Modulation of Cell-Cycle Progression by Hydrogen Peroxide-Mediated Cross-Linking and Degradation of Cell-Adhesive Hydrogels
Abstract
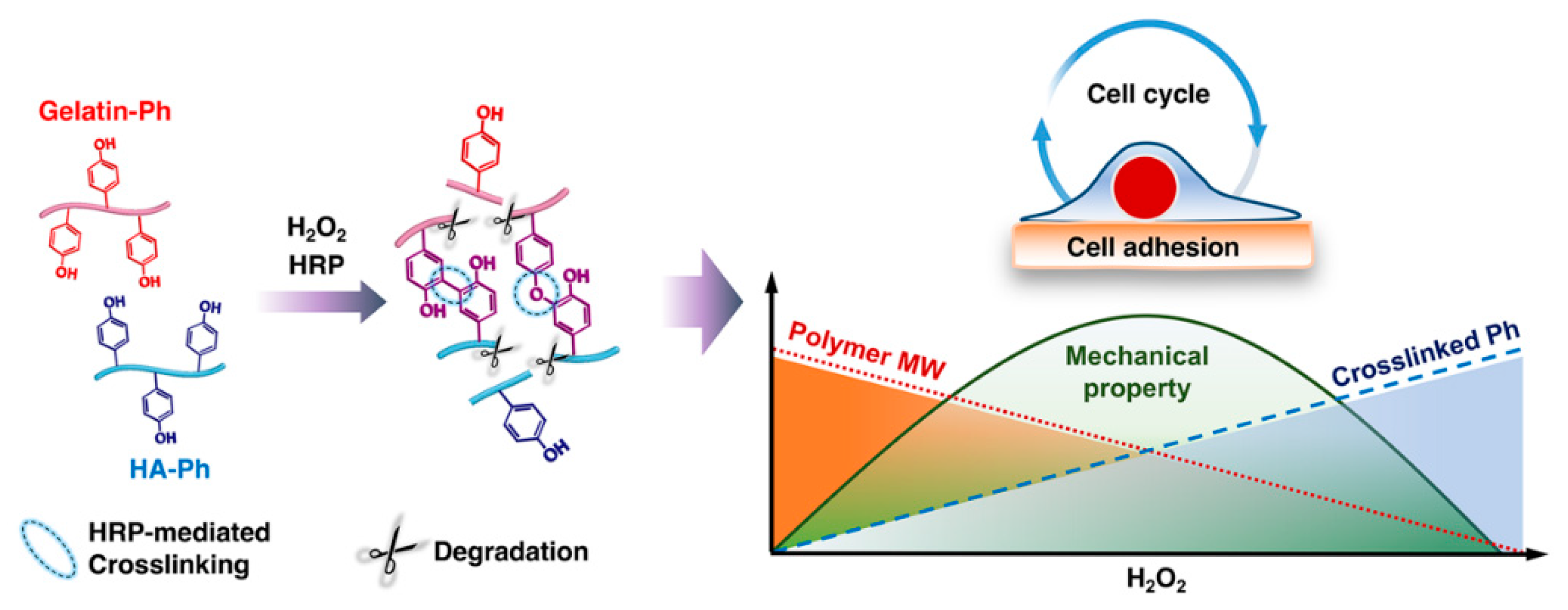
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Polymer–Ph Preparation
2.3. Cell Culture
2.4. Hydrogel Mechanical Properties Measurement
2.5. Cell Adhesion and Cell-Cycle Analysis
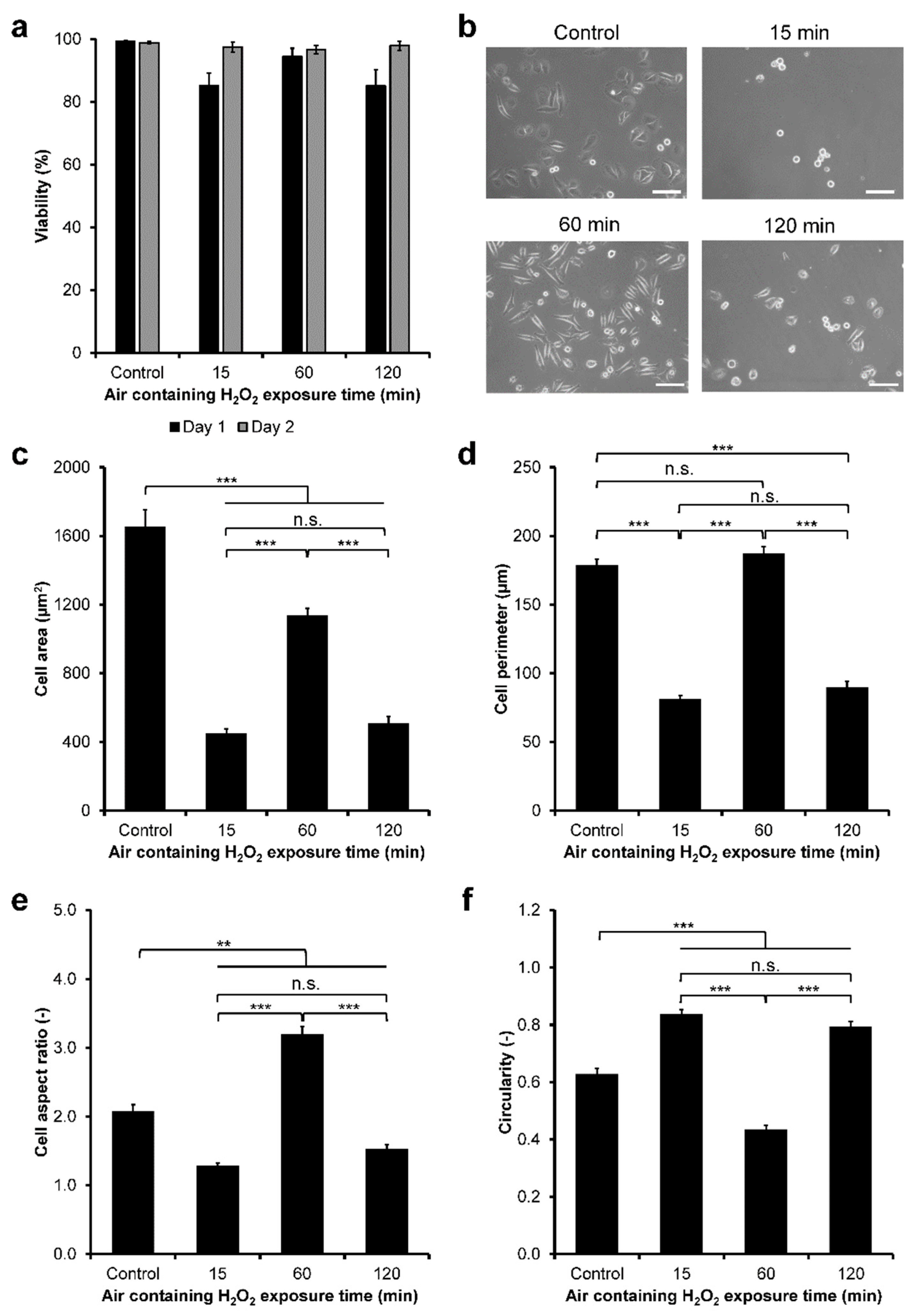
2.6. Viability Analysis
2.7. F-Actin Analysis
2.8. Statistical Analysis
3. Results
3.1. Mechanical Properties
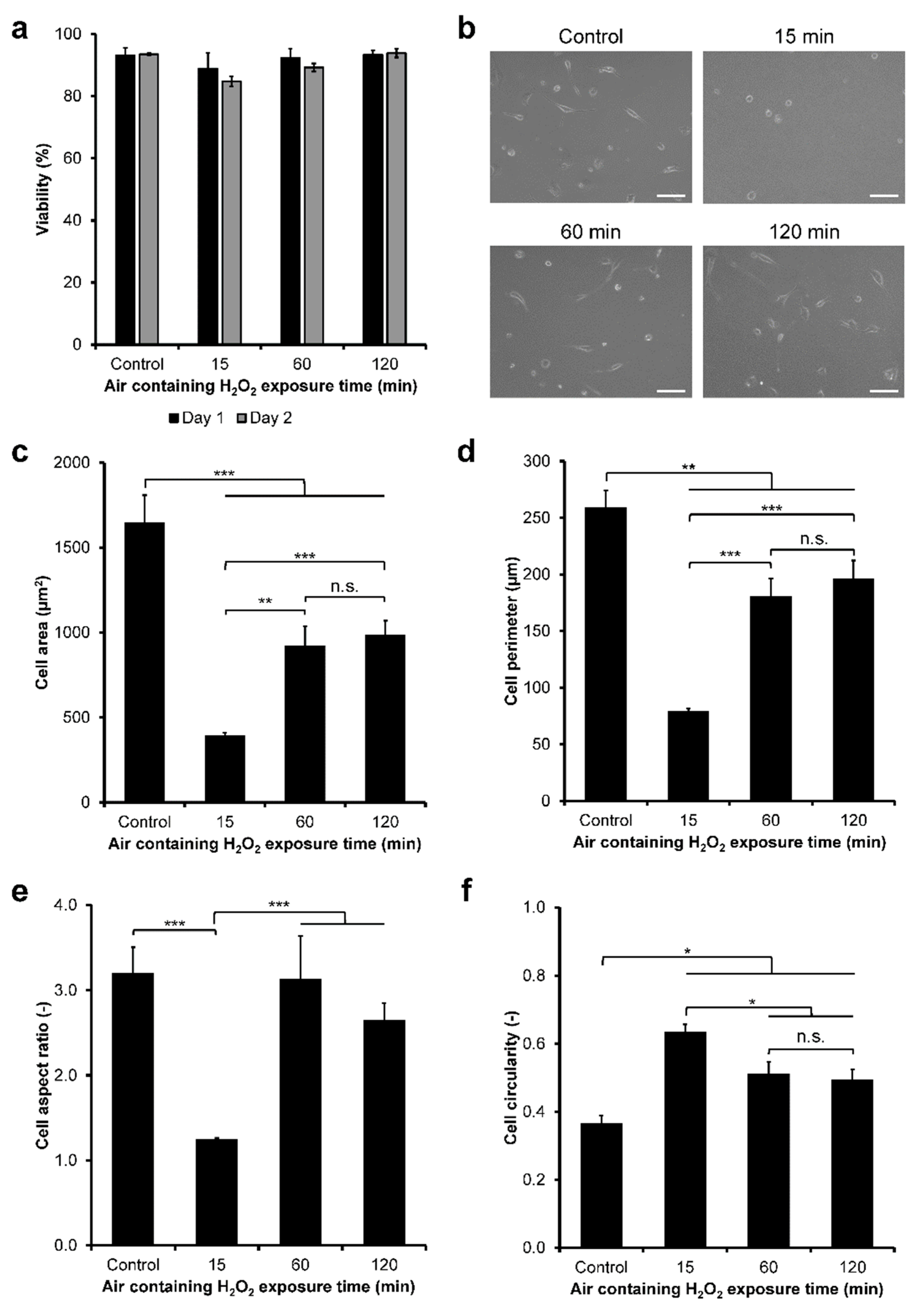
3.2. HeLa/Fucci2 Adhesion
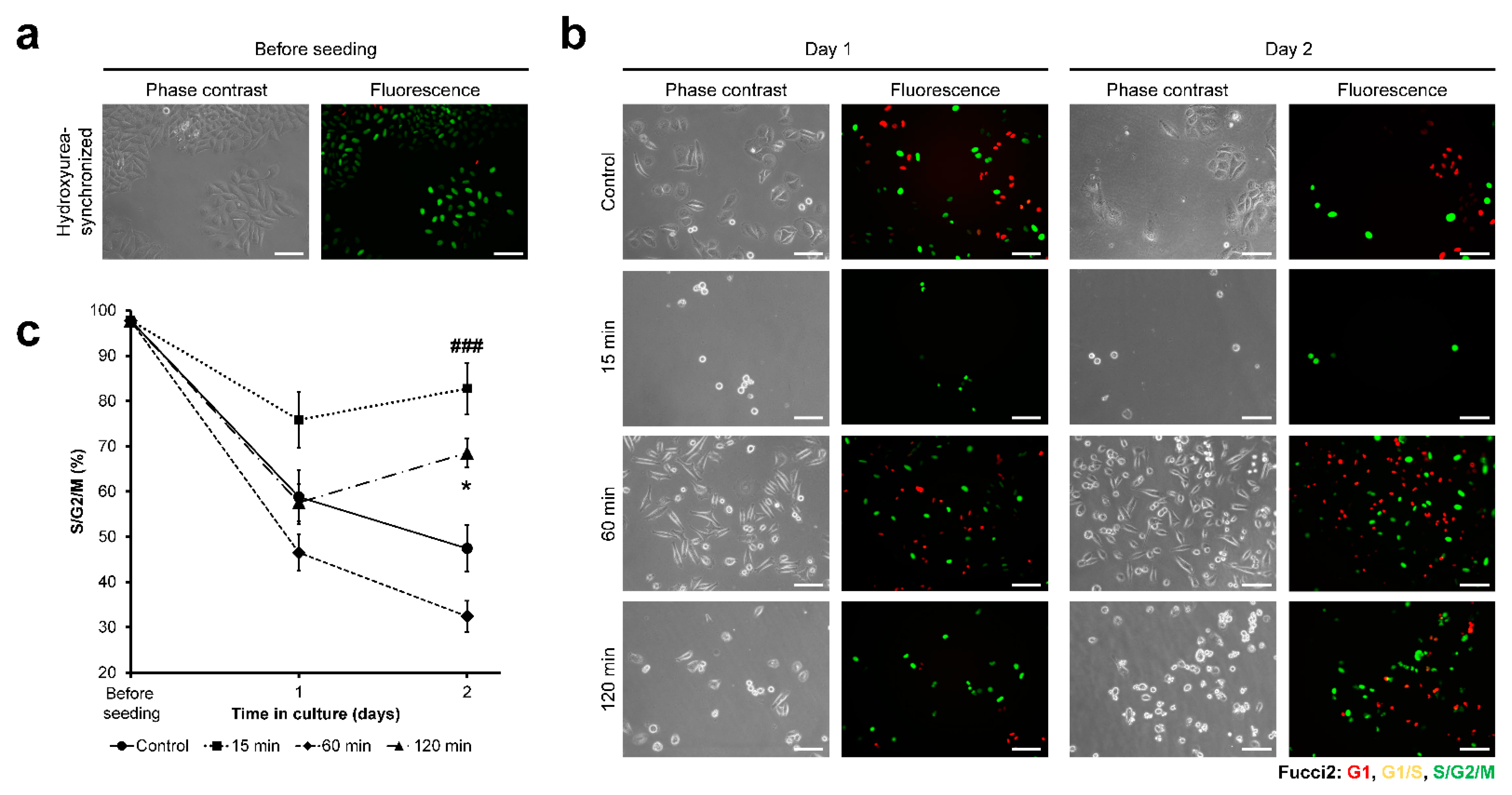
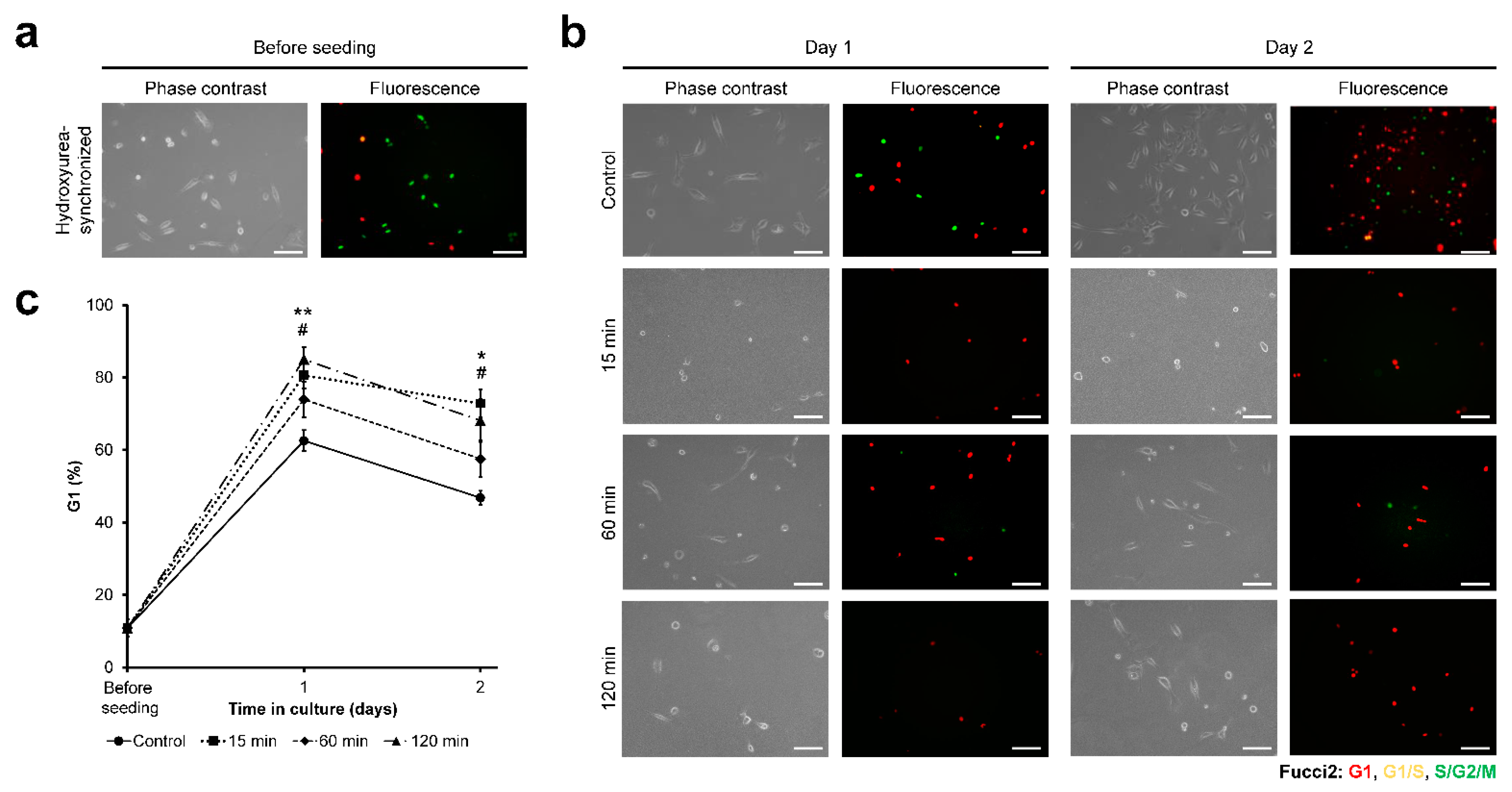
3.3. HeLa/Fucci2 Cell-Cycle Progression
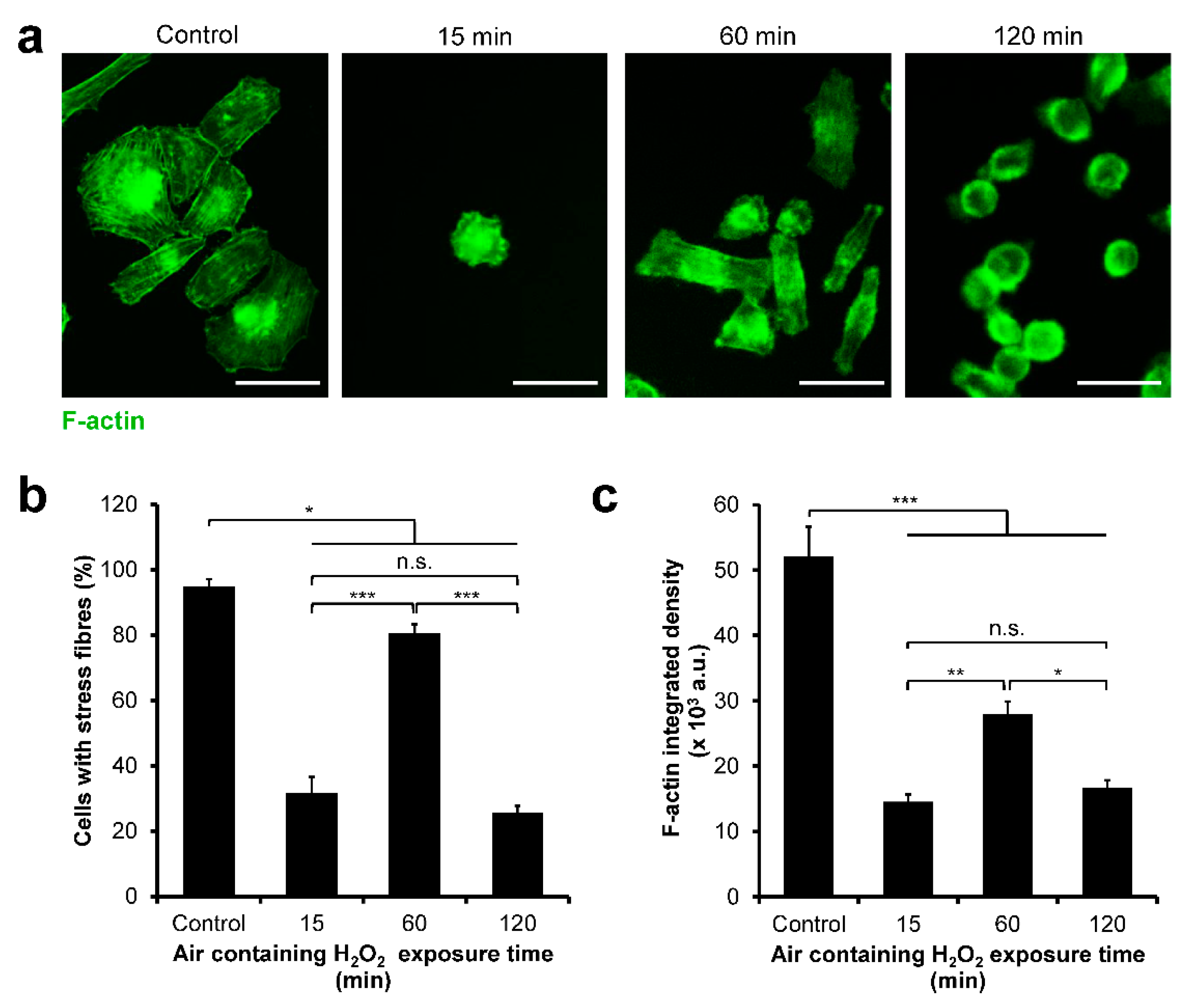
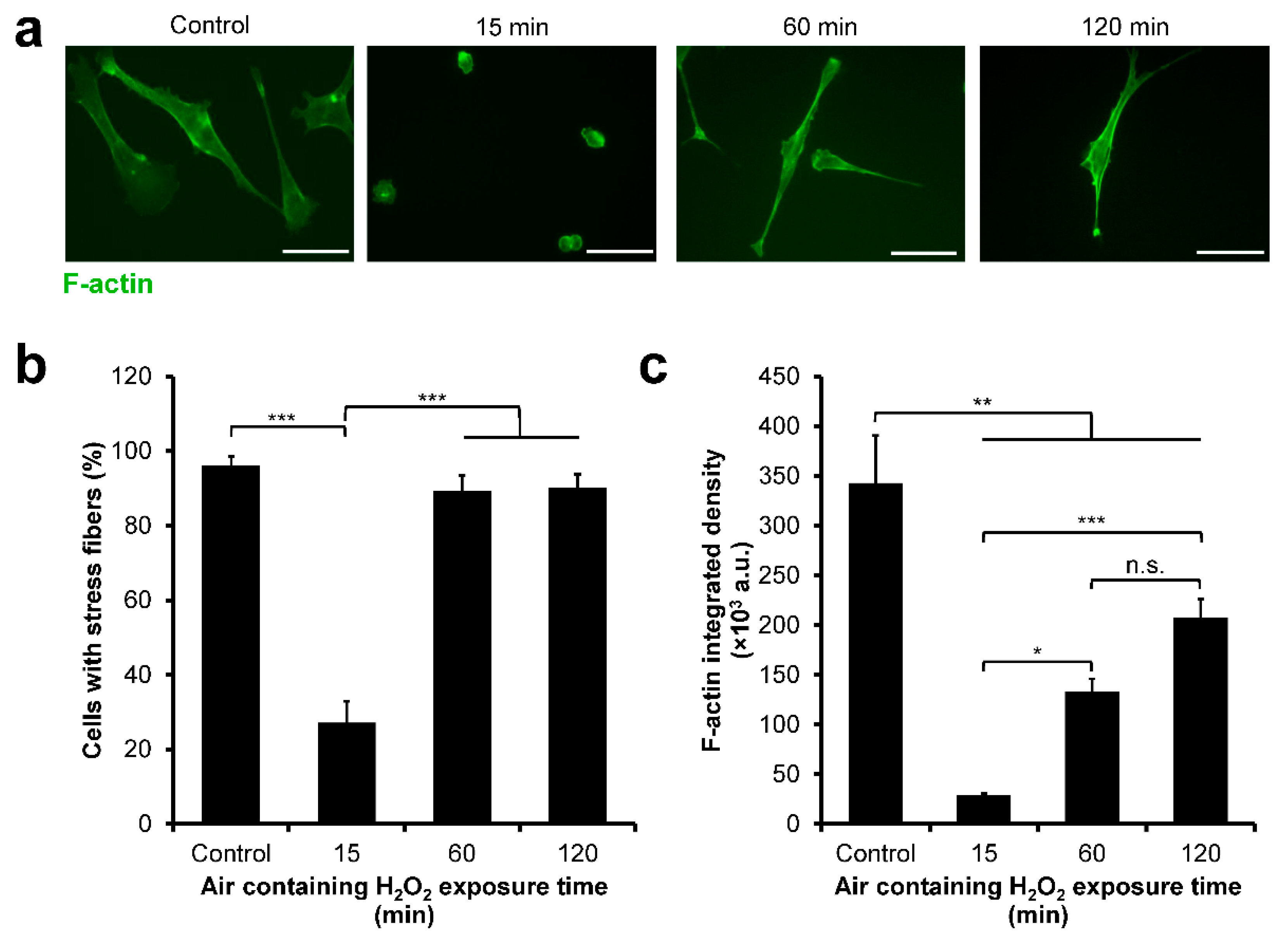
3.4. HeLa/Fucci2 F-Actin Reorganization
3.5. NMuMG/Fucci2 Adhesion
3.6. NMuMG/Fucci2 Cell-Cycle Progression
3.7. NMuMG/Fucci2 F-Actin Reorganization
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jingwen, B.; Yaochen, L.; Guojun, Z. Cell cycle regulation and anticancer drug discovery. Cancer Biol. Med. 2017, 14, 348–362. [Google Scholar] [CrossRef]
- Stewart, Z.A.; Westfall, M.D.; Pietenpol, J.A. Cell-cycle dysregulation and anticancer therapy. Trends Pharmacol. Sci. 2003, 24, 139–145. [Google Scholar] [CrossRef]
- Ni, Y.; Chiang, M.Y.M. Cell morphology and migration linked to substrate rigidity. Soft Matter 2007, 3, 1285–1292. [Google Scholar] [CrossRef]
- Sun, M.; Chi, G.; Li, P.; Lv, S.; Xu, J.; Xu, Z.; Xia, Y.; Tan, Y.; Xu, J.; Li, L.; et al. Effects of Matrix Stiffness on the Morphology, Adhesion, Proliferation and Osteogenic Differentiation of Mesenchymal Stem Cells. Int. J. Med. Sci. 2018, 15, 257–268. [Google Scholar] [CrossRef]
- Hadjipanayi, E.; Mudera, V.; Brown, R.A. Close dependence of fibroblast proliferation on collagen scaffold matrix stiffness. J. Tissue Eng. Regen. Med. 2009, 3, 77–84. [Google Scholar] [CrossRef]
- Yeh, Y.-T.; Hur, S.S.; Chang, J.; Wang, K.-C.; Chiu, J.-J.; Li, Y.-S.; Chien, S. Matrix Stiffness Regulates Endothelial Cell Proliferation through Septin 9. PLoS ONE 2012, 7, e46889. [Google Scholar] [CrossRef]
- Kalli, M.; Stylianopoulos, T. Defining the Role of Solid Stress and Matrix Stiffness in Cancer Cell Proliferation and Metastasis. Front. Oncol. 2018, 8, 55. [Google Scholar] [CrossRef]
- Lebl, M.D.A.; Martins, J.R.M.; Nader, H.B.; Simões, M.D.J.; De Biase, N. Concentration and Distribution of Hyaluronic Acid in Human Vocal Folds. Laryngoscope 2007, 117, 595–599. [Google Scholar] [CrossRef]
- Lee, S.-W.; Kim, J.; Do, M.J.; Namkoong, E.; Lee, H.; Ryu, J.H.; Park, K. Developmental role of hyaluronic acid and its application in salivary gland tissue engineering. Acta Biomater. 2020, 115, 275–287. [Google Scholar] [CrossRef]
