Androgen Receptor-Mediated Transcription in Prostate Cancer
Abstract
1. Androgen Receptor in Prostate Cancer
2. Androgen Receptor-Mediated Gene Transcription
3. Pioneer Factors and DNA Binding
4. Plasticity of the AR Cistrome in Prostate Cancer Progression
5. Impact of Motifs on AR Binding and Activity
6. Impact of Chromatin Modifying Enzymes on AR Activity
7. AR-Coregulators and Gene Transcription
8. AR Enhancers in Gene Transcription
9. 3D Genome Organization
10. Enhancer CRE Mutations
11. Targeting AR and Coregulators
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Lamont, K.R.; Tindall, D.J. Androgen Regulation of Gene Expression. Adv. Cancer Res. 2010, 107, 137–162. [Google Scholar] [CrossRef] [PubMed]
- Huggins, C.; Hodges, C.V. Studies on prostatic cancer i. the effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1941, 1, 293–297. [Google Scholar]
- Crawford, E.D.; Hou, A.H. The role of LHRH antagonists in the treatment of prostate cancer. Oncology 2009, 23, 626–630. [Google Scholar]
- Kirby, M.; Hirst, C.; Crawford, E.D. Characterising the castration-resistant prostate cancer population: A systematic review. Int. J. Clin. Pract. 2011, 65, 1180–1192. [Google Scholar] [CrossRef] [PubMed]
- Hay, C.W.; McEwan, I.J. The Impact of Point Mutations in the Human Androgen Receptor: Classification of Mutations on the Basis of Transcriptional Activity. PLoS ONE 2012, 7, e32514. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Attard, G.; Balk, S.P.; Bevan, C.; Burnstein, K.; Cato, L.; Cherkasov, A.; De Bono, J.S.; Dong, Y.; Gao, A.C.; et al. Role of Androgen Receptor Variants in Prostate Cancer: Report from the 2017 Mission Androgen Receptor Variants Meeting. Eur. Urol. 2018, 73, 715–723. [Google Scholar] [CrossRef]
- Zhang, A.; Zhao, J.C.; Kim, J.; Fong, K.-W.; Yang, Y.A.; Chakravarti, D.; Mo, Y.-Y.; Yu, J. LncRNA HOTAIR Enhances the Androgen-Receptor-Mediated Transcriptional Program and Drives Castration-Resistant Prostate Cancer. Cell Rep. 2015, 13, 209–221. [Google Scholar] [CrossRef]
- Barbieri, C.; Baca, S.C.; Lawrence, M.S.; Demichelis, F.; Blattner, M.; Theurillat, J.-P.; White, T.A.; Stojanov, P.; Van Allen, E.; Stransky, N.; et al. Exome sequencing identifies recurrent SPOP, FOXA1 and MED12 mutations in prostate cancer. Nat. Genet. 2012, 44, 685–689. [Google Scholar] [CrossRef]
- Robinson, D.; Van Allen, E.M.; Wu, Y.-M.; Schultz, N.; Lonigro, R.J.; Mosquera, J.-M.; Montgomery, B.; Taplin, M.-E.; Pritchard, C.C.; Attard, G.; et al. Integrative Clinical Genomics of Advanced Prostate Cancer. Cell 2015, 162, 454. [Google Scholar] [CrossRef]
- Taylor, B.S.; Schultz, N.; Hieronymus, H.; Gopalan, A.; Xiao, Y.; Carver, B.S.; Arora, V.K.; Kaushik, P.; Cerami, E.; Reva, B.; et al. Integrative Genomic Profiling of Human Prostate Cancer. Cancer Cell 2010, 18, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Mangelsdorf, D.J.; Thummel, C.; Beato, M.; Herrlich, P.; Schütz, G.; Umesono, K.; Blumberg, B.; Kastner, P.; Mark, M.; Chambon, P.; et al. The nuclear receptor superfamily: The second decade. Cell 1995, 83, 835–839. [Google Scholar] [CrossRef]
- Chang, C.; Saltzman, A.; Yeh, S.; Young, W.; Keller, E.; Lee, H.-J.; Wang, C.; Mizokami, A. Androgen Receptor: An Overview. Crit. Rev. Eukaryot. Gene Expr. 1995, 5, 97–125. [Google Scholar] [CrossRef] [PubMed]
- Davey, R.A.; Grossmann, M. Androgen Receptor Structure, Function and Biology: From Bench to Bedside. Clin. Biochem. Rev. 2016, 37, 3–15. [Google Scholar]
- Heemers, H.V.; Tindall, D.J. Androgen Receptor (AR) Coregulators: A Diversity of Functions Converging on and Regulating the AR Transcriptional Complex. Endocr. Rev. 2007, 28, 778–808. [Google Scholar] [CrossRef]
- Menon, T.; Yates, J.A.; Bochar, D.A. Regulation of Androgen-Responsive Transcription by the Chromatin Remodeling Factor CHD8. Mol. Endocrinol. 2010, 24, 1165–1174. [Google Scholar] [CrossRef]
- Jin, F.; Claessens, F.; Fondell, J.D. Regulation of Androgen Receptor-dependent Transcription by Coactivator MED1 Is Mediated through a Newly Discovered Noncanonical Binding Motif. J. Biol. Chem. 2012, 287, 858–870. [Google Scholar] [CrossRef]
- Tan, P.Y.; Chang, C.W.; Chng, K.R.; Wansa, K.S.A.; Sung, W.-K.; Cheung, E. Integration of Regulatory Networks by NKX3-1 Promotes Androgen-Dependent Prostate Cancer Survival. Mol. Cell. Biol. 2012, 32, 399–414. [Google Scholar] [CrossRef]
- Meyer, R.; Wolf, S.S.; Obendorf, M. PRMT2, a member of the protein arginine methyltransferase family, is a coactivator of the androgen receptor. J. Steroid Biochem. Mol. Biol. 2007, 107, 1–14. [Google Scholar] [CrossRef]
- Vélot, L.; Lessard, F.; Bérubé-Simard, F.-A.; Tav, C.; Neveu, B.; Teyssier, V.; Boudaoud, I.; Dionne, U.; Lavoie, N.; Bilodeau, S.; et al. Proximity-dependent Mapping of the Androgen Receptor Identifies Kruppel-like Factor 4 as a Functional Partner. Mol. Cell. Proteom. 2021, 20, 100064. [Google Scholar] [CrossRef]
- Shang, Y.; Myers, M.; Brown, M. Formation of the Androgen Receptor Transcription Complex. Mol. Cell 2002, 9, 601–610. [Google Scholar] [CrossRef]
- Gao, L.; Schwartzman, J.; Gibbs, A.; Lisac, R.; Kleinschmidt, R.; Wilmot, B.; Bottomly, D.; Coleman, I.; Nelson, P.; McWeeney, S.; et al. Androgen Receptor Promotes Ligand-Independent Prostate Cancer Progression through c-Myc Upregulation. PLoS ONE 2013, 8, e63563. [Google Scholar] [CrossRef]
- Cai, C.; Hsieh, C.-L.; Omwancha, J.; Zheng, Z.; Chen, S.-Y.; Baert, J.-L.; Shemshedini, L. ETV1 Is a Novel Androgen Receptor-Regulated Gene that Mediates Prostate Cancer Cell Invasion. Mol. Endocrinol. 2007, 21, 1835–1846. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Li, P.; Huang, H.; Ye, X.; Chen, W.; Xu, G.; Zhang, F. Androgen receptor regulates eIF5A2 expression and promotes prostate cancer metastasis via EMT. Cell Death Discov. 2021, 7, 373. [Google Scholar] [CrossRef] [PubMed]
- Paltoglou, S.; Das, R.; Townley, S.L.; Hickey, T.; Tarulli, G.; Coutinho, I.; Fernandes, R.; Hanson, A.R.; Denis, I.; Carroll, J.; et al. Novel Androgen Receptor Coregulator GRHL2 Exerts Both Oncogenic and Antimetastatic Functions in Prostate Cancer. Cancer Res. 2017, 77, 3417–3430. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.G.; Nam, Y.; Shin, K.J.; Yoon, S.; Park, W.S.; Joung, J.Y.; Seo, J.K.; Jang, J.; Lee, S.; Nam, D.; et al. Androgen-induced expression of DRP1 regulates mitochondrial metabolic reprogramming in prostate cancer. Cancer Lett. 2020, 471, 72–87. [Google Scholar] [CrossRef] [PubMed]
- Yu, I.-C.; Lin, H.-Y.; Sparks, J.D.; Yeh, S.; Chang, C. Androgen Receptor Roles in Insulin Resistance and Obesity in Males: The Linkage of Androgen-Deprivation Therapy to Metabolic Syndrome. Diabetes 2014, 63, 3180–3188. [Google Scholar] [CrossRef]
- Wu, Y.; Chhipa, R.R.; Cheng, J.; Zhang, H.; Mohler, J.L.; Ip, C. Androgen receptor-mTOR crosstalk is regulated by testosterone availability: Implication for prostate cancer cell survival. Anticancer Res. 2010, 30, 3895–3901. [Google Scholar]
- Carter, S.L.; Centenera, M.M.; Tilley, W.D.; Selth, L.A.; Butler, L.M. IκBα mediates prostate cancer cell death induced by combinatorial targeting of the androgen receptor. BMC Cancer 2016, 16, 141. [Google Scholar] [CrossRef]
