Abstract
Small noncoding RNAs, as post-translational regulators of many target genes, are not only markers of neoplastic disease initiation and progression, but also markers of response to anticancer therapy. Hundreds of miRNAs have been identified as biomarkers of drug resistance, and many have demonstrated the potential to sensitize cancer cells to therapy. Their properties of modulating the response of cells to therapy have made them a promising target for overcoming drug resistance. Several methods have been developed for the delivery of miRNAs to cancer cells, including introducing synthetic miRNA mimics, DNA plasmids containing miRNAs, and small molecules that epigenetically alter endogenous miRNA expression. The results of studies in animal models and preclinical studies for solid cancers and hematological malignancies have confirmed the effectiveness of treatment protocols using microRNA. Nevertheless, the use of miRNAs in anticancer therapy is not without limitations, including the development of a stable nanoconstruct, delivery method choices, and biodistribution. The aim of this review was to summarize the role of miRNAs in cancer treatment and to present new therapeutic concepts for these molecules. Supporting anticancer therapy with microRNA molecules has been verified in numerous clinical trials, which shows great potential in the treatment of cancer.
1. Introduction
MicroRNAs are an abundant class of endogenous small RNA molecules (18–22 nucleotides in length) that are noncoding post-transcriptional modulators of gene expression [1,2,3]. The human genome produces nearly 2000 miRNAs. Approximately 1900 precursors and 2600 mature human miRNA sequences are indexed in the miRBase database (http://www.mirbase.org/, accessed on 25 October 2021) [4]. The majority of miRNAs still await discovery, but some of them may be cancer-specific markers. They regulate gene expression by suppressing mRNA translation, mRNA cleavage, and mRNA decay initiated by miRNA-guided rapid deadenylation and reducing mRNA stability [5,6]. Each miRNA can control hundreds of target genes, so identifying key miRNA targets for cancer research is an important aspect.
Research on identifying single or sets of miRNAs, as regulators of cell proliferation and apoptosis processes, is currently one of the most promising areas of research. The role of these molecules is varied (Table 1). Some miRs exert negative control over the expression of many oncoproteins in normal cells, and therefore their deregulation is believed to be a significant mechanism underlying the development and progression of cancer. Due to their role in the process, there are three categories of microRNA: oncogenic miRNAs (oncomiRs), tumor suppressor miRNAs, and metastatic miRNAs (metastamiRs) (Table 1). The consequence of the overexpression of oncomiRs is the initiation, development, progression, and invasion of neoplastic disease. The miRNA classification is not clear, because many of the same miRNAs (e.g., miR-7 [7,8,9], miR-125b [10,11], miR-155 [12,13,14], and miR-30b/30d [15,16]) function as oncogenes and also as tumor suppressor genes [3,17]. This property, however, increases the therapeutic potential of these molecules.

Table 1.
Classes of miRNAs in cancer.
In many studies, it has been confirmed that microRNAs play important roles in the pathogenesis of human cancers. Identification of the expression patterns associated with specific tumor cell phenotypes may open new possibilities for the early diagnosis and therapy of cancer. Specific miRs expression patterns were identified for lung [31,32,33,34], breast [18,24,35,36,37], brain [38,39,40,41,42], liver [43,44,45,46,47,48], and colorectal cancer [49,50,51,52,53,54], and leukemia [2,55,56,57,58]. MiRNA signatures can be useful in developing new cancer prevention strategies and also new cancer therapy options. Although the role of miRNA in the pathogenesis of human cancers is proven, the exact mechanisms of regulation of the multiple stages of pathogenesis (initiation, promotion, malignant conversion, progression, and metastasis) are still unknown [5]. MicroRNAs are interesting biomarkers for several reasons, but the most important of them are the different expression patterns associated with the type of cancer, their remarkable stability, and their easy and noninvasive identification due to the presence in the blood and other body fluids [59].
2. MicroRNA’s Correlations with Therapy
The association between changes in the level of microRNA expression and therapy has been demonstrated for several years, including the relationship with drug resistance and modulation of the response to cancer therapy, as well as the development of new cancer therapy regimens and new targeted drugs. However, the introduction of miRNAs into therapy requires a holistic approach that takes into account both the multiplicity of miRs in cancer cells and the interactions of miRNAs with the immune system, tumor stromal cells, cancer therapies, and other factors extrinsic to the cancer cells themselves [17].
2.1. MicroRNA and Drug Resistance
The development of resistance to anticancer drugs is one of the most serious causes of therapy failure. Over the past few decades, this complex and multifactorial process of cancer cells acquiring resistance has been intensively studied, leading to the identification of various genetic biomarkers and mechanisms responsible for the phenomenon. Nevertheless, the interrelationships between specific cancer subtypes as well as the specific biomarkers and disturbances in the molecular pathways are still not fully understood and described. In the context of this research, in recent years, microRNAs have become an object of interest because of their ability to regulate the expression of genes involved in cell responses to drugs. Hundreds of miRNAs have been identified as biomarkers of drug resistance, and many have demonstrated the potential to sensitize cancer cells to therapy. Based on an analysis of their expression signatures, microRNAs playing a role in regulating the sensitivity of cells to anticancer therapy were selected [60,61,62,63,64] (Table 2). As resistance biomarkers, miRNAs can be useful in patient stratification and aid in the individualization of therapy.
MicroRNAs are primarily involved in the regulation of the expression of the efflux pumps of the ABC (ATP-binding cassette) transporter family, and thus significantly mediate their participation in the processes of absorption, distribution, and elimination of drugs and the development of resistance to them. By modulating the genes of ABC transporters, microRNAs are involved in the emergence of multidrug resistance, as well as MDR-related mechanisms such as apoptosis, autophagy, drug metabolism, and redox changes [65,66,67]. The P-glycoprotein (P-gp), encoded by the ABCB1 gene, also known as the multidrug-resistance gene (MDR1), is one of the most significant ABC type transporters and is responsible for resistance to a wide range of chemotherapy drugs. It has been experimentally shown that an increased level of P-gp in neoplastic cells may be a consequence of miR-451 and miR-27a overexpression (in the case of MDR and ovarian cancer cell lines) [68,69] or the negative regulatory role of miR-451 (in the case of breast cancer [70], leukemia cell lines [71], and hepatocellular carcinoma [72]). MicroRNAs associated with decreased P-glycoprotein expression have been identified: miR-137 (in the breast cancer cell line MCF-7) [73], miR-145 (in the colon carcinoma cell line Caco-2) [74], miR-200c (in breast cancer cell lines and tissue) [75], miR-223 (in HCC cells resistant to anticancer drugs) [76], miR-298 (in doxorubicin-resistant breast cancer cells [77]) miR-331-5p (in leukemia cell lines) [71], and miR-1253 (in the breast cancer cell line MDA-MB-231) [77]. Regulators responsible for the increase in P-gp expression levels are: miR-27a (in gastric and ovarian cell lines) [70,71], miR-138 (in adriamycin-resistant leukemia cell lines) [78], and miR-451 (in the ovarian cancer cell line A2780) [68]. Regulation of ABCB1 expression may also be associated with regulation of ABCB1 activity; consequently, MDR modulation may also be a consequence of the differential expression of miR-381, miR-495 [79], miR-9 [80], miR-122 [81], miR-873 [82], and miR-508-5p [83]. Markers of miRNAs have also been identified for other members of the ABC transporter family, including ABCG2/BCRP and ABCC1/MRP1 [84].
The consequence of the search for miRNA markers responsible for multidrug resistance in cancer cells is the development of the concept of breaking this resistance. Shang et al. [85] found that controlling the expression of two synergistic miRs, i.e., miR-508-5p and miR-27b, may be of therapeutic benefit in patients with gastric cancer. The study showed that multidrug resistance can be reversed by targeting ABCB1 and ZNRD1. The miR-27b/CCNG1/p53/miR-508-5p axis plays an important role in sensitizing chemoresistant tumors to in vivo and in vitro therapy. Equally promising observations of breaking down the resistance to therapy were made by Bitarte at al. [86] in colorectal cancer stem cells. It has been observed that transfection of the miR-451 precursor leads to a decreased potential for tumorigenicity and self-renewal of colon spheres. The study also showed a decreased level of cyclooxygenase-2 and P-gp, factors that inhibit macrophage migration. On the basis of the obtained results, it was proven that miR-451 can be considered a marker for predicting the response to irinotecan in patients with colon cancer, as a lower expression of miR-451 was characteristic of cells that did not show sensitivity to first-line therapy based on this drug. Pogribny et al. [9] showed the effects of changes in the expression level of miRs on the formation of a cisplatin-resistant phenotype by breast adenocarcinoma cells. The study identified 103 differentially expressed miRs correlated with the MCF-7 cell’s drug resistance. Of these, the largest changes in expression levels were found for miR-146a, miR-10a, miR-221/222, miR-345, miR-200b, and miR-200c. In addition, miR-345 and miR-7 have been shown to suppress drug efflux transporters, including human multidrug resistance-associated protein 1 (MRP1) [9]. These observations are interesting because of the dual nature of miR-7, which functions as both a tumor suppressor [7] and an oncomiR [8] for different types of cancer. Hence, it was noted that the inhibition of miR-7 in cancers for which it is an oncomiR can have a general harmful effect by increasing chemoresistance, despite slowing the growth of cancer cells. One of the most widely reported mechanisms for the regulation of epigenetic expression of genes associated with cisplatin resistance through miRNAs is the ovarian cancer model. For this type of cancer, selected marker microRNAs have been associated with a number of cell pathways and processes towards neoplasm, including abnormalities in the course of apoptosis (miR-27a-5p [87], miR-142-5p [88], miR-146a-5p [89], miR-424-3p [90], and miR-454 [91]), cell cycle disturbances and changes in the DNA repair pathway (miR-770-5p [92,93], miR-98-5p [94], and miR-409-3p [95]), abnormalities in signaling pathways (miR-7 [96], miR-106a [97], miR-205-5p [98], and miR-548e [99]), and also disturbances in the distribution of therapeutic particles as well as intensification and detoxification (miR-139-5p [100], miR-194-5p [101], miR-514 [102], and miR-595 [103]). Numerous in vivo and in vitro studies of ovarian tumor cells have demonstrated the possibility of restoring cisplatin sensitivity through targeted microRNA expression, and thus the effectiveness of chemotherapeutic treatment and patient survival. An example is overcoming resistance to this chemotherapeutic drug using miRNAs targeting ABC transporters. A decrease in the level of ABCB1 expression was made possible by introducing miR-186 [104] or miR-595 [103]. Such modulation towards ABCB1 suppression had an effect on the inhibition of tumor cell proliferation, metastasis, and drug resistance. A similar effect was observed for miR-130a [105] and miR-873 [82]. A slightly broader spectrum of influence was demonstrated for miR-514, which, through changes in the expression of ABCA1, ABCA10, and ABCF2 genes, inhibited the proliferation of ovarian cancer cells and increased sensitivity to cisplatin [102]. The relationship between changes in microRNA expression and the resistance of cancer cells to cisplatin was also demonstrated in the case of many solid and hematological cancers (Table 2).

Table 2.
Marker miRNAs involved in anticancer drug insensitivity mechanisms in human solid tumors.
Table 2.
Marker miRNAs involved in anticancer drug insensitivity mechanisms in human solid tumors.
| Drug | Cancer | MicroRNA |
|---|---|---|
| Cisplatin | Non-small-cell lung cancer | miR-21 [106,107], miR-107 [108], miR-200c [109], miR-451 [110] |
| Lung adenocarcinoma | miR-27a [111], miRNA-378 [112] | |
| Hepatocellular carcinoma | miR-101 [113], miR-130a [114], miR-182 [115], miR-199a-5p [116] | |
| Gastric cancer | miR-424 [117], miR-181a-2-3p [118], miR-3180-3p, miR-124-3p [119] | |
| Ovarian cancer | miR-21 [120,121], miR-125b, miR-133a [122], miR-15, miR-16 [123] | |
| Osteosarcoma | miR-21 [124], miR-16-5p [125] | |
| Neuroblastoma | miR-21 [126], miR-141 [127], miR-155 [128] | |
| 5-Fluorouracil | Hepatocellular carcinoma | miR-193a-3p [129] |
| Colorectal cancer | miR-587 [130], miR-125b-5p [131], miR-375-3p [132], miR-149 [133], miR-135, miR-182 [134], miR-3135b [135] | |
| Gastric cancer | miR-204 [136], miR-195 [137], miR-30a [138] | |
| Osteosarcoma | miR-140 [139] | |
| Lung cancer | miR-27a, miR-27b, miR-134 and miR-582-5p [140] | |
| Methotrexate | Colorectal adenocarcinoma | miR-770-5p [141], miR-24-3p [142], miR-505 [143] |
| Lung cancer | miR-200c [144] | |
| Osteosarcoma | miR-494-3p [145], miR-192 [146] | |
| Doxorubicin | Ovarian cancer | miR-146b-5p, miR-205 and miR-875-3p [147] |
| Gastric cancer | miR-494 [148] | |
| Neuroblastoma | miR-137 [149,150], miR-99b-5p, miR-380-3p, and miR-485-3p [151] | |
| Breast cancer | miR-200b, miR-17 [152], miR-127, miR-34a, miR-27b, miR-206, miR-21, miR-214, miR-28 and miR-451 [70], miR-200c [153] | |
| Paclitaxel | Ovarian cancer | miR-29a, miR-363, miR-18 and miR-20b [147], miR-130a, miR-30c, miR-335, miR-125b and let-7e [154] |
| Prostate cancer | miR-100-5p, miR-200b-3p, miR-34b-3p and miR-375 [155], miR-34a [156,157] | |
| Breast cancer | miR-21 [158] | |
| Non-small-cell lung cancer | miR-421 [159], miR-199-5a [160] | |
| Gefitinib | Non-small-cell lung cancer | miR-342-3p [161], miR-506-3p [162], miR-34a [163], miR-564 or miR-658 [164] |
| Docetaxel | Breast cancer | miRNA-452 [165], miR-34a [166] |
| Prostate cancer | miR-181a [167], miR-21 [168,169], miR-134 [170], miR-200 family [171,172] | |
| Gastric cancer | miR-15b, miR-16 [173] | |
| Oxaliplatin | Colon cancer | miR-137 [174], miR-519d, miR-545, miR-618 and miR-98 [175] |
| Colorectal cancer | miR-34a, miR-143, miR-153, miR-27a, miR-218, and miR-520 [176] | |
| Hepatocellular carcinoma | miR-125b [177] | |
| Topotecan | Ovarian cancer | miR-29a, miR-363, miR-31, miR-18 and miR-20b [147] |
| Renal cell carcinoma | miR-21 [178] | |
| Breast cancer | miR-21 [179] | |
| Fulvestrant | Breast cancer | let-7i, miR-346, miR-638, miR-181a, miR-191, miR-199b, miR-204, miR-211, miR-212, miR-216, miR-328, miR-373, miR-424, miR-768-3p, miR-221/222 [180] |
| Fludarabine | Leukemia | miR-21 and miR-222 [181], miR-29a, miR-181a, and miR-221 [182], miR-34a [183] |
| Etoposide | Neuroblastoma | miR-204 [184], miR-520f [185] |
| Gastric cancer | miR-15b, miR-16 [173] | |
| Lung cancer | miR-101 [186] | |
| Breast cancer | miR-132-3p [187] | |
| Tamoxifen | Breast cancer | miR-221/222 [188,189], miR-449a [190] |
| Mitoxantron | Breast cancer | miR-155, miR-206 [191], miR-328 [192] |
Activation of multidrug resistance is one of the factors of insensitivity to cancer cell therapy. The following are also responsible for the chemoresistance phenotype: abnormalities in autophagic induction (vesicle nucleation, vesicle elongation); changes in the activity of enzymes responsible for drug metabolism (P450 superfamily (CYP) metabolic enzymes); disturbances in the DNA repair pathway, cell cycle, and apoptosis; and changes in the levels of expression of drug targets [84]. Each of these factors has been experimentally shown to be modulated by microRNAs. The effectiveness of anticancer therapy is largely dependent on the proper metabolism of drugs, which is the responsibility of the P450 superfamily, the expression of which is regulated by microRNAs [193]. The results of in vitro and in silico analyses have indicated that as many as 56 CYP enzymes can be regulated by miRNAs [194], directly (by affecting the mRNA of the target cytochrome) or indirectly (by interacting with nuclear receptors (NRs), the constitutive androstane receptor (CAR), or vitamin D receptors) [195]. Cytochromes differ significantly in their extent of regulation, and the length of the mRNA 3′UTR is one of the reasons for this. Enzymes such as CYP1A1, 1A2, 1B1, 2B6, and 3A4 are the target of numerous miRNAs. In turn, CYP2A6, 2D6, 2E1, and 3A5 are regulated by a few specific miRNAs [196]. Among the regulators of the cytochrome superfamily, studies have mentioned miR-214-3p, miR-552, miR-570, and miR-378a-5p [193], and miR-378* for CYP2E1 [197]; miR-892a for CYP1A1 [198]; let-7b for CYP2J2 [199]; and miR-27a/b [195,200], miR-627 [201], miR-122, miR-378a-5p [202], and miR-148a [203] for CYP3A4.
