Characterization of Collapsin Response Mediator Protein 2 in Colorectal Cancer Progression in Subjects with Diabetic Comorbidity
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Study Subjects
2.3. Immunohistochemical Staining of CRMP2
2.4. Cell Culture
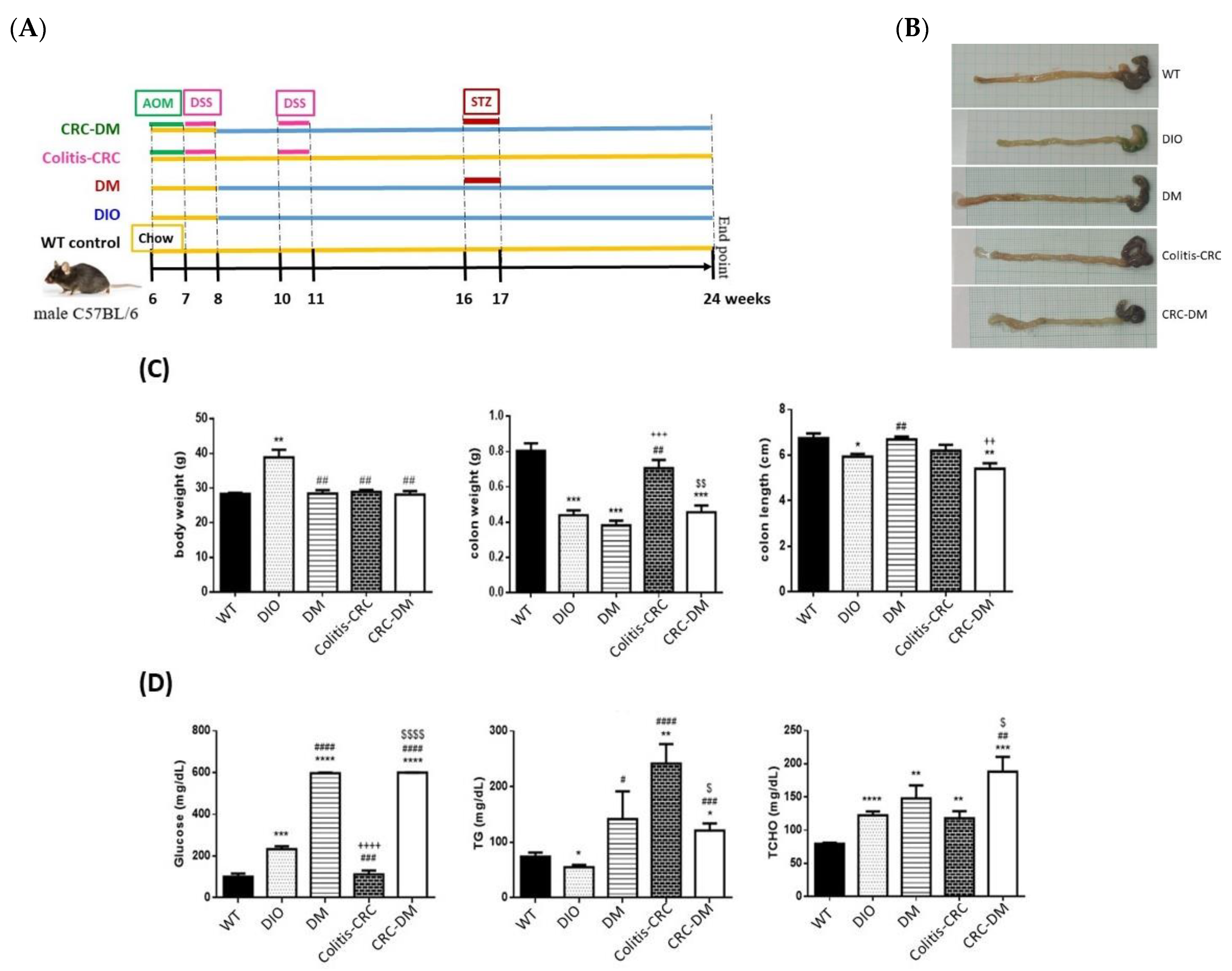
2.5. Animal Experiments
2.6. Western Blot Analysis
2.7. RNA Extraction and RT-PCR
2.8. Proliferation and Wound Healing Assay
2.9. CRMP2 Knockdown and Overexpression
2.10. Statistical Analysis
3. Results
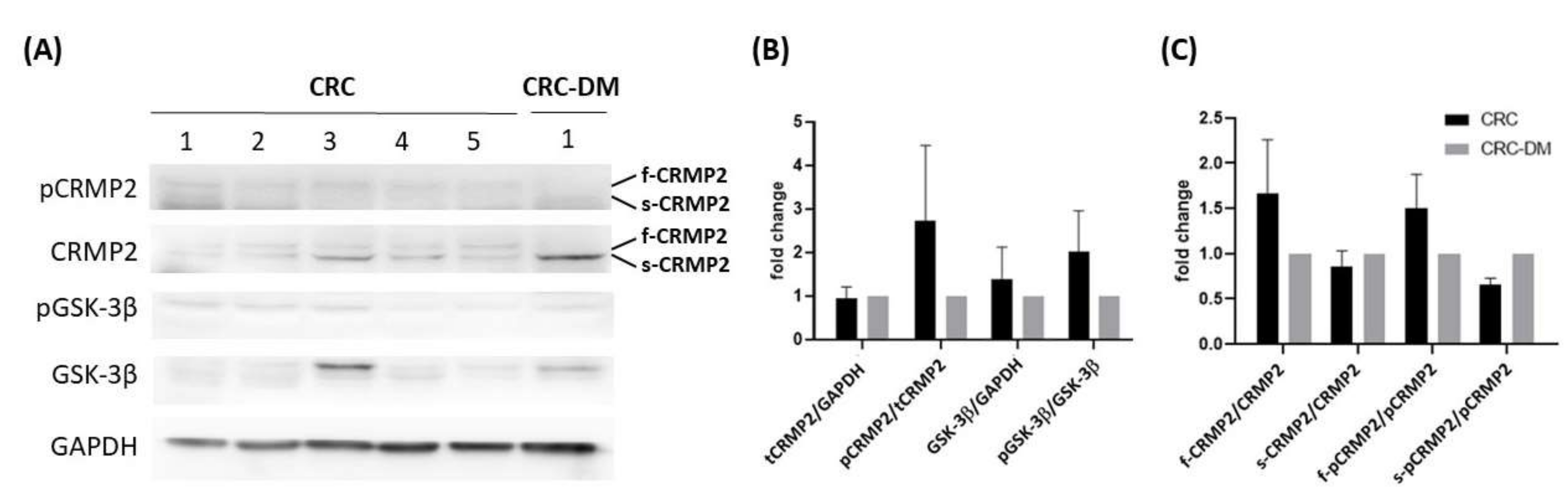
3.1. Significant Association between CRMP2 and Diabetic Comorbidity among CRC Patients
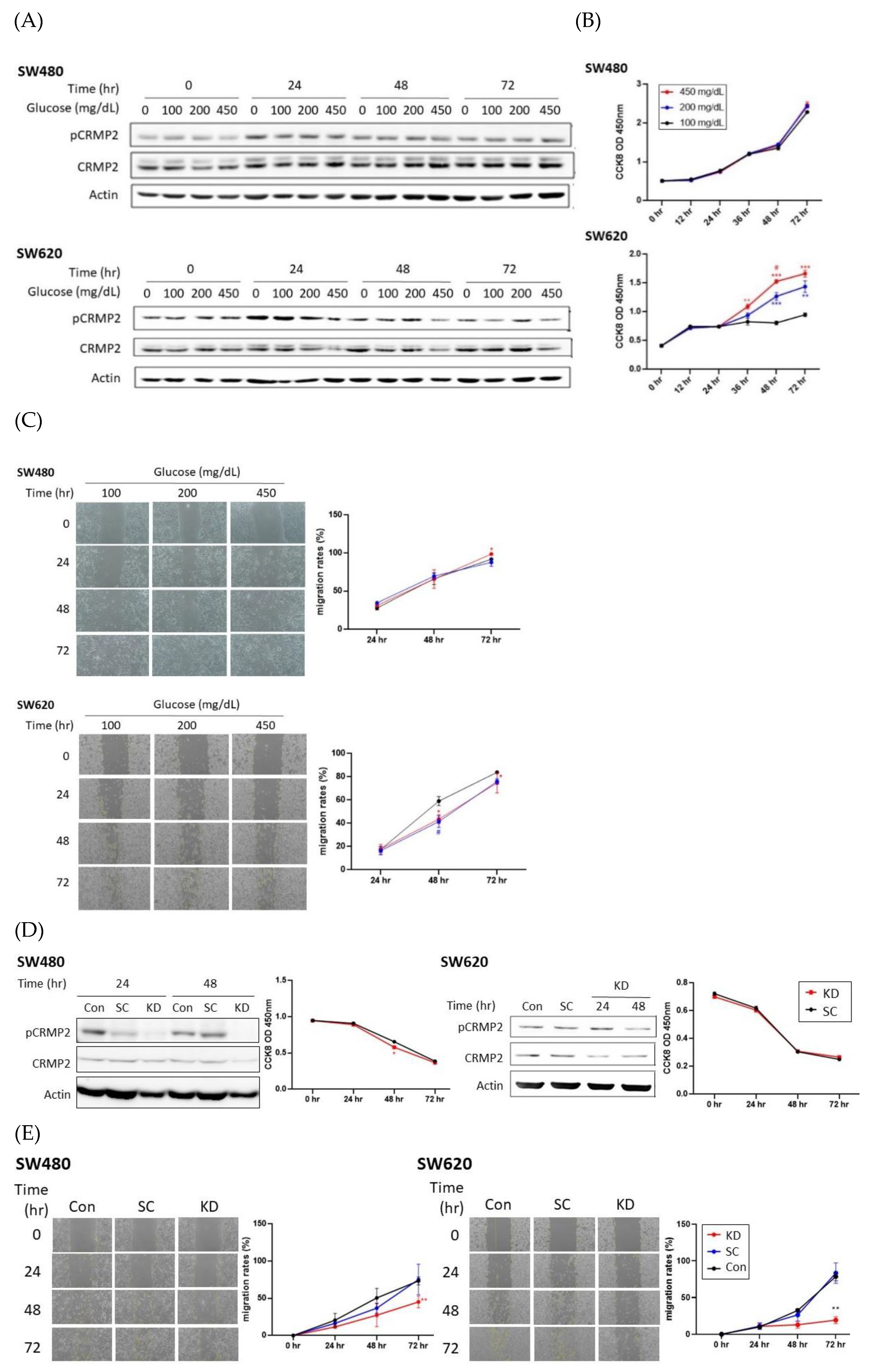
3.2. Effects of Glucose on CRMP2 Profiles and Malignant Traits of Colorectal Cancer Cells
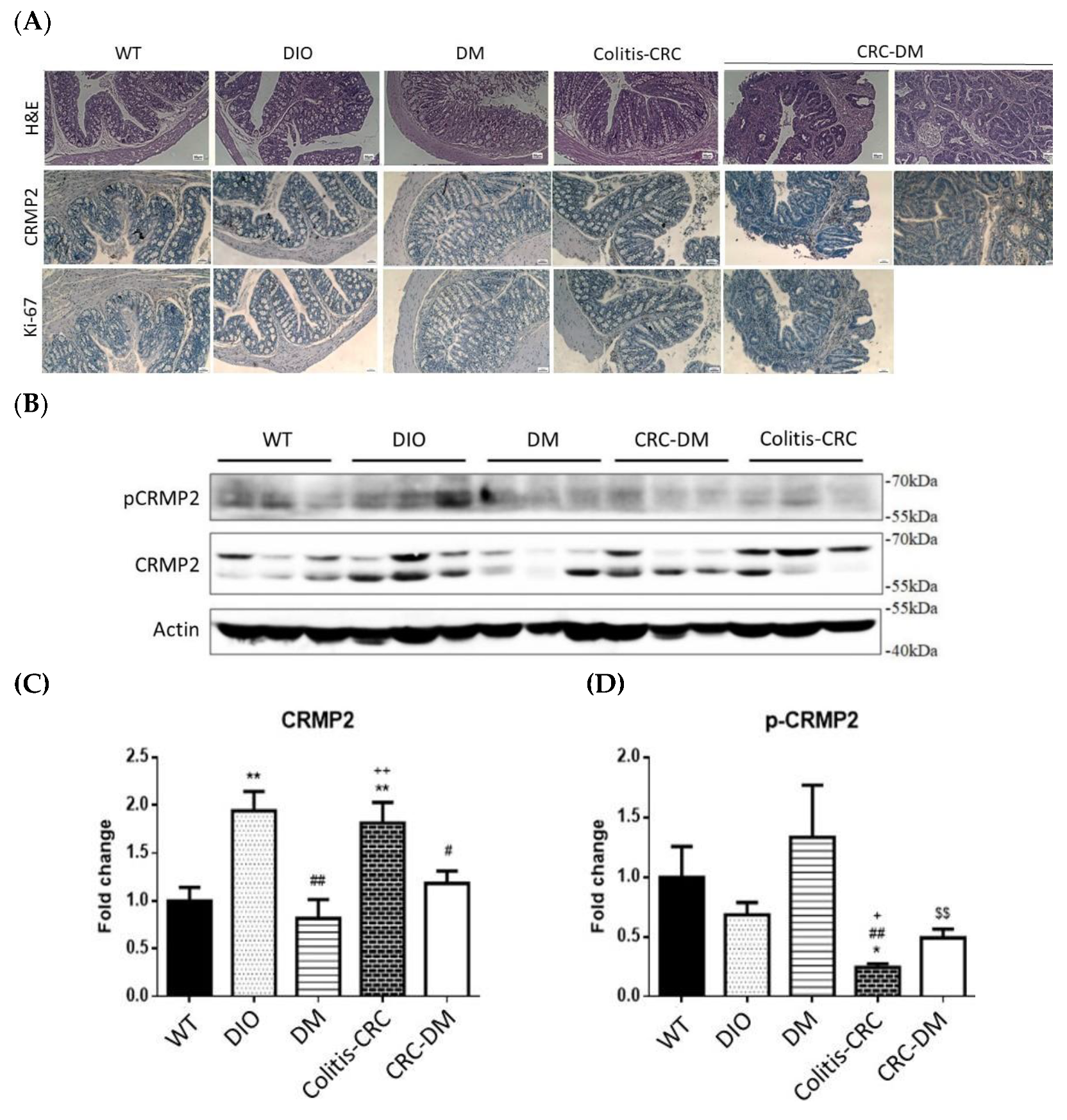
3.3. Regulation of In Vivo CRMP2 Expression Profile by Diabetic and Colorectal Comorbidity
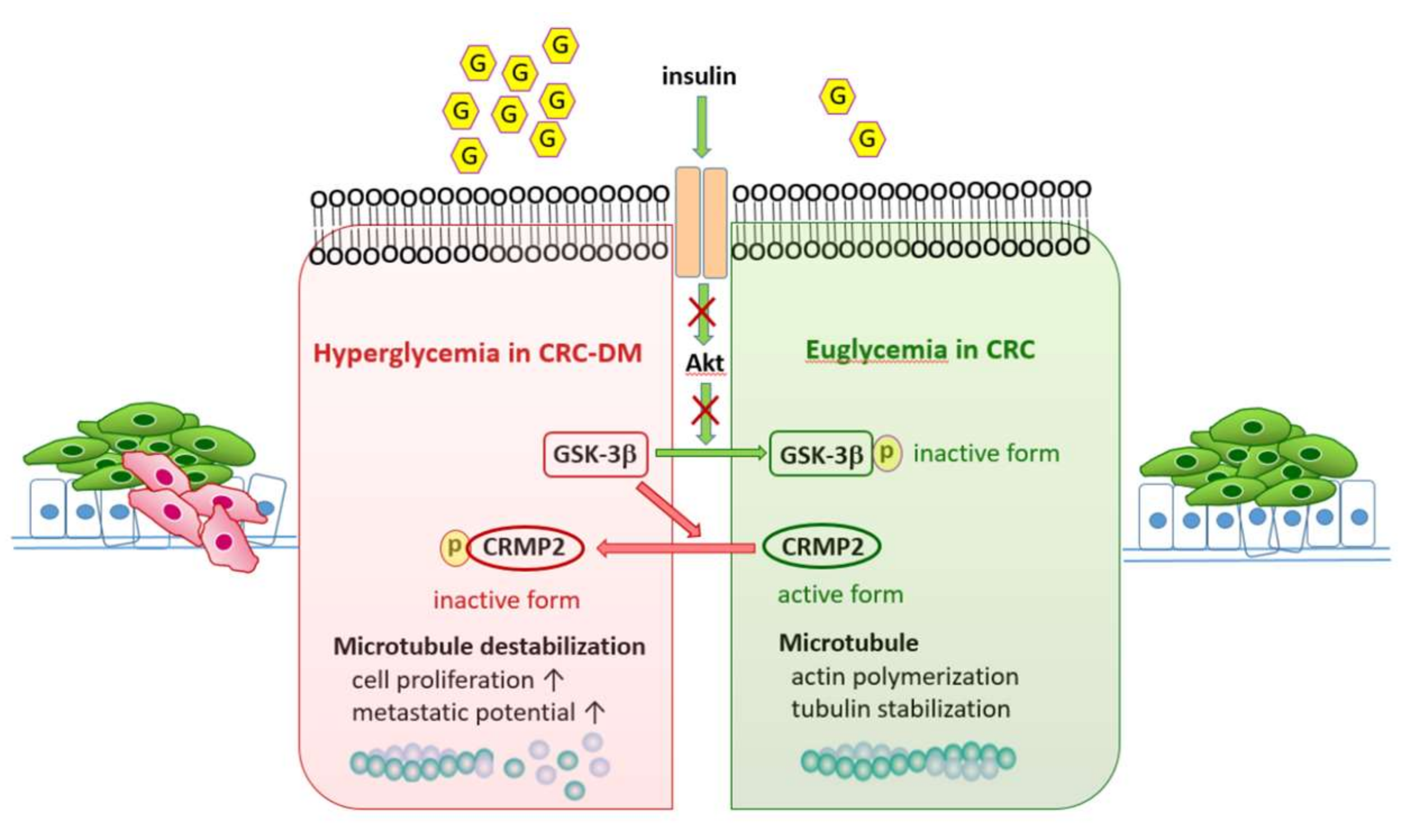
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, S.; Earle, C.C.; Bae, S.J.; Fischer, H.D.; Yun, L.; Austin, P.C.; Rochon, P.A.; Anderson, G.M.; Lipscombe, L. Incidence of diabetes in colorectal cancer survivors. J. Natl. Cancer Inst. 2016, 108, djv402. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.; Jin, C.; Huang, D.; Liu, C.; Sun, D.; Tan, C.; Zhu, X.; Fei, Y. Changes in serum IGF-1 level and tumor VEGF expression in mice with colorectal cancer under hyperglycemic conditions. Mol. Med. Rep. 2013, 7, 1361–1365. [Google Scholar] [CrossRef] [PubMed]
