Plant E3 Ligases and Their Role in Abiotic Stress Response
Abstract
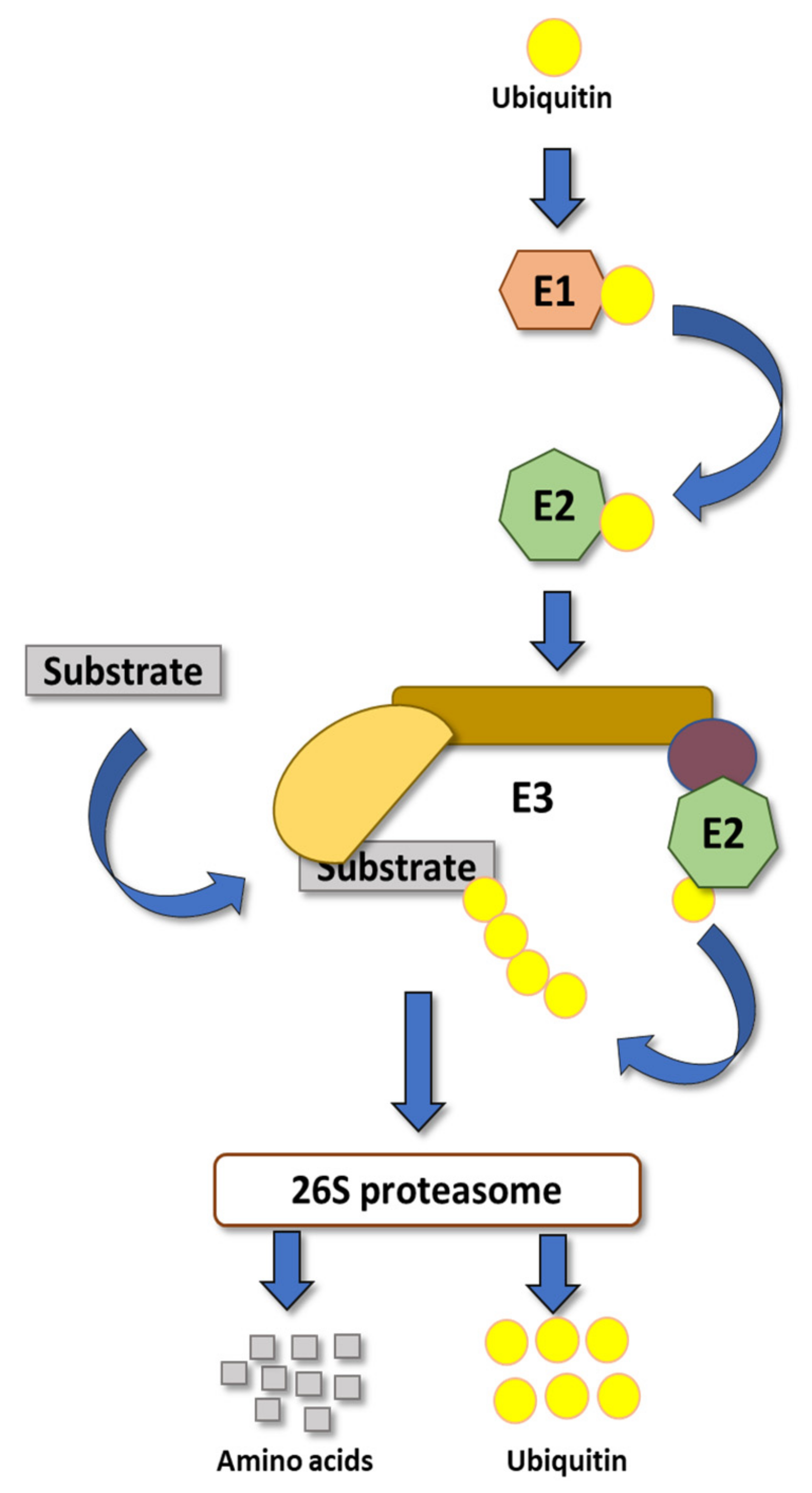
1. Introduction
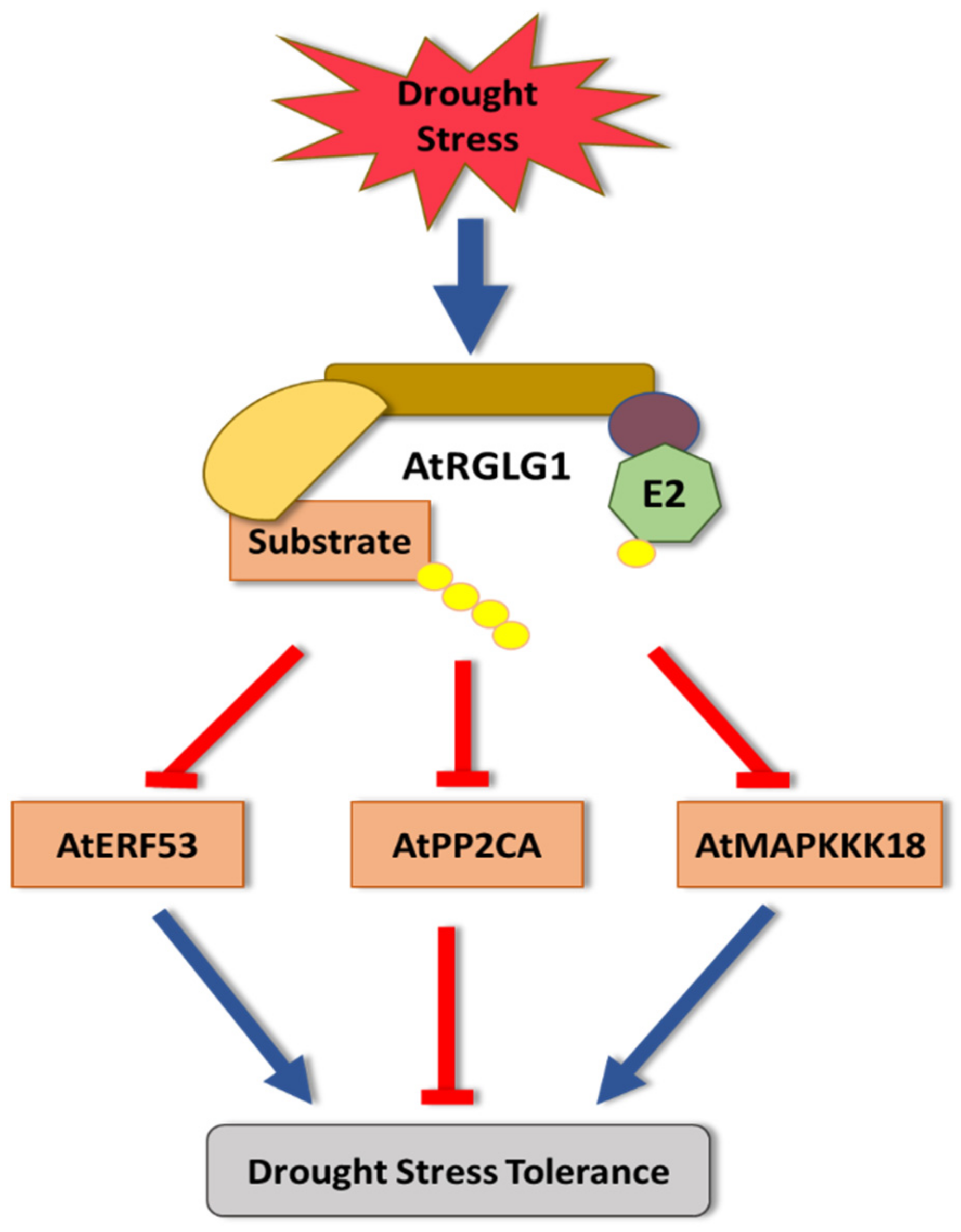
2. Drought Stress
3. Salt Stress
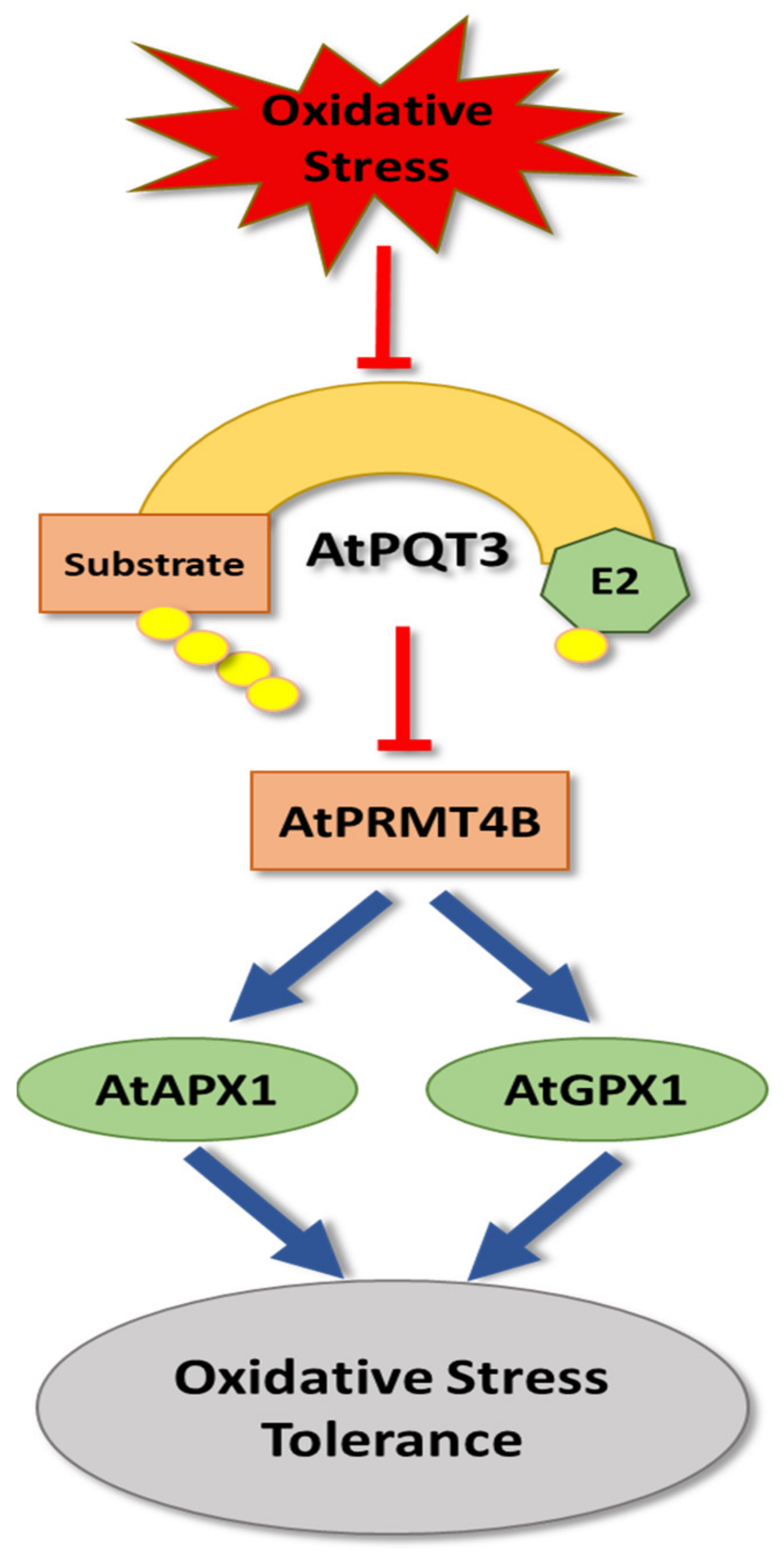
4. Oxidative Stress
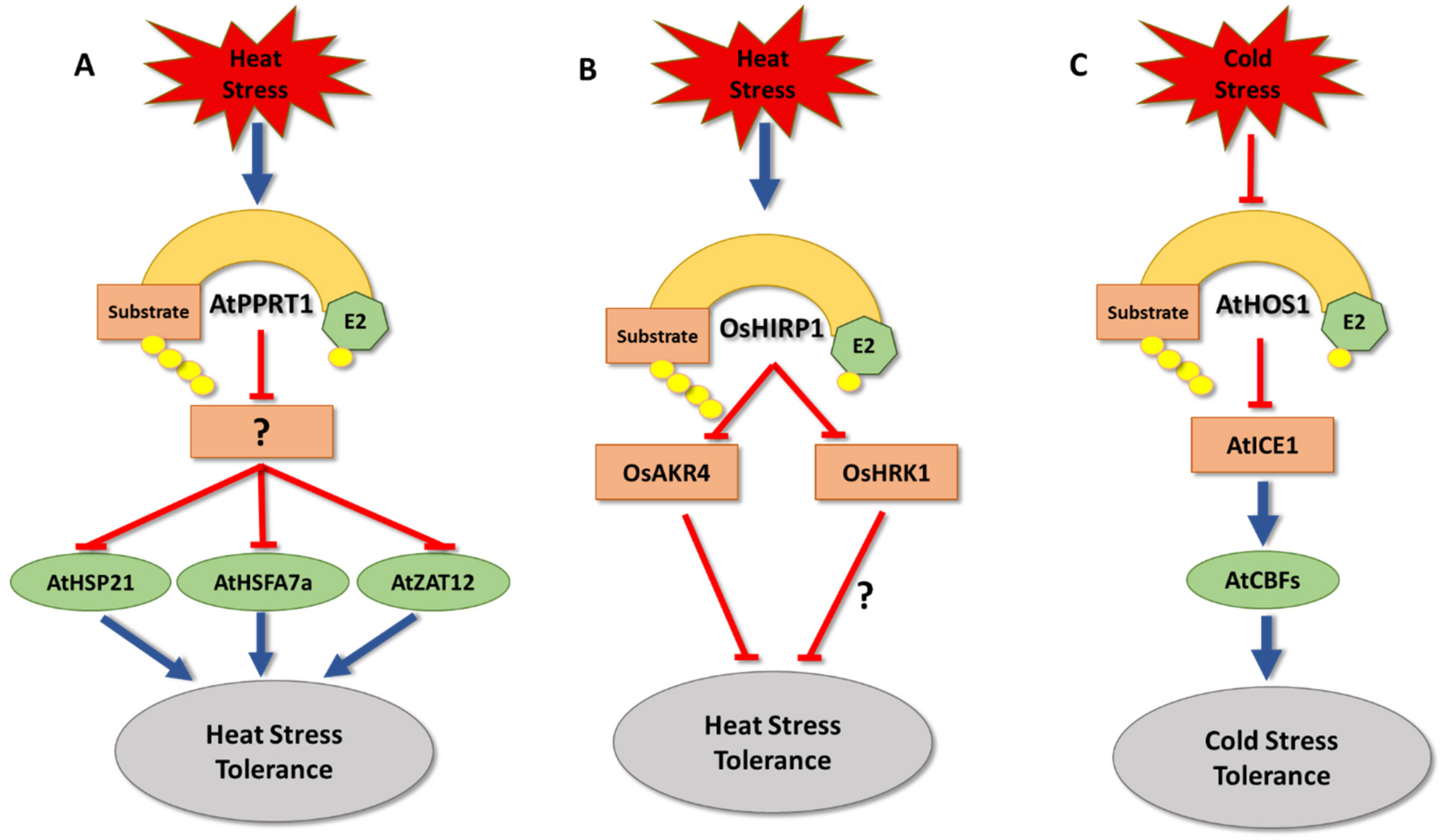
5. Temperature Stress
6. Heavy Metal Stress
7. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ABC1P11 | ABC domain containing protein |
| AKR4 | aldo/keto reductase 4 |
| APX1 | ascorbate peroxidase 1 |
| BiFC | bimolecular fluorescence complementation |
| BTB/POZ | broad-complex, tramtrack, and bric-à-brac/poxvirus and zinc finger |
| CAT | catalase |
| CBF | C-repeat binding factors |
| CLC 6 | chloride channel 6 |
| CRL3 | cullin3 ring e3 ligases |
| CRL4 | cullin4 ring e3 ligases |
| DOR | drought tolerance repressor |
| DRE | dehydration responsive elements |
| DREB2A | dehydration-responsive element binding protein2a |
| DRIP1 | DREB2A interacting protein1 |
| ERF53 | ethylene response factor53 |
| FBA1 | F-box- antagonist of mitotic exit network protein 1 |
| GPX1 | glutathione peroxidase 1 |
| HECT | homology to the E6-associated protein c-terminus |
| HIR1 | heavy metal induced ring e3 ligase 1 |
| HIRP1 | heat-induced ring finger protein 1 |
| HMS | heavy metals |
| HOS1 | high expression of osmotically responsive genes 1 |
| HRK1 | hirp1-regulated kinase1 |
| HSFA7A | heat shock transcription factor a7a |
| HSP21 | heat shock protein 21 |
| IAA17 | indole-3-acetic acid inducible 17 |
| ICE1 | inducer of cbf expression 1 |
| IRT1 | iron-regulated transporter 1 |
| KIN7 | kinase 7 |
| LRR1 | leucine rich repeat protein 1 |
| MADS70 | mads-box gene 70 |
| MAPKKK18 | mitogen activated protein kinase kinase kinase 18 |
| MIEL1 | myb30-interacting e3 ligase 1 |
| PEX11-1 | peroxisomal biogenesis factor 11-1 |
| POD | Peroxidase |
| PP2CA | protein phosphatase 2ca |
| PPRT1 | protein with the ring domain and tmemb1 |
| PQT3 | paraquat tolerance 3 |
| PRMT4B | protein arginine methyltransferase 4b |
| PUB | plant u box |
| RGLG1 | ring domain ligase1 |
| RING | really interesting new gene |
| ROS | reactive oxygen species |
| RPT2A | regulatory particle aaa-atpase 2a |
| SAP5 | stress-associated protein 5 |
| SARP1 | salt-associated ring finger protein |
| SCF | skip1-cullin1-f-box |
| SINA | seven in absentia |
| SIRP | salt-induced ring protein |
| SOD | superoxide dismutase |
| Split-LUC | Split-luciferase assay |
| SRFP1 | stress-related ring finger protein 1 |
| STRF1 | salt tolerance ring finger 1 |
| TIP4;1 | tonoplast intrinsic protein 4;1 |
| TKL1 | transketolase 1 |
| UBQ | Ubiquitin |
| UPP | ubiquitin proteasome pathway |
| ZAT12 | zinc-finger protein 12 |
References
- Kajla, M.; Yadav, V.K.; Khokhar, J.; Singh, S.; Chhokar, R.; Meena, R.P.; Sharma, R. Increase in wheat production through management of abiotic stresses: A review. J. Appl. Nat. Sci. 2015, 7, 1070–1080. [Google Scholar] [CrossRef]
- Iizumi, T.; Shen, Z.; Furuya, J.; Koizumi, T.; Furuhashi, G.; Kim, W.; Nishimori, M. Climate change adaptation cost and residual damage to global crop production. Clim. Res. 2020, 80, 203–218. [Google Scholar] [CrossRef]
- He, M.; He, C.Q.; Ding, N.Z. Abiotic Stresses: General Defenses of Land Plants and Chances for Engineering Multistress Tolerance. Front. Plant Sci. 2018, 9, 1771. [Google Scholar] [CrossRef]
- Jmii, S.; Cappadocia, L. Plant SUMO E3 Ligases: Function, Structural Organization, and Connection With DNA. Front. Plant Sci. 2021, 12, 652170. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Kim, W.T. Regulation of abiotic stress signal transduction by E3 ubiquitin ligases in Arabidopsis. Mol. Cells 2011, 31, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Hellmann, H. Plant E3 ligases: Flexible enzymes in a sessile world. Mol. Plant 2013, 6, 1388–1404. [Google Scholar] [CrossRef]
- Al-Saharin, R.; Mooney, S.; Hellmann, H. Chapter 11 Plant E3 Ligases as Versatile Tools for Novel Drug Development and Plant Bioengineering. In Protein Degradation with New Chemical Modalities: Successful Strategies in Drug Discovery and Chemical Biology; Weinmann, H., Crews, C., Eds.; The Royal Society of Chemistry: London, UK, 2020; pp. 212–233. [Google Scholar]
- Hatfield, P.M.; Gosink, M.M.; Carpenter, T.B.; Vierstra, R.D. The ubiquitin-activating enzyme (E1) gene family in Arabidopsis thaliana. Plant J. Cell Mol. Biol. 1997, 11, 213–226. [Google Scholar] [CrossRef]
- Bej, S.; Neeraja, C.N.; Kanth, K.T.; Suman, K.; Barbadikar, K.M.; Voleti, S.R. Correlation of expressional pattern of Ubiquitin activating gene with grain Fe content in rice. Oryza 2020, 57, 251–259. [Google Scholar] [CrossRef]
- E, Z.; Zhang, Y.; Li, T.; Wang, L.; Zhao, H. Characterization of the Ubiquitin-Conjugating Enzyme Gene Family in Rice and Evaluation of Expression Profiles under Abiotic Stresses and Hormone Treatments. PLoS ONE 2015, 10, e0122621. [Google Scholar] [CrossRef]
- Kraft, E.; Stone, S.L.; Ma, L.; Su, N.; Gao, Y.; Lau, O.S.; Deng, X.W.; Callis, J. Genome analysis and functional characterization of the E2 and RING-type E3 ligase ubiquitination enzymes of Arabidopsis. Plant Physiol. 2005, 139, 1597–1611. [Google Scholar] [CrossRef]
- Mazzucotelli, E.; Belloni, S.; Marone, D.; de Leonardis, A.; Guerra, D.; di Fonzo, N.; Cattivelli, L.; Mastrangelo, A. The e3 ubiquitin ligase gene family in plants: Regulation by degradation. Curr. Genom. 2006, 7, 509–522. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Zhou, X.; Li, L.; Su, Z. plantsUPS: A database of plants’ Ubiquitin Proteasome System. BMC Genom. 2009, 10, 227. [Google Scholar] [CrossRef] [PubMed]
- Hellmann, H.; Hobbie, L.; Chapman, A.; Dharmasiri, S.; Dharmasiri, N.; del Pozo, C.; Reinhardt, D.; Estelle, M. Arabidopsis AXR6 encodes CUL1 implicating SCF E3 ligases in auxin regulation of embryogenesis. EMBO J. 2003, 22, 3314–3325. [Google Scholar] [CrossRef]
- Hotton, S.K.; Callis, J. Regulation of cullin RING ligases. Annu. Rev. Plant Biol. 2008, 59, 467–489. [Google Scholar] [CrossRef] [PubMed]
- Choi, C.M.; Gray, W.M.; Mooney, S.; Hellmann, H. Composition, roles, and regulation of cullin-based ubiquitin e3 ligases. Arab. Book 2014, 12, e0175. [Google Scholar] [CrossRef]
- del Pozo, J.C.; Estelle, M. F-box proteins and protein degradation: An emerging theme in cellular regulation. Plant Mol. Biol. 2000, 44, 123–128. [Google Scholar] [CrossRef]
- Craig, K.L.; Tyers, M. The F-box: A new motif for ubiquitin dependent proteolysis in cell cycle regulation and signal transduction. Prog. Biophys. Mol. Biol. 1999, 72, 299–328. [Google Scholar] [CrossRef]
- Pham, V.N.; Kathare, P.K.; Huq, E. Phytochromes and Phytochrome Interacting Factors. Plant Physiol. 2018, 176, 1025–1038. [Google Scholar] [CrossRef]
- Salehin, M.; Bagchi, R.; Estelle, M. SCFTIR1/AFB-based auxin perception: Mechanism and role in plant growth and development. Plant Cell 2015, 27, 9–19. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, X.; Xiong, G.; Liu, H.; Chen, F.; Wang, L.; Meng, X.; Liu, G.; Yu, H.; Yuan, Y.; et al. DWARF 53 acts as a repressor of strigolactone signalling in rice. Nature 2013, 504, 401–405. [Google Scholar] [CrossRef] [PubMed]
- Gusti, A.; Baumberger, N.; Nowack, M.; Pusch, S.; Eisler, H.; Potuschak, T.; de Veylder, L.; Schnittger, A.; Genschik, P. The Arabidopsis thaliana F-box protein FBL17 is essential for progression through the second mitosis during pollen development. PLoS ONE 2009, 4, e4780. [Google Scholar] [CrossRef] [PubMed]
- Dohmann, E.M.; Levesque, M.P.; Isono, E.; Schmid, M.; Schwechheimer, C. Auxin responses in mutants of the Arabidopsis Constitutive Photomorphogenic9 signalosome. Plant Physiol. 2008, 147, 1369–1379. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.S.; Koo, A.J.; Gao, X.; Jayanty, S.; Thines, B.; Jones, A.D.; Howe, G.A. Regulation and function of Arabidopsis Jasmonate ZIM-domain genes in response to wounding and herbivory. Plant Physiol. 2008, 146, 952–964. [Google Scholar] [CrossRef] [PubMed]
- Thines, B.; Katsir, L.; Melotto, M.; Niu, Y.; Mandaokar, A.; Liu, G.; Nomura, K.; He, S.Y.; Howe, G.A.; Browse, J. JAZ repressor proteins are targets of the SCF(COI1) complex during jasmonate signalling. Nature 2007, 448, 661–665. [Google Scholar] [CrossRef]
- Han, L.; Mason, M.; Risseeuw, E.P.; Crosby, W.L.; Somers, D.E. Formation of an SCF(ZTL) complex is required for proper regulation of circadian timing. Plant J. Cell Mol. Biol. 2004, 40, 291–301. [Google Scholar] [CrossRef]
- McGinnis, K.M.; Thomas, S.G.; Soule, J.D.; Strader, L.C.; Zale, J.M.; Sun, T.P.; Steber, C.M. The Arabidopsis SLEEPY1 gene encodes a putative F-box subunit of an SCF E3 ubiquitin ligase. Plant Cell 2003, 15, 1120–1130. [Google Scholar] [CrossRef]
