S1P Signalling Axis Is Necessary for Adiponectin-Directed Regulation of Electrophysiological Properties and Oxidative Metabolism in C2C12 Myotubes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Culture
2.3. Quantitative Real-Time Reverse Transcription PCR
2.4. Western Blot Analysis
2.5. Liquid Chromatography Tandem-Mass Spectrometry
2.6. Electrophysiology
2.7. Oxygen Consumption Assay
2.8. NMR-Based Metabolomic Analyses
2.9. Statistical Analysis
3. Results
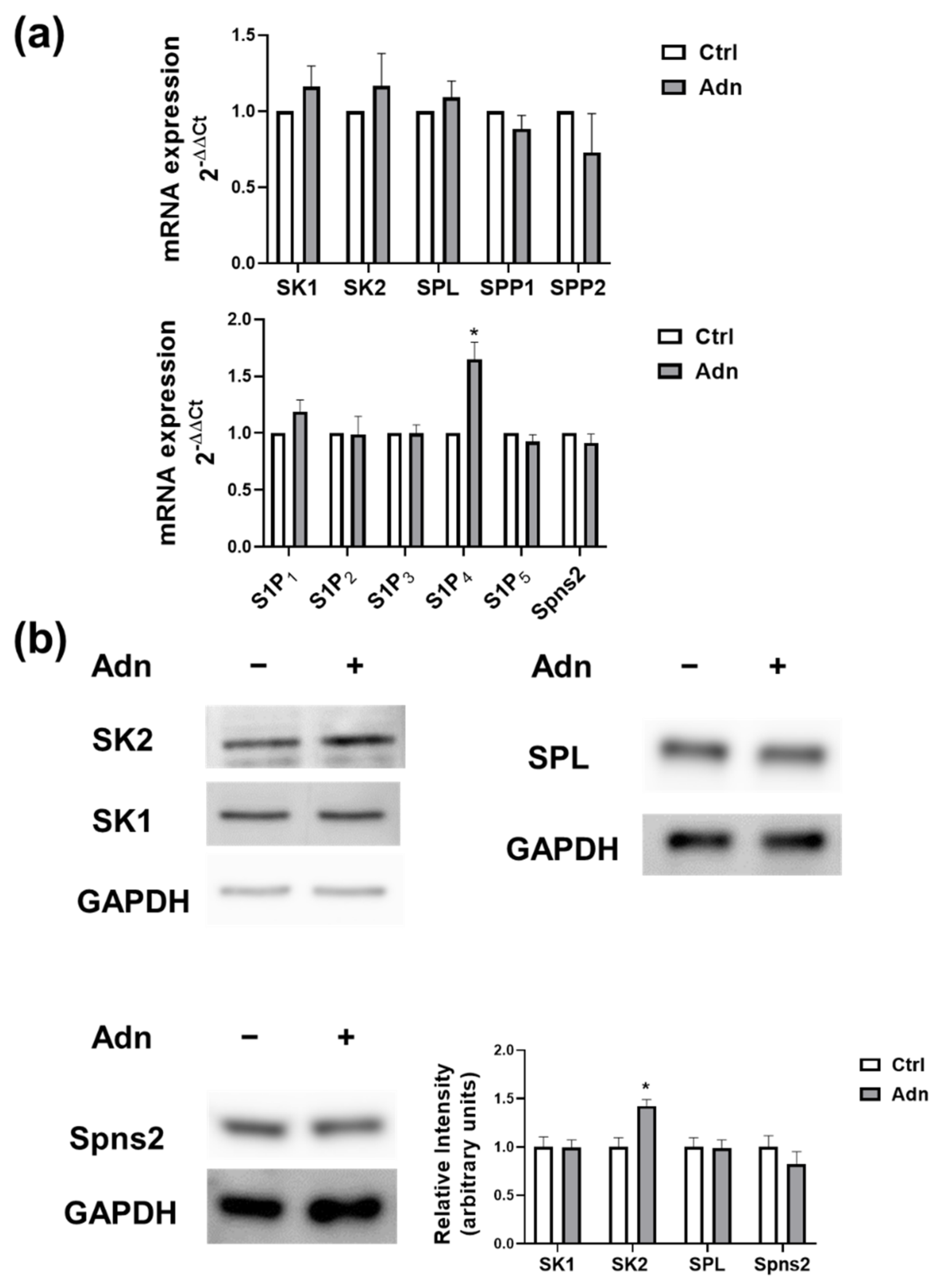
3.1. Adiponectin Modulates S1P Signalling in Myotubes
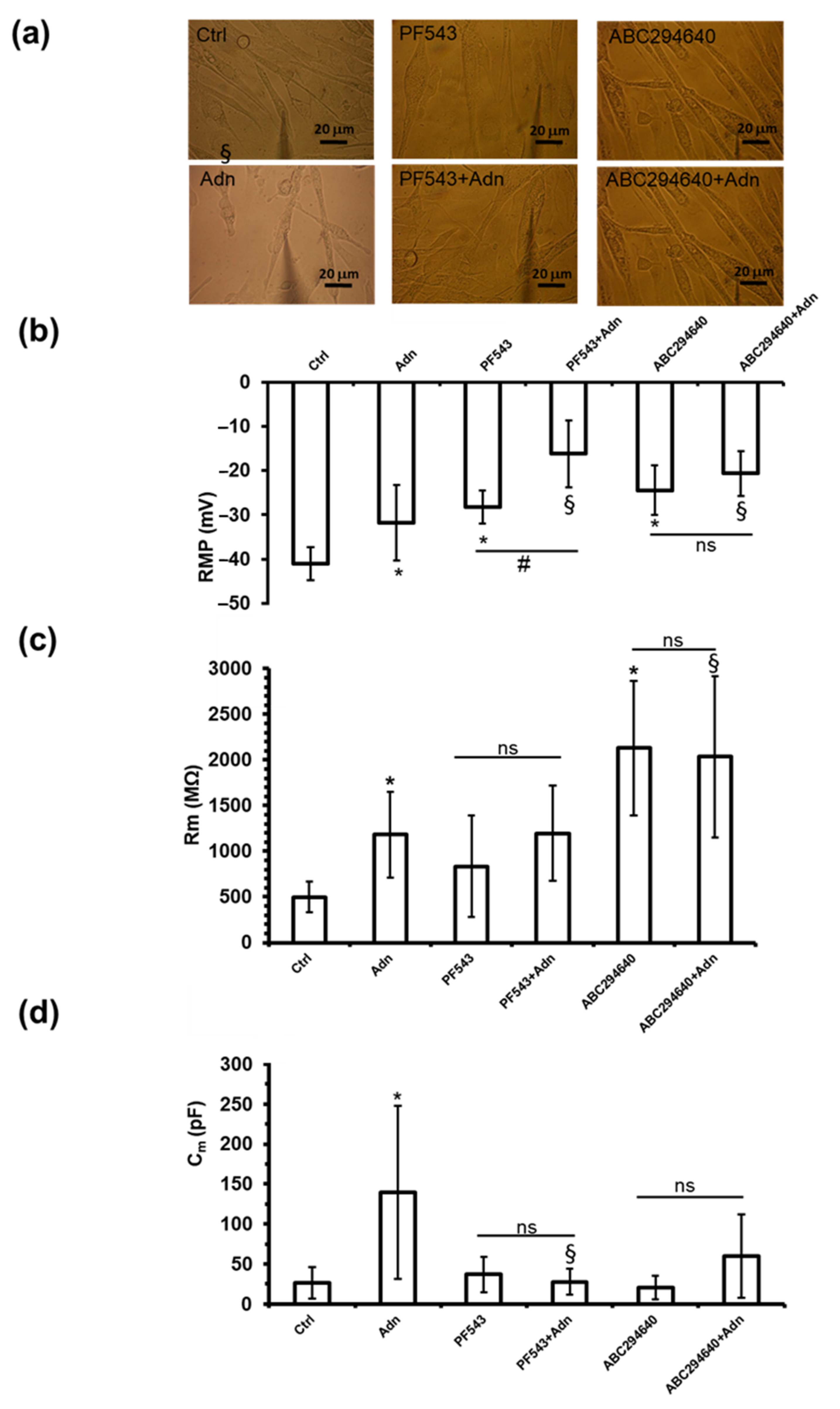
3.2. Role of S1P Biosynthesis in the Effect of Adn on Myotube Electrophysiological Properties
3.3. Role of S1P Biosynthesis in the Effect of Adn on Mitochondrial Function in Myotubes
3.4. Role of S1P Biosynthesis in the Metabolome of Adn-Treated C2C12 Myotubes
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bonaldo, P.; Sandri, M. Cellular and Molecular Mechanisms of Muscle Atrophy. Dis. Model. Mech. 2013, 6, 25–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phielix, E.; Mensink, M. Type 2 Diabetes Mellitus and Skeletal Muscle Metabolic Function. Physiol. Behav. 2008, 94, 252–258. [Google Scholar] [CrossRef] [PubMed]
- Erekat, N.S. Apoptotic Mediators Are Upregulated in the Skeletal Muscle of Chronic/Progressive Mouse Model of Parkinson’s Disease. Anat. Rec. 2015, 298, 1472–1478. [Google Scholar] [CrossRef]
- Calise, S.; Blescia, S.; Cencetti, F.; Bernacchioni, C.; Donati, C.; Bruni, P. Sphingosine 1-Phosphate Stimulates Proliferation and Migration of Satellite Cells: Role of S1P Receptors. Biochim. Biophys. Acta BBA Mol. Cell Res. 2012, 1823, 439–450. [Google Scholar] [CrossRef] [Green Version]
- Hotta, K.; Funahashi, T.; Arita, Y.; Takahashi, M.; Matsuda, M.; Okamoto, Y.; Iwahashi, H.; Kuriyama, H.; Ouchi, N.; Maeda, K.; et al. Plasma Concentrations of a Novel, Adipose-Specific Protein, Adiponectin, in Type 2 Diabetic Patients. Arter. Thromb. Vasc. Biol. 2000, 20, 1595–1599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scherer, P.E.; Williams, S.; Fogliano, M.; Baldini, G.; Lodish, H.F. A Novel Serum Protein Similar to C1q, Produced Exclusively in Adipocytes. J. Biol. Chem. 1995, 270, 26746–26749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiaschi, T.; Magherini, F.; Gamberi, T.; Modesti, P.A.; Modesti, A. Adiponectin as a Tissue Regenerating Hormone: More than a Metabolic Function. Cell. Mol. Life Sci. 2014, 71, 1917–1925. [Google Scholar] [CrossRef] [PubMed]
- Waki, H.; Yamauchi, T.; Kamon, J.; Kita, S.; Ito, Y.; Hada, Y.; Uchida, S.; Tsuchida, A.; Takekawa, S.; Kadowaki, T. Generation of Globular Fragment of Adiponectin by Leukocyte Elastase Secreted by Monocytic Cell Line THP-1. Endocrinology 2005, 146, 790–796. [Google Scholar] [CrossRef]
- Yamauchi, T.; Kamon, J.; Ito, Y.; Tsuchida, A.; Yokomizo, T.; Kita, S.; Sugiyama, T.; Miyagishi, M.; Hara, K.; Tsunoda, M.; et al. Cloning of Adiponectin Receptors That Mediate Antidiabetic Metabolic Effects. Nature 2003, 423, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Tishinsky, J.M.; Robinson, L.E.; Dyck, D.J. Insulin-Sensitizing Properties of Adiponectin. Biochimie 2012, 94, 2131–2136. [Google Scholar] [CrossRef]