- Kushlinskii, N.E.; Gershtein, E.S.; Nikolaev, A.A.; Delektorskaya, V.V.; Korotkova, E.A.; Dvorova, E.K.; Kostyleva, O.I. Insulin-like growth factors (IGF), IGF-binding proteins (IGFBP), and vascular endothelial growth factor (VEGF) in blood serum of patients with colorectal cancer. Bull. Exp. Biol. Med. 2014, 156, 684–688. [Google Scholar] [CrossRef] [PubMed]
- Han, X.; Hou, S.; Yang, A. Correlation between IGFs-related proteins expression and incidence of colorectal cancer in diabetic patients and related mechanisms. Med. Sci. Monit. 2016, 22, 848–854. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Larsson, S.C.; Orsini, N.; Wolk, A. Diabetes mellitus and risk of colorectal cancer: A meta-analysis. J. Natl. Cancer Inst. 2005, 97, 1679–1687. [Google Scholar] [CrossRef]
- Yin, S.; Bai, H.; Jing, D. Insulin therapy and colorectal cancer risk among type 2 diabetes mellitus patients: A systemic review and meta-analysis. Diagn. Pathol. 2014, 9, 91. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Fei, Q.; Li, J.; Zhang, C.; Sun, Y.; Zhu, C.; Wang, F.; Sun, Y. 2-Deoxyglucose reverses the promoting effect of insulin on colorectal cancer cells in vitro. PLoS ONE 2016, 11, e0151115. [Google Scholar] [CrossRef]
- Flavin, R.; Zadra, G.; Loda, M. Metabolic alterations and targeted therapies in prostate cancer. J. Pathol. 2011, 223, 283–294. [Google Scholar] [CrossRef]
- Biswas, S.; Lunec, J.; Bartlett, K. Non-glucose metabolism in cancer cells—is it all in the fat? Cancer Metastasis Rev. 2012, 31, 689–698. [Google Scholar] [CrossRef]
- Yang, I.P.; Tsai, H.L.; Huang, C.W.; Lu, C.Y.; Miao, Z.F.; Chang, S.F.; Hank Juo, S.F.; Wang, J.Y. High blood sugar levels significantly impact the prognosis of colorectal cancer patients through down-regulation of microRNA-16 by targeting Myb and VEGFR2. Oncotarget 2016, 7, 18837–18850. [Google Scholar] [CrossRef]