- Abd-Hamid, N.-A.; Ahmad-Fauzi, M.-I.; Zainal, Z.; Ismail, I. Diverse and dynamic roles of F-box proteins in plant biology. Planta 2020, 251, 68. [Google Scholar] [CrossRef]
- Nguyen, K.M.; Busino, L. The biology of F-box proteins: The SCF family of E3 ubiquitin ligases. In Cullin-RING Ligases Protein Neddylation; Springer: Singapore, 2020; Volume 1217, pp. 111–122. [Google Scholar]
- Gingerich, D.J.; Gagne, J.M.; Salter, D.W.; Hellmann, H.; Estelle, M.; Ma, L.; Vierstra, R.D. Cullins 3a and 3b assemble with members of the broad complex/tramtrack/bric-a-brac (BTB) protein family to form essential ubiquitin-protein ligases (E3s) in Arabidopsis. J. Biol. Chem. 2005, 280, 18810–18821. [Google Scholar] [CrossRef]
- Weber, H.; Bernhardt, A.; Dieterle, M.; Hano, P.; Mutlu, A.; Estelle, M.; Genschik, P.; Hellmann, H. Arabidopsis AtCUL3a and AtCUL3b form complexes with members of the BTB/POZ-MATH protein family. Plant Physiol. 2005, 137, 83–93. [Google Scholar] [CrossRef]
- Juranic, M.; Dresselhaus, T. Phylogenetic analysis of the expansion of the MATH-BTB gene family in the grasses. Plant Signal. Behav. 2014, 9, e28242. [Google Scholar] [CrossRef]
- Gingerich, D.J.; Hanada, K.; Shiu, S.H.; Vierstra, R.D. Large-scale, lineage-specific expansion of a bric-a-brac/tramtrack/broad complex ubiquitin-ligase gene family in rice. Plant Cell 2007, 19, 2329–2348. [Google Scholar] [CrossRef] [PubMed]
- Stogios, P.J.; Downs, G.S.; Jauhal, J.J.; Nandra, S.K.; Prive, G.G. Sequence and structural analysis of BTB domain proteins. Genome Biol. 2005, 6, R82. [Google Scholar] [CrossRef] [PubMed]
- Chico, J.M.; Lechner, E.; Fernandez-Barbero, G.; Canibano, E.; Garcia-Casado, G.; Franco-Zorrilla, J.M.; Hammann, P.; Zamarreno, A.M.; Garcia-Mina, J.M.; Rubio, V.; et al. CUL3(BPM) E3 ubiquitin ligases regulate MYC2, MYC3, and MYC4 stability and JA responses. Proc. Natl. Acad. Sci. USA 2020, 117, 6205–6215. [Google Scholar] [CrossRef] [PubMed]
- Mooney, S.; Al-Saharin, R.; Choi, C.M.; Tucker, K.; Beathard, C.; Hellmann, H.A. Characterization of Brassica rapa RAP2.4-Related Proteins in Stress Response and as CUL3-Dependent E3 Ligase Substrates. Cells 2019, 8, 336. [Google Scholar] [CrossRef]
- Julian, J.; Coego, A.; Lozano-Juste, J.; Lechner, E.; Wu, Q.; Zhang, X.; Merilo, E.; Belda-Palazon, B.; Park, S.Y.; Cutler, S.R.; et al. The MATH-BTB BPM3 and BPM5 subunits of Cullin3-RING E3 ubiquitin ligases target PP2CA and other clade A PP2Cs for degradation. Proc. Natl. Acad. Sci. USA 2019, 116, 15725–15734. [Google Scholar] [CrossRef]
- Morimoto, K.; Ohama, N.; Kidokoro, S.; Mizoi, J.; Takahashi, F.; Todaka, D.; Mogami, J.; Sato, H.; Qin, F.; Kim, J.S.; et al. BPM-CUL3 E3 ligase modulates thermotolerance by facilitating negative regulatory domain-mediated degradation of DREB2A in Arabidopsis. Proc. Natl. Acad. Sci. USA 2017, 114, E8528–E8536. [Google Scholar] [CrossRef]
- Chen, L.; Bernhardt, A.; Lee, J.; Hellmann, H. Identification of Arabidopsis MYB56 as a novel substrate for CRL3(BPM) E3 ligases. Mol. Plant 2015, 8, 242–250. [Google Scholar] [CrossRef]
- Chen, L.; Lee, J.H.; Weber, H.; Tohge, T.; Witt, S.; Roje, S.; Fernie, A.R.; Hellmann, H. Arabidopsis BPM proteins function as substrate adaptors to a cullin3-based E3 ligase to affect fatty acid metabolism in plants. Plant Cell 2013, 25, 2253–2264. [Google Scholar] [CrossRef]
- Weber, H.; Hellmann, H. Arabidopsis thaliana BTB/POZ-MATH proteins interact with members of the ERF/AP2 transcription factor family. FEBS J. 2009, 276, 6624–6635. [Google Scholar] [CrossRef]
- Figueroa, P.; Gusmaroli, G.; Serino, G.; Habashi, J.; Ma, L.; Shen, Y.; Feng, S.; Bostick, M.; Callis, J.; Hellmann, H.; et al. Arabidopsis has two redundant Cullin3 proteins that are essential for embryo development and that interact with RBX1 and BTB proteins to form multisubunit E3 ubiquitin ligase complexes in vivo. Plant Cell 2005, 17, 1180–1195. [Google Scholar] [CrossRef]
- Beathard, C.; Mooney, S.; Al-Saharin, R.; Goyer, A.; Hellmann, H. Characterization of Arabidopsis thaliana R2R3 S23 MYB Transcription Factors as Novel Targets of the Ubiquitin Proteasome-Pathway and Regulators of Salt Stress and Abscisic Acid Response. Front. Plant Sci. 2021, 12, 629208. [Google Scholar] [CrossRef] [PubMed]
- Biedermann, S.; Hellmann, H. WD40 and CUL4-based E3 ligases: Lubricating all aspects of life. Trends Plant Sci. 2011, 16, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Bian, S.; Li, X.; Mainali, H.; Chen, L.; Dhaubhadel, S. Genome-wide analysis of DWD proteins in soybean (Glycine max): Significance of Gm08DWD and GmMYB176 interaction in isoflavonoid biosynthesis. PLoS ONE 2017, 12, e0178947. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Terzaghi, W.; Gusmaroli, G.; Charron, J.B.; Yoon, H.J.; Chen, H.; He, Y.J.; Xiong, Y.; Deng, X.W. Characterization of Arabidopsis and rice DWD proteins and their roles as substrate receptors for CUL4-RING E3 ubiquitin ligases. Plant Cell 2008, 20, 152–167. [Google Scholar] [CrossRef]
- Li, D.; Zhang, L.; Li, X.; Kong, X.; Wang, X.; Li, Y.; Liu, Z.; Wang, J.; Li, X.; Yang, Y. AtRAE1 is involved in degradation of ABA receptor RCAR1 and negatively regulates ABA signalling in Arabidopsis. Plant Cell Environ. 2018, 41, 231–244. [Google Scholar] [CrossRef]
- Chen, H.; Huang, X.; Gusmaroli, G.; Terzaghi, W.; Lau, O.S.; Yanagawa, Y.; Zhang, Y.; Li, J.; Lee, J.H.; Zhu, D.; et al. Arabidopsis CULLIN4-damaged DNA binding protein 1 interacts with CONSTITUTIVELY PHOTOMORPHOGENIC1-SUPPRESSOR OF PHYA complexes to regulate photomorphogenesis and flowering time. Plant Cell 2010, 22, 108–123. [Google Scholar] [CrossRef]
- Biedermann, S.; Hellmann, H. The DDB1a interacting proteins ATCSA-1 and DDB2 are critical factors for UV-B tolerance and genomic integrity in Arabidopsis thaliana. Plant J. Cell Mol. Biol. 2010, 62, 404–415. [Google Scholar] [CrossRef]
- Saleme, M.D.L.S.; Andrade, I.R.; Eloy, N.B. The Role of Anaphase-Promoting Complex/Cyclosome (APC/C) in Plant Reproduction. Front. Plant Sci. 2021, 12, 269. [Google Scholar] [CrossRef]
- Volpe, M.; Levinton, N.; Rosenstein, N.; Prag, G.; Ben-Aroya, S. Regulation of the anaphase promoting complex/cyclosome by the degradation of its unassembled catalytic subunit, Apc11. FASEB J. 2019, 33, 9752–9761. [Google Scholar] [CrossRef]
- Jimenez-Lopez, D.; Munoz-Belman, F.; Gonzalez-Prieto, J.M.; Aguilar-Hernandez, V.; Guzman, P. Repertoire of plant RING E3 ubiquitin ligases revisited: New groups counting gene families and single genes. PLoS ONE 2018, 13, e0203442. [Google Scholar] [CrossRef]
- Ohi, M.D.; Vander Kooi, C.W.; Rosenberg, J.A.; Chazin, W.J.; Gould, K.L. Structural insights into the U-box, a domain associated with multi-ubiquitination. Nat. Struct. Biol. 2003, 10, 250–255. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Kang, K.K.; Cho, Y.G. Molecular and Functional Analysis of U-box E3 Ubiquitin Ligase Gene Family in Rice (Oryzasativa). Int. J. Mol. Sci. 2021, 22, 12088. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Song, B.; Dai, Y.; Zhang, S.; Huang, X. Genome-wide identification and functional analysis of U-box E3 ubiquitin ligases gene family related to drought stress response in Chinese white pear (Pyrus bretschneideri). BMC Plant Biol. 2021, 21, 235. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Lee, Y.J.; Hong, M.J.; Kim, J.H.; Seo, Y.W. Genome Wide Analysis of U-Box E3 Ubiquitin Ligases in Wheat (Triticum aestivum L.). Int. J. Mol. Sci. 2021, 22, 2699. [Google Scholar] [CrossRef]
- Yee, D.; Goring, D.R. The diversity of plant U-box E3 ubiquitin ligases: From upstream activators to downstream target substrates. J. Exp. Bot. 2009, 60, 1109–1121. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Xing, Y.; Lou, Q.; Feng, P.; Liu, S.; Zhu, M.; Yin, W.; Fang, S.; Lin, Y.; Zhang, T.; et al. Dwarf and short grain 1, encoding a putative U-box protein regulates cell division and elongation in rice. J. Plant Physiol. 2017, 209, 84–94. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, Y.; Yu, B.; Yin, Z.; Xia, Y. Extra-Large G Proteins Interact with E3 Ligases PUB4 and PUB2 and Function in Cytokinin and Developmental Processes. Plant Physiol. 2017, 173, 1235–1246. [Google Scholar] [CrossRef]
- Zhang, Y.; Xia, G.; Zhu, Q. Conserved and Unique Roles of Chaperone-Dependent E3 Ubiquitin Ligase CHIP in Plants. Front. Plant Sci. 2021, 12, 699756. [Google Scholar] [CrossRef]
- Lin, Y.; Hu, Q.; Zhou, J.; Yin, W.; Yao, D.; Shao, Y.; Zhao, Y.; Guo, B.; Xia, Y.; Chen, Q.; et al. Phytophthora sojae effector Avr1d functions as an E2 competitor and inhibits ubiquitination activity of GmPUB13 to facilitate infection. Proc. Natl. Acad. Sci. USA 2021, 118, 10. [Google Scholar] [CrossRef]
