C-C Chemokine Receptor 7 in Cancer
Abstract
:1. Introduction
2. Breast Cancers
3. CCR7 in Genitourinary Cancers
3.1. Gynecologic Cancers
3.2. Prostate Cancer
4. The Roles of CCR7 in Gastrointestinal Cancers
4.1. Colorectal Cancer
4.2. Esophageal Cancer
4.3. Gastric Cancers
4.4. Pancreatic Cancer
5. Head and Neck Cancers
5.1. Oral
5.2. Non-Oral
5.3. Thyroid Cancer
6. Tumors of Surface Epithelia
6.1. Lung Cancer
| Cancer | Observation | Reference |
|---|---|---|
| Lung | CCR7 in A549 reduces tumor size in the presence of CCL21 | [203,204] |
| Lu99 metastasized to LN via α4β1-integrins | [204] | |
| A549 cells grow when activated with CCL21 via upregulation of cyclin A, cyclin B1 and cyclin-dependent kinase 1 | [205] | |
| CCL21/CCR7 reduced apoptosis via upregulation of Bcl-2 and downregulation of Bax/Caspase-3 | [206] | |
| CCR7 inhibition in A549 induced EMT and suppressed inflammation | [207] | |
| CCR7 induced EMT via SP1 and heparinase to cleave ECM to facilitate EMT and metastasis | [208] | |
| CCL21 promoted E-cadherin and mesenchymal markers vimentin, Slug and ERK and correlated with clinical samples | [209] | |
| CCR7 mediated lymphangiogenesis via Akt, ERK1/2 and p38 | ||
| Skin | CCR7 may play a role in metastasis of non-melanoma skin cancers | [222] |
| B16 transduced with CCR7/CCL21 enhanced tumorigenesis and increased metastasis to 50% of mice compared to 5% of controls | [223,224,225] | |
| A375 malignant melanoma migrate to CCL21 in vitro and in vivo | [31,223,225,226] | |
| CCL21 is produced by melanoma, and promotes immune tolerance | [227] | |
| CCL21 levels higher in patients with non-metastatic tumors | [228] | |
| HDAC inhibitors increase expression of CCR7 | [229] | |
| CCR7 expression in primary melanoma and sentinel lymph nodes | [230] | |
| Footpad injection of B16 ± CCR7 had no effect on tumor growth, but reduced anti-tumor immunity | [231] | |
| VEGF-C induced CCL21/CCR7-mediated lymphangiogenesis which promoted the entry of naïve T cells into melanomas | [232] | |
| CCR7 overexpression shortened survival times/normal CCR7 increased overall survival | [233] | |
| CCR7 overexpression correlated with metal-binding protein | [234] | |
| CCR7 is typically cytoplasmic with <2% in the membrane | [235] | |
| PD-L1 and galectin-9 co-expression with CCR7 correlates with increased metastases | [236] | |
| Elevated expression of CCR7 in uveal melanoma did not affect LN metastasis. Observed elevated liver metastases correlated with CCR7 | [237,238] |
6.2. Skin Cancer
7. Bone and Blood Cancers
7.1. Bone Cancer
7.2. Leukemia
7.3. Lymphoma
| Cancer | Observation | Reference |
|---|---|---|
| Bone | CCR7 expression is low (expressed in 43% of patient samples); high CCR7 reduced survival | [242] |
| Osteosarcoma—CCR7 expression is rare/low lymph metastases. | [243] | |
| Ewing sarcoma CCR7 or CCL21 expressed in ~4% of patient samples | [244] | |
| More than 80% of chondrosarcoma tumors co-expressed CCR7/Slug (EMT marker); reduced 5 year survival. SW1353 chondrosarcoma cells phosphorylated ERK, AKT and expressed Slug and N-cadherin following CCL21 stimulation | [245] | |
| Adult T-cell lymphoblastic leukemia/lymphoma | HTLV-1-infected T cells had increased CCR7 levels compared to uninfected T cells, and were more likely to traffic to lymph nodes | [246,247,248] |
| SNPs common in CCR7 or epigenetic modifications may promote T-cell chemotaxis | [249] | |
| CCR7 expression and GOF CCR7 mutations linked to more aggressive disease | [250,251]. | |
| Acute lymphoblastic leukemia (ALL) | CCR7 expressed in B cells from B-ALL patients, but not in control cord blood B cells | [252] |
| B cells activated with CCL19 blocked TNF-α-induced apoptosis | [252] | |
| Childhood B-cell lymphoblastic leukemia cells, pre-B cells express CCR7 and migrate to CCL19. Pro-B cells required CD40 to be CCL19 responsive | [253] | |
| CCR7 expression in pediatric T-ALL necessary and sufficient for CNS invasion | [80] | |
| In 130 B-cell precursor ALL and 117 T-ALL patients, a correlation between ZAP70, elevated CCR7/CXCR4 levels and CNS invasion; Xenograft supported this observation | [256] | |
