Evaluation of Immunoregulatory Biomarkers on Plasma Small Extracellular Vesicles for Disease Progression and Early Therapeutic Response in Head and Neck Cancer
Abstract
1. Introduction
2. Materials and Methods
2.1. Plasma Specimens
2.2. sEV Preparation from Plasma by Size-Exclusion Chromatography
2.3. Transmission Electron Microscopy (TEM)
2.4. BCA Protein Assay
2.5. Western Blots
2.6. Nanoparticle Tracking Analysis (NTA)
2.7. Flow Cytometry of Immunosuppressive Markers on sEVs
2.8. sEV-Mediated Apoptosis Induction in CD8+ Jurkat Cells
2.9. Statistical Analysis
3. Results
3.1. Clinicopathological Characterization of the Study Participants
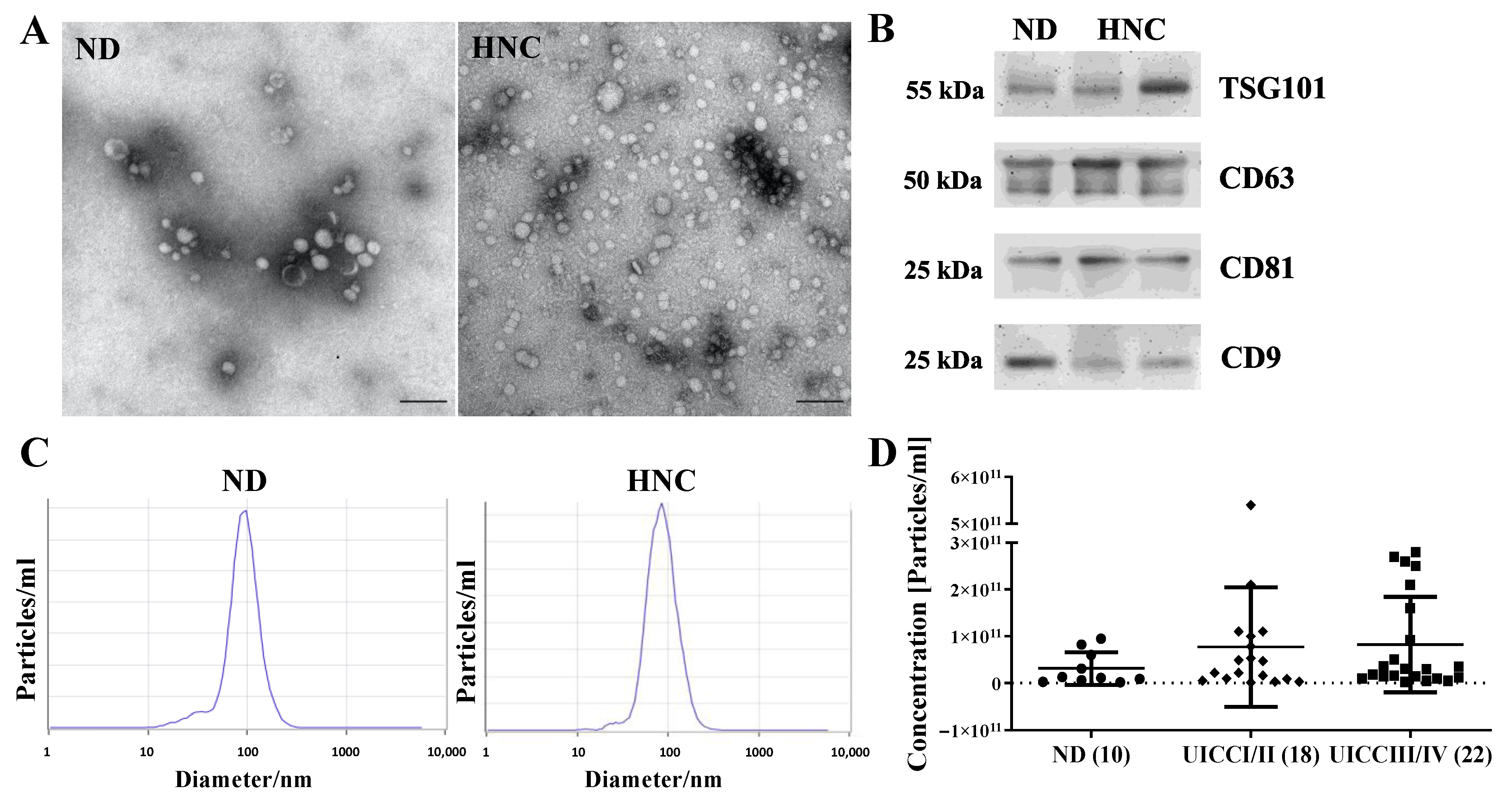
3.2. Morphology, Size Distribution, Concentration and Protein Content of Plasma sEVs
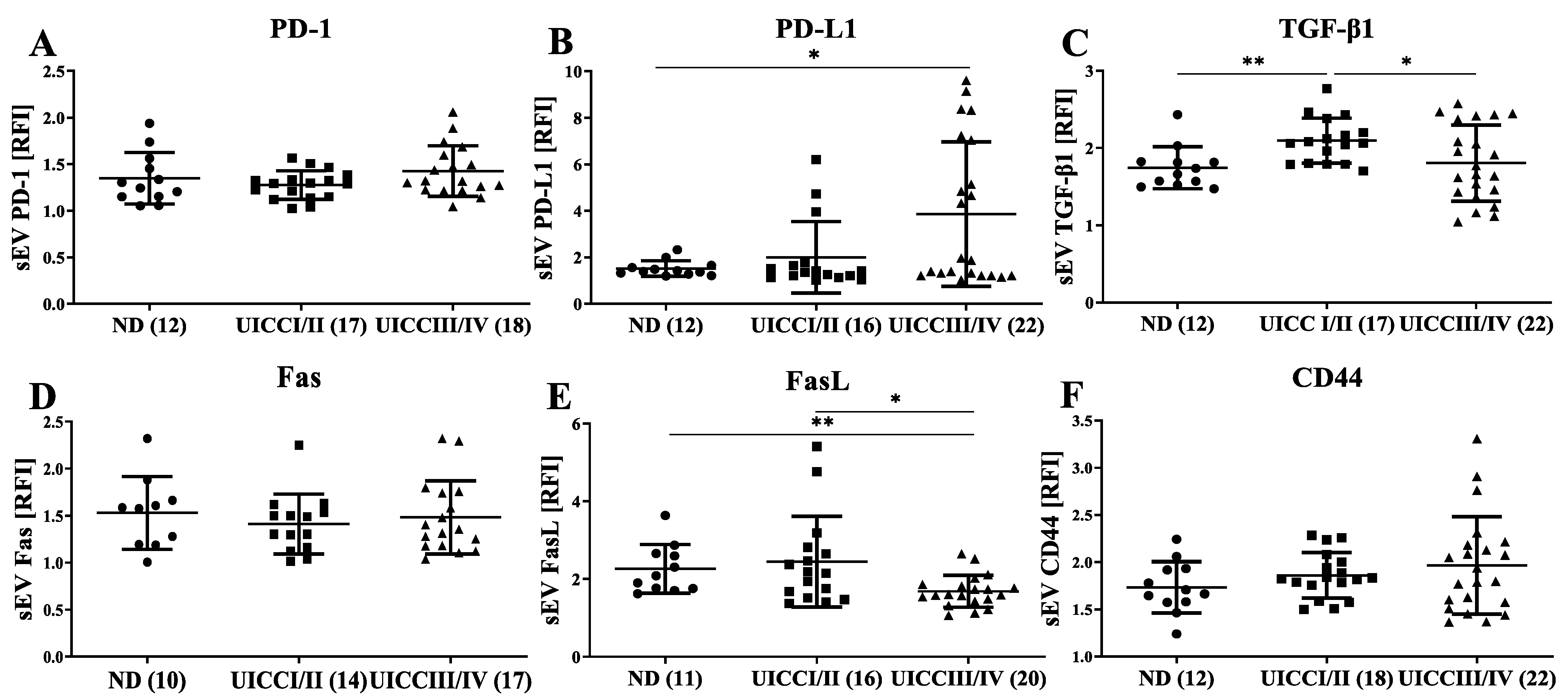
3.3. Immunomodulatory Surface Markers on sEVs from HNC Patients with Different Tumor Stages
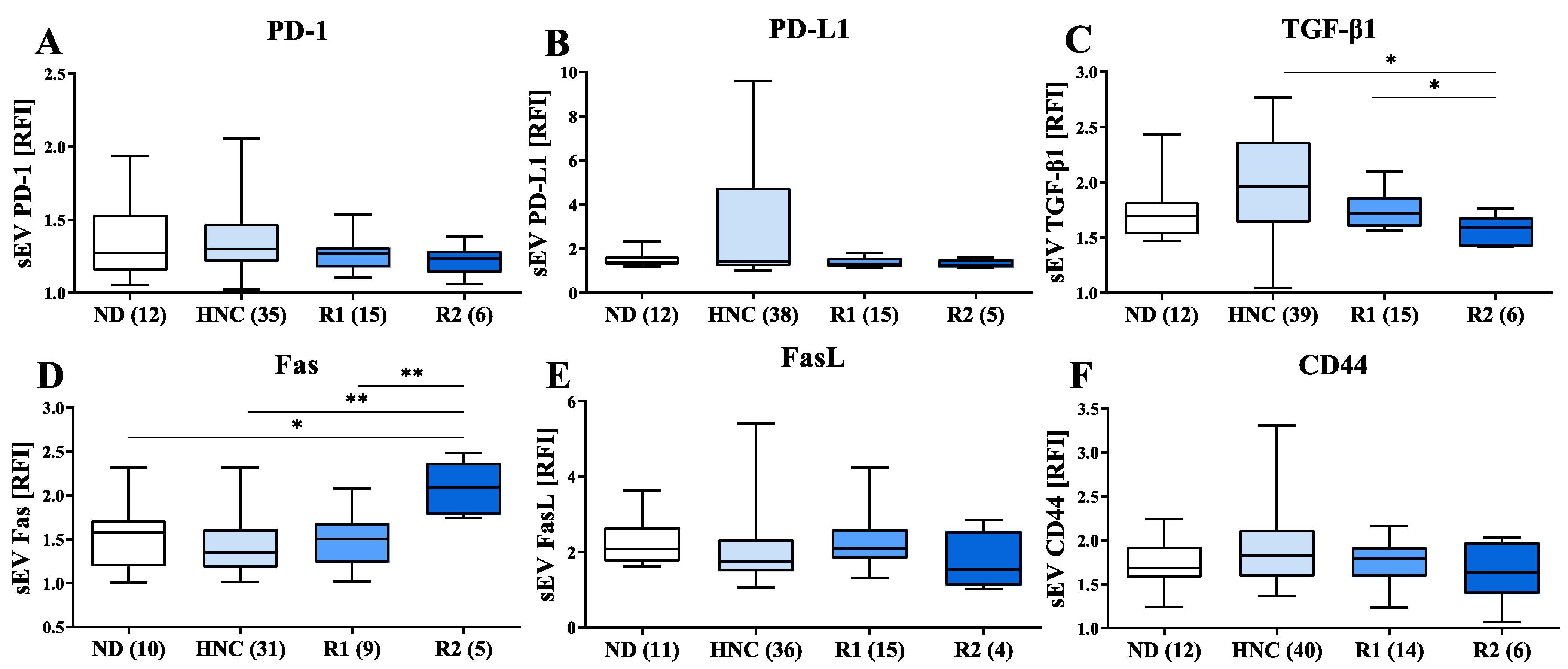
3.4. Modulation of Immunosuppressive Markers on sEVs of HNC Patients following Treatment
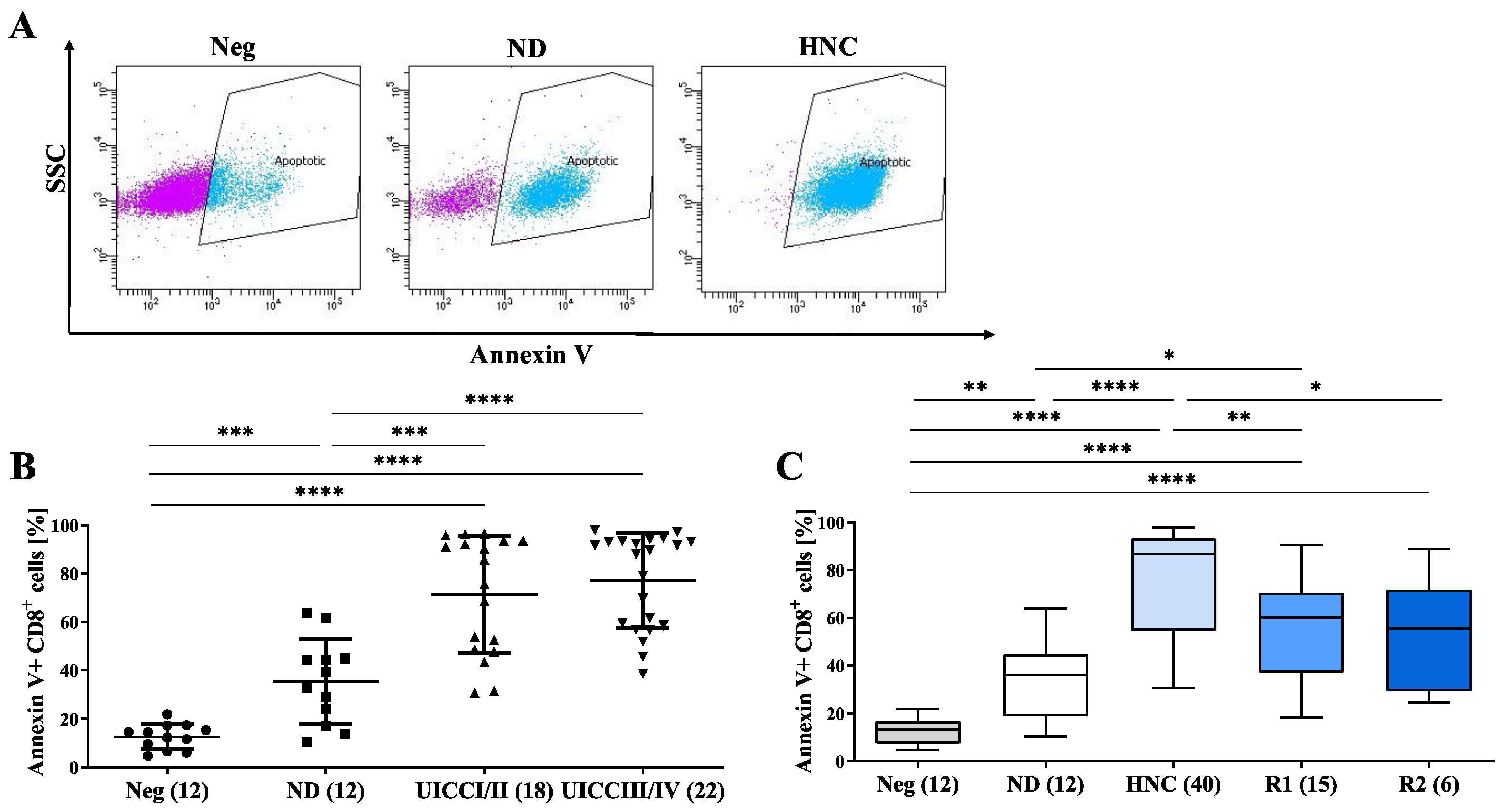
3.5. sEVs from HNC Patients Induce the Apoptosis of CD8+ T Cells
3.6. Five-Year Overall Survival (OS) of HNC Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Leemans, C.R.; Braakhuis, B.J.; Brakenhoff, R.H. The molecular biology of head and neck cancer. Nat. Rev. Cancer 2011, 11, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, T.L. Head and Neck Carcinoma Immunotherapy: Facts and Hopes. Clin. Cancer Res. 2018, 24, 6–13. [Google Scholar] [CrossRef]
- Gao, Y.; Qin, Y.; Wan, C.; Sun, Y.; Meng, J.; Huang, J.; Hu, Y.; Jin, H.; Yang, K. Small Extracellular Vesicles: A Novel Avenue for Cancer Management. Front. Oncol. 2021, 11, 638357. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367. [Google Scholar] [CrossRef] [PubMed]
