A Focused Review on Primary Graft Dysfunction after Clinical Lung Transplantation: A Multilevel Syndrome
Abstract
1. Introduction
2. PGD at Clinical Level
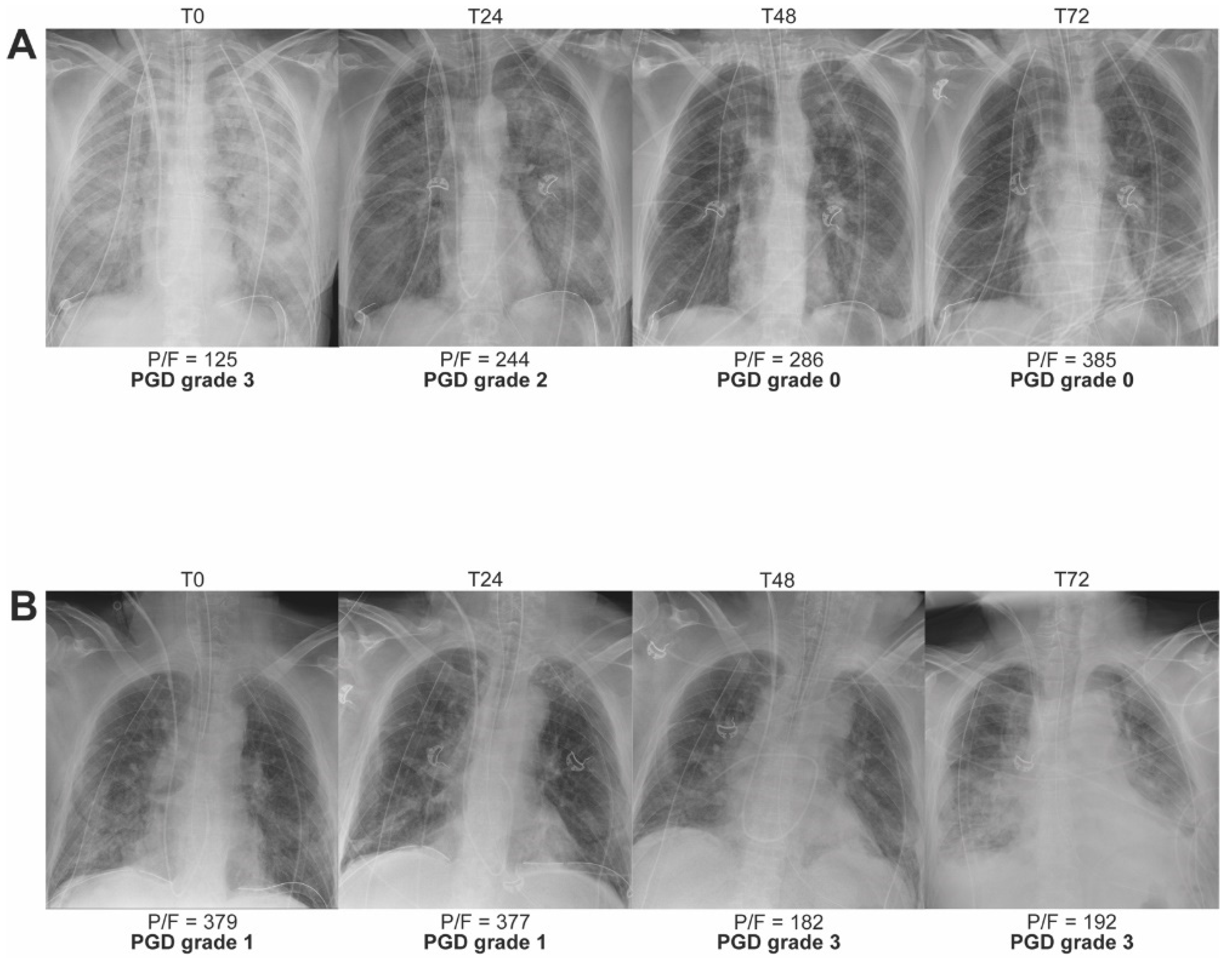
2.1. PGD Grading and Definition
2.2. Impact of PGD on Outcome
2.3. Clinical Phenotypes of High-Grade PGD
2.4. Risk Factors, Prevention and Treatment of PGD
3. PGD at Physiological Level
3.1. Lung Edema
3.2. Lung Compliance
3.3. Pulmonary Vascular Resistance
3.4. Hypoxemia
4. PGD at Radiological Level
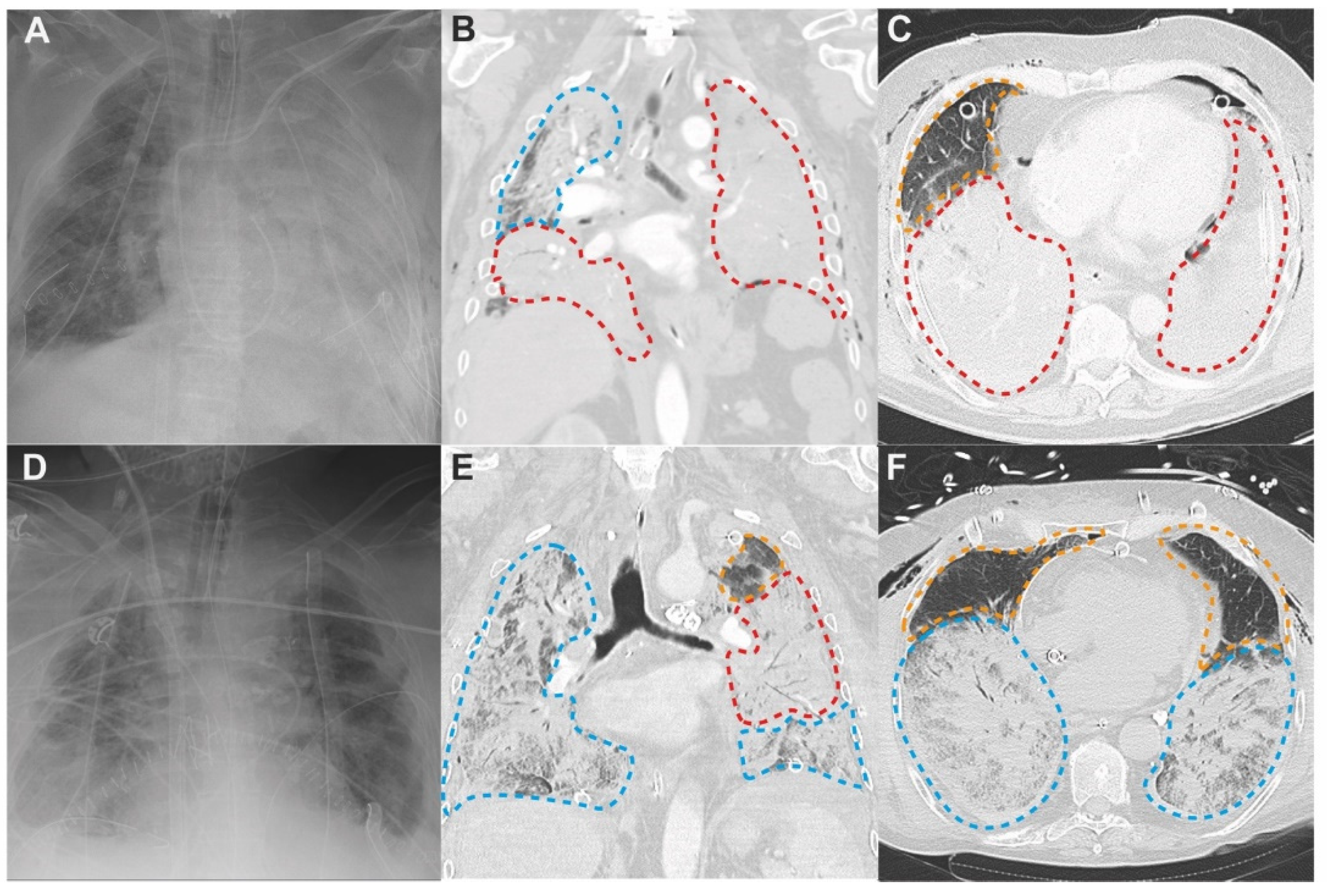
4.1. Patterns of PGD on Chest Computed Tomography
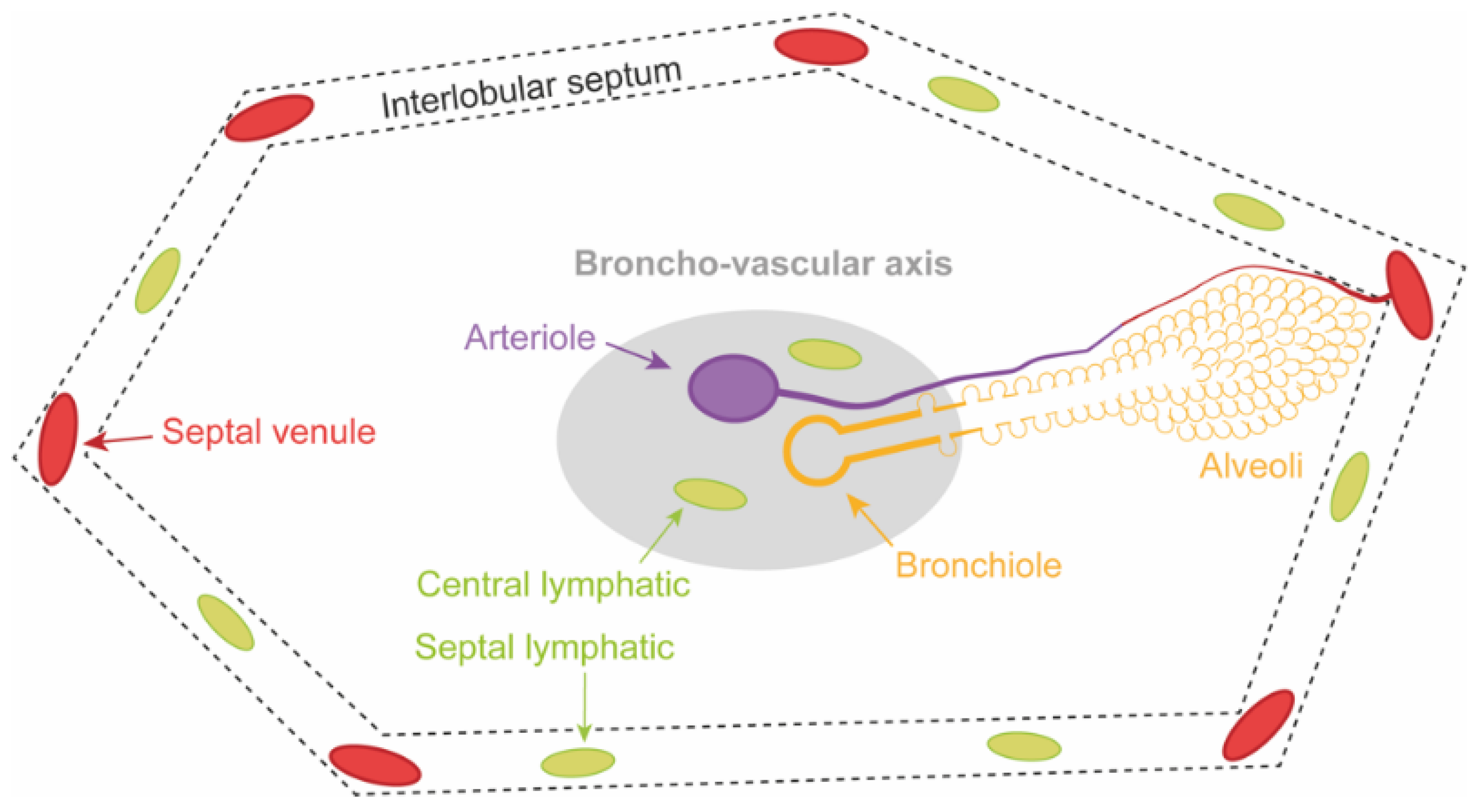
4.2. Three Lung Zones in PGD
5. PGD at Histological Level
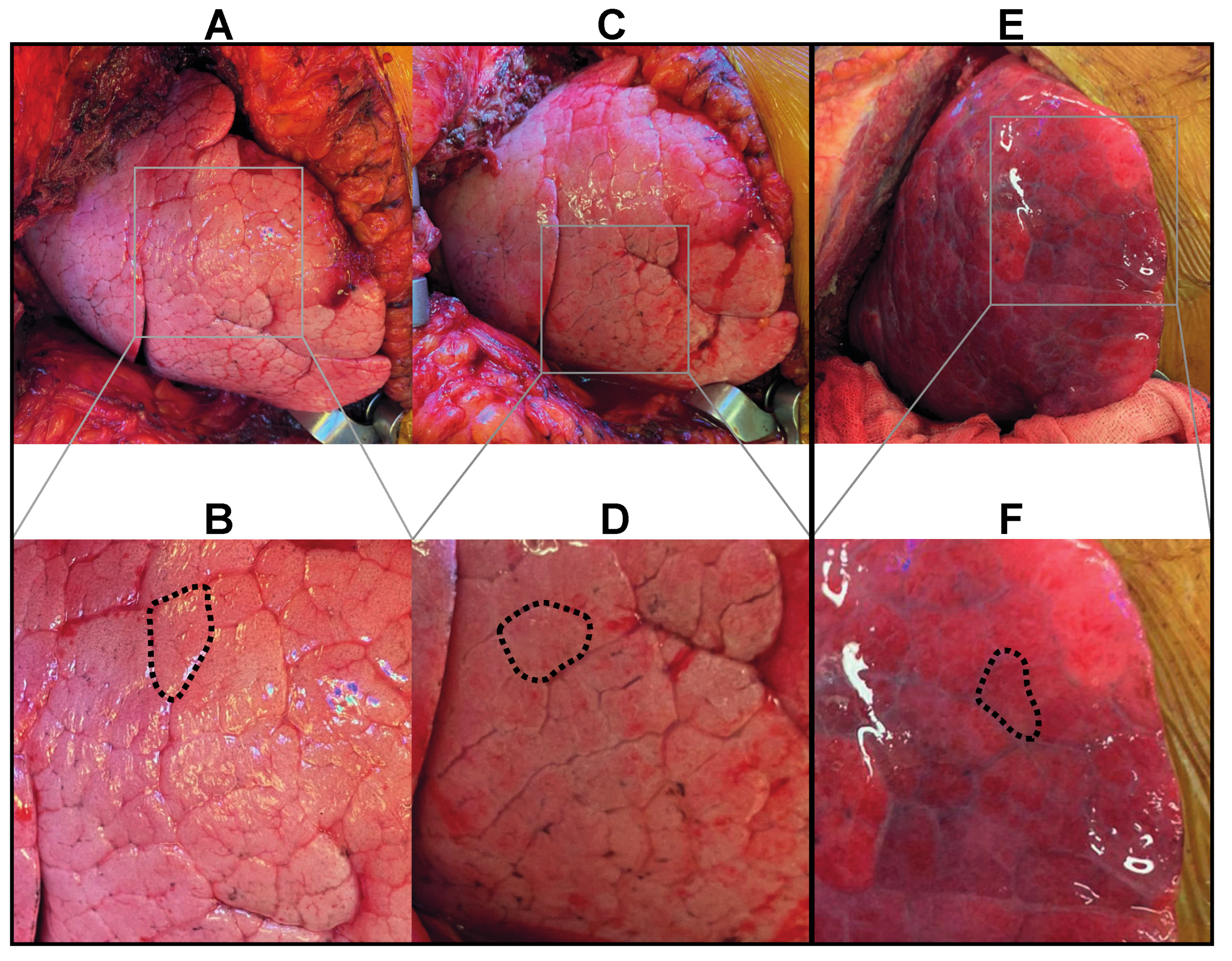
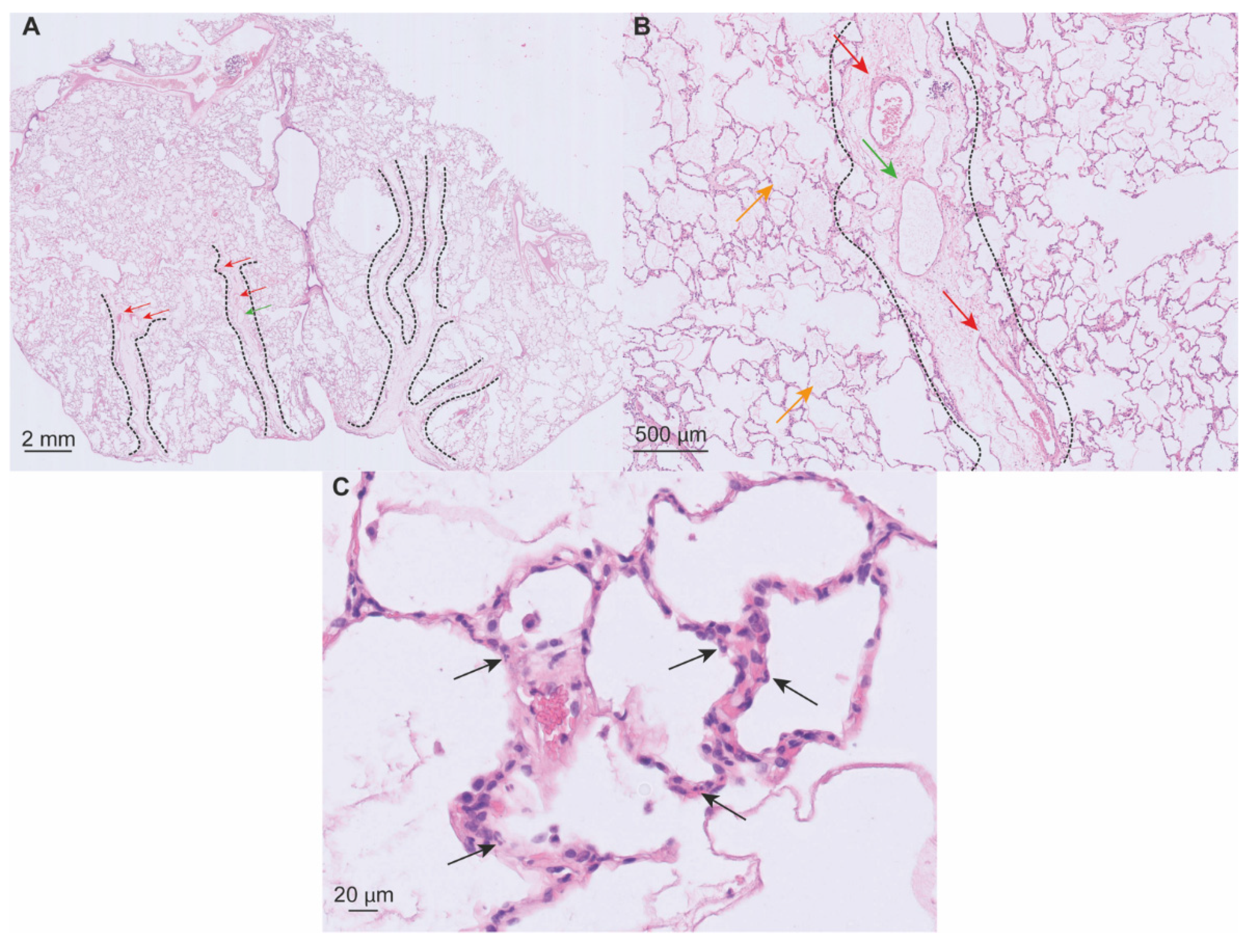
5.1. Macroscopy
5.2. Microscopy
6. PGD at Cellular Level
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Snell, G.I.; Yusen, R.D.; Weill, D.; Strueber, M.; Garrity, E.; Reed, A.; Pelaez, A.; Whelan, T.P.; Perch, M.; Bag, R.; et al. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction, part I: Definition and grading—A 2016 Consensus Group statement of the International Society for Heart and Lung Transplantation. J. Heart Lung Transplant. 2017, 36, 1097–1103. [Google Scholar] [CrossRef] [PubMed]
- Christie, J.D.; Carby, M.; Bag, R.; Corris, P.; Hertz, M.; Weill, D. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction Part II: Definition. A Consensus Statement of the International Society for Heart and Lung Transplantation. J. Heart Lung Transplant. 2005, 24, 1454–1459. [Google Scholar] [CrossRef] [PubMed]
- Porteous, M.K.; Lee, J.C. Primary Graft Dysfunction After Lung Transplantation. Clin. Chest Med. 2017, 38, 641–654. [Google Scholar] [CrossRef] [PubMed]
- Rosenheck, J.; Pietras, C.; Cantu, E. Early Graft Dysfunction after Lung Transplantation. Curr. Pulmonol. Rep. 2018, 7, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Cantu, E.; Christie, J.D. Primary graft dysfunction. Semin. Respir. Crit. Care Med. 2013, 34, 305–319. [Google Scholar] [CrossRef] [PubMed]
- Forgie, K.A.; Fialka, N.; Freed, D.H.; Nagendran, J. Lung Transplantation, Pulmonary Endothelial Inflammation, and Ex-Situ Lung Perfusion: A Review. Cells 2021, 10, 1417. [Google Scholar] [CrossRef]
- Talaie, T.; DiChiacchio, L.; Prasad, N.K.; Pasrija, C.; Julliard, W.; Kaczorowski, D.J.; Zhao, Y.; Lau, C.L. Ischemia-reperfusion Injury in the Transplanted Lung: A Literature Review. Transplant. Direct 2021, 7, e652. [Google Scholar] [CrossRef]
- Chen-Yoshikawa, T.F. Ischemia–Reperfusion Injury in Lung Transplantation. Cells 2021, 10, 1333. [Google Scholar] [CrossRef]
- den Hengst, W.A.; Gielis, J.F.; Lin, J.Y.; Van Schil, P.E.; De Windt, L.J.; Moens, A.L. Lung ischemia-reperfusion injury: A molecular and clinical view on a complex pathophysiological process. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H1283–H1299. [Google Scholar] [CrossRef]
- de Perrot, M.; Liu, M.; Waddell, T.K.; Keshavjee, S. Ischemia-reperfusion-induced lung injury. Am. J. Respir. Crit. Care Med. 2003, 167, 490–511. [Google Scholar] [CrossRef]
- Gelman, A.E.; Fisher, A.J.; Huang, H.J.; Baz, M.A.; Shaver, C.M.; Egan, T.M.; Mulligan, M.S. Report of the ISHLT Working Group on Primary Lung Graft Dysfunction Part III: Mechanisms: A 2016 Consensus Group Statement of the International Society for Heart and Lung Transplantation. J. Heart Lung Transplant. 2017, 36, 1114–1120. [Google Scholar] [CrossRef] [PubMed]
- Laubach, V.E.; Sharma, A.K. Mechanisms of lung ischemia-reperfusion injury. Curr. Opin. Organ. Transplant. 2016, 21, 246–252. [Google Scholar] [CrossRef] [PubMed]
- Thompson, B.T.; Chambers, R.C.; Liu, K.D. Acute Respiratory Distress Syndrome. N. Engl. J. Med. 2017, 377, 562–572. [Google Scholar] [CrossRef] [PubMed]
- Ashbaugh, D.; Boyd Bigelow, D.; Petty, T.; Levine, B. Acute Respiratory Distress in Adults. Lancet 1967, 290, 319–323. [Google Scholar] [CrossRef]