- Camci-Unal, G.; Cuttica, D.; Annabi, N.; Demarchi, D.; Khademhosseini, A. Synthesis and Characterization of Hybrid Hyaluronic Acid-Gelatin Hydrogels. Biomacromolecules 2013, 14, 1085–1092. [Google Scholar] [CrossRef]
- Fraser, J.R.E.; Laurent, T.C.; Laurent, U.B.G. Hyaluronan: Its nature, distribution, functions and turnover. J. Intern. Med. 1997, 242, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.; Liang, J.; Noble, P.W. Hyaluronan in Tissue Injury and Repair. Annu. Rev. Cell Dev. Biol. 2007, 23, 435–461. [Google Scholar] [CrossRef]
- Gómez-Guillén, M.; Giménez, B.; López-Caballero, M.; Montero, M. Functional and bioactive properties of collagen and gelatin from alternative sources: A review. Food Hydrocoll. 2011, 25, 1813–1827. [Google Scholar] [CrossRef]
- Rezaeeyazdi, M.; Colombani, T.; Memic, A.; Bencherif, S.A. Injectable Hyaluronic Acid-co-Gelatin Cryogels for Tissue-Engineering Applications. Materials 2018, 11, 1374. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; He, A.; Zheng, J.; Han, C.C. Gelatin and Gelatin-Hyaluronic Acid Nanofibrous Membranes Produced by Electrospinning of Their Aqueous Solutions. Biomacromolecules 2006, 7, 2243–2247. [Google Scholar] [CrossRef]
- Lam, T.; Dehne, T.; Krüger, J.P.; Hondke, S.; Endres, M.; Thomas, A.; Lauster, R.; Sittinger, M.; Kloke, L. Photopolymerizable gelatin and hyaluronic acid for stereolithographic 3D bioprinting of tissue-engineered cartilage. J. Biomed. Mater. Res. Part. B Appl. Biomater. 2019, 107, 2649–2657. [Google Scholar] [CrossRef]
- Nyman, E.; Huss, F.; Nyman, T.; Junker, J.; Kratz, G. Hyaluronic acid, an important factor in the wound healing properties of amniotic fluid:In vitrostudies of re-epithelialisation in human skin wounds. J. Plast. Surg. Hand Surg. 2013, 47, 89–92. [Google Scholar] [CrossRef] [PubMed]
- Termeer, C.; Benedix, F.; Sleeman, J.; Fieber, C.; Voith, U.; Ahrens, T.; Miyake, K.; Freudenberg, M.; Galanos, C.; Simon, J.C. Oligosaccharides of Hyaluronan Activate Dendritic Cells via Toll-like Receptor 4. J. Exp. Med. 2002, 195, 99–111. [Google Scholar] [CrossRef]
- Termeer, C.C.; Hennies, J.; Voith, U.; Ahrens, T.; Weiss, J.; Prehm, P.; Simon, J.C. Oligosaccharides of Hyaluronan Are Potent Activators of Dendritic Cells. J. Immunol. 2000, 165, 1863–1870. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Tolg, C.; Turley, E. Dissecting the Dual Nature of Hyaluronan in the Tumor Microenvironment. Front. Immunol. 2019, 10, 947. [Google Scholar] [CrossRef] [PubMed]