One of the better described mechanisms of regulation is the interaction between miR-27b and CYP1B1 mRNA, which is responsible for the metabolism of a wide range of drugs. Low expression of this microRNA contributes to the overexpression of CYP1B1 and thus the induction of resistance to a chemotherapeutic agent (e.g., docetaxel in breast, colon, lung, or pancreatic carcinoma cells) [204,205]. These observations prompted attempts to sensitize cancer cells to drugs by reducing the detoxification of drugs metabolized by CYP1B1 by activating p53-dependent apoptosis [206]. In their experiment, Tsuchiya et al. [205] used transfection with antisense 20-O-methyl oligoribonucleotides (ASO), which acted as an inhibitor of miR-27, and thus demonstrated miR-27-dependent control of CYP1B1 gene expression in human MCF-7 breast cancer cells.
2.2. MicroRNA and Modulation of Drug Activity
MicroRNAs are broad-spectrum molecules. The same miRs can both increase the proliferation of cancer cells but sensitize them to treatment at the same time, resulting in increased overall survival. As one of the first studies, Esquela-Kerscher et al. [207] confirmed their therapeutic potential in cancer using the suppressive properties of microRNA. They experimentally demonstrated that it is possible to reduce tumor weight in lung cancer (in an animal model) by modulating the expression of let-7. Gasparini et al. [208] have shown that miR-155 increases the sensitivity to ionizing radiation therapy in patients with breast cancer. The goal of such therapy is to induce double-stranded DNA breaks in cancer cells. Researchers have described the mechanism by which miR-155 directly suppresses the expression of RAD51, a key protein for homologous recombination of DNA, thus blocking the repair of double-stranded DNA breaks and sensitizing triple-negative breast cancer to ionizing radiotherapy. On the basis of in silico analysis and an experiment with colorectal cancer cell lines, Boni et al. [209] investigated the effects of miR-192 and miR-215 on 5-fluoruracil sensitivity. These molecules are modulators of the expression of thymidylate synthase (TS), the expression of which is a predictive biomarker of responses to 5-FU in gastrointestinal tumors. However, on the basis of an analysis of the obtained results, it was found that lowering TS levels with miRNAs does not significantly affect the sensitivity of tumor cells to 5-FU therapy, although the overexpression of miR-192 and miR-215 is associated with a decrease in cell proliferation. Changes in the cell cycle have been associated with p53 status and p21 and p27 activation. As noted, they may result in 5-FU resistance, independent of thymidylate synthase expression. Hirota et al. [140] identified miR-27a, miR-27b, miR-134, and miR-582-5p as regulators of the sensitivity of lung cancer cells to 5-FU. According to their concept, the mechanism of resistance to this chemotherapeutic agent involves post-transcriptional regulation of the expression of dihydropyrimidine dehydrogenase (DPD), which is involved in the metabolism of 5-fluorouracil. The high activity of DPD in cancer cells is an important factor in the efficacy and toxicity of 5-FU therapy. Overexpression of these four miRNAs reduced the DPD gene.
Analogous relationships have been described for the sensitivity of cells to gemcitabine therapy for tumor cells. The study by Maftouh et al. [210] showed that induction of miR-211 expression in cells increased the sensitivity to gemcitabine and decreased the expression of its target, ribonucleotide reductase 2 subunits (RRM2). The chemical resistance of pancreatic cancer to nucleoside analogs (e.g., gemcitabine) is a result of RRM2 overexpression. Researchers were able to inhibit the migration and invasion of pancreatic ductal adenocarcinoma cells through forced miR-211 overexpression. The chemosensitivity of neoplastic cells may also be regulated by let-7, which is a negative regulator of RRM2. Bhutia et al. [211] described the complicated mechanism of post-transcriptional regulation of RRM2 and chemotherapy sensitivity by let-7a, as well as the sensitization of PDACs to gemcitabine. These are just a few examples of the regulation of tumor cell responses to anti-cancer drugs available in the scientific literature (Table 2 and Table 3). Most chemotherapeutic agents have attempted to describe the mechanisms of changing the sensitivity profile through the interaction of miRNAs with the mRNA of the target genes involved in drug metabolism or that are effectors.

Table 3.
List of microRNAs with therapeutic potential in human cancers.
3. MiRNA Delivery Systems
One of the greatest challenges of microRNA therapy is the development of efficient methods of delivery to effector cells. The method must provide both protection against unwanted degradation, and delivery into the cell and uptake without inducing an immunogenic response. Nanoconstructs used for delivery systems must be biocompatible and made of biodegradable materials [261,262]. Due to the small size of the molecule, strategies for delivering microRNA to the cell are similar to those used for interference RNA (siRNA) [227]. The most commonly used carriers are viral and nonviral vectors [263], with viral vectors having lost their importance due to triggering an immune response. Hence, nonviral vectors (e.g., polymeric vectors, lipid-based carriers, and inorganic materials) may be of the greatest importance in anticancer therapy [264,265].
Several routes of introducing the therapeutic construct into the organism have been tested in in vivo studies. Among them, the most frequently chosen are:
- -
- Tail vein, e.g., cationic liposomes with miR-29 in lung cancer [214], PEI-PEG with miR-34a in hepatocellular carcinoma [266], and exosomes with miR-145 in lung cancer [267];
- -
- Intratumoral, e.g., cationic liposomes with miR-7 in lung cancer [268], polymeric micelles with miR-205 in pancreatic cancer [269], and exosomes with miR-146b in glioma [270];
- -
- Intravenous, e.g., carbonate apatite with miR-4711-5p in colon cancer [271], atelocollagen with miR-16 in prostate cancer [272], and exosome-GE11 peptides with let-7 in breast cancer [273];
- -
- Subcutaneous, e.g., ionizable liposomes with miR-200c in lung cancer [274], PEI with miR-203 in basal cell carcinoma [275], and atelocollagen with mir-34a in colon cancer [246];
- -
- Intraperitoneal, e.g., PEI with miR-145 in colon carcinoma [249] and exosomes with miR-122 in hepatocellular carcinoma [276].
4. Therapeutic Approaches Using miRNA
The goal of miRNA-based therapies is to restore the normal function of deregulated cell pathways. There are two possible approaches, including through inhibition of oncogenic microRNA activity (miRNA inhibitors) or by restoring the function of tumor suppressor microRNAs (miRNA mimics). Oncogenic miRNAs can be blocked by using antisense oligonucleotides (ASO), and locked nucleic acids (LNA) such as antimir, anti-mir oligonucleotides (AMO), and antagomirs [4,277]. Another popular strategy for restoring miRNA activity includes the introduction of miRNA mimics or microRNAs coded by expression vectors (Figure 1). The development of an effective therapy requires not only the selection of the correct expression-modulating molecules but also the development of an appropriate cell delivery strategy. Viral and nonviral vectors (polymers and liposomes) and nanoconstructs have been used in the group of carriers. Methods based on nanotechnology are being developed and tested in terms of their potential clinical application in solid tumors. Tumor suppressor microRNAs are the most frequently used in supporting anticancer therapy. Their introduction into the cell reactivates cellular protherapeutic pathways. This approach is known as “miRNA replacement therapy”.
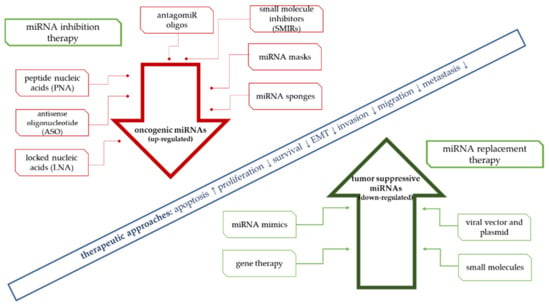
Figure 1.
Approaches for miRNA-based therapies.
MicroRNAs, as regulators of genes that are important for cancer progression, are increasingly being used in developing new therapeutic concepts in oncology. Analogous to the use of antisense mRNA and RNAi, miRs can be used to regulate the expression of the genes involved in carcinogenesis. High expression of most oncogenes is one of the key factors in the initiation of cancer (Figure 1). According to this assumption, artificial miRNAs are designed to block their expression based on the complementary miRNA properties of their target mRNAs, targeting silencing selected oncogenes. An example is the study of He et al. [27], in which it was confirmed in a mouse model that the induced expression of the miR-17-92 cluster resulted in strong inhibition of c-myc-induced apoptosis. The consequence of these changes was intensification of the tumor process.
It has been estimated that such therapy is less toxic compared with other anticancer drugs. For this reason, nanoconstructs containing synthetic antisense oligonucleotides coding complementary sequences to deliver mature oncogenic miRNAs (anti-miRNA oligonucleotides (AMOs)) are being developed [3]. The goal of such therapy is to effectively inactivate the overexpressed miRNAs in cancer cells and, consequently, slow their growth. Clinical trials have confirmed the ability of this class of drugs to significantly suppress the target genes’ expression. Silencing of targeted miRNAs in vivo could be achieved by using antisense oligonucleotides with various nucleic acid analogs involving LNA, AMO, PNAs, or nanoencapsulated PNAs [278,279,280,281]. Many antimiR delivery and targeting strategies have been described (Table 3).
There have been numerous experimental and preclinical attempts to develop therapy protocols. In a mouse model, Krützfeldt et al. [282] showed the possibility of effective inhibition of miRNA activity in various organs via antagomirs in the form of cholesterol-conjugated AMO. Interesting observations were also made by Dickins et al. [283], who found that miR-30 based shRNA (shRNA-miRs) inhibits gene expression under the control of Pol II promoters. Researchers also observed that with Trp53 knockdown using tetracycline-based systems and gene knockdown by the expression of shRNA-miRs (similar to the overexpression of protein-coding cDNAs), it was possible to control tumor growth. Another approach is to use miRNA overexpression techniques, based on transient expression systems, that function as tumor suppressors (e.g., encoded by the let-7 family). This method uses viruses or liposomes, which supply large amounts of miRNA. The construct uses the flanking sequences of pre-miRNA under the control of tissue-specific promoters, thanks to which, it is possible to stimulate and control the endogenous expression of selected molecules in the target cells. Nevertheless, it has been experimentally shown that an immune response may be an obstacle to achieving a therapeutic effect [284,285]. Gokito et al. [286] confirmed the therapeutic efficacy of forced expression of mimic miR-634 in a mouse model. In their study, they used a system of lipid nanoparticles (ionizable lipids: L021-LNP) that they administered to subjects intravenously. After introducing the constructs into the system, they observed the pro-apoptotic effect of the therapeutic agent, resulting in inhibition of pancreatic tumor growth. The disadvantage of this solution was, however, moderate hepatotoxicity. Wang et al. [287] analyzed the effect of miR-16-5p expression in breast cancer cell lines on ANLN inhibition. In their model, they used mimic miR-16-5p and si-ANLN, thanks to which, they demonstrated the effect of this marker miRNA on slowing down the proliferation and inhibition of cell migration and invasion. After miR-16-5p overexpression, breast cancer cells were arrested in the G2/M phase. These observations were consistent with those previously published by Magnusson et al. [288], who also demonstrated activation of the apoptotic processes of tumor cells after miR-16-5p overexpression by mimic miRNAs, resulting in subsequent suppression of ANLN expression.
The effectiveness of antimiR-based therapies in vivo is hampered by physiological and cellular barriers to delivery to the target cells. Cheng et al. [289] attempted to overcome the barriers of the tumor microenvironment in order to effectively provide therapeutic antisense oligomers. Researchers have developed a platform that targets the acidic tumor microenvironment, evades systemic clearance by the liver, and facilitates cell entry via a non-endocytic pathway. For this purpose, they developed a model targeted therapy, based on a construct containing the peptide nucleic acid (PNA) antimiRs and a peptide with a low pH-induced transmembrane structure (pHLIP) [289]. Researchers first tested platforms containing miR-155 (pHLIP-anti155) delivered to A549 cells and Toledo diffuse large-B cell lymphoma (DLBCL) cells. Nevertheless, they confirmed that the method can be effective for other miRNA molecules (e.g., miR-182, miR-21, and miR-210) and for many other types of cancer cells. The condition is the course of endocytosis, taking the transport properties of pHLIP into account. Brognara et al. [290] tested the biological activity of PNA directed against miR-221 in U251, U373, and T98G human glioma cell lines, using a PNA construct conjugated to an arginine peptide tail.
5. Limitations of Replacement Therapy
One of the key challenges in implementing miRNA therapy is the development of clinically cost-effective and effective delivery materials. MicroRNA therapies appear to be effective, especially when mimic molecules are used as endogenous miRNAs to restore tumor suppressor function [264,291]. Nevertheless, the systemic introduction of microRNA mimics carries the risk of integrating their function not only in neoplastic cells but also in properly functioning cells [292,293]. Despite the promising data from in vitro experiments and animal models for breast, intestine, gastric, lung, and hematological neoplasms confirming their therapeutic efficacy, side effects in the form of toxicity and induction of immune and inflammatory responses have also been noticed [294].
Despite the promising results of scientific and preclinical research, attempts to implement therapies with the use of the miRNA nanostructure in oncological clinical trials have been unsuccessful (Table 4). The main reasons are the technical barriers to the effective introduction of therapeutic molecules into the body, especially degradation by nucleases and an unfavorable immune reaction. One of these disadvantages was noted at the stage of Phase I clinical trials in an attempt to introduce miRNA replacement therapy with MRX34, the aim of which was to restore miR-34 expression in cancer patients [295]. Due to the strong immune responses that resulted in the death of four patients, the trials were stopped.

Table 4.
Clinical trials of miRNA therapy in oncology (based on https://clinicaltrials.gov, accessed on 10 January 2022).
Quite promising results of studies on the introduction of miRNA into clinical practice were obtained by van Zandwijk et al. [296] in a Phase I study, which checked the safety and activity of miR-16-loaded bacterial minicells (TargomiR) in the treatment of patients with recurrent malignant pleural mesothelioma. The nanoconstruct targeted EGFR (a mesothelioma overexpression receptor), and the main targets were genes involved in the progression of this cancer, e.g., BCL2, CDK1, and JUN. The use of the miR-16 mimic is a new therapeutic approach for this cancer, especially as palliative chemotherapy is the only available course for the majority of patients [297]. The developed nanoconstruct had to overcome the fibrous nature of the tumor barrier and to protect the nucleic acid from degradation in the peripheral line after intravenous administration. However, the authors failed to confirm the effective delivery of miR16-mimetics to tumor sites in vivo. In addition, many side effects were observed, the most serious of which was increased inflammation. The bacterial origin of the carrier was indicated as the probable cause of the induction of inflammation [296,297,298].
6. Conclusions
MiRNom analyses provided insights into the underlying mechanisms of oncogenesis. As strategic regulators of gene expression, small microRNAs are believed to be relatively simple for designing therapeutic agents as compared with antisense oligonucleotides, DNA and mRNA vaccines, or gene therapy vectors. The advantage of miRNAs is that as natural cell components, they should not cause undesirable effects and toxicity. The importance of microRNAs as therapeutic agents in recent years has increased, along with growing knowledge about the changes in cancer miRNAs affecting the response to therapy. It is therefore not surprising that there have been more and more attempts to include miRNAs in therapeutic protocols as part of targeted drugs. The promising results of experimental studies have proven the high effectiveness and low antigenicity of such personalized therapy. However, the clinical use of miRs in cancer therapy primarily requires the identification of specific miRNAs in a particular type of cancer and understanding their mechanisms of action. The next step is to develop a range of therapeutic manipulation methods and a way to deliver miRNA to target cells/tissues that has efficacy after overcoming immune barriers, as well as maintaining their stabilization and continuous activity. There is also no lack of scientific evidence that nanoconstructs containing mimics or antagomirs face barriers related to immunosuppression. Therefore, despite the promising results, the introduction of miRNAs as routine therapy in clinical practice is significantly difficult.