- Ferguson, N.D.; Fan, E.; Camporota, L.; Antonelli, M.; Anzueto, A.; Beale, R.; Brochard, L.; Brower, R.; Esteban, A.; Gattinoni, L.; et al. The Berlin definition of ARDS: An expanded rationale, justification, and supplementary material. Intensive Care Med. 2012, 38, 1573–1582. [Google Scholar] [CrossRef]
- Christie, J.D.; Bavaria, J.E.; Palevsky, H.I.; Litzky, L.; Blumenthal, N.P.; Kaiser, L.R.; Kotloff, R.M. Primary graft failure following lung transplantation. Chest 1998, 114, 51–60. [Google Scholar] [CrossRef]
- Khan, S.U.; Salloum, J.; O’Donovan, P.B.; Mascha, E.J.; Mehta, A.C.; Matthay, M.A.; Arroliga, A.C. Acute Pulmonary Edema After Lung Transplantation: The Pulmonary Reimplantation Response. Chest 1999, 116, 187–194. [Google Scholar] [CrossRef]
- Diamond, J.M.; Lee, J.C.; Kawut, S.M.; Shah, R.J.; Localio, A.R.; Bellamy, S.L.; Lederer, D.J.; Cantu, E.; Kohl, B.A.; Lama, V.N.; et al. Clinical Risk Factors for Primary Graft Dysfunction after Lung Transplantation. Am. J. Respir. Crit. Care Med. 2013, 187, 527–534. [Google Scholar] [CrossRef]
- Shah, R.J.; Bellamy, S.L.; Localio, A.R.; Wickersham, N.; Diamond, J.M.; Weinacker, A.; Lama, V.N.; Bhorade, S.; Belperio, J.A.; Crespo, M.; et al. A panel of lung injury biomarkers enhances the definition of primary graft dysfunction (PGD) after lung transplantation. J. Heart Lung Transplant. 2012, 31, 942–949. [Google Scholar] [CrossRef]
- Shah, R.J.; Diamond, J.M.; Cantu, E.; Lee, J.C.; Lederer, D.J.; Lama, V.N.; Orens, J.; Weinacker, A.; Wilkes, D.S.; Bhorade, S.; et al. Latent Class Analysis Identifies Distinct Phenotypes of Primary Graft Dysfunction After Lung Transplantation. Chest 2013, 144, 616–622. [Google Scholar] [CrossRef]
- Christie, J.D.; Sager, J.S.; Kimmel, S.E.; Ahya, V.N.; Gaughan, C.; Blumenthal, N.P.; Kotloff, R.M. Impact of Primary Graft Failure on Outcomes Following Lung Transplantation. Chest 2005, 127, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Prekker, M.E.; Nath, D.S.; Walker, A.R.; Johnson, A.C.; Hertz, M.I.; Herrington, C.S.; Radosevich, D.M.; Dahlberg, P.S. Validation of the Proposed International Society for Heart and Lung Transplantation Grading System for Primary Graft Dysfunction After Lung Transplantation. J. Heart Lung Transplant. 2006, 25, 371–378. [Google Scholar] [CrossRef] [PubMed]
- Christie, J.D.; Kotloff, R.M.; Ahya, V.N.; Tino, G.; Pochettino, A.; Gaughan, C.; DeMissie, E.; Kimmel, S.E. The Effect of Primary Graft Dysfunction on Survival after Lung Transplantation. Am. J. Respir. Crit. Care Med. 2005, 171, 1312–1316. [Google Scholar] [CrossRef] [PubMed]
- Kreisel, D.; Krupnick, A.S.; Puri, V.; Guthrie, T.J.; Trulock, E.P.; Meyers, B.F.; Patterson, G.A. Short- and long-term outcomes of 1000 adult lung transplant recipients at a single center. J. Thorac. Cardiovasc. Surg. 2011, 141, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Whitson, B.A.; Prekker, M.E.; Herrington, C.S.; Whelan, T.P.M.; Radosevich, D.M.; Hertz, M.I.; Dahlberg, P.S. Primary Graft Dysfunction and Long-term Pulmonary Function After Lung Transplantation. J. Heart Lung Transplant. 2007, 26, 1004–1011. [Google Scholar] [CrossRef]
- Huang, H.J.; Yusen, R.D.; Meyers, B.F.; Walter, M.J.; Mohanakumar, T.; Patterson, G.A.; Trulock, E.P.; Hachem, R.R. Late Primary Graft Dysfunction after Lung Transplantation and Bronchiolitis Obliterans Syndrome. Am. J. Transplant. 2008, 8, 2454–2462. [Google Scholar] [CrossRef]
- Daud, S.A.; Yusen, R.D.; Meyers, B.F.; Chakinala, M.M.; Walter, M.J.; Aloush, A.A.; Patterson, G.A.; Trulock, E.P.; Hachem, R.R. Impact of Immediate Primary Lung Allograft Dysfunction on Bronchiolitis Obliterans Syndrome. Am. J. Respir. Crit. Care Med. 2007, 175, 507–513. [Google Scholar] [CrossRef]
- Bharat, A.; Kuo, E.; Steward, N.; Aloush, A.; Hachem, R.; Trulock, E.P.; Patterson, G.A.; Meyers, B.F.; Mohanakumar, T. Immunological link between primary graft dysfunction and chronic lung allograft rejection. Ann. Thorac. Surg. 2008, 86, 189–195; discussion 187–196. [Google Scholar] [CrossRef]