- Inagaki, N.; Chihara, K.; Arimura, N.; Menager, C.; Kawano, Y.; Matsuo, N.; Kaibuchi, K. CRMP-2 induces axons in cultured hippocampal neurons. Nat. Neurosci. 2001, 4, 781–782. [Google Scholar] [CrossRef] [PubMed]
- Fukata, Y.; Itoh, T.J.; Kimura, T.; Menager, C.; Nishimura, T.; Shiromizu, T.; Watanabe, H.; Inagaki, N.; Iwamatsu, A.; Hotani, H.; et al. CRMP-2 binds to tubulin heterodimers to promote microtubule assembly. Nat. Cell Biol. 2002, 4, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Arimura, N.; Menager, C.; Fukata, Y.; Kaibuchi, K. Role of CRMP-2 in neuronal polarity. J. Neurobiol. 2004, 58, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Tan, F.; Thiele, C.J.; Li, Z. Collapsin response mediator proteins: Potential diagnostic and prognostic biomarkers in cancers. Oncol. Lett. 2014, 7, 1333–1340. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Chen, H.C.; Chen, S.J.; Liu, H.P.; Hsieh, Y.Y.; Yu, C.J.; Tang, P.; Hsieh, L.L.; Yu, J.S.; Chang, Y.S. Identification of collapsin response mediator protein-2 as a potential marker of colorectal carcinoma by comparative analysis of cancer cell secretomes. Proteomics 2008, 8, 316–332. [Google Scholar] [CrossRef]
- Brown, M.; Jacobs, T.; Eickholt, B.; Ferrari, G.; Teo, M.; Monfries, C.; Qi, R.Z.; Leung, T.; Lim, L.; Hall, C. Alpha2-chimaerin, cyclin-dependent Kinase 5/p35, and its target collapsin response mediator protein-2 are essential components in semaphorin 3A-induced growth-cone collapse. J. Neurosci. 