- Yu, Y.; Meng, X.; Guo, D.; Yang, S.; Zhang, G.; Liang, Z. Grapevine U-Box E3 Ubiquitin Ligase VlPUB38 Negatively Regulates Fruit Ripening by Facilitating Abscisic-Aldehyde Oxidase Degradation. Plant Cell Physiol. 2021, 61, 2043–2054. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Gao, Z.; Xu, X.; Wang, Y.; Lin, Y.; Ye, P.; Huang, T. Plant U-box E3 ligases PUB25 and PUB26 control organ growth in Arabidopsis. New Phytol. 2021, 229, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Somssich, M.; Je, B.I.; Simon, R.; Jackson, D. Clavata-Wuschel signaling in the shoot meristem. Development 2016, 143, 3238–3248. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.H.; Lee, A.; Yu, S.G.; Cui, L.H.; Min, H.J.; Lee, S.E.; Cho, N.H.; Kim, S.; Bae, H.; Kim, W.T. OsPUB41, a U-box E3 ubiquitin ligase, acts as a negative regulator of drought stress response in rice (Oryza sativa L.). Plant Mol. Biol. 2021, 106, 463–477. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhao, L.; Zhang, M.; Zafar, S.A.; Fang, J.; Li, M.; Zhang, W.; Li, X. Arabidopsis E3 ubiquitin ligases PUB22 and PUB23 negatively regulate drought tolerance by targeting ABA receptor PYL9 for degradation. Int. J. Mol. Sci. 2017, 18, 1841. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, T.; Rehman, A.U.; Wang, Y.; Qi, J.; Li, Z.; Song, C.; Wang, B.; Yang, S.; Gong, Z. Arabidopsis U--box E3 ubiquitin ligase PUB11 negatively regulates drought tolerance by degrading the receptor--like protein kinases LRR1 and KIN7. J. Integr. Plant Biol. 2021, 63, 494–509. [Google Scholar] [CrossRef]
- Alam, I.; Cui, D.L.; Batool, K.; Yang, Y.Q.; Lu, Y.H. Comprehensive Genomic Survey, Characterization and Expression Analysis of the HECT Gene Family in Brassica rapa L. and Brassica oleracea L. Genes 2019, 10, 400. [Google Scholar] [CrossRef]
- Li, Y.; Zhai, L.; Fan, J.; Ren, J.; Gong, W.; Wang, X.; Huang, J. Genome-wide identification, phylogenetic and expression analysis of the maize HECT E3 ubiquitin ligase genes. Genetica 2019, 147, 391–400. [Google Scholar] [CrossRef]
- Lan, W.; Ma, W.; Miao, Y. Role of HECT ubiquitin protein ligases in Arabidopsis thaliana. J Plant SSci Phytopathol. 2018, 2, 20–30. [Google Scholar] [CrossRef]
- Kamadurai, H.B.; Qiu, Y.; Deng, A.; Harrison, J.S.; Macdonald, C.; Actis, M.; Rodrigues, P.; Miller, D.J.; Souphron, J.; Lewis, S.M.; et al. Mechanism of ubiquitin ligation and lysine prioritization by a HECT E3. Elife 2013, 2, e00828. [Google Scholar] [CrossRef]
- Furniss, J.J.; Grey, H.; Wang, Z.; Nomoto, M.; Jackson, L.; Tada, Y.; Spoel, S.H. Proteasome-associated HECT-type ubiquitin ligase activity is required for plant immunity. PLoS ONE Pathog. 2018, 14, e1007447. [Google Scholar] [CrossRef]
- Bray, E.A. Plant responses to water deficit. Trends Plant Sci. 1997, 2, 48–54. [Google Scholar] [CrossRef]
- Hussain, S.; Hussain, S.; Qadir, T.; Khaliq, A.; Ashraf, U.; Parveen, A.; Saqib, M.; Rafiq, M. Drought stress in plants: An overview on implications, tolerance mechanisms and agronomic mitigation strategies. Plant Sci. Today 2019, 6, 389–402. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Manivannan, P.; Wahid, A.; Farooq, M.; Al-Juburi, H.J.; Somasundaram, R.; Panneerselvam, R. Drought stress in plants: A review on morphological characteristics and pigments composition. Int. J. Agric. Biol 2009, 11, 100–105. [Google Scholar]
- Cheng, M.-C.; Hsieh, E.-J.; Chen, J.-H.; Chen, H.-Y.; Lin, T.-P. Arabidopsis RGLG2, functioning as a RING E3 ligase, interacts with AtERF53 and negatively regulates the plant drought stress response. Plant Physiol. 2012, 158, 363–375. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.E.; Xu, W.; Li, Z.; Deng, X.W.; Wu, W.; Xue, Y. F-box protein DOR functions as a novel inhibitory factor for abscisic acid-induced stomatal closure under drought stress in Arabidopsis. Plant Physiol. 2008, 148, 2121–2133. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Song, E.; Kim, Y.; Choi, E.; Hwang, J.; Lee, J.H. Loss-of-function of Arabidopsis F--BOX PROTEIN HYPERSENSITIVE TO ABA 1 enhances drought tolerance and delays germination. Physiol. Plant. 2021, 173, 2376–2389. [Google Scholar] [CrossRef] [PubMed]
- Li, B.W.; Gao, S.; Yang, Z.M.; Song, J.B. The F-box E3 ubiquitin ligase AtSDR is involved in salt and drought stress responses in Arabidopsis. Gene 2022, 809, 146011. [Google Scholar] [CrossRef]
- Kang, M.; Fokar, M.; Abdelmageed, H.; Allen, R.D. Arabidopsis SAP5 functions as a positive regulator of stress responses and exhibits E3 ubiquitin ligase activity. Plant Mol. Biol. 2011, 75, 451–466. [Google Scholar] [CrossRef] [PubMed]
- Ryu, M.Y.; Cho, S.K.; Kim, W.T. The Arabidopsis C3H2C3-type RING E3 ubiquitin ligase AtAIRP1 is a positive regulator of an abscisic acid-dependent response to drought stress. Plant Physiol. 2010, 154, 1983–1997. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.K.; Ryu, M.Y.; Seo, D.H.; Kang, B.G.; Kim, W.T. The Arabidopsis RING E3 ubiquitin ligase AtAIRP2 plays combinatory roles with AtAIRP1 in abscisic acid-mediated drought stress responses. Plant Physiol. 2011, 157, 2240–2257. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, W.T. The Arabidopsis RING E3 ubiquitin ligase AtAIRP3/LOG2 participates in positive regulation of high-salt and drought stress responses. Plant Physiol. 2013, 162, 1733–1749. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Huang, S.; Liu, Y.; Bian, M.; Shi, W.; Zuo, Z.; Yang, Z. Overexpression of A RING finger ubiquitin ligase gene AtATRF1 enhances aluminium tolerance in Arabidopsis thaliana. J. Plant Biol. 2017, 60, 66–74. [Google Scholar] [CrossRef]
- Gao, W.; Liu, W.; Zhao, M.; Li, W.-X. NERF encodes a RING E3 ligase important for drought resistance and enhances the expression of its antisense gene NFYA5 in Arabidopsis. Nucleic Acids Res. 2015, 43, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Han, Y.; Zhao, Q.; Li, C.; Xie, Q.; Chong, K.; Xu, Y. The E3 ligase AtRDUF1 positively regulates salt stress responses in Arabidopsis thaliana. PLoS ONE 2013, 8, e71078. [Google Scholar]
- Luo, C.; Cai, X.-T.; Du, J.; Zhao, T.-L.; Wang, P.-F.; Zhao, P.-X.; Liu, R.; Xie, Q.; Cao, X.-F.; Xiang, C.-B. Paraquat Tolerance3 is an E3 ligase and acts as a negative regulator of oxidative stress response. bioRxiv 2016, 1, 040543. [Google Scholar]
- Li, Y.; Jia, F.; Yu, Y.; Luo, L.; Huang, J.; Yang, G.; Wu, C.; Zheng, C. The SCF E3 ligase AtPP2-B11 plays a negative role in response to drought stress in Arabidopsis. Plant Mol. Biol. Rep. 2014, 32, 943–956. [Google Scholar] [CrossRef]
- Jia, F.; Wang, C.; Huang, J.; Yang, G.; Wu, C.; Zheng, C. SCF E3 ligase PP2-B11 plays a positive role in response to salt stress in Arabidopsis. J. Exp. Bot. 2015, 66, 4683–4697. [Google Scholar] [CrossRef]
- Liu, Y.-C.; Wu, Y.-R.; Huang, X.-H.; Sun, J.; Xie, Q. AtPUB19, a U-box E3 ubiquitin ligase, negatively regulates abscisic acid and drought responses in Arabidopsis thaliana. Mol. Plant 2011, 4, 938–946. [Google Scholar] [CrossRef]
- Hwang, J.H.; Seo, D.H.; Kang, B.G.; Kwak, J.M.; Kim, W.T. Suppression of Arabidopsis AtPUB30 resulted in increased tolerance to salt stress during germination. Plant Cell Rep. 2015, 34, 277–289. [Google Scholar] [CrossRef]
- Adler, G.; Mishra, A.K.; Maymon, T.; Raveh, D.; Bar-Zvi, D. Overexpression of Arabidopsis ubiquitin ligase AtPUB46 enhances tolerance to drought and oxidative stress. Plant Sci. 2018, 276, 220–228. [Google Scholar] [CrossRef]
- Adler, G.; Konrad, Z.; Zamir, L.; Mishra, A.K.; Raveh, D.; Bar-Zvi, D. The Arabidopsis paralogs, PUB46 and PUB48, encoding U-box E3 ubiquitin ligases, are essential for plant response to drought stress. BMC Plant Biol. 2017, 17, 8. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Wan, X.; Huang, K.; Pei, L.; Xiong, J.; Li, X.; Wang, J. AtPUB48 E3 ligase plays a crucial role in the thermotolerance of Arabidopsis. Biochem. Biophys. Res. Commun. 2019, 509, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yang, C.; Li, Y.; Zheng, N.; Chen, H.; Zhao, Q.; Gao, T.; Guo, H.; Xie, Q. SDIR1 is a RING finger E3 ligase that positively regulates stress-responsive abscisic acid signaling in Arabidopsis. Plant Cell 2007, 19, 1912–1929. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Wang, H.; Su, T.; Wu, W.-H.; Chen, Y.-F. The ubiquitin E3 ligase PRU1 regulates WRKY6 degradation to modulate phosphate homeostasis in response to low-pi stress in Arabidopsis. Plant Cell 2018, 30, 1062–1076. [Google Scholar] [CrossRef]
- Lee, H.; Xiong, L.; Gong, Z.; Ishitani, M.; Stevenson, B.; Zhu, J.-K. The Arabidopsis HOS1 gene negatively regulates cold signal transduction and encodes a RING finger protein that displays cold-regulated nucleo–cytoplasmic partitioning. Genes Dev. 2001, 15, 912–924. [Google Scholar] [CrossRef]