Author Contributions
J.S.: concept, article design, literature interpretation, manuscript preparation and revision; M.S.: selection of literature and consultation on the graphic side; A.T.: substantive consultation and approval of the final version. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Szczepanek, J. Role of microRNA dysregulation in childhood acute leukemias: Diagnostics, monitoring and therapeutics: A comprehensive review. World J. Clin. Oncol. 2020, 11, 348–369. [Google Scholar] [CrossRef]
- Esquela-Kerscher, A.; Slack, F.J. Oncomirs—microRNAs with a role in cancer. Nat. Rev. Cancer 2006, 6, 259–269. [Google Scholar] [CrossRef]
- Tessitore, A.; Cicciarelli, G.; Mastroiaco, V.; Del Vecchio, F.; Capece, D.; Verzella, D.; Fischietti, M.; Vecchiotti, D.; Zazzeroni, F.; Alesse, E. Therapeutic Use of MicroRNAs in Cancer. Anti-Cancer Agents Med. Chem. 2015, 16, 7–19. [Google Scholar] [CrossRef]
- Zhang, B.; Pan, X.; Cobb, G.P.; Anderson, T.A. MicroRNAs as oncogenes and tumor suppressors. Dev. Biol. 2007, 302, 1–12. [Google Scholar] [CrossRef]
- Cho, W.C. OncomiRs: The discovery and progress of microRNAs in cancers. Mol. Cancer 2007, 6, 60. [Google Scholar] [CrossRef]
- Kalinowski, F.C.; Brown, R.A.; Ganda, C.; Giles, K.M.; Epis, M.R.; Horsham, J.; Leedman, P.J. microRNA-7: A tumor suppressor miRNA with therapeutic potential. Int. J. Biochem. Cell Biol. 2014, 54, 312–317. [Google Scholar] [CrossRef]
- Chou, Y.T.; Lin, H.H.; Lien, Y.C.; Wang, Y.H.; Hong, C.F.; Kao, Y.R.; Lin, S.C.; Chang, Y.C.; Lin, S.Y.; Chen, S.J.; et al. EGFR promotes lung tumorigenesis by activating miR-7 through a Ras/ERK/Myc pathway that targets the Ets2 transcriptional repressor ERF. Cancer Res. 2010, 70, 8822–8831. [Google Scholar] [CrossRef]
- Pogribny, I.P.; Filkowski, J.N.; Tryndyak, V.P.; Golubov, A.; Shpyleva, S.I.; Kovalchuk, O. Alterations of microRNAs and their targets are associated with acquired resistance of MCF-7 breast cancer cells to cisplatin. Int. J. Cancer 2010, 127, 1785–1794. [Google Scholar] [CrossRef]
- Shaham, L.; Binder, V.; Gefen, N.; Borkhardt, A.; Izraeli, S. MiR-125 in normal and malignant hematopoiesis. Leukemia 2012, 26, 2011–2018. [Google Scholar] [CrossRef]
- Tili, E.; Michaille, J.J.; Luo, Z.; Volinia, S.; Rassenti, L.Z.; Kipps, T.J.; Croce, C.M. The down-regulation of miR-125b in chronic lymphocytic leukemias leads to metabolic adaptation of cells to a transformed state. Blood 2012, 120, 2631–2638. [Google Scholar] [CrossRef]
- Li, C.L.; Nie, H.; Wang, M.; Su, L.P.; Li, J.F.; Yu, Y.Y.; Yan, M.; Qu, Q.L.; Zhu, Z.G.; Liu, B.Y. microRNA-155 is downregulated in gastric cancer cells and involved in cell metastasis. Oncol. Rep. 2012, 27, 1960–1966. [Google Scholar] [CrossRef]
- Palma, C.A.; Al Sheikha, D.; Lim, T.K.; Bryant, A.; Vu, T.T.; Jayaswal, V.; Ma, D.D. MicroRNA-155 as an inducer of apoptosis and cell differentiation in Acute Myeloid Leukaemia. Mol. Cancer 2014, 13, 79. [Google Scholar] [CrossRef]
- Qin, W.; Ren, Q.; Liu, T.; Huang, Y.; Wang, J. MicroRNA-155 is a novel suppressor of ovarian cancer-initiating cells that targets CLDN1. FEBS Lett. 2013, 587, 1434–1439. [Google Scholar] [CrossRef]
- Kao, C.J.; Martiniez, A.; Shi, X.B.; Yang, J.; Evans, C.P.; Dobi, A.; DeVere White, R.W.; Kung, H.J. miR-30 as a tumor suppressor connects EGF/Src signal to ERG and EMT. Oncogene 2014, 33, 2495–2503. [Google Scholar] [CrossRef]
- Gaziel-Sovran, A.; Segura, M.F.; Di Micco, R.; Collins, M.K.; Hanniford, D.; Vega-Saenz de Miera, E.; Rakus, J.F.; Dankert, J.F.; Shang, S.; Kerbel, R.S.; et al. miR-30b/30d regulation of GalNAc transferases enhances invasion and immunosuppression during metastasis. Cancer Cell 2011, 20, 104–118. [Google Scholar] [CrossRef]
- Svoronos, A.A.; Engelman, D.M.; Slack, F.J. OncomiR or Tumor Suppressor? The Duplicity of MicroRNAs in Cancer. Cancer Res. 2016, 76, 3666–3670. [Google Scholar] [CrossRef]
- Blenkiron, C.; Goldstein, L.D.; Thorne, N.P.; Spiteri, I.; Chin, S.-F.; Dunning, M.J.; Barbosa-Morais, N.L.; Teschendorff, A.E.; Green, A.R.; Ellis, I.O.; et al. MicroRNA expression profiling of human breast cancer identifies new markers of tumor subtype. Genome Biol. 2007, 8, R214. [Google Scholar] [CrossRef]
- Sachdeva, M.; Mito, J.K.; Lee, C.L.; Zhang, M.; Li, Z.; Dodd, R.D.; Cason, D.; Luo, L.; Ma, Y.; Van Mater, D.; et al. MicroRNA-182 drives metastasis of primary sarcomas by targeting multiple genes. J. Clin. Investig. 2014, 124, 4305–4319. [Google Scholar] [CrossRef]
- Kim, J.; Yao, F.; Xiao, Z.; Sun, Y.; Ma, L. MicroRNAs and metastasis: Small RNAs play big roles. Cancer Metastasis Rev. 2018, 37, 5–15. [Google Scholar] [CrossRef]
- Lopez-Camarillo, C.; Marchat, L.A.; Arechaga-Ocampo, E.; Perez-Plasencia, C.; Del Moral-Hernandez, O.; Castaneda-Ortiz, E.J.; Rodriguez-Cuevas, S. MetastamiRs: Non-coding MicroRNAs driving cancer invasion and metastasis. Int. J. Mol. Sci. 2012, 13, 1347–1379. [Google Scholar] [CrossRef]
- Takamizawa, J.; Konishi, H.; Yanagisawa, K.; Tomida, S.; Osada, H.; Endoh, H.; Harano, T.; Yatabe, Y.; Nagino, M.; Nimura, Y.; et al. Reduced Expression of thelet-7MicroRNAs in Human Lung Cancers in Association with Shortened Postoperative Survival. Cancer Res. 2004, 64, 3753–3756. [Google Scholar] [CrossRef]
- Johnson, S.M.; Grosshans, H.; Shingara, J.; Byrom, M.; Jarvis, R.; Cheng, A.; Labourier, E.; Reinert, K.L.; Brown, D.; Slack, F.J. RAS Is Regulated by the let-7 MicroRNA Family. Cell 2005, 120, 635–647. [Google Scholar] [CrossRef]
- Iorio, M.V.; Ferracin, M.; Liu, C.G.; Veronese, A.; Spizzo, R.; Sabbioni, S.; Magri, E.; Pedriali, M.; Fabbri, M.; Campiglio, M.; et al. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005, 65, 7065–7070. [Google Scholar] [CrossRef]
- Metzler, M.; Wilda, M.; Busch, K.; Viehmann, S.; Borkhardt, A. High expression of precursor microRNA-155/BIC RNA in children with Burkitt lymphoma. Genes Chromosomes Cancer 2004, 39, 167–169. [Google Scholar] [CrossRef]
- Kluiver, J.; Haralambieva, E.; De Jong, D.; Blokzijl, T.; Jacobs, S.; Kroesen, B.J.; Poppema, S.; Van den Berg, A. Lack of BIC and microRNA miR-155 expression in primary cases of Burkitt lymphoma. Genes Chromosomes Cancer 2006, 45, 147–153. [Google Scholar] [CrossRef]
- He, L.; Thomson, J.M.; Hemann, M.T.; Hernando-Monge, E.; Mu, D.; Goodson, S.; Powers, S.; Cordon-Cardo, C.; Lowe, S.W.; Hannon, G.J.; et al. A microRNA polycistron as a potential human oncogene. Nature 2005, 435, 828–833. [Google Scholar] [CrossRef]
- Hayashita, Y.; Osada, H.; Tatematsu, Y.; Yamada, H.; Yanagisawa, K.; Tomida, S.; Yatabe, Y.; Kawahara, K.; Sekido, Y.; Takahashi, T. A Polycistronic MicroRNA Cluster,miR-17-92, Is Overexpressed in Human Lung Cancers and Enhances Cell Proliferation. Cancer Res. 2005, 65, 9628–9632. [Google Scholar] [CrossRef]
- Zhang, C.Z.; Zhang, J.X.; Zhang, A.L.; Shi, Z.D.; Han, L.; Jia, Z.F.; Yang, W.D.; Wang, G.X.; Jiang, T.; You, Y.P.; et al. MiR-221 and miR-222 target PUMA to induce cell survival in glioblastoma. Mol. Cancer 2010, 9, 229. [Google Scholar] [CrossRef]
- Chun-Zhi, Z.; Lei, H.; An-Ling, Z.; Yan-Chao, F.; Xiao, Y.; Guang-Xiu, W.; Zhi-Fan, J.; Pei-Yu, P.; Qing-Yu, Z.; Chun-Sheng, K. MicroRNA-221 and microRNA-222 regulate gastric carcinoma cell proliferation and radioresistance by targeting PTEN. BMC Cancer 2010, 10, 367. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Arora, S.; Prakasam, G.; Calin, G.A.; Syed, M.A. MicroRNA in lung cancer: Role, mechanisms, pathways and therapeutic relevance. Mol. Asp. Med. 2019, 70, 3–20. [Google Scholar] [CrossRef]
- Wu, K.-L.; Tsai, Y.-M.; Lien, C.-T.; Kuo, P.-L.; Hung, J.-Y. The Roles of MicroRNA in Lung Cancer. Int. J. Mol. Sci. 2019, 20, 1611. [Google Scholar] [CrossRef]
- Wu, S.-G.; Chang, T.-H.; Liu, Y.-N.; Shih, J.-Y. MicroRNA in Lung Cancer Metastasis. Cancers 2019, 11, 265. [Google Scholar] [CrossRef]
- Castro, D.; Moreira, M.; Gouveia, A.M.; Pozza, D.H.; De Mello, R.A. MicroRNAs in lung cancer. Oncotarget 2017, 8, 81679–81685. [Google Scholar] [CrossRef]
- Heneghan, H.M.; Miller, N.; Lowery, A.J.; Sweeney, K.J.; Newell, J.; Kerin, M.J. Circulating microRNAs as Novel Minimally Invasive Biomarkers for Breast Cancer. Ann. Surg. 2010, 251, 499–505. [Google Scholar] [CrossRef]
- Dvinge, H.; Git, A.; Gräf, S.; Salmon-Divon, M.; Curtis, C.; Sottoriva, A.; Zhao, Y.; Hirst, M.; Armisen, J.; Miska, E.A.; et al. The shaping and functional consequences of the microRNA landscape in breast cancer. Nature 2013, 497, 378–382. [Google Scholar] [CrossRef]
- Negrini, M.; Calin, G.A. Breast cancer metastasis: A microRNA story. Breast Cancer Res. 2008, 10, 303. [Google Scholar] [CrossRef]
- Banelli, B.; Forlani, A.; Allemanni, G.; Morabito, A.; Pistillo, M.P.; Romani, M. MicroRNA in Glioblastoma: An Overview. Int. J. Genom. 2017, 2017, 7639084. [Google Scholar] [CrossRef]
- Mathupala, S.P.; Mittal, S.; Guthikonda, M.; Sloan, A.E. MicroRNA and Brain Tumors: A Cause and a Cure? DNA Cell Biol. 2007, 26, 301–310. [Google Scholar] [CrossRef]
- Petrescu, G.E.D.; Sabo, A.A.; Torsin, L.I.; Calin, G.A.; Dragomir, M.P. MicroRNA based theranostics for brain cancer: Basic principles. J. Exp. Clin. Cancer Res. 2019, 38, 231. [Google Scholar] [CrossRef]
- Zhou, X.; Kang, C.; Pu, P. MicroRNA and brain tumors. Chin. J. Clin. Oncol. 2007, 4, 355–359. [Google Scholar] [CrossRef]
- Turner, J.D.; Williamson, R.; Almefty, K.K.; Nakaji, P.; Porter, R.; Tse, V.; Kalani, M.Y.S. The many roles of microRNAs in brain tumor biology. Neurosurg. Focus 2010, 28, E3. [Google Scholar] [CrossRef]
- Callegari, E.; Gramantieri, L.; Domenicali, M.; D’Abundo, L.; Sabbioni, S.; Negrini, M. MicroRNAs in liver cancer: A model for investigating pathogenesis and novel therapeutic approaches. Cell Death Differ. 2014, 22, 46–57. [Google Scholar] [CrossRef]
- Braconi, C.; Henry, J.C.; Kogure, T.; Schmittgen, T.; Patel, T. The Role of MicroRNAs in Human Liver Cancers. Semin. Oncol. 2011, 38, 752–763. [Google Scholar] [CrossRef]
- Lin, H.; Lin-Hui, L.; Xiang-Huo, H. Role of microRNAs in inflammation-associated liver cancer. Cancer Biol. Med. 2016, 13, 407. [Google Scholar] [CrossRef]
- Tao, J.; Jiang, L.; Chen, X. Roles of microRNA in liver cancer. Liver Res. 2018, 2, 61–72. [Google Scholar] [CrossRef]
- Crunkhorn, S. microRNA suppresses liver cancer. Nat. Rev. Cancer 2009, 9, 532. [Google Scholar] [CrossRef]
- Onishi, M.; Ochiya, T.; Tanaka, Y. MicroRNA and liver cancer. Cancer Drug Resist. 2020, 3, 386–400. [Google Scholar] [CrossRef]
- Ding, L.; Lan, Z.; Xiong, X.; Ao, H.; Feng, Y.; Gu, H.; Yu, M.; Cui, Q. The Dual Role of MicroRNAs in Colorectal Cancer Progression. Int. J. Mol. Sci. 2018, 19, 2791. [Google Scholar] [CrossRef]
- Chen, B.; Xia, Z.; Deng, Y.-N.; Yang, Y.; Zhang, P.; Zhu, H.; Xu, N.; Liang, S. Emerging microRNA biomarkers for colorectal cancer diagnosis and prognosis. Open Biol. 2019, 9, 180212. [Google Scholar] [CrossRef]
- Zhu, J.; Xu, Y.; Liu, S.; Qiao, L.; Sun, J.; Zhao, Q. MicroRNAs Associated With Colon Cancer: New Potential Prognostic Markers and Targets for Therapy. Front. Bioeng. Biotechnol. 2020, 8, 176. [Google Scholar] [CrossRef]
- Baran, B.; Ozupek, N.-M.; Calibasi-Kocal, G.; Basbinar, Y. MicroRNAs (miRNAs) in Colorectal Cancer. In Oncogenes and Carcinogenesis; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Fadaka, A.O.; Pretorius, A.; Klein, A. Biomarkers for Stratification in Colorectal Cancer: MicroRNAs. Cancer Control 2019, 26, 2046–2053. [Google Scholar] [CrossRef]
- Roemer, K.; Gmerek, L.; Martyniak, K.; Horbacka, K.; Krokowicz, P.; Scierski, W.; Golusinski, P.; Golusinski, W.; Schneider, A.; Masternak, M.M. MicroRNA regulation in colorectal cancer tissue and serum. PLoS ONE 2019, 14, e0222013. [Google Scholar] [CrossRef]
- Wallace, J.A.; O’Connell, R.M. MicroRNAs and acute myeloid leukemia: Therapeutic implications and emerging concepts. Blood 2017, 130, 1290–1301. [Google Scholar] [CrossRef]
- Trino, S.; Lamorte, D.; Caivano, A.; Laurenzana, I.; Tagliaferri, D.; Falco, G.; Del Vecchio, L.; Musto, P.; De Luca, L. MicroRNAs as New Biomarkers for Diagnosis and Prognosis, and as Potential Therapeutic Targets in Acute Myeloid Leukemia. Int. J. Mol. Sci. 2018, 19, 460. [Google Scholar] [CrossRef]