- Tewari, A.K.; Yardimci, G.G.; Shibata, Y.; Sheffield, N.C.; Song, L.; Taylor, B.S.; Georgiev, S.G.; Coetzee, G.A.; Ohler, U.; Furey, T.S.; et al. Chromatin accessibility reveals insights into androgen receptor activation and transcriptional specificity. Genome Biol. 2012, 13, R88. [Google Scholar] [CrossRef]
- Lupien, M.; Eeckhoute, J.; Meyer, C.A.; Wang, Q.; Zhang, Y.; Li, W.; Carroll, J.S.; Liu, X.S.; Brown, M. FoxA1 Translates Epigenetic Signatures into Enhancer-Driven Lineage-Specific Transcription. Cell 2008, 132, 958–970. [Google Scholar] [CrossRef] [PubMed]
- Sahu, B.; Laakso, M.; Ovaska, K.; Mirtti, T.; Lundin, J.; Rannikko, A.; Sankila, A.; Turunen, J.-P.; Lundin, M.; Konsti, J.; et al. Dual role of FoxA1 in androgen receptor binding to chromatin, androgen signalling and prostate cancer. EMBO J. 2011, 30, 3962–3976. [Google Scholar] [CrossRef]
- Garcia, M.F.; Moore, C.D.; Schulz, K.N.; Alberto, O.; Donague, G.; Harrison, M.M.; Zhu, H.; Zaret, K.S. Structural Features of Transcription Factors Associating with Nucleosome Binding. Mol. Cell 2019, 75, 921–932.e6. [Google Scholar] [CrossRef] [PubMed]
- Polach, K.; Widom, J. Mechanism of Protein Access to Specific DNA Sequences in Chromatin: A Dynamic Equilibrium Model for Gene Regulation. J. Mol. Biol. 1995, 254, 130–149. [Google Scholar] [CrossRef]
- Anderson, J.; Widom, J. Sequence and position-dependence of the equilibrium accessibility of nucleosomal DNA target sites. J. Mol. Biol. 2000, 296, 979–987. [Google Scholar] [CrossRef]
- Sunkel, B.D.; Stanton, B.Z. Pioneer factors in development and cancer. iScience 2021, 24, 103132. [Google Scholar] [CrossRef] [PubMed]
- Strahl, B.D.; Ohba, R.; Cook, R.G.; Allis, C.D. Methylation of histone H3 at lysine 4 is highly conserved and correlates with transcriptionally active nuclei in Tetrahymena. Proc. Natl. Acad. Sci. USA 1999, 96, 14967–14972. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhuang, J.; Iyer, S.; Lin, X.; Whitfield, T.W.; Greven, M.C.; Pierce, B.G.; Dong, X.; Kundaje, A.; Cheng, Y.; et al. Sequence features and chromatin structure around the genomic regions bound by 119 human transcription factors. Genome Res. 2012, 22, 1798–1812. [Google Scholar] [CrossRef]
- Struhl, K. Histone acetylation and transcriptional regulatory mechanisms. Genes Dev. 1998, 12, 599–606. [Google Scholar] [CrossRef]
- Creyghton, M.P.; Cheng, A.W.; Welstead, G.G.; Kooistra, T.; Carey, B.W.; Steine, E.J.; Hanna, J.; Lodato, M.A.; Frampton, G.M.; Sharp, P.A.; et al. Histone H3K27ac separates active from poised enhancers and predicts developmental state. Proc. Natl. Acad. Sci. USA 2010, 107, 21931–21936. [Google Scholar] [CrossRef]
- Rougeulle, C.; Chaumeil, J.; Sarma, K.; Allis, C.D.; Reinberg, D.; Avner, P.; Heard, E. Differential Histone H3 Lys-9 and Lys-27 Methylation Profiles on the X Chromosome. Mol. Cell. Biol. 2004, 24, 5475–5484. [Google Scholar] [CrossRef] [PubMed]
- Iwafuchi-Doi, M.; Zaret, K.S. Cell fate control by pioneer transcription factors. Development 2016, 143, 1833–1837. [Google Scholar] [CrossRef]
- Ballare, C.; Castellano, G.; Gaveglia, L.; Althammer, S.; González-Vallinas, J.; Eyras, E.; Le Dily, F.; Zaurin, R.; Soronellas, D.; Vicent, G.P.; et al. Nucleosome-Driven Transcription Factor Binding and Gene Regulation. Mol. Cell 2013, 49, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Swinstead, E.E.; Miranda, T.B.; Paakinaho, V.; Baek, S.; Goldstein, I.; Hawkins, M.; Karpova, T.; Ball, D.; Mazza, D.; Lavis, L.; et al. Steroid Receptors Reprogram FoxA1 Occupancy through Dynamic Chromatin Transitions. Cell 2016, 165, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Swinstead, E.E.; Paakinaho, V.; Presman, D.M.; Hager, G.L. Pioneer factors and ATP-dependent chromatin remodeling factors interact dynamically: A new perspective: Multiple transcription factors can effect chromatin pioneer functions through dynamic interactions with ATP-dependent chromatin remodeling factors. Bioessays 2016, 38, 1150–1157. [Google Scholar] [CrossRef] [PubMed]
- Zaret, K.S.; Carroll, J.S. Pioneer transcription factors: Establishing competence for gene expression. Genes Dev. 2011, 25, 2227–2241. [Google Scholar] [CrossRef] [PubMed]
- Hankey, W.; Chen, Z.; Wang, Q. Shaping Chromatin States in Prostate Cancer by Pioneer Transcription Factors. Cancer Res. 2020, 80, 2427–2436. [Google Scholar] [CrossRef]
- Teng, M.; Zhou, S.; Cai, C.; Lupien, M.; He, H.H. Pioneer of prostate cancer: Past, present and the future of FOXA1. Protein Cell 2021, 12, 29–38. [Google Scholar] [CrossRef]
- Clark, K.L.; Halay, E.D.; Lai, E.; Burley, S.K. Co-crystal structure of the HNF-3/fork head DNA-recognition motif resembles histone H5. Nature 1993, 364, 412–420. [Google Scholar] [CrossRef]
- Cirillo, L.A.; McPherson, C.E.; Bossard, P.; Stevens, K.; Cherian, S.; Shim, E.Y.; Clark, K.L.; Burley, S.; Zaret, K.S. Binding of the winged-helix transcription factor HNF3 to a linker histone site on the nucleosome. EMBO J. 1998, 17, 244–254. [Google Scholar] [CrossRef]
- Iwafuchi, M.; Donahue, G.; Kakumanu, A.; Watts, J.A.; Mahony, S.; Pugh, B.F.; Lee, D.; Kaestner, K.H.; Zaret, K.S. The Pioneer Transcription Factor FoxA Maintains an Accessible Nucleosome Configuration at Enhancers for Tissue-Specific Gene Activation. Mol. Cell 2016, 62, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Cirillo, L.A.; Lin, F.R.; Cuesta, I.; Friedman, D.; Jarnik, M.; Zaret, K.S. Opening of Compacted Chromatin by Early Developmental Transcription Factors HNF3 (FoxA) and GATA-4. Mol. Cell 2002, 9, 279–289. [Google Scholar] [CrossRef]
- Pomerantz, M.M.; Li, F.; Takeda, D.Y.; Lenci, R.; Chonkar, A.; Chabot, M.S.; Cejas, P.; Vazquez, F.; Cook, J.; Shivdasani, R.A.; et al. The androgen receptor cistrome is extensively reprogrammed in human prostate tumorigenesis. Nat. Genet. 2015, 47, 1346–1351. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Zhao, J.C.; Wu, L.; Kim, J.; Yu, J. Cooperativity and equilibrium with FOXA1 define the androgen receptor transcriptional program. Nat. Commun. 2014, 5, 3972. [Google Scholar] [CrossRef]
- Casciello, F.; Al-Ejeh, F.; Kelly, G.; Brennan, D.J.; Ngiow, S.F.; Young, A.; Stoll, T.; Windloch, K.; Hill, M.M.; Smyth, M.J.; et al. G9a drives hypoxia-mediated gene repression for breast cancer cell survival and tumorigenesis. Proc. Natl. Acad. Sci. USA 2017, 114, 7077–7082. [Google Scholar] [CrossRef]
- Wang, Q.; Li, W.; Liu, X.S.; Carroll, J.; Jänne, O.A.; Keeton, E.K.; Chinnaiyan, A.M.; Pienta, K.; Brown, M. A Hierarchical Network of Transcription Factors Governs Androgen Receptor-Dependent Prostate Cancer Growth. Mol. Cell 2007, 27, 380–392. [Google Scholar] [CrossRef]
- Krum, S.A.; Miranda-Carboni, G.A.; Lupien, M.; Eeckhoute, J.; Carroll, J.S.; Brown, M. Unique ERα Cistromes Control Cell Type-Specific Gene Regulation. Mol. Endocrinol. 2008, 22, 2393–2406. [Google Scholar] [CrossRef]
- Sanalkumar, R.; Johnson, K.D.; Gao, X.; Boyer, M.E.; Chang, Y.-I.; Hewitt, K.J.; Zhang, J.; Bresnick, E.H. Mechanism governing a stem cell-generating cis-regulatory element. Proc. Natl. Acad. Sci. USA 2014, 111, E1091–E1100. [Google Scholar] [CrossRef]
- Wu, D.; Sunkel, B.; Chen, Z.; Liu, X.; Ye, Z.; Li, Q.; Grenade, C.; Ke, J.; Zhang, C.; Chen, H.; et al. Three-tiered role of the pioneer factor GATA2 in promoting androgen-dependent gene expression in prostate cancer. Nucleic Acids Res. 2014, 42, 3607–3622. [Google Scholar] [CrossRef]
