Maintenance of Chronological Aging Features in Culture of Normal Human Dermal Fibroblasts from Old Donors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
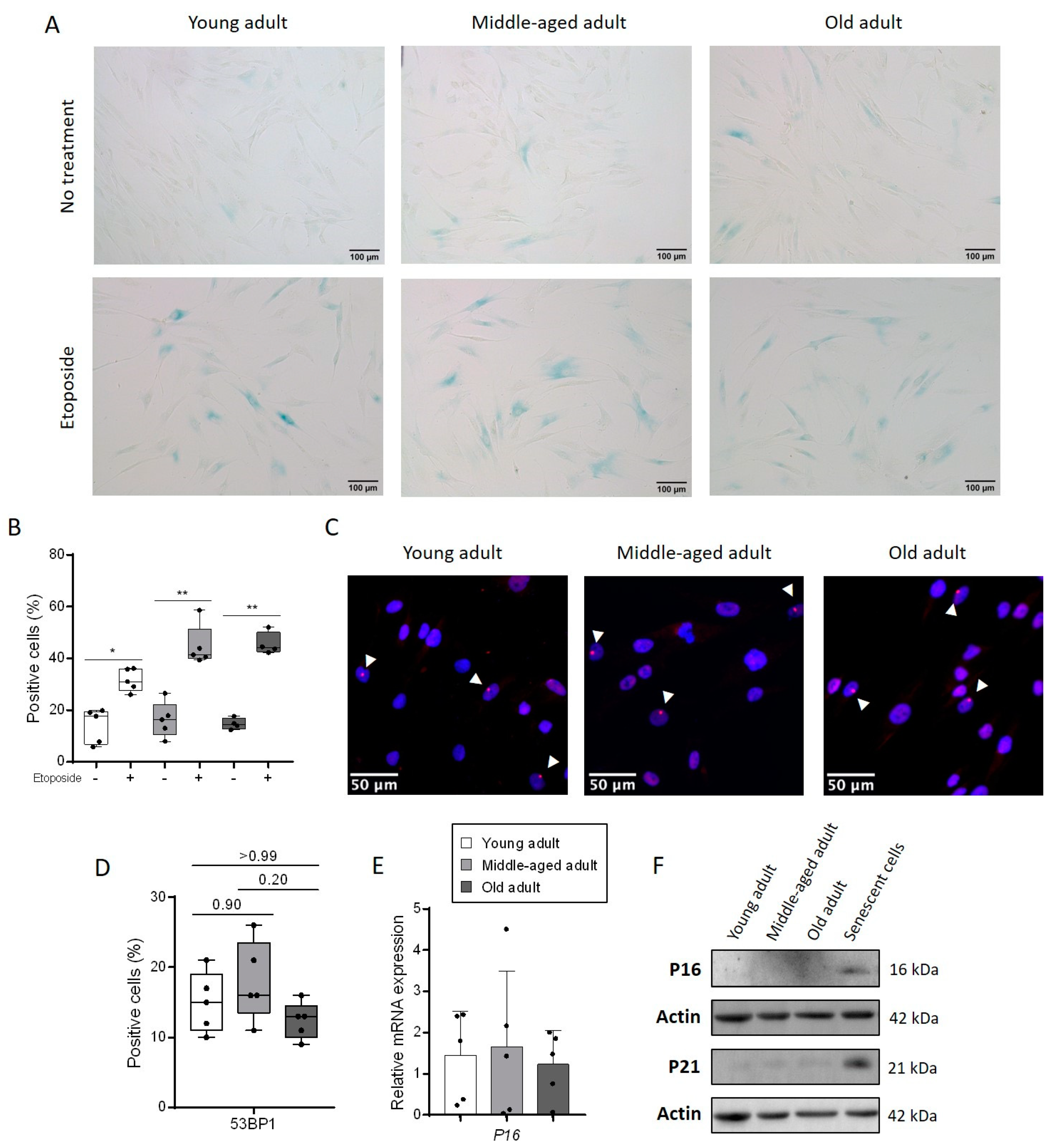
2.2. SA-β-Galactosidase Staining
2.3. 53BP1 Immunostaining
2.4. Protein Extraction and Immunoblotting
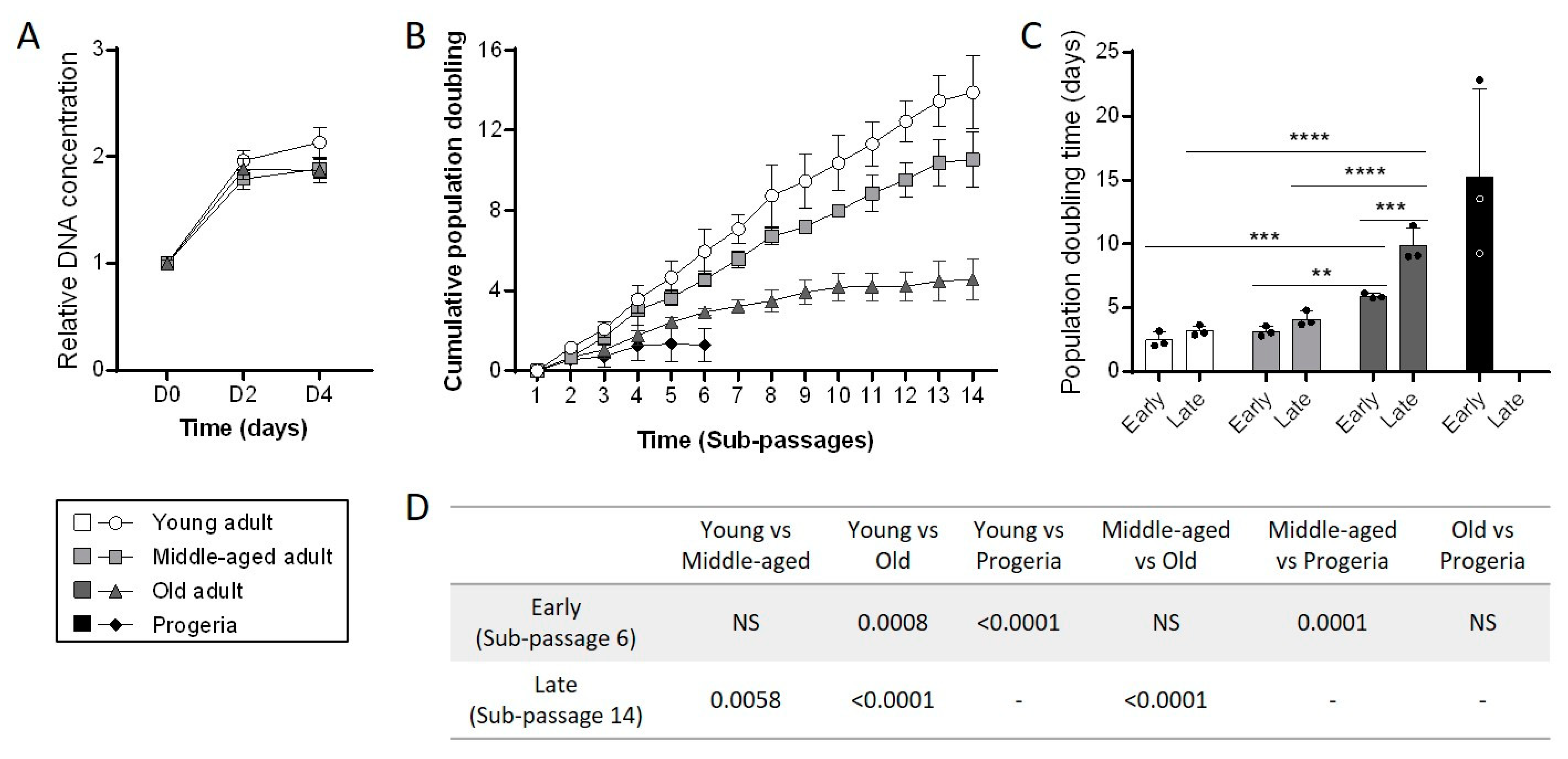
2.5. Population Doubling Assay
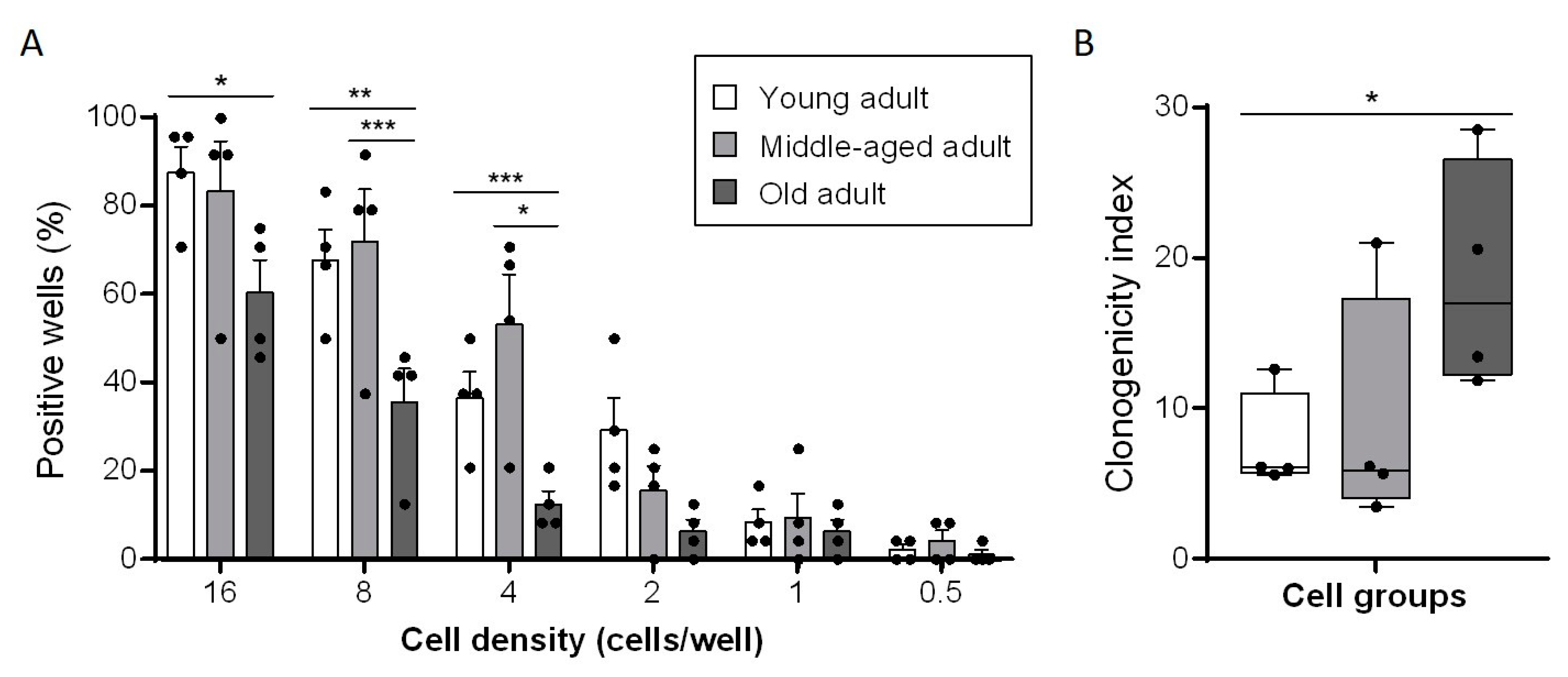
2.6. Clonogenicity Frequency
2.7. Proliferation Assay
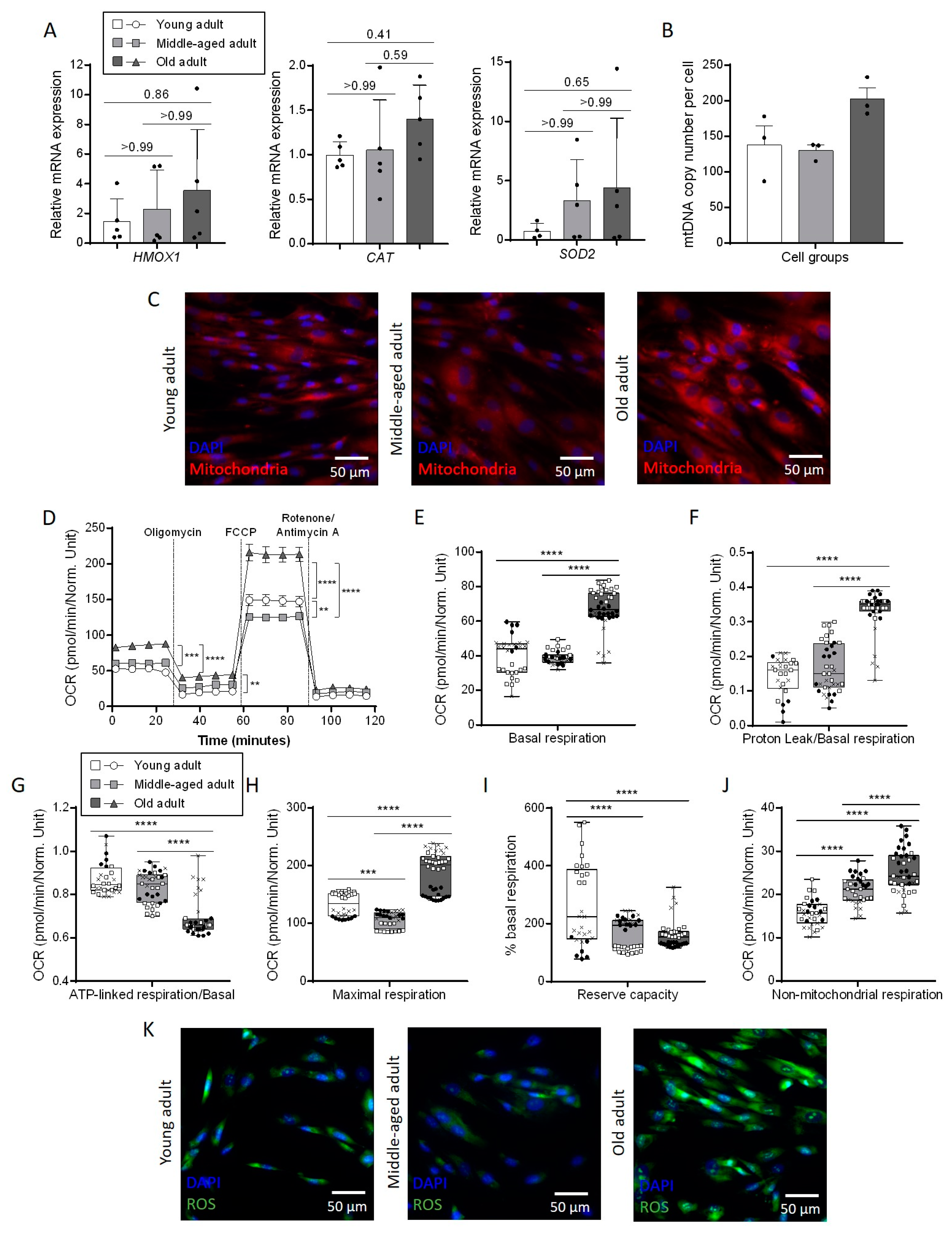
2.8. Analysis of Energy Metabolism
2.9. Mitochondria and Reactive Oxygen Species (ROS) Content
2.10. MMP12 Elastase Activity Assay
2.11. Elastin, Fibronectin and Collagen Content
2.12. Gene Expression Analysis and Estimation of mtDNA Content
2.13. Statistical Analysis
3. Results
3.1. Aging Does Not Change the Rate of Senescent Cells in a Culture of Primary Fibroblasts
3.2. Aging Negatively Impacts Primary Fibroblasts Proliferation
3.3. Aging Decreases Clonogenic Abilities of Primary Fibroblasts
3.4. Aging Affects Mitochondrial Metabolism in Primary Fibroblasts
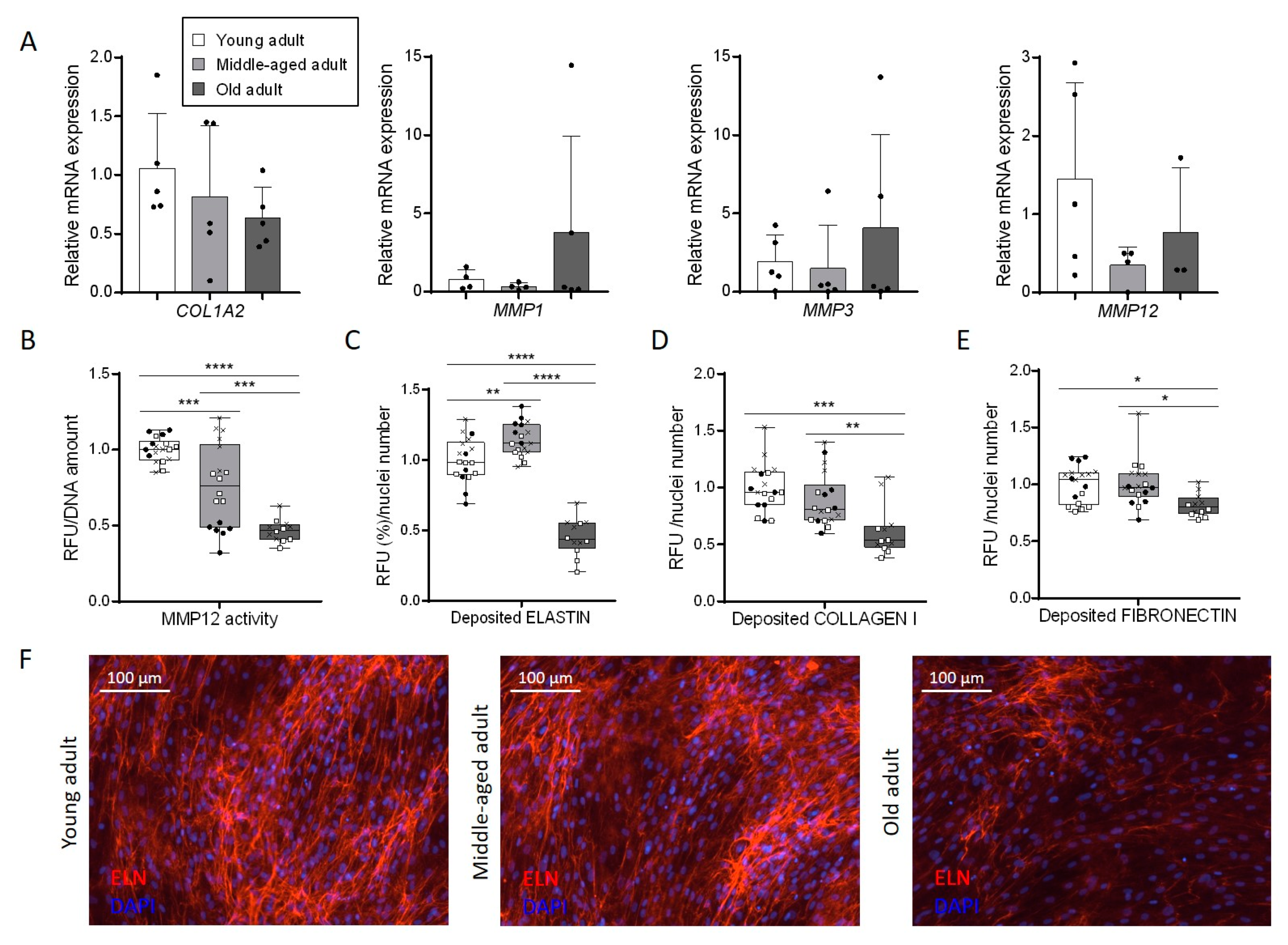
3.5. Aging Disturbs Extracellular Matrix Composition Secreted by Primary Fibroblasts
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rinnerthaler, M.; Duschl, J.; Steinbacher, P.; Salzmann, M.; Bischof, J.; Schuller, M.; Wimmer, H.; Peer, T.; Bauer, J.W.; Richter, K. Age-Related Changes in the Composition of the Cornified Envelope in Human Skin. Exp. Dermatol. 2013, 22, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Rorteau, J.; Chevalier, F.P.; Fromy, B.; Lamartine, J. Vieillissement et intégrité de la peau—De la biologie cutanée aux stratégies anti-âge. Med. Sci. 2020, 36, 1155–1162. [Google Scholar] [CrossRef]