- Whiteside, T.L. Exosomes and tumor-mediated immune suppression. J. Clin. Investig. 2016, 126, 1216–1223. [Google Scholar] [CrossRef]
- Theodoraki, M.N.; Yerneni, S.S.; Hoffmann, T.K.; Gooding, W.E.; Whiteside, T.L. Clinical Significance of PD-L1(+) Exosomes in Plasma of Head and Neck Cancer Patients. Clin. Cancer Res. 2018, 24, 896–905. [Google Scholar] [CrossRef]
- Ludwig, S.; Floros, T.; Theodoraki, M.N.; Hong, C.S.; Jackson, E.K.; Lang, S.; Whiteside, T.L. Suppression of lymphocyte functions by plasma exosomes correlates with disease activity in patients with head and neck cancer. Clin. Cancer Res. 2017, 23, 4843–4854. [Google Scholar] [CrossRef]
- Thery, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Hong, C.S.; Funk, S.; Muller, L.; Boyiadzis, M.; Whiteside, T.L. Isolation of biologically active and morphologically intact exosomes from plasma of patients with cancer. J. Extracell Vesicles 2016, 5, 29289. [Google Scholar] [CrossRef]
- Boing, A.N.; van der Pol, E.; Grootemaat, A.E.; Coumans, F.A.; Sturk, A.; Nieuwland, R. Single-step isolation of extracellular vesicles by size-exclusion chromatography. J. Extracell Vesicles 2014, 3, 23430. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, N.; Hong, C.S.; Ludwig, S.; Azambuja, J.H.; Sharma, P.; Theodoraki, M.N.; Whiteside, T.L. Isolation and Analysis of Tumor-Derived Exosomes. Curr. Protoc. Immunol. 2019, 127, e91. [Google Scholar] [CrossRef] [PubMed]
- Theodoraki, M.N.; Laban, S.; Jackson, E.K.; Lotfi, R.; Schuler, P.J.; Brunner, C.; Hoffmann, T.K.; Whiteside, T.L.; Hofmann, L. Changes in circulating exosome molecular profiles following surgery/(chemo)radiotherapy: Early detection of response in head and neck cancer patients. Br. J. Cancer 2021, 125, 1677–1686. [Google Scholar] [CrossRef] [PubMed]
- Theodoraki, M.N.; Hong, C.S.; Donnenberg, V.S.; Donnenberg, A.D.; Whiteside, T.L. Evaluation of Exosome Proteins by on-Bead Flow Cytometry. Cytom. A 2021, 99, 372–381. [Google Scholar] [CrossRef]