- Mann, R.S.; Affolter, M. Hox proteins meet more partners. Curr. Opin. Genet. Dev. 1998, 8, 423–429. [Google Scholar] [CrossRef]
- Yin, Y.; Morgunova, E.; Jolma, A.; Kaasinen, E.; Sahu, B.; Khund-Sayeed, S.; Das, P.K.; Kivioja, T.; Dave, K.; Zhong, F.; et al. Impact of cytosine methylation on DNA binding specificities of human transcription factors. Science 2017, 356, eaaj2239. [Google Scholar] [CrossRef]
- Norris, J.D.; Chang, C.-Y.; Wittmann, B.M.; Kunder, R.S.; Cui, H.; Fan, D.; Joseph, J.D.; McDonnell, D.P. The Homeodomain Protein HOXB13 Regulates the Cellular Response to Androgens. Mol. Cell 2009, 36, 405–416. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Lanz, R.B.; Fiskus, W.; Geng, C.; Yi, P.; Hartig, S.M.; Rajapakshe, K.; Shou, J.; Wei, L.; Shah, S.S.; et al. GATA2 facilitates steroid receptor coactivator recruitment to the androgen receptor complex. Proc. Natl. Acad. Sci. USA 2014, 111, 18261–18266. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Chen, S.; Han, D.; Wang, Z.; Li, M.; Han, W.; Besschetnova, A.; Liu, M.; Zhou, F.; Barrett, D.; et al. Chromatin binding of FOXA1 is promoted by LSD1-mediated demethylation in prostate cancer. Nat. Genet. 2020, 52, 1011–1017. [Google Scholar] [CrossRef]
- Claessens, F.; Rushmere, N.; Davies, P.; Celis, L.; Peeters, B.; Rombauts, W. Sequence-specific binding of androgen-receptor complexes to prostatic binding protein genes. Mol. Cell. Endocrinol. 1990, 74, 203–212. [Google Scholar] [CrossRef]
- Rushmere, N.; Parker, M.; Davies, P. Androgen receptor-binding regions of an androgen-responsive gene. Mol. Cell. Endocrinol. 1987, 51, 259–265. [Google Scholar] [CrossRef]
- Rennie, P.S.; Bruchovsky, N.; Leco, K.J.; Sheppard, P.C.; McQueen, S.A.; Cheng, H.; Snoek, R.; Hamel, A.; Bock, M.E.; Macdonald, B.S.; et al. Characterization of two cis-acting DNA elements involved in the androgen regulation of the probasin gene. Mol. Endocrinol. 1993, 7, 23–36. [Google Scholar] [CrossRef]
- Luke, M.C.; Coffey, D.S. Human androgen receptor binding to the androgen response element of prostate specific antigen. J. Androl. 1994, 15, 41–51. [Google Scholar] [PubMed]
- Riegman, P.H.J.; Vlietstra, R.J.; Van Der Korput, J.A.G.M.; Brinkmann, A.O.; Trapman, J. The Promoter of the Prostate-Specific Antigen Gene Contains a Functional Androgen Responsive Element. Mol. Endocrinol. 1991, 5, 1921–1930. [Google Scholar] [CrossRef]
- Yamamoto, K.R.; Alberts, B. On the Specificity of the Binding of the Estradiol Receptor Protein to Deoxyribonucleic Acid. J. Biol. Chem. 1974, 249, 7076–7086. [Google Scholar] [CrossRef]
- Gronemeyer, H.; Pongs, O. Localization of ecdysterone on polytene chromosomes of Drosophila melanogaster. Proc. Natl. Acad. Sci. USA 1980, 77, 2108–2112. [Google Scholar] [CrossRef] [PubMed]
- Benoist, C.; Chambon, P. In vivo sequence requirements of the SV40 early promoter region. Nature 1981, 290, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Cleutjens, K.B.; van der Korput, H.A.; van Eekelen, C.C.; van Rooij, H.C.; Faber, P.W.; Trapman, J. An androgen response element in a far upstream enhancer region is essential for high, androgen-regulated activity of the prostate-specific antigen promoter. Mol. Endocrinol. 1997, 11, 148–161. [Google Scholar] [CrossRef]
- Watt, F.; Martorana, A.; Brookes, D.E.; Ho, T.; Kingsley, E.; O’Keefe, D.S.; Russell, P.J.; Hestond, W.D.W.; Molloy, P.L. A tissue-specific enhancer of the prostate-specific membrane antigen gene, FOLH1. Genomics 2001, 73, 243–254. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Liu, M.; Epner, D.E.; Tsai, S.Y.; Tsai, M.J. Androgen regulation of the cyclin-dependent kinase inhibitor p21 gene through an androgen response element in the proximal promoter. Mol. Endocrinol. 1999, 13, 376–384. [Google Scholar] [CrossRef][Green Version]
- Yu, J.; Yu, J.; Mani, R.; Cao, Q.; Brenner, C.J.; Cao, X.; Wang, X.; Wu, L.; Li, J.; Hu, M.; et al. An Integrated Network of Androgen Receptor, Polycomb, and TMPRSS2-ERG Gene Fusions in Prostate Cancer Progression. Cancer Cell 2010, 17, 443–454. [Google Scholar] [CrossRef]
- Massie, C.L.E.; Adryan, B.; Barbosa-Morais, N.; Lynch, A.; Tran, M.G.B.; Neal, D.; Mills, I.G. New androgen receptor genomic targets show an interaction with the ETS1 transcription factor. EMBO Rep. 2007, 8, 871–878. [Google Scholar] [CrossRef]
- Wilson, S.; Qi, J.; Filipp, F.V. Refinement of the androgen response element based on ChIP-Seq in androgen-insensitive and androgen-responsive prostate cancer cell lines. Sci. Rep. 2016, 6, 32611. [Google Scholar] [CrossRef]
- Pomerantz, M.M.; Qiu, X.; Zhu, Y.; Takeda, D.Y.; Pan, W.; Baca, S.C.; Gusev, A.; Korthauer, K.; Severson, T.M.; Ha, G.; et al. Prostate cancer reactivates developmental epigenomic programs during metastatic progression. Nat. Genet. 2020, 52, 790–799. [Google Scholar] [CrossRef]
- Urbanucci, A.; Sahu, B.; Seppälä, J.; Larjo, A.; Latonen, L.M.; Waltering, K.K.; Tammela, T.L.J.; Vessella, R.L.; Lähdesmäki, H.; Jänne, O.A.; et al. Overexpression of androgen receptor enhances the binding of the receptor to the chromatin in prostate cancer. Oncogene 2012, 31, 2153–2163. [Google Scholar] [CrossRef]
- Urbanucci, A.; Barfeld, S.J.; Kytölä, V.; Itkonen, H.M.; Coleman, I.M.; Vodák, D.; Sjöblom, L.; Sheng, X.; Tolonen, T.; Minner, S.; et al. Androgen Receptor Deregulation Drives Bromodomain-Mediated Chromatin Alterations in Prostate Cancer. Cell Rep. 2017, 19, 2045–2059. [Google Scholar] [CrossRef] [PubMed]
- Stelloo, S.; Nevedomskaya, E.; Poel, H.G.; Jong, J.; Leenders, G.J.; Jenster, G.; Wessels, L.F.A.; Bergman, A.M.; Zwart, W. Androgen receptor profiling predicts prostate cancer outcome. EMBO Mol. Med. 2015, 7, 1450–1464. [Google Scholar] [CrossRef] [PubMed]
- Roche, P.J.; Hoare, S.A.; Parker, M.G. A consensus DNA-binding site for the androgen receptor. Mol. Endocrinol. 1992, 6, 2229–2235. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Martini, G.D.; Rube, H.T.; Kribelbauer, J.F.; Rastogi, C.; Fitzpatrick, V.D.; Houtman, J.; Bussemaker, H.J.; Pufall, M.A. SelexGLM differentiates androgen and glucocorticoid receptor DNA-binding preference over an extended binding site. Genome Res. 2018, 28, 111–121. [Google Scholar] [CrossRef]
- Deblois, G.; Giguère, V. Nuclear Receptor Location Analyses in Mammalian Genomes: From Gene Regulation to Regulatory Networks. Mol. Endocrinol. 2008, 22, 1999–2011. [Google Scholar] [CrossRef]
- Bolton, E.C.; So, A.Y.; Chaivorapol, C.; Haqq, C.M.; Li, H.; Yamamoto, K.R. Cell- and gene-specific regulation of primary target genes by the androgen receptor. Genes Dev. 2007, 21, 2005–2017. [Google Scholar] [CrossRef]
- Denayer, S.; Helsen, C.; Thorrez, L.; Haelens, A.; Claessens, F. The Rules of DNA Recognition by the Androgen Receptor. Mol. Endocrinol. 2010, 24, 898–913. [Google Scholar] [CrossRef]
- Chen, Z.; Lan, X.; Thomas-Ahner, J.; Wu, D.; Liu, X.; Ye, Z.; Wang, L.; Sunkel, B.; Grenade, C.; Chen, J.; et al. Agonist and antagonist switch DNA motifs recognized by human androgen receptor in prostate cancer. EMBO J. 2015, 34, 502–516. [Google Scholar] [CrossRef]