| In a mouse model, CCL19 and CCR7 promote CNS invasion of lymphoma | [292] | |
| mTORC2 promotes T-cell invasion, cell migration and survival | [257] | |
| CCR7, CXCR4 and CXCR5 were highly expressed in B-CLL | [262,263] | |
| B-CLL active via CCR7/CCL19 blocked TNFα-induced apoptosis to promote survival | [252] | |
| In B-CLL CCR7 signals through PI3K/Rho to promote migration and survival | [264] | |
| CCL19/CCR7 promotes Cdc42 activation during chemotaxis in JVM3 B-CLL cells | [265] | |
| trisomy 12 B-CLL demonstrates preferential migration to LN | [267] | |
| CCL21/CCR7 activity increased MMP-9 production in B-CLL | [269] | |
| CCRL2 competitively inhibits CCR7/CCL19-induced B-cell migration | [270] | |
| p66Shc controlled expression of CCR7, promoted retention in LN | [276,277] | |
| CML has low CCR7 levels that correlate with abnormal trafficking of CML | [280] | |
| CML signals via CCL19/CCL21 to promote cell growth | [281] | |
| Lymphoma | HL expressed CCR7 via NF-kB and promoted migration to interfollicular zone of LN | [283] |
| T-cell non-HL expressed high levels of CCR7 and MMP-9 | [284] | |
| Cutaneous T-cell lymphoma (HuT78) expresses high levels of CCR7 compared to adult T-cell lymphoma (Jurkat) | [284] | |
| Mantle cell lymphoma express CCR7 and migrate to CCL19 | [286] | |
| Mediastinal large B-cell lymphoma responds to CCR7 signaling | [299] | |
| CCR7 directed chemotaxis of Eµ-Myc B-cell lymphoma promotes survival | [28] | |
| CCR7 is mutated in ~11% of Epstein–Barr virus B-cell lymphoproliferative disorders. | [288,289] | |
| 100% of primary CNS lymphoma cells expressed cytoplasmic CCR7, while CCR7 was membrane and cytoplasmic in B-CLL; deleting CCR7 or CCL19 prevented CNS lymphoma | [291,292] | |
| Cutaneous T-cell lymphoma (Sézary syndrome) cells expressed CCR7, correlates with presence in epidermis | [295,297] | |
| In MyLa cells, CCL21/CCR7 increased migration and enhanced mTOR activation | [298] |
8. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Birkenbach, M.; Josefsen, K.; Yalamanchili, R.; Lenoir, G.; Kieff, E. Epstein-Barr virus-induced genes: First lymphocyte-specific G protein-coupled peptide receptors. J. Virol. 1993, 67, 2209–2220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burgstahler, R.; Kempkes, B.; Steube, K.; Lipp, M. Expression of the chemokine receptor BLR2/EBI1 is specifically transactivated by Epstein-Barr virus nuclear antigen 2. Biochem. Biophys. Res. Commun. 1995, 215, 737–743. [Google Scholar] [CrossRef] [PubMed]
- Ngo, V.N.; Tang, H.L.; Cyster, J.G. Epstein-Barr virus-induced molecule 1 ligand chemokine is expressed by dendritic cells in lymphoid tissues and strongly attracts naive T cells and activated B cells. J. Exp. Med. 1998, 188, 181–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sallusto, F.; Palermo, B.; Lenig, D.; Miettinen, M.; Matikainen, S.; Julkunen, I.; Forster, R.; Burgstahler, R.; Lipp, M.; Lanzavecchia, A. Distinct patterns and kinetics of chemokine production regulate dendritic cell function. Eur. J. Immunol. 1999, 29, 1617–1625. [Google Scholar] [CrossRef]
- Kim, C.H.; Pelus, L.M.; White, J.R.; Applebaum, E.; Johanson, K.; Broxmeyer, H.E. CK beta-11/macrophage inflammatory protein-3 beta/EBI1-ligand chemokine is an efficacious chemoattractant for T and B cells. J. Immunol. 1998, 160, 2418–2424. [Google Scholar]
- Yoshida, R.; Nagira, M.; Imai, T.; Baba, M.; Takagi, S.; Tabira, Y.; Akagi, J.; Nomiyama, H.; Yoshie, O. EBI1-ligand chemokine (ELC) attracts a broad spectrum of lymphocytes: Activated T cells strongly up-regulate CCR7 and efficiently migrate toward ELC. Int. Immunol. 1998, 10, 901–910. [Google Scholar] [CrossRef] [Green Version]
- Nagira, M.; Imai, T.; Hieshima, K.; Kusuda, J.; Ridanpää, M.; Takagi, S.; Nishimura, M.; Kakizaki, M.; Nomiyama, H.; Yoshie, O. Molecular cloning of a novel human CC chemokine secondary lymphoid-tissue chemokine that is a potent chemoattractant for lymphocytes and mapped to chromosome 9p13. J. Biol. Chem. 1997, 272, 19518–19524. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, R.; Imai, T.; Hieshima, K.; Kusuda, J.; Baba, M.; Kitaura, M.; Nishimura, M.; Kakizaki, M.; Nomiyama, H.; Yoshie, O. Molecular cloning of a novel human CC chemokine EBI1-ligand chemokine that is a specific functional ligand for EBI1, CCR7. J. Biol. Chem. 1997, 272, 13803–13809. [Google Scholar] [CrossRef] [Green Version]