- Roman-Gomez, J.; Agirre, X.; Jiménez-Velasco, A.; Arqueros, V.; Vilas-Zornoza, A.; Rodriguez-Otero, P.; Martin-Subero, I.; Garate, L.; Cordeu, L.; San José-Eneriz, E.; et al. Epigenetic Regulation of MicroRNAs in Acute Lymphoblastic Leukemia. J. Clin. Oncol. 2009, 27, 1316–1322. [Google Scholar] [CrossRef]
- Schotte, D.; De Menezes, R.X.; Moqadam, F.A.; Khankahdani, L.M.; Lange-Turenhout, E.; Chen, C.; Pieters, R.; Den Boer, M.L. MicroRNA characterize genetic diversity and drug resistance in pediatric acute lymphoblastic leukemia. Haematologica 2011, 96, 703–711. [Google Scholar] [CrossRef]
- Krutovskikh, V.A.; Herceg, Z. Oncogenic microRNAs (OncomiRs) as a new class of cancer biomarkers. Bioessays 2010, 32, 894–904. [Google Scholar] [CrossRef]
- Hummel, R.; Hussey, D.J.; Haier, J. MicroRNAs: Predictors and modifiers of chemo- and radiotherapy in different tumour types. Eur. J. Cancer 2010, 46, 298–311. [Google Scholar] [CrossRef]
- Sarkar, F.H.; Li, Y.; Wang, Z.; Kong, D.; Ali, S. Implication of microRNAs in drug resistance for designing novel cancer therapy. Drug Resist. Updat. 2010, 13, 57–66. [Google Scholar] [CrossRef]
- Kim, J.G.; Park, M.T.; Heo, K.; Yang, K.M.; Yi, J.M. Epigenetics meets radiation biology as a new approach in cancer treatment. Int. J. Mol. Sci. 2013, 14, 15059–15073. [Google Scholar] [CrossRef] [PubMed]
- Pan, B.; Yi, J.; Song, H. MicroRNA-mediated autophagic signaling networks and cancer chemoresistance. Cancer Biother. Radiopharm. 2013, 28, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Migliore, C.; Giordano, S. Resistance to targeted therapies: A role for microRNAs? Trends Mol. Med. 2013, 19, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Blower, P.E.; Chung, J.H.; Verducci, J.S.; Lin, S.; Park, J.K.; Dai, Z.; Liu, C.G.; Schmittgen, T.D.; Reinhold, W.C.; Croce, C.M.; et al. MicroRNAs modulate the chemosensitivity of tumor cells. Mol. Cancer Ther. 2008, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, M.; Li, Y.; Han, B. The Role of MicroRNAs in the Chemoresistance of Breast Cancer. Drug Dev. Res. 2015, 76, 368–374. [Google Scholar] [CrossRef]
- Gupta, S.; Silveira, D.A.; Mombach, J.C.M. Towards DNA-damage induced autophagy: A Boolean model of p53-induced cell fate mechanisms. DNA Repair 2020, 96, 102971. [Google Scholar] [CrossRef]
- Zhu, H.; Wu, H.; Liu, X.; Evans, B.R.; Medina, D.J.; Liu, C.G.; Yang, J.M. Role of MicroRNA miR-27a and miR-451 in the regulation of MDR1/P-glycoprotein expression in human cancer cells. Biochem. Pharmacol. 2008, 76, 582–588. [Google Scholar] [CrossRef]
- Li, Z.; Hu, S.; Wang, J.; Cai, J.; Xiao, L.; Yu, L.; Wang, Z. MiR-27a modulates MDR1/P-glycoprotein expression by targeting HIPK2 in human ovarian cancer cells. Gynecol. Oncol. 2010, 119, 125–130. [Google Scholar] [CrossRef]
- Kovalchuk, O.; Filkowski, J.; Meservy, J.; Ilnytskyy, Y.; Tryndyak, V.P.; Chekhun, V.F.; Pogribny, I.P. Involvement of microRNA-451 in resistance of the MCF-7 breast cancer cells to chemotherapeutic drug doxorubicin. Mol. Cancer Ther. 2008, 7, 2152–2159. [Google Scholar] [CrossRef]
- Feng, D.D.; Zhang, H.; Zhang, P.; Zheng, Y.S.; Zhang, X.J.; Han, B.W.; Luo, X.Q.; Xu, L.; Zhou, H.; Qu, L.H.; et al. Down-regulated miR-331-5p and miR-27a are associated with chemotherapy resistance and relapse in leukaemia. J. Cell Mol. Med. 2011, 15, 2164–2175. [Google Scholar] [CrossRef]
- Chen, Z.; Ma, T.; Huang, C.; Zhang, L.; Lv, X.; Xu, T.; Hu, T.; Li, J. MiR-27a modulates the MDR1/P-glycoprotein expression by inhibiting FZD7/beta-catenin pathway in hepatocellular carcinoma cells. Cell Signal 2013, 25, 2693–2701. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Li, Y.; Shen, H.; Li, H.; Long, L.; Hui, L.; Xu, W. miR-137 restoration sensitizes multidrug-resistant MCF-7/ADM cells to anticancer agents by targeting YB-1. Acta Biochim. Biophys. Sin. 2013, 45, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Uchino, K.; Ochiya, T.; Takeshita, F. RNAi therapeutics and applications of microRNAs in cancer treatment. Jpn. J. Clin. Oncol. 2013, 43, 596–607. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Tian, W.; Cai, H.; He, H.; Deng, Y. Down-regulation of microRNA-200c is associated with drug resistance in human breast cancer. Med. Oncol. 2012, 29, 2527–2534. [Google Scholar] [CrossRef]
- Yang, T.; Zheng, Z.M.; Li, X.N.; Li, Z.F.; Wang, Y.; Geng, Y.F.; Bai, L.; Zhang, X.B. MiR-223 modulates multidrug resistance via downregulation of ABCB1 in hepatocellular carcinoma cells. Exp. Biol. Med. 2013, 238, 1024–1032. [Google Scholar] [CrossRef]
- Bao, L.; Hazari, S.; Mehra, S.; Kaushal, D.; Moroz, K.; Dash, S. Increased expression of P-glycoprotein and doxorubicin chemoresistance of metastatic breast cancer is regulated by miR-298. Am. J. Pathol. 2012, 180, 2490–2503. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, L.; Hu, J.; Ruan, J. miR-138 might reverse multidrug resistance of leukemia cells. Leuk. Res. 2010, 34, 1078–1082. [Google Scholar] [CrossRef]
- Xu, Y.; Ohms, S.J.; Li, Z.; Wang, Q.; Gong, G.; Hu, Y.; Mao, Z.; Shannon, M.F.; Fan, J.Y. Changes in the expression of miR-381 and miR-495 are inversely associated with the expression of the MDR1 gene and development of multi-drug resistance. PLoS ONE 2013, 8, e82062. [Google Scholar] [CrossRef]
- Munoz, J.L.; Bliss, S.A.; Greco, S.J.; Ramkissoon, S.H.; Ligon, K.L.; Rameshwar, P. Delivery of Functional Anti-miR-9 by Mesenchymal Stem Cell-derived Exosomes to Glioblastoma Multiforme Cells Conferred Chemosensitivity. Mol. Ther. Nucleic Acids 2013, 2, e126. [Google Scholar] [CrossRef]
- Lin, C.J.; Gong, H.Y.; Tseng, H.C.; Wang, W.L.; Wu, J.L. miR-122 targets an anti-apoptotic gene, Bcl-w, in human hepatocellular carcinoma cell lines. Biochem. Biophys. Res. Commun. 2008, 375, 315–320. [Google Scholar] [CrossRef]
- Wu, D.D.; Li, X.S.; Meng, X.N.; Yan, J.; Zong, Z.H. MicroRNA-873 mediates multidrug resistance in ovarian cancer cells by targeting ABCB1. Tumour Biol. 2016, 37, 10499–10506. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Zhang, Z.; Liu, Z.; Feng, B.; Ren, G.; Li, K.; Zhou, L.; Sun, Y.; Li, M.; Zhou, J.; et al. miR-508-5p regulates multidrug resistance of gastric cancer by targeting ABCB1 and ZNRD1. Oncogene 2014, 33, 3267–3276. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Sarmiento, C.; Tan, T.; Zhu, H. Regulation of multidrug resistance by microRNAs in anti-cancer therapy. Acta Pharm. Sin. B 2017, 7, 38–51. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.; Feng, B.; Zhou, L.; Ren, G.; Zhang, Z.; Fan, X.; Sun, Y.; Luo, G.; Liang, J.; Wu, K.; et al. The miR27b-CCNG1-P53-miR-508-5p axis regulates multidrug resistance of gastric cancer. Oncotarget 2016, 7, 538–549. [Google Scholar] [CrossRef]
- Bitarte, N.; Bandres, E.; Boni, V.; Zarate, R.; Rodriguez, J.; Gonzalez-Huarriz, M.; Lopez, I.; Javier Sola, J.; Alonso, M.M.; Fortes, P.; et al. MicroRNA-451 is involved in the self-renewal, tumorigenicity, and chemoresistance of colorectal cancer stem cells. Stem Cells 2011, 29, 1661–1671. [Google Scholar] [CrossRef]
- Wambecke, A.; Ahmad, M.; Morice, P.M.; Lambert, B.; Weiswald, L.B.; Vernon, M.; Vigneron, N.; Abeilard, E.; Brotin, E.; Figeac, M.; et al. The lncRNA ‘UCA1’ modulates the response to chemotherapy of ovarian cancer through direct binding to miR-27a-5p and control of UBE2N levels. Mol. Oncol. 2021, 15, 3659–3678. [Google Scholar] [CrossRef]
- Li, X.; Chen, W.; Jin, Y.; Xue, R.; Su, J.; Mu, Z.; Li, J.; Jiang, S. miR-142-5p enhances cisplatin-induced apoptosis in ovarian cancer cells by targeting multiple anti-apoptotic genes. Biochem. Pharmacol. 2019, 161, 98–112. [Google Scholar] [CrossRef]
- Li, X.; Jin, Y.; Mu, Z.; Chen, W.; Jiang, S. MicroRNA146a5p enhances cisplatininduced apoptosis in ovarian cancer cells by targeting multiple antiapoptotic genes. Int. J. Oncol. 2017, 51, 327–335. [Google Scholar] [CrossRef]
- Bieg, D.; Sypniewski, D.; Nowak, E.; Bednarek, I. MiR-424-3p suppresses galectin-3 expression and sensitizes ovarian cancer cells to cisplatin. Arch. Gynecol. Obstet. 2019, 299, 1077–1087. [Google Scholar] [CrossRef]
- Wang, D.Y.; Li, N.; Cui, Y.L. Long Non-coding RNA CCAT1 Sponges miR-454 to Promote Chemoresistance of Ovarian Cancer Cells to Cisplatin by Regulation of Surviving. Cancer Res. Treat. 2020, 52, 798–814. [Google Scholar] [CrossRef]
- Zhu, M.; Yang, L.; Wang, X. NEAT1 Knockdown Suppresses the Cisplatin Resistance in Ovarian Cancer by Regulating miR-770-5p/PARP1 Axis. Cancer Manag. Res. 2020, 12, 7277–7289. [Google Scholar] [CrossRef]
- Zhao, H.; Yu, X.; Ding, Y.; Zhao, J.; Wang, G.; Wu, X.; Jiang, J.; Peng, C.; Guo, G.Z.; Cui, S. MiR-770-5p inhibits cisplatin chemoresistance in human ovarian cancer by targeting ERCC2. Oncotarget 2016, 7, 53254–53268. [Google Scholar] [CrossRef]
- Guo, H.; Ha, C.; Dong, H.; Yang, Z.; Ma, Y.; Ding, Y. Cancer-associated fibroblast-derived exosomal microRNA-98-5p promotes cisplatin resistance in ovarian cancer by targeting CDKN1A. Cancer Cell. Int. 2019, 19, 347. [Google Scholar] [CrossRef]
- Cheng, Y.; Ban, R.; Liu, W.; Wang, H.; Li, S.; Yue, Z.; Zhu, G.; Zhuan, Y.; Wang, C. MiRNA-409-3p enhances cisplatin-sensitivity of ovarian cancer cells by blocking the autophagy mediated by Fip200. Oncol. Res. 2018, 28, 7–8. [Google Scholar] [CrossRef]
- Jiang, X.; Cheng, Y.; He, Y.; Cong, S.; Sun, L.; Wu, D.; Wu, H.; Zhang, G. LNC00115 Mediates Cisplatin Resistance by Regulating the miR-7/ERK Signalling Pathway in Ovarian Cancer. Cancer Manag. Res. 2021, 13, 3817–3826. [Google Scholar] [CrossRef]
- Li, L.; Li, L.; Hu, L.; Li, T.; Xie, D.; Liu, X. Long noncoding RNA HAND2AS1/miR106a/PTEN axis resensitizes cisplatinresistant ovarian cells to cisplatin treatment. Mol. Med. Rep. 2021, 24, 762. [Google Scholar] [CrossRef]
- Shi, X.; Xiao, L.; Mao, X.; He, J.; Ding, Y.; Huang, J.; Peng, C.; Xu, Z. miR-205-5p Mediated Downregulation of PTEN Contributes to Cisplatin Resistance in C13K Human Ovarian Cancer Cells. Front. Genet. 2018, 9, 555. [Google Scholar] [CrossRef]
- Zhang, J.; Quan, L.N.; Meng, Q.; Wang, H.Y.; Wang, J.; Yu, P.; Fu, J.T.; Li, Y.J.; Chen, J.; Cheng, H.; et al. miR-548e Sponged by ZFAS1 Regulates Metastasis and Cisplatin Resistance of OC by Targeting CXCR4 and let-7a/BCL-XL/S Signaling Axis. Mol. Ther. Nucleic Acids 2020, 20, 621–638. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, T.; Xia, L.; Zhang, M. LncRNA WDFY3-AS2 promotes cisplatin resistance and the cancer stem cell in ovarian cancer by regulating hsa-miR-139-5p/SDC4 axis. Cancer Cell. Int. 2021, 21, 284. [Google Scholar] [CrossRef]
- Wu, J.; Zhang, L.; Wu, S.; Yi, X.; Liu, Z. miR-194-5p inhibits SLC40A1 expression to induce cisplatin resistance in ovarian cancer. Pathol. Res. Pract. 2020, 216, 152979. [Google Scholar] [CrossRef]
- Xiao, S.; Zhang, M.; Liu, C.; Wang, D. MiR-514 attenuates proliferation and increases chemoresistance by targeting ATP binding cassette subfamily in ovarian cancer. Mol. Genet. Genom. 2018, 293, 1159–1167. [Google Scholar] [CrossRef]
- Tian, S.; Zhang, M.; Chen, X.; Liu, Y.; Lou, G. MicroRNA-595 sensitizes ovarian cancer cells to cisplatin by targeting ABCB1. Oncotarget 2016, 7, 87091–87099. [Google Scholar] [CrossRef]
- Sun, K.X.; Jiao, J.W.; Chen, S.; Liu, B.L.; Zhao, Y. MicroRNA-186 induces sensitivity of ovarian cancer cells to paclitaxel and cisplatin by targeting ABCB1. J. Ovarian Res. 2015, 8, 80. [Google Scholar] [CrossRef]
- Yang, L.; Li, N.; Wang, H.; Jia, X.; Wang, X.; Luo, J. Altered microRNA expression in cisplatin-resistant ovarian cancer cells and upregulation of miR-130a associated with MDR1/P-glycoprotein-mediated drug resistance. Oncol. Rep. 2012, 28, 592–600. [Google Scholar] [CrossRef]
- Xu, L.; Huang, Y.; Chen, D.; He, J.; Zhu, W.; Zhang, Y.; Liu, X. Downregulation of miR-21 increases cisplatin sensitivity of non-small-cell lung cancer. Cancer Genet. 2014, 207, 214–220. [Google Scholar] [CrossRef]
- Shen, H.; Zhu, F.; Liu, J.; Xu, T.; Pei, D.; Wang, R.; Qian, Y.; Li, Q.; Wang, L.; Shi, Z.; et al. Alteration in Mir-21/PTEN expression modulates gefitinib resistance in non-small cell lung cancer. PLoS ONE 2014, 9, e103305. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, L.; Yin, Z.Y.; Fan, X.L.; Hu, B.; Wang, L.Q.; Zhang, D. miR-107 regulates cisplatin chemosensitivity of A549 non small cell lung cancer cell line by targeting cyclin dependent kinase 8. Int. J. Clin. Exp. Pathol. 2014, 7, 7236–7241. [Google Scholar]