- McDowell, I.C.; Barrera, A.; D’Ippolito, A.M.; Vockley, C.M.; Hong, L.K.; Leichter, S.M.; Bartelt, L.C.; Majoros, W.H.; Song, L.; Safi, A.; et al. Glucocorticoid receptor recruits to enhancers and drives activation by motif-directed binding. Genome Res. 2018, 28, 1272–1284. [Google Scholar] [CrossRef]
- Huang, C.-C.F.; Lingadahalli, S.; Morova, T.; Ozturan, D.; Hu, E.; Yu, I.P.L.; Linder, S.; Hoogstraat, M.; Stelloo, S.; Sar, F.; et al. Functional mapping of androgen receptor enhancer activity. Genome Biol. 2021, 22, 149. [Google Scholar] [CrossRef]
- Cucchiara, V.; Yang, J.C.; Mirone, V.; Gao, A.C.; Rosenfeld, M.G.; Evans, C.P. Epigenomic Regulation of Androgen Receptor Signaling: Potential Role in Prostate Cancer Therapy. Cancers 2017, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Varambally, S.; Dhanasekaran, S.M.; Zhou, M.; Barrette, T.R.; Kumar-Sinha, C.; Sanda, M.G.; Ghosh, D.; Pienta, K.J.; Sewalt, R.G.; Otte, A.P.; et al. The polycomb group protein EZH2 is involved in progression of prostate cancer. Nature 2002, 419, 624–629. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Yu, J.; Rhodes, D.R.; Tomlins, S.; Cao, X.; Chen, G.; Mehra, R.; Wang, X.; Ghosh, D.; Shah, R.B.; et al. A Polycomb Repression Signature in Metastatic Prostate Cancer Predicts Cancer Outcome. Cancer Res. 2007, 67, 10657–10663. [Google Scholar] [CrossRef] [PubMed]
- Davies, A.; Nouruzi, S.; Ganguli, D.; Namekawa, T.; Thaper, D.; Linder, S.; Karaoğlanoğlu, F.; Omur, M.E.; Kim, S.; Kobelev, M.; et al. An androgen receptor switch underlies lineage infidelity in treatment-resistant prostate cancer. Nat. Cell Biol. 2021, 23, 1023–1034. [Google Scholar] [CrossRef] [PubMed]
- Gaughan, L.; Stockley, J.; Wang, N.; McCracken, S.R.; Treumann, A.; Armstrong, K.; Shaheen, F.; Watt, K.; McEwan, I.J.; Wang, C.; et al. Regulation of the androgen receptor by SET9-mediated methylation. Nucleic Acids Res. 2011, 39, 1266–1279. [Google Scholar] [CrossRef] [PubMed]
- Malik, R.; Khan, A.P.; Asangani, I.; Cieślik, M.; Prensner, J.; Wang, X.; Iyer, M.K.; Jiang, X.; Borkin, D.; Escara-Wilke, J.; et al. Targeting the MLL complex in castration-resistant prostate cancer. Nat. Med. 2015, 21, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Anzick, S.L.; Kononen, J.; Walker, R.L.; Azorsa, D.O.; Tanner, M.M.; Guan, X.-Y.; Sauter, G.; Kallioniemi, O.-P.; Trent, J.M.; Meltzer, P.S. AIB1, a Steroid Receptor Coactivator Amplified in Breast and Ovarian Cancer. Science 1997, 277, 965–968. [Google Scholar] [CrossRef]
- Chen, H.; Lin, R.J.; Schiltz, R.; Chakravarti, D.; Nash, A.; Nagy, L.; Privalsky, M.L.; Nakatani, Y.; Evans, R.M. Nuclear Receptor Coactivator ACTR Is a Novel Histone Acetyltransferase and Forms a Multimeric Activation Complex with P/CAF and CBP/p300. Cell 1997, 90, 569–580. [Google Scholar] [CrossRef]
- Hong, H.; Kohli, K.; Trivedi, A.; Johnson, D.L.; Stallcup, M.R. GRIP1, a novel mouse protein that serves as a transcriptional coactivator in yeast for the hormone binding domains of steroid receptors. Proc. Natl. Acad. Sci. USA 1996, 93, 4948–4952. [Google Scholar] [CrossRef]
- Li, H.; Gomes, P.J.; Chen, J.D. RAC3, a steroid/nuclear receptor-associated coactivator that is related to SRC-1 and TIF2. Proc. Natl. Acad. Sci. USA 1997, 94, 8479–8484. [Google Scholar] [CrossRef]
- Oñate, S.A.; Tsai, S.Y.; Tsai, M.-J.; O’Malley, B.W. Sequence and Characterization of a Coactivator for the Steroid Hormone Receptor Superfamily. Science 1995, 270, 1354–1357. [Google Scholar] [CrossRef] [PubMed]
- Chakravarti, D.; Lamorte, V.J.; Nelson, M.C.; Nakajima, T.; Schulman, I.G.; Juguilon, H.; Montminy, M.; Evans, R. Role of CBP/P300 in nuclear receptor signalling. Nature 1996, 383, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Hanstein, B.; Eckner, R.; DiRenzo, J.; Halachmi, S.; Liu, H.; Searcy, B.; Kurokawa, R.; Brown, M. p300 is a component of an estrogen receptor coactivator complex. Proc. Natl. Acad. Sci. USA 1996, 93, 11540–11545. [Google Scholar] [CrossRef]
- Blanco, J.C.; Minucci, S.; Lu, J.; Yang, X.-J.; Walker, K.K.; Chen, H.; Evans, R.M.; Nakatani, Y.; Ozato, K. The histone acetylase PCAF is a nuclear receptor coactivator. Genes Dev. 1998, 12, 1638–1651. [Google Scholar] [CrossRef]
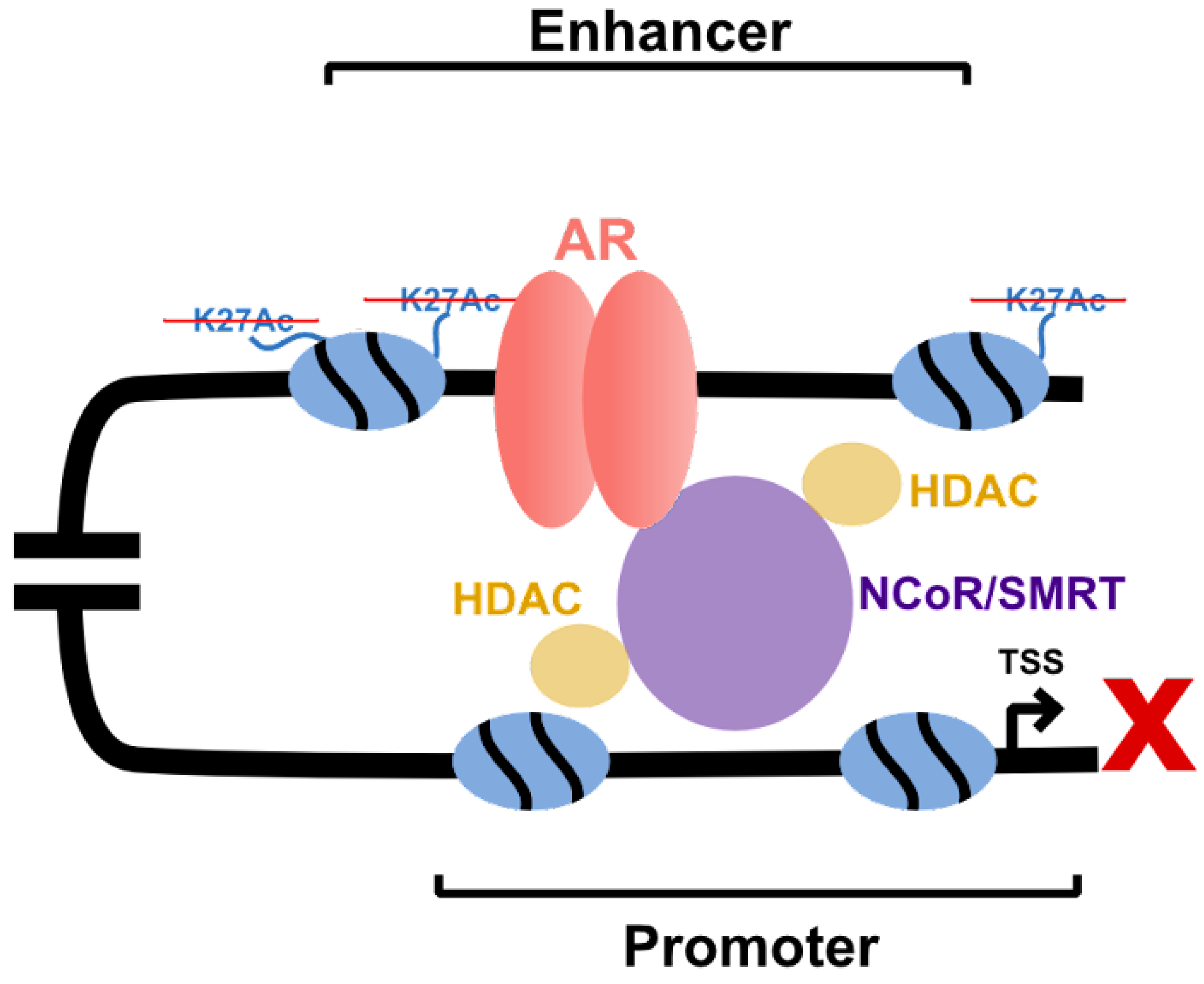
- Abbas, A.; Gupta, S. The role of histone deacetylases in prostate cancer. Epigenetics 2008, 3, 300–309. [Google Scholar] [CrossRef] [PubMed]
- Rana, Z.; Diermeier, S.; Hanif, M.; Rosengren, R.J. Understanding Failure and Improving Treatment Using HDAC Inhibitors for Prostate Cancer. Biomedecines 2020, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.-Q.; Li, J.; Sachs, L.M.; Cole, P.A.; Wong, J. A role for cofactor-cofactor and cofactor-histone interactions in targeting p300, SWI/SNF and Mediator for transcription. EMBO J. 2003, 22, 2146–2155. [Google Scholar] [CrossRef]
- Marshall, T.W.; Link, K.A.; Petre-Draviam, C.E.; Knudsen, K.E. Differential Requirement of SWI/SNF for Androgen Receptor Activity. J. Biol. Chem. 2003, 278, 30605–30613. [Google Scholar] [CrossRef]
- Jozwik, K.M.; Chernukhin, I.; Serandour, A.A.; Nagarajan, S.; Carroll, J.S. FOXA1 Directs H3K4 Monomethylation at Enhancers via Recruitment of the Methyltransferase MLL3. Cell Rep. 2016, 17, 2715–2723. [Google Scholar] [CrossRef]
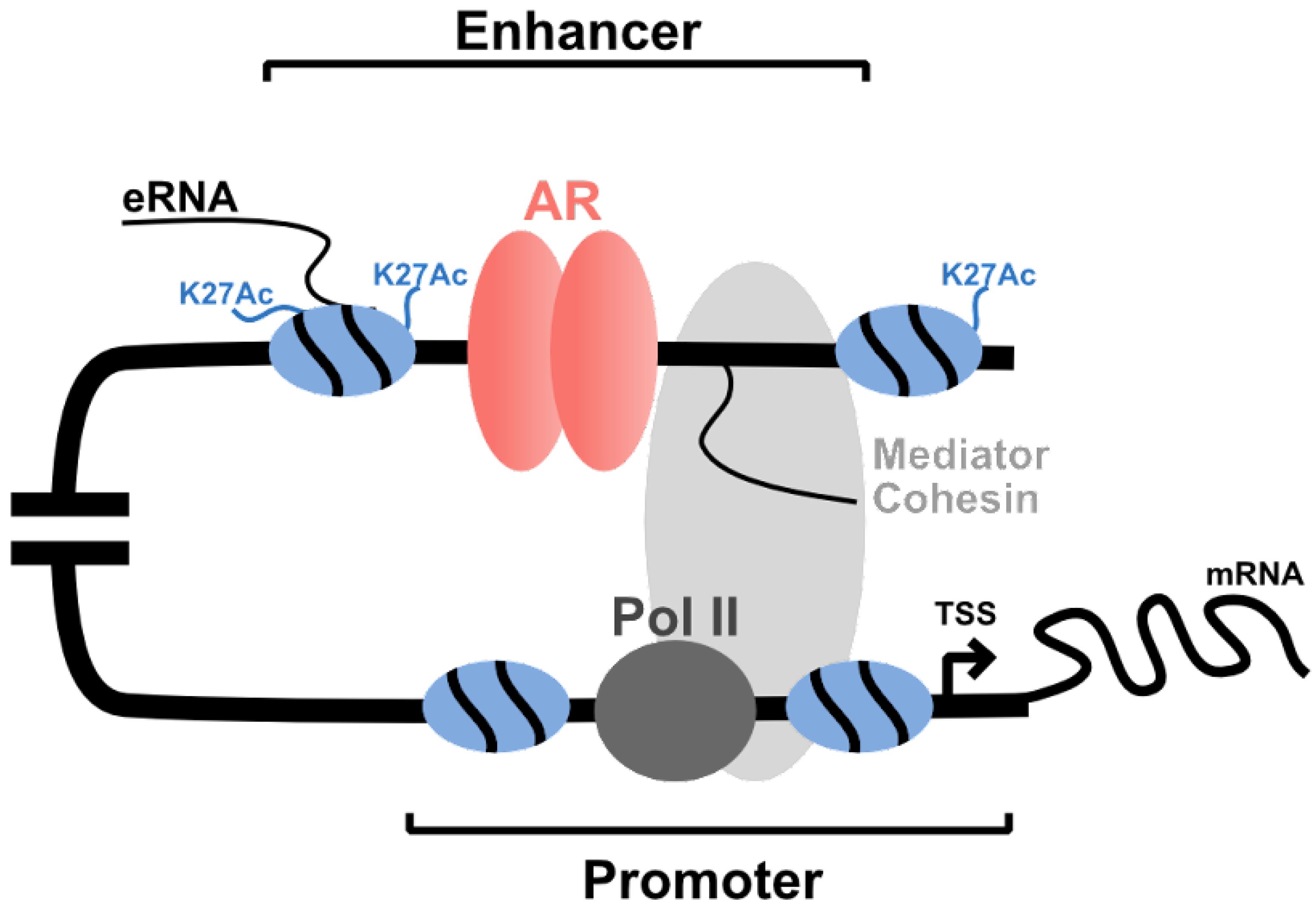
- Wang, Q.; Carroll, J.; Brown, M. Spatial and Temporal Recruitment of Androgen Receptor and Its Coactivators Involves Chromosomal Looping and Polymerase Tracking. Mol. Cell 2005, 19, 631–642. [Google Scholar] [CrossRef]
- Green, K.A.; Carroll, J. Oestrogen-receptor-mediated transcription and the influence of co-factors and chromatin state. Nat. Cancer 2007, 7, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Cato, L.; Neeb, A.; Sharp, A.; Buzón, V.; Ficarro, S.B.; Yang, L.; Muhle-Goll, C.; Kuznik, N.C.; Riisnaes, R.; Rodrigues, D.N.; et al. Development of Bag-1L as a therapeutic target in androgen receptor-dependent prostate cancer. eLife 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Coffey, K.; Rogerson, L.; Ryan-Munden, C.; Alkharaif, D.; Stockley, J.; Heer, R.; Sahadevan, K.; O’Neill, D.; Jones, D.; Darby, S.; et al. The lysine demethylase, KDM4B, is a key molecule in androgen receptor signalling and turnover. Nucleic Acids Res. 2013, 41, 4433–4446. [Google Scholar] [CrossRef] [PubMed]
- DePriest, A.D.; Fiandalo, M.; Schlanger, S.; Heemers, F.; Mohler, J.L.; Liu, S.; Heemers, H.V. Regulators of Androgen Action Resource: A one-stop shop for the comprehensive study of androgen receptor action. Database 2016, 2016. [Google Scholar] [CrossRef]
- Zhang, F.; Wong, S.; Lee, J.; Lingadahalli, S.; Wells, C.; Saxena, N.; Sanchez, C.; Sun, B.; Parra-Nuñez, A.K.; Chan, N.; et al. Dynamic phase separation of the androgen receptor and its coactivators to regulate gene expression. bioRxiv 2021. [Google Scholar] [CrossRef]
- Hnisz, D.; Abraham, B.; Lee, T.I.; Lau, A.; Saint-André, V.; Sigova, A.A.; Hoke, H.A.; Young, R.A. Super-Enhancers in the Control of Cell Identity and Disease. Cell 2013, 155, 934–947. [Google Scholar] [CrossRef]
- Wen, S.; He, Y.; Wang, L.; Zhang, J.; Quan, C.; Niu, Y.; Huang, H. Aberrant activation of super enhancer and choline metabolism drive antiandrogen therapy resistance in prostate cancer. Oncogene 2020, 39, 6556–6571. [Google Scholar] [CrossRef]