- Shin, J.-W.; Kwon, S.-H.; Choi, J.-Y.; Na, J.-I.; Huh, C.-H.; Choi, H.-R.; Park, K.-C. Molecular Mechanisms of Dermal Aging and Antiaging Approaches. Int. J. Mol. Sci. 2019, 20, 2126. [Google Scholar] [CrossRef] [Green Version]
- Poon, F.; Kang, S.; Chien, A.L. Mechanisms and Treatments of Photoaging. Photodermatol. Photoimmunol. Photomed. 2015, 31, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Campisi, J. Aging, Cellular Senescence, and Cancer. Annu. Rev. Physiol. 2013, 75, 685–705. [Google Scholar] [CrossRef] [Green Version]
- Hernandez-Segura, A.; Nehme, J.; Demaria, M. Hallmarks of Cellular Senescence. Trends Cell Biol. 2018, 28, 436–453. [Google Scholar] [CrossRef]
- Lopes-Paciencia, S.; Saint-Germain, E.; Rowell, M.-C.; Ruiz, A.F.; Kalegari, P.; Ferbeyre, G. The Senescence-Associated Secretory Phenotype and Its Regulation. Cytokine 2019, 117, 15–22. [Google Scholar] [CrossRef]
- Terlecki-Zaniewicz, L.; Lämmermann, I.; Latreille, J.; Bobbili, M.R.; Pils, V.; Schosserer, M.; Weinmüllner, R.; Dellago, H.; Skalicky, S.; Pum, D.; et al. Small Extracellular Vesicles and Their MiRNA Cargo Are Anti-Apoptotic Members of the Senescence-Associated Secretory Phenotype. Aging 2018, 10, 1103–1132. [Google Scholar] [CrossRef]