- Theodoraki, M.N.; Hoffmann, T.K.; Whiteside, T.L. Separation of plasma-derived exosomes into CD3((+)) and CD3((–)) fractions allows for association of immune cell and tumour cell markers with disease activity in HNSCC patients. Clin. Exp. Immunol. 2018, 192, 271–283. [Google Scholar] [CrossRef]
- Beccard, I.J.; Hofmann, L.; Schroeder, J.C.; Ludwig, S.; Laban, S.; Brunner, C.; Lotfi, R.; Hoffmann, T.K.; Jackson, E.K.; Schuler, P.J.; et al. Immune Suppressive Effects of Plasma-Derived Exosome Populations in Head and Neck Cancer. Cancers 2020, 12, 1997. [Google Scholar] [CrossRef]
- Chen, C.; Zhao, S.; Karnad, A.; Freeman, J.W. The biology and role of CD44 in cancer progression: Therapeutic implications. J. Hematol. Oncol. 2018, 11, 64. [Google Scholar] [CrossRef]
- White, R.A.; Malkoski, S.P.; Wang, X.J. TGFbeta signaling in head and neck squamous cell carcinoma. Oncogene 2010, 29, 5437–5446. [Google Scholar] [CrossRef]
- Hu, J.; Li, G.; Zhang, P.; Zhuang, X.; Hu, G. A CD44v(+) subpopulation of breast cancer stem-like cells with enhanced lung metastasis capacity. Cell Death Dis. 2017, 8, e2679. [Google Scholar] [CrossRef]
- Slomiany, M.G.; Grass, G.D.; Robertson, A.D.; Yang, X.Y.; Maria, B.L.; Beeson, C.; Toole, B.P. Hyaluronan, CD44, and emmprin regulate lactate efflux and membrane localization of monocarboxylate transporters in human breast carcinoma cells. Cancer Res. 2009, 69, 1293–1301. [Google Scholar] [CrossRef]
- Reategui, E.P.; de Mayolo, A.A.; Das, P.M.; Astor, F.C.; Singal, R.; Hamilton, K.L.; Goodwin, W.J.; Carraway, K.L.; Franzmann, E.J. Characterization of CD44v3-containing isoforms in head and neck cancer. Cancer Biol. Ther. 2006, 5, 1163–1168. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wang, S.J.; Wreesmann, V.B.; Bourguignon, L.Y. Association of CD44 V3-containing isoforms with tumor cell growth, migration, matrix metalloproteinase expression, and lymph node metastasis in head and neck cancer. Head Neck 2007, 29, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Theodoraki, M.N.; Matsumoto, A.; Beccard, I.; Hoffmann, T.K.; Whiteside, T.L. CD44v3 protein-carrying tumor-derived exosomes in HNSCC patients’ plasma as potential noninvasive biomarkers of disease activity. Oncoimmunology 2020, 9, 1747732. [Google Scholar] [CrossRef] [PubMed]
- Weeks, B.H.; He, W.; Olson, K.L.; Wang, X.J. Inducible expression of transforming growth factor beta1 in papillomas causes rapid metastasis. Cancer Res. 2001, 61, 7435–7443. [Google Scholar]
- Lu, S.L.; Reh, D.; Li, A.G.; Woods, J.; Corless, C.L.; Kulesz-Martin, M.; Wang, X.J. Overexpression of transforming growth factor beta1 in head and neck epithelia results in inflammation, angiogenesis, and epithelial hyperproliferation. Cancer Res. 2004, 64, 4405–4410. [Google Scholar] [CrossRef]
- Chatterjee, S.; Chatterjee, A.; Jana, S.; Dey, S.; Roy, H.; Das, M.K.; Alam, J.; Adhikary, A.; Chowdhury, A.; Biswas, A.; et al. Transforming growth factor beta orchestrates PD-L1 enrichment in tumor-derived exosomes and mediates CD8 T-cell dysfunction regulating early phosphorylation of TCR signalome in breast cancer. Carcinogenesis 2021, 42, 38–47. [Google Scholar] [CrossRef]
- Daassi, D.; Mahoney, K.M.; Freeman, G.J. The importance of exosomal PDL1 in tumour immune evasion. Nat. Rev. Immunol. 2020, 20, 209–215. [Google Scholar] [CrossRef]
- Zhou, K.; Guo, S.; Li, F.; Sun, Q.; Liang, G. Exosomal PD-L1: New Insights Into Tumor Immune Escape Mechanisms and Therapeutic Strategies. Front. Cell Dev. Biol. 2020, 8, 569219. [Google Scholar] [CrossRef]