- Hsieh, C.-L.; Fei, T.; Chen, Y.; Li, T.; Gao, Y.; Wang, X.; Sun, T.; Sweeney, C.J.; Lee, G.-S.M.; Chen, S.; et al. Enhancer RNAs participate in androgen receptor-driven looping that selectively enhances gene activation. Proc. Natl. Acad. Sci. USA 2014, 111, 7319–7324. [Google Scholar] [CrossRef]
- Lee, N.; Steitz, J.A. Noncoding RNA-guided recruitment of transcription factors: A prevalent but undocumented mechanism? BioEssays 2015, 37, 936–941. [Google Scholar] [CrossRef]
- Ferreira, L.B.; Palumbo, A.; De Mello, K.D.; Sternberg, C.; Caetano, M.S.; De Oliveira, F.L.; Neves, A.F.; Nasciutti, L.E.; Goulart, L.R.; Gimba, E.R.P. PCA3 noncoding RNA is involved in the control of prostate-cancer cell survival and modulates androgen receptor signaling. BMC Cancer 2012, 12, 507. [Google Scholar] [CrossRef]
- Zhang, Y.; Pitchiaya, S.; Cieślik, M.; Niknafs, Y.S.; Tien, J.C.-Y.; Hosono, Y.; Iyer, M.K.; Yazdani, S.; Subramaniam, S.; Shukla, S.; et al. Analysis of the androgen receptor–regulated lncRNA landscape identifies a role for ARLNC1 in prostate cancer progression. Nat. Genet. 2018, 50, 814–824. [Google Scholar] [CrossRef]
- Prensner, J.R.; Iyer, M.K.; Sahu, A.; Asangani, I.A.; Cao, Q.; Patel, L.; Vergara, I.A.; Davicioni, E.; Erho, N.; Ghadessi, M.; et al. The long noncoding RNA SChLAP1 promotes aggressive prostate cancer and antagonizes the SWI/SNF complex. Nat. Genet. 2013, 45, 1392–1398. [Google Scholar] [CrossRef] [PubMed]
- Simon, T.W.; Budinsky, R.A.; Rowlands, J.C. A Model for Aryl Hydrocarbon Receptor-Activated Gene Expression Shows Potency and Efficacy Changes and Predicts Squelching Due to Competition for Transcription Co-Activators. PLoS ONE 2015, 10, e0127952. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Tse, A.K.-W.; Li, P.; Ma, Q.; Xiang, S.; Nicosia, S.V.; Seto, E.; Zhang, X.; Bai, W. Inhibition of androgen receptor activity by histone deacetylase 4 through receptor SUMOylation. Oncogene 2011, 30, 2207–2218. [Google Scholar] [CrossRef] [PubMed]
- Carascossa, S.; Gobinet, J.; Georget, V.; Lucas, A.; Badia, E.; Castet, A.; White, R.; Nicolas, J.-C.; Cavailles, V.; Jalaguier, S.S. Receptor-Interacting Protein 140 Is a Repressor of the Androgen Receptor Activity. Mol. Endocrinol. 2006, 20, 1506–1518. [Google Scholar] [CrossRef]
- Fernandes, I.; Bastien, Y.; Wai, T.; Nygard, K.; Lin, R.; Cormier, O.; Lee, H.S.; Eng, F.; Bertos, N.R.; Pelletier, N.; et al. Ligand-Dependent Nuclear Receptor Corepressor LCoR Functions by Histone Deacetylase-Dependent and -Independent Mechanisms. Mol. Cell 2003, 11, 139–150. [Google Scholar] [CrossRef]
- Asim, M.; Bin Hafeez, B.; Siddiqui, I.A.; Gerlach, C.; Patz, M.; Mukhtar, H.; Baniahmad, A. Ligand-dependent Corepressor Acts as a Novel Androgen Receptor Corepressor, Inhibits Prostate Cancer Growth, and Is Functionally Inactivated by the Src Protein Kinase. J. Biol. Chem. 2011, 286, 37108–37117. [Google Scholar] [CrossRef]
- Nguyen, M.M.; Dincer, Z.; Wade, J.R.; Alur, M.; Michalak, M.; DeFranco, D.B.; Wang, Z. Cytoplasmic localization of the androgen receptor is independent of calreticulin. Mol. Cell. Endocrinol. 2009, 302, 65–72. [Google Scholar] [CrossRef]
- Dedhar, S.; Rennie, P.S.; Shago, M.; Hagesteijn, C.-Y.L.; Yang, H.; Filmus, J.; Hawley, R.G.; Bruchovsky, N.; Cheng, H.; Matusik, R.J.; et al. Inhibition of nuclear hormone receptor activity by calreticulin. Nature 1994, 367, 480–483. [Google Scholar] [CrossRef]
- Simons, S.S. Structure and Function of the Steroid and Nuclear Receptor Ligand Binding Domain. In Molecular Biology of Steroid and Nuclear Hormone Receptors; Freedman, L.P., Ed.; Birkhäuser Boston: Boston, MA, USA, 1998; pp. 35–104. [Google Scholar]
- Chen, C.; Bhalala, H.V.; Vessella, R.L.; Dong, J.-T. KLF5 is frequently deleted and down-regulated but rarely mutated in prostate cancer. Prostate 2003, 55, 81–88. [Google Scholar] [CrossRef]
- Ni, M.; Chen, Y.; Fei, T.; Li, D.; Lim, E.; Liu, X.S.; Brown, M. Amplitude modulation of androgen signaling by c-MYC. Genes Dev. 2013, 27, 734–748. [Google Scholar] [CrossRef]
- Barfeld, S.J.; Urbanucci, A.; Itkonen, H.; Fazli, L.; Hicks, J.L.; Thiede, B.; Rennie, P.S.; Yegnasubramanian, S.; DeMarzo, A.M.; Mills, I.G. c-Myc Antagonises the Transcriptional Activity of the Androgen Receptor in Prostate Cancer Affecting Key Gene Networks. EBioMedicine 2017, 18, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Wu, Y.; Nouri, M.; Spisak, S.; Russo, J.W.; Sowalsky, A.G.; Pomerantz, M.M.; Wei, Z.; Korthauer, K.; Seo, J.-H.; et al. Androgen receptor and MYC equilibration centralizes on developmental super-enhancer. Nat. Commun. 2021, 12, 7308. [Google Scholar] [CrossRef] [PubMed]
- Vatapalli, R.; Sagar, V.; Rodriguez, Y.; Zhao, J.C.; Unno, K.; Pamarthy, S.; Lysy, B.; Anker, J.; Han, H.; Yoo, Y.A.; et al. Histone methyltransferase DOT1L coordinates AR and MYC stability in prostate cancer. Nat. Commun. 2020, 11, 4153. [Google Scholar] [CrossRef] [PubMed]
- Alimirah, F.; Panchanathan, R.; Chen, J.; Zhang, X.; Ho, S.-M.; Choubey, D. Expression of Androgen Receptor Is Negatively Regulated By p53. Neoplasia 2007, 9, 1152–1159. [Google Scholar] [CrossRef] [PubMed]
- Rokhlin, O.W.; Taghiyev, A.F.; Guseva, N.V.; Glover, R.A.; Chumakov, P.; Kravchenko, J.E.; Cohen, M.B. Androgen regulates apoptosis induced by TNFR family ligands via multiple signaling pathways in LNCaP. Oncogene 2005, 24, 6773–6784. [Google Scholar] [CrossRef]
- Wise, H.M.; Hermida, M.A.; Leslie, N.R. Prostate cancer, PI3K, PTEN and prognosis. Clin. Sci. 2017, 131, 197–210. [Google Scholar] [CrossRef]
- Wang, Y.; Romigh, T.; He, X.; Tan, M.-H.; Orloff, M.S.; Silverman, R.H.; Heston, W.D.; Eng, C. Differential regulation of PTEN expression by androgen receptor in prostate and breast cancers. Oncogene 2011, 30, 4327–4338. [Google Scholar] [CrossRef]
- Li, Q.; Liu, B.; Chao, H.-P.; Ji, Y.; Lu, Y.; Mehmood, R.; Jeter, C.; Chen, T.; Moore, J.R.; Li, W.; et al. LRIG1 is a pleiotropic androgen receptor-regulated feedback tumor suppressor in prostate cancer. Nat. Commun. 2019, 10, 5495. [Google Scholar] [CrossRef]
- Thomasson, M.; Wang, B.; Hammarsten, P.; Dahlman, A.; Persson, J.L.; Josefsson, A.; Stattin, P.; Granfors, T.; Egevad, L.; Henriksson, R.; et al. LRIG1 and the liar paradox in prostate cancer: A study of the expression and clinical significance of LRIG1 in prostate cancer. Int. J. Cancer 2011, 128, 2843–2852. [Google Scholar] [CrossRef]
- Gareau, J.R.; Lima, C.D. The SUMO pathway: Emerging mechanisms that shape specificity, conjugation and recognition. Nat. Rev. Mol. Cell Biol. 2010, 11, 861–871. [Google Scholar] [CrossRef] [PubMed]
- Jones, P.L.; Shi, Y.B. N-CoR-HDAC corepressor complexes: Roles in transcriptional regulation by nuclear hormone receptors. Curr. Top Microbiol. Immunol. 2003, 274, 237–268. [Google Scholar] [PubMed]
- Shashikant, T.; Ettensohn, C.A. Genome-wide analysis of chromatin accessibility using ATAC-seq. Methods Cell Biol. 2019, 151, 219–235. [Google Scholar] [CrossRef]