- Kawashima, M.; Juvet, S.C. The role of innate immunity in the long-term outcome of lung transplantation. Ann. Transl. Med. 2020, 8, 412. [Google Scholar] [CrossRef]
- Shepherd, H.M.; Gauthier, J.M.; Li, W.; Krupnick, A.S.; Gelman, A.E.; Kreisel, D. Innate immunity in lung transplantation. J. Heart Lung Transplant. 2021, 40, 562–568. [Google Scholar] [CrossRef]
- Martinu, T.; Chen, D.-F.; Palmer, S.M. Acute Rejection and Humoral Sensitization in Lung Transplant Recipients. Proc. Am. Thorac. Soc. 2009, 6, 54–65. [Google Scholar] [CrossRef] [PubMed]
- Derhovanessian, A.; Weigt, S.S.; Palchevskiy, V.; Shino, M.Y.; Sayah, D.M.; Gregson, A.L.; Noble, P.W.; Palmer, S.M.; Fishbein, M.C.; Kubak, B.M.; et al. The Role of TGF-β in the Association Between Primary Graft Dysfunction and Bronchiolitis Obliterans Syndrome. Am. J. Transplant. 2016, 16, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Christie, J.D.; Bellamy, S.; Ware, L.B.; Lederer, D.; Hadjiliadis, D.; Lee, J.; Robinson, N.; Localio, A.R.; Wille, K.; Lama, V.; et al. Construct validity of the definition of primary graft dysfunction after lung transplantation. J. Heart Lung Transplant. 2010, 29, 1231–1239. [Google Scholar] [CrossRef] [PubMed]
- Van Raemdonck, D.; Hartwig, M.G.; Hertz, M.I.; Davis, R.D.; Cypel, M.; Hayes, D., Jr.; Ivulich, S.; Kukreja, J.; Lease, E.D.; Loor, G.; et al. Report of the ISHLT Working Group on primary lung graft dysfunction Part IV: Prevention and treatment: A 2016 Consensus Group statement of the International Society for Heart and Lung Transplantation. J. Heart Lung Transplant. 2017, 36, 1121–1136. [Google Scholar] [CrossRef]
- Van Raemdonck, D.; Rega, F.; Rex, S.; Neyrinck, A. Machine perfusion of thoracic organs. J. Thorac. Dis. 2018, 10, S910–S923. [Google Scholar] [CrossRef]
- Copeland, H.; Hayanga, J.W.A.; Neyrinck, A.; Macdonald, P.; Dellgren, G.; Bertolotti, A.; Khuu, T.; Burrows, F.; Copeland, J.G.; Gooch, D.; et al. Donor heart and lung procurement: A consensus statement. J. Heart Lung Transplant. 2020, 39, 501–517. [Google Scholar] [CrossRef]
- Leard, L.E.; Holm, A.M.; Valapour, M.; Glanville, A.R.; Attawar, S.; Aversa, M.; Campos, S.V.; Christon, L.M.; Cypel, M.; Dellgren, G.; et al. Consensus document for the selection of lung transplant candidates: An update from the International Society for Heart and Lung Transplantation. J. Heart Lung Transplant. 2021, 40, 1349–1379. [Google Scholar] [CrossRef]
- West, J.B.; Luks, A.M. West’s Pulmonary Pathophysiology: The Essentials, 9th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2013; pp. 120–145. [Google Scholar]
- De Wever, W.; Verschakelen, J. Computed Tomography of the Lung: A Pattern Approach, 2nd ed.; Springer: Heidelberg, Germany, 2018; pp. 3–19. [Google Scholar]
- Huppert, L.A.; Matthay, M.A. Alveolar Fluid Clearance in Pathologically Relevant Conditions: In Vitro and In Vivo Models of Acute Respiratory Distress Syndrome. Front. Immunol. 2017, 8, 371. [Google Scholar] [CrossRef]
- Chatterjee, S. Endothelial Mechanotransduction, Redox Signaling and the Regulation of Vascular Inflammatory Pathways. Front. Physiol. 2018, 9, 524. [Google Scholar] [CrossRef]
- Ius, F.; Sommer, W.; Tudorache, I.; Avsar, M.; Siemeni, T.; Salman, J.; Molitoris, U.; Gras, C.; Juettner, B.; Puntigam, J.; et al. Five-year experience with intraoperative extracorporeal membrane oxygenation in lung transplantation: Indications and midterm results. J. Heart Lung Transplant. 2016, 35, 49–58. [Google Scholar] [CrossRef]
- Hoetzenecker, K.; Schwarz, S.; Muckenhuber, M.; Benazzo, A.; Frommlet, F.; Schweiger, T.; Bata, O.; Jaksch, P.; Ahmadi, N.; Muraközy, G.; et al. Intraoperative extracorporeal membrane oxygenation and the possibility of postoperative prolongation improve survival in bilateral lung transplantation. J. Thorac. Cardiovasc. Surg. 2018, 155, 2193–2206.e3. [Google Scholar] [CrossRef] [PubMed]
- Ius, F.; Tudorache, I.; Warnecke, G. Extracorporeal support, during and after lung transplantation: The history of an idea. J. Thorac. Dis. 2018, 10, 5131–5148. [Google Scholar] [CrossRef]