- Dong, C.-H.; Agarwal, M.; Zhang, Y.; Xie, Q.; Zhu, J.-K. The negative regulator of plant cold responses, HOS1, is a RING E3 ligase that mediates the ubiquitination and degradation of ICE1. Proc. Natl. Acad. Sci. USA 2006, 103, 8281–8286. [Google Scholar] [CrossRef]
- Kim, S.J.; Ryu, M.Y.; Kim, W.T. Suppression of Arabidopsis RING-DUF1117 E3 ubiquitin ligases, AtRDUF1 and AtRDUF2, reduces tolerance to ABA-mediated drought stress. Biochem. Biophys. Res. Commun. 2012, 420, 141–147. [Google Scholar] [CrossRef]
- Kim, S.J.; Kim, W.T. Suppression of Arabidopsis RING E3 ubiquitin ligase AtATL78 increases tolerance to cold stress and decreases tolerance to drought stress. FEBS Lett. 2013, 587, 2584–2590. [Google Scholar] [CrossRef]
- Yang, R.; Wang, T.; Shi, W.; Li, S.; Liu, Z.; Wang, J.; Yang, Y. E3 ubiquitin ligase ATL61 acts as a positive regulator in abscisic acid mediated drought response in Arabidopsis. Biochem. Biophys. Res. Commun. 2020, 528, 292–298. [Google Scholar] [CrossRef]
- Tian, M.; Lou, L.; Liu, L.; Yu, F.; Zhao, Q.; Zhang, H.; Wu, Y.; Tang, S.; Xia, R.; Zhu, B. The RING finger E3 ligase STRF1 is involved in membrane trafficking and modulates salt--stress response in Arabidopsis thaliana. Plant J. 2015, 82, 81–92. [Google Scholar] [CrossRef]
- Yu, S.G.; Kim, J.H.; Cho, N.H.; Oh, T.R.; Kim, W.T. Arabidopsis RING E3 ubiquitin ligase JUL1 participates in ABA--mediated microtubule depolymerization, stomatal closure, and tolerance response to drought stress. Plant J. 2020, 103, 824–842. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Jiang, Y.; Tang, H.; Tong, S.; Lou, S.; Shao, C.; Zhang, J.; Song, Y.; Chen, N.; Bi, H. The ubiquitin E3 ligase SR1 modulates the submergence response by degrading phosphorylated WRKY33 in Arabidopsis. Plant Cell 2021, 33, 1771–1789. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Pei, L.; Xiao, S.; Peng, L.; Liu, Z.; Li, X.; Yang, Y.; Wang, J. AtPPRT1 negatively regulates salt stress response in Arabidopsis seedlings. Plant Signal. Behav. 2020, 15, 1732103. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Xiao, S.; Sun, H.; Pei, L.; Liu, Y.; Peng, L.; Gao, X.; Wang, J. AtPPRT1, an E3 ubiquitin ligase, enhances the thermotolerance in Arabidopsis. Plants 2020, 9, 1074. [Google Scholar] [CrossRef] [PubMed]
- Pei, L.; Peng, L.; Wan, X.; Xiong, J.; Liu, Z.; Li, X.; Yang, Y.; Wang, J. Expression pattern and function analysis of AtPPRT1, a novel negative regulator in ABA and drought stress responses in Arabidopsis. Int. J. Mol. Sci. 2019, 20, 394. [Google Scholar] [CrossRef]
- Li, H.; Jiang, H.; Bu, Q.; Zhao, Q.; Sun, J.; Xie, Q.; Li, C. The Arabidopsis RING finger E3 ligase RHA2b acts additively with RHA2a in regulating abscisic acid signaling and drought response. Plant Physiol. 2011, 156, 550–563. [Google Scholar] [CrossRef]
- Zheng, Y.; Chen, Z.; Ma, L.; Liao, C. The ubiquitin E3 ligase RHA2b promotes degradation of MYB30 in abscisic acid signaling. Plant Physiol. 2018, 178, 428–440. [Google Scholar] [CrossRef]
- Ding, S.; Zhang, B.; Qin, F. Arabidopsis RZFP34/CHYR1, a ubiquitin E3 ligase, regulates stomatal movement and drought tolerance via SnRK2. 6-mediated phosphorylation. Plant Cell 2015, 27, 3228–3244. [Google Scholar] [CrossRef]
- Li, Q.; Serio, R.J.; Schofield, A.; Liu, H.; Rasmussen, S.R.; Hofius, D.; Stone, S.L. Arabidopsis RING--type E3 ubiquitin ligase XBAT35. 2 promotes proteasome--dependent degradation of ACD11 to attenuate abiotic stress tolerance. Plant J. 2020, 104, 1712–1723. [Google Scholar] [CrossRef]
- Kim, J.H.; Seo, H.U.; Lee, J.E.; Lim, S.D.; Jang, C.S. Heterogeneous Overexpression of Two Oryza sativa Arsenic-Induced RING E3 Ligase4 (OsAIR4. 1 and 4.2) Transcripts Enhances Plant Tolerance to Arsenic Stress. J. Plant Biol. 2021, 1–12. [Google Scholar] [CrossRef]
- Choi, J.; Lee, W.; An, G.; Kim, S.-R. OsCBE1, a Substrate Receptor of Cullin4-Based E3 Ubiquitin Ligase, Functions as a Regulator of Abiotic Stress Response and Productivity in Rice. Int. J. Mol. Sci. 2021, 22, 2487. [Google Scholar] [CrossRef] [PubMed]
- Park, G.-G.; Park, J.-J.; Yoon, J.; Yu, S.-N.; An, G. A RING finger E3 ligase gene, Oryza sativa Delayed Seed Germination 1 (OsDSG1), controls seed germination and stress responses in rice. Plant Mol. Biol. 2010, 74, 467–478. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.; Kim, S.K.; Cho, S.K.; Kang, B.G.; Kim, W.T. Overexpression of OsRDCP1, a rice RING domain-containing E3 ubiquitin ligase, increased tolerance to drought stress in rice (Oryza sativa L.). Plant Sci. 2011, 180, 775–782. [Google Scholar] [CrossRef]
- Ning, Y.; Jantasuriyarat, C.; Zhao, Q.; Zhang, H.; Chen, S.; Liu, J.; Liu, L.; Tang, S.; Park, C.H.; Wang, X. The SINA E3 ligase OsDIS1 negatively regulates drought response in rice. Plant Physiol. 2011, 157, 242–255. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.D.; Hwang, J.G.; Han, A.R.; Park, Y.C.; Lee, C.; Ok, Y.S.; Jang, C.S. Positive regulation of rice RING E3 ligase OsHIR1 in arsenic and cadmium uptakes. Plant Mol. Biol. 2014, 85, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Lim, S.D.; Jang, C.S. Oryza sativa heat-induced RING finger protein 1 (OsHIRP1) positively regulates plant response to heat stress. Plant Mol. Biol. 2019, 99, 545–559. [Google Scholar] [CrossRef]
- Park, Y.C.; Chapagain, S.; Jang, C.S. The microtubule-associated RING finger protein 1 (OsMAR1) acts as a negative regulator for salt-stress response through the regulation of OCPI2 (O. sativa chymotrypsin protease inhibitor 2). Planta 2018, 247, 875–886. [Google Scholar] [CrossRef]
- Zeng, D.-E.; Hou, P.; Xiao, F.; Liu, Y. Overexpressing a novel RING-H2 finger protein gene, OsRHP1, enhances drought and salt tolerance in rice (Oryza sativa L.). J. Plant Biol. 2014, 57, 357–365. [Google Scholar] [CrossRef]
- Gao, T.; Wu, Y.; Zhang, Y.; Liu, L.; Ning, Y.; Wang, D.; Tong, H.; Chen, S.; Chu, C.; Xie, Q. OsSDIR1 overexpression greatly improves drought tolerance in transgenic rice. Plant Mol. Biol. 2011, 76, 145–156. [Google Scholar] [CrossRef]
- Chapagain, S.; Jang, C.S. Heterogeneous overexpression of Oryza sativa salt induced RING Finger protein OsSIRF1 positively regulates salt and osmotic stress in transgenic Arabidopsis. In Proceedings of the Korean Society of Crop Science Conference, Jeju, Korea, 4–7 June 2017; p. 150. [Google Scholar]
- Hwang, S.G.; Kim, J.J.; Lim, S.D.; Park, Y.C.; Moon, J.C.; Jang, C.S. Molecular dissection of Oryza sativa salt--induced RING Finger Protein 1 (OsSIRP1): Possible involvement in the sensitivity response to salinity stress. Physiol. Plant. 2016, 158, 168–179. [Google Scholar] [CrossRef]
- Chapagain, S.; Park, Y.C.; Kim, J.H.; Jang, C.S. Oryza sativa salt-induced RING E3 ligase 2 (OsSIRP2) acts as a positive regulator of transketolase in plant response to salinity and osmotic stress. Planta 2018, 247, 925–939. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.C.; Moon, J.-C.; Chapagain, S.; Oh, D.G.; Kim, J.J.; Jang, C.S. Role of salt-induced RING finger protein 3 (OsSIRP3), a negative regulator of salinity stress response by modulating the level of its target proteins. Environ. Exp. Bot. 2018, 155, 21–30. [Google Scholar] [CrossRef]
- Kim, J.H.; Jang, C.S. E3 ligase, the Oryza sativa salt-induced RING finger protein 4 (OsSIRP4), negatively regulates salt stress responses via degradation of the OsPEX11-1 protein. Plant Mol. Biol. 2021, 105, 231–245. [Google Scholar] [CrossRef]
- Park, Y.C.; Lim, S.D.; Moon, J.C.; Jang, C.S. A rice really interesting new gene H 2--type E 3 ligase, OsSIRH2--14, enhances salinity tolerance via ubiquitin/26 S proteasome--mediated degradation of salt--related proteins. Plant Cell Environ. 2019, 42, 3061–3076. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Meng, Q.; Xu, J.; Tang, H.; Tang, S.; Zhang, H.; Huang, J. Knock-down of stress inducible OsSRFP1 encoding an E3 ubiquitin ligase with transcriptional activation activity confers abiotic stress tolerance through enhancing antioxidant protection in rice. Plant Mol. Biol. 2015, 87, 441–458. [Google Scholar] [CrossRef] [PubMed]
- Byun, M.Y.; Cui, L.H.; Oh, T.K.; Jung, Y.-J.; Lee, A.; Park, K.Y.; Kang, B.G.; Kim, W.T. Homologous U-box E3 ubiquitin ligases OsPUB2 and OsPUB3 are involved in the positive regulation of low temperature stress response in rice (Oryza sativa L.). Front. Plant Sci. 2017, 8, 16. [Google Scholar] [CrossRef]
- Park, J.J.; Yi, J.; Yoon, J.; Cho, L.H.; Ping, J.; Jeong, H.J.; Cho, S.K.; Kim, W.T.; An, G. OsPUB15, an E3 ubiquitin ligase, functions to reduce cellular oxidative stress during seedling establishment. Plant J. 2011, 65, 194–205. [Google Scholar] [CrossRef]
