perspectives for prevention and treatment. Arq. Bras. Cardiol. 2017, 108, 74. [Google Scholar] [CrossRef]
- Yin, J.; Lu, X.; Qian, Z.; Xu, W.; Zhou, X. New insights into the pathogenesis and treatment of sarcopenia in chronic heart failure. Theranostics 2019, 9, 4019–4029. [Google Scholar] [CrossRef]
- Bauer, J.; Biolo, G.; Cederholm, T.; Cesari, M.; Cruz-Jentoft, A.J.; Morley, J.E.; Phillips, S.; Sieber, C.; Stehle, P.; Teta, D.; et al. Evidence-based recommendations for optimal dietary protein intake in older people: A position paper from the PROT-AGE Study Group. J. Am. Med. Dir. Assoc. 2013, 14, 542–559. [Google Scholar] [CrossRef] [PubMed]
- Mijan de la Torre, A.; de Mateo Silleras, B.; Perez García, A.M. Nutricion e insuficiencia cardiaca. In Tratado de Nutrición. Tomo 5: Nutricion y Enfermedad, 3rd ed.; Gil, A., Ed.; Editorial Medica Panamericana: Madrid, Spain, 2017; p. 705e28. [Google Scholar]
- von Haehling, S.; Ebner, N.; Dos Santos, M.R.; Springer, J.; Anker, S.D. Muscle wasting and cachexia in heart failure: Mechanisms and therapies. Nat. Rev. Cardiol. 2017, 14, 323–341. [Google Scholar] [CrossRef] [PubMed]
- Santarpia, L.; Contaldo, F.; Pasanisi, F. Dietary protein content for an optimal diet: A clinical view. J. Cachexia Sarcopenia Muscle 2017, 8, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Howard, P. Basics in clinical nutrition: Enteral nutrition. E-SPEN 2009, 4, e223–e225. [Google Scholar] [CrossRef][Green Version]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Colvin, M.M.; Drazner, M.H.; Filippatos, G.; Fonarow, G.C.; Givertz, M.M.; et al. 2016 ACC/AHA/HFSA focused update on new pharmacological therapy for heart failure: An update of the 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J. Am. Coll. Cardiol. 2016, 68, 1476–1488. [Google Scholar] [CrossRef]
- Otten, J.J.; Hellwig, J.P.; Meyers, L.D. Dietary Reference Intakes: The Essential Guide to Nutrient Requirements; National Academies Press: Washington, DC, USA, 2006. [Google Scholar]
- Cheung, W.W.; Ding, W.; Hoffman, H.M.; Wang, Z.; Hao, S.; Zheng, R.; Gonzalez, A.; Zhan, J.Y.; Zhou, P.; Li, S.; et al. Vitamin D ameliorates adipose browning in chronic kidney disease cachexia. Sci. Rep. 2020, 10, 14175. [Google Scholar] [CrossRef]
- Pfeifer, M.; Begerow, B.; Minne, H.W.; Nachtigall, D.; Hansen, C. Effects of a short-term vitamin D(3) and calcium supplementation on blood pressure and parathyroid hormone levels in elderly women. J. Clin. Endocrinol. Metab. 2001, 86, 1633–163730. [Google Scholar]
- Sugden, J.A.; Davies, J.I.; Witham, M.D.; Morris, A.D.; Struthers, A.D. VitaminD improves endothelial function in patients with type 2 diabetes mellitus and low vitamin D levels. Diab. Med. 2008, 25, 320–325. [Google Scholar] [CrossRef]
- Schleithoff, S.S.; Zittermann, A.; Tenderich, G.; Berthold, H.K.; Stehle, P.; Koerfer, R. Vitamin D supplementation improves cytokine profiles in patients with congestive heart failure: A double-blind, randomized, placebo-controlled trial. Am. J. Clin. Nutr. 2006, 83, 754–759. [Google Scholar] [CrossRef]
- Witham, M.D.; Crighton, L.J.; Gillespie, N.D.; Struthers, A.D.; McMurdo, M.E. The effects of vitamin D supplementation on physical function and quality of life in older patients with heart failure: A randomized controlled trial. Circ. Heart Fail. 2010, 3, 195–201. [Google Scholar] [CrossRef]