2004, 24, 8994–9004. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, T.; Kawano, Y.; Arimura, N.; Kawabata, S.; Kikuchi, A.; Kaibuchi, K. GSK-3beta regulates phosphorylation of CRMP-2 and neuronal polarity. Cell 2005, 120, 137–149. [Google Scholar] [CrossRef]
- Chang, Y.H.; Tsai, J.N.; Chang, S.W.; Hsu, W.T.; Yang, C.P.; Hsiao, C.W.; Shiau, M.Y. Regulation of adipogenesis and lipid deposits by collapsin response mediator protein 2. Int. J. Mol. Sci. 2020, 21, 2172. [Google Scholar] [CrossRef]
- Frame, S.; Cohen, P.; Biondi, R.M. A common phosphate binding site explains the unique substrate specificity of GSK3 and its inactivation by phosphorylation. Mol. Cell. 2001, 7, 1321–1327. [Google Scholar] [CrossRef]
- Roach, P.J. Glycogen and its metabolism. Curr. Mol. Med. 2002, 2, 101–120. [Google Scholar] [CrossRef]
- Bian, J.; Dannappel, M.; Wan, C.; Firestein, R. Transcriptional regulation of Wnt/β-catenin pathway in colorectal cancer. Cells 2020, 19, 2125. [Google Scholar] [CrossRef] [PubMed]
- Wolff, A.C.; Hammond, M.E.H.; Schwartz, J.N.; Hagerty, K.L.; Allred, D.C.; Cote, R.J.; Dowsett, M.; Fitzgibbons, P.L.; Hanna, W.M.; Langer, A.; et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. Arch. Pathol. Lab. Med. 2007, 131, 18–43. [Google Scholar] [CrossRef] [PubMed]
- Hwang, W.L.; Yang, M.H.; Tsai, M.L.; Lan, H.Y.; Su, S.H.; Chang, S.C.; Teng, H.W.; Yang, S.H.; Lan, Y.T.; Chiou, S.H.; et al. SNAIL regulates interleukin-8 expression, stem cell-like activity, and tumorigenicity of human colorectal carcinoma cells. Gastroenterology 2011, 141, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Song, G.; Lu, Y.; Yu, Z.; Xu, L.; Liu, J.; Chen, K.; Chang, P. The inhibitory effect of polysaccharide from Rhizopus nigricans on colitis-associated colorectal cancer. Biomed. Pharmacother. 2019, 112, 108593. [Google Scholar] [CrossRef]