- Ceppi, P.; Mudduluru, G.; Kumarswamy, R.; Rapa, I.; Scagliotti, G.V.; Papotti, M.; Allgayer, H. Loss of miR-200c expression induces an aggressive, invasive, and chemoresistant phenotype in non-small cell lung cancer. Mol. Cancer Res. 2010, 8, 1207–1216. [Google Scholar] [CrossRef]
- Bian, H.B.; Pan, X.; Yang, J.S.; Wang, Z.X.; De, W. Upregulation of microRNA-451 increases cisplatin sensitivity of non-small cell lung cancer cell line (A549). J. Exp. Clin. Cancer Res. 2011, 30, 20. [Google Scholar] [CrossRef]
- Li, J.; Wang, Y.; Song, Y.; Fu, Z.; Yu, W. miR-27a regulates cisplatin resistance and metastasis by targeting RKIP in human lung adenocarcinoma cells. Mol. Cancer 2014, 13, 193. [Google Scholar] [CrossRef]
- Chen, X.; Jiang, Y.; Huang, Z.; Li, D.; Chen, X.; Cao, M.; Meng, Q.; Pang, H.; Sun, L.; Zhao, Y.; et al. miRNA-378 reverses chemoresistance to cisplatin in lung adenocarcinoma cells by targeting secreted clusterin. Sci. Rep. 2016, 6, 19455. [Google Scholar] [CrossRef]
- Xu, Y.; An, Y.; Wang, Y.; Zhang, C.; Zhang, H.; Huang, C.; Jiang, H.; Wang, X.; Li, X. miR-101 inhibits autophagy and enhances cisplatin-induced apoptosis in hepatocellular carcinoma cells. Oncol. Rep. 2013, 29, 2019–2024. [Google Scholar] [CrossRef]
- Xu, N.; Shen, C.; Luo, Y.; Xia, L.; Xue, F.; Xia, Q.; Zhang, J. Upregulated miR-130a increases drug resistance by regulating RUNX3 and Wnt signaling in cisplatin-treated HCC cell. Biochem. Biophys. Res. Commun. 2012, 425, 468–472. [Google Scholar] [CrossRef]
- Qin, J.; Luo, M.; Qian, H.; Chen, W. Upregulated miR-182 increases drug resistance in cisplatin-treated HCC cell by regulating TP53INP1. Gene 2014, 538, 342–347. [Google Scholar] [CrossRef]
- Xu, N.; Zhang, J.; Shen, C.; Luo, Y.; Xia, L.; Xue, F.; Xia, Q. Cisplatin-induced downregulation of miR-199a-5p increases drug resistance by activating autophagy in HCC cell. Biochem. Biophys. Res. Commun. 2012, 423, 826–831. [Google Scholar] [CrossRef]
- Lu, L.; Wu, M.; Lu, Y.; Zhao, Z.; Liu, T.; Fu, W.; Li, W. MicroRNA-424 regulates cisplatin resistance of gastric cancer by targeting SMURF1 based on GEO database and primary validation in human gastric cancer tissues. OncoTargets Ther. 2019, 12, 7623–7636. [Google Scholar] [CrossRef]
- Jin, L.; Ma, X.; Zhang, N.; Zhang, Q.; Chen, X.; Zhang, Z.; Ding, G.; Yu, H. Targeting Oncogenic miR-181a-2-3p Inhibits Growth and Suppresses Cisplatin Resistance of Gastric Cancer. Cancer Manag. Res. 2021, 13, 8599–8609. [Google Scholar] [CrossRef]
- Jin, L.; Zhang, Z. Serum miR-3180-3p and miR-124-3p may Function as Noninvasive Biomarkers of Cisplatin Resistance in Gastric Cancer. Clin. Lab. 2020, 66. [Google Scholar] [CrossRef]
- Yu, X.; Chen, Y.; Tian, R.; Li, J.; Li, H.; Lv, T.; Yao, Q. miRNA-21 enhances chemoresistance to cisplatin in epithelial ovarian cancer by negatively regulating PTEN. Oncol. Lett. 2017, 14, 1807–1810. [Google Scholar] [CrossRef]
- Pink, R.C.; Samuel, P.; Massa, D.; Caley, D.P.; Brooks, S.A.; Carter, D.R. The passenger strand, miR-21-3p, plays a role in mediating cisplatin resistance in ovarian cancer cells. Gynecol. Oncol. 2015, 137, 143–151. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, X.; Hu, C.-F.; Xu, Q.; Zhu, H.-X.; Xu, N.-Z. MicroRNA-mRNA functional pairs for cisplatin resistance in ovarian cancer cells. Chin. J. Cancer 2014, 33, 285–294. [Google Scholar] [CrossRef]
- Schwarzenbach, H. Clinical significance of miR-15 and miR-16 in ovarian cancer. Transl. Cancer Res. 2016, 5, S50–S53. [Google Scholar] [CrossRef]
- Vanas, V.; Haigl, B.; Stockhammer, V.; Sutterluty-Fall, H. MicroRNA-21 Increases Proliferation and Cisplatin Sensitivity of Osteosarcoma-Derived Cells. PLoS ONE 2016, 11, e0161023. [Google Scholar] [CrossRef]
- Gu, Z.; Li, Z.; Xu, R.; Zhu, X.; Hu, R.; Xue, Y.; Xu, W. miR-16-5p Suppresses Progression and Invasion of Osteosarcoma via Targeting at Smad3. Front. Pharmacol. 2020, 11, 1324. [Google Scholar] [CrossRef]
- Chen, Y.; Tsai, Y.H.; Fang, Y.; Tseng, S.H. Micro-RNA-21 regulates the sensitivity to cisplatin in human neuroblastoma cells. J. Pediatr. Surg. 2012, 47, 1797–1805. [Google Scholar] [CrossRef]
- Wang, Z.; Lei, H.; Sun, Q. MicroRNA-141 and its associated gene FUS modulate proliferation, migration and cisplatin chemosensitivity in neuroblastoma cell lines. Oncol. Rep. 2016, 35, 2943–2951. [Google Scholar] [CrossRef]
- Challagundla, K.B.; Wise, P.M.; Neviani, P.; Chava, H.; Murtadha, M.; Xu, T.; Kennedy, R.; Ivan, C.; Zhang, X.; Vannini, I.; et al. Exosome-Mediated Transfer of microRNAs Within the Tumor Microenvironment and Neuroblastoma Resistance to Chemotherapy. JNCI J. Natl. Cancer Inst. 2015, 107, djv135. [Google Scholar] [CrossRef]
- Ma, K.; He, Y.; Zhang, H.; Fei, Q.; Niu, D.; Wang, D.; Ding, X.; Xu, H.; Chen, X.; Zhu, J. DNA methylation-regulated miR-193a-3p dictates resistance of hepatocellular carcinoma to 5-fluorouracil via repression of SRSF2 expression. J. Biol. Chem. 2012, 287, 5639–5649. [Google Scholar] [CrossRef]
- Zhang, Y.; Talmon, G.; Wang, J. MicroRNA-587 antagonizes 5-FU-induced apoptosis and confers drug resistance by regulating PPP2R1B expression in colorectal cancer. Cell Death Dis. 2015, 6, e1845. [Google Scholar] [CrossRef]
- Yu, X.; Shi, W.; Zhang, Y.; Wang, X.; Sun, S.; Song, Z.; Liu, M.; Zeng, Q.; Cui, S.; Qu, X. CXCL12/CXCR4 axis induced miR-125b promotes invasion and confers 5-fluorouracil resistance through enhancing autophagy in colorectal cancer. Sci. Rep. 2017, 7, 42226. [Google Scholar] [CrossRef]
- Xu, F.; Ye, M.L.; Zhang, Y.P.; Li, W.J.; Li, M.T.; Wang, H.Z.; Qiu, X.; Xu, Y.; Yin, J.W.; Hu, Q.; et al. MicroRNA-375-3p enhances chemosensitivity to 5-fluorouracil by targeting thymidylate synthase in colorectal cancer. Cancer Sci. 2020, 111, 1528–1541. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Xie, T.; Mao, X.; Xue, L.; Chu, X.; Chen, L. MicroRNA-149 Increases the Sensitivity of Colorectal Cancer Cells to 5-Fluorouracil by Targeting Forkhead Box Transcription Factor FOXM1. Cell Physiol. Biochem. 2016, 39, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Liu, Y.; Zhao, L.; Pan, Y.; Shan, Y.; Li, Y.; Jia, L. Upregulation of microRNA-135b and microRNA-182 promotes chemoresistance of colorectal cancer by targeting ST6GALNAC2 via PI3K/AKT pathway. Mol. Carcinog. 2017, 56, 2669–2680. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, X.; Ma, S.; Zhang, H. Decreased expression of miR-3135b reduces sensitivity to 5-fluorouracil in colorectal cancer by direct repression of PIM1. Exp. Ther. Med. 2021, 22, 1151. [Google Scholar] [CrossRef]
- Li, L.Q.; Pan, D.; Chen, Q.; Zhang, S.W.; Xie, D.Y.; Zheng, X.L.; Chen, H. Sensitization of Gastric Cancer Cells to 5-FU by MicroRNA-204 Through Targeting the TGFBR2-Mediated Epithelial to Mesenchymal Transition. Cell. Physiol. Biochem. 2018, 47, 1533–1545. [Google Scholar] [CrossRef]
- Wang, C.Q. MiR-195 reverses 5-FU resistance through targeting HMGA1 in gastric cancer cells. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 3771–3778. [Google Scholar] [CrossRef]
- Wang, T.; Ji, R.; Liu, G.; Ma, B.; Wang, Z.; Wang, Q. Lactate induces aberration in the miR-30a-DBF4 axis to promote the development of gastric cancer and weakens the sensitivity to 5-Fu. Cancer Cell Int. 2021, 21, 602. [Google Scholar] [CrossRef]
- Song, B.; Wang, Y.; Xi, Y.; Kudo, K.; Bruheim, S.; Botchkina, G.I.; Gavin, E.; Wan, Y.; Formentini, A.; Kornmann, M.; et al. Mechanism of chemoresistance mediated by miR-140 in human osteosarcoma and colon cancer cells. Oncogene 2009, 28, 4065–4074. [Google Scholar] [CrossRef]
- Hirota, T.; Date, Y.; Nishibatake, Y.; Takane, H.; Fukuoka, Y.; Taniguchi, Y.; Burioka, N.; Shimizu, E.; Nakamura, H.; Otsubo, K.; et al. Dihydropyrimidine dehydrogenase (DPD) expression is negatively regulated by certain microRNAs in human lung tissues. Lung Cancer 2012, 77, 16–23. [Google Scholar] [CrossRef]
- Zhang, D.; Li, Y.; Sun, P. miR-770-5p modulates resistance to methotrexate in human colorectal adenocarcinoma cells by downregulating HIPK1. Exp. Ther. Med. 2019, 19, 339–346. [Google Scholar] [CrossRef]
- Zhang, H.W.; Shi, Y.; Liu, J.B.; Wang, H.M.; Wang, P.Y.; Wu, Z.J.; Li, L.; Gu, L.P.; Cao, P.S.; Wang, G.R.; et al. Cancer-associated fibroblast-derived exosomal microRNA-24-3p enhances colon cancer cell resistance to MTX by down-regulating CDX2/HEPH axis. J. Cell. Mol. Med. 2021, 25, 3699–3713. [Google Scholar] [CrossRef]
- Chen, Y.; Bian, L.; Zhang, Y. MiR-505 mediates methotrexate resistance in colorectal cancer by targeting RASSF8. J. Pharm. Pharmacol. 2018, 70, 937–951. [Google Scholar] [CrossRef] [PubMed]
- Shan, W.; Zhang, X.; Li, M.; Deng, F.; Zhang, J. Over expression of miR-200c suppresses invasion and restores methotrexate sensitivity in lung cancer A549 cells. Gene 2016, 593, 265–271. [Google Scholar] [CrossRef]
- Wei, W.; Ji, L.; Duan, W.; Zhu, J. Circular RNA circ_0081001 knockdown enhances methotrexate sensitivity in osteosarcoma cells by regulating miR-494-3p/TGM2 axis. J. Orthop. Surg. Res. 2021, 16, 50. [Google Scholar] [CrossRef]
- Bazavar, M.; Fazli, J.; Valizadeh, A.; Ma, B.; Mohammadi, E.; Asemi, Z.; Alemi, F.; Maleki, M.; Xing, S.; Yousefi, B. miR-192 enhances sensitivity of methotrexate drug to MG-63 osteosarcoma cancer cells. Pathol. Res. Pract. 2020, 216, 153176. [Google Scholar] [CrossRef]
- Kazmierczak, D.; Jopek, K.; Sterzynska, K.; Ginter-Matuszewska, B.; Nowicki, M.; Rucinski, M.; Januchowski, R. The Significance of MicroRNAs Expression in Regulation of Extracellular Matrix and Other Drug Resistant Genes in Drug Resistant Ovarian Cancer Cell Lines. Int. J. Mol. Sci. 2020, 21, 2619. [Google Scholar] [CrossRef]
- Peng, Q.P.; Du, D.B.; Ming, Q.; Hu, F.; Wu, Z.B.; Qiu, S. MicroRNA 494 increases chemosensitivity to doxorubicin in gastric cancer cells by targeting phosphodiesterases 4D. Cell. Mol. Biol. 2018, 64, 62–66. [Google Scholar] [CrossRef]
- Takwi, A.A.; Wang, Y.M.; Wu, J.; Michaelis, M.; Cinatl, J.; Chen, T. miR-137 regulates the constitutive androstane receptor and modulates doxorubicin sensitivity in parental and doxorubicin-resistant neuroblastoma cells. Oncogene 2014, 33, 3717–3729. [Google Scholar] [CrossRef]
- Zhao, G.; Wang, G.; Bai, H.; Li, T.; Gong, F.; Yang, H.; Wen, J.; Wang, W. Targeted inhibition of HDAC8 increases the doxorubicin sensitivity of neuroblastoma cells via up regulation of miR-137. Eur. J. Pharmacol. 2017, 802, 20–26. [Google Scholar] [CrossRef]
- Holliday, H.; Yang, J.; Dodson, E.; Nikolic, I.; Kamili, A.; Wheatley, M.; Deng, N.; Alexandrou, S.; Davis, T.P.; Kavallaris, M.; et al. miR-99b-5p, miR-380-3p, and miR-485-3p are novel chemosensitizing miRNAs in high-risk neuroblastoma. Mol. Ther. 2022, 30, 1119–1134. [Google Scholar] [CrossRef]
- Salter, K.H.; Acharya, C.R.; Walters, K.S.; Redman, R.; Anguiano, A.; Garman, K.S.; Anders, C.K.; Mukherjee, S.; Dressman, H.K.; Barry, W.T.; et al. An integrated approach to the prediction of chemotherapeutic response in patients with breast cancer. PLoS ONE 2008, 3, e1908. [Google Scholar] [CrossRef] [PubMed]
- Safaei, S.; Amini, M.; Najjary, S.; Mokhtarzadeh, A.; Bolandi, N.; Saeedi, H.; Alizadeh, N.; Javadrashid, D.; Baradaran, B. miR-200c increases the sensitivity of breast cancer cells to Doxorubicin through downregulating MDR1 gene. Exp. Mol. Pathol. 2022, 125, 104753. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, A.; Liu, C.G.; Addario, A.; Peschle, C.; Scambia, G.; Ferlini, C. Role of microRNAs in drug-resistant ovarian cancer cells. Gynecol. Oncol. 2008, 111, 478–486. [Google Scholar] [CrossRef]
- Samli, H.; Samli, M.; Vatansever, B.; Ardicli, S.; Aztopal, N.; Dincel, D.; Sahin, A.; Balci, F. Paclitaxel resistance and the role of miRNAs in prostate cancer cell lines. World J. Urol. 2019, 37, 1117–1126. [Google Scholar] [CrossRef]
- Kojima, K.; Fujita, Y.; Nozawa, Y.; Deguchi, T.; Ito, M. MiR-34a attenuates paclitaxel-resistance of hormone-refractory prostate cancer PC3 cells through direct and indirect mechanisms. Prostate 2010, 70, 1501–1512. [Google Scholar] [CrossRef]