- Ye, L.; Zhu, Z.; Chen, X.; Zhang, H.; Huang, J.; Gu, S.; Zhao, X. The Importance of Exosomal PD-L1 in Cancer Progression and Its Potential as a Therapeutic Target. Cells 2021, 10, 3427. [Google Scholar] [CrossRef]
- Schneider, S.; Kadletz, L.; Wiebringhaus, R.; Kenner, L.; Selzer, E.; Fureder, T.; Rajky, O.; Berghoff, A.S.; Preusser, M.; Heiduschka, G. PD-1 and PD-L1 expression in HNSCC primary cancer and related lymph node metastasis—Impact on clinical outcome. Histopathology 2018, 73, 573–584. [Google Scholar] [CrossRef]
- Sanchez-Canteli, M.; Granda-Diaz, R.; Del Rio-Ibisate, N.; Allonca, E.; Lopez-Alvarez, F.; Agorreta, J.; Garmendia, I.; Montuenga, L.M.; Garcia-Pedrero, J.M.; Rodrigo, J.P. PD-L1 expression correlates with tumor-infiltrating lymphocytes and better prognosis in patients with HPV-negative head and neck squamous cell carcinomas. Cancer Immunol. Immunother 2020, 69, 2089–2100. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.W.; Li, S.H.; Shi, D.B.; Jiang, W.M.; Song, M.; Yang, A.K.; Li, Y.D.; Bei, J.X.; Chen, W.K.; Zhang, Q. Expression of PD-1/PD-L1 in head and neck squamous cell carcinoma and its clinical significance. Int. J. Biol. Markers 2019, 34, 398–405. [Google Scholar] [CrossRef] [PubMed]
- De Meulenaere, A.; Vermassen, T.; Aspeslagh, S.; Huvenne, W.; Van Dorpe, J.; Ferdinande, L.; Rottey, S. Turning the tide: Clinical utility of PD-L1 expression in squamous cell carcinoma of the head and neck. Oral Oncol. 2017, 70, 34–42. [Google Scholar] [CrossRef]
- Yang, W.F.; Wong, M.C.M.; Thomson, P.J.; Li, K.Y.; Su, Y.X. The prognostic role of PD-L1 expression for survival in head and neck squamous cell carcinoma: A systematic review and meta-analysis. Oral Oncol. 2018, 86, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Huang, A.C.; Zhang, W.; Zhang, G.; Wu, M.; Xu, W.; Yu, Z.; Yang, J.; Wang, B.; Sun, H.; et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature 2018, 560, 382–386. [Google Scholar] [CrossRef]
