Abstract
The sarcoplasmic reticulum (SR) in cardiac muscle is suggested to act as a dynamic storage for Zn2+ release and reuptake, albeit it is primarily implicated in the Ca2+ signaling required for the cardiac cycle. A large Ca2+ release from the SR is mediated by the cardiac ryanodine receptor (RYR2), and while this has a prominent conductance for Ca2+ in vivo, it also conducts other divalent cations in vitro. Since Zn2+ and permeant Mg2+ have similar physical properties, we tested if the RYR2 channel also conducts Zn2+. Using the method of planar lipid membranes, we evidenced that the RYR2 channel is permeable to Zn2+ with a considerable conductance of 81.1 ± 2.4 pS, which was significantly lower than the values for Ca2+ (127.5 ± 1.8 pS) and Mg2+ (95.3 ± 1.4 pS), obtained under the same asymmetric conditions. Despite similar physical properties, the intrinsic Zn2+ permeability (PCa/PZn = 2.65 ± 0.19) was found to be ~2.3-fold lower than that of Mg2+ (PCa/PMg = 1.146 ± 0.071). Further, we assessed whether the channel itself could be a direct target of the Zn2+ current, having the Zn2+ finger extended into the cytosolic vestibular portion of the permeation pathway. We attempted to displace Zn2+ from the RYR2 Zn2+ finger to induce its structural defects, which are associated with RYR2 dysfunction. Zn2+ chelators were added to the channel cytosolic side or strongly competing cadmium cations (Cd2+) were allowed to permeate the RYR2 channel. Only the Cd2+ current was able to cause the decay of channel activity, presumably as a result of Zn2+ to Cd2+ replacement. Our findings suggest that the RYR2 channel can provide a suitable pathway for rapid Zn2+ escape from the cardiac SR; thus, the channel may play a role in local and/or global Zn2+ signaling in cardiomyocytes.
1. Introduction
Zinc (Zn2+) is one of the most abundant metal cations in mammalian cells, with diverse functions in numerous physiological processes important for differentiation, growth and survival (reviewed in [1,2]). Although, Zn2+ has been generally considered to have moderate biological importance, significant advances in understanding Zn2+ biology in the past decade have changed this view. Reflecting the physiological relevance, both Zn2+ deficiency and excess have been documented in a wide range of pathological conditions, including cancer, diabetes and inflammatory, cardiovascular and neurodegenerative diseases, which have been the topic of several review articles [3,4,5,6,7,8,9]. While catalytic and structural functions of Zn2+ are well-established in a great number of metalloproteins [10,11], Zn2+ signaling capacities have only recently received extensive attention, mainly in a cellular and molecular context.
The Zn2+ affinity to protein metal binding sites is highly competitive towards Mg2+ and Ca2+, therefore, free intracellular Zn2+ concentration is tightly controlled and fluctuates in an extraordinarily narrow range (pM–nM), depending on the cell type [12,13,14,15]. In addition to cytosolic Zn2+-chelating proteins (reviewed in [16]), various Zn2+ transporters (carrier-type) are involved in controlling intracellular Zn2+, such as ZnT proteins exporting Zn2+ from the cytoplasm and ZIP proteins with the opposite function (reviewed in [17,18,19]).
ZnT and ZIP proteins have a distinct subcellular location, depending on their contribution to Zn2+ mobilization. In mammals, they are mostly localized to the plasma membrane [20,21,22,23,24,25,26] or in membranes of intracellular organelles, such as the ER and Golgi apparatus [27,28,29,30,31,32,33]. All these intracellular compartments obviously accumulate Zn2+ [34,35,36], but only some could be implicated in Zn2+ signaling, exemplified by Zn2+ transients and waves [35,37]. Although, there is no clear evidence, it can be proposed that the SR in cardiomyocytes acts as a dynamic storage for Zn2+ cations, readily available for release [8,38]. These results strongly imply the presence of Zn2+ transporters, and indeed ZIP and ZnT proteins have been evidenced in the heart tissue [27,39,40]. Moreover, their predominant subcellular localization in the SR membrane has been demonstrated [41].
It is well established that the release and accumulation of Ca2+ by the SR in cardiomyocytes is fundamental to the excitation-contraction coupling [42,43,44]. The cyclic release of Ca2+ required for contraction is mediated by the cardiac ryanodine receptor (RYR2) displaying a remarkable conductance for Ca2+ [45,46] compared to other Ca2+-permeable channels [47,48]. However, the RYR2 channel discriminates only slightly between divalent cations [46], and thus has been shown to be also permeable to Mg2+, Sr2+ and Ba2+ [46,49,50,51]. Since we noticed that both Mg2+ and Zn2+ possess similar physical properties relevant to permeation through ion channels [52,53,54], we examined whether the RYR2 channel provides a suitable permeation pathway for Zn2+. At the single-channel level, we established that Zn2+ greatly permeated the RYR2 channel in the lumen-to-cytosol direction, although with a lower conductance and intrinsic permeability than Ca2+ and even Mg2+. Our results indicate that the RYR2 channel may play a role in Zn2+ signaling in cardiomyocytes, and the channel itself could be a direct target for the localized Zn2+ increase, with the Zn2+ finger, a well-known Zn2+-binding site, extending into the cytosolic vestibular portion of the RYR2 permeation pathway [55].
2. Materials and Methods
2.1. Single-Channel Recordings
The sarcoplasmic reticulum (SR) microsomes enriched in RYR2 channels isolated from rat ventricular muscle were used for single-channel recordings as described previously [51] with some modifications. Planar lipid membranes (BLMs) were formed across a 50–70 μm aperture in the wall of a polystyrene cup separating two compartments, cytosolic and luminal.
The cytosolic compartment was filled with 1 mL of 50 mM KCl, 10 mM Tris and 20 mM HEPES (pH = 7.35). The free cytosolic Ca2+ concentration of 90 nM was obtained by including 1 mM ethylene glycol-bis(β-aminoethylether)–N,N,N′,N′-tetraacetic acid (EGTA) and 0.587 mM CaCl2. In some cases, N,N,N′,N′-tetrakis(2-pyridylmethyl)-ethylene diamine (TPEN) or nitrilotriacetic acid (NTA) was used to chelate Zn2+ impurities in the cytosolic solutions. The free Zn2+ ([Zn2+]C) and Ca2+([Ca2+]C) concentrations were determined by WinMaxc32 version 2.50 (http://www.stanford.edu/~cpatton/maxc.html, accessed on 24 April 2021). In control experiments, the luminal compartment was filled with 1 mL of 1–8 mM Ca(OH)2, 50 mM KCl, 10 mM Tris and 22–43 mM HEPES (pH = 7.35). In mole-fraction experiments for luminal Mg2+ or Ca2+, prior to formation of the BLM, 4–7 mM of MgCl2 or CaCl2 was added to the luminal solution to obtain varied mixtures with 1–4 mM Ca(OH)2 while maintaining the total divalent concentration at 8 mM. For pure Mg2+ or Ca2+, the luminal compartment was filled with 1 mL of 8 mM MgCl2 or CaCl2, 50 mM KCl, 10 mM Tris and 20 mM HEPES (pH = 7.35). The fusion of SR microsomes with the BLM was not successful enough when Zn2+ was present on the BLM luminal side, therefore, the RYR2 channel was exposed to 8 mM ZnCl2, 50 mM KCl, 10 mM Tris and 20 mM HEPES (pH = 6.83) by perfusing the luminal compartment after channel reconstitution under control conditions. In mole-fraction experiments, 4–7 mM ZnCl2 was also added to 1–4 mM Ca(OH)2 after RYR2 reconstitution. In addition, mixtures of 8 mM Ca(OH)2 with 8 mM ZnCl2 or 8 mM CdCl2 were also tested. In the control and for the MCl2 additions (4–7 mM), 1–2 mM cytosolic caffeine was used to moderately activate RYR2 channels (M: Mg, Zn or Ca). Similarly, these caffeine concentrations were sufficient for the activation of channels exposed to 8 mM Ca(OH)2 mixed with 8 mM ZnCl2 or 8 mM CdCl2. When ZnCl2 or MgCl2 was present in the luminal solution alone, caffeine concentration was increased to 6–7 mM to achieve a moderate RYR2 activity. The channel was incorporated into the BLM in a fixed orientation with the cytosolic side always exposed to the cytosolic solution because all RYR2 channels were sensitive to cytosolic caffeine [55,56,57,58]. At the end of experiments, the addition of ryanodine causing the characteristic transition to a half-conducting state was used to validate the channel identity [59].
2.2. Acquisition and Analysis of Single-Channel Recordings
Data acquisition and analysis of the open probability (PO), frequency of opening (F), average open (TO) and closed times (TC) were performed as detailed in [51,60]. The conductance (G) and the reversal potential (Erev) were determined from a linear regression of the current-voltage relationship, acquired by applying membrane voltage varying from −20 mV to +20 mV. The slope of the fitted line was equal to G, and Erev was taken as the intersection of the voltage axis with the linear fit. To avoid misinterpretation of Erev measurements, the Erev value was further corrected for the measured offset voltage at the end of the experiment and liquid junction potentials (LJPs). The LJP values were determined for all tested luminal solutions according to Barry et al. [61] and were in the range from −0.18 mV to +0.76 mV. The current amplitude at 0 mV (I0) was consequently corrected. For tested M2+ (M2+: Mg2+, Zn2+ or Ca2+), the dependence of Erev on a composition of M2+/Ca2+ mixture on the RYR2 luminal face was fitted by the following equation to determine the relative Ca2+/M2+ permeability coefficient (PCa/PM), referred to as intrinsic permeability [62]:
Equation (1) is derived from the well-known Goldman—Hodgkin—Katz (GHK) equation [63,64]; where F is Faraday’s constant, R is the universal gas constant and T is the absolute temperature. At room temperature (25 °C), RT/F is ~25.693 mV. The values of PCa/PTris and PCa/PK were, respectively, 29.545 [65] and 6.5 [46,66]. The subscripts C and L denote a cytosolic or luminal quantity, respectively. Similarly, the Erev plotted against pure luminal Ca2+ ([Ca2+]L) was fitted. For visualization purposes, the dependences of G and I0 on [Ca2+]L were fitted by the Michaelis—Menten equation.
Ion diffusion theory [67] was used to relate the local [Zn2+], Zn2+ current amplitude at 0 mV (I0,Zn), and distance from an open channel pore (r) (Equation (2)):
Here, DZn = 7.03 × 10−10 m2 s−1 [68] is the diffusion coefficient for Zn2+, k is the rate for Zn2+ binding to the buffer B, [B] = 1 mM is the buffer concentration, F = 96,500 C mol−1 is the Faraday constant. The only Zn2+ buffer present in most of our experiments was EGTA and its rate of Zn2+ binding, k, was 2.6 × 106 M−1 s−1 [69].
The results are reported as the average ± SEM or the fitted value ± SEM. Statistical comparison of differences in mole-fraction experiments (I0 and G) were made by two-way ANOVA with Tukey’s post hoc test. We also used one-way ANOVA with Tukey’s post hoc test to statistically compare changes in the pH of solutions when luminal M2+ was added and changes in the values of TO, TC, and F caused by luminal Zn2+. Unpaired Student’s t tests were performed to detect significant changes in the latency of RYR2 activity decay when [Cd2+]L was added and differences in I0, Erev, or G values when pH was changed. Paired Student’s t tests were utilized for statistical comparison of differences in I0, Erev, or G values when 8 mM [Zn2+]L or 8 mM [Cd2+]L was mixed with 8 mM [Ca2+]L, and changes in PO when NTA or TPEN was added to chelate Zn2+ in the cytosolic solution. Differences were regarded to be statistically significant at p < 0.05.