- Gruber, F.; Kremslehner, C.; Eckhart, L.; Tschachler, E. Cell Aging and Cellular Senescence in Skin Aging—Recent Advances in Fibroblast and Keratinocyte Biology. Exp. Gerontol. 2020, 130, 110780. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The Hallmarks of Aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [Green Version]
- Debacq-Chainiaux, F.; Erusalimsky, J.D.; Campisi, J.; Toussaint, O. Protocols to Detect Senescence-Associated Beta-Galactosidase (SA-Betagal) Activity, a Biomarker of Senescent Cells in Culture and in Vivo. Nat. Protoc. 2009, 4, 1798–1806. [Google Scholar] [CrossRef] [PubMed]
- Fazekas de St Groth, null The Evaluation of Limiting Dilution Assays. J. Immunol. Methods 1982, 49, R11–R23. [CrossRef]
- Assenmacher, M.; Avraham, H.K.; Avraham, S.; Bala, S.; Barnett, J.; Basketter, D.; Ben-David, Y.; Berek, C.; Blümel, J.; Bolliger, A.P.; et al. Limiting Dilution Analysis. In Encyclopedic Reference of Immunotoxicology; Vohr, H.-W., Ed.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 385–387. ISBN 978-3-540-27806-1. [Google Scholar]
- Tigges, J.; Krutmann, J.; Fritsche, E.; Haendeler, J.; Schaal, H.; Fischer, J.W.; Kalfalah, F.; Reinke, H.; Reifenberger, G.; Stühler, K.; et al. The Hallmarks of Fibroblast Ageing. Mech. Ageing Dev. 2014, 138, 26–44. [Google Scholar] [CrossRef] [PubMed]
- Waldera Lupa, D.M.; Kalfalah, F.; Safferling, K.; Boukamp, P.; Poschmann, G.; Volpi, E.; Götz-Rösch, C.; Bernerd, F.; Haag, L.; Huebenthal, U.; et al. Characterization of Skin Aging-Associated Secreted Proteins (SAASP) Produced by Dermal Fibroblasts Isolated from Intrinsically Aged Human Skin. J. Investig. Dermatol. 2015, 135, 1954–1968. [Google Scholar] [CrossRef] [Green Version]