- Fujita, Y.; Kojima, K.; Hamada, N.; Ohhashi, R.; Akao, Y.; Nozawa, Y.; Deguchi, T.; Ito, M. Effects of miR-34a on cell growth and chemoresistance in prostate cancer PC3 cells. Biochem. Biophys. Res. Commun. 2008, 377, 114–119. [Google Scholar] [CrossRef]
- Zhao, Z.L.; Cai, Y.; Wang, Y.Y.; Xia, C.L.; Li, C.X.; Chen, S.L.; Yang, Q.L.; Chen, C.J. Effects of miRNA-21 on paclitaxel-resistance in human breast cancer cells. Zhejiang Da Xue Xue Bao Yi Xue Ban 2015, 44, 400–409. [Google Scholar]
- Duan, F.-G.; Wang, M.-F.; Cao, Y.-B.; Dan, L.; Li, R.-Z.; Fan, X.-X.; Khan, I.; Lai, H.-L.; Zhang, Y.-Z.; Hsiao, W.W.-L.; et al. MicroRNA-421 confers paclitaxel resistance by binding to the KEAP1 3′UTR and predicts poor survival in non-small cell lung cancer. Cell Death Dis. 2019, 10, 821. [Google Scholar] [CrossRef]
- Zeng, T.; Xu, M.; Zhang, W.; Gu, X.; Zhao, F.; Liu, X.; Zhang, X. Autophagy inhibition and microRNA199a5p upregulation in paclitaxelresistant A549/T lung cancer cells. Oncol. Rep. 2021, 46, 149. [Google Scholar] [CrossRef]
- Zheng, F.; Zhang, H.; Lu, J. Identification of potential microRNAs and their targets in promoting gefitinib resistance by integrative network analysis. J. Thorac. Dis. 2019, 11, 5535–5546. [Google Scholar] [CrossRef]
- Zhu, J.; Tao, L.; Jin, L. MicroRNA-506-3p reverses gefitinib resistance in non-small cell lung cancer by targeting Yes-associated protein 1. Mol. Med. Rep. 2018, 19, 1331–1339. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.Y.; Chen, X.; Zhao, J.; Bao, Z.; Chen, X.; Zhang, P.; Liu, Z.F.; Zhou, J.Y. MicroRNA-34a overcomes HGF-mediated gefitinib resistance in EGFR mutant lung cancer cells partly by targeting MET. Cancer Lett. 2014, 351, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Azuma, Y.; Yokobori, T.; Mogi, A.; Yajima, T.; Kosaka, T.; Iijima, M.; Shimizu, K.; Shirabe, K.; Kuwano, H. Cancer exosomal microRNAs from gefitinib-resistant lung cancer cells cause therapeutic resistance in gefitinib-sensitive cells. Surg. Today 2020, 50, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Chen, W.X.; Zhong, S.L.; Zhang, J.Y.; Ma, T.F.; Ji, H.; Lv, M.M.; Tang, J.H.; Zhao, J.H. MicroRNA-452 contributes to the docetaxel resistance of breast cancer cells. Tumour Biol. 2014, 35, 6327–6334. [Google Scholar] [CrossRef]
- Sharma, S.; Pukale, S.; Sahel, D.K.; Singh, P.; Mittal, A.; Chitkara, D. Folate targeted hybrid lipo-polymeric nanoplexes containing docetaxel and miRNA-34a for breast cancer treatment. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 128, 112305. [Google Scholar] [CrossRef]
- Armstrong, C.M.; Liu, C.; Lou, W.; Lombard, A.P.; Evans, C.P.; Gao, A.C. MicroRNA-181a promotes docetaxel resistance in prostate cancer cells. Prostate 2017, 77, 1020–1028. [Google Scholar] [CrossRef]
- Shi, G.H.; Ye, D.W.; Yao, X.D.; Zhang, S.L.; Dai, B.; Zhang, H.L.; Shen, Y.J.; Zhu, Y.; Zhu, Y.P.; Xiao, W.J.; et al. Involvement of microRNA-21 in mediating chemo-resistance to docetaxel in androgen-independent prostate cancer PC3 cells. Acta Pharmacol. Sin. 2010, 31, 867–873. [Google Scholar] [CrossRef]
- Zhang, H.L.; Yang, L.F.; Zhu, Y.; Yao, X.D.; Zhang, S.L.; Dai, B.; Zhu, Y.P.; Shen, Y.J.; Shi, G.H.; Ye, D.W. Serum miRNA-21: Elevated levels in patients with metastatic hormone-refractory prostate cancer and potential predictive factor for the efficacy of docetaxel-based chemotherapy. Prostate 2011, 71, 326–331. [Google Scholar] [CrossRef]
- Xu, B.; Niu, X.; Zhang, X.; Tao, J.; Wu, D.; Wang, Z.; Li, P.; Zhang, W.; Wu, H.; Feng, N.; et al. miR-143 decreases prostate cancer cells proliferation and migration and enhances their sensitivity to docetaxel through suppression of KRAS. Mol. Cell. Biochem. 2011, 350, 207–213. [Google Scholar] [CrossRef]
- Yu, J.; Lu, Y.; Cui, D.; Li, E.; Zhu, Y.; Zhao, Y.; Zhao, F.; Xia, S. miR-200b suppresses cell proliferation, migration and enhances chemosensitivity in prostate cancer by regulating Bmi-1. Oncol. Rep. 2014, 31, 910–918. [Google Scholar] [CrossRef]
- Puhr, M.; Hoefer, J.; Schafer, G.; Erb, H.H.; Oh, S.J.; Klocker, H.; Heidegger, I.; Neuwirt, H.; Culig, Z. Epithelial-to-mesenchymal transition leads to docetaxel resistance in prostate cancer and is mediated by reduced expression of miR-200c and miR-205. Am. J. Pathol. 2012, 181, 2188–2201. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Zhang, D.; Du, R.; Pan, Y.; Zhao, L.; Sun, S.; Hong, L.; Liu, J.; Fan, D. miR-15b and miR-16 modulate multidrug resistance by targeting BCL2 in human gastric cancer cells. Int. J. Cancer 2008, 123, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Pang, Y.; Gao, X.; Zhao, M.; Zhang, X.; Zhang, H.; Xuan, B.; Wang, Y. MicroRNA-137 chemosensitizes colon cancer cells to the chemotherapeutic drug oxaliplatin (OXA) by targeting YBX1. Cancer Biomark. 2017, 18, 1–9. [Google Scholar] [CrossRef]
- Evert, J.; Pathak, S.; Sun, X.F.; Zhang, H. A Study on Effect of Oxaliplatin in MicroRNA Expression in Human Colon Cancer. J. Cancer 2018, 9, 2046–2053. [Google Scholar] [CrossRef] [PubMed]
- Moradi Marjaneh, R.; Khazaei, M.; Ferns, G.A.; Avan, A.; Aghaee-Bakhtiari, S.H. MicroRNAs as potential therapeutic targets to predict responses to oxaliplatin in colorectal cancer: From basic evidence to therapeutic implication. IUBMB Life 2019, 71, 1428–1441. [Google Scholar] [CrossRef]
- Ren, W.W.; Li, D.D.; Chen, X.; Li, X.L.; He, Y.P.; Guo, L.H.; Liu, L.N.; Sun, L.P.; Zhang, X.P. MicroRNA-125b reverses oxaliplatin resistance in hepatocellular carcinoma by negatively regulating EVA1A mediated autophagy. Cell Death Dis. 2018, 9, 547. [Google Scholar] [CrossRef]
- Naro, Y.; Ankenbruck, N.; Thomas, M.; Tivon, Y.; Connelly, C.M.; Gardner, L.; Deiters, A. Small Molecule Inhibition of MicroRNA miR-21 Rescues Chemosensitivity of Renal-Cell Carcinoma to Topotecan. J. Med. Chem. 2018, 61, 5900–5909. [Google Scholar] [CrossRef]
- Si, M.L.; Zhu, S.; Wu, H.; Lu, Z.; Wu, F.; Mo, Y.Y. miR-21-mediated tumor growth. Oncogene 2007, 26, 2799–2803. [Google Scholar] [CrossRef]
- Xin, F.; Li, M.; Balch, C.; Thomson, M.; Fan, M.; Liu, Y.; Hammond, S.M.; Kim, S.; Nephew, K.P. Computational analysis of microRNA profiles and their target genes suggests significant involvement in breast cancer antiestrogen resistance. Bioinformatics 2009, 25, 430–434. [Google Scholar] [CrossRef]
- Ferracin, M.; Zagatti, B.; Rizzotto, L.; Cavazzini, F.; Veronese, A.; Ciccone, M.; Saccenti, E.; Lupini, L.; Grilli, A.; De Angeli, C.; et al. MicroRNAs involvement in fludarabine refractory chronic lymphocytic leukemia. Mol. Cancer 2010, 9, 123. [Google Scholar] [CrossRef]
- Moussay, E.; Palissot, V.; Vallar, L.; Poirel, H.A.; Wenner, T.; El Khoury, V.; Aouali, N.; Van Moer, K.; Leners, B.; Bernardin, F.; et al. Determination of genes and microRNAs involved in the resistance to fludarabine in vivo in chronic lymphocytic leukemia. Mol. Cancer 2010, 9, 115. [Google Scholar] [CrossRef] [PubMed]
- Mraz, M.; Cerna, K.; Mayerova, V.; Musilova, K.; Plevova, K.; Pavlova, S.; Tichy, B.; Doubek, M.; Brychtova, Y.; Malcikova, J.; et al. Microrna-34a As a Marker for Fludarabine Resistance and Impairment of p53-Pathway in Chronic Lymphocytic Leukemia. Blood 2012, 120, 3883. [Google Scholar] [CrossRef]
- Ryan, J.; Tivnan, A.; Fay, J.; Bryan, K.; Meehan, M.; Creevey, L.; Lynch, J.; Bray, I.M.; O’Meara, A.; Tracey, L.; et al. MicroRNA-204 increases sensitivity of neuroblastoma cells to cisplatin and is associated with a favourable clinical outcome. Br. J. Cancer 2012, 107, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Harvey, H.; Piskareva, O.; Creevey, L.; Alcock, L.C.; Buckley, P.G.; O’Sullivan, M.J.; Segura, M.F.; Gallego, S.; Stallings, R.L.; Bray, I.M. Modulation of chemotherapeutic drug resistance in neuroblastoma SK-N-AS cells by the neural apoptosis inhibitory protein and miR-520f. Int. J. Cancer 2015, 136, 1579–1588. [Google Scholar] [CrossRef]
- Shahverdi, M.; Amri, J.; Karami, H.; Baazm, M. Knockdown of Myeloid Cell Leukemia-1 by MicroRNA-101 Increases Sensitivity of A549 Lung Cancer Cells to Etoposide. Iran. J. Med. Sci. 2021, 46, 298–307. [Google Scholar] [CrossRef]
- Xu, C.; Du, Z.; Ren, S.; Pian, Y. Downregulation of GSK3B by miR-132-3p Enhances Etoposide-Induced Breast Cancer Cell Apoptosis. Ann. Clin. Lab. Sci. 2021, 51, 285–294. [Google Scholar]
- Miller, T.E.; Ghoshal, K.; Ramaswamy, B.; Roy, S.; Datta, J.; Shapiro, C.L.; Jacob, S.; Majumder, S. MicroRNA-221/222 confers tamoxifen resistance in breast cancer by targeting p27Kip1. J. Biol. Chem. 2008, 283, 29897–29903. [Google Scholar] [CrossRef]
- Zhao, J.J.; Lin, J.; Yang, H.; Kong, W.; He, L.; Ma, X.; Coppola, D.; Cheng, J.Q. MicroRNA-221/222 negatively regulates estrogen receptor alpha and is associated with tamoxifen resistance in breast cancer. J. Biol. Chem. 2016, 291, 22859. [Google Scholar] [CrossRef]
- Li, J.; Lu, M.; Jin, J.; Lu, X.; Xu, T.; Jin, S. miR-449a Suppresses Tamoxifen Resistance in Human Breast Cancer Cells by Targeting ADAM22. Cell. Physiol. Biochem. 2018, 50, 136–149. [Google Scholar] [CrossRef]
- Chen, G.Q.; Zhao, Z.W.; Zhou, H.Y.; Liu, Y.J.; Yang, H.J. Systematic analysis of microRNA involved in resistance of the MCF-7 human breast cancer cell to doxorubicin. Med. Oncol. 2010, 27, 406–415. [Google Scholar] [CrossRef]
- Pan, Y.Z.; Morris, M.E.; Yu, A.M. MicroRNA-328 negatively regulates the expression of breast cancer resistance protein (BCRP/ABCG2) in human cancer cells. Mol. Pharmacol. 2009, 75, 1374–1379. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Tolleson, W.H.; Yu, D.; Chen, S.; Guo, L.; Xiao, W.; Tong, W.; Ning, B. Regulation of cytochrome P450 expression by microRNAs and long noncoding RNAs: Epigenetic mechanisms in environmental toxicology and carcinogenesis. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2019, 37, 180–214. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.; Chen, S. Epigenetic Regulation of Cytochrome P450 Enzymes and Clinical Implication. Curr. Drug Metab. 2015, 16, 86–96. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.Z.; Gao, W.; Yu, A.M. MicroRNAs regulate CYP3A4 expression via direct and indirect targeting. Drug Metab. Dispos. 2009, 37, 2112–2117. [Google Scholar] [CrossRef]
- Ramamoorthy, A.; Skaar, T.C. In silico identification of microRNAs predicted to regulate the drug metabolizing cytochrome P450 genes. Drug Metab. Lett. 2011, 5, 126–131. [Google Scholar] [CrossRef]
- Mohri, T.; Nakajima, M.; Fukami, T.; Takamiya, M.; Aoki, Y.; Yokoi, T. Human CYP2E1 is regulated by miR-378. Biochem. Pharmacol. 2010, 79, 1045–1052. [Google Scholar] [CrossRef]
- Choi, Y.M.; An, S.; Lee, E.M.; Kim, K.; Choi, S.J.; Kim, J.S.; Jang, H.H.; An, I.S.; Bae, S. CYP1A1 is a target of miR-892a-mediated post-transcriptional repression. Int. J. Oncol. 2012, 41, 331–336. [Google Scholar] [CrossRef][Green Version]
- Chen, F.; Chen, C.; Yang, S.; Gong, W.; Wang, Y.; Cianflone, K.; Tang, J.; Wang, D.W. Let-7b inhibits human cancer phenotype by targeting cytochrome P450 epoxygenase 2J2. PLoS ONE 2012, 7, e39197. [Google Scholar] [CrossRef]
- Shi, Y.; Liu, Y.; Wei, Z.; Zhang, Y.; Zhang, L.; Jiang, S.; Xiong, Y.; Shen, L.; He, L.; Xing, Q.; et al. Hsa-miR-27a is involved in the regulation of CYP3A4 expression in human livers from Chinese Han population. Pharmacogenomics 2015, 16, 1379–1386. [Google Scholar] [CrossRef]
- Sun, M.; Zhang, Q.; Yang, X.; Qian, S.Y.; Guo, B. Vitamin D Enhances the Efficacy of Irinotecan through miR-627-Mediated Inhibition of Intratumoral Drug Metabolism. Mol. Cancer Ther. 2016, 15, 2086–2095. [Google Scholar] [CrossRef]
- Gill, P.; Bhattacharyya, S.; McCullough, S.; Letzig, L.; Mishra, P.J.; Luo, C.; Dweep, H.; James, L. MicroRNA regulation of CYP 1A2, CYP3A4 and CYP2E1 expression in acetaminophen toxicity. Sci. Rep. 2017, 7, 12331. [Google Scholar] [CrossRef] [PubMed]
- Takagi, S.; Nakajima, M.; Mohri, T.; Yokoi, T. Post-transcriptional regulation of human pregnane X receptor by micro-RNA affects the expression of cytochrome P450 3A4. J. Biol. Chem. 2008, 283, 9674–9680. [Google Scholar] [CrossRef] [PubMed]