- Barile, M. Pulmonary Edema: A Pictorial Review of Imaging Manifestations and Current Understanding of Mechanisms of Disease. Eur. J. Radiol. Open 2020, 7, 100274. [Google Scholar] [CrossRef] [PubMed]
- Malik, A.B. Mechanisms of neurogenic pulmonary edema. Circ. Res. 1985, 57, 1–18. [Google Scholar] [CrossRef] [PubMed]
- West, J.B.; Luks, A.M. West’s Respiratory Physiology: The Essentials, 11th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2021; pp. 116–150. [Google Scholar]
- Caironi, P.; Carlesso, E.; Gattinoni, L. Radiological Imaging in Acute Lung Injury and Acute Respiratory Distress Syndrome. Semin. Respir. Crit. Care Med. 2006, 27, 404–415. [Google Scholar] [CrossRef]
- Slutsky, A.S.; Ranieri, V.M. Ventilator-Induced Lung Injury. N. Engl. J. Med. 2013, 369, 2126–2136. [Google Scholar] [CrossRef]
- Hughes, K.T.; Beasley, M.B. Pulmonary Manifestations of Acute Lung Injury: More Than Just Diffuse Alveolar Damage. Arch. Pathol. Lab. Med. 2017, 141, 916–922. [Google Scholar] [CrossRef]
- Konstam, M.A.; Kiernan, M.S.; Bernstein, D.; Bozkurt, B.; Jacob, M.; Kapur, N.K.; Kociol, R.D.; Lewis, E.F.; Mehra, M.R.; Pagani, F.D.; et al. Evaluation and Management of Right-Sided Heart Failure: A Scientific Statement From the American Heart Association. Circulation 2018, 137, e578–e622. [Google Scholar] [CrossRef]
- Pinsky, M.R. The right ventricle: Interaction with the pulmonary circulation. Crit. Care 2016, 20, 266. [Google Scholar] [CrossRef]
- Zompatori, M.; Ciccarese, F.; Fasano, L. Overview of current lung imaging in acute respiratory distress syndrome. Eur. Respir. Rev. 2014, 23, 519–530. [Google Scholar] [CrossRef]
- Belmaati, E.; Jensen, C.; Kofoed, K.F.; Iversen, M.; Steffensen, I.; Nielsen, M.B. Primary graft dysfunction; possible evaluation by high resolution computed tomography, and suggestions for a scoring system. Interact. Cardiovasc. Thorac. Surg. 2009, 9, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Belmaati, E.O.; Steffensen, I.; Jensen, C.; Kofoed, K.F.; Mortensen, J.; Nielsen, M.B.; Iversen, M. Radiological patterns of primary graft dysfunction after lung transplantation evaluated by 64-multi-slice computed tomography: A descriptive study. Interact. Cardiovasc. Thorac. Surg. 2012, 14, 785–791. [Google Scholar] [CrossRef] [PubMed]
- Katzenstein, A.L.; Bloor, C.M.; Leibow, A.A. Diffuse alveolar damage--the role of oxygen, shock, and related factors. A review. Am. J. Pathol. 1976, 85, 209–228. [Google Scholar] [PubMed]
- Patel, S.R.; Karmpaliotis, D.; Ayas, N.T.; Mark, E.J.; Wain, J.; Thompson, B.T.; Malhotra, A. The role of open-lung biopsy in ARDS. Chest 2004, 125, 197–202. [Google Scholar] [CrossRef]
- Chaparro, C.; Chamberlain, D.; Maurer, J.; De Hoyos, A.; Winton, T.; Kesten, S. Acute lung injury in lung allografts. J. Heart Lung Transplant. 1995, 14, 267–273. [Google Scholar]
- Fisher, A.J.; Wardle, J.; Dark, J.H.; Corris, P.A. Non-immune acute graft injury after lung transplantation and the risk of subsequent bronchiolitis obliterans syndrome (BOS). J. Heart Lung Transplant. 2002, 21, 1206–1212. [Google Scholar] [CrossRef]
- Sato, M.; Hwang, D.M.; Ohmori-Matsuda, K.; Chaparro, C.; Waddell, T.K.; Singer, L.G.; Hutcheon, M.A.; Keshavjee, S. Revisiting the pathologic finding of diffuse alveolar damage after lung transplantation. J. Heart Lung Transplant. 2012, 31, 354–363. [Google Scholar] [CrossRef]
- Gammie, J.S.; Stukus, D.R.; Pham, S.M.; Hattler, B.G.; McGrath, M.F.; McCurry, K.R.; Griffith, B.P.; Keenan, R.J. Effect of ischemic time on survival in clinical lung transplantation. Ann. Thorac. Surg. 1999, 68, 2015–2019; discussion 2019–2020. [Google Scholar] [CrossRef]
- Burton, C.M.; Iversen, M.; Carlsen, J.; Andersen, C.B. Interstitial inflammatory lesions of the pulmonary allograft: A retrospective analysis of 2697 transbronchial biopsies. Transplantation 2008, 86, 811–819. [Google Scholar] [CrossRef]