- Brun, C.; Jean-Louis, F.; Oddos, T.; Bagot, M.; Bensussan, A.; Michel, L. Phenotypic and Functional Changes in Dermal Primary Fibroblasts Isolated from Intrinsically Aged Human Skin. Exp. Dermatol. 2016, 25, 113–119. [Google Scholar] [CrossRef]
- Cao, L.; Xu, X.; Bunting, S.F.; Liu, J.; Wang, R.-H.; Cao, L.L.; Wu, J.J.; Peng, T.-N.; Chen, J.; Nussenzweig, A.; et al. A Selective Requirement for 53BP1 in the Biological Response to Genomic Instability Induced by Brca1 Deficiency. Mol. Cell 2009, 35, 534–541. [Google Scholar] [CrossRef] [Green Version]
- Lago, J.C.; Puzzi, M.B. The Effect of Aging in Primary Human Dermal Fibroblasts. PLoS ONE 2019, 14, e0219165. [Google Scholar] [CrossRef] [Green Version]
- Aliper, A.M.; Csoka, A.B.; Buzdin, A.; Jetka, T.; Roumiantsev, S.; Moskalev, A.; Zhavoronkov, A. Signaling Pathway Activation Drift during Aging: Hutchinson-Gilford Progeria Syndrome Fibroblasts Are Comparable to Normal Middle-Age and Old-Age Cells. Aging 2015, 7, 26–37. [Google Scholar] [CrossRef] [Green Version]
- Bainbridge, P. Wound Healing and the Role of Fibroblasts. J. Wound Care 2013, 22, 407–411. [Google Scholar] [CrossRef]
- Haydont, V.; Bernard, B.A.; Fortunel, N.O. Age-Related Evolutions of the Dermis: Clinical Signs, Fibroblast and Extracellular Matrix Dynamics. Mech. Ageing Dev. 2019, 177, 150–156. [Google Scholar] [CrossRef]