- Campbell, J.J.; Hedrick, J.; Zlotnik, A.; Siani, M.A.; Thompson, D.A.; Butcher, E.C. Chemokines and the arrest of lymphocytes rolling under flow conditions. Science 1998, 279, 381–384. [Google Scholar] [CrossRef]
- Kellermann, S.A.; Hudak, S.; Oldham, E.R.; Liu, Y.J.; McEvoy, L.M. The CC chemokine receptor-7 ligands 6Ckine and macrophage inflammatory protein-3 beta are potent chemoattractants for in vitro- and in vivo-derived dendritic cells. J. Immunol. 1999, 162, 3859–3864. [Google Scholar]
- Campbell, J.J.; Bowman, E.P.; Murphy, K.; Youngman, K.R.; Siani, M.A.; Thompson, D.A.; Wu, L.; Zlotnik, A.; Butcher, E.C. 6-C-kine (SLC), a lymphocyte adhesion-triggering chemokine expressed by high endothelium, is an agonist for the MIP-3beta receptor CCR7. J. Cell Biol. 1998, 141, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.H.; Pelus, L.M.; Appelbaum, E.; Johanson, K.; Anzai, N.; Broxmeyer, H.E. CCR7 ligands, SLC/6Ckine/Exodus2/TCA4 and CKbeta-11/MIP-3beta/ELC, are chemoattractants for CD56(+)CD16(−) NK cells and late stage lymphoid progenitors. Cell Immunol. 1999, 193, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Förster, R.; Schubel, A.; Breitfeld, D.; Kremmer, E.; Renner-Müller, I.; Wolf, E.; Lipp, M. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell 1999, 99, 23–33. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.H.; Pelus, L.M.; White, J.R.; Broxmeyer, H.E. Macrophage-inflammatory protein-3 beta/EBI1-ligand chemokine/CK beta-11, a CC chemokine, is a chemoattractant with a specificity for macrophage progenitors among myeloid progenitor cells. J. Immunol. 1998, 161, 2580–2585. [Google Scholar]
- Ueno, T.; Saito, F.; Gray, D.H.; Kuse, S.; Hieshima, K.; Nakano, H.; Kakiuchi, T.; Lipp, M.; Boyd, R.L.; Takahama, Y. CCR7 signals are essential for cortex-medulla migration of developing thymocytes. J. Exp. Med. 2004, 200, 493–505. [Google Scholar] [CrossRef] [Green Version]
- Ueno, T.; Hara, K.; Willis, M.S.; Malin, M.A.; Höpken, U.E.; Gray, D.H.; Matsushima, K.; Lipp, M.; Springer, T.A.; Boyd, R.L.; et al. Role for CCR7 ligands in the emigration of newly generated T lymphocytes from the neonatal thymus. Immunity 2002, 16, 205–218. [Google Scholar] [CrossRef] [Green Version]
- Zlotnik, A.; Yoshie, O. Chemokines: A new classification system and their role in immunity. Immunity 2000, 12, 121–127. [Google Scholar] [CrossRef] [Green Version]
- Nagira, M.; Imai, T.; Yoshida, R.; Takagi, S.; Iwasaki, M.; Baba, M.; Tabira, Y.; Akagi, J.; Nomiyama, H.; Yoshie, O. A lymphocyte-specific CC chemokine, secondary lymphoid tissue chemokine (SLC), is a highly efficient chemoattractant for B cells and activated T cells. Eur. J. Immunol. 1998, 28, 1516–1523. [Google Scholar] [CrossRef]
- Förster, R.; Davalos-Misslitz, A.C.; Rot, A. CCR7 and its ligands: Balancing immunity and tolerance. Nat. Rev. Immunol. 2008, 8, 362–371. [Google Scholar] [CrossRef]
- Schweickart, V.L.; Raport, C.J.; Godiska, R.; Byers, M.G.; Eddy, R.L.; Shows, T.B.; Gray, P.W. Cloning of human and mouse EBI1, a lymphoid-specific G-protein-coupled receptor encoded on human chromosome 17q12-q21.2. Genomics 1994, 23, 643–650. [Google Scholar] [CrossRef]
- Comerford, I.; Harata-Lee, Y.; Bunting, M.D.; Gregor, C.; Kara, E.E.; McColl, S.R. A myriad of functions and complex regulation of the CCR7/CCL19/CCL21 chemokine axis in the adaptive immune system. Cytokine Growth Factor Rev. 2013, 24, 269–283. [Google Scholar] [CrossRef] [PubMed]
- Link, A.; Vogt, T.K.; Favre, S.; Britschgi, M.R.; Acha-Orbea, H.; Hinz, B.; Cyster, J.G.; Luther, S.A. Fibroblastic reticular cells in lymph nodes regulate the homeostasis of naive T cells. Nat. Immunol. 2007, 8, 1255–1265. [Google Scholar] [CrossRef] [PubMed]