3. Results
RYR2 channels isolated from rat hearts were incorporated into the BLMs and recorded under voltage clamp conditions. The goal here was to measure the RYR2 current carried by Zn2+ in the lumen-to-cytosol direction. Data are presented that define permeation and gating properties including the intrinsic Zn2+ permeability of the RYR2 channel. Additionally, a potential role of the RYR2 Zn2+ current in local Zn2+ signaling was assessed by computing Zn2+ accumulation near potential molecular targets, such as neighboring RYR2 channels and the Zn2+ finger domain extending into the cytosolic vestibular portion of the RYR2 permeation pathway [55]. Finally, the ability of Cd2+ current in the lumen-to-cytosol direction to displace Zn2+ from the RYR2 Zn2+ finger was investigated.
Working with Zn2+ compounds is usually difficult, because they show a low solubility in water at the physiologically-relevant pH range [70,71]. We therefore tested more Zn2+ compounds and found that the chloride salt of Zn2+ was most appropriate for our purpose. In addition, 8 mM ZnCl2 was the maximal concentration available for testing to avoid formation of precipitates and thus prevent overestimation of the Zn2+ content. Of note, K+ and Tris+ cations were also added to the solutions, although both permeate the RYR2 channel [65] and could potentially interfere with RYR2 permeation properties for Zn2+ cations. However, they were added symmetrically to both RYR2 sides to diminish their contributions. Since only the chloride salt of Zn2+ could have been used, a small Cl− gradient (50 mM vs. 58–66 mM) was inevitably generated at 0 mV by Zn2+ additions to the luminal solution, thus providing a driving force for Cl− transport through the BLM. Despite the presence of Cl− channels in cardiac SR microsomes, we did not observe their activity under the conditions utilized in our study. The reason for this probably lies in the fact that Cl− gradient was not sufficient to produce recordable Cl− currents. Another possibility is that Cl− channels were not incorporated into the BLMs together with RYR2 channels.
3.1. The RYR2 Conductance for Zn2+
Our initial aim was to directly record the Zn2+ current by building the Zn2+ gradient across the BLM. However, after several unsuccessful attempts to reconstitute RYR2 channels with Zn2+ in the luminal solution, we modified our experimental approach. The luminal compartment was perfused with the Zn2+ solution after RYR2 channels were incorporated into the BLMs in the presence of 8 mM [Ca2+]L. Representative RYR2 recordings measured in the presence of 8 mM [Zn2+]L are shown in Figure 1A. The channels were solely activated by 6–7 mM cytosolic caffeine in the presence of non-activating free [Ca2+]C (90 nM) to record the longer and fully resolved open and closed channel events required for the unbiased determination of the Zn2+ current values (IZn). When the membrane voltage changed in 5 mV incremental steps from −10 mV to +10 mV, the RYR2 IZn increased from 0.31 ± 0.15 pA to 1.86 ± 0.16 pA. At 0 mV, the typical value of I0,Zn was 0.927 ± 0.093 pA. To estimate Zn2+ conductance (GZn), linear regression was performed on the current-voltage relationship (Figure 1B). Under asymmetrical conditions when the pure Zn2+ current was driven by the 8 mM gradient, the value of GZn was 81.1 ± 2.4 pS. Moreover, the reversal potential (Erev) was −11.8 ± 1.1 mV.
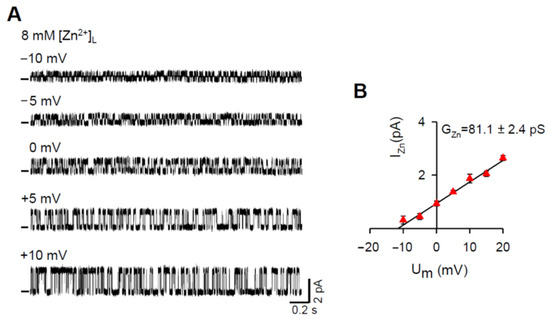
Figure 1.
The RYR2 channel is permeable to Zn2+. (A) Representative RYR2 currents (digitally filtered at 250–400 Hz), shown as upward deflections from the marked zero-current level, in the presence of 8 mM [Zn2+]L when Zn2+ was only the charge carrier. Current recordings were conducted at −10, −5, 0, +5, +10 mV and are from the same channel. RYR2 channels were activated by 6–7 mM caffeine in the presence of 90 nM free [Ca2+]C. (B) Current-voltage relationship of the RYR2 channel was well-fitted by a straight line with the GZn value of 81.1 ± 2.4 pS. Data shown are average ± SEM of 5–7 experiments and error bars are shown only when SEM is larger than symbol size.
3.2. Mole-Fraction Experiments under Non-Saturating Conditions
To evaluate the Zn2+ permeability coefficient relative to Ca2+, we investigated the mole-fraction behavior of I0, Erev, and G in the following Zn2+/Ca2+ mixtures on RYR2 luminal face. Particularly, 4, 5, 6 and 7 mM [Zn2+]L was mixed with 4, 3, 2 and 1 mM [Ca2+]L, respectively. We focused on the luminal solutions with the highest mole fraction of Zn2+ because if there were differences from the control (1–8 mM [Ca2+]L), they should be the most pronounced. Figure 2 depicts the representative RYR2 recordings at 0 mV in the control and when luminal Zn2+ was present. RYR2 channels were moderately activated by 1–2 mM caffeine and only for 8 mM [Zn2+]L, caffeine concentration was increased to 6–7 mM because luminal Ca2+, in contrast with other divalent cations, has been shown to be a strong sensitizer of the RYR2 channel to caffeine [50,51]. Since RYR2 gating is also strongly affected by luminal Ca2+ [51], the RYR2 channel exhibited a much faster gating, manifested by the increased number of open and closed events at the similar channel activity when only 8 mM [Zn2+]L was present (Figure 2). At the quantitative level, this finding was supported by gating parameter calculations. Results collected for the frequency of opening (F), average open (TO) and closed (TC) times are summarized in Table 1. The F value was increased more than three-fold when 8 mM [Ca2+]L was completely replaced by 8 mM [Zn2+]L. Because F is the reciprocal of a summation of TO and TC, a significant shortening in both TO and TC was observed. As we expected, the presence of even 1 mM [Ca2+]L precluded any change in RYR2 gating behavior when luminal Zn2+ was added. This is fully consistent with findings gained for various mixtures of luminal Mg2+ or Ba2+ with Ca2+ [51], thus further highlighting a specific ability of luminal Ca2+ to slow down RYR2 gating. Moreover, no appreciable differences were noted in all three gating parameters calculated for 8 mM [Zn2+]L and 8 mM [Mg2+]L, similar to that found previously for luminal Mg2+ and Ba2+ [51].

Figure 2.
Luminal Zn2+ mixed with Ca2+ increases RYR2 currents under non-saturating conditions. Representative RYR2 currents (digitally filtered at 250–400 Hz), shown as upward deflections from the marked zero-current level, in the absence (control, left five traces) and after luminal Zn2+ exposure (right five traces). In total, 4, 5, 6, 7 mM [Zn2+]L was added to 4, 3, 2, 1 mM [Ca2+]L (control), respectively, to keep the total divalent concentration constant at 8 mM. Representative RYR2 recordings for pure 8 mM [Ca2+]L (control) and 8 mM [Zn2+]L are also shown. RYR2 channels were activated by 1–2 mM caffeine in the presence of 90 nM free [Ca2+]C. In the presence of 8 mM [Zn2+]L, caffeine concentration was increased to 6–7 mM. Current recordings were conducted at 0 mV and are from different channels.

Table 1.
Effects of luminal M2+ (M2+: Mg2+, Zn2+ or Ca2+) on gating parameters of the RYR2 channel at PO ~ 0.5.
Further, it is evident from the raw current traces that for all tested Zn2+ additions the I0 substantially increased (Figure 2). The averaged results are presented in Figure 3A (middle panel). In the control, the I0 grew with increasing [Ca2+]L. The dependence of I0 on [Ca2+]L was well fitted by the Michaelis—Menten equation. The significant increase in I0 by ~45% and ~100% was caused by the addition of 6 mM and 7 mM [Zn2+]L, respectively. For lower [Zn2+]L, an increasing trend was also detected; however, it did not achieve significance. To demonstrate the validity and reliability of our Zn2+ measurements, we generated the same dataset for Mg2+ and Ca2+ (chloride salts). Mg2+ and Zn2+ share the intrinsic physical properties such as ionic size, charge density and hydration enthalpy (Table 2) relevant to permeation through ion channels [52,53,54]; therefore, we would expect similar passage of these cations through the RYR2 pore. As the positive control, varied CaCl2 concentrations were added to the luminal RYR2 side to reach the total 8 mM [Ca2+]L. Here, M2+ stands for Mg2+, Zn2+ or Ca2+ for simplicity. Luminal Zn2+ had less ability to affect I0 carried by Ca2+ than luminal Mg2+. This was particularly noted when 7 mM [Mg2+]L caused more than three-fold increase in the RYR2 current driven by the 1 mM Ca2+ gradient (Figure 3A, left panel). Lower [Mg2+]L had a smaller but still significant impact. Because the RYR2 I0 was considerably lower when 8 mM [Ca2+]L was replaced by 8 mM [Mg2+]L, the dominance of Mg2+ (6–7 mM) in the mixture with Ca2+ was not sufficient to reach the value of I0 with pure 8 mM [Ca2+]L. Therefore, only the Ca2+ additions (chloride salt) caused such a large increase that the I0 values became similar to those obtained for both 8 mM Ca(OH)2 (2.835 ± 0.094 pA) and 8 mM CaCl2 (2.69 ± 0.11 pA) (Figure 3A, right panel). I0 data indicate that the RYR2 channel apparently has a lower conductance and intrinsic permeability for Zn2+ than for Ca2+ and Mg2+.
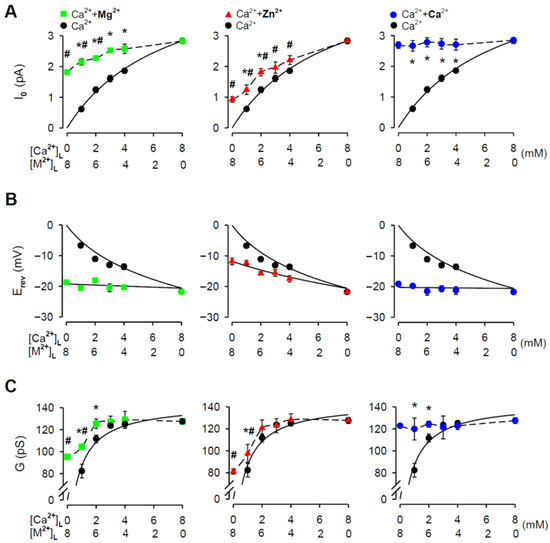
Figure 3.
M2+/Ca2+ mole-fraction experiments under non-saturating conditions. The I0 (A), Erev (B), and G (C) plotted against a composition of M2+/Ca2+ mixture on the RYR2 luminal face (M2+: Mg2+–green square, Zn2+–red triangle, or Ca2+–blue circle). In total, 4, 5, 6, 7 mM [M2+]L was added to 4, 3, 2, 1 mM [Ca2+]L, respectively, to keep the total divalent concentration constant at 8 mM. Data obtained for pure 8 mM [M2+]L are also included. As the control, the [Ca2+]L-dependence of Erev, G and I0 is shown (black circle). * Significantly different from the respective control values; # significantly different from the control value obtained for 8 mM [Ca2+]L (two-way ANOVA with Tukey’s post hoc test). The solid lines in (B) are the best fits using Equation (1) and in (A) and (C) using the Michaelis—Menten equation. The dashed lines in (A) and (C) are drawn point to point. Data shown are average ± SEM of 5–15 experiments and error bars are shown only when SEM is larger than symbol size.

Table 2.
Physical characteristics of divalent cations implicated in permeation through ion channels.