- Ooki, T.; Murata-Kamiya, N.; Kanemitsu, A.; Wu, W.; Hatakeyama, M. High-Molecular-Weight Hyaluronan Is a Hippo Pathway Ligand Directing Cell Density-Dependent Growth Inhibition via PAR1b. Dev. Cell 2019, 49, 590–604.e9. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, K.; Hippe, A.; Schmaus, A.; Homey, B.; Sleeman, J.; Orian-Rousseau, V. Opposing effects of high- and low-molecular weight hyaluronan on CXCL12-induced CXCR4 signaling depend on CD44. Cell Death Dis. 2013, 4, e819. [Google Scholar] [CrossRef]
- Chen, H.; Qin, J.; Hu, Y. Efficient Degradation of High-Molecular-Weight Hyaluronic Acid by a Combination of Ultrasound, Hydrogen Peroxide, and Copper Ion. Molecules 2019, 24, 617. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, Y.; He, H.; Ping, W. Hydrogen peroxide-induced degradation of type I collagen fibers of tilapia skin. Food Struct. 2014, 2, 41–48. [Google Scholar] [CrossRef]
- Li, X.; Xu, A.; Xie, H.; Yu, W.; Xie, W.; Ma, X. Preparation of low molecular weight alginate by hydrogen peroxide depolymerization for tissue engineering. Carbohydr. Polym. 2010, 79, 660–664. [Google Scholar] [CrossRef]
- Chang, K.L.B.; Tai, M.-C.; Cheng, F.-H. Kinetics and Products of the Degradation of Chitosan by Hydrogen Peroxide. J. Agric. Food Chem. 2001, 49, 4845–4851. [Google Scholar] [CrossRef]
- Park, J.; Baranov, P.; Aydin, A.; Abdelgawad, H.; Singh, D.; Niu, W.; Kurisawa, M.; Spector, M.; Young, M.J. In Situ Cross-linking Hydrogel as a Vehicle for Retinal Progenitor Cell Transplantation. Cell Transplant. 2019, 28, 596–606. [Google Scholar] [CrossRef]
- Kondo, D.; Ogino, Y.; Ayukawa, Y.; Sakai, S.; Kawakami, K.; Koyano, K. Bone regeneration of tibial defects in rats with enzymatic hydrogelation of gelatin derivative and recombinant human platelet-derived growth factor-BB complex. Int. J. Oral Maxillofac. Implant. 2013, 28, 1377–1385. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yao, M.; Zhang, J.; Gao, F.; Chen, Y.; Ma, S.; Zhang, K.; Liu, H.; Guan, F. New BMSC-Laden Gelatin Hydrogel Formed in Situ by Dual-Enzymatic Cross-Linking Accelerates Dermal Wound Healing. ACS Omega 2019, 4, 8334–8340. [Google Scholar] [CrossRef]
- Sakai, S.; Ueda, K.; Gantumur, E.; Taya, M.; Nakamura, M. Drop-On-Drop Multimaterial 3D Bioprinting Realized by Peroxidase-Mediated Cross-Linking. Macromol. Rapid Commun. 2017, 39. [Google Scholar] [CrossRef]
- Mubarok, W.; Qu, Y.; Sakai, S. Influence of Hydrogen Peroxide-Mediated Cross-Linking and Degradation on Cell-Adhesive Gelatin Hydrogels. ACS Appl. Bio Mater. 2021, 4, 4184–4190. [Google Scholar] [CrossRef]
- Gérard, C.; Goldbeter, A. The balance between cell cycle arrest and cell proliferation: Control by the extracellular matrix and by contact inhibition. Interface Focus 2014, 4, 20130075. [Google Scholar] [CrossRef] [PubMed]