- Hardtke, S.; Ohl, L.; Förster, R. Balanced expression of CXCR5 and CCR7 on follicular T helper cells determines their transient positioning to lymph node follicles and is essential for efficient B-cell help. Blood 2005, 106, 1924–1931. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sallusto, F.; Lenig, D.; Förster, R.; Lipp, M.; Lanzavecchia, A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature 1999, 401, 708–712. [Google Scholar] [CrossRef] [PubMed]
- Luther, S.A.; Tang, H.L.; Hyman, P.L.; Farr, A.G.; Cyster, J.G. Coexpression of the chemokines ELC and SLC by T zone stromal cells and deletion of the ELC gene in the plt/plt mouse. Proc. Natl. Acad. Sci. USA 2000, 97, 12694–12699. [Google Scholar] [CrossRef] [Green Version]
- Menzel, L.; Höpken, U.E.; Rehm, A. Angiogenesis in Lymph Nodes Is a Critical Regulator of Immune Response and Lymphoma Growth. Front. Immunol. 2020, 11, 591741. [Google Scholar] [CrossRef]
- Balkwill, F.R. The chemokine system and cancer. J. Pathol. 2012, 226, 148–157. [Google Scholar] [CrossRef]
- Rehm, A.; Mensen, A.; Schradi, K.; Gerlach, K.; Wittstock, S.; Winter, S.; Büchner, G.; Dörken, B.; Lipp, M.; Höpken, U.E. Cooperative function of CCR7 and lymphotoxin in the formation of a lymphoma-permissive niche within murine secondary lymphoid organs. Blood 2011, 118, 1020–1033. [Google Scholar] [CrossRef]
- Zlotnik, A.; Burkhardt, A.M.; Homey, B. Homeostatic chemokine receptors and organ-specific metastasis. Nat. Rev. Immunol. 2011, 11, 597–606. [Google Scholar] [CrossRef]
- Weber, M.; Hauschild, R.; Schwarz, J.; Moussion, C.; de Vries, I.; Legler, D.F.; Luther, S.A.; Bollenbach, T.; Sixt, M. Interstitial dendritic cell guidance by haptotactic chemokine gradients. Science 2013, 339, 328–332. [Google Scholar] [CrossRef] [Green Version]
- Shields, J.D.; Fleury, M.E.; Yong, C.; Tomei, A.A.; Randolph, G.J.; Swartz, M.A. Autologous chemotaxis as a mechanism of tumor cell homing to lymphatics via interstitial flow and autocrine CCR7 signaling. Cancer Cell 2007, 11, 526–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, A.; Homey, B.; Soto, H.; Ge, N.; Catron, D.; Buchanan, M.E.; McClanahan, T.; Murphy, E.; Yuan, W.; Wagner, S.N.; et al. Involvement of chemokine receptors in breast cancer metastasis. Nature 2001, 410, 50–56. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, H.D.; Shannon, L.A.; Calloway, P.A.; Fassold, B.C.; Dunwiddie, I.; Vielhauer, G.; Zhang, M.; Vines, C.M. Expression of the C-C chemokine receptor 7 mediates metastasis of breast cancer to the lymph nodes in mice. Transl. Oncol. 2010, 3, 354–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mihály, Z.; Szász, A.M.; Győrffy, B. Predicting the chance of relapse after tamoxifen treatment in breast cancer. Biomark Med. 2014, 8, 77–79. [Google Scholar] [CrossRef]
- American Cancer Society. American Cancer Society. Available online: https://www.cancer.org/ (accessed on 18 October 2021).
- Wilson, J.L.; Burchell, J.; Grimshaw, M.J. Endothelins induce CCR7 expression by breast tumor cells via endothelin receptor A and hypoxia-inducible factor-1. Cancer Res. 2006, 66, 11802–11807. [Google Scholar] [CrossRef] [Green Version]
- Davenport, A.P.; Hyndman, K.A.; Dhaun, N.; Southan, C.; Kohan, D.E.; Pollock, J.S.; Pollock, D.M.; Webb, D.J.; Maguire, J.J. Endothelin. Pharmacol. Rev. 2016, 68, 357–418. [Google Scholar] [CrossRef] [Green Version]
- Maguire, J.J.; Davenport, A.P. Endothelin receptors and their antagonists. Semin. Nephrol. 2015, 35, 125–136. [Google Scholar] [CrossRef] [Green Version]
- Scandella, E.; Men, Y.; Gillessen, S.; Förster, R.; Groettrup, M. Prostaglandin E2 is a key factor for CCR7 surface expression and migration of monocyte-derived dendritic cells. Blood 2002, 100, 1354–1361. [Google Scholar] [CrossRef]
- Côté, S.C.; Pasvanis, S.; Bounou, S.; Dumais, N. CCR7-specific migration to CCL19 and CCL21 is induced by PGE(2) stimulation in human monocytes: Involvement of EP(2)/EP(4) receptors activation. Mol. Immunol. 2009, 46, 2682–2693. [Google Scholar] [CrossRef]
- Legler, D.F.; Krause, P.; Scandella, E.; Singer, E.; Groettrup, M. Prostaglandin E2 is generally required for human dendritic cell migration and exerts its effect via EP2 and EP4 receptors. J. Immunol. 2006, 176, 966–973. [Google Scholar] [CrossRef] [Green Version]