We tested this possibility by determining Erev and G from current-voltage relationships when the membrane voltage changed in 5 mV incremental steps from −20 mV to +20 mV. All collected current-voltage plots were Ohmic (data not shown) and well fitted by a straight line. In the control, the Erev values substantially decreased with raising [Ca2+]L from 1 mM to 8 mM (Figure 3B). Equation (1) well reproduces the luminal Ca2+-dependence of Erev, thus validating the accuracy of our Erev determination. When luminal Zn2+ was added, the Erev decreased, as expected for the addition of permeant cation, while Erev for pure 8 mM [Zn2+]L was −11.8 ± 1.1 mV (Figure 3B, middle panel). To estimate the intrinsic Zn2+ permeability of the RYR2 channel, the dependence of Erev on a composition of Zn2+/Ca2+ mixture was then fitted by Equation (1) yielding the relative Ca2+/Zn2+ permeability coefficient of 2.65 ± 0.19 (PCa/PZn). In comparison, the Erev data collected for Mg2+/Ca2+ mixtures were well fitted with Equation (1) when PCa/PMg was 1.146 ± 0.071 (Figure 3B, left panel). This is in good agreement with the intrinsic Mg2+ permeability reported for the RYR2 channel [46]. As expected, the Erev measured in Ca2+/Ca2+ mixtures did not depend on a mixture composition because 8 mM [Ca2+]L was always present on the RYR2 luminal face. The fitted value for PCa/PCa was equal to 1.035 ± 0.061 (Figure 3B, right panel).
In general, ion translocation through the channel pore is characterized by a specific conductance, therefore, we examined G as a function of the Zn2+/Ca2+ mixture composition (Figure 3C, middle panel). For the pure luminal Ca2+ solutions, the G values plotted against [Ca2+]L were fitted by the Michaelis—Menten equation with almost full saturation at 8 mM [Ca2+]L (Figure 3C). When luminal Zn2+ was added, we revealed a significant increase in G only for the addition of 7 mM [Zn2+]L to 1 mM [Ca2+]L (Figure 3C, middle panel), evidently because of the weaker intrinsic Zn2+ permeability (PCa/PZn = 2.65 ± 0.19) and a significantly lower RYR2 conductance for Zn2+ (81.1 ± 2.4 pS for 8 mM [Zn2+]L vs. 127.5 ± 1.8 pS for 8 mM [Ca2+]L). The situation for luminal Mg2+ was slightly different because the Mg2+ additions resulted in a greater change in the G values (Figure 3C, left panel). This was despite that the RYR2 channel exhibited a significantly lower G for Mg2+ than for Ca2+ when testing 8 mM gradients (95.3 ± 1.4 pS vs. 127.5 ± 1.8 pS, respectively). When luminal Ca2+ was added, two identical cations were mixed, and therefore the G values were not changed (Figure 3C, right panel). In summary, the I0, G and Erev results collectively indicate that the passage of Zn2+ through the RYR2 channel is less effective than even for Mg2+, despite having similar physical properties (Table 2).
3.3. Effects of pH When Luminal Zn2+ Was Added
The chloride salt of Zn2+ has been reported to change the pH of solutions because it is a weak acid [74]. Such changes could substantially affect measurements of the RYR2 G and intrinsic permeability [75,76]. To evaluate this potential source of inaccuracy, we first investigated the effects of the Zn2+ additions on the pH of the luminal solutions, while retaining the same volumes used for the single-channel recordings. Mg2+ and Ca2+ were examined in a similar manner. The summary data are plotted as a function of M2+/Ca2+ mixture content in Figure 4A. While Mg2+ and Ca2+ additions (4–7 mM) induced no significant changes in the pH from the control, luminal Zn2+ had a substantial effect with increasing its mole fraction. The pH of the luminal solution with no Ca2+ present (10 mM Tris, 20 mM HEPES, 50 mM KCl) dropped from 7.34 ± 0.007 to 6.83 ± 0.017 when 8 mM [Zn2+]L was added. In contrast, there was no pH change with 8 mM [Mg2+]L or [Ca2+]l. Importantly, experimental conditions with 8 mM [Zn2+]L were limited to a lower pH of 6.83 because, otherwise, Zn2+ precipitates occurred. To test if the large decrease in pH observed for the addition of 8 mM [Zn2+]L might produce a shift in I0, Erev and G values, we tested 8 mM [Ca2+]L (hydroxide) buffered to pH values of 6.83 and 7.35. Summary results presented in Figure 4B show that pH decrease was essentially without any significant effect. These data indicate that all results of mole-fraction experiments collected for luminal Zn2+ can be indeed interpreted as a manifestation of the intrinsic Zn2+ permeability displayed by the RYR2 channel.
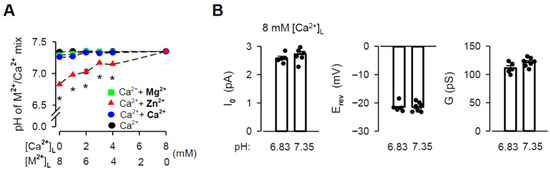
Figure 4.
Changes in pH caused by the Zn2+ additions have no effects on RYR2 permeation properties. (A) Effects of the M2+ additions on pH of M2+/Ca2+ mixture (M2+: Mg2+−green square, Zn2+–red triangle, or Ca2+–blue circle). In total, 4–7 mM [M2+]L was added to 1–4 mM [Ca2+]L, keeping the total divalent concentration constant at 8 mM. As the control, the [Ca2+]L-dependence of pH is shown (black circle). Data obtained for the pure 8 mM [M2+]L (chloride salt) or 8 mM [Ca2+]L (hydroxide) are also included. * Significantly different from the respective control values (one-way ANOVA with Tukey’s post hoc test). (B) The I0, Erev and G values obtained for pH of 6.83 and 7.35 when 8 mM [Ca2+]L (hydroxide) was present (unpaired Student’s t test). Data shown are average ± SEM of 5–7 experiments, and error bars in (A) are shown only when SEM is larger than symbol size. Data points shown in (B) are individual measurements.
3.4. Effects of Luminal Zn2+ on RYR2 Permeation Properties under Near-Saturating Conditions
As seen in Figure 3C, the [Ca2+]L-dependence of the RYR2 G almost saturated at 8 mM. This indicates that all mole-fraction experiments shown in Figure 3 were performed at [Ca2+]L, which is not at the saturating portion of the G-[Ca2+]L curve. Under such conditions, luminal Zn2+ would not strongly compete with Ca2+ for occupancy of the RYR2 pore, if at all [77,78]. To further examine the mechanisms of Zn2+ permeation, we extended the I0, Erev and G measurements to include near-saturating conditions. Because 8 mM concentration was the highest possible [Zn2+]L available for testing, we could use only a mixture with 8 mM [Ca2+]L to allow a reasonable competition between these cations [79]. Figure 5A shows representative current traces of the same caffeine-activated RYR2 channel recorded at 0 mV in the absence and presence of luminal Zn2+. One can see that 8 mM [Zn2+]L slightly reduced the current amplitude carried by 8 mM [Ca2+]L. Average results are presented in Figure 5B (left panel). While the reduction in the I0 was small but significant, Erev changed only negligibly by ~−1.35 mV (Figure 5B, middle panel). This small shift toward more negative values was also predicted by Equation (1) for the estimated PCa/PZn of 2.65 ± 0.19 (−23.97 mV theoretical vs. −23.59 ± 1.03 mV experimental). Inevitably, the G significantly decreased (Figure 5B, right panel). Of note, the aforementioned changes were not related to pH, although it dropped from 7.35 ± 0.04 to 7.058 ± 0.029 by the luminal addition of 8 mM [Zn2+]L. As shown in Figure 4B, an even larger decrease in pH from 7.35 to 6.83 did not produce any significant effect. Taken together, the decrease in I0 and G values by Zn2+ addition to almost saturating [Ca2+]L could indeed be interpreted as a consequence of competition between two permeant cations inside the RYR2 pore, which exhibits a different intrinsic permeability and/or conductance for each cation.
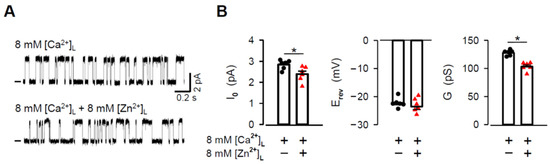
Figure 5.
Luminal Zn2+ decreases the RYR2 current under near-saturating conditions. (A) Representative RYR2 currents, shown as upward deflections from the marked zero-current level, for 8 mM [Ca2+]L (control, top trace) and when 8 mM [Zn2+]L was added (bottom trace). RYR2 channels were activated by 1–2 mM caffeine in the presence of 90 nM free [Ca2+]C. Current recordings were conducted at 0 mV and are from the same channel. (B) The I0, Erev and G values obtained in the absence and after luminal Zn2+ exposure. * Significantly different from the control values when only luminal Ca2+ was present (paired Student’s t test). Data shown are average ± SEM of 6 experiments. Data points shown in (B) are individual measurements (Ca2+–black circle and Zn2+/Ca2+–red triangle).
3.5. The Zn2+ Finger Located within the C-Terminus of the RYR2 Channel as a Potential Target of the Zn2+ Current
In an attempt to identify a potential target of the RYR2 Zn2+ current, we focused on small protein domains termed the Zn2+ fingers whose structure is highly organized upon Zn2+ complexation [80]. C2H2 type with two Cysteines (Cys or C) followed by a pair of Histidines (His or H) is one of the most ubiquitous coordination sites for Zn2+ (Figure 6A). This configuration has been recognized within the C-terminal tail of the RYR2 channel [55] and the inositol-1,4,5-trisphosphate receptor isoform-1 (IP3R1) [81,82]. The IP3R1 channel participates in Ca2+ transport across the ER membrane in all cell types (reviewed in [83]). The classical C2H2 topology has the consensus sequence C-X2–5-C-X12-H-X3–4-H (X is for any amino acid) (reviewed in [84]). To determine and compare spacing in the C2H2 Zn2+ fingers of RYR2 and IP3R1 channels, we computed the alignment of their C-terminal sequences using the ClustalX method (Figure 6B; [85]). Instead of focusing on rat channels, we extended our analysis to other mammalians such as mouse, dog and human, which are commonly used as a source of RYR2 and IP3R1 channels for BLM experiments. Within this set, the first Cys and the second Cys are separated by two residues and this spacing is well conserved across mammalian RYR2 and IP3R1 channels. Further, the interhistidine spacing of four residues is conserved as well. Up to here, the topology of RYR2 and IP3R1 Zn2+ fingers matches the classical C2H2 pattern. However, we revealed one deviation in the distance between Cys-Cys and His-His pairs. The spacing of 16 residues is uncommon (Figure 6B). According to the consensus sequence, 12 residues are allowed, albeit, it has been shown that 10–14 residues could also be tolerated (atypical C2H2, Figure 6A). In the RYR2 sequence, specifically, we located one additional conserved His that is 11 residues away from the Cys-Cys pair and four residues from the following His. It is thus conceivable that this newly-identified His residue could act as the third Zn2+ ligand matching the overall spacing of the atypical C2H2 topology.
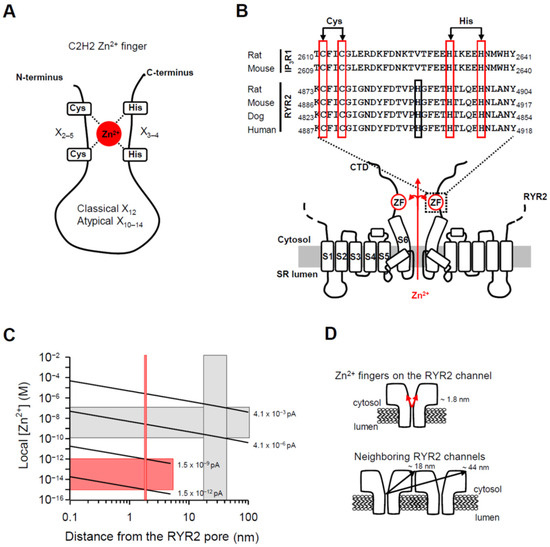
Figure 6.