- Kadowaki, T.; Yamauchi, T.; Kubota, N.; Hara, K.; Ueki, K.; Tobe, K. Adiponectin and Adiponectin Receptors in Insulin Resistance, Diabetes, and the Metabolic Syndrome. J. Clin. Investig. 2006, 116, 1784–1792. [Google Scholar] [CrossRef] [Green Version]
- Yamauchi, T.; Kamon, J.; Waki, H.; Terauchi, Y.; Kubota, N.; Hara, K.; Mori, Y.; Ide, T.; Murakami, K.; Tsuboyama-Kasaoka, N.; et al. The Fat-Derived Hormone Adiponectin Reverses Insulin Resistance Associated with Both Lipoatrophy and Obesity. Nat. Med. 2001, 7, 941–946. [Google Scholar] [CrossRef]
- Fiaschi, T.; Cirelli, D.; Comito, G.; Gelmini, S.; Ramponi, G.; Serio, M.; Chiarugi, P. Globular Adiponectin Induces Differentiation and Fusion of Skeletal Muscle Cells. Cell Res. 2009, 19, 584–597. [Google Scholar] [CrossRef]
- Idrizaj, E.; Garella, R.; Nistri, S.; Dell’Accio, A.; Cassioli, E.; Rossi, E.; Castellini, G.; Ricca, V.; Squecco, R.; Baccari, M.C. Adiponectin Exerts Peripheral Inhibitory Effects on the Mouse Gastric Smooth Muscle through the AMPK Pathway. Int. J. Mol. Sci. 2020, 21, 9617. [Google Scholar] [CrossRef]
- Holland, W.L.; Miller, R.A.; Wang, Z.V.; Sun, K.; Barth, B.M.; Bui, H.H.; Davis, K.E.; Bikman, B.T.; Halberg, N.; Rutkowski, J.M.; et al. Receptor-Mediated Activation of Ceramidase Activity Initiates the Pleiotropic Actions of Adiponectin. Nat. Med. 2011, 17, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Maceyka, M.; Harikumar, K.B.; Milstien, S.; Spiegel, S. Sphingosine-1-Phosphate Signaling and Its Role in Disease. Trends Cell Biol. 2012, 22, 50–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, D.R.; Pyne, S.; Pyne, N.J. Structure-Function Analysis of Lipid Substrates and Inhibitors of Sphingosine Kinases. Cell. Signal. 2020, 76, 109806. [Google Scholar] [CrossRef]
- Blaho, V.A.; Hla, T. An Update on the Biology of Sphingosine 1-Phosphate Receptors. J. Lipid. Res. 2014, 55, 1596–1608. [Google Scholar] [CrossRef] [Green Version]
- Kawahara, A.; Nishi, T.; Hisano, Y.; Fukui, H.; Yamaguchi, A.; Mochizuki, N. The Sphingolipid Transporter Spns2 Functions in Migration of Zebrafish Myocardial Precursors. Science 2009, 323, 524–527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vu, T.M.; Ishizu, A.-N.; Foo, J.C.; Toh, X.R.; Zhang, F.; Whee, D.M.; Torta, F.; Cazenave-Gassiot, A.; Matsumura, T.; Kim, S.; et al. Mfsd2b Is Essential for the Sphingosine-1-Phosphate Export in Erythrocytes and Platelets. Nature 2017, 550, 524–528. [Google Scholar] [CrossRef] [PubMed]
- Sato, K.; Malchinkhuu, E.; Horiuchi, Y.; Mogi, C.; Tomura, H.; Tosaka, M.; Yoshimoto, Y.; Kuwabara, A.; Okajima, F. Critical Role of ABCA1 Transporter in Sphingosine 1-Phosphate Release from Astrocytes. J. Neurochem. 2007, 103, 2610–2619. [Google Scholar] [CrossRef]