- Weyker, P.D.; Webb, C.A.; Kiamanesh, D.; Flynn, B.C. Lung ischemia reperfusion injury: A bench-to-bedside review. Semin. Cardiothorac. Vasc. Anesth. 2013, 17, 28–43. [Google Scholar] [CrossRef]
- Tao, J.Q.; Sorokina, E.M.; Vazquez Medina, J.P.; Mishra, M.K.; Yamada, Y.; Satalin, J.; Nieman, G.F.; Nellen, J.R.; Beduhn, B.; Cantu, E.; et al. Onset of Inflammation With Ischemia: Implications for Donor Lung Preservation and Transplant Survival. Am. J. Transplant. 2016, 16, 2598–2611. [Google Scholar] [CrossRef] [PubMed]
- Jungraithmayr, W. Novel Strategies for Endothelial Preservation in Lung Transplant Ischemia-Reperfusion Injury. Front. Physiol. 2020, 11, 1420. [Google Scholar] [CrossRef] [PubMed]
- Eltzschig, H.K.; Collard, C.D. Vascular ischaemia and reperfusion injury. Br. Med. Bull. 2004, 70, 71–86. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Charles, E.J.; Zhao, Y.; Narahari, A.K.; Baderdinni, P.K.; Good, M.E.; Lorenz, U.M.; Kron, I.L.; Bayliss, D.A.; Ravichandran, K.S.; et al. Pannexin-1 channels on endothelial cells mediate vascular inflammation during lung ischemia-reperfusion injury. Am. J. Physiol. Lung Cell Mol. 2018, 315, L301–L312. [Google Scholar] [CrossRef] [PubMed]
- Dixon, J.T.; Gozal, E.; Roberts, A.M. Platelet-mediated vascular dysfunction during acute lung injury. Arch. Physiol. Biochem. 2012, 118, 72–82. [Google Scholar] [CrossRef]
- Sayah, D.M.; Mallavia, B.; Liu, F.; Ortiz-Muñoz, G.; Caudrillier, A.; DerHovanessian, A.; Ross, D.J.; Lynch, J.P.; Saggar, R.; Ardehali, A.; et al. Neutrophil extracellular traps are pathogenic in primary graft dysfunction after lung transplantation. Am. J. Respir. Crit. Care Med. 2015, 191, 455–463. [Google Scholar] [CrossRef]
- Sladden, T.M.; Yerkovich, S.; Grant, M.; Zhang, F.; Liu, X.; Trotter, M.; Hopkins, P.; Linhardt, R.J.; Chambers, D.C. Endothelial Glycocalyx Shedding Predicts Donor Organ Acceptability and Is Associated With Primary Graft Dysfunction in Lung Transplant Recipients. Transplantation 2019, 103, 1277–1285. [Google Scholar] [CrossRef]
- Chappell, D.; Dörfler, N.; Jacob, M.; Rehm, M.; Welsch, U.; Conzen, P.; Becker, B.F. Glycocalyx protection reduces leukocyte adhesion after ischemia/reperfusion. Shock 2010, 34, 133–139. [Google Scholar] [CrossRef]
- Annecke, T.; Fischer, J.; Hartmann, H.; Tschoep, J.; Rehm, M.; Conzen, P.; Sommerhoff, C.P.; Becker, B.F. Shedding of the coronary endothelial glycocalyx: Effects of hypoxia/reoxygenation vs ischaemia/reperfusion. Br. J. Anaesth. 2011, 107, 679–686. [Google Scholar] [CrossRef]
- Weinbaum, S.; Tarbell, J.M.; Damiano, E.R. The Structure and Function of the Endothelial Glycocalyx Layer. Annu. Rev. Biomed. Eng. 2007, 9, 121–167. [Google Scholar] [CrossRef]
- Brettner, F.; von Dossow, V.; Chappell, D. The endothelial glycocalyx and perioperative lung injury. Curr. Opin. Anaesthesiol. 2017, 30, 36–41. [Google Scholar] [CrossRef]
- Granger, D.N.; Kvietys, P.R. Reperfusion injury and reactive oxygen species: The evolution of a concept. Redox Biol. 2015, 6, 524–551. [Google Scholar] [CrossRef]
- Pak, O.; Sydykov, A.; Kosanovic, D.; Schermuly, R.T.; Dietrich, A.; Schröder, K.; Brandes, R.P.; Gudermann, T.; Sommer, N.; Weissmann, N. Lung Ischaemia-Reperfusion Injury: The Role of Reactive Oxygen Species. Adv. Exp. Med. Biol. 2017, 967, 195–225. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Nieman, G.F.; Christie, J.D.; Fisher, A.B. Shear stress-related mechanosignaling with lung ischemia: Lessons from basic research can inform lung transplantation. Am. J. Physiol. Lung Cell Mol. 2014, 307, L668–L680. [Google Scholar] [CrossRef] [PubMed]
- Schröder, K. NADPH oxidases in redox regulation of cell adhesion and migration. Antioxid. Redox Signal. 2014, 20, 2043–2058. [Google Scholar] [CrossRef] [PubMed]
- Briot, R.; Frank, J.A.; Uchida, T.; Lee, J.W.; Calfee, C.S.; Matthay, M.A. Elevated Levels of the Receptor for Advanced Glycation End Products, a Marker of Alveolar Epithelial Type I Cell Injury, Predict Impaired Alveolar Fluid Clearance in Isolated Perfused Human Lungs. Chest 2009, 135, 269–275. [Google Scholar] [CrossRef] [PubMed]