The RYR2 Zn2+ finger might be locally controlled by the Zn2+ current. (A) A schematic representation of the C2H2 Zn2+ finger consisting of two Cysteines (Cys or C) and two Histidines (His or H), which are coordinated by Zn2+ cation. Usually, 12 residues separate the last Cys and the first His residues (classical C2H2 pattern). In atypical C2H2 Zn2+ fingers which still retain the ability to bind Zn2+, 10–14 residues are tolerated. (B) Sequence alignment of the Zn2+ finger motifs identified within the C-terminal tail of the rat and mouse IP3R1 channels [81,82] and the RYR2 channel [55] from indicated mammals. The sequences were taken from the UniProtKB database. The Cys and His residues of the Zn2+ finger domains with uncommon spacing (16 residues separating the Cys-Cys pair from the His-His pair) are boxed in red. The conserved His in the RYR2 sequences located 11 residues from the Cys-Cys pair, but not in the IP3R1 sequence, is highlighted in black. The membrane topology model of two opposing RYR2 subunits with six transmembrane domains was generated from recent work on the near-atomic resolution structure of the RYR2 isoform [55]. The putative Zn2+-finger domains (ZF) within the C-terminal tails might be supplied with luminal Zn2+ emanating from the RYR2 pore in the lumen-to-cytosol direction when the channel is open. (C) The theoretical prediction of local Zn2+ in respect to a distance from the Zn2+ exit site (the open RYR2 pore). Zn2+ accumulation profile was calculated with buffer present (1 mM EGTA). The red vertical bar indicates the Zn2+ finger distance from a Zn2+ point source (~1.8 nm). The gray vertical box indicates the center-to-corner distances covering the cytosolic domain of the neighboring RYR2 channel (~18–44 nm). At a distance of ~1.8 nm, the I0,Zn of 1.5 × 10−12–1.5 × 10−9 pA is required to elevate [Zn2+] up to the femtomolar range (horizontal red bar), sufficient to saturate the Zn2+ fingers. At a distance of ~18–44 nm, the I0,Zn of 4.1 × 10−6–4.1 × 10−3 pA is required to reach activating [Zn2+] from 100 pM to 100 nM (horizontal gray bar). (D) Schematic depicting distances of the RYR2 Zn2+ fingers and cytosolic domain of the neighboring RYR2 channel from the Zn2+ exit site to help place the values on the X axis, shown in (C), in context.
Despite a little doubt about the composition of the RYR2 Zn2+ finger, its location at the joint between the CTD (C-terminal domain) and the last transmembrane segment (S6) remains indisputable [55]. Since the Zn2+ finger domain is oriented toward the channel permeation pathway it is feasible to test the possibility that this domain is directly targeted by the Zn2+ current (Figure 6B). We began by calculating the free [Zn2+] profile around an open RYR2 pore. Considering the extremely high binding affinities of numerous Zn2+ fingers in the femtomolar range [86,87,88], Figure 6C shows that an extremely small Zn2+ current (from 1.5 × 10−12 pA to 1.5 × 10−9 pA) would be sufficient to achieve femtomolar [Zn2+] around the Zn2+ finger located ~1.8 nm from the channel pore (Figure 6D, top panel; [55]). Since the RYR2 channel has been shown to be sensitive to cytosolic Zn2+ [89,90] and the channel itself seems to be resistant to its own current [91], we extended our calculations also to the neighboring RYR2 channels that might be targeted via Zn2+ diffusion. This scenario should also receive attention because in the heart, RYR2 channels operate in small packed clusters [92,93] and at the single channel level, two or more RYR2 channels can be occasionally reconstituted, perhaps at their normal cellular spacing. Recent BLM studies have presented evidence that Ca2+-activated RYR2 channels are substantially modulated by cytosolic Zn2+ (100 pM–100 nM) [89,90]. To reach this magnitude change over the entire cytosolic domain of the neighboring channel, where Zn2+ binding sites must be located, the I0,Zn from 4.1 × 10−6 pA to 4.1 × 10−3 pA is required (Figure 6C). Center-to-corner distances of ~18 nm and ~44 nm were taken from Liu et al. [91] and are indicated in Figure 6D (bottom panel). Such I0,Zn values are 122- to 122,000-fold lower in comparison to the physiologically relevant amplitude of the Ca2+ current (~0.5 pA) flowing through the RYR2 channel [77,78]. Thus, our calculations suggest that RYR2 function might be significantly affected by almost negligible Zn2+ current of more than or equal to 4.1 × 10−6 pA, which could be reachable in cardiomyocytes.
3.6. Displacement of Zn2+ from the RYR2 Zn2+ Finger
To further test the idea that the Zn2+ current supplies Zn2+ cations essential for the stabilization of the RYR2 Zn2+ finger, we assessed the accessibility of this Zn2+ binding site from either channel side (cytosolic and luminal) by displaying Zn2+ from its structure. As a nondestructive readout for this event, we monitored RYR2 PO because it has been evidenced that the Zn2+ finger is fundamental to the channel activation by caffeine [55]. First, we tested whether Zn2+ chelating agents such as NTA or TPEN could compete for Zn2+ when applied to the RYR2 cytosolic side. Based on the purity assays of ultrapure chemicals (Sigma-Aldrich) and the strong binding affinity of EGTA to Zn2+, we estimated that ~2.13 pM [Zn2+]C should have always been present in the cytosolic solution. When 5 mM NTA was added, [Zn2+]C dropped down to 0.5 pM. During 20-min incubation, the RYR2 PO did not change significantly (Figure 7A, left panel). Although, 5 µM TPEN decreased free [Zn2+]C to the attomolar range (7.2 aM = 0.0072 fM), no significant change in the channel activity was observed during 20-min exposure (Figure 7A, right panel). Insufficiently high Zn2+ affinities of NTA and TPEN appear to be unlikely because binding affinities of numerous Zn2+ fingers are in the femtomolar range [86,87,88]. Presumably, a substantially longer incubation (it could reach hours) was needed to remove obviously tightly bound Zn2+ in the RYR2 Zn2+ finger, which could also be less approachable sitting deep in the RYR2 cytosolic vestibule. A short BLM lifetime, however, limited such demanding conditions. In a further attempt to displace Zn2+, we assessed whether a well-known competition between Zn2+ and Cd2+ in different eukaryotic Zn2+ fingers [94,95,96,97] could be a more direct approach. Given the comparable physical properties of Cd2+ with those of permeant Ca2+ and Mg2+ (Table 2), Cd2+ passage through the RYR2 channel might be expected. Indeed, we found that the luminal addition of 8 mM [Cd2+]L to 8 mM [Ca2+]L significantly decreased the RYR2 G, thus reflecting Cd2+ permeation (Figure 7B), as we evidenced for Zn2+ under the same conditions (Figure 5). This finding was crucial because it allowed us to approach the Zn2+ finger from the RYR2 luminal side by the Cd2+ current in the lumen-to-cytosol direction. Importantly, a drop in pH from 7.350 ± 0.010 to 7.234 ± 0.016 when 8 mM [Cd2+]L was added did not contribute to a decrease in G because a much larger pH change from 7.35 to 6.83 was essentially without effect (Figure 4B). In Figure 7C (top trace), the Ca2+ current was only passing the caffeine-activated RYR2 channel in the presence of 8 mM [Ca2+]L. After the addition of 8 mM [Cd2+]L, a sudden decay of channel activity occurred after 8-min incubation and persisted within 20 min of exposure (Figure 7C, bottom two traces). Figure 7D shows that the latency of RYR2 activity decay varied considerably with [Cd2+]L. As [Cd2+]L increased from 8 mM to 16 mM, the channel activity was suppressed within a shorter time due to a greater ability of Cd2+ to substitute for Zn2+. Importantly, RYR2 activity was not recovered when caffeine concentration was substantially increased to 6–7 mM. This indicates that RYR2 sensitivity to caffeine was not attenuated as a result of Cd2+/Ca2+ competition directly at the RYR2 luminal side, as previously shown for Sr2+, Mg2+, or Ba2+ (when competing with 1 mM luminal Ca2+) [51]. Rather, the Cd2+ current destabilized the Zn2+ finger by replacing Zn2+ and thus promoting the decay of channel activity.
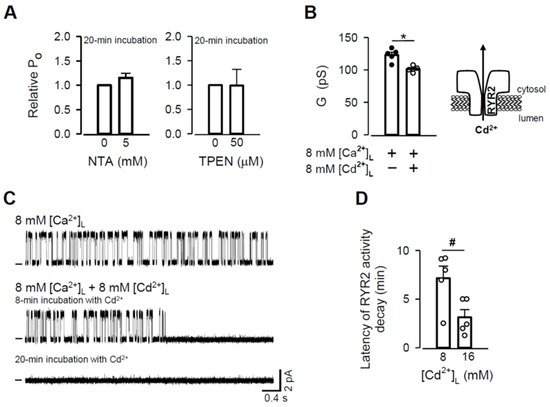
Figure 7.
Zn2+ displacement from the RYR2 Zn2+ finger. (A) Effects of NTA (left panel) and TPEN (right panel) added to the cytosolic solution on RYR2 PO as a consequence of a decrease in [Zn2+]C (paired Student’s t test). (B) The effect of Cd2+ addition (8 mM) on the RYR2 G under near-saturating conditions when 8 mM [Ca2+]L was present. * Significantly different from the control values when only luminal Ca2+ was present (paired Student’s t test). (C) Representative RYR2 currents, shown as upward deflections from the marked zero-current level, for 8 mM [Ca2+]L (control, top trace) and when 8 mM [Cd2+]L was added (two bottom traces). RYR2 channels were activated by 1–2 mM caffeine in the presence of 90 nM free [Ca2+]C. Current recordings were conducted at 0 mV and are from the same channel. (D) Latency of RYR2 activity decay as a function of [Cd2+]L. # Significantly different from 8 mM [Cd2+]L (unpaired Student’s t test). Data shown are average ± SEM of 5–7 experiments. Data points shown in (B) and (D) are individual measurements (Ca2+–black circle and Cd2+/Ca2+–white circle).
4. Discussion
There has been a rapidly growing interest in the field of Zn2+ biology in the last ten years because of significant advances in the knowledge of Zn2+ chemistry and biochemistry. Moreover, defective Zn2+ cellular homeostasis has been implicated in the pathophysiology of cancer and diabetes, common diseases which greatly affect global health. This has further stimulated an intensive research effort to understand the importance of Zn2+ in a diverse spectrum of biological functions. In mammalian cells, large amounts of Zn2+ accumulate in various cellular compartments and organelles [34,35,36], mainly as a structural or catalytic factor directly regulating a great number of protein functions. To be candidates for Zn2+ signaling, intracellular Zn2+ storages must have proteins for both Zn2+ release and reuptake. Although, Zn2+ transporters such as ZIP and ZnT with subcellular distribution have been identified in various mammalian cells (reviewed in [17,18,19]), a limited number of studies have been conducted in cardiomyocytes. However, at least one member of the ZIP family responsible for Zn2+ release to the cytoplasm and one member of the ZnT family mediating Zn2+ transport in the opposite direction have been found [27,40] and localized to the cardiac SR membrane [41]. Although, these transporters assumingly participated in Zn2+ signaling originating from the SR [8,38], we put forward the possibility that the cardiac ryanodine receptor (RYR2) located in the SR membrane also contributes. It has been known for many years that the RYR2 channel is responsible for a massive release of Ca2+ from the SR required for muscle contraction (reviewed in [98]). Its role in Zn2+ signaling has however been overlooked. Tuncay et al. [8] revealed a significant suppression of Zn2+ transients by ryanodine in electrically stimulated cardiomyocytes. Ryanodine is a plant alkaloid that binds to all three isoforms of the RYR channel with high affinity and either holds the channel open in a state of reduced conductance or causes a complete inhibition, depending on its concentration [59,99,100,101]. According to Tuncay et al. [8], Zn2+ transients mostly resulted from an increase in [Ca2+]C and subsequent Zn2+ displacement from intracellular binding sites. In such a scenario, Zn2+ transients would be suppressed in the presence of ryanodine, because Ca2+ release from the SR mediated by the RYR2 channel would be compromised. However, considering similar physical properties of Zn2+ with those of permeant Mg2+ (Table 2), the Zn2+ release through the RYR2 channel which would inevitably accompany Ca2+ release, could be an alternative explanation. Our study was therefore designed to investigate whether Zn2+ permeates the RYR2 channel reconstituted into the BLM.