- Wang, Q.; Li, W.; Zhang, Y.; Yuan, X.; Xu, K.; Yu, J.; Chen, Z.; Beroukhim, R.; Wang, H.; Lupien, M.; et al. Androgen Receptor Regulates a Distinct Transcription Program in Androgen-Independent Prostate Cancer. Cell 2009, 138, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Barozzi, I.; Simonatto, M.; Bonifacio, S.; Yang, L.; Rohs, R.; Ghisletti, S.; Natoli, G. Coregulation of Transcription Factor Binding and Nucleosome Occupancy through DNA Features of Mammalian Enhancers. Mol. Cell 2014, 54, 844–857. [Google Scholar] [CrossRef]
- Hallikas, O.; Palin, K.; Sinjushina, N.; Rautiainen, R.; Partanen, J.; Ukkonen, E.; Taipale, J. Genome-wide Prediction of Mammalian Enhancers Based on Analysis of Transcription-Factor Binding Affinity. Cell 2006, 124, 47–59. [Google Scholar] [CrossRef]
- Hah, N.; Murakami, S.; Nagari, A.; Danko, C.G.; Kraus, W.L. Enhancer transcripts mark active estrogen receptor binding sites. Genome Res. 2013, 23, 1210–1223. [Google Scholar] [CrossRef]
- Koch, F.; Jourquin, F.; Ferrier, P.; Andrau, J.-C. Genome-wide RNA polymerase II: Not genes only! Trends Biochem. Sci. 2008, 33, 265–273. [Google Scholar] [CrossRef]
- Cheng, J.-H.; Pan, D.Z.-C.; Tsai, Z.T.-Y.; Tsai, H.-K. Genome-wide analysis of enhancer RNA in gene regulation across 12 mouse tissues. Sci. Rep. 2015, 5, 12648. [Google Scholar] [CrossRef]
- Arner, E.; Daub, C.O.; Vitting-Seerup, K.; Andersson, R.; Lilje, B.; Drabløs, F.; Lennartsson, A.; Rönnerblad, M.; Hrydziuszko, O.; Vitezic, M.; et al. Transcribed enhancers lead waves of coordinated transcription in transitioning mammalian cells. Science 2015, 347, 1010–1014. [Google Scholar] [CrossRef]
- Kouno, T.; Moody, J.; Kwon, A.T.-J.; Shibayama, Y.; Kato, S.; Huang, Y.; Böttcher, M.; Motakis, E.; Mendez, M.; Severin, J.; et al. C1 CAGE detects transcription start sites and enhancer activity at single-cell resolution. Nat. Commun. 2019, 10, 360. [Google Scholar] [CrossRef]
- Xie, S.; Duan, J.; Li, B.; Zhou, P.; Hon, G.C. Multiplexed Engineering and Analysis of Combinatorial Enhancer Activity in Single Cells. Mol. Cell 2017, 66, 285–299.e5. [Google Scholar] [CrossRef] [PubMed]
- Zacher, B.; Michel, M.; Schwalb, B.; Cramer, P.; Tresch, A.; Gagneur, J. Accurate Promoter and Enhancer Identification in 127 ENCODE and Roadmap Epigenomics Cell Types and Tissues by GenoSTAN. PLoS ONE 2017, 12, e0169249. [Google Scholar] [CrossRef]
- Zhang, T.; Zhang, Z.; Dong, Q.; Xiong, J.; Zhu, B. Histone H3K27 acetylation is dispensable for enhancer activity in mouse embryonic stem cells. Genome Biol. 2020, 21, 45. [Google Scholar] [CrossRef] [PubMed]
- Inoue, F.; Kircher, M.; Martin, B.; Cooper, G.M.; Witten, D.M.; McManus, M.T.; Ahituv, N.; Shendure, J. A systematic comparison reveals substantial differences in chromosomal versus episomal encoding of enhancer activity. Genome Res. 2017, 27, 38–52. [Google Scholar] [CrossRef] [PubMed]
- Arnold, C.D.; Gerlach, D.; Stelzer, C.; Boryń, L.M.; Rath, M.; Stark, A. Genome-Wide Quantitative Enhancer Activity Maps Identified by STARR-seq. Science 2013, 339, 1074–1077. [Google Scholar] [CrossRef] [PubMed]
- Neumayr, C.; Pagani, M.; Stark, A.; Arnold, C.D. STARR-seq and UMI-STARR-seq: Assessing Enhancer Activities for Genome-Wide-, High-, and Low-Complexity Candidate Libraries. Curr. Protoc. Mol. Biol. 2019, 128, e105. [Google Scholar] [CrossRef]
- Liu, Y.; Yuwen, L.; Dhiman, V.K.; Brunetti, T.; Eckart, H.; White, K.P. Functional assessment of human enhancer activities using whole-genome STARR-sequencing. Genome Biol. 2017, 18, 219. [Google Scholar] [CrossRef]
- Muerdter, F.; Boryń, Ł.M.; Arnold, C.D. STARR-seq—Principles and applications. Genomics 2015, 106, 145–150. [Google Scholar] [CrossRef]
- Chen, Z.; Song, X.; Li, Q.; Xie, L.; Guo, T.; Su, T.; Tang, C.; Chang, X.; Liang, B.; Huang, D.; et al. Androgen Receptor-Activated Enhancers Simultaneously Regulate Oncogene TMPRSS2 and lncRNA PRCAT38 in Prostate Cancer. Cells 2019, 8, 864. [Google Scholar] [CrossRef]
- Carleton, J.B.; Berrett, K.C.; Gertz, J. Multiplex Enhancer Interference Reveals Collaborative Control of Gene Regulation by Estrogen Receptor α-Bound Enhancers. Cell Syst. 2017, 5, 333–344.e5. [Google Scholar] [CrossRef]
- Proudhon, C.; Snetkova, V.; Raviram, R.; Lobry, C.; Badri, S.; Jiang, T.; Hao, B.; Trimarchi, T.; Kluger, Y.; Aifantis, I.; et al. Active and Inactive Enhancers Cooperate to Exert Localized and Long-Range Control of Gene Regulation. Cell Rep. 2016, 15, 2159–2169. [Google Scholar] [CrossRef] [PubMed]
- Carleton, J.B.; Ginley-Hidinger, M.; Berrett, K.C.; Layer, R.M.; Quinlan, A.R.; Gertz, J. Regulatory sharing between estrogen receptor α bound enhancers. Nucleic Acids Res. 2020, 48, 6597–6610. [Google Scholar] [CrossRef] [PubMed]
- Souaid, C.; Bloyer, S.; Noordermeer, D. 19—Promoter–Enhancer Looping and Regulatory Neighborhoods: Gene Regulation in the Framework of Topologically Associating Domains. In Nuclear Architecture and Dynamics; Lavelle, C., Victor, J.-M., Eds.; Academic Press: Boston, MA, USA, 2018; pp. 435–456. [Google Scholar]
- Rowley, M.J.; Corces, V.G. Organizational principles of 3D genome architecture. Nat. Rev. Genet. 2018, 19, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Dixon, J.R.; Selvaraj, S.; Yue, F.; Kim, A.; Li, Y.; Shen, Y.; Hu, M.; Liu, J.S.; Ren, B. Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature 2012, 485, 376–380. [Google Scholar] [CrossRef]
- Sexton, T.; Yaffe, E.; Kenigsberg, E.; Bantignies, F.; Leblanc, B.; Hoichman, M.; Parrinello, H.; Tanay, A.; Cavalli, G. Three-Dimensional Folding and Functional Organization Principles of the Drosophila Genome. Cell 2012, 148, 458–472. [Google Scholar] [CrossRef]
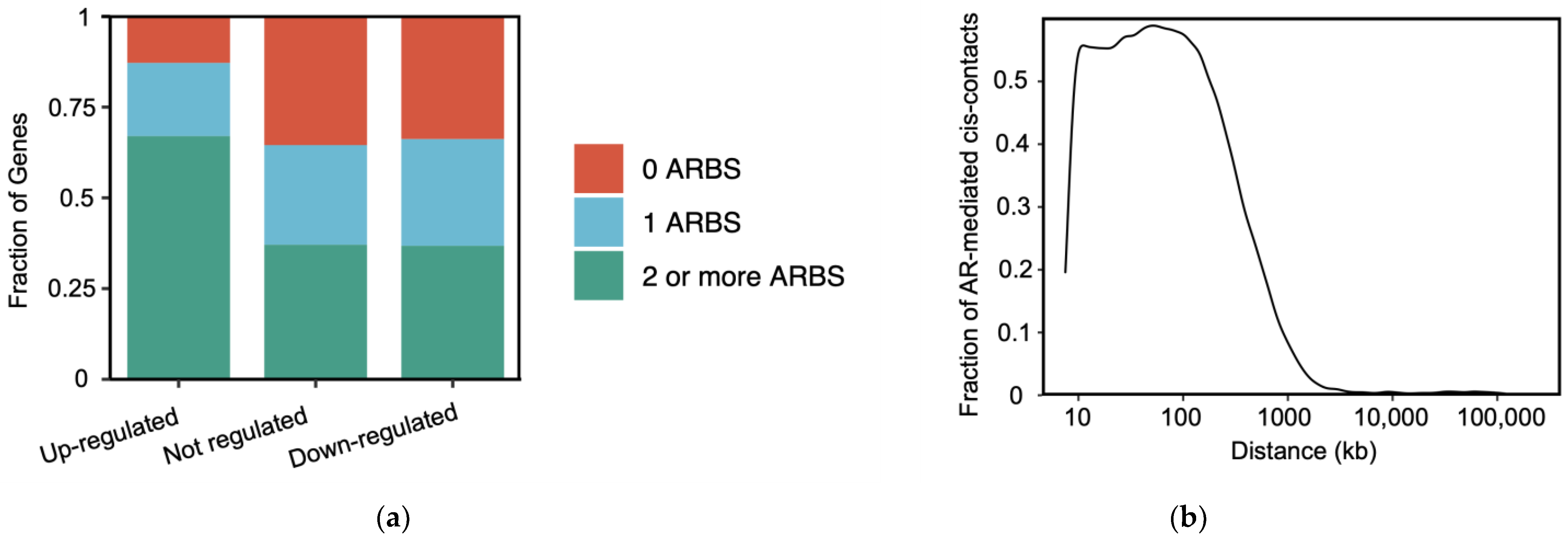
- Zhang, Z.; Chng, K.R.; Lingadahalli, S.; Chen, Z.; Liu, M.H.; Do, H.H.; Cai, S.; Rinaldi, N.; Poh, H.M.; Li, G.; et al. An AR-ERG transcriptional signature defined by long-range chromatin interactomes in prostate cancer cells. Genome Res. 2019, 29, 223–235. [Google Scholar] [CrossRef]
- Rhie, S.K.; Perez, A.; Lay, F.D.; Schreiner, S.; Shi, J.; Polin, J.; Farnham, P.J. A high-resolution 3D epigenomic map reveals insights into the creation of the prostate cancer transcriptome. Nat. Commun. 2019, 10, 4154. [Google Scholar] [CrossRef]
- Palstra, R.-J.; Tolhuis, B.; Splinter, E.; Nijmeijer, R.; Grosveld, F.; de Laat, W. The β-globin nuclear compartment in development and erythroid differentiation. Nat. Genet. 2003, 35, 190–194. [Google Scholar] [CrossRef]
- Lettice, L.A.; Heaney, S.J.; Purdie, L.A.; Li, L.; De Beer, P.; Oostra, B.A.; Goode, D.; Elgar, G.; Hill, R.E.; De Graaff, E. A long-range Shh enhancer regulates expression in the developing limb and fin and is associated with preaxial polydactyly. Hum. Mol. Genet. 2003, 12, 1725–1735. [Google Scholar] [CrossRef]
- Andrey, G.; Montavon, T.; Mascrez, B.; Gonzalez, F.; Noordermeer, D.; Leleu, M.; Trono, D.; Spitz, F.; Duboule, D. A Switch Between Topological Domains Underlies HoxD Genes Collinearity in Mouse Limbs. Science 2013, 340, 1234167. [Google Scholar] [CrossRef] [PubMed]
- Vernimmen, D.; De Gobbi, M.; Sloane-Stanley, J.A.; Wood, W.G.; Higgs, D.R. Long-range chromosomal interactions regulate the timing of the transition between poised and active gene expression. EMBO J. 2007, 26, 2041–2051. [Google Scholar] [CrossRef] [PubMed]
- Verheul, T.C.J.; Van Hijfte, L.; Perenthaler, E.; Barakat, T.S. The Why of YY1: Mechanisms of Transcriptional Regulation by Yin Yang 1. Front. Cell Dev. Biol. 2020, 8, 592164. [Google Scholar] [CrossRef] [PubMed]