- Feriancová, M.; Walter, I.; Singer, C.F.; Gazdarica, J.; Pohlodek, K. Expression of COX-2, p16, and Ki67 in the range from normal breast tissue to breast cancer. Neoplasma 2021, 68, 342–351. [Google Scholar] [CrossRef] [PubMed]
- de Souza, C.P.; Alves, B.; Waisberg, J.; Fonseca, F.; Carmo, A.O.; Gehrke, F. Detection of COX-2 in liquid biopsy in patients with breast cancer. J. Clin. Pathol. 2020, 73, 826–829. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Li, M.; Zhang, C.; Cui, J.; Liu, J.; Li, J.; Jiang, H. Clinicopathological and prognostic significance of COX-2 immunohistochemical expression in breast cancer: A meta-analysis. Oncotarget 2017, 8, 6003–6012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Misron, N.A.; Looi, L.M.; Nik Mustapha, N.R. Cyclooxygenase-2 expression in invasive breast carcinomas of no special type and correlation with pathological profiles suggest a role in tumorigenesis rather than cancer progression. Asian. Pac. J. Cancer Prev. 2015, 16, 1553–1558. [Google Scholar] [CrossRef] [Green Version]
- Bartova, M.; Ondrias, F.; Muy-Kheng, T.; Kastner, M.; Singer, C.; Pohlodek, K. COX-2, p16 and Ki67 expression in DCIS, microinvasive and early invasive breast carcinoma with extensive intraductal component. Bratisl. Lek. Listy 2014, 115, 445–451. [Google Scholar] [CrossRef] [Green Version]
- Chuang, C.W.; Pan, M.R.; Hou, M.F.; Hung, W.C. Cyclooxygenase-2 up-regulates CCR7 expression via AKT-mediated phosphorylation and activation of Sp1 in breast cancer cells. J. Cell. Physiol. 2013, 228, 341–348. [Google Scholar] [CrossRef]
- Pan, M.R.; Hou, M.F.; Chang, H.C.; Hung, W.C. Cyclooxygenase-2 up-regulates CCR7 via EP2/EP4 receptor signaling pathways to enhance lymphatic invasion of breast cancer cells. J. Biol. Chem. 2008, 283, 11155–11163. [Google Scholar] [CrossRef] [Green Version]
- Vosooghi, M.; Amini, M. The discovery and development of cyclooxygenase-2 inhibitors as potential anticancer therapies. Expert Opin. Drug Discov. 2014, 9, 255–267. [Google Scholar] [CrossRef]
- Fang, L.W.; Kao, Y.H.; Chuang, Y.T.; Huang, H.L.; Tai, T.S. Ets-1 enhances tumor migration through regulation of CCR7 expression. BMB Rep. 2019, 52, 548–553. [Google Scholar] [CrossRef] [Green Version]
- Weitzenfeld, P.; Kossover, O.; Körner, C.; Meshel, T.; Wiemann, S.; Seliktar, D.; Legler, D.F.; Ben-Baruch, A. Chemokine axes in breast cancer: Factors of the tumor microenvironment reshape the CCR7-driven metastatic spread of luminal-A breast tumors. J. Leukoc. Biol. 2016, 99, 1009–1025. [Google Scholar] [CrossRef] [Green Version]
- Strien, L.; Joensuu, K.; Heikkilä, P.; Leidenius, M.H. Different Expression Patterns of CXCR4, CCR7, Maspin and FOXP3 in Luminal Breast Cancers and Their Sentinel Node Metastases. Anticancer Res. 2017, 37, 175–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Li, L.; Liu, J.; Wang, Y.; Wang, Z.; Liu, W.; Zhou, Z.; Chen, C.; Liu, R.; Yang, R. CC chemokine receptor 7 promotes triple-negative breast cancer growth and metastasis. Acta Biochim. Biophys. Sin. (Shanghai) 2018, 50, 835–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, S.; Tiruthani, K.; Wang, Y.; Xu, L.; Hu, M.; Li, J.; Song, W.; Jiang, H.; Sun, J.; Liu, R.; et al. Locally Trapping the C-C Chemokine Receptor Type 7 by Gene Delivery Nanoparticle Inhibits Lymphatic Metastasis Prior to Tumor Resection. Small 2019, 15, e1805182. [Google Scholar] [CrossRef] [PubMed]
- Cabioglu, N.; Gong, Y.; Islam, R.; Broglio, K.R.; Sneige, N.; Sahin, A.; Gonzalez-Angulo, A.M.; Morandi, P.; Bucana, C.; Hortobagyi, G.N.; et al. Expression of growth factor and chemokine receptors: New insights in the biology of inflammatory breast cancer. Ann. Oncol. 2007, 18, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Cabioglu, N.; Sahin, A.A.; Morandi, P.; Meric-Bernstam, F.; Islam, R.; Lin, H.Y.; Bucana, C.D.; Gonzalez-Angulo, A.M.; Hortobagyi, G.N.; Cristofanilli, M. Chemokine receptors in advanced breast cancer: Differential expression in metastatic disease sites with diagnostic and therapeutic implications. Ann. Oncol. 2009, 20, 1013–1019. [Google Scholar] [CrossRef]