- de Carvalho-Neto, P.B.; dos Santos, M.; de Carvalho, M.B.; Mercante, A.M.; dos Santos, V.P.; Severino, P.; Tajara, E.H.; Louro, I.D.; da Silva-Conforti, A.M. FAS/FASL expression profile as a prognostic marker in squamous cell carcinoma of the oral cavity. PLoS ONE 2013, 8, e69024. [Google Scholar] [CrossRef]
- Asensio, C.; Zapata, A.; Garcia-Ahijado, J.; Gil, B.; Salvadores, P.; Schneider, J. Fas expression is associated with a better prognosis in laryngeal squamous cell carcinoma. Anticancer Res. 2007, 27, 4083–4086. [Google Scholar]
- Li, Q.; Peng, J.; Li, X.H.; Liu, T.; Liang, Q.C.; Zhang, G.Y. Clinical significance of Fas and FasL protein expression in gastric carcinoma and local lymph node tissues. World J. Gastroenterol. 2010, 16, 1274–1278. [Google Scholar] [CrossRef]
- Pryczynicz, A.; Guzinska-Ustymowicz, K.; Kemona, A. Fas/FasL expression in colorectal cancer. An immunohistochemical study. Folia Histochem. Cytobiol. 2010, 48, 425–429. [Google Scholar] [CrossRef]
- Watson, G.A.; Naran, S.; Zhang, X.; Stang, M.T.; Queiroz de Oliveira, P.E.; Hughes, S.J. Cytoplasmic overexpression of CD95L in esophageal adenocarcinoma cells overcomes resistance to CD95-mediated apoptosis. Neoplasia 2011, 13, 198–205. [Google Scholar] [CrossRef]
- Whiteside, T.L.; Rabinowich, H. The role of Fas/FasL in immunosuppression induced by human tumors. Cancer Immunol. Immunother 1998, 46, 175–184. [Google Scholar] [CrossRef]
- Abusamra, A.J.; Zhong, Z.; Zheng, X.; Li, M.; Ichim, T.E.; Chin, J.L.; Min, W.P. Tumor exosomes expressing Fas ligand mediate CD8+ T-cell apoptosis. Blood Cells Mol. Dis. 2005, 35, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Wang, H.D.; Guo, W.; Yang, K.; Zhao, Y.P.; Jiang, Y.G.; He, P. Up-regulation of Fas reverses cisplatin resistance of human small cell lung cancer cells. J. Exp. Clin. Cancer Res. 2010, 29, 49. [Google Scholar] [CrossRef] [PubMed]