4.1. Permeation Properties of the RYR2 Channel for Zn2+
In this study, the permeation properties of the rat RYR2 channel were examined under various ionic conditions. As shown in Figure 1, the Zn2+ currents through the RYR2 channel in the luminal-to-cytosol direction were recorded for the first time. Our results are compatible with several studies reporting a weak Zn2+ conductance for voltage-gated Ca2+ channels [102,103,104,105]. When 8 mM [Zn2+]L was present and used as a sole charge carrier, the RYR2 I0,Zn was 0.927 ± 0.093 pA. It is comparable to, but significantly smaller than, those obtained for luminal Ca2+ (2.835 ± 0.094 pA) or even Mg2+ (1.822 ± 0.079 pA) under similar conditions. Accordingly, the G values fall in the sequence Ca2+ (127.5 ± 1.8 pS) > Mg2+ (95.3 ± 1.4 pS) > Zn2+ (81.1 ± 2.4 pS). Such differences between Zn2+ and Mg2+ were unexpected as these cations have similar physical properties relevant to ion channel permeation (Table 2). To gain a better understanding, we evaluated the Zn2+ permeability coefficient relative to Ca2+. We performed mole-fraction experiments with a mixture of Zn2+ and Ca2+ on the luminal side (8 mM total concentration) and monitored the changes in RYR2 permeation properties. Zn2+ additions to the luminal solution resulted in specific changes when we used non-saturating concentrations of luminal Ca2+ in respect to the RYR2 conductance (<8 mM). The I0 and G were increased while the Erev was shifted towards more negative values (Figure 3). The changes were more pronounced in mixtures with the highest mole fractions of Zn2+. Under near-saturating conditions, where a competition between Zn2+ and Ca2+ could occur within the RYR2 permeation pathway [79], the I0 and G were significantly decreased, but no change was revealed for Erev when 8 mM [Zn2+]L was added to 8 mM [Ca2+]L (Figure 5). Since the I0 and G did not drop below the values obtained for pure luminal Zn2+, this phenomenon cannot be interpreted as the anomalous mole fraction effect occurring when the values of I0 or G are lower in a mixture of two ions than in the pure solutions of individual ions at the same total concentration. To make such conclusion, we assumed that the permeation properties of the RYR2 channel at 8 mM and 16 mM [Zn2+]L (the latter is not reachable at pH ~7.00) are similar. This was not an unreasonable proposal, as 8 mM concentration of divalent cations was found to be near-saturating.
In comparison to permeant Mg2+, Zn2+ displayed much less ability to contribute to permeation properties. The permeability coefficient for Zn2+ (PCa/PZn = 2.65 ± 0.19), estimated by fitting dependence of Erev on a composition of Zn2+/Ca2+ mixture by Equation (1), was found to be ~2.3-fold lower than that of Mg2+ (PCa/PMg = 1.146 ± 0.071). Because the permeability coefficient has been shown to be a diffusion component of channel selectivity and reflects how well particular ions pass through the channel pore [106], our results clearly indicate that the RYR2 channel can differentiate, albeit only moderately, between Zn2+ and Mg2+ or Ca2+, providing a slower path to Zn2+. In respect to G, it has been proven that this parameter contains information not only about ion movement throughout the channel pore, but it also measures how well particular ions enter and exit the channel [62,106]. Since Zn2+ and Mg2+ exhibit a similar hydration enthalpy (Table 2), critically implicated in ion entry into the narrowest region of the channel pore [52,62], and as Zn2+ moves through the RYR2 channel more slowly than Mg2+, a lower GZn is an inevitable consequence.
4.2. Physiological Implications
Ion channels are predominantly involved in rapid signal transduction because ion movement through ion channels is usually much faster than passive transport down the electrochemical gradient mediated by carrier-type transporters [107]. During each heartbeat, RYR2 channels are responsible for the release of a huge amount of Ca2+ from the SR lumen. Our study shows that the same route is also suitable for Zn2+ mobilization from the SR, and thus Zn2+ might contribute to the RYR2 current of ~0.5 pA [77,78] during cardiac excitation-contraction coupling, albeit primarily driven by a 10,000-fold Ca2+ gradient across the SR membrane (100 nM in the cytosol, 1 mM in the SR lumen [108,109]). This seems reasonable, if we consider the extremely low free Zn2+ level of ~100 pM in the cytosol of cardiomyocytes [3,110] and more than 60-fold higher free [Zn2+] in the cardiac SR lumen (>6 nM, [110]). The existence of a Zn2+ gradient, albeit ~166-fold smaller than for Ca2+, together with a considerable RYR2 permeability for Zn2+, is compatible with the biological significance of the Zn2+ current through the RYR2 channel. At the cellular level, Tuncay et al. [8] support this idea by visualizing ryanodine-sensitive Zn2+ transients in cardiomyocytes having similar kinetics to those of Ca2+. Furthermore, both Ca2+ and Zn2+ transients required a small Ca2+ influx from the extracellular space through the L-type Ca2+ channel in the plasma membrane. Thus, it seems reasonable to propose that the RYR2 channel has a real Zn2+ transport function in cardiomyocytes (Figure 8), but the cellular target for this Zn2+ release has not been established. Certainly, the release does not trigger contraction because Zn2+ only negligibly interacts with proteins of the contractile machinery [111]. However, 100 pM–100 nM Zn2+ has been shown to shape Ca2+ release by amplifying the Ca2+-induced RYR2 activity [89,90]. According to our simple calculations, the Zn2+ current of more than or equal to 4.1 × 10−6 pA (~0.0008% of the RYR2 current in cardiomyocytes) should be sufficient for the building up of activating [Zn2+] near neighboring RYR2 channels (Figure 6C). This estimation suggests that almost negligible Zn2+ current mediated by one RYR2 channel could amplify Ca2+ release through neighboring RYR2 channels. This regulatory process might be impaired in cardiovascular diseases, which are often associated with Zn2+ deficiency [39,112,113,114]. It may arise from low intake, malabsorptive syndromes, or the administration of various medications (reviewed in [115]). In Zn2+-deficient cardiomyocytes, intracellular Zn2+ stores including the SR have been indeed found to be substantially depleted of Zn2+ [116]. One would then expect that the Zn2+ gradient across the SR membrane will be reduced, resulting in a compromised Zn2+ release. This implies that appropriate Zn2+ supplementation might be beneficial in the management of cardiovascular diseases, directly affecting Ca2+ signaling fundamental to the cardiac contraction. So far, a few studies have reported an improvement in symptoms of heart failure with Zn2+ supplementation [116,117,118]. Some clues on the physiological relevance of the RYR2 Zn2+ current, can also be found in work of Atar et al. [104], where changes in free [Zn2+]C have been linked to the activation of transcription in electrically stimulated cardiomyocytes, given the catalytic and structural roles of Zn2+ in DNA- and RNA-binding proteins.
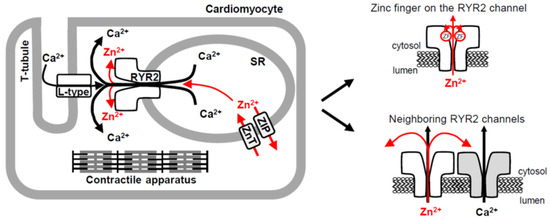
Figure 8.
The RYR2 channel provides a novel pathway for rapid Zn2+ transport in cardiomyocytes. Schematic illustrating three pathways (one novel) for Zn2+ transport across the cardiac SR membrane. Two Zn2+ transporter protein families, ZIP and ZnT, play an essential role in cardiac Zn2+ homeostasis and mediate a slow-rate transport. In the heart, Zn2+ release through the RYR2 channel, accompanying a robust Ca2+ release triggered by small Ca2+ entry through the L-type Ca2+ channel, could also contribute to global and/or local Zn2+ signaling which requires a fast-rate transport. At the local scale, the Zn2+ current flowing through the RYR2 channel might target its intrinsic Zn2+ finger situated within the C-terminal tail of all four RYR2 subunits. In addition, the RYR2 channel (colored gray) might be stimulated by Zn2+ cations emanating from the neighboring RYR2 channel (colored white). Arrows indicate the direction of Zn2+ (red) and Ca2+ (black) mobilization.
Numerous proteins with a variety of functions, including transcription, protein degradation, and DNA repair harbor small structured domains, the Zn2+ fingers, whose stability is dramatically improved by Zn2+ binding. The C2H2 Zn2+ finger with two Cys (C) followed by a pair of His residues (H) (Figure 6A) has also been identified within the C-terminal tail of structurally related IP3R1 [81,82] and RYR2 channels [55] (Figure 6B). When individual or combined Cys and His residues were mutated, IP3R1 function was completely abolished highlighting the critical role of the C-terminus in channel gating [81,82]. The C-terminal region is highly conserved in both the IP3R and RYR families [119] and therefore it is not surprising that similar results were obtained for the RYR2 channel. The channel completely lost its ability to gate when both Cys residues and only the first of a His-His pair were individually substituted [55]. Surprisingly, mutation of the final His residue caused no change and the RYR2 channel retained its function. This is in sharp contrast to the IP3R1 channel, where a critical importance of the final His residue in the Zn2+ finger sequence has been reported [82], albeit the spacing between the Cys-Cys and His-His pairs is uncommon (16 instead of classical 12 or atypical 10–14 residues, Figure 6B). The same deviation has also been demonstrated for the RYR2 channel [55], although we found one nearer conserved His, 11 residues away from the Cys-Cys pair (atypical C2H2 pattern), that could potentially serve as a third Zn2+ ligand when pairing with the following His (Figure 6B). To address such uncertainty in the composition of RYR2 Zn2+ finger, the mutation of the newly-identified His residue should be studied in the context of RYR2 function and Zn2+ binding properties.
The Zn2+ finger domain of each of the four RYR2 subunits impinges toward the central vertical axis of the channel permeation pathway, thus it may be well supplied with the Zn2+ current to ensure a proper channel function (Figure 8). In agreement with this prediction, the Cd2+ current passing the RYR2 channel in the lumen-to-cytosol direction caused a sudden decay of RYR2 activity. Since this action was specific for Cd2+ because the luminal additions of other divalent cations never had such detrimental effects [50,51], it was highly likely due to Zn2+ to Cd2+ replacement in the RYR2 Zn2+ finger. Obviously, Zn2+ displacement by Cd2+ was not structurally tolerated, and therefore RYR2 function was abolished. If the RYR2 Zn2+ current was fundamental to stabilization of the Zn2+ finger structure, one could ask why the channel function was not impaired in the absence of luminal Zn2+ when only Ca2+ or Mg2+ currents were carried by the channel. The answer could be found in Zn2+ impurities coming from otherwise ultrapure chemicals. We estimated that ~2.13 pM and ~0.8–1.0 nM free [Zn2+] was present in the cytosolic and luminal solutions, respectively. In generally, eukaryotic Zn2+ fingers possess the extremely high binding affinities in the femtomolar range [86,87,88], therefore, picomolar [Zn2+] on the RYR2 cytosolic side should have been sufficient to saturate the Zn2+ finger, albeit the deep position of this Zn2+-stabilized domain within the cytosolic portion of the RYR2 permeation pathway could considerably impair this process. We tested this idea by adding strong Zn2+ chelators such as NTA and TPEN to reach the atto-femtomolar free [Zn2+]C, and thus facilitate Zn2+ displacement from the channel. This strategy was, however, not successful. One reason was probably the specific binding properties of the RYR2 Zn2+ finger. It is reasonable to assume that Zn2+ has to be tightly bound even under conditions of a transient depletion in Zn2+ because the RYR2 channel plays a dominant role in cardiac excitation-contraction coupling [42,43,44], and thus a proper RYR2 function is of particular importance. On the other hand, the ~470-fold Zn2+ gradient across the BLM, which was always present due to Zn2+ impurities, also likely contributed. Our calculations suggest that almost negligible Zn2+ current (≥1.5 × 10−12 pA) is required to accumulate femtomolar [Zn2+] near the Zn2+ finger position (~1.8 nm from the pore). In cardiomyocytes, this extremely small current is seemingly also reachable, albeit Zn2+ movement will be driven by a smaller Zn2+ gradient compared to BLM experiments. Taken together, it appears that the RYR2 Zn2+ finger is more accessible from the luminal than the cytosolic side of the channel since Cd2+ current (from lumen to cytosol) replaced Zn2+ bound in the Zn2+ finger domain even in the presence of saturating [Zn2+]C in the cytosolic solution. In contrast, a substantial decrease in [Zn2+]C to the non-saturating range had no effect on Zn2+-RYR2 interactions when a subtle Zn2+ current in the lumen-to-cytosol direction occurred.