- Kase, H.; Hattori, Y.; Jojima, T.; Okayasu, T.; Tomizawa, A.; Suzuki, K.; Banba, N.; Monden, T.; Satoh, H.; Akimoto, K.; et al. Globular Adiponectin Induces Adhesion Molecule Expression through the Sphingosine Kinase Pathway in Vascular Endothelial Cells. Life Sci. 2007, 81, 939–943. [Google Scholar] [CrossRef]
- Botta, A.; Elizbaryan, K.; Tashakorinia, P.; Lam, N.H.; Sweeney, G. An Adiponectin-S1P Autocrine Axis Protects Skeletal Muscle Cells from Palmitate-Induced Cell Death. Lipids Health Dis. 2020, 19, 156. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, A.V.; Silva, V.R.R.; Pauli, J.R.; da Silva, A.S.R.; Cintra, D.E.; Moura, L.P.; Ropelle, E.R. The Role of Sphingosine-1-Phosphate in Skeletal Muscle: Physiology, Mechanisms, and Clinical Perspectives. J. Cell Physiol. 2019, 234, 10047–10059. [Google Scholar] [CrossRef] [PubMed]
- Donati, C.; Cencetti, F.; Bruni, P. Sphingosine 1-Phosphate Axis: A New Leader Actor in Skeletal Muscle Biology. Front. Physiol. 2013, 4, 338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donati, C.; Cencetti, F.; Bruni, P. New Insights into the Role of Sphingosine 1-Phosphate and Lysophosphatidic Acid in the Regulation of Skeletal Muscle Cell Biology. Biochim. Biophys. Acta 2013, 1831, 176–184. [Google Scholar] [CrossRef]
- Nagata, Y.; Partridge, T.A.; Matsuda, R.; Zammit, P.S. Entry of Muscle Satellite Cells into the Cell Cycle Requires Sphingolipid Signaling. J. Cell Biol. 2006, 174, 245–253. [Google Scholar] [CrossRef] [Green Version]
- Donati, C.; Meacci, E.; Nuti, F.; Becciolini, L.; Farnararo, M.; Bruni, P. Sphingosine 1-Phosphate Regulates Myogenic Differentiation: A Major Role for S1P2 Receptor. FASEB J. 2005, 19, 449–451. [Google Scholar] [CrossRef]
- Danieli-Betto, D.; Germinario, E.; Esposito, A.; Megighian, A.; Midrio, M.; Ravara, B.; Damiani, E.; Libera, L.D.; Sabbadini, R.A.; Betto, R. Sphingosine 1-Phosphate Protects Mouse Extensor Digitorum Longus Skeletal Muscle during Fatigue. Am. J. Physiol. Cell Physiol. 2005, 288, C1367–C1373. [Google Scholar] [CrossRef] [Green Version]
- Rapizzi, E.; Taddei, M.L.; Fiaschi, T.; Donati, C.; Bruni, P.; Chiarugi, P. Sphingosine 1-Phosphate Increases Glucose Uptake through Trans-Activation of Insulin Receptor. Cell. Mol. Life Sci. 2009, 66, 3207–3218. [Google Scholar] [CrossRef]
- Formigli, L.; Francini, F.; Meacci, E.; Vassalli, M.; Nosi, D.; Quercioli, F.; Tiribilli, B.; Bencini, C.; Piperio, C.; Bruni, P.; et al. Sphingosine 1-Phosphate Induces Ca2+ Transients and Cytoskeletal Rearrangement in C2C12 Myoblastic Cells. Am. J. Physiol. Cell Physiol. 2002, 282, C1361–C1373. [Google Scholar] [CrossRef] [Green Version]
- Bernacchioni, C.; Cencetti, F.; Ouro, A.; Bruno, M.; Gomez-Muñoz, A.; Donati, C.; Bruni, P. Lysophosphatidic Acid Signaling Axis Mediates Ceramide 1-Phosphate-Induced Proliferation of C2C12 Myoblasts. Int. J. Mol. Sci. 2018, 19, 139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruno, G.; Cencetti, F.; Bernacchioni, C.; Donati, C.; Blankenbach, K.V.; Thomas, D.; Meyer Zu Heringdorf, D.; Bruni, P. Bradykinin Mediates Myogenic Differentiation in Murine Myoblasts through the Involvement of SK1/Spns2/S1P2 Axis. Cell. Signal. 