- Tsao, C.H.; Shiau, M.Y.; Chuang, P.H.; Chang, Y.H.; Hwang, J. Interleukin-4 regulates lipid metabolism by inhibiting adipogenesis and promoting lipolysis. J. Lipid Res. 2014, 55, 385–397. [Google Scholar] [CrossRef]
- Chang, Y.H.; Ho, K.T.; Lu, S.H.; Huang, C.N.; Shiau, M.Y. Regulation of glucose/lipid metabolism and insulin sensitivity by interleukin-4. Int. J. Obes. 2012, 36, 993–998. [Google Scholar] [CrossRef]
- Oliemuller, E.; Pelaez, R.; Garasa, S.; Pajares, M.J.; Agorreta, J.; Pio, R.; Montuenga, L.M.; Teijeira, A.; Llanos, S.; Rouzaut, A. Phosphorylated tubulin adaptor protein crmp-2 as prognostic marker and candidate therapeutic target for nsclc. Int. J. Cancer 2013, 132, 1986–1995. [Google Scholar] [CrossRef]
- Shiau, M.Y.; Lee, P.S.; Huang, Y.J.; Yang, C.P.; Hsiao, C.W.; Chang, K.Y.; Chen, H.W.; Chang, Y.H. Role of PARL-PINK1-Parkin pathway in adipocyte differentiation. Metabolism 2017, 72, 1–17. [Google Scholar] [CrossRef]
- Tahimic, C.G.; Tomimatsu, N.; Nishigaki, R.; Fukuhara, A.; Toda, T.; Kaibuchi, K.; Shiota, G.; Oshimura, M.; Kurimasa, A. Evidence for a role of collapsin response mediator protein-2 in signaling pathways that regulate the proliferation of non-neuronal cells. Biochem. Biophys. Res. Commun. 2006, 340, 1244–1250. [Google Scholar] [CrossRef]
- Buck, K.B.; Zheng, J.Q. Growth cone turning induced by direct local modification of microtubule dynamics. J. Neurosci. 2002, 22, 9358–9367. [Google Scholar] [CrossRef]