- Van de Werken, H.J.G.; Landan, G.; Holwerda, S.J.B.; Hoichman, M.; Klous, P.; Chachik, R.; Splinter, E.; Valdes-Quezada, C.; Öz, Y.; Bouwman, B.A.M.; et al. Robust 4C-seq data analysis to screen for regulatory DNA interactions. Nat. Methods 2012, 9, 969–972. [Google Scholar] [CrossRef]
- Sanyal, A.; Lajoie, B.R.; Jain, G.; Dekker, J. The long-range interaction landscape of gene promoters. Nature 2012, 489, 109–113. [Google Scholar] [CrossRef]
- Misteli, T. Higher-order Genome Organization in Human Disease. Cold Spring Harb. Perspect. Biol. 2010, 2, a000794. [Google Scholar] [CrossRef]
- Martin, R.S.; Das, P.; Marques, R.D.R.; Xu, Y.; McCord, R.P. Alterations in chromosome spatial compartmentalization classify prostate cancer progression. bioRxiv 2021. [Google Scholar] [CrossRef]
- Taberlay, P.; Achinger-Kawecka, J.; Lun, A.; Buske, F.A.; Sabir, K.; Gould, C.M.; Zotenko, E.; Bert, S.A.; Giles, K.; Bauer, D.; et al. Three-dimensional disorganization of the cancer genome occurs coincident with long-range genetic and epigenetic alterations. Genome Res. 2016, 26, 719–731. [Google Scholar] [CrossRef]
- Hawley, J.R.; Zhou, S.; Arlidge, C.; Grillo, G.; Kron, K.J.; Hugh-White, R.; van der Kwast, T.H.; Fraser, M.; Boutros, P.C.; Bristow, R.G.; et al. Reorganization of the 3D Genome Pinpoints Noncoding Drivers of Primary Prostate Tumors. Cancer Res. 2021, 81, 5833–5848. [Google Scholar] [CrossRef]
- Metzger, E.; Willmann, D.; McMillan, J.; Forne, I.; Metzger, P.; Gerhardt, S.; Petroll, K.; Von Maessenhausen, A.; Urban, S.; Schott, A.-K.; et al. Assembly of methylated KDM1A and CHD1 drives androgen receptor–dependent transcription and translocation. Nat. Struct. Mol. Biol. 2016, 23, 132–139. [Google Scholar] [CrossRef]
- Malinen, M.; Niskanen, E.; Kaikkonen, M.; Palvimo, J.J. Crosstalk between androgen and pro-inflammatory signaling remodels androgen receptor and NF-κB cistrome to reprogram the prostate cancer cell transcriptome. Nucleic Acids Res. 2017, 45, 619–630. [Google Scholar] [CrossRef]
- Martincorena, I.; Campbell, P.J. Somatic mutation in cancer and normal cells. Science 2015, 349, 1483–1489. [Google Scholar] [CrossRef] [PubMed]
- Pan-cancer analysis of whole genomes. Nature 2020, 578, 82–93. [CrossRef]
- Frank, S.; Nelson, P.; Vasioukhin, V. Recent advances in prostate cancer research: Large-scale genomic analyses reveal novel driver mutations and DNA repair defects. F1000Research 2018, 7, 1173. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, C.; Bangma, C.H.; Bjartell, A.; Catto, J.; Culig, Z.; Grönberg, H.; Luo, J.; Visakorpi, T.; Rubin, M. The Mutational Landscape of Prostate Cancer. Eur. Urol. 2013, 64, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Lang, S.H.; Swift, S.L.; White, H.; Misso, K.; Kleijnen, J.; Quek, R.G. A systematic review of the prevalence of DNA damage response gene mutations in prostate cancer. Int. J. Oncol. 2019, 55, 597–616. [Google Scholar] [CrossRef] [PubMed]
- Elliott, K.; Larsson, E. Non-coding driver mutations in human cancer. Nat. Cancer 2021, 21, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Walavalkar, K.; Notani, D. Beyond the coding genome: Non-coding mutations and cancer. Front. Biosci. 2020, 25, 1828–1838. [Google Scholar]
- ENCODE Project Consortium. An integrated encyclopedia of DNA elements in the human genome. Nature 2012, 489, 57–74. [Google Scholar] [CrossRef]
- Li, Y.; PCAWG Structural Variation Working Group; Roberts, N.; Wala, J.A.; Shapira, O.; Schumacher, S.E.; Kumar, K.; Khurana, E.; Waszak, S.; Korbel, J.O.; et al. Patterns of somatic structural variation in human cancer genomes. Nature 2020, 578, 112–121. [Google Scholar] [CrossRef]
- Takeda, D.Y.; Spisák, S.; Seo, J.-H.; Bell, C.; O’Connor, E.; Korthauer, K.; Ribli, D.; Csabai, I.; Solymosi, N.; Szallasi, Z.; et al. A Somatically Acquired Enhancer of the Androgen Receptor Is a Noncoding Driver in Advanced Prostate Cancer. Cell 2018, 174, 422–432.e13. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, S.; Ha, G.; Hoff, A.M.; Wala, J.A.; Carrot-Zhang, J.; Whelan, C.; Haradhvala, N.J.; Freeman, S.; Reed, S.; Rhoades, J.; et al. Structural Alterations Driving Castration-Resistant Prostate Cancer Revealed by Linked-Read Genome Sequencing. Cell 2018, 174, 433–447.e19. [Google Scholar] [CrossRef] [PubMed]
- Weischenfeldt, J.; Dubash, T.; Drainas, A.P.; Mardin, B.R.; Chen, Y.; Stütz, A.M.; Waszak, S.M.; Bosco, G.; Halvorsen, A.R.; Raeder, B.; et al. Pan-cancer analysis of somatic copy-number alterations implicates IRS4 and IGF2 in enhancer hijacking. Nat. Genet. 2017, 49, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Spielmann, M.; Lupiáñez, D.G.; Mundlos, S. Structural variation in the 3D genome. Nat. Rev. Genet. 2018, 19, 453–467. [Google Scholar] [CrossRef]
- Wang, X.; Xu, J.; Zhang, B.; Hou, Y.; Song, F.; Lyu, H.; Yue, F. Genome-wide detection of enhancer-hijacking events from chromatin interaction data in rearranged genomes. Nat. Methods 2021, 18, 661–668. [Google Scholar] [CrossRef]
- Tomlins, S.A.; Rhodes, D.R.; Perner, S.; Dhanasekaran, S.M.; Mehra, R.; Sun, X.-W.; Varambally, S.; Cao, X.; Tchinda, J.; Kuefer, R.; et al. Recurrent Fusion of TMPRSS2 and ETS Transcription Factor Genes in Prostate Cancer. Science 2005, 310, 644–648. [Google Scholar] [CrossRef]
- St John, J.; Powell, K.; Conley-Lacomb, M.K.; Chinni, S.R. TMPRSS2-ERG Fusion Gene Expression in Prostate Tumor Cells and Its Clinical and Biological Significance in Prostate Cancer Progression. J. Cancer Sci. Ther. 2012, 4, 94–101. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Zhang, J.; Zhu, W.; Zhi, F.; Zhang, S.; Mao, D.; Zhang, Y.; Yuliang, W. Significance of the TMPRSS2:ERG gene fusion in prostate cancer. Mol. Med. Rep. 2017, 16, 5450–5458. [Google Scholar] [CrossRef]
- Baca, S.C.; Prandi, D.; Lawrence, M.S.; Mosquera, J.M.; Romanel, A.; Drier, Y.; Park, K.; Kitabayashi, N.; Macdonald, T.Y.; Ghandi, M.; et al. Punctuated Evolution of Prostate Cancer Genomes. Cell 2013, 153, 666–677. [Google Scholar] [CrossRef]
- Schuijers, J.; Manteiga, J.C.; Weintraub, A.S.; Day, D.S.; Zamudio, A.V.; Hnisz, D.; Lee, T.I.; Young, R.A. Transcriptional Dysregulation of MYC Reveals Common Enhancer-Docking Mechanism. Cell Rep. 2018, 23, 349–360. [Google Scholar] [CrossRef]
- Helmsauer, K.; Valieva, M.E.; Ali, S.; Chamorro González, R.; Schöpflin, R.; Röefzaad, C.; Bei, Y.; Dorado Garcia, H.; Rodriguez-Fos, E.; Puiggròs, M.; et al. Enhancer hijacking determines extrachromosomal circular MYCN amplicon architecture in neuroblastoma. Nat. Commun. 2020, 11, 5823. [Google Scholar] [CrossRef] [PubMed]
- Khoury, A.; Achinger-Kawecka, J.; Bert, S.A.; Smith, G.C.; French, H.J.; Luu, P.-L.; Peters, T.J.; Du, Q.; Parry, A.J.; Valdes-Mora, F.; et al. Constitutively bound CTCF sites maintain 3D chromatin architecture and long-range epigenetically regulated domains. Nat. Commun. 2020, 11, 54. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.H.S.; Cattoglio, C.; Slobodyanyuk, E.; Hansen, A.S. Enhancer-Promoter Interactions and Transcription Are Maintained upon Acute Loss of CTCF, Cohesin, WAPL, and YY1. bioRxiv 2021. Available online: https://www.biorxiv.org/content/10.1101/2021.07.14.452365.abstract (accessed on 16 January 2022).