- Walker, C.; Mojares, E.; del Río Hernández, A. Role of Extracellular Matrix in Development and Cancer Progression. Int. J. Mol. Sci. 2018, 19, 3028. [Google Scholar] [CrossRef] [PubMed]
- Greco, R.M.; Iocono, J.A.; Ehrlich, H.P. Hyaluronic acid stimulates human fibroblast proliferation within a collagen matrix. J. Cell. Physiol. 1998, 177, 465–473. [Google Scholar] [CrossRef]
- Arya, A.D.; Hallur, P.M.; Karkisaval, A.G.; Gudipati, A.; Rajendiran, S.; Dhavale, V.; Ramachandran, B.; Jayaprakash, A.; Gundiah, N.; Chaubey, A. Gelatin Methacrylate Hydrogels as Biomimetic Three-Dimensional Matrixes for Modeling Breast Cancer Invasion and Chemoresponse in Vitro. ACS Appl. Mater. Interfaces 2016, 8, 22005–22017. [Google Scholar] [CrossRef] [PubMed]
- Mao, J.S.; Cui, Y.L.; Wang, X.H.; Sun, Y.; Yin, Y.J.; Zhao, H.M.; De Yao, K. A preliminary study on chitosan and gelatin polyelectrolyte complex cytocompatibility by cell cycle and apoptosis analysis. Biomaterials 2004, 25, 3973–3981. [Google Scholar] [CrossRef] [PubMed]
- Sheu, B.-C.; Lien, H.-C.; Ho, H.-N.; Lin, H.-H.; Chow, S.-N.; Huang, S.-C.; Hsu, S.-M. Increased expression and activation of gelatinolytic matrix metalloproteinases is associated with the progression and recurrence of human cervical cancer. Cancer Res. 2003, 63, 6537–6542. [Google Scholar] [PubMed]
- Vu, M.; Yu, J.; Awolude, O.A.; Chuang, L. Cervical cancer worldwide. Curr. Probl. Cancer 2018, 42, 457–465. [Google Scholar] [CrossRef] [PubMed]
- Canfell, K. Towards the global elimination of cervical cancer. Papillomavirus Res. 2019, 8, 100170. [Google Scholar] [CrossRef] [PubMed]
- Qian, Q.; Wang, D.; Shi, L.; Zhang, Z.; Qian, J.; Shen, J.; Yu, C.; Zhu, X. A pure molecular drug hydrogel for post-surgical cancer treatment. Biomaterials 2020, 265, 120403. [Google Scholar] [CrossRef] [PubMed]
- Lan, T.; Pang, J.; Wu, Y.; Zhu, M.; Yao, X.; Wu, M.; Qian, H.; Zhang, Z.; Gao, J.; Chen, Y. Cross-linked hyaluronic acid gel inhibits metastasis and growth of gastric and hepatic cancer cells: In vitro and in vivo studies. Oncotarget 2016, 7, 65418–65428. [Google Scholar] [CrossRef]
- Ma, H.T.; Poon, R.Y.C. Synchronization of HeLa Cells. In Methods in Molecular Biology (Methods and Protocols); Banfalvi, G., Ed.; Humana Press: New York, NY, USA, 2011; Volume 761, pp. 151–161. ISBN 978-1-61779-181-9. [Google Scholar]
- Sakaue-Sawano, A.; Kobayashi, T.; Ohtawa, K.; Miyawaki, A. Drug-induced cell cycle modulation leading to cell-cycle arrest, nuclear mis-segregation, or endoreplication. BMC Cell Biol. 2011, 12, 2. [Google Scholar] [CrossRef]