- Ware, L.B.; Golden, J.A.; Finkbeiner, W.E.; Matthay, M.A. Alveolar epithelial fluid transport capacity in reperfusion lung injury after lung transplantation. Am. J. Respir. Crit. Care Med. 1999, 159, 980–988. [Google Scholar] [CrossRef]
- Ferrari, R.S.; Andrade, C.F. Oxidative Stress and Lung Ischemia-Reperfusion Injury. Oxid. Med. Cell. Longev. 2015, 2015, 590987. [Google Scholar] [CrossRef]
- Kalogeris, T.; Bao, Y.; Korthuis, R.J. Mitochondrial reactive oxygen species: A double edged sword in ischemia/reperfusion vs. preconditioning. Redox Biol. 2014, 2, 702–714. [Google Scholar] [CrossRef]
- Deng, C.; Zhai, Z.; Wu, D.; Lin, Q.; Yang, Y.; Yang, M.; Ding, H.; Cao, X.; Zhang, Q.; Wang, C. Inflammatory response and pneumocyte apoptosis during lung ischemia-reperfusion injury in an experimental pulmonary thromboembolism model. J. Thromb. Thrombolysis 2015, 40, 42–53. [Google Scholar] [CrossRef]
- Fei, L.; Jingyuan, X.; Fangte, L.; Huijun, D.; Liu, Y.; Ren, J.; Jinyuan, L.; Linghui, P. Preconditioning with rHMGB1 ameliorates lung ischemia-reperfusion injury by inhibiting alveolar macrophage pyroptosis via the Keap1/Nrf2/HO-1 signaling pathway. J. Transl. Med. 2020, 18, 301. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Feng, G.; Gauthier, J.M.; Lokshina, I.; Higashikubo, R.; Evans, S.; Liu, X.; Hassan, A.; Tanaka, S.; Cicka, M.; et al. Ferroptotic cell death and TLR4/Trif signaling initiate neutrophil recruitment after heart transplantation. J. Clin. Investig. 2019, 129, 2293–2304. [Google Scholar] [CrossRef]
- Xu, Y.; Li, X.; Cheng, Y.; Yang, M.; Wang, R. Inhibition of ACSL4 attenuates ferroptotic damage after pulmonary ischemia-reperfusion. FASEB J. 2020, 34, 16262–16275. [Google Scholar] [CrossRef] [PubMed]
- Almeida, F.M.; Oliveira-Junior, M.C.; Souza, R.A.; Petroni, R.C.; Soto, S.F.; Soriano, F.G.; Carvalho, P.T.; Albertini, R.; Damaceno-Rodrigues, N.R.; Lopes, F.D.; et al. Creatine supplementation attenuates pulmonary and systemic effects of lung ischemia and reperfusion injury. J. Heart Lung Transplant. 2016, 35, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Linkermann, A.; Hackl, M.J.; Kunzendorf, U.; Walczak, H.; Krautwald, S.; Jevnikar, A.M. Necroptosis in Immunity and Ischemia-Reperfusion Injury. Am. J. Transplant. 2013, 13, 2797–2804. [Google Scholar] [CrossRef] [PubMed]
- Harris, H.E.; Andersson, U.; Pisetsky, D.S. HMGB1: A multifunctional alarmin driving autoimmune and inflammatory disease. Nat. Rev. Rheumatol. 2012, 8, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Y.; Nuñez, G. Sterile inflammation: Sensing and reacting to damage. Nat. Rev. Immunol. 2010, 10, 826–837. [Google Scholar] [CrossRef]
- Matzinger, P. Tolerance, Danger, and the Extended Family. Annu. Rev. Immunol. 1994, 12, 991–1045. [Google Scholar] [CrossRef]
- Van Beijnum, J.R.; Buurman, W.A.; Griffioen, A.W. Convergence and amplification of toll-like receptor (TLR) and receptor for advanced glycation end products (RAGE) signaling pathways via high mobility group B1 (HMGB1). Angiogenesis 2008, 11, 91–99. [Google Scholar] [CrossRef]
- Zanotti, G.; Casiraghi, M.; Abano, J.B.; Tatreau, J.R.; Sevala, M.; Berlin, H.; Smyth, S.; Funkhouser, W.K.; Burridge, K.; Randell, S.H.; et al. Novel critical role of Toll-like receptor 4 in lung ischemia-reperfusion injury and edema. Am. J. Physiol. Lung Cell Mol. 2009, 297, L52–L63. [Google Scholar] [CrossRef]
- Gallucci, S.; Lolkema, M.; Matzinger, P. Natural adjuvants: Endogenous activators of dendritic cells. Nat. Med. 1999, 5, 1249–1255. [Google Scholar] [CrossRef]
- Aggarwal, N.R.; King, L.S.; D’Alessio, F.R. Diverse macrophage populations mediate acute lung inflammation and resolution. Am. J. Physiol. Lung Cell Mol. 2014, 306, L709–L725. [Google Scholar] [CrossRef] [PubMed]