5. Conclusions
Here, we identify the RYR2 channel as a novel Zn2+ transporting protein in the SR membrane that might play a role in local and/or global Zn2+ signaling in cardiomyocytes, considering much faster ion movement through ion channels than the passive transport mediated by carrier-type transporters. The results demonstrate that the RYR2 channel itself could be regulated by its own Zn2+ current. Since Zn2+-binding domains, such as the Zn2+ fingers, often display extremely high binding affinities in the femtomolar range a subtle Zn2+ current in the lumen-to-cytosol direction was predicted to be sufficient for the saturation of the Zn2+ finger situated within the C-terminal tail of each of the four RYR2 subunits. It is not an unreasonable proposal, because the RYR2 Zn2+ finger is directed toward the permeation pathway, and thus it will inevitably be targeted by the Zn2+ current.
Author Contributions
Conceptualization, J.G. and M.G.; methodology, J.G. and M.G.; software, J.G.; validation, J.G. and M.G.; formal analysis, J.G. and M.G.; investigation, J.G. and M.G.; resources, J.G. and M.G.; data curation, J.G. and M.G.; writing—original draft preparation, J.G. and M.G.; writing—review and editing, M.G.; visualization, M.G.; project administration, J.G.; funding acquisition, J.G. and M.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Grant Agency of the Ministry of Education, Science, Research and Sport of the Slovak Republic and the Slovak Academy of Sciences (grants VEGA 2/0018/21 and VEGA 2/0008/20) and the Research & Development Operational Program (ITMS 26230120009) funded by the European Fund for Regional Development.
Institutional Review Board Statement
The animal study protocol was approved by the State Veterinary and Food Administration of the Slovak Republic (permit number: 2721/17-221 and 2100/19-221 approved 4 July 2017).
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated for this study are available on request to the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- MacDonald, R.S. The role of zinc in growth and cell proliferation. J. Nutr. 2000, 130, 1500S–1508S. [Google Scholar] [CrossRef] [PubMed]
- Maret, W. Zinc in cellular regulation: The nature and significance of “zinc signals”. Int. J. Mol. Sci. 2017, 18, 2285. [Google Scholar] [CrossRef] [PubMed]
- Turan, B.; Fliss, H.; Désilets, M. Oxidants increase intracellular free Zn2+ concentration in rabbit ventricular myocytes. Am. J. Physiol. 1997, 272, H2095–H2106. [Google Scholar] [CrossRef] [PubMed]
- Prasad, A.S. Impact of the discovery of human zinc deficiency on health. J. Am. Coll. Nutr. 2009, 28, 257–265. [Google Scholar] [CrossRef]
- Prasad, A.S.; Beck, F.W.; Snell, D.C.; Kucuk, O. Zinc in cancer prevention. Nutr. Cancer 2009, 61, 879–887. [Google Scholar] [CrossRef]
- Sensi, S.L.; Paoletti, P.; Bush, A.I.; Sekler, I. Zinc in the physiology and pathology of the CNS. Nat. Rev. Neurosci. 2009, 10, 780–791. [Google Scholar] [CrossRef]
- Little, P.J.; Bhattacharya, R.; Moreyra, A.E.; Korichneva, I.L. Zinc and cardiovascular disease. Nutrition 2010, 26, 1050–1057. [Google Scholar] [CrossRef]
- Tuncay, E.; Bilginoglu, A.; Sozmen, N.N.; Zeydanli, E.N.; Ugur, M.; Vassort, G.; Turan, B. Intracellular free zinc during cardiac excitation-contraction cycle: Calcium and redox dependencies. Cardiovasc. Res. 2011, 89, 634–642. [Google Scholar] [CrossRef]
- Krizkova, S.; Ryvolova, M.; Hrabeta, J.; Adam, V.; Stiborova, M.; Eckschlager, T.; Kizek, R. Metallothioneins and zinc in cancer diagnosis and therapy. Drug Metab. Rev. 2012, 44, 287–301. [Google Scholar] [CrossRef]
- Vallee, B.L.; Auld, D.S. Cocatalytic zinc motifs in enzyme catalysis. Proc. Natl. Acad. Sci. USA 1993, 90, 2715–2718. [Google Scholar] [CrossRef]
- Prasad, A.S. Zinc: An overview. Nutrition 1995, 11, 93–99. [Google Scholar] [PubMed]
- Peck, E.J., Jr.; Ray, W.J., Jr. Metal complexes of phosphoglucomutase in vivo. Alterations induced by insulin. J. Biol. Chem. 1971, 246, 1160–1167. [Google Scholar] [CrossRef]
- Simons, T.J.B. Intracellular free zinc and zinc buffering in human red blood cells. J. Membr. Biol. 1991, 123, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Benters, J.; Flögel, U.; Schäfer, T.; Leibfritz, D.; Hechtenberg, S.; Beyersmann, D. Study of the interactions of cadmium and zinc ions with cellular calcium homoeostasis using 19F-NMR spectroscopy. Biochem. J. 1997, 322, 793–799. [Google Scholar] [CrossRef]
- Bozym, R.A.; Thompson, R.B.; Stoddard, A.K.; Fierke, C.A. Measuring picomolar intracellular exchangeable zinc in PC-12 cells using a ratiometric fluorescence biosensor. ACS Chem. Biol. 2006, 1, 103–111. [Google Scholar] [CrossRef]
- Colvin, R.A.; Holmes, W.R.; Fontaine, C.P.; Maret, W. Cytosolic zinc buffering and muffling: Their role in intracellular zinc homeostasis. Metallomics 2010, 2, 306–317. [Google Scholar] [CrossRef]
- Lichten, L.A.; Cousins, R.J. Mammalian zinc transporters: Nutritional and physiologic regulation. Annu. Rev. Nutr. 2009, 29, 153–176. [Google Scholar] [CrossRef]
- Kambe, T.; Tsuji, T.; Hashimoto, A.; Itsumura, N. The physiological, biochemical, and molecular roles of zinc transporters in zinc homeostasis and metabolism. Physiol. Rev. 2015, 95, 749–784. [Google Scholar] [CrossRef]
- Hara, T.; Takeda, T.A.; Takagishi, T.; Fukue, K.; Kambe, T.; Fukada, T. Physiological roles of zinc transporters: Molecular and genetic importance in zinc homeostasis. J. Physiol. Sci. 2017, 67, 283–301. [Google Scholar] [CrossRef]
- Palmiter, R.D.; Findley, S.D. Cloning and functional characterization of a mammalian zinc transporter that confers resistance to zinc. EMBO J. 1995, 14, 639–649. [Google Scholar] [CrossRef]
- Bruinsma, J.J.; Jirakulaporn, T.; Muslin, A.J.; Kornfeld, K. Zinc ions and cation diffusion facilitator proteins regulate Ras-mediated signaling. Dev. Cell 2002, 2, 567–578. [Google Scholar] [CrossRef]
- Jirakulaporn, T.; Muslin, A.J. Cation diffusion facilitator proteins modulate Raf-1 activity. J. Biol. Chem. 2004, 279, 27807–27815. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bharadwaj, U.; Logsdon, C.D.; Chen, C.; Yao, Q.; Li, M. ZIP4 regulates pancreatic cancer cell growth by activating IL-6/STAT3 pathway through zinc finger transcription factor CREB. Clin. Cancer Res. 2010, 16, 1423–1430. [Google Scholar] [CrossRef] [PubMed]
- Hojyo, S.; Fukada, T.; Shimoda, S.; Ohashi, W.; Bin, B.H.; Koseki, H.; Hirano, T. The zinc transporter SLC39A14/ZIP14 controls G-protein coupled receptor-mediated signaling required for systemic growth. PLoS ONE 2011, 6, e18059. [Google Scholar] [CrossRef] [PubMed]
- Beker Aydemir, T.; Chang, S.M.; Guthrie, G.J.; Maki, A.B.; Ryu, M.S.; Karabiyik, A.; Cousins, R.J. Zinc transporter ZIP14 functions in hepatic zinc, iron and glucose homeostasis during the innate immune response (endotoxemia). PLoS ONE 2012, 7, e48679. [Google Scholar] [CrossRef]
- Chowanadisai, W.; Graham, D.M.; Keen, C.L.; Rucker, R.B.; Messerli, M.A. Neurulation and neurite extension require the zinc transporter ZIP12 (slc39a12). Proc. Natl. Acad. Sci. USA 2013, 110, 9903–9908. [Google Scholar] [CrossRef]
- Kirschke, C.P.; Huang, L. ZnT7, a novel mammalian zinc transporter, accumulates zinc in the Golgi apparatus. J. Biol. Chem. 2003, 278, 4096–4102. [Google Scholar] [CrossRef]
- Chi, Z.H.; Wang, X.; Wang, Z.Y.; Gao, H.L.; Dahlstrom, A.; Huang, L. Zinc transporter 7 is located in the cis-Golgi apparatus of mouse choroid epithelial cells. Neuroreport 2006, 17, 1807–1811. [Google Scholar] [CrossRef]
- Lazarczyk, M.; Pons, C.; Mendoza, J.A.; Cassonnet, P.; Jacob, Y.; Favre, M. Regulation of cellular zinc balance as a potential mechanism of EVER-mediated protection against pathogenesis by cutaneous oncogenic human papillomaviruses. J. Exp. Med. 2008, 205, 35–42. [Google Scholar] [CrossRef]
- Fukada, T.; Civic, N.; Furuichi, T.; Shimoda, S.; Mishima, K.; Higashiyama, H.; Idaira, Y.; Asada, Y.; Kitamura, H.; Yamasaki, S.; et al. The zinc transporter SLC39A13/ZIP13 is required for connective tissue development; its involvement in BMP/TGF-beta signaling pathways. PLoS ONE 2008, 3, e3642. [Google Scholar] [CrossRef]
- Hogstrand, C.; Kille, P.; Nicholson, R.I.; Taylor, K.M. Zinc transporters and cancer: A potential role for ZIP7 as a hub for tyrosine kinase activation. Trends Mol. Med. 2009, 15, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.M.; Hiscox, S.; Nicholson, R.I.; Hogstrand, C.; Kille, P. Protein kinase CK2 triggers cytosolic zinc signaling pathways by phosphorylation of zinc channel ZIP7. Sci. Signal. 2012, 5, ra11. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, M.; Fukunaka, A.; Hagihara, M.; Watanabe, K.; Kamino, S.; Kambe, T.; Enomoto, S.; Hiromura, M. Essential role of the zinc transporter ZIP9/SLC39A9 in regulating the activations of Akt and Erk in B-cell receptor signaling pathway in DT40 cells. PLoS ONE 2013, 8, e58022. [Google Scholar] [CrossRef] [PubMed]
- Bonnemaison, M.L.; Duffy, M.E.; Mains, R.E.; Vogt, S.; Eipper, B.A.; Ralle, M. Copper, zinc and calcium: Imaging and quantification in anterior pituitary secretory granules. Metallomics 2016, 8, 1012–1022. [Google Scholar] [CrossRef]
- Lu, Q.; Haragopal, H.; Slepchenko, K.G.; Stork, C.; Li, Y.V. Intracellular zinc distribution in mitochondria, ER and the Golgi apparatus. Int. J. Physiol. Pathophysiol. Pharmacol. 2016, 8, 35–43. [Google Scholar]