2018, 45, 110–121. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Gulbins, A.; Schumacher, F.; Becker, K.A.; Wilker, B.; Soddemann, M.; Boldrin, F.; Müller, C.P.; Edwards, M.J.; Goodman, M.; Caldwell, C.C.; et al. Antidepressants Act by Inducing Autophagy Controlled by Sphingomyelin-Ceramide. Mol. Psychiatry 2018, 23, 2324–2346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naser, E.; Kadow, S.; Schumacher, F.; Mohamed, Z.H.; Kappe, C.; Hessler, G.; Pollmeier, B.; Kleuser, B.; Arenz, C.; Becker, K.A.; et al. Characterization of the Small Molecule ARC39, a Direct and Specific Inhibitor of Acid Sphingomyelinase in Vitro. J. Lipid. Res. 2020, 61, 896–910. [Google Scholar] [CrossRef] [Green Version]
- Bernacchioni, C.; Ghini, V.; Squecco, R.; Idrizaj, E.; Garella, R.; Puliti, E.; Cencetti, F.; Bruni, P.; Donati, C. Role of Sphingosine 1-Phosphate Signalling Axis in Muscle Atrophy Induced by TNFα in C2C12 Myotubes. Int. J. Mol. Sci. 2021, 22, 1280. [Google Scholar] [CrossRef]
- Cencetti, F.; Bernacchioni, C.; Bruno, M.; Squecco, R.; Idrizaj, E.; Berbeglia, M.; Bruni, P.; Donati, C. Sphingosine 1-Phosphate-Mediated Activation of Ezrin-Radixin-Moesin Proteins Contributes to Cytoskeletal Remodeling and Changes of Membrane Properties in Epithelial Otic Vesicle Progenitors. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 554–565. [Google Scholar] [CrossRef]
- Squecco, R.; Carraro, U.; Kern, H.; Pond, A.; Adami, N.; Biral, D.; Vindigni, V.; Boncompagni, S.; Pietrangelo, T.; Bosco, G.; et al. A Subpopulation of Rat Muscle Fibers Maintains an Assessable Excitation-Contraction Coupling Mechanism after Long-Standing Denervation despite Lost Contractility. J. Neuropathol. Exp. Neurol. 2009, 68, 1256–1268. [Google Scholar] [CrossRef] [Green Version]
- Collins, C.A.; Rojas, E.; Suarez-Isla, B.A. Fast Charge Movements in Skeletal Muscle Fibres from Rana Temporaria. J. Physiol. 1982, 324, 319–345. [Google Scholar] [CrossRef] [Green Version]
- Squecco, R.; Chellini, F.; Idrizaj, E.; Tani, A.; Garella, R.; Pancani, S.; Pavan, P.; Bambi, F.; Zecchi-Orlandini, S.; Sassoli, C. Platelet-Rich Plasma Modulates Gap Junction Functionality and Connexin 43 and 26 Expression During TGF-Β1-Induced Fibroblast to Myofibroblast Transition: Clues for Counteracting Fibrosis. Cells 2020, 9, 1199. [Google Scholar] [CrossRef] [PubMed]
- Squecco, R.; Idrizaj, E.; Morelli, A.; Gallina, P.; Vannelli, G.B.; Francini, F. An Electrophysiological Study on the Effects of BDNF and FGF2 on Voltage Dependent Ca(2+) Currents in Developing Human Striatal Primordium. Mol. Cell. Neurosci. 2016, 75, 50–62. [Google Scholar] [CrossRef] [PubMed]
- Mannelli, M.; Gamberi, T.; Magherini, F.; Fiaschi, T. A Metabolic Change towards Fermentation Drives Cancer Cachexia in Myotubes. Biomedicines 2021, 9, 698. [Google Scholar] [CrossRef] [PubMed]
- Takis, P.G.; Ghini, V.; Tenori, L.; Turano, P.; Luchinat, C. Uniqueness of the NMR Approach to Metabolomics. TrAC Trends Anal. Chem. 2019, 120, 115300. [Google Scholar] [CrossRef]