- Phelan, P.; Merry, H.E.; Hwang, B.; Mulligan, M.S. Differential toll-like receptor activation in lung ischemia reperfusion injury. J. Thorac. Cardiovasc. Surg. 2015, 149, 1653–1661. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zheng, Z.; Chiu, S.; Akbarpour, M.; Sun, H.; Reyfman, P.A.; Anekalla, K.R.; Abdala-Valencia, H.; Edgren, D.; Li, W.; Kreisel, D.; et al. Donor pulmonary intravascular nonclassical monocytes recruit recipient neutrophils and mediate primary lung allograft dysfunction. Sci. Transl. Med. 2017, 9, eaal4508. [Google Scholar] [CrossRef]
- Charles, A.; Janeway, J.; Medzhitov, R. Innate Immune Recognition. Annu. Rev. Immunol. 2002, 20, 197–216. [Google Scholar] [CrossRef]
- Merry, H.E.; Phelan, P.; Doaks, M.; Zhao, M.; Mulligan, M.S. Functional roles of tumor necrosis factor-alpha and interleukin 1-Beta in hypoxia and reoxygenation. Ann. Thorac. Surg. 2015, 99, 1200–1205. [Google Scholar] [CrossRef] [PubMed]
- Capucetti, A.; Albano, F.; Bonecchi, R. Multiple Roles for Chemokines in Neutrophil Biology. Front. Immunol. 2020, 11, 1259. [Google Scholar] [CrossRef]
- Kreisel, D.; Nava, R.G.; Li, W.; Zinselmeyer, B.H.; Wang, B.; Lai, J.; Pless, R.; Gelman, A.E.; Krupnick, A.S.; Miller, M.J. In vivo two-photon imaging reveals monocyte-dependent neutrophil extravasation during pulmonary inflammation. Proc. Natl. Acad. Sci. USA 2010, 107, 18073. [Google Scholar] [CrossRef]
- Sharma, A.K.; Lapar, D.J.; Stone, M.L.; Zhao, Y.; Kron, I.L.; Laubach, V.E. Receptor for advanced glycation end products (RAGE) on iNKT cells mediates lung ischemia-reperfusion injury. Am. J. Transplant. 2013, 13, 2255–2267. [Google Scholar] [CrossRef]
- Sharma, A.K.; LaPar, D.J.; Zhao, Y.; Li, L.; Lau, C.L.; Kron, I.L.; Iwakura, Y.; Okusa, M.D.; Laubach, V.E. Natural killer T cell-derived IL-17 mediates lung ischemia-reperfusion injury. Am. J. Respir. Crit. Care Med. 2011, 183, 1539–1549. [Google Scholar] [CrossRef]
- Williams, A.E.; José, R.J.; Mercer, P.F.; Brealey, D.; Parekh, D.; Thickett, D.R.; O’Kane, C.; McAuley, D.F.; Chambers, R.C. Evidence for chemokine synergy during neutrophil migration in ARDS. Thorax 2017, 72, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Kreisel, D.; Goldstein, D.R. Innate immunity and organ transplantation: Focus on lung transplantation. Transpl. Int. 2013, 26, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Mcdonald, B.; Pittman, K.; Menezes, G.B.; Hirota, S.A.; Slaba, I.; Waterhouse, C.C.M.; Beck, P.L.; Muruve, D.A.; Kubes, P. Intravascular Danger Signals Guide Neutrophils to Sites of Sterile Inflammation. Science 2010, 330, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Grommes, J.; Soehnlein, O. Contribution of Neutrophils to Acute Lung Injury. Mol. Med. 2011, 17, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Müller-Redetzky, H. Targeting neutrophil extracellular traps in acute lung injury: A novel therapeutic approach in acute respiratory distress syndrome? Anesthesiology 2015, 122, 725–727. [Google Scholar] [CrossRef]
- Torii, K.; Iida, K.; Miyazaki, Y.; Saga, S.; Kondoh, Y.; Taniguchi, H.; Taki, F.; Takagi, K.; Matsuyama, M.; Suzuki, R. Higher concentrations of matrix metalloproteinases in bronchoalveolar lavage fluid of patients with adult respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 1997, 155, 43–46. [Google Scholar] [CrossRef]
- Ali, H.A.; Pavlisko, E.N.; Snyder, L.D.; Frank, M.; Palmer, S.M. Complement system in lung transplantation. Clin. Transplant. 2018, 32, e13208. [Google Scholar] [CrossRef]
- Kulkarni, H.S.; Liszewski, M.K.; Brody, S.L.; Atkinson, J.P. The complement system in the airway epithelium: An overlooked host defense mechanism and therapeutic target? J. Allergy Clin. Immunol. 2018, 141, 1582–1586.e1581. [Google Scholar] [CrossRef]
- Kulkarni, H.S.; Ramphal, K.; Ma, L.; Brown, M.; Oyster, M.; Speckhart, K.N.; Takahashi, T.; Byers, D.E.; Porteous, M.K.; Kalman, L.; et al. Local complement activation is associated with primary graft dysfunction after lung transplantation. JCI Insight 2020, 5. [Google Scholar] [CrossRef]
- Yang, Z.; Sharma, A.K.; Linden, J.; Kron, I.L.; Laubach, V.E. CD4+ T lymphocytes mediate acute pulmonary ischemia–reperfusion injury. J. Thorac. Cardiovasc. Surg. 2009, 137, 695–702. [Google Scholar] [CrossRef]