- Yan, Y.; Guo, G.; Huang, J.; Gao, M.; Zhu, Q.; Zeng, S.; Gong, Z.; Xu, Z. Current understanding of extrachromosomal circular DNA in cancer pathogenesis and therapeutic resistance. J. Hematol. Oncol. 2020, 13, 124. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Nguyen, N.-P.; Turner, K.; Wu, S.; Gujar, A.D.; Luebeck, J.; Liu, J.; Deshpande, V.; Rajkumar, U.; Namburi, S.; et al. Extrachromosomal DNA is associated with oncogene amplification and poor outcome across multiple cancers. Nat. Genet. 2020, 52, 891–897. [Google Scholar] [CrossRef] [PubMed]
- Hawley, J.R.; Zhou, S.; Arlidge, C.; Grillo, G.; Kron, K.J.; Hugh-White, R.; van der Kwast, T.H.; Fraser, M.; Boutros, P.C.; Bristow, R.G.; et al. Cis-regulatory Element Hijacking by Structural Variants Overshadows Higher-Order Topological Changes in Prostate Cancer. bioRxiv 2021. [Google Scholar] [CrossRef]
- Zhu, Y.; Gujar, A.D.; Wong, C.-H.; Tjong, H.; Ngan, C.Y.; Gong, L.; Chen, Y.-A.; Kim, H.; Liu, J.; Li, M.; et al. Oncogenic extrachromosomal DNA functions as mobile enhancers to globally amplify chromosomal transcription. Cancer Cell 2021, 39, 694–707.e7. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Shao, J.; Mitra, J.; Xiong, F.; D’Antonio, M.; Wang, R.; Garcia-Bassets, I.; Ma, Q.; Zhu, X.; Lee, J.-H.; et al. Enhancer release and retargeting activates disease-susceptibility genes. Nature 2021, 595, 735–740. [Google Scholar] [CrossRef]
- Mazrooei, P.; Kron, K.J.; Zhu, Y.; Zhou, S.; Grillo, G.; Mehdi, T.; Ahmed, M.; Severson, T.M.; Guilhamon, P.; Armstrong, N.S.; et al. Cistrome Partitioning Reveals Convergence of Somatic Mutations and Risk Variants on Master Transcription Regulators in Primary Prostate Tumors. Cancer Cell 2019, 36, 674–689.e6. [Google Scholar] [CrossRef]
- Bailey, S.D.; Desai, K.; Kron, K.J.; Mazrooei, P.; Sinnott-Armstrong, N.A.; Treloar, A.E.; Dowar, M.; Thu, K.L.; Cescon, D.W.; Silvester, J.; et al. Noncoding somatic and inherited single-nucleotide variants converge to promote ESR1 expression in breast cancer. Nat. Genet. 2016, 48, 1260–1266. [Google Scholar] [CrossRef]
- Hua, J.T.; Ahmed, M.; Guo, H.; Zhang, Y.; Chen, S.; Soares, F.; Lu, J.; Zhou, S.; Wang, M.; Li, H.; et al. Risk SNP-Mediated Promoter-Enhancer Switching Drives Prostate Cancer through lncRNA PCAT19. Cell 2018, 174, 564–575.e18. [Google Scholar] [CrossRef] [PubMed]
- Takayama, K.-I.; Suzuki, T.; Fujimura, T.; Urano, T.; Takahashi, S.; Homma, Y.; Inoue, S. CtBP2 Modulates the Androgen Receptor to Promote Prostate Cancer Progression. Cancer Res. 2014, 74, 6542–6553. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Xia, J.; Sipeky, C.; Dong, X.-M.; Zhang, Q.; Yang, Y.; Zhang, P.; Cruz, S.P.; Zhang, K.; Zhu, J.; et al. Biology and Clinical Implications of the 19q13 Aggressive Prostate Cancer Susceptibility Locus. Cell 2018, 174, 576–589.e18. [Google Scholar] [CrossRef] [PubMed]
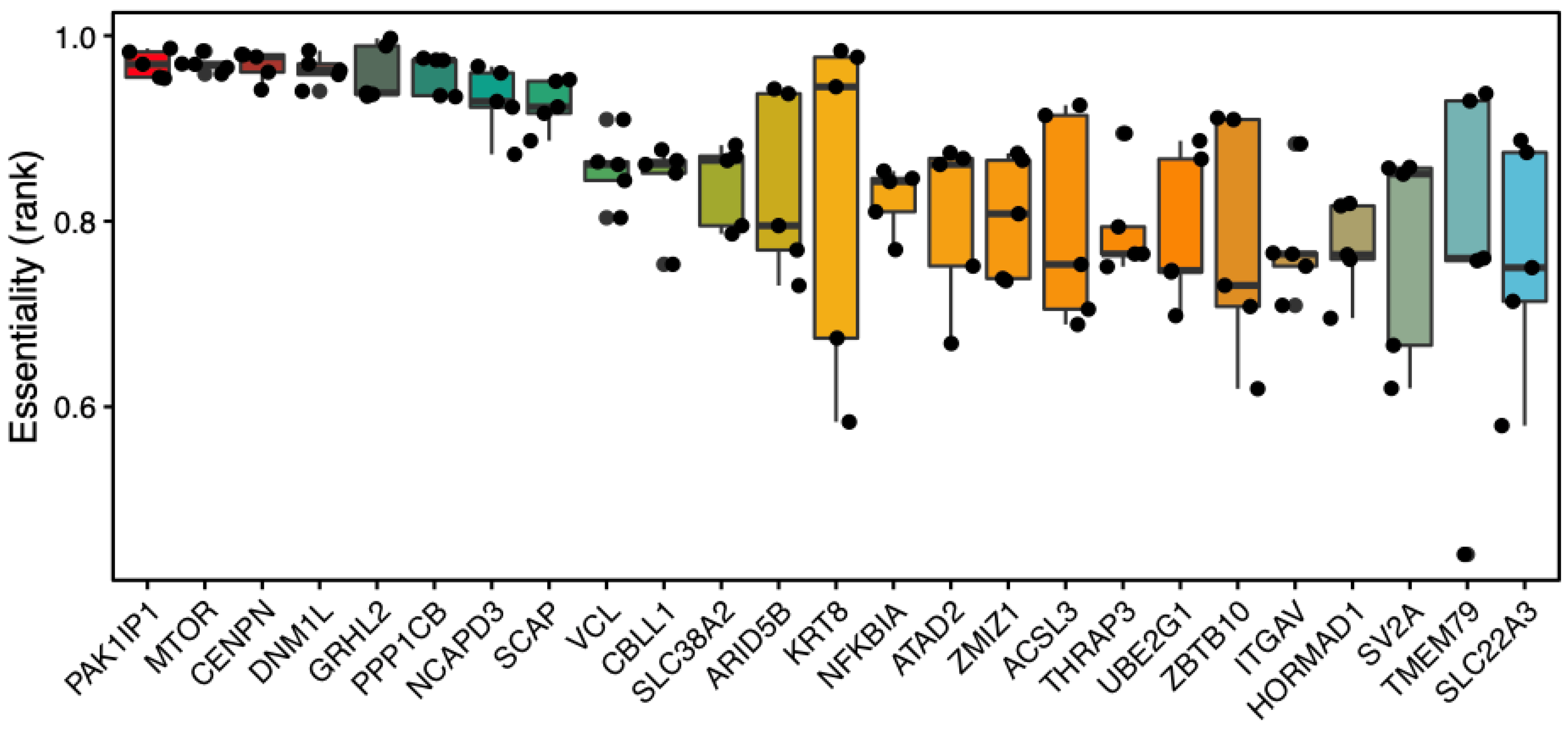
- Morova, T.; McNeill, D.R.; Lallous, N.; Gönen, M.; Dalal, K.; Wilson, D.M., 3rd; Gürsoy, A.; Keskin, Ö.; Lack, N.A. Androgen receptor-binding sites are highly mutated in prostate cancer. Nat Commun. 2020, 11, 832. [Google Scholar] [CrossRef]
- Gucalp, A.; Tolaney, S.; Isakoff, S.J.; Ingle, J.N.; Liu, M.C.; Carey, L.A.; Blackwell, K.; Rugo, H.; Nabell, L.; Forero, A.; et al. Phase II Trial of Bicalutamide in Patients with Androgen Receptor–Positive, Estrogen Receptor–Negative Metastatic Breast Cancer. Clin. Cancer Res. 2013, 19, 5505–5512. [Google Scholar] [CrossRef]
- Xu, D.; Chen, Q.; Liu, Y.; Wen, X. Baicalein suppresses the androgen receptor (AR)-mediated prostate cancer progression via inhibiting the AR N-C dimerization and AR-coactivators interaction. Oncotarget 2017, 8, 105561–105573. [Google Scholar] [CrossRef]
- ENCODE Project Consortium. The ENCODE (ENCyclopedia Of DNA Elements) Project. Science 2004, 306, 636–640. [Google Scholar] [CrossRef]
- Dekker, J.; Belmont, A.S.; Guttman, M.; Leshyk, V.O.; Lis, J.T.; Lomvardas, S.; Mirny, L.A.; O’Shea, C.C.; Park, P.J.; Ren, B.; et al. The 4D nucleome project. Nature 2017, 549, 219–226. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Özturan, D.; Morova, T.; Lack, N.A. Androgen Receptor-Mediated Transcription in Prostate Cancer. Cells 2022, 11, 898. https://doi.org/10.3390/cells11050898
Özturan D, Morova T, Lack NA. Androgen Receptor-Mediated Transcription in Prostate Cancer. Cells. 2022; 11(5):898. https://doi.org/10.3390/cells11050898
Chicago/Turabian StyleÖzturan, Doğancan, Tunç Morova, and Nathan A. Lack. 2022. "Androgen Receptor-Mediated Transcription in Prostate Cancer" Cells 11, no. 5: 898. https://doi.org/10.3390/cells11050898
APA StyleÖzturan, D., Morova, T., & Lack, N. A. (2022). Androgen Receptor-Mediated Transcription in Prostate Cancer. Cells, 11(5), 898. https://doi.org/10.3390/cells11050898