- Bernacchioni, C.; Ghini, V.; Cencetti, F.; Japtok, L.; Donati, C.; Bruni, P.; Turano, P. NMR Metabolomics Highlights Sphingosine Kinase-1 as a New Molecular Switch in the Orchestration of Aberrant Metabolic Phenotype in Cancer Cells. Mol. Oncol. 2017, 11, 517–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Alessandro, G.; Quaglio, D.; Monaco, L.; Lauro, C.; Ghirga, F.; Ingallina, C.; de Martino, M.; Fucile, S.; Porzia, A.; Di Castro, M.A.; et al. 1H-NMR Metabolomics Reveals the Glabrescione B Exacerbation of Glycolytic Metabolism beside the Cell Growth Inhibitory Effect in Glioma. Cell Commun. Signal. 2019, 17, 108. [Google Scholar] [CrossRef] [Green Version]
- Ghini, V.; Senzacqua, T.; Massai, L.; Gamberi, T.; Messori, L.; Turano, P. NMR Reveals the Metabolic Changes Induced by Auranofin in A2780 Cancer Cells: Evidence for Glutathione Dysregulation. Dalton Trans. 2021, 50, 6349–6355. [Google Scholar] [CrossRef] [PubMed]
- Vignoli, A.; Ghini, V.; Meoni, G.; Licari, C.; Takis, P.G.; Tenori, L.; Turano, P.; Luchinat, C. High-Throughput Metabolomics by 1D NMR. Angew. Chem. Int. Ed. Engl. 2019, 58, 968–994. [Google Scholar] [CrossRef] [PubMed]
- Pitson, S.M.; Xia, P.; Leclercq, T.M.; Moretti, P.A.B.; Zebol, J.R.; Lynn, H.E.; Wattenberg, B.W.; Vadas, M.A. Phosphorylation-Dependent Translocation of Sphingosine Kinase to the Plasma Membrane Drives Its Oncogenic Signalling. J. Exp. Med. 2004, 201, 49–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hait, N.C.; Bellamy, A.; Milstien, S.; Kordula, T.; Spiegel, S. Sphingosine Kinase Type 2 Activation by ERK-Mediated Phosphorylation. J. Biol. Chem. 2007, 282, 12058–12065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baskin, K.K.; Winders, B.R.; Olson, E.N. Muscle as a “Mediator” of Systemic Metabolism. Cell Metab. 2015, 21, 237–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernacchioni, C.; Cencetti, F.; Blescia, S.; Donati, C.; Bruni, P. Sphingosine Kinase/Sphingosine 1-Phosphate Axis: A New Player for Insulin-like Growth Factor-1-Induced Myoblast Differentiation. Skelet. Muscle 2012, 2, 15. [Google Scholar] [CrossRef] [Green Version]
- Nincheri, P.; Bernacchioni, C.; Cencetti, F.; Donati, C.; Bruni, P. Sphingosine Kinase-1/S1P1 Signalling Axis Negatively Regulates Mitogenic Response Elicited by PDGF in Mouse Myoblasts. Cell. Signal. 2010, 22, 1688–1699. [Google Scholar] [CrossRef] [PubMed]
- Donati, C.; Nincheri, P.; Cencetti, F.; Rapizzi, E.; Farnararo, M.; Bruni, P. Tumor Necrosis Factor-Alpha Exerts pro-Myogenic Action in C2C12 Myoblasts via Sphingosine Kinase/S1P2 Signaling. FEBS Lett. 2007, 581, 4384–4388. [Google Scholar] [CrossRef] [Green Version]
- Cencetti, F.; Bruno, G.; Bernacchioni, C.; Japtok, L.; Puliti, E.; Donati, C.; Bruni, P. Sphingosine 1-Phosphate Lyase Blockade Elicits Myogenic Differentiation of Murine Myoblasts Acting via Spns2/S1P2 Receptor Axis. Biochim. Biophys. Acta Mol. Cell. Biol. Lipids 2020, 1865, 158759. [Google Scholar] [CrossRef]