- Zhou, F.Q.; Cohan, C.S. How actin filaments and microtubules steer growth cones to their targets. J. Neurobiol. 2004, 58, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Shimada, K.; Ishikawa, T.; Nakamura, F.; Shimizu, D.; Chishima, T.; Ichikawa, Y.; Sasaki, T.; Endo, I.; Nagashima, Y.; Goshima, Y. Collapsin response mediator protein 2 is involved in regulating breast cancer progression. Breast Cancer 2014, 21, 715–723. [Google Scholar] [CrossRef] [PubMed]
- Shiau, M.Y.; Chuang, P.H.; Yang, C.P.; Hsiao, C.W.; Chang, S.W.; Chang, K.Y.; Liu, T.M.; Chen, H.W.; Chuang, C.C.; Yuan, S.Y.; et al. Mechanism of interleukin-4 reducing lipid deposit by regulating hormone-sensitive lipase. Sci. Rep. 2019, 9, 11974. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.P.; Shiau, M.Y.; Lai, Y.R.; Ho, K.T.; Hsiao, C.W.; Chen, C.J.; Lo, Y.L.; Chang, Y.H. Anti-inflammatory cytokine interleukin-4 boosts insulin-induced energy deposits by enhancing glucose uptake and lipogenesis in hepatocytes. Oxid. Med. Cell. Longev. 2018, 2018, 6923187. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.H.; Tsai, J.N.; Chen, T.Z.; Ho, K.T.; Cheng, H.Y.; Hsiao, C.W.; Shiau, M.Y. Interleukin-4 promotes myogenesis and boosts myocyte insulin efficacy. Mediat. Inflamm. 2019, 2019, 4182015. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.Y.; Yang, C.P.; Wang, Y.Y.; Hsiao, C.W.; Chen, W.Y.; Liao, S.L.; Lo, Y.L.; Chang, Y.H.; Hong, C.J.; Chen, C.J. Interleukin-4 improves metabolic abnormalities in leptin-deficient and high-fat diet mice. Int. J. Mol. Sci. 2020, 21, 4451. [Google Scholar] [CrossRef]