- Qi, Z.; Shi, W.; Zhao, Y.; Ji, X.; Liu, K.J. Zinc accumulation in mitochondria promotes ischemia-induced BBB disruption through Drp1-dependent mitochondria fission. Toxicol. Appl. Pharmacol. 2019, 377, 114601. [Google Scholar] [CrossRef]
- Stork, C.J.; Li, Y.V. Zinc release from thapsigargin/IP3-sensitive stores in cultured cortical neurons. J. Mol. Signal. 2010, 5, 5. [Google Scholar] [CrossRef]
- Palmer, B.M.; Vogt, S.; Chen, Z.Y.; Lachapelle, R.R.; LeWinter, M.M. Intracellular distributions of essential elements in cardiomyocytes. J. Struct. Biol. 2006, 155, 12–21. [Google Scholar] [CrossRef]
- Etzion, Y.; Ganiel, A.; Beharier, O.; Shalev, A.; Novack, V.; Volvich, L.; Abrahamov, D.; Matsa, M.; Sahar, G.; Moran, A.; et al. Correlation between atrial ZnT-1 expression and atrial fibrillation in humans: A pilot study. J. Cardiovasc. Electrophysiol. 2008, 19, 157–164. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Y.; Cui, X.; Yao, W.; Yu, X.; Cen, P.; Hodges, S.E.; Fisher, W.E.; Brunicardi, F.C.; Chen, C.; et al. Gene profile identifies Zinc transporters differentially expressed in normal human organs and human pancreatic cancer. Curr. Mol. Med. 2013, 13, 401–409. [Google Scholar]
- Tuncay, E.; Bitirim, V.C.; Durak, A.; Carrat, G.R.J.; Taylor, K.M.; Rutter, G.A.; Turan, B. Hyperglycemia-induced changes in ZIP7 and ZnT7 expression cause Zn2+ release from sarco(endo)plasmic reticulum and mediate ER-stress in the heart. Diabetes 2017, 66, 1346–1358. [Google Scholar] [CrossRef] [PubMed]
- Endo, M.; Tanaka, M.; Ogawa, Y. Calcium induced release of calcium from the sarcoplasmic reticulum of skinned skeletal muscle fibres. Nature 1970, 228, 34–36. [Google Scholar] [CrossRef] [PubMed]
- Fabiato, A. Calcium-induced release of calcium from the cardiac sarcoplasmic reticulum. Am. J. Physiol. 1983, 245, C1–C14. [Google Scholar] [CrossRef]
- Fabiato, A. Simulated calcium current can both cause calcium loading in and trigger calcium release from the sarcoplasmic reticulum of a skinned canine cardiac Purkinje cell. J. Gen. Physiol. 1985, 85, 291–320. [Google Scholar] [CrossRef]
- Lindsay, A.R.G.; Williams, A.J. Functional-characterization of the ryanodine receptor purified from sheep cardiac-muscle sarcoplasmic-reticulum. Biochim. Biophys. Acta 1991, 1064, 89–102. [Google Scholar] [CrossRef]
- Tinker, A.; Williams, A.J. Divalent-cation conduction in the ryanodine receptor channel of sheep cardiac-muscle sarcoplasmic-reticulum. J. Gen. Physiol. 1992, 100, 479–493. [Google Scholar] [CrossRef] [PubMed]
- Hess, P.; Lansman, J.B.; Tsien, R.W. Calcium channel selectivity for divalent and monovalent cations. Voltage and concentration dependence of single channel current in ventricular heart cells. J. Gen. Physiol. 1986, 88, 293–319. [Google Scholar] [CrossRef]
- Bezprozvanny, I.; Ehrlich, B.E. Inositol (1,4,5)-trisphosphate (InsP3)-gated Ca2+ channels from cerebellum: Conduction properties for divalent cations and regulation by intraluminal calcium. J. Gen. Physiol. 1994, 104, 821–856. [Google Scholar] [CrossRef]
- Zoghbi, M.E.; Copello, J.A.; Villalba-Galea, C.A.; Vélez, P.; Diaz Sylvester, P.L.; Bolaños, P.; Marcano, A.; Fill, M.; Escobar, A.L. Differential Ca2+ and Sr2+ regulation of intracellular divalent cations release in ventricular myocytes. Cell Calcium 2004, 36, 119–134. [Google Scholar] [CrossRef]
- Diaz-Sylvester, P.L.; Porta, M.; Copello, J.A. Modulation of cardiac ryanodine receptor channels by alkaline earth cations. PLoS ONE 2011, 6, e26693. [Google Scholar] [CrossRef]
- Gaburjakova, J.; Gaburjakova, M. Cardiac ryanodine receptor: Selectivity for alkaline earth metal cations points to the EF-hand nature of luminal binding sites. Bioelectrochemistry 2016, 109, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Morais-Cabral, J.H.; Kaufman, A.; Mackinnon, R. Chemistry of ion coordination and hydration revealed by a K+ channel-Fab complex at 2.0 Å resolution. Nature 2001, 414, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, D. Energetics of divalent selectivity in a calcium channel: The ryanodine receptor case study. Biophys. J. 2008, 94, 1169–1184. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, D.; Xu, L.; Meissner, G. Selecting ions by size in a calcium channel: The ryanodine receptor case study. Biophys. J. 2014, 107, 2263–2273. [Google Scholar] [CrossRef][Green Version]
- Peng, W.; Shen, H.; Wu, J.; Guo, W.; Pan, X.; Wang, R.; Chen, S.R.W.; Yan, N. Structural basis for the gating mechanism of the type 2 ryanodine receptor RyR2. Science 2016, 354, aah5324. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, E.; Meissner, G. Single cardiac sarcoplasmic reticulum Ca2+-release channel: Activation by caffeine. Am. J. Physiol. 1989, 256, H328–H333. [Google Scholar] [CrossRef]
- Sitsapesan, R.; Williams, A.J. Mechanisms of caffeine activation of single calcium-release channels of sheep cardiac sarcoplasmic reticulum. J. Physiol. 1990, 423, 425–439. [Google Scholar] [CrossRef]
- Porta, M.; Zima, A.V.; Nani, A.; Diaz-Sylvester, P.L.; Copello, J.A.; Ramos-Franco, J.; Blatter, L.A.; Fill, M. Single ryanodine receptor channel basis of caffeine’s action on Ca2+ sparks. Biophys. J. 2011, 100, 931–938. [Google Scholar] [CrossRef]
- Rousseau, E.; Smith, J.S.; Meissner, G. Ryanodine modifies conductance and gating behavior of single Ca2+ release channel. Am. J. Physiol. 1987, 253, C364–C368. [Google Scholar] [CrossRef]
- Gaburjakova, J.; Gaburjakova, M. Comparison of the effects exerted by luminal Ca2+ on the sensitivity of the cardiac ryanodine receptor to caffeine and cytosolic Ca2+. J. Membr. Biol. 2006, 212, 17–28. [Google Scholar] [CrossRef]
- Barry, P.H.; Lewis, T.M.; Moorhouse, A.J. An optimised 3 M KCl salt-bridge technique used to measure and validate theoretical liquid junction potential values in patch-clamping and electrophysiology. Eur. Biophys. J. 2013, 42, 631–646. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dawson, D.C. Permeability and conductance of ion channels a primer. In Molecular Biology of Membrane Transport Disorders; Schultz, S.G., Andreoli, T.E., Brown, A.M., Fambrough, D.M., Hoffman, J.F., Welsh, M.J., Eds.; Springer: Boston, MA, USA, 1996; pp. 87–110. [Google Scholar]
- Goldman, D.E. Potential, impedance, and rectification in membranes. J. Gen. Physiol. 1943, 27, 37–60. [Google Scholar] [CrossRef] [PubMed]
- Hodgkin, A.L.; Katz, B. The effect of sodium ions on the electrical activity of the giant axon of the squid. J. Physiol. 1949, 108, 37–77. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, A.R.; Manning, S.D.; Williams, A.J. Monovalent cation conductance in the ryanodine receptor-channel of sheep cardiac muscle sarcoplasmic reticulum. J. Physiol. 1991, 439, 463–480. [Google Scholar] [CrossRef] [PubMed]
- Tinker, A.; Lindsay, A.R.G.; Williams, A.J. A model for ionic conduction in the ryanodine receptor channel of sheep cardiac muscle sarcoplasmic reticulum. J. Gen. Physiol. 1992, 100, 495–517. [Google Scholar] [CrossRef]
- Stern, M.D. Buffering of calcium in the vicinity of a channel pore. Cell Calcium 1992, 13, 183–192. [Google Scholar] [CrossRef]
- Buffle, J.; Zhang, Z.; Startchev, K. Metal flux and dynamic speciation at (bio)interfaces. Part I: Critical evaluation and compilation of physicochemical parameters for complexes with simple ligands and fulvic/humic substances. Environ. Sci. Technol. 2007, 41, 7609–7620. [Google Scholar] [CrossRef]
- Tanaka, M.; Funahashi, S.; Shirai, K. Kinetics of the ligand substitution reaction of the zinc(II)-4-(2-pyridylazo)resorcinol complex with (ethylene glycol)bis(2-aminoethyl ether)-N,N,N′,N′-tetraacetic acid. Inorg. Chem. 1968, 7, 573–578. [Google Scholar] [CrossRef]
- Takada, T.; Kiyama, M.; Torii, H.; Asai, T.; Takano, M.; Nakanishi, N. Effect of pH values on the formation and solubility of Zinc compounds. Bull. Inst. Chem. Res. Kyoto Univ. 1978, 56, 242–246. [Google Scholar]
- Reichle, R.A.; Mccurdy, K.G.; Hepler, L.G. Zinc hydroxide: Solubility product and hydroxy-complex stability constants from 12.5 °C–75 °C. Can. J. Chem. 1975, 53, 3841–3845. [Google Scholar] [CrossRef]
- Brown, I.D. What factors determine cation coordination numbers? Acta Cryst. 1988, B44, 545–553. [Google Scholar] [CrossRef]
- Smith, D.W. Ionic hydration enthalpies. J. Chem. Educ. 1977, 54, 540–542. [Google Scholar] [CrossRef]
- Krezel, A.; Maret, W. The biological inorganic chemistry of zinc ions. Arch. Biochem. Biophys. 2016, 611, 3–19. [Google Scholar] [CrossRef]
- Xu, L.; Mann, G.; Meissner, G. Regulation of cardiac Ca2+ release channel (ryanodine receptor) by Ca2+, H+, Mg2+, and adenine nucleotides under normal and simulated ischemic conditions. Circ. Res. 1996, 79, 1100–1109. [Google Scholar] [CrossRef]
- Laver, D.R.; Eager, K.R.; Taoube, L.; Lamb, G.D. Effects of cytoplasmic and luminal pH on Ca2+ release channels from rabbit skeletal muscle. Biophys. J. 2000, 78, 1835–1851. [Google Scholar] [CrossRef]
- Kettlun, C.; Gonzales, A.; Rios, E.; Fill, M. Unitary Ca2+ current through mammalian cardiac and amphibian skeletal muscle ryanodine receptor channel under near-physiological ionic conditions. J. Gen. Physiol. 2003, 122, 407–417. [Google Scholar] [CrossRef]