- Becciolini, L.; Meacci, E.; Donati, C.; Cencetti, F.; Rapizzi, E.; Bruni, P. Sphingosine 1-Phosphate Inhibits Cell Migration in C2C12 Myoblasts. Biochim. Biophys. Acta 2006, 1761, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Cencetti, F.; Bernacchioni, C.; Tonelli, F.; Roberts, E.; Donati, C.; Bruni, P. TGFβ1 Evokes Myoblast Apoptotic Response via a Novel Signaling Pathway Involving S1P4 Transactivation Upstream of Rho-Kinase-2 Activation. FASEB J. 2013, 27, 4532–4546. [Google Scholar] [CrossRef]
- Cencetti, F.; Bernacchioni, C.; Nincheri, P.; Donati, C.; Bruni, P. Transforming Growth Factor-Beta1 Induces Transdifferentiation of Myoblasts into Myofibroblasts via up-Regulation of Sphingosine Kinase-1/S1P3 Axis. Mol. Biol. Cell 2010, 21, 1111–1124. [Google Scholar] [CrossRef] [Green Version]
- Botta, A.; Liu, Y.; Wannaiampikul, S.; Tungtrongchitr, R.; Dadson, K.; Park, T.-S.; Sweeney, G. An Adiponectin-S1P Axis Protects against Lipid Induced Insulin Resistance and Cardiomyocyte Cell Death via Reduction of Oxidative Stress. Nutr. Metab. 2019, 16, 14. [Google Scholar] [CrossRef]
- Ikeda, Y.; Ohashi, K.; Shibata, R.; Pimentel, D.R.; Kihara, S.; Ouchi, N.; Walsh, K. Cyclooxygenase-2 Induction by Adiponectin Is Regulated by a Sphingosine Kinase-1 Dependent Mechanism in Cardiac Myocytes. FEBS Lett. 2008, 582, 1147–1150. [Google Scholar] [CrossRef] [Green Version]
- Cuvillier, O.; Pirianov, G.; Kleuser, B.; Vanek, P.G.; Coso, O.A.; Gutkind, S.; Spiegel, S. Suppression of Ceramide-Mediated Programmed Cell Death by Sphingosine-1-Phosphate. Nature 1996, 381, 800–803. [Google Scholar] [CrossRef] [PubMed]
- Hait, N.C.; Oskeritzian, C.A.; Paugh, S.W.; Milstien, S.; Spiegel, S. Sphingosine Kinases, Sphingosine 1-Phosphate, Apoptosis and Diseases. Biochim. Biophys. Acta 2006, 1758, 2016–2026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasiliauskaité-Brooks, I.; Sounier, R.; Rochaix, P.; Bellot, G.; Fortier, M.; Hoh, F.; de Colibus, L.; Bechara, C.; Saied, E.M.; Arenz, C.; et al. Structural Insights into Adiponectin Receptors Suggest Ceramidase Activity. Nature 2017, 544, 120–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alemany, R.; van Koppen, C.J.; Danneberg, K.; ter Braak, M.; Meyer zu Heringdorf, D. Regulation and Functional Roles of Sphingosine Kinases. Naunyn-Schmied Arch. Pharmacol. 2007, 374, 413–428. [Google Scholar] [CrossRef]
- Laurenzana, A.; Cencetti, F.; Serratì, S.; Bruno, G.; Japtok, L.; Bianchini, F.; Torre, E.; Fibbi, G.; Del Rosso, M.; Bruni, P.; et al. Endothelial Sphingosine Kinase/SPNS2 Axis Is Critical for Vessel-like Formation by Human Mesoangioblasts. J. Mol. Med. 2015, 93, 1145–1157. [Google Scholar] [CrossRef] [PubMed]
- Mizugishi, K.; Yamashita, T.; Olivera, A.; Miller, G.F.; Spiegel, S.; Proia, R.L. Essential Role for Sphingosine Kinases in Neural and Vascular Development. Mol. Cell. Biol. 2005, 25, 11113–11121. [Google Scholar] [CrossRef] [Green Version]
- Adiponectin Ameliorates Doxorubicin-Induced Cardiotoxicity through Akt Protein-Dependent Mechanism—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/21784858/ (accessed on 11 February 2022).