- Park, Y.C.; Chapagain, S.; Jang, C.S. A negative regulator in response to salinity in rice: Oryza sativa salt-, ABA-and drought-induced RING finger protein 1 (OsSADR1). Plant Cell Physiol. 2018, 59, 575–589. [Google Scholar] [CrossRef]
- Lourenço, T.; Sapeta, H.; Figueiredo, D.D.; Rodrigues, M.; Cordeiro, A.; Abreu, I.A.; Saibo, N.J.; Oliveira, M.M. Isolation and characterization of rice (Oryza sativa L.) E3-ubiquitin ligase OsHOS1 gene in the modulation of cold stress response. Plant Mol. Biol. 2013, 83, 351–363. [Google Scholar] [CrossRef]
- Cui, L.H.; Min, H.J.; Byun, M.Y.; Oh, H.G.; Kim, W.T. OsDIRP1, a putative RING E3 ligase, plays an opposite role in drought and cold stress responses as a negative and positive factor, respectively, in rice (Oryza sativa L.). Front. Plant Sci. 2018, 9, 1797. [Google Scholar] [CrossRef]
- Kim, J.H.; Lim, S.D.; Jang, C.S. Oryza sativa drought-, heat-, and salt-induced RING finger protein 1 (OsDHSRP1) negatively regulates abiotic stress-responsive gene expression. Plant Mol. Biol. 2020, 103, 235–252. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, L.; Zhang, L.; Lv, Q.; Zhao, Y.; Li, X. Isolation and identification of wheat gene TaDIS1 encoding a RING finger domain protein, which negatively regulates drought stress tolerance in transgenic Arabidopsis. Plant Sci. 2018, 275, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Wang, W.; Wang, W.; Zhang, G.; Liu, Y.; Wang, Y.; Wang, W. Wheat F-box protein gene TaFBA1 is involved in plant tolerance to heat stress. Front. Plant Sci. 2018, 9, 521. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Sun, X.; Yin, S.; Kong, X.; Zhou, S.; Xu, Y.; Luo, Y.; Wang, W. The role of the F-box gene TaFBA1 from wheat (Triticum aestivum L.) in drought tolerance. Plant Physiol. Biochem. 2014, 84, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.-M.; Kong, X.-Z.; Kang, H.-H.; Sun, X.-D.; Wang, W. The involvement of wheat F-box protein gene TaFBA1 in the oxidative stress tolerance of plants. PLoS ONE 2015, 10, e0122117. [Google Scholar] [CrossRef]
- Wang, W.; Wang, W.; Wu, Y.; Li, Q.; Zhang, G.; Shi, R.; Yang, J.; Wang, Y.; Wang, W. The involvement of wheat U--box E3 ubiquitin ligase TaPUB1 in salt stress tolerance. J. Integr. Plant Biol. 2020, 62, 631–651. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, M.; Zhao, Z.; Ren, Y.; Li, Q.; Wang, W. Wheat TaPUB1 modulates plant drought stress resistance by improving antioxidant capability. Sci. Rep. 2017, 7, 7549. [Google Scholar] [CrossRef]
- Zhang, G.; Yang, J.; Zhang, M.; Li, Q.; Wu, Y.; Zhao, X.; Zhang, H.; Wang, Y.; Wu, J.; Wang, W. Wheat TaPUB1 Regulates Cd Uptake and Tolerance by Promoting the Degradation of TaIRT1 and TaIAA17. J. Agric. Food Chem. 2021, 69, 5818–5829. [Google Scholar] [CrossRef]
- Li, Q.; Li, B.; Wang, J.; Chang, X.; Mao, X.; Jing, R. TaPUB15, a U--Box E3 ubiquitin ligase gene from wheat, enhances salt tolerance in rice. Food Energy Secur. 2021, 10, e250. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, W.; Li, Q.; Zhang, G.; Zhao, X.; Li, G.; Li, Y.; Wang, Y.; Wang, W. The wheat E3 ligase TaPUB26 is a negative regulator in response to salt stress in transgenic Brachypodium distachyon. Plant Sci. 2020, 294, 110441. [Google Scholar] [CrossRef]
- Zhang, N.; Yin, Y.; Liu, X.; Tong, S.; Xing, J.; Zhang, Y.; Pudake, R.N.; Izquierdo, E.M.; Peng, H.; Xin, M. The E3 ligase TaSAP5 alters drought stress responses by promoting the degradation of DRIP proteins. Plant Physiol. 2017, 175, 1878–1892. [Google Scholar] [CrossRef]
- Agarwal, P.; Khurana, P. TaZnF, a C3HC4 type RING zinc finger protein from Triticum aestivum is involved in dehydration and salinity stress. J. Plant Biochem. Biotechnol. 2020, 29, 395–406. [Google Scholar] [CrossRef]
- Joo, H.; Lim, C.W.; Lee, S.C. A pepper RING--type E3 ligase, CaASRF1, plays a positive role in drought tolerance via modulation of CaAIBZ1 stability. Plant J. 2019, 98, 5–18. [Google Scholar] [CrossRef] [PubMed]
- Baek, W.; Lim, C.W.; Lee, S.C. Pepper E3 ligase CaAIRE1 promotes ABA sensitivity and drought tolerance by degradation of protein phosphatase CaAITP1. J. Exp. Bot. 2021, 72, 4520–4534. [Google Scholar] [CrossRef] [PubMed]
- Park, C.; Lim, C.W.; Baek, W.; Lee, S.C. RING type E3 ligase CaAIR1 in pepper acts in the regulation of ABA signaling and drought stress response. Plant Cell Physiol. 2015, 56, 1808–1819. [Google Scholar] [CrossRef][Green Version]
- Joo, H.; Lim, C.W.; Lee, S.C. Identification and functional expression of the pepper RING type E3 ligase, CaDTR1, involved in drought stress tolerance via ABA-mediated signalling. Sci. Rep. 2016, 6, 30097. [Google Scholar] [CrossRef]
- Joo, H.; Lim, C.W.; Lee, S.C. The pepper RING--type E3 ligase, CaATIR1, positively regulates abscisic acid signalling and drought response by modulating the stability of CaATBZ1. Plant Cell Environ. 2020, 43, 1911–1924. [Google Scholar] [CrossRef]
- Lim, C.W.; Park, C.; Kim, J.-H.; Joo, H.; Hong, E.; Lee, S.C. Pepper CaREL1, a ubiquitin E3 ligase, regulates drought tolerance via the ABA-signalling pathway. Sci. Rep. 2017, 7, 477. [Google Scholar] [CrossRef]
- Joo, H.; Lim, C.W.; Han, S.-W.; Lee, S.C. The pepper RING finger E3 ligase, CaDIR1, regulates the drought stress response via ABA-mediated signaling. Front. Plant Sci. 2017, 8, 690. [Google Scholar] [CrossRef]
- Lim, C.W.; Baek, W.; Lee, S.C. The pepper RING-type E3 ligase CaAIRF1 regulates ABA and drought signaling via CaADIP1 protein phosphatase degradation. Plant Physiol. 2017, 173, 2323–2339. [Google Scholar] [CrossRef]
- Yang, L.; Wu, L.; Chang, W.; Li, Z.; Miao, M.; Li, Y.; Yang, J.; Liu, Z.; Tan, J. Overexpression of the maize E3 ubiquitin ligase gene ZmAIRP4 enhances drought stress tolerance in Arabidopsis. Plant Physiol. Biochem. 2018, 123, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Xia, Z.; Liu, Q.; Wu, J.; Ding, J. ZmRFP1, the putative ortholog of SDIR1, encodes a RING-H2 E3 ubiquitin ligase and responds to drought stress in an ABA-dependent manner in maize. Gene 2012, 495, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zhang, X.; Zhu, S.; Zhang, H.; Li, Y.; Zhang, T.; Sun, J. Overexpression of GhSARP1 encoding a E3 ligase from cotton reduce the tolerance to salt in transgenic Arabidopsis. Biochem. Biophys. Res. Commun. 2016, 478, 1491–1496. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Li, C.X.; Li, X.; Liu, A.; Chen, S.; Zhou, J. Overexpression of tomato RING E3 ubiquitin ligase gene SlRING1 confers cadmium tolerance by attenuating cadmium accumulation and oxidative stress. Physiol. Plant. 2021, 173, 449–459. [Google Scholar] [CrossRef]
- Zhang, R.; Chen, H.; Duan, M.; Zhu, F.; Wen, J.; Dong, J.; Wang, T. Medicago falcata MfSTMIR, an E3 ligase of endoplasmic reticulum--associated degradation, is involved in salt stress response. Plant J. 2019, 98, 680–696. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.; Lin, Q.; Zhu, H.; Gao, F.; Zhang, W.; Hua, X. The RING finger E3 ligase SpRing is a positive regulator of salt stress signaling in salt-tolerant wild tomato species. Plant Cell Physiol. 2016, 57, 528–539. [Google Scholar] [CrossRef]
- An, J.-P.; Liu, X.; Song, L.-Q.; You, C.-X.; Wang, X.-F.; Hao, Y.-J. Apple RING finger E3 ubiquitin ligase MdMIEL1 negatively regulates salt and oxidative stresses tolerance. J. Plant Biol. 2017, 60, 137–145. [Google Scholar] [CrossRef]
- Wang, N.; Liu, Y.; Cong, Y.; Wang, T.; Zhong, X.; Yang, S.; Li, Y.; Gai, J. Genome-wide identification of soybean U-box E3 ubiquitin ligases and roles of GmPUB8 in negative regulation of drought stress response in Arabidopsis. Plant Cell Physiol. 2016, 57, 1189–1209. [Google Scholar] [CrossRef]
- Wang, N.; Liu, Y.; Cai, Y.; Tang, J.; Li, Y.; Gai, J. The soybean U-box gene GmPUB6 regulates drought tolerance in Arabidopsis thaliana. Plant Physiol. Biochem. 2020, 155, 284–296. [Google Scholar] [CrossRef]
- Yu, J.; Kang, L.; Li, Y.; Wu, C.; Zheng, C.; Liu, P.; Huang, J. RING finger protein RGLG1 and RGLG2 negatively modulate MAPKKK18 mediated drought stress tolerance in Arabidopsis. J. Integr. Plant Biol. 2021, 63, 484–493. [Google Scholar] [CrossRef]
- Wu, Q.; Zhang, X.; Peirats-Llobet, M.; Belda-Palazon, B.; Wang, X.; Cui, S.; Yu, X.; Rodriguez, P.L.; An, C. Ubiquitin ligases RGLG1 and RGLG5 regulate abscisic acid signaling by controlling the turnover of phosphatase PP2CA. Plant Cell 2016, 28, 2178–2196. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, E.-J.; Cheng, M.-C.; Lin, T.-P. Functional characterization of an abiotic stress-inducible transcription factor AtERF53 in Arabidopsis thaliana. Plant Mol. Biol. 2013, 82, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Tajdel, M.; Mituła, F.; Ludwików, A. Regulation of Arabidopsis MAPKKK18 by ABI1 and SnRK2, components of the ABA signaling pathway. Plant Signal. Behav. 2016, 11, e1139277. [Google Scholar] [CrossRef]