- Ochando, J.; Ordikhani, F.; Boros, P.; Jordan, S. The innate immune response to allotransplants: Mechanisms and therapeutic potentials. Cell. Mol. Immunol. 2019, 16, 350–356. [Google Scholar] [CrossRef]
- Persson, C.G.A.; Uller, L. Increased lung neutrophil apoptosis and inflammation resolution. Eur. Respir. J. 2012, 39, 789. [Google Scholar] [CrossRef]
- D’Alessio, F.R.; Tsushima, K.; Aggarwal, N.R.; West, E.E.; Willett, M.H.; Britos, M.F.; Pipeling, M.R.; Brower, R.G.; Tuder, R.M.; McDyer, J.F.; et al. CD4+CD25+Foxp3+ Tregs resolve experimental lung injury in mice and are present in humans with acute lung injury. J. Clin. Investig. 2009, 119, 2898–2913. [Google Scholar] [CrossRef] [PubMed]
- Mock, J.R.; Garibaldi, B.T.; Aggarwal, N.R.; Jenkins, J.; Limjunyawong, N.; Singer, B.D.; Chau, E.; Rabold, R.; Files, D.C.; Sidhaye, V.; et al. Foxp3+ regulatory T cells promote lung epithelial proliferation. Mucosal Immunol. 2014, 7, 1440–1451. [Google Scholar] [CrossRef] [PubMed]
- Dodd-o, J.M.; Lendermon, E.A.; Miller, H.L.; Zhong, Q.; John, E.R.; Jungraithmayr, W.M.; D’Alessio, F.R.; McDyer, J.F. CD154 Blockade Abrogates Allospecific Responses and Enhances CD4+ Regulatory T-Cells in Mouse Orthotopic Lung Transplant. Am. J. Transplant. 2011, 11, 1815–1824. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Millar, F.R.; Summers, C.; Griffiths, M.J.; Toshner, M.R.; Proudfoot, A.G. The pulmonary endothelium in acute respiratory distress syndrome: Insights and therapeutic opportunities. Thorax 2016, 71, 462–473. [Google Scholar] [CrossRef] [PubMed]


| Grade | Bilateral Alveolar Infiltrates on Chest X-ray | PaO2/FiO2 Ratio |
|---|---|---|
| PGD grade 0 | No | Any |
| PGD grade 1 | Yes | >300 |
| PGD grade 2 | Yes | 200–300 |
| PGD grade 3 | Yes | <200 |
| Category | Risk Factors |
|---|---|
| Donor | Age > 45 years or <21 years |
| Female sex | |
| History of smoking | |
| Mechanisms of death: aspiration, head trauma | |
| Hemodynamic instability after brain death | |
| Prolonged mechanical ventilation | |
| Recipient | BMI > 25 |
| Female sex | |
| Diagnosis: idiopathic pulmonary fibrosis, idiopathic pulmonary hypertension, secondary pulmonary hypertension, sarcoidosis | |
| Elevated pulmonary artery pressure at time of surgery | |
| Procedure | Prolonged ischemic time |
| Single lung transplantation | |
| Use of cardiopulmonary bypass | |
| Administration > 1 L packed red blood cells | |
| FiO2 > 0.4 at reperfusion |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Van Slambrouck, J.; Van Raemdonck, D.; Vos, R.; Vanluyten, C.; Vanstapel, A.; Prisciandaro, E.; Willems, L.; Orlitová, M.; Kaes, J.; Jin, X.; et al. A Focused Review on Primary Graft Dysfunction after Clinical Lung Transplantation: A Multilevel Syndrome. Cells 2022, 11, 745. https://doi.org/10.3390/cells11040745
Van Slambrouck J, Van Raemdonck D, Vos R, Vanluyten C, Vanstapel A, Prisciandaro E, Willems L, Orlitová M, Kaes J, Jin X, et al. A Focused Review on Primary Graft Dysfunction after Clinical Lung Transplantation: A Multilevel Syndrome. Cells. 2022; 11(4):745. https://doi.org/10.3390/cells11040745
Chicago/Turabian StyleVan Slambrouck, Jan, Dirk Van Raemdonck, Robin Vos, Cedric Vanluyten, Arno Vanstapel, Elena Prisciandaro, Lynn Willems, Michaela Orlitová, Janne Kaes, Xin Jin, and et al. 2022. "A Focused Review on Primary Graft Dysfunction after Clinical Lung Transplantation: A Multilevel Syndrome" Cells 11, no. 4: 745. https://doi.org/10.3390/cells11040745
APA StyleVan Slambrouck, J., Van Raemdonck, D., Vos, R., Vanluyten, C., Vanstapel, A., Prisciandaro, E., Willems, L., Orlitová, M., Kaes, J., Jin, X., Jansen, Y., Verleden, G. M., Neyrinck, A. P., Vanaudenaerde, B. M., & Ceulemans, L. J. (2022). A Focused Review on Primary Graft Dysfunction after Clinical Lung Transplantation: A Multilevel Syndrome. Cells, 11(4), 745. https://doi.org/10.3390/cells11040745