- Sassoli, C.; Formigli, L.; Bini, F.; Tani, A.; Squecco, R.; Battistini, C.; Zecchi-Orlandini, S.; Francini, F.; Meacci, E. Effects of S1P on Skeletal Muscle Repair/Regeneration during Eccentric Contraction. J. Cell. Mol. Med. 2011, 15, 2498–2511. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, Y.; Kita, S.; Nishizawa, H.; Fukuda, S.; Fujishima, Y.; Obata, Y.; Nagao, H.; Masuda, S.; Nakamura, Y.; Shimizu, Y.; et al. Adiponectin Promotes Muscle Regeneration through Binding to T-Cadherin. Sci. Rep. 2019, 9, 16. [Google Scholar] [CrossRef] [Green Version]
- Christé, G.; Bonvallet, R.; Chouabe, C. Accounting for Cardiac T-Tubule Increase with Age and Myocyte Volume to Improve Measurements of Its Membrane Area and Ionic Current Densities. Prog. Biophys. Mol. Biol. 2020, 157, 40–53. [Google Scholar] [CrossRef]
- Yamauchi, T.; Kamon, J.; Minokoshi, Y.; Ito, Y.; Waki, H.; Uchida, S.; Yamashita, S.; Noda, M.; Kita, S.; Ueki, K.; et al. Adiponectin Stimulates Glucose Utilization and Fatty-Acid Oxidation by Activating AMP-Activated Protein Kinase. Nat. Med. 2002, 8, 1288–1295. [Google Scholar] [CrossRef]
- Iwabu, M.; Yamauchi, T.; Okada-Iwabu, M.; Sato, K.; Nakagawa, T.; Funata, M.; Yamaguchi, M.; Namiki, S.; Nakayama, R.; Tabata, M.; et al. Adiponectin and AdipoR1 Regulate PGC-1alpha and Mitochondria by Ca(2+) and AMPK/SIRT1. Nature 2010, 464, 1313–1319. [Google Scholar] [CrossRef] [PubMed]
- Inoki, K.; Kim, J.; Guan, K.-L. AMPK and MTOR in Cellular Energy Homeostasis and Drug Targets. Annu. Rev. Pharmacol. Toxicol. 2012, 52, 381–400. [Google Scholar] [CrossRef] [PubMed]
- Fiaschi, T.; Tedesco, F.S.; Giannoni, E.; Diaz-Manera, J.; Parri, M.; Cossu, G.; Chiarugi, P. Globular Adiponectin as a Complete Mesoangioblast Regulator: Role in Proliferation, Survival, Motility, and Skeletal Muscle Differentiation. Mol. Biol. Cell 2010, 21, 848–859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goto, A.; Ohno, Y.; Ikuta, A.; Suzuki, M.; Ohira, T.; Egawa, T.; Sugiura, T.; Yoshioka, T.; Ohira, Y.; Goto, K. Up-Regulation of Adiponectin Expression in Antigravitational Soleus Muscle in Response to Unloading Followed by Reloading, and Functional Overloading in Mice. PLoS ONE 2013, 8, e81929. [Google Scholar] [CrossRef] [Green Version]
- Inoue, A.; Cheng, X.W.; Huang, Z.; Hu, L.; Kikuchi, R.; Jiang, H.; Piao, L.; Sasaki, T.; Itakura, K.; Wu, H.; et al. Exercise Restores Muscle Stem Cell Mobilization, Regenerative Capacity and Muscle Metabolic Alterations via Adiponectin/AdipoR1 Activation in SAMP10 Mice. J. Cachexia Sarcopenia Muscle 2017, 8, 370–385. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.K.; Shree, S.; Chattopadhyay, S.; Kumar, S.; Gurjar, A.; Kushwaha, S.; Kumar, H.; Trivedi, A.K.; Chattopadhyay, N.; Maurya, R.; et al. Small Molecule Adiponectin Receptor Agonist GTDF Protects against Skeletal Muscle Atrophy. Mol. Cell. Endocrinol. 2017, 439, 273–285. [Google Scholar] [CrossRef]