- Bonté, F.; Girard, D.; Archambault, J.-C.; Desmoulière, A. Skin Changes During Ageing. Subcell Biochem. 2019, 91, 249–280. [Google Scholar] [CrossRef] [PubMed]
- Allsopp, R.C.; Vaziri, H.; Patterson, C.; Goldstein, S.; Younglai, E.V.; Futcher, A.B.; Greider, C.W.; Harley, C.B. Telomere Length Predicts Replicative Capacity of Human Fibroblasts. Proc. Natl. Acad. Sci. USA 1992, 89, 10114–10118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaji, K.; Ohta, T.; Horie, N.; Naru, E.; Hasegawa, M.; Kanda, N. Donor Age Reflects the Replicative Lifespan of Human Fibroblasts in Culture. Hum. Cell 2009, 22, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Schneider, E.L.; Mitsui, Y. The Relationship between in Vitro Cellular Aging and in Vivo Human Age. Proc. Natl. Acad. Sci. USA 1976, 73, 3584–3588. [Google Scholar] [CrossRef] [Green Version]
- Zouboulis, C.C.; Picardo, M.; Ju, Q.; Kurokawa, I.; Törőcsik, D.; Bíró, T.; Schneider, M.R. Beyond Acne: Current Aspects of Sebaceous Gland Biology and Function. Rev. Endocr. Metab. Disord. 2016, 17, 319–334. [Google Scholar] [CrossRef]
- Chen, F.G.; Zhang, W.J.; Bi, D.; Liu, W.; Wei, X.; Chen, F.F.; Zhu, L.; Cui, L.; Cao, Y. Clonal Analysis of Nestin– Vimentin+ Multipotent Fibroblasts Isolated from Human Dermis. J. Cell Sci. 2007, 120, 2875–2883. [Google Scholar] [CrossRef] [Green Version]
- Mine, S.; Fortunel, N.O.; Pageon, H.; Asselineau, D. Aging Alters Functionally Human Dermal Papillary Fibroblasts but Not Reticular Fibroblasts: A New View of Skin Morphogenesis and Aging. PLoS ONE 2008, 3, e4066. [Google Scholar] [CrossRef] [Green Version]