- Hill, D.P.; Harper, A.; Malcolm, J.; McAndrews, M.S.; Mockus, S.M.; Patterson, S.E.; Reynolds, T.; Baker, E.J.; Bult, C.J.; Chesler, E.J.; et al. Cisplatin-resistant triple-negative breast cancer subtypes: Multiple mechanisms of resistance. BMC Cancer 2019, 19, 1039. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Sanmamed, M.F.; Chen, L. A Paradigm Shift in Cancer Immunotherapy: From Enhancement to Normalization. Cell 2018, 175, 313–326. [Google Scholar] [CrossRef]
- Quezada, S.A.; Peggs, K.S. Exploiting CTLA-4, PD-1 and PD-L1 to reactivate the host immune response against cancer. Br. J. Cancer 2013, 108, 1560–1565. [Google Scholar] [CrossRef]
- Cramer, J.D.; Burtness, B.; Ferris, R.L. Immunotherapy for head and neck cancer: Recent advances and future directions. Oral Oncol. 2019, 99, 104460. [Google Scholar] [CrossRef]


| N | % | |
|---|---|---|
| Age (mean±SD; years) | ||
| ≤63.7 ± 9.1 | 18 | 45.0 |
| >63.7 ± 9.1 | 22 | 55.0 |
| Gender | ||
| Male | 29 | 72.5 |
| Female | 11 | 27.5 |
| Tumor site | ||
| Oral cavity | 9 | 22.5 |
| Oropharynx | 13 | 32.5 |
| Larynx | 16 | 40.0 |
| Hypopharynx | 2 | 5.0 |
| HPV status 1 | ||
| Negative | 10 | 76.9 |
| Positive | 3 | 23.1 |
| Smoking | ||
| Never | 8 | 20.0 |
| Former | 6 | 15.0 |
| Current | 26 | 65.0 |
| Alcohol consumption | ||
| None | 18 | 45.0 |
| Former | 7 | 18.5 |
| Current | 15 | 37.5 |
| Tumor size | ||
| T1 | 10 | 25.0 |
| T2 | 15 | 37.5 |
| T3 | 11 | 27.5 |
| T4 | 4 | 10.0 |
| Lymph node status | ||
| N0 | 21 | 52.5 |
| N+ | 19 | 47.5 |
| Metastases | ||
| M0 | 40 | 100.0 |
| UICC stages 2 | ||
| I | 8 | 20.0 |
| II | 10 | 25.0 |
| III | 9 | 22.5 |
| IV | 13 | 32.5 |
| Grading | ||
| G1 | 1 | 2.5 |
| G2 | 28 | 70.0 |
| G3 | 10 | 25.0 |
| Missing | 1 | 2.5 |