- Martinez, V.G.; O’Connor, R.; Liang, Y.; Clynes, M. CYP1B1 expression is induced by docetaxel: Effect on cell viability and drug resistance. Br. J. Cancer 2008, 98, 564–570. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, Y.; Nakajima, M.; Takagi, S.; Taniya, T.; Yokoi, T. MicroRNA regulates the expression of human cytochrome P450 1B1. Cancer Res. 2006, 66, 9090–9098. [Google Scholar] [CrossRef] [PubMed]
- Mu, W.; Hu, C.; Zhang, H.; Qu, Z.; Cen, J.; Qiu, Z.; Li, C.; Ren, H.; Li, Y.; He, X.; et al. miR-27b synergizes with anticancer drugs via p53 activation and CYP1B1 suppression. Cell Res. 2015, 25, 477–495. [Google Scholar] [CrossRef]
- Esquela-Kerscher, A.; Trang, P.; Wiggins, J.F.; Patrawala, L.; Cheng, A.; Ford, L.; Weidhaas, J.B.; Brown, D.; Bader, A.G.; Slack, F.J. The let-7 microRNA reduces tumor growth in mouse models of lung cancer. Cell Cycle 2008, 7, 759–764. [Google Scholar] [CrossRef]
- Gasparini, P.; Lovat, F.; Fassan, M.; Casadei, L.; Cascione, L.; Jacob, N.K.; Carasi, S.; Palmieri, D.; Costinean, S.; Shapiro, C.L.; et al. Protective role of miR-155 in breast cancer through RAD51 targeting impairs homologous recombination after irradiation. Proc. Natl. Acad. Sci. USA 2014, 111, 4536–4541. [Google Scholar] [CrossRef]
- Boni, V.; Bitarte, N.; Cristobal, I.; Zarate, R.; Rodriguez, J.; Maiello, E.; Garcia-Foncillas, J.; Bandres, E. miR-192/miR-215 influence 5-fluorouracil resistance through cell cycle-mediated mechanisms complementary to its post-transcriptional thymidilate synthase regulation. Mol. Cancer Ther. 2010, 9, 2265–2275. [Google Scholar] [CrossRef]
- Maftouh, M.; Avan, A.; Funel, N.; Frampton, A.E.; Fiuji, H.; Pelliccioni, S.; Castellano, L.; Galla, V.; Peters, G.J.; Giovannetti, E. miR-211 modulates gemcitabine activity through downregulation of ribonucleotide reductase and inhibits the invasive behavior of pancreatic cancer cells. Nucleosides Nucleotides Nucleic Acids 2014, 33, 384–393. [Google Scholar] [CrossRef]
- Bhutia, Y.D.; Hung, S.W.; Krentz, M.; Patel, D.; Lovin, D.; Manoharan, R.; Thomson, J.M.; Govindarajan, R. Differential processing of let-7a precursors influences RRM2 expression and chemosensitivity in pancreatic cancer: Role of LIN-28 and SET oncoprotein. PLoS ONE 2013, 8, e53436. [Google Scholar] [CrossRef]
- Stahlhut, C.; Slack, F.J. Combinatorial Action of MicroRNAs let-7 and miR-34 Effectively Synergizes with Erlotinib to Suppress Non-small Cell Lung Cancer Cell Proliferation. Cell Cycle 2015, 14, 2171–2180. [Google Scholar] [CrossRef] [PubMed]
- Trang, P.; Wiggins, J.F.; Daige, C.L.; Cho, C.; Omotola, M.; Brown, D.; Weidhaas, J.B.; Bader, A.G.; Slack, F.J. Systemic delivery of tumor suppressor microRNA mimics using a neutral lipid emulsion inhibits lung tumors in mice. Mol. Ther. 2011, 19, 1116–1122. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Crawford, M.; Mao, Y.; Lee, R.J.; Davis, I.C.; Elton, T.S.; Lee, L.J.; Nana-Sinkam, S.P. Therapeutic Delivery of MicroRNA-29b by Cationic Lipoplexes for Lung Cancer. Mol. Ther. Nucleic Acids 2013, 2, e84. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Crawford, M.; Yu, B.; Mao, Y.; Nana-Sinkam, S.P.; Lee, L.J. MicroRNA delivery by cationic lipoplexes for lung cancer therapy. Mol. Pharm. 2011, 8, 1381–1389. [Google Scholar] [CrossRef]
- Kasinski, A.L.; Slack, F.J. miRNA-34 prevents cancer initiation and progression in a therapeutically resistant K-ras and p53-induced mouse model of lung adenocarcinoma. Cancer Res. 2012, 72, 5576–5587. [Google Scholar] [CrossRef]
- Wiggins, J.F.; Ruffino, L.; Kelnar, K.; Omotola, M.; Patrawala, L.; Brown, D.; Bader, A.G. Development of a lung cancer therapeutic based on the tumor suppressor microRNA-34. Cancer Res. 2010, 70, 5923–5930. [Google Scholar] [CrossRef]
- Chiou, G.Y.; Cherng, J.Y.; Hsu, H.S.; Wang, M.L.; Tsai, C.M.; Lu, K.H.; Chien, Y.; Hung, S.C.; Chen, Y.W.; Wong, C.I.; et al. Cationic polyurethanes-short branch PEI-mediated delivery of Mir145 inhibited epithelial-mesenchymal transdifferentiation and cancer stem-like properties and in lung adenocarcinoma. J. Control. Release 2012, 159, 240–250. [Google Scholar] [CrossRef]
- Ma, L.; Reinhardt, F.; Pan, E.; Soutschek, J.; Bhat, B.; Marcusson, E.G.; Teruya-Feldstein, J.; Bell, G.W.; Weinberg, R.A. Therapeutic silencing of miR-10b inhibits metastasis in a mouse mammary tumor model. Nat. Biotechnol. 2010, 28, 341–347. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Z.; Chen, C.; Liu, Y.; Si, Q.; Chuang, T.H.; Li, N.; Gomez-Cabrero, A.; Reisfeld, R.A.; Xiang, R.; et al. MicroRNA-19a-3p inhibits breast cancer progression and metastasis by inducing macrophage polarization through downregulated expression of Fra-1 proto-oncogene. Oncogene 2014, 33, 3014–3023. [Google Scholar] [CrossRef]
- Li, L.; Xie, X.; Luo, J.; Liu, M.; Xi, S.; Guo, J.; Kong, Y.; Wu, M.; Gao, J.; Xie, Z.; et al. Targeted expression of miR-34a using the T-VISA system suppresses breast cancer cell growth and invasion. Mol. Ther. 2012, 20, 2326–2334. [Google Scholar] [CrossRef]
- Kim, S.J.; Oh, J.S.; Shin, J.Y.; Lee, K.D.; Sung, K.W.; Nam, S.J.; Chun, K.H. Development of microRNA-145 for therapeutic application in breast cancer. J. Control. Release 2011, 155, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, M.; Zhu, S.; Wu, F.; Wu, H.; Walia, V.; Kumar, S.; Elble, R.; Watabe, K.; Mo, Y.Y. p53 represses c-Myc through induction of the tumor suppressor miR-145. Proc. Natl. Acad. Sci. USA 2009, 106, 3207–3212. [Google Scholar] [CrossRef] [PubMed]
- Liang, Z.; Wu, H.; Xia, J.; Li, Y.; Zhang, Y.; Huang, K.; Wagar, N.; Yoon, Y.; Cho, H.T.; Scala, S.; et al. Involvement of miR-326 in chemotherapy resistance of breast cancer through modulating expression of multidrug resistance-associated protein 1. Biochem. Pharmacol. 2010, 79, 817–824. [Google Scholar] [CrossRef]
- Lan, F.F.; Wang, H.; Chen, Y.C.; Chan, C.Y.; Ng, S.S.; Li, K.; Xie, D.; He, M.L.; Lin, M.C.; Kung, H.F. Hsa-let-7g inhibits proliferation of hepatocellular carcinoma cells by downregulation of c-Myc and upregulation of p16(INK4A). Int. J. Cancer 2011, 128, 319–331. [Google Scholar] [CrossRef] [PubMed]
- Bao, L.; Yan, Y.; Xu, C.; Ji, W.; Shen, S.; Xu, G.; Zeng, Y.; Sun, B.; Qian, H.; Chen, L.; et al. MicroRNA-21 suppresses PTEN and hSulf-1 expression and promotes hepatocellular carcinoma progression through AKT/ERK pathways. Cancer Lett. 2013, 337, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Kota, J.; Chivukula, R.R.; O’Donnell, K.A.; Wentzel, E.A.; Montgomery, C.L.; Hwang, H.W.; Chang, T.C.; Vivekanandan, P.; Torbenson, M.; Clark, K.R.; et al. Therapeutic microRNA delivery suppresses tumorigenesis in a murine liver cancer model. Cell 2009, 137, 1005–1017. [Google Scholar] [CrossRef]
- Xiong, Y.; Fang, J.H.; Yun, J.P.; Yang, J.; Zhang, Y.; Jia, W.H.; Zhuang, S.M. Effects of microRNA-29 on apoptosis, tumorigenicity, and prognosis of hepatocellular carcinoma. Hepatology 2010, 51, 836–845. [Google Scholar] [CrossRef]
- Su, H.; Yang, J.R.; Xu, T.; Huang, J.; Xu, L.; Yuan, Y.; Zhuang, S.M. MicroRNA-101, down-regulated in hepatocellular carcinoma, promotes apoptosis and suppresses tumorigenicity. Cancer Res. 2009, 69, 1135–1142. [Google Scholar] [CrossRef]
- Hatziapostolou, M.; Polytarchou, C.; Aggelidou, E.; Drakaki, A.; Poultsides, G.A.; Jaeger, S.A.; Ogata, H.; Karin, M.; Struhl, K.; Hadzopoulou-Cladaras, M.; et al. An HNF4alpha-miRNA inflammatory feedback circuit regulates hepatocellular oncogenesis. Cell 2011, 147, 1233–1247. [Google Scholar] [CrossRef]
- Hsu, S.H.; Wang, B.; Kota, J.; Yu, J.; Costinean, S.; Kutay, H.; Yu, L.; Bai, S.; La Perle, K.; Chivukula, R.R.; et al. Essential metabolic, anti-inflammatory, and anti-tumorigenic functions of miR-122 in liver. J. Clin. Investig. 2012, 122, 2871–2883. [Google Scholar] [CrossRef]
- Bai, S.; Nasser, M.W.; Wang, B.; Hsu, S.H.; Datta, J.; Kutay, H.; Yadav, A.; Nuovo, G.; Kumar, P.; Ghoshal, K. MicroRNA-122 inhibits tumorigenic properties of hepatocellular carcinoma cells and sensitizes these cells to sorafenib. J. Biol. Chem. 2009, 284, 32015–32027. [Google Scholar] [CrossRef] [PubMed]
- Tsai, W.C.; Hsu, P.W.; Lai, T.C.; Chau, G.Y.; Lin, C.W.; Chen, C.M.; Lin, C.D.; Liao, Y.L.; Wang, J.L.; Chau, Y.P.; et al. MicroRNA-122, a tumor suppressor microRNA that regulates intrahepatic metastasis of hepatocellular carcinoma. Hepatology 2009, 49, 1571–1582. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, S.; Hu, T.; Liu, S.; He, Y.; Sun, S. Up-regulated microRNA-143 transcribed by nuclear factor kappa B enhances hepatocarcinoma metastasis by repressing fibronectin expression. Hepatology 2009, 50, 490–499. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhou, X.; Wang, B.; Yung, B.C.; Lee, L.J.; Ghoshal, K.; Lee, R.J. Lactosylated gramicidin-based lipid nanoparticles (Lac-GLN) for targeted delivery of anti-miR-155 to hepatocellular carcinoma. J. Control. Release 2013, 168, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Lin, L.; Zhou, W.; Wang, Z.; Ding, G.; Dong, Q.; Qin, L.; Wu, X.; Zheng, Y.; Yang, Y.; et al. Identification of miRNomes in human liver and hepatocellular carcinoma reveals miR-199a/b-3p as therapeutic target for hepatocellular carcinoma. Cancer Cell 2011, 19, 232–243. [Google Scholar] [CrossRef]
- Callegari, E.; Elamin, B.K.; D’Abundo, L.; Falzoni, S.; Donvito, G.; Moshiri, F.; Milazzo, M.; Altavilla, G.; Giacomelli, L.; Fornari, F.; et al. Anti-tumor activity of a miR-199-dependent oncolytic adenovirus. PLoS ONE 2013, 8, e73964. [Google Scholar] [CrossRef]
- Ji, Q.; Hao, X.; Meng, Y.; Zhang, M.; Desano, J.; Fan, D.; Xu, L. Restoration of tumor suppressor miR-34 inhibits human p53-mutant gastric cancer tumorspheres. BMC Cancer 2008, 8, 266. [Google Scholar] [CrossRef]
- Feng, R.; Chen, X.; Yu, Y.; Su, L.; Yu, B.; Li, J.; Cai, Q.; Yan, M.; Liu, B.; Zhu, Z. miR-126 functions as a tumour suppressor in human gastric cancer. Cancer Lett. 2010, 298, 50–63. [Google Scholar] [CrossRef]
- Takei, Y.; Takigahira, M.; Mihara, K.; Tarumi, Y.; Yanagihara, K. The metastasis-associated microRNA miR-516a-3p is a novel therapeutic target for inhibiting peritoneal dissemination of human scirrhous gastric cancer. Cancer Res. 2011, 71, 1442–1453. [Google Scholar] [CrossRef]
- Akao, Y.; Nakagawa, Y.; Naoe, T. let-7 microRNA functions as a potential growth suppressor in human colon cancer cells. Biol. Pharm. Bull. 2006, 29, 903–906. [Google Scholar] [CrossRef]
- Dai, L.; Wang, W.; Zhang, S.; Jiang, Q.; Wang, R.; Dai, L.; Cheng, L.; Yang, Y.; Wei, Y.Q.; Deng, H.X. Vector-based miR-15a/16-1 plasmid inhibits colon cancer growth in vivo. Cell Biol. Int. 2012, 36, 765–770. [Google Scholar] [CrossRef] [PubMed]
- Idogawa, M.; Sasaki, Y.; Suzuki, H.; Mita, H.; Imai, K.; Shinomura, Y.; Tokino, T. A single recombinant adenovirus expressing p53 and p21-targeting artificial microRNAs efficiently induces apoptosis in human cancer cells. Clin. Cancer Res. 2009, 15, 3725–3732. [Google Scholar] [CrossRef] [PubMed]
- Bao, Y.; Chen, Z.; Guo, Y.; Feng, Y.; Li, Z.; Han, W.; Wang, J.; Zhao, W.; Jiao, Y.; Li, K.; et al. Tumor suppressor microRNA-27a in colorectal carcinogenesis and progression by targeting SGPP1 and Smad2. PLoS ONE 2014, 9, e105991. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Wu, X.; Wu, D.; Wu, P.; Ni, C.; Zhang, Z.; Chen, Z.; Qiu, F.; Xu, J.; Huang, J. miRNA-27b targets vascular endothelial growth factor C to inhibit tumor progression and angiogenesis in colorectal cancer. PLoS ONE 2013, 8, e60687. [Google Scholar] [CrossRef]
- Tazawa, H.; Tsuchiya, N.; Izumiya, M.; Nakagama, H. Tumor-suppressive miR-34a induces senescence-like growth arrest through modulation of the E2F pathway in human colon cancer cells. Proc. Natl. Acad. Sci. USA 2007, 104, 15472–15477. [Google Scholar] [CrossRef]
- Dong, Y.; Zhao, J.; Wu, C.W.; Zhang, L.; Liu, X.; Kang, W.; Leung, W.W.; Zhang, N.; Chan, F.K.; Sung, J.J.; et al. Tumor suppressor functions of miR-133a in colorectal cancer. Mol. Cancer Res. 2013, 11, 1051–1060. [Google Scholar] [CrossRef]
- Akao, Y.; Nakagawa, Y.; Hirata, I.; Iio, A.; Itoh, T.; Kojima, K.; Nakashima, R.; Kitade, Y.; Naoe, T. Role of anti-oncomirs miR-143 and -145 in human colorectal tumors. Cancer Gene Ther. 2010, 17, 398–408. [Google Scholar] [CrossRef]