- Rydlova, M.; Holubec, L.; Ludvikova, M.; Kalfert, D.; Franekova, J.; Povysil, C.; Ludvikova, M. Biological Activity and Clinical Implications of the Matrix Metalloproteinases. Anticancer Res. 2008, 28, 1389–1397. [Google Scholar]
- Zhang, X.; Chen, C.T.; Bhargava, M.; Torzilli, P.A. A Comparative Study of Fibronectin Cleavage by MMP-1, -3, -13, and -14. Cartilage 2012, 3, 267–277. [Google Scholar] [CrossRef] [Green Version]
- Varani, J.; Dame, M.K.; Rittie, L.; Fligiel, S.E.G.; Kang, S.; Fisher, G.J.; Voorhees, J.J. Decreased Collagen Production in Chronologically Aged Skin: Roles of Age-Dependent Alteration in Fibroblast Function and Defective Mechanical Stimulation. Am. J. Pathol. 2006, 168, 1861–1868. [Google Scholar] [CrossRef] [Green Version]
- Yokose, U.; Hachiya, A.; Sriwiriyanont, P.; Fujimura, T.; Visscher, M.O.; Kitzmiller, W.J.; Bello, A.; Tsuboi, R.; Kitahara, T.; Kobinger, G.P.; et al. The Endogenous Protease Inhibitor TIMP-1 Mediates Protection and Recovery from Cutaneous Photodamage. J. Investig. Dermatol. 2012, 132, 2800–2809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sherratt, M.J. Tissue Elasticity and the Ageing Elastic Fibre. Age 2009, 31, 305–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mora Huertas, A.C.; Schmelzer, C.E.H.; Hoehenwarter, W.; Heyroth, F.; Heinz, A. Molecular-Level Insights into Aging Processes of Skin Elastin. Biochimie 2016, 128–129, 163–173. [Google Scholar] [CrossRef]