- Andre, F.; Cabioglu, N.; Assi, H.; Sabourin, J.C.; Delaloge, S.; Sahin, A.; Broglio, K.; Spano, J.P.; Combadiere, C.; Bucana, C.; et al. Expression of chemokine receptors predicts the site of metastatic relapse in patients with axillary node positive primary breast cancer. Ann. Oncol. 2006, 17, 945–951. [Google Scholar] [CrossRef]
- Sonbul, S.N.; Gorringe, K.L.; Aleskandarany, M.A.; Mukherjee, A.; Green, A.R.; Ellis, I.O.; Rakha, E.A. Chemokine (C-C motif) receptor 7 (CCR7) associates with the tumour immune microenvironment but not progression in invasive breast carcinoma. J. Pathol. Clin. Res. 2017, 3, 105–114. [Google Scholar] [CrossRef] [Green Version]
- Cassier, P.A.; Treilleux, I.; Bachelot, T.; Ray-Coquard, I.; Bendriss-Vermare, N.; Ménétrier-Caux, C.; Trédan, O.; Goddard-Léon, S.; Pin, J.J.; Mignotte, H.; et al. Prognostic value of the expression of C-Chemokine Receptor 6 and 7 and their ligands in non-metastatic breast cancer. BMC Cancer 2011, 11, 213. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Ji, R.; Li, J.; Gu, Q.; Zhao, X.; Sun, T.; Wang, J.; Du, Q.; Sun, B. Correlation effect of EGFR and CXCR4 and CCR7 chemokine receptors in predicting breast cancer metastasis and prognosis. J. Exp. Clin. Cancer Res. 2010, 29, 16. [Google Scholar] [CrossRef] [Green Version]
- Gurgel, D.C.; Wong, D.V.T.; Bandeira, A.M.; Pereira, J.F.B.; Gomes-Filho, J.V.; Pereira, A.C.; Barros Silva, P.G.; Távora, F.R.F.; Pereira, A.F.; Lima-Júnior, R.C.P.; et al. Cytoplasmic CCR7 (CCR7c) immunoexpression is associated with local tumor recurrence in triple-negative breast cancer. Pathol. Res. Pract. 2020, 216, 153265. [Google Scholar] [CrossRef]
- Zeillinger, R.; Kury, F.; Czerwenka, K.; Kubista, E.; Sliutz, G.; Knogler, W.; Huber, J.; Zielinski, C.; Reiner, G.; Jakesz, R. HER-2 amplification, steroid receptors and epidermal growth factor receptor in primary breast cancer. Oncogene 1989, 4, 109–114. [Google Scholar] [PubMed]
- Lamy, P.J.; Fina, F.; Bascoul-Mollevi, C.; Laberenne, A.C.; Martin, P.M.; Ouafik, L.; Jacot, W. Quantification and clinical relevance of gene amplification at chromosome 17q12-q21 in human epidermal growth factor receptor 2-amplified breast cancers. Breast Cancer Res. 2011, 13, R15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- El-Ghonaimy, E.A.; El-Shinawi, M.; Ibrahim, S.A.; El-Ghazaly, H.; Abd-El-Tawab, R.; Nouh, M.A.; El-Mamlouk, T.; Mohamed, M.M. Positive lymph-node breast cancer patients―Activation of NF-κB in tumor-associated leukocytes stimulates cytokine secretion that promotes metastasis via C-C chemokine receptor CCR7. FEBS J. 2015, 282, 271–282. [Google Scholar] [CrossRef] [PubMed]
- Cabioglu, N.; Yazici, M.S.; Arun, B.; Broglio, K.R.; Hortobagyi, G.N.; Price, J.E.; Sahin, A. CCR7 and CXCR4 as novel biomarkers predicting axillary lymph node metastasis in T1 breast cancer. Clin. Cancer Res. 2005, 11, 5686–5693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byers, M.A.; Calloway, P.A.; Shannon, L.; Cunningham, H.D.; Smith, S.; Li, F.; Fassold, B.C.; Vines, C.M. Arrestin 3 mediates endocytosis of CCR7 following ligation of CCL19 but not CCL21. J. Immunol. 2008, 181, 4723–4732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaeuble, K.; Hauser, M.A.; Rippl, A.V.; Bruderer, R.; Otero, C.; Groettrup, M.; Legler, D.F. Ubiquitylation of the chemokine receptor CCR7 enables efficient receptor recycling and cell migration. J. Cell Sci. 2012, 125, 4463–4474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tutunea-Fatan, E.; Majumder, M.; Xin, X.; Lala, P.K. The role of CCL21/CCR7 chemokine axis in breast cancer-induced lymphangiogenesis. Mol. Cancer 2015, 14, 35. [Google Scholar] [CrossRef] [Green Version]
- Issa, A.; Le, T.X.; Shoushtari, A.N.; Shields, J.D.; Swartz, M.A. Vascular endothelial growth factor-C and C-C chemokine receptor 7 in tumor cell-lymphatic cross-talk promote invasive phenotype. Cancer Res. 2009, 69, 349–357. [Google Scholar] [CrossRef] [Green Version]
- Kochetkova, M.; Kumar, S.; McColl, S.R. Chemokine receptors CXCR4 and CCR7 promote metastasis by preventing anoikis in cancer cells. Cell Death Differ. 2009, 16, 664–673. [Google Scholar] [CrossRef] [Green Version]