- Shin, S.; Buel, G.R.; Nagiec, M.J.; Han, M.-J.; Roux, P.P.; Blenis, J.; Yoon, S.-O. ERK2 regulates epithelial-to-mesenchymal plasticity through DOCK10-dependent Rac1/FoxO1 activation. Proc. Natl. Acad. Sci. USA 2019, 116, 2967–2976. [Google Scholar] [CrossRef]
- Meyer-Schaller, N.; Cardner, M.; Diepenbruck, M.; Saxena, M.; Tiede, S.; Lüönd, F.; Ivanek, R.; Beerenwinkel, N.; Christofori, G. A Hierarchical Regulatory Landscape during the Multiple Stages of EMT. Dev. Cell 2019, 48, 539–553.e6. [Google Scholar] [CrossRef] [PubMed]
- Sakai, S.; Hirose, K.; Taguchi, K.; Ogushi, Y.; Kawakami, K. An injectable, in situ enzymatically gellable, gelatin derivative for drug delivery and tissue engineering. Biomaterials 2009, 30, 3371–3377. [Google Scholar] [CrossRef] [PubMed]
- Sakai, S.; Ueda, K.; Taya, M. Peritoneal adhesion prevention by a biodegradable hyaluronic acid-based hydrogel formed in situ through a cascade enzyme reaction initiated by contact with body fluid on tissue surfaces. Acta Biomater. 2015, 24, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Apraiz, A.; Mitxelena, J.; Zubiaga, A. Studying Cell Cycle-regulated Gene Expression by Two Complementary Cell Synchronization Protocols. J. Vis. Exp. 2017. [Google Scholar] [CrossRef] [PubMed]
- Sakai, S.; Ohi, H.; Taya, M. Gelatin/Hyaluronic Acid Content in Hydrogels Obtained through Blue Light-Induced Gelation Affects Hydrogel Properties and Adipose Stem Cell Behaviors. Biomolecules 2019, 9, 342. [Google Scholar] [CrossRef]
- Matthews, H.K.; Bertoli, C.; de Bruin, R.A.M. Cell cycle control in cancer. Nat. Rev. Mol. Cell Biol. 2021, 23, 74–88. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, G.K.; Shah, M.A. Targeting the Cell Cycle: A New Approach to Cancer Therapy. J. Clin. Oncol. 2005, 23, 9408–9421. [Google Scholar] [CrossRef] [PubMed]
- Baynton, K.J.; Bewtra, J.K.; Biswas, N.; Taylor, K.E. Inactivation of horseradish peroxidase by phenol and hydrogen peroxide: A kinetic investigation. Biochim. Biophys. Acta (BBA) Protein Struct. Mol. Enzym. 1994, 1206, 272–278. [Google Scholar] [CrossRef]
- Arnao, M.; Acosta, M.; Del Rio, J.; Varón, R.; Garcia-Canovas, F. A kinetic study on the suicide inactivation of peroxidase by hydrogen peroxide. Biochim. Biophys. Acta (BBA) Protein Struct. Mol. Enzym. 1990, 1041, 43–47. [Google Scholar] [CrossRef]
- Takahashi, S.; Itoh, N.; Kawamura, Y.; Hayashi, R. Physical and chemical changes of gelatins by oxidation treatment. Bull. Soc. Sci. Photogr. Jpn. 1998, 51, 22–28. (In Japanese) [Google Scholar] [CrossRef]
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular principles of metastasis: A hallmark of cancer revisited. Signal. Transduct. Target. Ther. 2020, 5, 28. [Google Scholar] [CrossRef]