- Kozieł, R.; Greussing, R.; Maier, A.B.; Declercq, L.; Jansen-Dürr, P. Functional Interplay between Mitochondrial and Proteasome Activity in Skin Aging. J. Investig. Dermatol. 2011, 131, 594–603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, D.-F.; Chiao, Y.A.; Marcinek, D.J.; Szeto, H.H.; Rabinovitch, P.S. Mitochondrial Oxidative Stress in Aging and Healthspan. Longev. Healthspan 2014, 3, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pesce, V.; Cormio, A.; Fracasso, F.; Vecchiet, J.; Felzani, G.; Lezza, A.M.; Cantatore, P.; Gadaleta, M.N. Age-Related Mitochondrial Genotypic and Phenotypic Alterations in Human Skeletal Muscle. Free Radic. Biol. Med. 2001, 30, 1223–1233. [Google Scholar] [CrossRef]
- Oblong, J.E.; Bowman, A.; Rovito, H.A.; Jarrold, B.B.; Sherrill, J.D.; Black, M.R.; Nelson, G.; Kimball, A.B.; Birch-Machin, M.A. Metabolic Dysfunction in Human Skin: Restoration of Mitochondrial Integrity and Metabolic Output by Nicotinamide (Niacinamide) in Primary Dermal Fibroblasts from Older Aged Donors. Aging Cell 2020, 19, e13248. [Google Scholar] [CrossRef]
- Okada, A.A.; Dice, J.F. Altered Degradation of Intracellular Proteins in Aging Human Fibroblasts. Mech. Ageing Dev. 1984, 26, 341–356. [Google Scholar] [CrossRef]
- Kammeyer, A.; Luiten, R.M. Oxidation Events and Skin Aging. Ageing Res. Rev. 2015, 21, 16–29. [Google Scholar] [CrossRef]
- Rinnerthaler, M.; Bischof, J.; Streubel, M.K.; Trost, A.; Richter, K. Oxidative Stress in Aging Human Skin. Biomolecules 2015, 5, 545–589. [Google Scholar] [CrossRef] [Green Version]