- Li, Y.; Cai, H.; Liu, P.; Wang, C.; Gao, H.; Wu, C.; Yan, K.; Zhang, S.; Huang, J.; Zheng, C. Arabidopsis MAPKKK18 positively regulates drought stress resistance via downstream MAPKK3. Biochem. Biophys. Res. Commun. 2017, 484, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, N.; Sarkeshik, A.; Nito, K.; Park, S.Y.; Wang, A.; Carvalho, P.C.; Lee, S.; Caddell, D.F.; Cutler, S.R.; Chory, J. PYR/PYL/RCAR family members are major in--vivo ABI1 protein phosphatase 2C--interacting proteins in Arabidopsis. Plant J. 2010, 61, 290–299. [Google Scholar] [CrossRef] [PubMed]
- Belda--Palazon, B.; Julian, J.; Coego, A.; Wu, Q.; Zhang, X.; Batistic, O.; Alquraishi, S.A.; Kudla, J.; An, C.; Rodriguez, P.L. ABA inhibits myristoylation and induces shuttling of the RGLG 1 E3 ligase to promote nuclear degradation of PP 2 CA. Plant J. 2019, 98, 813–825. [Google Scholar] [CrossRef] [PubMed]
- Qin, F.; Sakuma, Y.; Tran, L.-S.P.; Maruyama, K.; Kidokoro, S.; Fujita, Y.; Fujita, M.; Umezawa, T.; Sawano, Y.; Miyazono, K.-i. Arabidopsis DREB2A-interacting proteins function as RING E3 ligases and negatively regulate plant drought stress–responsive gene expression. Plant Cell 2008, 20, 1693–1707. [Google Scholar] [CrossRef]
- Sakuma, Y.; Maruyama, K.; Osakabe, Y.; Qin, F.; Seki, M.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Functional analysis of an Arabidopsis transcription factor, DREB2A, involved in drought-responsive gene expression. Plant Cell 2006, 18, 1292–1309. [Google Scholar] [CrossRef]
- Wu, F.; Chi, Y.; Jiang, Z.; Xu, Y.; Xie, L.; Huang, F.; Wan, D.; Ni, J.; Yuan, F.; Wu, X. Hydrogen peroxide sensor HPCA1 is an LRR receptor kinase in Arabidopsis. Nature 2020, 578, 577–581. [Google Scholar] [CrossRef]
- Isner, J.C.; Begum, A.; Nuehse, T.; Hetherington, A.M.; Maathuis, F.J. KIN7 kinase regulates the vacuolar TPK1 K+ channel during stomatal closure. Curr. Biol. 2018, 28, 466–472. [Google Scholar] [CrossRef]
- Um, T.Y.; Lee, S.; Kim, J.-K.; Jang, G.; Do Choi, Y. Chloride Channel 1 promotes drought tolerance in rice, leading to increased grain yield. Plant Biotechnol. Rep. 2018, 12, 283–293. [Google Scholar] [CrossRef]
- Chinnusamy, V.; Zhu, J.; Zhu, J.-K. Salt stress signaling and mechanisms of plant salt tolerance. Genet. Eng. 2006, 27, 141–177. [Google Scholar]
- Isayenkov, S. Physiological and molecular aspects of salt stress in plants. Cytol. Genet. 2012, 46, 302–318. [Google Scholar] [CrossRef]
- Muchate, N.S.; Nikalje, G.C.; Rajurkar, N.S.; Suprasanna, P.; Nikam, T.D. Plant salt stress: Adaptive responses, tolerance mechanism and bioengineering for salt tolerance. Bot. Rev. 2016, 82, 371–406. [Google Scholar] [CrossRef]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef]
- Ashraf, M.; Harris, P.J. Photosynthesis under stressful environments: An overview. Photosynthetica 2013, 51, 163–190. [Google Scholar] [CrossRef]
- Khozaei, M.; Fisk, S.; Lawson, T.; Gibon, Y.; Sulpice, R.; Stitt, M.; Lefebvre, S.C.; Raines, C.A. Overexpression of plastid transketolase in tobacco results in a thiamine auxotrophic phenotype. Plant Cell 2015, 27, 432–447. [Google Scholar] [CrossRef]
- Suzuki, Y.; Kondo, E.; Makino, A. Effects of co-overexpression of the genes of Rubisco and transketolase on photosynthesis in rice. Photosynth. Res. 2017, 131, 281–289. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Hossain, M.A.; da Silva, J.A.T.; Fujita, M. Plant response and tolerance to abiotic oxidative stress: Antioxidant defense is a key factor. In Crop Stress and its Management: Perspectives and Strategies; Springer: Berlin/Heidelberg, Germany, 2012; pp. 261–315. [Google Scholar]
- Xie, X.; He, Z.; Chen, N.; Tang, Z.; Wang, Q.; Cai, Y. The roles of environmental factors in regulation of oxidative stress in plant. BioMed Res. Int. 2019, 2019, 9732325. [Google Scholar] [CrossRef]
- Sharma, P.; Dubey, R. Involvement of oxidative stress and role of antioxidative defense system in growing rice seedlings exposed to toxic concentrations of aluminum. Plant Cell Rep. 2007, 26, 2027–2038. [Google Scholar] [CrossRef]
- Luo, C.; Cai, X.-T.; Du, J.; Zhao, T.-L.; Wang, P.-F.; Zhao, P.-X.; Liu, R.; Xie, Q.; Cao, X.-F.; Xiang, C.-B. Paraquat Tolerance3 is an E3 ligase that switches off activated oxidative response by targeting histone-modifying Protein Methyltransferase4b. PLoS ONE Genet. 2016, 12, e1006332. [Google Scholar] [CrossRef] [PubMed]
- Koussevitzky, S.; Suzuki, N.; Huntington, S.; Armijo, L.; Sha, W.; Cortes, D.; Shulaev, V.; Mittler, R. Ascorbate peroxidase 1 plays a key role in the response of Arabidopsis thaliana to stress combination. J. Biol. Chem. 2008, 283, 34197–34203. [Google Scholar] [CrossRef] [PubMed]
- Roxas, V.P.; Lodhi, S.A.; Garrett, D.K.; Mahan, J.R.; Allen, R.D. Stress tolerance in transgenic tobacco seedlings that overexpress glutathione S-transferase/glutathione peroxidase. Plant Cell Physiol. 2000, 41, 1229–1234. [Google Scholar] [CrossRef]
- Zinn, K.E.; Tunc-Ozdemir, M.; Harper, J.F. Temperature stress and plant sexual reproduction: Uncovering the weakest links. J. Exp. Bot. 2010, 61, 1959–1968. [Google Scholar] [CrossRef] [PubMed]
- DiPaola, J.; Beard, J. Physiological effects of temperature stress. Turfgrass 1992, 32, 231–267. [Google Scholar]
- Shah, F.; Huang, J.; Cui, K.; Nie, L.; Shah, T.; Chen, C.; Wang, K. Impact of high-temperature stress on rice plant and its traits related to tolerance. J. Agric. Sci. 2011, 149, 545–556. [Google Scholar] [CrossRef]
- Qu, A.-L.; Ding, Y.-F.; Jiang, Q.; Zhu, C. Molecular mechanisms of the plant heat stress response. Biochem. Biophys. Res. Commun. 2013, 432, 203–207. [Google Scholar] [CrossRef]
- Beck, E.H.; Heim, R.; Hansen, J. Plant resistance to cold stress: Mechanisms and environmental signals triggering frost hardening and dehardening. J. Biosci. 2004, 29, 449–459. [Google Scholar] [CrossRef]
- Adam, S.; Murthy, S. Effect of cold stress on photosynthesis of plants and possible protection mechanisms. In Approaches to Plant Stress and Their Management; Springer: Berlin/Heidelberg, Germany, 2014; pp. 219–226. [Google Scholar]
- Larkindale, J.; Vierling, E. Core genome responses involved in acclimation to high temperature. Plant Physiol. 2008, 146, 748–761. [Google Scholar] [CrossRef]
- Sedaghatmehr, M.; Mueller-Roeber, B.; Balazadeh, S. The plastid metalloprotease FtsH6 and small heat shock protein HSP21 jointly regulate thermomemory in Arabidopsis. Nat. Commun. 2016, 7, 12439. [Google Scholar] [CrossRef]
- Davletova, S.; Schlauch, K.; Coutu, J.; Mittler, R. The zinc-finger protein Zat12 plays a central role in reactive oxygen and abiotic stress signaling in Arabidopsis. Plant Physiol. 2005, 139, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Turóczy, Z.; Kis, P.; Török, K.; Cserháti, M.; Lendvai, A.; Dudits, D.; Horváth, G.V. Overproduction of a rice aldo–keto reductase increases oxidative and heat stress tolerance by malondialdehyde and methylglyoxal detoxification. Plant Mol. Biol. 2011, 75, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.-H.; Cao, P.; Seo, Y.-S.; Dardick, C.; Ronald, P.C. The Rice Kinase Phylogenomics Database: A guide for systematic analysis of the rice kinase super-family. Trends Plant Sci. 2010, 15, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Vazquez-Hernandez, M.; Romero, I.; Escribano, M.I.; Merodio, C.; Sanchez-Ballesta, M.T. Deciphering the role of CBF/DREB transcription factors and dehydrins in maintaining the quality of table grapes cv. autumn royal treated with high CO2 levels and stored at 0 C. Front. Plant Sci. 2017, 8, 1591. [Google Scholar] [CrossRef] [PubMed]
- Robison, J.D.; Yamasaki, Y.; Randall, S.K. The ethylene signaling pathway negatively impacts CBF/DREB-regulated cold response in soybean (Glycine max). Front. Plant Sci. 2019, 10, 121. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Ding, Y.; Yang, S. Molecular regulation of CBF signaling in cold acclimation. Trends Plant Sci. 2018, 23, 623–637. [Google Scholar] [CrossRef]
- Chinnusamy, V.; Ohta, M.; Kanrar, S.; Lee, B.-h.; Hong, X.; Agarwal, M.; Zhu, J.-K. ICE1: A regulator of cold-induced transcriptome and freezing tolerance in Arabidopsis. Genes Dev. 2003, 17, 1043–1054. [Google Scholar] [CrossRef]
- Fan, Z.-Q.; Chen, J.-Y.; Kuang, J.-F.; Lu, W.-J.; Shan, W. The banana fruit SINA ubiquitin ligase MaSINA1 regulates the stability of MaICE1 to be negatively involved in cold stress response. Front. Plant Sci. 2017, 8, 995. [Google Scholar] [CrossRef]