- Mejía-Alvarez, R.; Kettlun, C.; Ríos, E.; Stern, M.; Fill, M. Unitary Ca2+ current through cardiac ryanodine receptor channels under quasi-physiological ionic conditions. J. Gen. Physiol. 1999, 113, 177–186. [Google Scholar] [CrossRef]
- Gillespie, D.; Chen, H.Y.; Fill, M. Is ryanodine receptor a calcium or magnesium channel? Roles of K+ and Mg2+ during Ca2+ release. Cell Calcium 2012, 51, 427–433. [Google Scholar] [CrossRef]
- Krishna, S.S.; Majumdar, I.; Grishin, N.V. Structural classification of zinc fingers: Survey and summary. Nucleic Acids Res. 2003, 31, 532–550. [Google Scholar] [CrossRef]
- Uchida, K.; Miyauchi, H.; Furuichi, T.; Michikawa, T.; Mikoshiba, K. Critical regions for activation gating of the inositol 1,4,5-trisphosphate receptor. J. Biol. Chem. 2003, 278, 16551–16560. [Google Scholar] [CrossRef]
- Bhanumathy, C.; Da Fonseca, P.C.A.; Morris, E.P.; Joseph, S.K. Identification of functionally critical residues in the channel domain of inositol trisphosphate receptors. J. Biol. Chem. 2012, 287, 43674–43684. [Google Scholar] [CrossRef]
- Serysheva, I.I. Toward a high-resolution structure of IP3R channel. Cell Calcium 2014, 56, 125–132. [Google Scholar] [CrossRef]
- Wolfe, S.A.; Nekludova, L.; Pabo, C.O. DNA recognition by Cys2His2 zinc finger proteins. Annu. Rev. Biophys. Biomol. Struct. 2000, 29, 183–212. [Google Scholar] [CrossRef]
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettingan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef]
- Witkiewicz-Kucharczyk, A.; Bal, W. Damage of zinc fingers in DNA repair proteins, a novel molecular mechanism in carcinogenesis. Toxicol. Lett. 2006, 162, 29–42. [Google Scholar] [CrossRef]
- Sénѐque, O.; Bonnet, E.; Joumas, F.L.; Latour, J.M. Cooperative metal binding and helical folding in model peptides of treble-clef zinc fingers. Chem. Eur. J. 2009, 15, 4798–4810. [Google Scholar] [CrossRef]
- Sénѐque, O.; Latour, J.-M. Coordination properties of zinc finger peptides revisited: Ligand competition studies reveal higher affinities for zinc and cobalt. J. Am. Chem. Soc. 2010, 132, 17760–17774. [Google Scholar] [CrossRef]
- Woodier, J.; Rainbow, R.D.; Stewart, A.J.; Pitt, S.J. Intracellular zinc modulates cardiac ryanodine receptor-mediated calcium release. J. Biol. Chem. 2015, 290, 17599–17610. [Google Scholar] [CrossRef]
- Reilly-O’Donnell, B.; Robertson, G.B.; Karumbi, A.; McIntyre, C.; Bal, W.; Nishi, M.; Takeshima, H.; Stewart, A.J.; Pitt, S.J. Dysregulated Zn2+ homeostasis impairs cardiac type-2 ryanodine receptor and mitsugumin 23 functions, leading to sarcoplasmic reticulum Ca2+ leakage. J. Biol. Chem. 2017, 292, 13361–13373. [Google Scholar] [CrossRef]
- Liu, Y.; Porta, M.; Qin, J.; Ramos, J.; Nani, A.; Shannon, T.R.; Fill, M. Flux regulation of cardiac ryanodine receptor channels. J. Gen. Physiol. 2010, 135, 15–27. [Google Scholar] [CrossRef]
- Baddeley, D.; Jayasinghe, I.D.; Lam, L.; Rossberger, S.; Cannell, M.B.; Soeller, C. Optical single-channel resolution imaging of the ryanodine receptor distribution in rat cardiac myocytes. Proc. Natl. Acad. Sci. USA 2009, 106, 22275–22280. [Google Scholar] [CrossRef]
- Hayashi, T.; Martone, M.E.; Yu, Z.; Thor, A.; Doi, M.; Holst, M.J.; Ellisman, M.H.; Hoshijima, M. Three-dimensional electron microscopy reveals new details of membrane systems for Ca2+ signaling in the heart. J. Cell Sci. 2009, 122, 1005–1013. [Google Scholar] [CrossRef]
- Petering, D.H.; Huang, M.; Moteki, S.; Shaw, C.F., 3rd. Cadmium and lead interactions with transcription factor IIIA from Xenopus laevis: A model for zinc finger protein reactions with toxic metal ions and metallothionein. Mar. Environ. Res. 2000, 50, 89–92. [Google Scholar] [CrossRef]
- Huang, M.; Krepkiy, D.; Hu, W.; Petering, D.H. Zn-, Cd-, and Pb-transcription factor IIIA: Properties, DNA binding, and comparison with TFIIIA-finger 3 metal complexes. J. Inorg. Biochem. 2004, 98, 775–785. [Google Scholar] [CrossRef]
- Kothinti, R.; Blodgett, A.; Tabatabai, N.M.; Petering, D.H. Zinc finger transcription factor Zn3-Sp1 reactions with Cd2+. Chem. Res. Toxicol. 2010, 23, 405–412. [Google Scholar] [CrossRef][Green Version]
- Malgieri, G.; Zaccaro, L.; Leone, M.; Bucci, E.; Esposito, S.; Baglivo, I.; Del Gatto, A.; Russo, L.; Scandurra, R.; Pedone, P.V.; et al. Zinc to cadmium replacement in the A. thaliana SUPERMAN Cys2His2 zinc finger induces structural rearrangements of typical DNA base determinant positions. Biopolymers 2011, 95, 801–810. [Google Scholar] [CrossRef]
- Ebashi, S. Excitation-contraction coupling and the mechanism of muscle contraction. Ann. Rev. Physiol. 1991, 53, 1–17. [Google Scholar] [CrossRef]
- Flockerzi, V.; Oeken, H.-J.; Hofmarm, F. Purification of a functional receptor for calcium-channel blockers from rabbit skeletal-muscle microsomes. Eur. J. Biochem. 1986, 161, 217–224. [Google Scholar] [CrossRef]
- Pessah, I.N.; Zimanyi, I. Characterization of multiple [3H]ryanodine binding sites on the Ca2+ release channel of sarcoplasmic reticulum from skeletal and cardiac muscle: Evidence for a sequential mechanism in ryanodine action. Mol. Pharmacol. 1991, 39, 679–689. [Google Scholar]
- Lai, F.A.; Misra, M.; Xu, L.; Smith, H.A.; Meissner, G. The ryanodine receptor-Ca2+ release channel complex of skeletal muscle sarcoplasmic reticulum. Evidence for a cooperatively coupled, negatively charged homotetramer. J. Biol. Chem. 1989, 264, 16776–16785. [Google Scholar] [CrossRef]
- Fukuda, J.; Kawa, K. Permeation of manganese, cadmium, zinc, and beryllium through calcium channels of an insect muscle membrane. Science 1977, 196, 309–311. [Google Scholar] [CrossRef]
- Kawa, K. Zinc-dependent action potentials in giant neurons of the snail, Euhadra quaestia. J. Membr. Biol. 1979, 49, 325–344. [Google Scholar] [CrossRef]
- Atar, D.; Backx, P.H.; Appel, M.M.; Gao, W.D.; Marban, E. Excitation-transcription coupling mediated by zinc influx through voltage-dependent calcium channels. J. Biol. Chem. 1995, 270, 2473–2477. [Google Scholar] [CrossRef]
- Kerchner, G.A.; Canzoniero, L.M.T.; Yu, S.P.; Ling, C.; Choi, D.W. Zn2+ current is mediated by voltage-gated Ca2+ channels and enhanced by extracellular acidity in mouse cortical neurones. J. Physiol. 2000, 528, 39–52. [Google Scholar] [CrossRef]
- Gillespie, D.; Eisenberg, R.S. Physical descriptions of experimental selectivity measurements in ion channels. Eur. Biophys. J. 2002, 31, 454–466. [Google Scholar] [CrossRef]
- Dubyak, G.R. Ion homeostasis, channels, and transporters: An update on cellular mechanisms. Adv. Physiol. Educ. 2004, 28, 143–154. [Google Scholar] [CrossRef]
- Chen, W.; Steenberg, C.; Levy, L.A.; Vance, J.; London, R.E.; Murphy, E. Measurements of free Ca2+ on sarcoplasmic reticulum in perfuse rabbit heart loaded with 1,2-bis (2-amino-5,6-difluorophenoxy)ethane-N,N,N′,N′-tetraacetic acid by 19F NMR. J. Biol. Chem. 1996, 271, 7398–7403. [Google Scholar] [CrossRef]
- Shannon, T.R.; Bers, D.M. Assessment of intra-SR free [Ca] and buffering in rat heart. Biophys. J. 1997, 73, 1524–1531. [Google Scholar] [CrossRef]
- Chabosseau, P.; Tuncay, E.; Meur, G.; Bellomo, E.A.; Hessels, A.; Hughes, S.; Johnson, P.R.; Bugliani, M.; Marchetti, P.; Turan, B.; et al. Mitochondrial and ER-targeted eCALWY probes reveal high levels of free Zn2+. ACS Chem. Biol. 2014, 9, 2111–2120. [Google Scholar] [CrossRef]
- Fuchs, F. Ion exchange properties of the calcium receptor site of troponin. Biochim. Biophys. Acta Bioenerg. 1971, 245, 221–229. [Google Scholar] [CrossRef]
- Witte, K.K.; Clark, A.L.; Cleland, J.G. Chronic heart failure and micronutrients. J. Am. Coll. Cardiol. 2001, 37, 1765–1774. [Google Scholar] [CrossRef]
- Shokrzadeh, M.; Ghaemian, A.; Salehifar, E.; Aliakbari, S.; Saravi, S.S.; Ebrahimi, P. Serum zinc and copper levels in ischemic cardiomyopathy. Biol. Trace Elem. Res. 2009, 127, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, B.; Wang, Y.; Tong, Q.; Liu, Q.; Sun, J.; Zheng, Y.; Cai, L. Zinc prevents the development of diabetic cardiomyopathy in db/db mice. Int. J. Mol. Sci. 2017, 18, 580. [Google Scholar] [CrossRef] [PubMed]
- Cohen, N.; Golik, A. Zinc balance and medications commonly used in the management of heart failure. Heart Fail. Rev. 2006, 11, 19–24. [Google Scholar] [CrossRef]
- Karagulova, G.; Yue, Y.; Moreyra, A.E.; Boutjdir, M.; Korichneva, I. Protective role of intracellular zinc in myocardial ischemia/reperfusion is associated with preservation of protein kinase C isoforms. J. Pharmacol. Exp. Ther. 2007, 321, 517–525. [Google Scholar] [CrossRef]
- Frustaci, A.; Sabbioni, E.; Fortaner, S.; Massimo, F.; del Torchio, R.; Tafani, M.; Morgante, E.; Ciriolo, M.R.; Russo, M.A.; Chimenti, C. Selenium- and zincdeficient cardiomyopathy in human intestinal malabsorption: Preliminary results of selenium/zinc infusion. Eur. J. Heart Fail. 2012, 14, 202–210. [Google Scholar] [CrossRef]
- Rosenblum, H.; Bikdeli, B.; Wessler, J.; Gupta, A.; Jacoby, D.L. Zinc deficiency as a reversible cause of heart failure. Tex. Heart Inst. J. 2020, 47, 152–154. [Google Scholar] [CrossRef]
- Furuichi, T.; Yoshikawa, S.; Miyawaki, A.; Wada, K.; Maeda, N.; Mikoshiba, K. Primary structure and functional expression of the inositol 1,4,5-trisphosphate-binding protein P400. Nature 1989, 342, 32–38. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).