- Dalbeni, A.; Scaturro, G.; Degan, M.; Minuz, P.; Delva, P. Effects of six months of vitamin D supplementation in patients with heart failure: A randomized double-blind controlled trial. Nutr. Metab. Cardiovasc. Dis 2014, 24, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Witte, K.K.; Byrom, R.; Gierula, J.; Paton, M.F.; Jamil, H.A.; Lowry, J.E.; Gillott, R.G.; Barnes, S.A.; Chumun, H.; Kearney, L.C.; et al. Effects of vitamin D on cardiac function in patients with chronic HF: The VINDICATE study. J. Am. Coll. Cardiol. 2016, 67, 2593–2603. [Google Scholar] [CrossRef]
- Zittermann, A.; Ernst, J.B.; Prokop, S.; Fuchs, U.; Dreier, J.; Kuhn, J.; Knabbe, C.; Birschmann, I.; Schulz, U.; Berthold, H.K.; et al. Effect of vitamin D on all-cause mortality in heart failure (EVITA): A 3-year randomized clinical trial with 4000 IU vitamin D daily. Eur. Heart J. 2017, 38, 2279–2286. [Google Scholar] [CrossRef]
- Zittermann, A.; Ernst, J.B.; Prokop, S.; Fuchs, U.; Gruszka, A.; Dreier, J.; Knabbe, C.; Berthold, H.K.; Gouni-Berthold, I.; Gummert, J.F.; et al. Vitamin D supplementation of 4000 IU daily and cardiac function in patients with advanced heart failure: The EVITA trial. Int. J. Cardiol. 2019, 280, 117–123. [Google Scholar] [CrossRef]
- Keith, M.E.; Jeejeebhoy, K.N.; Langer, A.; Kurian, R.; Barr, A.; O’Kelly, B.; Sole, M.J. A controlled clinical trial of vitamin E supplementation in patients with congestive heart failure. Am. J. Clin. Nutr. 2001, 73, 219–224. [Google Scholar] [CrossRef] [PubMed]
- Ghatak, A.; Brar, M.J.; Agarwal, A.; Goel, N.; Rastogi, A.K.; Vaish, A.K.; Sircar, A.R.; Chandra, M. Oxy free radical system in heart failure and therapeutic role of oral vitamin E. Int. J. Cardiol. 1996, 57, 119–127. [Google Scholar] [CrossRef]
- Shimon, I.; Almog, S.; Vered, Z.; Seligmann, H.; Shefi, M.; Peleg, E.; Rosenthal, T.; Motro, M.; Halkin, H.; Ezra, D. Improved left ventricular function after thiamine supplementation in patients with congestive heart failure receiving long-term furosemide therapy. Am. J. Med. 1995, 98, 485–490. [Google Scholar] [CrossRef]
- Schoenenberger, A.W.; Schoenenberger-Berzins, R.; der Maur, C.A.; Suter, P.M.; Vergopoulos, A.; Erne, P. Thiamine supplementation in symptomatic chronic heart failure: A randomized, double-blind, placebo-controlled, cross-over pilot study. Clin. Res. Cardiol. 2012, 101, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Keith, M.; Quach, S.; Ahmed, M.; Azizi-Namini, P.; Al-Hesayen, A.; Azevedo, E.; James, R.; Leong-Poi, H.; Ong, G.; Desjardins, S.; et al. Thiamin supplementation does not improve left ventricular ejection fraction in ambulatory heart failure patients: A randomized controlled trial. Am. J. Clin. Nutr. 2019, 110, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Andersson, S.E.; Edvinsson, M.L.; Edvinsson, L. Reduction of homocysteine in elderly with heart failure improved vascular function and blood pressure control but did not affect inflammatory activity. Basic Clin. Pharmacol. Toxicol. 2005, 97, 306–310. [Google Scholar] [CrossRef]
- Shinke, T.; Shite, J.; Takaoka, H.; Hata, K.; Inoue, N.; Yoshikawa, R.; Matsumoto, H.; Masai, H.; Watanabe, S.; Ozawa, T.; et al. Vitamin C restores the contractile response to dobutamine and improves myocardial efficiency in patients with heart failure after anterior myocardial infarction. Am. Heart J. 2007, 154, 645.e1–645.e8. [Google Scholar] [CrossRef] [PubMed]