- Su, M.L.; Chang, T.M.; Chiang, C.H.; Chang, H.C.; Hou, M.F.; Li, W.S.; Hung, W.C. Inhibition of chemokine (C-C motif) receptor 7 sialylation suppresses CCL19-stimulated proliferation, invasion and anti-anoikis. PLoS ONE 2014, 9, e98823. [Google Scholar] [CrossRef]
- Huang, H.L.; Chiang, C.H.; Hung, W.C.; Hou, M.F. Targeting of TGF-β-activated protein kinase 1 inhibits chemokine (C-C motif) receptor 7 expression, tumor growth and metastasis in breast cancer. Oncotarget 2015, 6, 995–1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mihaly, S.R.; Ninomiya-Tsuji, J.; Morioka, S. TAK1 control of cell death. Cell Death Differ. 2014, 21, 1667–1676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cowin, P.; Welch, D.R. Breast cancer progression: Controversies and consensus in the molecular mechanisms of metastasis and EMT. J. Mammary Gland Biol. Neoplasia 2007, 12, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Zou, Z.; Suo, N.; Zhang, Z.; Wan, F.; Zhong, G.; Qu, Y.; Ntaka, K.S.; Tian, H. CCL21/CCR7 axis activating chemotaxis accompanied with epithelial-mesenchymal transition in human breast carcinoma. Med. Oncol. 2014, 31, 180. [Google Scholar] [CrossRef]
- Xu, B.; Zhou, M.; Qiu, W.; Ye, J.; Feng, Q. CCR7 mediates human breast cancer cell invasion, migration by inducing epithelial-mesenchymal transition and suppressing apoptosis through AKT pathway. Cancer Med. 2017, 6, 1062–1071. [Google Scholar] [CrossRef]
- Pang, Y.; Mao, S.S.; Yao, R.; He, J.Y.; Zhou, Z.Z.; Feng, L.; Zhang, K.T.; Cheng, S.J.; Sun, W. TGF-β induced epithelial-mesenchymal transition in an advanced cervical tumor model by 3D printing. Biofabrication 2018, 10, 044102. [Google Scholar] [CrossRef]
- Boyle, S.T.; Ingman, W.V.; Poltavets, V.; Faulkner, J.W.; Whitfield, R.J.; McColl, S.R.; Kochetkova, M. The chemokine receptor CCR7 promotes mammary tumorigenesis through amplification of stem-like cells. Oncogene 2016, 35, 105–115. [Google Scholar] [CrossRef] [Green Version]
- Boyle, S.T.; Gieniec, K.A.; Gregor, C.E.; Faulkner, J.W.; McColl, S.R.; Kochetkova, M. Interplay between CCR7 and Notch1 axes promotes stemness in MMTV-PyMT mammary cancer cells. Mol. Cancer 2017, 16, 19. [Google Scholar] [CrossRef] [Green Version]
- Buonamici, S.; Trimarchi, T.; Ruocco, M.G.; Reavie, L.; Cathelin, S.; Mar, B.G.; Klinakis, A.; Lukyanov, Y.; Tseng, J.C.; Sen, F.; et al. CCR7 signalling as an essential regulator of CNS infiltration in T-cell leukaemia. Nature 2009, 459, 1000–1004. [Google Scholar] [CrossRef] [Green Version]
- Bernstein, E.; Caudy, A.A.; Hammond, S.M.; Hannon, G.J. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature 2001, 409, 363–366. [Google Scholar] [CrossRef]
- Zeng, Y.; Cullen, B.R. Sequence requirements for micro RNA processing and function in human cells. RNA 2003, 9, 112–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.J.; Shin, J.Y.; Lee, K.D.; Bae, Y.K.; Sung, K.W.; Nam, S.J.; Chun, K.H. MicroRNA let-7a suppresses breast cancer cell migration and invasion through downregulation of C-C chemokine receptor type 7. Breast Cancer Res. 2012, 14, R14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, O.Y.; Chang, H.W.; Lin, Y.D.; Chuang, L.Y.; Hou, M.F.; Yang, C.H. Breast cancer-associated high-order SNP-SNP interaction of CXCL12/CXCR4-related genes by an improved multifactor dimensionality reduction (MDR-ER). Oncol. Rep. 2016, 36, 1739–1747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gracio, F.; Burford, B.; Gazinska, P.; Mera, A.; Mohd Noor, A.; Marra, P.; Gillett, C.; Grigoriadis, A.; Pinder, S.; Tutt, A.; et al. Splicing imbalances in basal-like breast cancer underpin perturbation of cell surface and oncogenic pathways and are associated with patients’ survival. Sci. Rep. 2017, 7, 40177. [Google Scholar] [CrossRef]
- von Hardenberg, J.; Martini, T.; Knauer, A.; Ströbel, P.; Becker, A.; Herrmann, E.; Schubert, C.; Steidler, A.; Bolenz, C. Expression and predictive value of lymph-specific markers in urothelial carcinoma of the bladder. Urol. Oncol. 2014, 32, 54.e9–54.e17. [Google Scholar] [CrossRef]