- Amm, I.; Sommer, T.; Wolf, D.H. Protein quality control and elimination of protein waste: The role of the ubiquitin–proteasome system. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2014, 1843, 182–196. [Google Scholar] [CrossRef]
- Goldberg, A.L. Protein degradation and protection against misfolded or damaged proteins. Nature 2003, 426, 895–899. [Google Scholar] [CrossRef]
- Tamás, M.J.; Sharma, S.K.; Ibstedt, S.; Jacobson, T.; Christen, P. Heavy metals and metalloids as a cause for protein misfolding and aggregation. Biomolecules 2014, 4, 252–267. [Google Scholar] [CrossRef] [PubMed]
- Farid, M.; Shakoor, M.B.; Ehsan, S.; Ali, S.; Zubair, M.; Hanif, M. Morphological, physiological and biochemical responses of different plant species to Cd stress. Int. J. Chem. Biochem. Sci. 2013, 3, 53–60. [Google Scholar]
- Bielen, A.; Remans, T.; Vangronsveld, J.; Cuypers, A. The influence of metal stress on the availability and redox state of ascorbate, and possible interference with its cellular functions. Int. J. Mol. Sci. 2013, 14, 6382–6413. [Google Scholar] [CrossRef]
- Emamverdian, A.; Ding, Y.; Mokhberdoran, F.; Xie, Y. Heavy metal stress and some mechanisms of plant defense response. Sci. World J. 2015, 2015, 756120. [Google Scholar] [CrossRef] [PubMed]
- Schutzendubel, A.; Polle, A. Plant responses to abiotic stresses: Heavy metal--induced oxidative stress and protection by mycorrhization. J. Exp. Bot. 2002, 53, 1351–1365. [Google Scholar] [CrossRef] [PubMed]
- Rascio, N.; Navari-Izzo, F. Heavy metal hyperaccumulating plants: How and why do they do it? And what makes them so interesting? Plant Sci. 2011, 180, 169–181. [Google Scholar] [CrossRef] [PubMed]
- Xiang, M.; Li, Y.; Yang, J.; Lei, K.; Li, Y.; Li, F.; Zheng, D.; Fang, X.; Cao, Y. Heavy metal contamination risk assessment and correlation analysis of heavy metal contents in soil and crops. Environ. Pollut. 2021, 278, 116911. [Google Scholar] [CrossRef]
- Kaur, R.; Das, S.; Bansal, S.; Singh, G.; Sardar, S.; Dhar, H.; Ram, H. Heavy metal stress in rice: Uptake, transport, signaling, and tolerance mechanisms. Physiol. Plant. 2021, 173, 430–448. [Google Scholar] [CrossRef]
- Taiz, L. The plant vacuole. J. Exp. Biol. 1992, 172, 113–122. [Google Scholar] [CrossRef]
- Li, G.-W.; Peng, Y.-H.; Yu, X.; Zhang, M.-H.; Cai, W.-M.; Sun, W.-N.; Su, W.-A. Transport functions and expression analysis of vacuolar membrane aquaporins in response to various stresses in rice. J. Plant Physiol. 2008, 165, 1879–1888. [Google Scholar] [CrossRef]
- Meharg, A.A.; Jardine, L. Arsenite transport into paddy rice (Oryza sativa) roots. New Phytol. 2003, 157, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Vert, G.; Grotz, N.; Dédaldéchamp, F.; Gaymard, F.; Guerinot, M.L.; Briat, J.-F.; Curie, C. IRT1, an Arabidopsis transporter essential for iron uptake from the soil and for plant growth. Plant Cell 2002, 14, 1223–1233. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.K.; Fang, X.Z.; Guan, M.Y.; Ye, Y.Q.; Lin, X.Y.; Du, S.T.; Jin, C.W. Exogenous abscisic acid application decreases cadmium accumulation in Arabidopsis plants, which is associated with the inhibition of IRT1-mediated cadmium uptake. Front. Plant Sci. 2014, 5, 721. [Google Scholar] [CrossRef]
- Han, M.; Park, Y.; Kim, I.; Kim, E.-H.; Yu, T.-K.; Rhee, S.; Suh, J.-Y. Structural basis for the auxin-induced transcriptional regulation by Aux/IAA17. Proc. Natl. Acad. Sci. USA 2014, 111, 18613–18618. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Qin, S.; Rengel, Z.; Gao, W.; Nie, Z.; Liu, H.; Li, C.; Zhao, P. Cadmium stress increases antioxidant enzyme activities and decreases endogenous hormone concentrations more in Cd-tolerant than Cd-sensitive wheat varieties. Ecotoxicol. Environ. Saf. 2019, 172, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.M.; Huang, X. Inhibition of root meristem growth by cadmium involves nitric oxide--mediated repression of auxin accumulation and signalling in Arabidopsis. Plant Cell Environ. 2016, 39, 120–135. [Google Scholar] [CrossRef]
- Corguinha, A.P.B.; de Souza, G.A.; Gonçalves, V.C.; de Andrade Carvalho, C.; de Lima, W.E.A.; Martins, F.A.D.; Yamanaka, C.H.; Francisco, E.A.B.; Guilherme, L.R.G. Assessing arsenic, cadmium, and lead contents in major crops in Brazil for food safety purposes. J. Food Compos. Anal. 2015, 37, 143–150. [Google Scholar] [CrossRef]
- Khan, M.U.; Malik, R.N.; Muhammad, S. Human health risk from heavy metal via food crops consumption with wastewater irrigation practices in Pakistan. Chemosphere 2013, 93, 2230–2238. [Google Scholar] [CrossRef]
- Struk, S.; Jacobs, A.; Sánchez Martín--Fontecha, E.; Gevaert, K.; Cubas, P.; Goormachtig, S. Exploring the protein–protein interaction landscape in plants. Plant Cell Environ. 2019, 42, 387–409. [Google Scholar] [CrossRef]



| Species | Name | Kind | Abiotic Stress Function | Citation |
|---|---|---|---|---|
| Arabidopsis | RGLG1 RGLG2 RING DOMAIN LIGASE | RING finger domain E3 ubiquitin ligase | Negatively regulates drought stress tolerance | [76] |
| DOR DROUGHT TOLERANCE REPRESSOR | Skp1–Cullin–F-box (SCF) RING finger E3 ligase | Negatively regulates ABA-dependent drought tolerance | [77] | |
| AFA1 ARABIDOPSIS F-BOX PROTEIN HYPERSENSITIVE TO ABA 1 | Skp1–Cullin–F-box (SCF) RING finger E3 ligase | Negatively regulates ABA-dependent drought tolerance | [78] | |
| SDR SALT AND DROUGHT RESPONSIVENESS | Skp1–Cullin–F-box (SCF) RING finger E3 ligase | Positively regulate salt stress tolerance, negatively regulate drought stress tolerance | [79] | |
| SAP5 STRESS ASSOCIATED PROTEIN 5 | RING finger domain E3 ubiquitin ligase | Positively regulates salt, drought, and osmotic stress tolerance | [80] | |
| AIRP1 AIRP2 ABA-INSENSITIVE RING PROTEIN | C3H2C3-type RING finger E3 ubiquitin ligase | Positively regulates ABA-dependent drought tolerance | [81,82] | |
| AIRP3/LOG2 ABA-INSENSITIVE RING PROTEIN3/LOSS OF GLUTAMINE DUMPER 2 | RING finger domain E3 ubiquitin ligase | Positively regulates the ABA-mediated drought and salt stress tolerance | [83] | |
| ATRF1 ALUMINUM TOLERANCE RING FINGER 1 | C3H2C3-type RING finger E3 ubiquitin ligase | Positively regulates aluminum tolerance | [84] | |
| NERF NFYA5 ENHANCING RING FINGER | RING finger domain E3 ubiquitin ligase | Positively regulates drought stress response | [85] | |
| RDUF1 RDUF2 RING DOMAIN OF UNKNOWN FUNCTION | RING finger domain E3 ubiquitin ligase | Positively regulates salt stress responses and ABA-dependent drought stress responses in arabidopsis | [84,86] | |
| PQ3 PARAQUAT TOLERANCE 3 | U-box E3 ubiquitin ligase | Negatively regulates oxidative stress response | [87] | |
| PP2-B11 PHLOEM PROTEIN 2-B11 | Skp1–Cullin–F-box (SCF) RING finger E3 ligase | Negatively regulates drought stress and positively regulate salt stress | [88,89] | |
| PUB11 PLANT U-BOX 11 | U-box E3 ubiquitin ligase | Negatively regulates drought tolerance | [67] | |
| PUB19 PLANT U-BOX 19 | U-box E3 ubiquitin ligase | Negatively regulates drought stress tolerance | [90] | |
| PUB22 PUB23 PLANT U-BOX | U-box E3 ubiquitin ligase | Negatively regulates drought stress tolerance | [66] | |
| PUB30 PLANT U-BOX 30 | U-box E3 ubiquitin ligase | Negatively regulates the salt stress tolerance during germination | [91] | |
| PUB46 PLANT U-BOX | U-box E3 ubiquitin ligase | Positively regulates oxidative stress tolerance | [92] | |
| PUB46 PUB48 PLANT U-BOX | U-box E3 ubiquitin ligase | Positively regulate drought stress tolerance | [93] | |
| PUB48 PLANT U-BOX 48 | U-box E3 ubiquitin ligase | Positively regulates heat tolerance | [94] | |
| SDIR1 SALT- AND DROUGHT-INDUCED RING FINGER1 | RING finger domain E3 ubiquitin ligase | Positively regulates ABA dependent salt and drought stress tolerance | [95] | |
| PRU1 PHOSPHATE RESPONSE UBIQUITIN E3 LIGASE1 | RING finger domain E3 ubiquitin ligase | Modulates Pi homeostasis in response to low-Pi stress in Arabidopsis | [96] | |
| HOS1 HIGH EXPRESSION OF OSMOTICALLY RESPONSIVE GENE 1 | RING finger domain E3 ubiquitin ligase | Negatively regulates the cold stress response in Arabidopsis | [97,98] | |
| DUF1 DUF2 DOMAIN OF UNKNOWN FUNCTION | RING finger domain E3 ubiquitin ligase | Positively regulates ABA dependent drought stress response | [99] | |
| ATL61 ATL78 ARABIDOPSIS TÓXICOS EN LEVADURA | C3H2C3 RING finger domain E3 ubiquitin ligase | Negatively regulates cold stress response and a positively regulates drought stress response | [100,101] | |
| STRF1 SALT TOLERANCE RING FINGER 1 | RING finger domain E3 ubiquitin ligase | Positively regulates salt stress | [102] | |
| JUL1 JAV1-ASSOCIATED UBIQUITIN LIGASE1 | C3H2C3 RING finger domain E3 ubiquitin ligase | Positively regulates drought stress | [103] | |
| SR1 SUBMERGENCE RESISTANT1 | RING finger domain E3 ubiquitin ligase | Negatively regulates submergence tolerance | [104] | |