- Keogh, A.; Fenton, S.; Leslie, C.; Aboyoun, C.; Macdonald, P.; Zhao, Y.C.; Bailey, M.; Rosenfeldt, F. Randomised double-blind, placebo-controlled trial of coenzyme Q, therapy in class II and III systolic heart failure. Heart Lung Circ. 2003, 12, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Berman, M.; Erman, A.; Ben-Gal, T.; Dvir, D.; Georghiou, G.P.; Stamler, A.; Vered, Y.; Vidne, B.A.; Aravot, D. Coenzyme Q10 in patients with end-stage heart failure awaiting cardiac transplantation: A randomized, placebo-controlled study. Clin. Cardiol. 2004, 27, 295–299. [Google Scholar] [CrossRef] [PubMed]
- Morisco, C.; Trimarco, B.; Condorelli, M. Effect of coenzyme Q10 therapy in patients with congestive heart failure: A long-term multicenter randomized study. Clin. Investig. 1993, 71 (Suppl. 8), S134–S136. [Google Scholar] [CrossRef] [PubMed]
- Ponikowski, P.; van Veldhuisen, D.J.; Comin-Colet, J.; Ertl, G.; Komajda, M.; Mareev, V.; McDonagh, T.; Parkhomenko, A.; Tavazzi, L.; Levesque, V.; et al. CONFIRM-HF Investigators. Beneficial effects of long-term intravenous iron therapy with ferric carboxymaltose in patients with symptomatic heart failure and iron deficiency. Eur. Heart J. 2015, 36, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Lewis, G.D.; Malhotra, R.; Hernandez, A.F.; McNulty, S.E.; Smith, A.; Felker, G.M.; Tang, W.H.W.; LaRue, S.J.; Redfield, M.M.; Semigran, M.J.; et al. NHLBI Heart Failure Clinical Research Network. Effect of oral iron repletion on exercise capacity in patients with heart failure with reduced ejection fraction and iron deficiency: The IRONOUT HF randomized clinical trial. J. Am. Med. Assoc. 2017, 317, 1958–1966. [Google Scholar] [CrossRef]
- Harrington, D.; Chua, T.P.; Coats, A.J. The effect of salbutamol on skeletal muscle in chronic heart failure. Int. J. Cardiol. 2000, 73, 257–265. [Google Scholar] [CrossRef]
- Piepoli, M.F.; Davos, C.; Francis, D.P.; Coats, A.J.; ExTraMATCH Collaborative. Exercise training meta-analysis of trials in patients with chronic heart failure (ExTraMATCH). BMJ 2004, 328, 189. [Google Scholar] [CrossRef]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.; Coats, A.J.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. Authors/Task Force Members, Document Reviewers. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2016, 18, 891–975. [Google Scholar] [CrossRef]
- Estruch, R.; Ros, E.; Salas-Salvado, J.; Covas, M.I.; Corella, D.; Aros, F.; Gómez-Gracia, E.; Ruiz-Gutiérrez, V.; Fiol, M.; Lapetra, J.; et al. PREDIMED Study Investigators. Primary prevention of cardiovascular disease with a Mediterranean diet supplemented with extra-virgin olive oil or nuts. N. Engl. J. Med. 2018, 378, e34. [Google Scholar] [CrossRef]
- Nagaya, N.; Moriya, J.; Yasumura, Y.; Uematsu, M.; Ono, F.; Shimizu, W.; Ueno, K.; Kitakaze, M.; Miyatake, K.; Kangawa, K. Effects of ghrelin administration on left ventricular function, exercise capacity, and muscle wasting in patients with chronic heart failure. Circulation. 2004, 110, 3674–3679. [Google Scholar] [CrossRef] [PubMed]
- Hryniewicz, K.; Androne, A.S.; Hudaihed, A.; Katz, S.D. Partial reversal of cachexia by beta-adrenergic receptor blocker therapy in patients with chronic heart failure. J. Card. Fail. 2003, 9, 464–468. [Google Scholar] [CrossRef]