| Therapy | ||
| Surgery | 12 | 30.0 |
| Surgery plus CRT | 24 | 60.0 |
| Primary CRT | 4 | 10.0 |
| Disease course after therapy | ||
| No evident disease (NED) | 30 | 75 |
| Local recurrence | 2 | 5 |
| Distant metastasis | 1 | 2.5 |
| Second carcinoma | 1 | 2.5 |
| Deceased | 4 | 10.0 |
| Unknown/Loss of follow-up | 3 | 7.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jablonska, J.; Rist, M.; Spyra, I.; Tengler, L.; Domnich, M.; Kansy, B.; Giebel, B.; Thakur, B.K.; Rotter, N.; Lang, S.; et al. Evaluation of Immunoregulatory Biomarkers on Plasma Small Extracellular Vesicles for Disease Progression and Early Therapeutic Response in Head and Neck Cancer. Cells 2022, 11, 902. https://doi.org/10.3390/cells11050902
Jablonska J, Rist M, Spyra I, Tengler L, Domnich M, Kansy B, Giebel B, Thakur BK, Rotter N, Lang S, et al. Evaluation of Immunoregulatory Biomarkers on Plasma Small Extracellular Vesicles for Disease Progression and Early Therapeutic Response in Head and Neck Cancer. Cells. 2022; 11(5):902. https://doi.org/10.3390/cells11050902
Chicago/Turabian StyleJablonska, Jadwiga, Malwina Rist, Ilona Spyra, Luisa Tengler, Maksim Domnich, Benjamin Kansy, Bernd Giebel, Basant Kumar Thakur, Nicole Rotter, Stephan Lang, and et al. 2022. "Evaluation of Immunoregulatory Biomarkers on Plasma Small Extracellular Vesicles for Disease Progression and Early Therapeutic Response in Head and Neck Cancer" Cells 11, no. 5: 902. https://doi.org/10.3390/cells11050902
APA StyleJablonska, J., Rist, M., Spyra, I., Tengler, L., Domnich, M., Kansy, B., Giebel, B., Thakur, B. K., Rotter, N., Lang, S., & Ludwig, S. (2022). Evaluation of Immunoregulatory Biomarkers on Plasma Small Extracellular Vesicles for Disease Progression and Early Therapeutic Response in Head and Neck Cancer. Cells, 11(5), 902. https://doi.org/10.3390/cells11050902