- Murakami, K.; Eguchi, J.; Hida, K.; Nakatsuka, A.; Katayama, A.; Sakurai, M.; Choshi, H.; Furutani, M.; Ogawa, D.; Takei, K.; et al. Antiobesity action of ACAM by modulating the dynamics of cell adhesion and actin polymerization in adipocytes. Diabetes 2016, 65, 1255–1267. [Google Scholar] [CrossRef]
- Vassaux, G.; Gaillard, D.; Ailhaud, G.; Ne’grel, R. Prostacyclin is a specific effector of adipose cell differentiation. Its dual role as a cAMP- and Ca2+-elevating agent. J. Biol. Chem. 1992, 267, 11092–11097. [Google Scholar] [CrossRef]
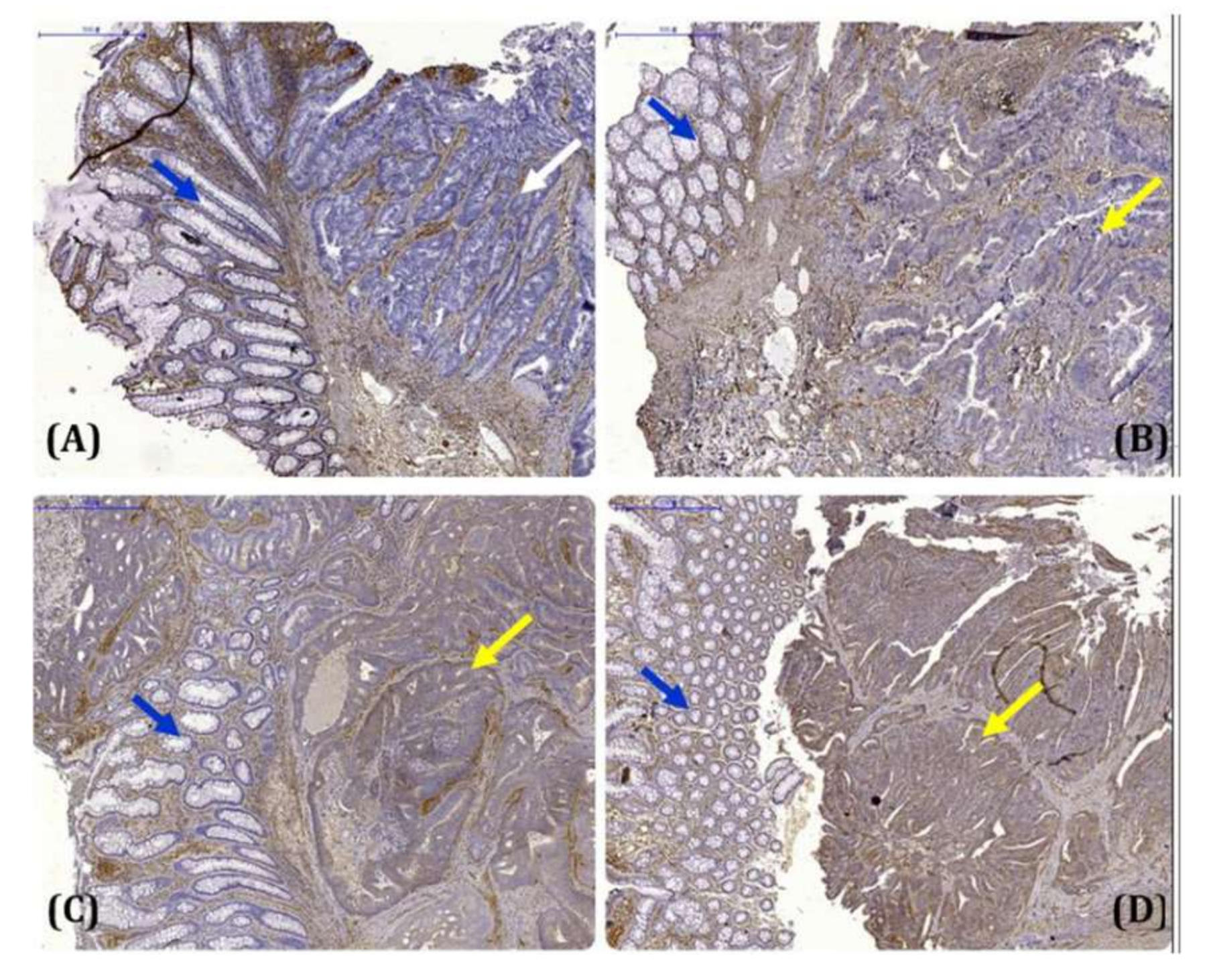

| Subject Characteristics | CRC (61) | CRC-DM (46) | p |
|---|---|---|---|
| Gender | |||
| Male | 41 (67.2) | 29 (63.0) | 0.653 # |
| Female | 20 (32.8) | 17 (37.0) | |
| Hypertension | |||
| Yes | 26 (42.6) | 28 (60.9) | 0.062 # |
| No | 35 (57.4) | 18 (39.1) | |
| Stage | |||
| Ⅰ | 11 (19.0) | 6 (14.6) | 0.487 # |
| Ⅱ | 28 (48.3) | 16 (39.0) | |
| Ⅲ | 16 (27.6) | 14 (34.1) | |
| Ⅳ | 3 (5.2) | 5 (12.2) | |
| CRMP2 expression ‡ | |||
| Low | 17 (27.9) | 36 (78.3) | <0.0001 # |
| High | 44 (72.1) | 10 (21.7) | |
| Age | 68.2 ± 13.6 | 73.5 ± 11.5 | 0.012 ¶ |
| Body mass index | 25.1 ± 3.9 | 25.5 ± 4.7 | 0.607 ¶ |
| Total cholesterol (125–240 mg/dL) † | 170.2 ± 38.8 | 171.4 ± 36.0 | 0.867 ¶ |
| Triglycerides (20–200 mg/dL) † | 91.1 ± 45.8 | 127.3 ± 76.0 | 0.007 ¶ |
| CRMP2 Expression # | CRC/n (%) ‡ | CRC-DM/n (%) ‡ | p * | Odds Ratio (95%CI) |
|---|---|---|---|---|
| 0+ | 4 (6.6%) | 12 (26.1%) | - | - |
| 1+ | 13 (21.3%) | 24 (52.2%) | 0.470 | 0.615 (0.165~2.298) |