| PPRT1 PROTEIN WITH THE RING DOMAIN AND TMEMB 1 | C3H2C3 RING finger domain E3 ubiquitin ligase | Negatively regulates salt stress response. positive role in regulating the high temperature. Negative role in ABA and drought stress responses | [105,106,107] | |
| RHA2B RHA2A RING-H2 FINGER PROTEIN 2B/2A | RING finger domain E3 ubiquitin ligase | Positively regulates ABA-dependent drought response | [108,109] | |
| RZP34/CHYR1 RING ZINC-FINGER PROTEIN34/CHY ZINC-FINGER AND RING PROTEIN1 | RING finger domain E3 ubiquitin ligase | Positively regulates drought stress tolerant | [110] | |
| XBAT35.2 XB3 ORTHOLOG 5 IN ARABIDOPSIS THALIANA | RING finger domain E3 ubiquitin ligase | Negatively regulates the drought and salt stress response | [111] | |
| Rice Oryza sativa | AIR4.1 AIR4.2 ARSENIC-INDUCED RING E3 LIGASE4 | RING finger domain E3 ubiquitin ligase | Positively regulates Arsenic stress tolerance | [112] |
| CBE1 CULLIN4-BASED E3 UBIQUITIN LIGASE1 | Cullin4-Based E3 Ubiquitin Ligase | Negatively regulates abiotic stress tolerance | [113] | |
| DSG1 DELAYED SEED GERMINATION 1 | RING finger domain E3 ubiquitin ligase | Negatively regulates salt and drought stress tolerance | [114] | |
| RDCPS RING DOMAIN-CONTAINING PROTEINS | RING finger domain E3 ubiquitin ligase | Positively regulates drought stress tolerant | [115] | |
| DIS1 DROUGHT-INDUCED SINA PROTEIN 1 | C3HC4 RING finger domain E3 ubiquitin ligase | Negatively Regulates drought response | [116] | |
| HIR1 HEAVY METAL INDUCED RING E3 LIGASE 1 | RING finger domain E3 ubiquitin ligase | Positively regulates heavy metal tolerance | [117] | |
| HIRP1 HEAT INDUCED RING FINGER PROTEIN 1 | RING finger domain E3 ubiquitin ligase | Positively regulates plant response to heat stress | [118] | |
| MAR1 MICROTUBULE-ASSOCIATED RING FINGER PROTEIN 1 | RING finger domain E3 ubiquitin ligase | Negatively regulates the salt-stress response | [119] | |
| RHP1 RING-H2 FINGER PROTEIN 1 | RING finger domain E3 ubiquitin ligase | Positively regulates salt and drought stress tolerance | [120] | |
| SDIR1 SALT-AND DROUGHT-INDUCED RING FINGER 1 | RING finger domain E3 ubiquitin ligase | Positively regulates drought stress tolerance | [121] | |
| SIRF1 SALT INDUCED RING FINGER PROTEIN | RING finger domain E3 ubiquitin ligase | Positively regulates salt and osmotic stress | [122] | |
| SIRP1 SALT-INDUCED RING PROTEIN 1 | RING finger domain E3 ubiquitin ligase | Negatively regulates of salinity stress tolerance | [123] | |
| SIRP2 SALT-INDUCED RING PROTEIN 2 | RING finger domain E3 ubiquitin ligase | Positively regulates of salt and osmotic stress tolerance | [124] | |
| SIRP3 SALT-INDUCED RING PROTEIN 3 | RING finger domain E3 ubiquitin ligase | Negatively regulates salinity stress response | [125] | |
| SIRP4 SALT-INDUCED RING PROTEIN 4 | RING finger domain E3 ubiquitin ligase | Negatively regulates salt stress responses | [126] | |
| SIRH2- 14 SALT-INSENSITIVE RING-H2 TYPE 14 | RING finger domain E3 ubiquitin ligase | Positively regulates salt stress tolerance | [127] | |
| SRFP1 STRESS-RELATED RING FINGER PROTEIN 1 | RING finger domain E3 ubiquitin ligase | Negatively regulates salt, cold and oxidative stresses | [128] | |
| PUB2 PUB3 PLANT U-BOX 2AND 3 | U-box E3 ubiquitin ligase | Positively regulates the response to cold stress | [129] | |
| PUB15 PLANT U-BOX 15 | U-box E3 ubiquitin ligase | Positively regulates oxidative stress tolerance | [130] | |
| PUB41 PLANT U-BOX41 | U-box E3 ubiquitin ligase | Negatively regulates drought stress response | [65] | |
| SADR1 SALT, ABA, AND DROUGHT STRESS-INDUCED RING FINGER PROTEIN 1 | RING finger domain E3 ubiquitin ligase | Negatively Regulates Response to Salinity and drought stress | [131] | |
| HOS1 HIGH EXPRESSION OF OSMOTICALLY RESPONSIVE GENES 1 | RING finger domain E3 ubiquitin ligase | Negatively regulates the cold stress response | [132] | |
| DIRP1 DROUGHT-INDUCED RING PROTEIN 1 | RING finger domain E3 ubiquitin ligase | Negatively regulates drought and salt stress, and positively regulates cold stress response in rice | [133] | |
| DHSRP1 DROUGHT-HEAT-SALT INDUCED RING FINGER PROTEIN 1 | RING finger domain E3 ubiquitin ligase | Negatively regulates drought, heat, and salt stress tolerance | [134] | |
| Wheat Triticum aestivum | DIS1 DROUGHT-INDUCED SINA PROTEIN 1 | C3HC4 RING finger domain E3 ubiquitin ligase | Negatively regulates drought stress tolerance | [135] |
| FBA1 FBOX-AMN1 | Skp1–Cullin–F-box (SCF) RING finger E3 ligase | Positively regulates heat and drought stress in wheat. Positively regulates oxidative stress | [136,137,138] | |
| PUB1 PLANT U-BOX 1 | U-box E3 ubiquitin ligase | Positively regulates salt and drought stress tolerance in wheat. Positively regulate Cadmium stress tolerance | [139,140,141] | |
| PUB15 PLANT U-BOX 15 | U-box E3 ubiquitin ligase | Positively regulates salt stress tolerance in wheat | [142] | |
| PUB26 PLANT U-BOX 26 | U-box E3 ubiquitin ligase | Negatively regulates salt stress in wheat | [143] | |
| SAP5 STRESS-ASSOCIATED PROTEIN | RING finger domain E3 ubiquitin ligase | Positively regulates drought stress | [144] | |
| ZNF ZINC-FINGER PROTEIN | C3HC4 RING finger domain E3 ubiquitin ligase | Positively regulates salt and drought stress | [145] | |
| Pepper Capsicum annuum | ASRF1 ABA SENSITIVE RING FINGER E3 LIGASE 1 | RING finger domain E3 ubiquitin ligase | Positively regulates drought stress tolerance in pepper | [146] |
| AIRE1 ABA INDUCED RING-TYPE E3 LIGASE 1 | RING finger domain E3 ubiquitin ligase | Positively regulates the drought stress response in pepper | [147] | |
| AIR1 ABA-INSENSITIVE RING PROTEIN 1 GENE | RING finger domain E3 ubiquitin ligase | Negatively regulates the ABA-mediated drought stress tolerance mechanism | [148] | |
| DTR1 DROUGHT TOLERANCE RING 1 | RING finger domain E3 ubiquitin ligase | Positively regulates the drought stress response in pepper | [149] | |
| ATIR1 ATBZ1 INTERACTING RING FINGER PROTEIN 1 | RING finger domain E3 ubiquitin ligase | Positively regulates abscisic acid signaling and drought response | [150] | |
| REL1 RING TYPE E3 LIGASE 1 GENE | RING finger domain E3 ubiquitin ligase | Negatively regulates ABA-mediated drought stress tolerance | [151] | |
| DIR1 DROUGHT INDUCED RING TYPE E3 LIGASE 1 | RING finger domain E3 ubiquitin ligase | Negatively regulates the drought stress response via ABA-mediated signaling | [152] | |
| AIRF1 ADIP1 INTERACTING RING FINGER PROTEIN 1 | RING finger domain E3 ubiquitin ligase | Positively regulates the drought stress response in pepper | [153] | |
| Corn Zea maiz | AIRP4: ZEA MAYS ABA INSENSITIVE RING PROTEIN 4 | RING finger domain E3 ubiquitin ligase | Positively regulates the drought tolerance response pathway | [154] |
| RFP1 RING FINGER PROTEIN 1 | RING finger domain E3 ubiquitin ligase | Positively regulates salt and drought stress tolerance | [155] | |
| Cotton Gossypium hirsutum | SARP1 SALT-ASSOCIATED RING FINGER PROTEIN | C3H2C3 RING finger domain E3 ubiquitin ligase | Negatively regulate the response to salt stress | [156] |
| Tomato Solanum lycopersicum | RING1REALLY INTERESTING NEW GENE 1 | RING finger domain E3 ubiquitin ligase | Positively regulates Cd tolerance | [157] |
| Sickle medick Medicago falcata | STMIR SALT TUNICAMYCIN-INDUCED RING FINGER PROTEIN | RING finger domain E3 ubiquitin ligase | Positively regulates salt stress | [158] |
| Wild tomato Solanum pimpinellifolium | RING REALLY INTERESTING NEW GENE | RING finger domain E3 ubiquitin ligase | Positively regulates salt stress in wild tomato species | [159] |
| Apple Malus domestica | MIEL MYB30-INTERACTING E3 LIGASE 1 | RING finger domain E3 ubiquitin ligase | Negatively regulates salt and oxidative stresses tolerance | [160] |
| Soybean Glycine max | PUB6 PUB8 PLANT U-BOX | U-box E3 ubiquitin ligase | Negative regulator of drought stress response in Arabidopsis | [161,162] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Saharin, R.; Hellmann, H.; Mooney, S. Plant E3 Ligases and Their Role in Abiotic Stress Response. Cells 2022, 11, 890. https://doi.org/10.3390/cells11050890
Al-Saharin R, Hellmann H, Mooney S. Plant E3 Ligases and Their Role in Abiotic Stress Response. Cells. 2022; 11(5):890. https://doi.org/10.3390/cells11050890
Chicago/Turabian StyleAl-Saharin, Raed, Hanjo Hellmann, and Sutton Mooney. 2022. "Plant E3 Ligases and Their Role in Abiotic Stress Response" Cells 11, no. 5: 890. https://doi.org/10.3390/cells11050890
APA StyleAl-Saharin, R., Hellmann, H., & Mooney, S. (2022). Plant E3 Ligases and Their Role in Abiotic Stress Response. Cells, 11(5), 890. https://doi.org/10.3390/cells11050890