- Ecker, B.L.; Kaur, A.; Douglass, S.M.; Webster, M.R.; Almeida, F.; Marino, G.; Sinnamon, A.J.; Neuwirth, M.G.; Alicea, G.M.; Ndoye, A.; et al. Age-Related Changes in HAPLN1 Increase Lymphatic Permeability and Affect Routes of Melanoma Metastasis. Cancer Discov. 2018, 9, 82–95. [Google Scholar] [CrossRef] [PubMed]
- Nakahata, M.; Gantumur, E.; Furuno, K.; Sakai, S.; Taya, M. Versatility of hydrogelation by dual-enzymatic reactions with oxidases and peroxidase. Biochem. Eng. J. 2018, 131, 1–8. [Google Scholar] [CrossRef]
- Le Thi, P.; Lee, Y.; Nguyen, D.H.; Park, K.D. In situ forming gelatin hydrogels by dual-enzymatic cross-linking for enhanced tissue adhesiveness. J. Mater. Chem. B 2016, 5, 757–764. [Google Scholar] [CrossRef] [PubMed]
- Previtera, M.L.; Trout, K.; Verma, D.; Chippada, U.; Schloss, R.S.; Langrana, N.A. Fibroblast Morphology on Dynamic Softening of Hydrogels. Ann. Biomed. Eng. 2011, 40, 1061–1072. [Google Scholar] [CrossRef]
- Paszek, M.J.; Zahir, N.; Johnson, K.R.; Lakins, J.N.; Rozenberg, G.I.; Gefen, A.; Reinhart-King, C.A.; Margulies, S.S.; Dembo, M.; Boettiger, D.; et al. Tensional homeostasis and the malignant phenotype. Cancer Cell 2005, 8, 241–254. [Google Scholar] [CrossRef]
- Wall, S.J.; Zhong, Z.-D.; DeClerck, Y. The Cyclin-dependent Kinase Inhibitors p15INK4B and p21CIP1 Are Critical Regulators of Fibrillar Collagen-induced Tumor Cell Cycle Arrest. J. Biol. Chem. 2007, 282, 24471–24476. [Google Scholar] [CrossRef]
- Klein, E.A.; Yin, L.; Kothapalli, D.; Castagnino, P.; Byfield, F.J.; Xu, T.; Levental, I.; Hawthorne, E.; Janmey, P.A.; Assoian, R.K. Cell-Cycle Control by Physiological Matrix Elasticity and In Vivo Tissue Stiffening. Curr. Biol. 2009, 19, 1511–1518. [Google Scholar] [CrossRef] [PubMed]
- Kunda, P.; Baum, B. The actin cytoskeleton in spindle assembly and positioning. Trends Cell Biol. 2009, 19, 174–179. [Google Scholar] [CrossRef]
- Reshetnikova, G.; Barkan, R.; Popov, B.; Nikolsky, N.; Chang, L.-S. Disruption of the Actin Cytoskeleton Leads to Inhibition of Mitogen-Induced Cyclin E Expression, Cdk2 Phosphorylation, and Nuclear Accumulation of the Retinoblastoma Protein-Related p107 Protein. Exp. Cell Res. 2000, 259, 35–53. [Google Scholar] [CrossRef] [PubMed]
- An, W.G.; Shin, I.J.; Ahn, Y.-T.; Kim, Y.; Kim, J.-M. Actin disruption agents induce phosphorylation of histone H2AX in human breast adenocarcinoma MCF-7 cells. Oncol. Rep. 2011, 25, 1313–1319. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, D.; Choi, D.; Song, K. Actin Dysfunction Induces Cell Cycle Delay at G2/M with Sustained ERK and RSK Activation in IMR-90 Normal Human Fibroblasts. Mol. Cells 2018, 41, 436–443. [Google Scholar] [CrossRef]