- Caminiti, G.; Volterrani, M.; Iellamo, F.; Marazzi, G.; Massaro, R.; Miceli, M.; Mammi, C.; Piepoli, M.; Fini, M.; Rosano, G.M. Effect of long-acting testosterone treatment on functional exercise apacity, skeletal muscle performance, insulin resistance, and baroreflex sensitivity in elderly patients with chronic heart failure a double-blind, placebo-controlled, randomized study. J. Am. Coll. Cardiol. 2009, 54, 919–927. [Google Scholar] [CrossRef] [PubMed]
- Au, D.H.; Udris, E.M.; Fan, V.S.; Curtis, J.R.; McDonell, M.B.; Fihn, S.D. Risk of mortality and heart failure exacerbations associated with inhaled beta-adrenoceptor agonists among patients with known left ventricular systolic dysfunction. Chest. 2003, 123, 1964–1969. [Google Scholar] [CrossRef]
- Mitja, L.; Keber, I.; Anker, S.D. Body composition changes in patients with systolic heart failure treated with beta blockers: A pilot study. Int. J. Cardiol. 2006, 106, 319–322. [Google Scholar] [CrossRef]
- Kamalakkannan, G.; Petrilli, C.M.; George, I.; LaManca, J.; McLaughlin, B.T.; Shane, E.; Mancini, D.M.; Maybaum, S. Clenbuterol increases lean muscle mass but not endurance in patients with chronic heart failure. J. Heart Lung Transplant. 2008, 27, 457–461. [Google Scholar] [CrossRef]
- Clark, A.L.; Coats, A.J.S.; Krum, H.; Katus, H.A.; Mohacsi, P.; Salekin, D.; Schultz, M.K.; Packer, M.; Anker, S.D. Effect of beta-adrenergic blockade with carvedilol on cachexia in severe chronic heart failure: Results from the COPERNICUS trial. J. Cachexia Sarcopenia Muscle 2017, 8, 549–556. [Google Scholar] [CrossRef]
- von Haehling, S.; Lainscak, M.; Springer, J.; Anker, S.D. Cardiac cachexia: A systematic overview. Pharmacol. Ther. 2009, 121, 227–252. [Google Scholar] [CrossRef]
- Honors, M.A.; Kinzig, K.P. The role of insulin resistance in the development of muscle wasting during cancer cachexia. J. Cachexia Sarcopenia Muscle 2012, 3, 5–11. [Google Scholar] [CrossRef]
- Bora, V.; Patel, B.M. Investigation into the role of anti-diabetic agents in cachexia associated with metastatic cancer. Life Sci. 2021, 274, 119329. [Google Scholar] [CrossRef]
- Kang, M.J.; Moon, J.W.; Lee, J.O.; Kim, J.H.; Jung, E.J.; Kim, S.J.; Oh, J.Y.; Wu, S.W.; Lee, P.R.; Park, S.H.; et al. Metformin induces muscle atrophy by transcriptional regulation of myostatin via HDAC6 and FoxO3a. J. Cachexia Sarcopenia Muscle 2021, 13, 605–620. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soto, M.E.; Pérez-Torres, I.; Rubio-Ruiz, M.E.; Manzano-Pech, L.; Guarner-Lans, V. Interconnection between Cardiac Cachexia and Heart Failure—Protective Role of Cardiac Obesity. Cells 2022, 11, 1039. https://doi.org/10.3390/cells11061039
Soto ME, Pérez-Torres I, Rubio-Ruiz ME, Manzano-Pech L, Guarner-Lans V. Interconnection between Cardiac Cachexia and Heart Failure—Protective Role of Cardiac Obesity. Cells. 2022; 11(6):1039. https://doi.org/10.3390/cells11061039
Chicago/Turabian StyleSoto, María Elena, Israel Pérez-Torres, María Esther Rubio-Ruiz, Linaloe Manzano-Pech, and Verónica Guarner-Lans. 2022. "Interconnection between Cardiac Cachexia and Heart Failure—Protective Role of Cardiac Obesity" Cells 11, no. 6: 1039. https://doi.org/10.3390/cells11061039
APA StyleSoto, M. E., Pérez-Torres, I., Rubio-Ruiz, M. E., Manzano-Pech, L., & Guarner-Lans, V. (2022). Interconnection between Cardiac Cachexia and Heart Failure—Protective Role of Cardiac Obesity. Cells, 11(6), 1039. https://doi.org/10.3390/cells11061039