- Ibrahim, A.F.; Weirauch, U.; Thomas, M.; Grunweller, A.; Hartmann, R.K.; Aigner, A. MicroRNA replacement therapy for miR-145 and miR-33a is efficacious in a model of colon carcinoma. Cancer Res. 2011, 71, 5214–5224. [Google Scholar] [CrossRef]
- Liang, G.; Zhu, Y.; Jing, A.; Wang, J.; Hu, F.; Feng, W.; Xiao, Z.; Chen, B. Cationic microRNA-delivering nanocarriers for efficient treatment of colon carcinoma in xenograft model. Gene Ther. 2016, 23, 829–838. [Google Scholar] [CrossRef]
- Gregersen, L.H.; Jacobsen, A.B.; Frankel, L.B.; Wen, J.; Krogh, A.; Lund, A.H. MicroRNA-145 targets YES and STAT1 in colon cancer cells. PLoS ONE 2010, 5, e8836. [Google Scholar] [CrossRef]
- Zhai, H.; Song, B.; Xu, X.; Zhu, W.; Ju, J. Inhibition of autophagy and tumor growth in colon cancer by miR-502. Oncogene 2013, 32, 1570–1579. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Schwind, S.; Yu, B.; Santhanam, R.; Wang, H.; Hoellerbauer, P.; Mims, A.; Klisovic, R.; Walker, A.R.; Chan, K.K.; et al. Targeted delivery of microRNA-29b by transferrin-conjugated anionic lipopolyplex nanoparticles: A novel therapeutic strategy in acute myeloid leukemia. Clin. Cancer Res. 2013, 19, 2355–2367. [Google Scholar] [CrossRef] [PubMed]
- Craig, V.J.; Tzankov, A.; Flori, M.; Schmid, C.A.; Bader, A.G.; Muller, A. Systemic microRNA-34a delivery induces apoptosis and abrogates growth of diffuse large B-cell lymphoma in vivo. Leukemia 2012, 26, 2421–2424. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; Fiori, M.E.; Albini, S.; Cifaldi, L.; Giovinazzi, S.; Forloni, M.; Boldrini, R.; Donfrancesco, A.; Federici, V.; Giacomini, P.; et al. Antagomir-17-5p abolishes the growth of therapy-resistant neuroblastoma through p21 and BIM. PLoS ONE 2008, 3, e2236. [Google Scholar] [CrossRef] [PubMed]
- Tivnan, A.; Orr, W.S.; Gubala, V.; Nooney, R.; Williams, D.E.; McDonagh, C.; Prenter, S.; Harvey, H.; Domingo-Fernandez, R.; Bray, I.M.; et al. Inhibition of neuroblastoma tumor growth by targeted delivery of microRNA-34a using anti-disialoganglioside GD2 coated nanoparticles. PLoS ONE 2012, 7, e38129. [Google Scholar] [CrossRef] [PubMed]
- Swarbrick, A.; Woods, S.L.; Shaw, A.; Balakrishnan, A.; Phua, Y.; Nguyen, A.; Chanthery, Y.; Lim, L.; Ashton, L.J.; Judson, R.L.; et al. miR-380-5p represses p53 to control cellular survival and is associated with poor outcome in MYCN-amplified neuroblastoma. Nat. Med. 2010, 16, 1134–1140. [Google Scholar] [CrossRef]
- Ji, Q.; Hao, X.; Zhang, M.; Tang, W.; Yang, M.; Li, L.; Xiang, D.; Desano, J.T.; Bommer, G.T.; Fan, D.; et al. MicroRNA miR-34 inhibits human pancreatic cancer tumor-initiating cells. PLoS ONE 2009, 4, e6816. [Google Scholar] [CrossRef]
- Pramanik, D.; Campbell, N.R.; Karikari, C.; Chivukula, R.; Kent, O.A.; Mendell, J.T.; Maitra, A. Restitution of tumor suppressor microRNAs using a systemic nanovector inhibits pancreatic cancer growth in mice. Mol. Cancer Ther. 2011, 10, 1470–1480. [Google Scholar] [CrossRef]
- Dong, Q.; Meng, P.; Wang, T.; Qin, W.; Qin, W.; Wang, F.; Yuan, J.; Chen, Z.; Yang, A.; Wang, H. MicroRNA let-7a inhibits proliferation of human prostate cancer cells in vitro and in vivo by targeting E2F2 and CCND2. PLoS ONE 2010, 5, e10147. [Google Scholar] [CrossRef]
- Aagaard, L.; Rossi, J.J. RNAi therapeutics: Principles, prospects and challenges. Adv. Drug Deliv. Rev. 2007, 59, 75–86. [Google Scholar] [CrossRef]
- Yang, H.W.; Huang, C.Y.; Lin, C.W.; Liu, H.L.; Huang, C.W.; Liao, S.S.; Chen, P.Y.; Lu, Y.J.; Wei, K.C.; Ma, C.C. Gadolinium-functionalized nanographene oxide for combined drug and microRNA delivery and magnetic resonance imaging. Biomaterials 2014, 35, 6534–6542. [Google Scholar] [CrossRef] [PubMed]
- Bakhshandeh, B.; Soleimani, M.; Hafizi, M.; Ghaemi, N. A comparative study on nonviral genetic modifications in cord blood and bone marrow mesenchymal stem cells. Cytotechnology 2012, 64, 523–540. [Google Scholar] [CrossRef] [PubMed]
- Hosseinahli, N.; Aghapour, M.; Duijf, P.H.G.; Baradaran, B. Treating cancer with microRNA replacement therapy: A literature review. J. Cell. Physiol. 2018, 233, 5574–5588. [Google Scholar] [CrossRef]
- Yin, H.; Kanasty, R.L.; Eltoukhy, A.A.; Vegas, A.J.; Dorkin, J.R.; Anderson, D.G. Non-viral vectors for gene-based therapy. Nat. Rev. Genet. 2014, 15, 541–555. [Google Scholar] [CrossRef]
- Hu, H.; Li, Y.; Zhou, Q.; Ao, Y.; Yu, C.; Wan, Y.; Xu, H.; Li, Z.; Yang, X. Redox-Sensitive Hydroxyethyl Starch-Doxorubicin Conjugate for Tumor Targeted Drug Delivery. ACS Appl. Mater. Interfaces 2016, 8, 30833–30844. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Rios, A.J.; Molina-Crespo, A.; Bouzo, B.L.; Lopez-Lopez, R.; Moreno-Bueno, G.; De la Fuente, M. Exosome-mimetic nanoplatforms for targeted cancer drug delivery. J. Nanobiotechnol. 2019, 17, 85. [Google Scholar] [CrossRef] [PubMed]
- Rai, K.; Takigawa, N.; Ito, S.; Kashihara, H.; Ichihara, E.; Yasuda, T.; Shimizu, K.; Tanimoto, M.; Kiura, K. Liposomal delivery of MicroRNA-7-expressing plasmid overcomes epidermal growth factor receptor tyrosine kinase inhibitor-resistance in lung cancer cells. Mol. Cancer Ther. 2011, 10, 1720–1727. [Google Scholar] [CrossRef]
- Mittal, A.; Chitkara, D.; Behrman, S.W.; Mahato, R.I. Efficacy of gemcitabine conjugated and miRNA-205 complexed micelles for treatment of advanced pancreatic cancer. Biomaterials 2014, 35, 7077–7087. [Google Scholar] [CrossRef]
- Katakowski, M.; Buller, B.; Zheng, X.; Lu, Y.; Rogers, T.; Osobamiro, O.; Shu, W.; Jiang, F.; Chopp, M. Exosomes from marrow stromal cells expressing miR-146b inhibit glioma growth. Cancer Lett. 2013, 335, 201–204. [Google Scholar] [CrossRef]
- Morimoto, Y.; Mizushima, T.; Wu, X.; Okuzaki, D.; Yokoyama, Y.; Inoue, A.; Hata, T.; Hirose, H.; Qian, Y.; Wang, J.; et al. miR-4711-5p regulates cancer stemness and cell cycle progression via KLF5, MDM2 and TFDP1 in colon cancer cells. Br. J. Cancer 2020, 122, 1037–1049. [Google Scholar] [CrossRef]
- Takeshita, F.; Patrawala, L.; Osaki, M.; Takahashi, R.U.; Yamamoto, Y.; Kosaka, N.; Kawamata, M.; Kelnar, K.; Bader, A.G.; Brown, D.; et al. Systemic delivery of synthetic microRNA-16 inhibits the growth of metastatic prostate tumors via downregulation of multiple cell-cycle genes. Mol. Ther. 2010, 18, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Ohno, S.; Takanashi, M.; Sudo, K.; Ueda, S.; Ishikawa, A.; Matsuyama, N.; Fujita, K.; Mizutani, T.; Ohgi, T.; Ochiya, T.; et al. Systemically injected exosomes targeted to EGFR deliver antitumor microRNA to breast cancer cells. Mol. Ther. 2013, 21, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Cortez, M.A.; Valdecanas, D.; Zhang, X.; Zhan, Y.; Bhardwaj, V.; Calin, G.A.; Komaki, R.; Giri, D.K.; Quini, C.C.; Wolfe, T.; et al. Therapeutic delivery of miR-200c enhances radiosensitivity in lung cancer. Mol. Ther. 2014, 22, 1494–1503. [Google Scholar] [CrossRef] [PubMed]
- Sonkoly, E.; Loven, J.; Xu, N.; Meisgen, F.; Wei, T.; Brodin, P.; Jaks, V.; Kasper, M.; Shimokawa, T.; Harada, M.; et al. MicroRNA-203 functions as a tumor suppressor in basal cell carcinoma. Oncogenesis 2012, 1, e3. [Google Scholar] [CrossRef]
- Lou, G.; Song, X.; Yang, F.; Wu, S.; Wang, J.; Chen, Z.; Liu, Y. Exosomes derived from miR-122-modified adipose tissue-derived MSCs increase chemosensitivity of hepatocellular carcinoma. J. Hematol. Oncol. 2015, 8, 122. [Google Scholar] [CrossRef]
- Zhang, S.; Chen, L.; Jung, E.J.; Calin, G.A. Targeting microRNAs with small molecules: From dream to reality. Clin. Pharmacol. Ther. 2010, 87, 754–758. [Google Scholar] [CrossRef]
- Hutvagner, G.; Simard, M.J.; Mello, C.C.; Zamore, P.D. Sequence-specific inhibition of small RNA function. PLoS Biol. 2004, 2, E98. [Google Scholar] [CrossRef]
- Meister, G.; Landthaler, M.; Dorsett, Y.; Tuschl, T. Sequence-specific inhibition of microRNA- and siRNA-induced RNA silencing. RNA 2004, 10, 544–550. [Google Scholar] [CrossRef]
- Babar, I.A.; Cheng, C.J.; Booth, C.J.; Liang, X.; Weidhaas, J.B.; Saltzman, W.M.; Slack, F.J. Nanoparticle-based therapy in an in vivo microRNA-155 (miR-155)-dependent mouse model of lymphoma. Proc. Natl. Acad. Sci. USA 2012, 109, E1695–E1704. [Google Scholar] [CrossRef]
- Elmen, J.; Lindow, M.; Schutz, S.; Lawrence, M.; Petri, A.; Obad, S.; Lindholm, M.; Hedtjarn, M.; Hansen, H.F.; Berger, U.; et al. LNA-mediated microRNA silencing in non-human primates. Nature 2008, 452, 896–899. [Google Scholar] [CrossRef]
- Krutzfeldt, J.; Rajewsky, N.; Braich, R.; Rajeev, K.G.; Tuschl, T.; Manoharan, M.; Stoffel, M. Silencing of microRNAs in vivo with ‘antagomirs’. Nature 2005, 438, 685–689. [Google Scholar] [CrossRef] [PubMed]
- Dickins, R.A.; Hemann, M.T.; Zilfou, J.T.; Simpson, D.R.; Ibarra, I.; Hannon, G.J.; Lowe, S.W. Probing tumor phenotypes using stable and regulated synthetic microRNA precursors. Nat. Genet. 2005, 37, 1289–1295. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo, M. Short interfering RNAs as a tool for cancer gene therapy. Cancer Gene Ther. 2005, 12, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Gleave, M.E.; Monia, B.P. Antisense therapy for cancer. Nat. Rev. Cancer 2005, 5, 468–479. [Google Scholar] [CrossRef]
- Gokita, K.; Inoue, J.; Ishihara, H.; Kojima, K.; Inazawa, J. Therapeutic Potential of LNP-Mediated Delivery of miR-634 for Cancer Therapy. Mol. Ther. Nucleic Acids 2020, 19, 330–338. [Google Scholar] [CrossRef]
- Wang, Z.; Hu, S.; Li, X.; Liu, Z.; Han, D.; Wang, Y.; Wei, L.; Zhang, G.; Wang, X. MiR-16-5p suppresses breast cancer proliferation by targeting ANLN. BMC Cancer 2021, 21, 1188. [Google Scholar] [CrossRef]
- Magnusson, K.; Gremel, G.; Ryden, L.; Ponten, V.; Uhlen, M.; Dimberg, A.; Jirstrom, K.; Ponten, F. ANLN is a prognostic biomarker independent of Ki-67 and essential for cell cycle progression in primary breast cancer. BMC Cancer 2016, 16, 904. [Google Scholar] [CrossRef]
- Cheng, C.J.; Bahal, R.; Babar, I.A.; Pincus, Z.; Barrera, F.; Liu, C.; Svoronos, A.; Braddock, D.T.; Glazer, P.M.; Engelman, D.M.; et al. MicroRNA silencing for cancer therapy targeted to the tumour microenvironment. Nature 2015, 518, 107–110. [Google Scholar] [CrossRef]
- Brognara, E.; Fabbri, E.; Bazzoli, E.; Montagner, G.; Ghimenton, C.; Eccher, A.; Cantu, C.; Manicardi, A.; Bianchi, N.; Finotti, A.; et al. Uptake by human glioma cell lines and biological effects of a peptide-nucleic acids targeting miR-221. J. Neurooncol. 2014, 118, 19–28. [Google Scholar] [CrossRef]
- Braicu, C.; Tomuleasa, C.; Monroig, P.; Cucuianu, A.; Berindan-Neagoe, I.; Calin, G.A. Exosomes as divine messengers: Are they the Hermes of modern molecular oncology? Cell Death Differ. 2015, 22, 34–45. [Google Scholar] [CrossRef]
- Jansson, M.D.; Lund, A.H. MicroRNA and cancer. Mol. Oncol. 2012, 6, 590–610. [Google Scholar] [CrossRef] [PubMed]
- Ekin, A.; Karatas, O.F.; Culha, M.; Ozen, M. Designing a gold nanoparticle-based nanocarrier for microRNA transfection into the prostate and breast cancer cells. J. Gene Med. 2014, 16, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Chen, W.; Yu, W.; Huang, W.; Deng, W. Small interfering RNA-based molecular therapy of cancers. Chin. J. Cancer 2013, 32, 488–493. [Google Scholar] [CrossRef] [PubMed]
- Hong, D.S.; Kang, Y.K.; Borad, M.; Sachdev, J.; Ejadi, S.; Lim, H.Y.; Brenner, A.J.; Park, K.; Lee, J.L.; Kim, T.Y.; et al. Phase 1 study of MRX34, a liposomal miR-34a mimic, in patients with advanced solid tumours. Br. J. Cancer 2020, 122, 1630–1637. [Google Scholar] [CrossRef]
- Van Zandwijk, N.; Pavlakis, N.; Kao, S.C.; Linton, A.; Boyer, M.J.; Clarke, S.; Huynh, Y.; Chrzanowska, A.; Fulham, M.J.; Bailey, D.L.; et al. Safety and activity of microRNA-loaded minicells in patients with recurrent malignant pleural mesothelioma: A first-in-man, phase 1, open-label, dose-escalation study. Lancet Oncol. 2017, 18, 1386–1396. [Google Scholar] [CrossRef]
- Viteri, S.; Rosell, R. An innovative mesothelioma treatment based on miR-16 mimic loaded EGFR targeted minicells (TargomiRs). Transl. Lung Cancer Res. 2018, 7, S1–S4. [Google Scholar] [CrossRef]
- Van Zandwijk, N.; McDiarmid, J.; Brahmbhatt, H.; Reid, G. Response to “An innovative mesothelioma treatment based on mir-16 mimic loaded EGFR targeted minicells (TargomiRs)”. Transl. Lung Cancer Res. 2018, 7, S60–S61. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).