- Scott, R.A.; Robinson, K.G.; Kiick, K.L.; Akins, R.E. Human Adventitial Fibroblast Phenotype Depends on the Progression of Changes in Substrate Stiffness. Adv. Heal. Mater. 2020, 9, e1901593. [Google Scholar] [CrossRef]
- Case, L.; Ditlev, J.A.; Rosen, M.K. Regulation of Transmembrane Signaling by Phase Separation. Annu. Rev. Biophys. 2019, 48, 465–494. [Google Scholar] [CrossRef]
- Kouvidi, K.; Berdiaki, A.; Nikitovic, D.; Katonis, P.; Afratis, N.; Hascall, V.C.; Karamanos, N.; Tzanakakis, G. Role of Receptor for Hyaluronic Acid-mediated Motility (RHAMM) in Low Molecular Weight Hyaluronan (LMWHA)-mediated Fibrosarcoma Cell Adhesion. J. Biol. Chem. 2011, 286, 38509–38520. [Google Scholar] [CrossRef]
- Tolg, C.; Yuan, H.; Flynn, S.M.; Basu, K.; Ma, J.; Tse, K.C.K.; Kowalska, B.; Vulkanesku, D.; Cowman, M.K.; McCarthy, J.B.; et al. Hyaluronan modulates growth factor induced mammary gland branching in a size dependent manner. Matrix Biol. 2017, 63, 117–132. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Sun, Q.; Li, X.; Feng, J.; Ao, Z.; Li, X.; Wang, J. Substrate Stiffness Modulates the Growth, Phenotype, and Chemoresistance of Ovarian Cancer Cells. Front. Cell Dev. Biol. 2021, 9. [Google Scholar] [CrossRef] [PubMed]
- Jariyal, H.; Gupta, C.; Srivastava, A. Hyaluronic acid induction on breast cancer stem cells unfolds subtype specific variations in stemness and epithelial-to-mesenchymal transition. Int. J. Biol. Macromol. 2020, 160, 1078–1089. [Google Scholar] [CrossRef]
- Vega, S.; Morales, A.V.; Ocaña, O.H.; Valdés, F.; Fabregat, I.; Nieto, M.A. Snail blocks the cell cycle and confers resistance to cell death. Genes Dev. 2004, 18, 1131–1143. [Google Scholar] [CrossRef]
- Prakash, V.; Carson, B.B.; Feenstra, J.M.; Dass, R.A.; Sekyrova, P.; Hoshino, A.; Petersen, J.; Guo, Y.; Parks, M.M.; Kurylo, C.M.; et al. Ribosome biogenesis during cell cycle arrest fuels EMT in development and disease. Nat. Commun. 2019, 10, 2110. [Google Scholar] [CrossRef]
- Lovisa, S.; LeBleu, V.S.; Tampe, B.; Sugimoto, H.; Vadnagara, K.; Carstens, J.L.; Wu, C.-C.; Hagos, Y.; Burckhardt, B.C.; Pentcheva-Hoang, T.; et al. Epithelial-to-mesenchymal transition induces cell cycle arrest and parenchymal damage in renal fibrosis. Nat. Med. 2015, 21, 998–1009. [Google Scholar] [CrossRef]
- Assani, G.; Zhou, Y. Effect of modulation of epithelial-mesenchymal transition regulators Snail1 and Snail2 on cancer cell radiosensitivity by targeting of the cell cycle, cell apoptosis and cell migration/invasion (Review). Oncol. Lett. 2018, 17, 23–30. [Google Scholar] [CrossRef]