- Tavallaie, M.; Voshtani, R.; Deng, X.; Qiao, Y.; Jiang, F.; Collman, J.P.; Fu, L. Moderation of Mitochondrial Respiration Mitigates Metabolic Syndrome of Aging. Proc. Natl. Acad. Sci. USA 2020, 117, 9840–9850. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rorteau, J.; Chevalier, F.P.; Bonnet, S.; Barthélemy, T.; Lopez-Gaydon, A.; Martin, L.S.; Bechetoille, N.; Lamartine, J. Maintenance of Chronological Aging Features in Culture of Normal Human Dermal Fibroblasts from Old Donors. Cells 2022, 11, 858. https://doi.org/10.3390/cells11050858
Rorteau J, Chevalier FP, Bonnet S, Barthélemy T, Lopez-Gaydon A, Martin LS, Bechetoille N, Lamartine J. Maintenance of Chronological Aging Features in Culture of Normal Human Dermal Fibroblasts from Old Donors. Cells. 2022; 11(5):858. https://doi.org/10.3390/cells11050858
Chicago/Turabian StyleRorteau, Julie, Fabien P. Chevalier, Sébastien Bonnet, Théo Barthélemy, Amandine Lopez-Gaydon, Lisa S. Martin, Nicolas Bechetoille, and Jérôme Lamartine. 2022. "Maintenance of Chronological Aging Features in Culture of Normal Human Dermal Fibroblasts from Old Donors" Cells 11, no. 5: 858. https://doi.org/10.3390/cells11050858
APA StyleRorteau, J., Chevalier, F. P., Bonnet, S., Barthélemy, T., Lopez-Gaydon, A., Martin, L. S., Bechetoille, N., & Lamartine, J. (2022). Maintenance of Chronological Aging Features in Culture of Normal Human Dermal Fibroblasts from Old Donors. Cells, 11(5), 858. https://doi.org/10.3390/cells11050858