| 2+ | 25 (41.0%) | 8 (17.4%) | 0.002 | 0.107 (0.027~0.426) |
| 3+ | 19 (31.1%) | 2 (4.3%) | <0.0001 | 0.035 (0.006~0.222) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.-H.; Yang, H.-J.; Chen, H.-W.; Hsiao, C.-W.; Hsieh, Y.-C.; Chan, Y.-W.; Chang, S.-W.; Hwang, W.-L.; Chen, W.-S.; Cheng, H.-H.; et al. Characterization of Collapsin Response Mediator Protein 2 in Colorectal Cancer Progression in Subjects with Diabetic Comorbidity. Cells 2022, 11, 727. https://doi.org/10.3390/cells11040727
Chang Y-H, Yang H-J, Chen H-W, Hsiao C-W, Hsieh Y-C, Chan Y-W, Chang S-W, Hwang W-L, Chen W-S, Cheng H-H, et al. Characterization of Collapsin Response Mediator Protein 2 in Colorectal Cancer Progression in Subjects with Diabetic Comorbidity. Cells. 2022; 11(4):727. https://doi.org/10.3390/cells11040727
Chicago/Turabian StyleChang, Yih-Hsin, Hui-Ju Yang, Huan-Wen Chen, Chiao-Wan Hsiao, Yi-Chen Hsieh, Yu-Wei Chan, Shu-Wen Chang, Wei-Lun Hwang, Wei-Shone Chen, Hou-Hsuan Cheng, and et al. 2022. "Characterization of Collapsin Response Mediator Protein 2 in Colorectal Cancer Progression in Subjects with Diabetic Comorbidity" Cells 11, no. 4: 727. https://doi.org/10.3390/cells11040727
APA StyleChang, Y.-H., Yang, H.-J., Chen, H.-W., Hsiao, C.-W., Hsieh, Y.-C., Chan, Y.-W., Chang, S.-W., Hwang, W.-L., Chen, W.-S., Cheng, H.-H., Chou, T.-Y., Chang, F.-P., Ho, H.-L., Chu, F.-Y., Lo, Y.-L., Chen, C.-J., Tsai, H.-F., & Shiau, M.-Y. (2022). Characterization of Collapsin Response Mediator Protein 2 in Colorectal Cancer Progression in Subjects with Diabetic Comorbidity. Cells, 11(4), 727. https://doi.org/10.3390/cells11040727