- Gantumur, E.; Kimura, M.; Taya, M.; Horie, M.; Nakamura, M.; Sakai, S. Inkjet micropatterning through horseradish peroxidase-mediated hydrogelation for controlled cell immobilization and microtissue fabrication. Biofabrication 2019, 12, 011001. [Google Scholar] [CrossRef]
- Pierantoni, L.; Ribeiro, V.P.; Costa, L.; Pina, S.; Morais, A.D.S.; Silva-Correia, J.; Kundu, S.C.; Motta, A.; Reis, R.L.; Oliveira, J.M. Horseradish Peroxidase-Crosslinked Calcium-Containing Silk Fibroin Hydrogels as Artificial Matrices for Bone Cancer Research. Macromol. Biosci. 2021, 21, 2000425. [Google Scholar] [CrossRef]

| Abbreviation | Full Form |
|---|---|
| CLSM | Confocal laser-scanning microscope |
| DMEM | Dulbecco’s Modified Eagle Medium |
| DMF | N,N-dimethylformamide |
| ECM | Extracellular matrix |
| EGF | Epidermal growth factor |
| EMT | Epithelial-to-mesenchymal transition |
| FBS | Fetal bovine serum |
| Fucci | Fluorescent ubiquitination-based cell-cycle indicator |
| HA | Hyaluronic acid |
| hASCs | Human adipose-derived stem cells |
| HEPES | 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid |
| HRP | Horseradish peroxidase |
| LMW-HA | Low-molecular-weight hyaluronic acid |
| MW | Molecular weight |
| NHS | N-hydroxysuccinimide |
| PBS | Phosphate buffered saline |
| PFA | Paraformaldehyde |
| Ph | Phenolic hydroxyl moieties |
| PI | Propidium iodide |
| RGD | Tripeptide arginine–glycine–aspartic acid |
| RHAMM | Receptor for hyaluronic acid-mediated motility |
| WSCD·HCl | Water-soluble carbodiimide hydrochloride |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mubarok, W.; Elvitigala, K.C.M.L.; Nakahata, M.; Kojima, M.; Sakai, S. Modulation of Cell-Cycle Progression by Hydrogen Peroxide-Mediated Cross-Linking and Degradation of Cell-Adhesive Hydrogels. Cells 2022, 11, 881. https://doi.org/10.3390/cells11050881
Mubarok W, Elvitigala KCML, Nakahata M, Kojima M, Sakai S. Modulation of Cell-Cycle Progression by Hydrogen Peroxide-Mediated Cross-Linking and Degradation of Cell-Adhesive Hydrogels. Cells. 2022; 11(5):881. https://doi.org/10.3390/cells11050881
Chicago/Turabian StyleMubarok, Wildan, Kelum Chamara Manoj Lakmal Elvitigala, Masaki Nakahata, Masaru Kojima, and Shinji Sakai. 2022. "Modulation of Cell-Cycle Progression by Hydrogen Peroxide-Mediated Cross-Linking and Degradation of Cell-Adhesive Hydrogels" Cells 11, no. 5: 881. https://doi.org/10.3390/cells11050881
APA StyleMubarok, W., Elvitigala, K. C. M. L., Nakahata, M., Kojima, M., & Sakai, S. (2022). Modulation of Cell-Cycle Progression by Hydrogen Peroxide-Mediated Cross-Linking and Degradation of Cell-Adhesive Hydrogels. Cells, 11(5), 881. https://doi.org/10.3390/cells11050881