- Ito, R.; Higa, M.; Goto, A.; Aoshima, M.; Ikuta, A.; Ohashi, K.; Yokoyama, S.; Ohno, Y.; Egawa, T.; Miyata, H.; et al. Activation of Adiponectin Receptors Has Negative Impact on Muscle Mass in C2C12 Myotubes and Fast-Type Mouse Skeletal Muscle. PLoS ONE 2018, 13, e0205645. [Google Scholar] [CrossRef] [Green Version]
- Harada, H.; Kai, H.; Shibata, R.; Niiyama, H.; Nishiyama, Y.; Murohara, T.; Yoshida, N.; Katoh, A.; Ikeda, H. New Diagnostic Index for Sarcopenia in Patients with Cardiovascular Diseases. PLoS ONE 2017, 12, e0178123. [Google Scholar] [CrossRef] [Green Version]
- Malik, F.A.; Meissner, A.; Semenkov, I.; Molinski, S.; Pasyk, S.; Ahmadi, S.; Bui, H.H.; Bear, C.E.; Lidington, D.; Bolz, S.-S. Sphingosine-1-Phosphate Is a Novel Regulator of Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Activity. PLoS ONE 2015, 10, e0130313. [Google Scholar] [CrossRef] [Green Version]
- Rapizzi, E.; Donati, C.; Cencetti, F.; Pinton, P.; Rizzuto, R.; Bruni, P. Sphingosine 1-Phosphate Receptors Modulate Intracellular Ca2+ Homeostasis. Biochem. Biophys. Res. Commun. 2007, 353, 268–274. [Google Scholar] [CrossRef]
- Yang, S.J.; Choi, J.M.; Kim, L.; Park, S.E.; Rhee, E.J.; Lee, W.Y.; Oh, K.W.; Park, S.W.; Park, C.-Y. Nicotinamide Improves Glucose Metabolism and Affects the Hepatic NAD-Sirtuin Pathway in a Rodent Model of Obesity and Type 2 Diabetes. J. Nutr. Biochem. 2014, 25, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Goody, M.F.; Henry, C.A. A Need for NAD+ in Muscle Development, Homeostasis, and Aging. Skelet. Muscle 2018, 8, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernacchioni, C.; Squecco, R.; Gamberi, T.; Ghini, V.; Schumacher, F.; Mannelli, M.; Garella, R.; Idrizaj, E.; Cencetti, F.; Puliti, E.; et al. S1P Signalling Axis Is Necessary for Adiponectin-Directed Regulation of Electrophysiological Properties and Oxidative Metabolism in C2C12 Myotubes. Cells 2022, 11, 713. https://doi.org/10.3390/cells11040713
Bernacchioni C, Squecco R, Gamberi T, Ghini V, Schumacher F, Mannelli M, Garella R, Idrizaj E, Cencetti F, Puliti E, et al. S1P Signalling Axis Is Necessary for Adiponectin-Directed Regulation of Electrophysiological Properties and Oxidative Metabolism in C2C12 Myotubes. Cells. 2022; 11(4):713. https://doi.org/10.3390/cells11040713
Chicago/Turabian StyleBernacchioni, Caterina, Roberta Squecco, Tania Gamberi, Veronica Ghini, Fabian Schumacher, Michele Mannelli, Rachele Garella, Eglantina Idrizaj, Francesca Cencetti, Elisa Puliti, and et al. 2022. "S1P Signalling Axis Is Necessary for Adiponectin-Directed Regulation of Electrophysiological Properties and Oxidative Metabolism in C2C12 Myotubes" Cells 11, no. 4: 713. https://doi.org/10.3390/cells11040713
APA StyleBernacchioni, C., Squecco, R., Gamberi, T., Ghini, V., Schumacher, F., Mannelli, M., Garella, R., Idrizaj, E., Cencetti, F., Puliti, E., Bruni, P., Turano, P., Fiaschi, T., & Donati, C. (2022). S1P Signalling Axis Is Necessary for Adiponectin-Directed Regulation of Electrophysiological Properties and Oxidative Metabolism in C2C12 Myotubes. Cells, 11(4), 713. https://doi.org/10.3390/cells11040713