- Mo, M.; Zhou, M.; Wang, L.; Qi, L.; Zhou, K.; Liu, L.F.; Chen, Z.; Zu, X.B. CCL21/CCR7 enhances the proliferation, migration, and invasion of human bladder cancer T24 cells. PLoS ONE 2015, 10, e0119506. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.; Wang, S.; Hu, L.; Liu, F.; Zhang, Q.; Zhang, D. miR-199a-5p suppresses human bladder cancer cell metastasis by targeting CCR7. BMC Urol. 2016, 16, 64. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Liu, S.; Zhou, B.; Wang, J.; Ping, H.; Xing, N. RRBP1 is highly expressed in bladder cancer and is associated with migration and invasion. Oncol. Lett. 2020, 20, 203. [Google Scholar] [CrossRef]
- Xiong, Y.; Shi, X.; Hu, Q.; Wu, X.; Long, E.; Bian, Y. A Nomogram for Predicting Survival in Patients With Breast Cancer Liver Metastasis: A Population-Based Study. Front. Oncol. 2021, 11, 600768. [Google Scholar] [CrossRef]
- Shannon, L.A.; Calloway, P.A.; Welch, T.P.; Vines, C.M. CCR7/CCL21 migration on fibronectin is mediated by phospholipase Cgamma1 and ERK1/2 in primary T lymphocytes. J. Biol. Chem. 2010, 285, 38781–38787. [Google Scholar] [CrossRef] [Green Version]
- Arbyn, M.; Weiderpass, E.; Bruni, L.; de Sanjosé, S.; Saraiya, M.; Ferlay, J.; Bray, F. Estimates of incidence and mortality of cervical cancer in 2018: A worldwide analysis. Lancet Glob. Health 2020, 8, e191–e203. [Google Scholar] [CrossRef] [Green Version]
- Kodama, J.; Hasengaowa; Kusumoto, T.; Seki, N.; Matsuo, T.; Ojima, Y.; Nakamura, K.; Hongo, A.; Hiramatsu, Y. Association of CXCR4 and CCR7 chemokine receptor expression and lymph node metastasis in human cervical cancer. Ann. Oncol. 2007, 18, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Y.; Cao, Y.; Zhao, H. Increased CCL19 expression is associated with progression in cervical cancer. Oncotarget 2017, 8, 73817–73825. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, S.; Han, L.; Guo, J.; Yang, Q.; Zhou, J.; Yang, X. The essential roles of CCR7 in epithelial-to-mesenchymal transition induced by hypoxia in epithelial ovarian carcinomas. Tumour. Biol. 2014, 35, 12293–12298. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Li, J.; Li, H.; Yuan Wu, N.Y.; Ou-Yang, P.; Liu, S.; Cai, J.; Wang, J. Integrative Bioinformatics Approaches to Screen Potential Prognostic Immune-Related Genes and Drugs in the Cervical Cancer Microenvironment. Front. Genet. 2020, 11, 727. [Google Scholar] [CrossRef]
- Zhao, M.M.; Yang, W.L.; Yang, F.Y.; Zhang, L.; Huang, W.J.; Hou, W.; Fan, C.F.; Jin, R.H.; Feng, Y.M.; Wang, Y.C.; et al. Cathepsin L plays a key role in SARS-CoV-2 infection in humans and humanized mice and is a promising target for new drug development. Signal Transduct. Target. Ther. 2021, 6, 134. [Google Scholar] [CrossRef]
- Tian, W.J.; Feng, P.H.; Wang, J.; Yan, T.; Qin, Q.F.; Li, D.L.; Liang, W.T. Has Potential to Be a Prognosis Marker for Cervical Squamous Cell Carcinoma and an Index for Tumor Microenvironment Change. Front. Mol. Biosci. 2021, 8, 583028. [Google Scholar] [CrossRef]
- Xu, F.; Shen, J.; Xu, S. Multi-Omics Data Analyses Construct a Six Immune-Related Genes Prognostic Model for Cervical Cancer in Tumor Microenvironment. Front. Genet. 2021, 12, 663617. [Google Scholar] [CrossRef]
- Cheng, S.; Guo, J.; Yang, Q.; Yang, X. Crk-like adapter protein regulates CCL19/CCR7-mediated epithelial-to-mesenchymal transition via ERK signaling pathway in epithelial ovarian carcinomas. Med. Oncol. 2015, 32, 47. [Google Scholar] [CrossRef]
- Heresi, G.A.; Wang, J.; Taichman, R.; Chirinos, J.A.; Regalado, J.J.; Lichtstein, D.M.; Rosenblatt, J.D. Expression of the chemokine receptor CCR7 in prostate cancer presenting with generalized lymphadenopathy: Report of a case, review of the literature, and analysis of chemokine receptor expression. Urol. Oncol. 2005, 23, 261–267. [Google Scholar] [CrossRef]
- Dalgleish, A.; Featherstone, P.; Vlassov, V.; Rogosnitzky, M. Rituximab for treating CD20+ prostate cancer with generalized lymphadenopathy: A case report and review of the literature. Investig. New Drugs 2014, 32, 1048–1052. [Google Scholar] [CrossRef] [PubMed]
- Chi, B.J.; Du, C.L.; Fu, Y.F.; Zhang, Y.N.; Wang, R.W. Silencing of CCR7 inhibits the growth, invasion and migration of prostate cancer cells induced by VEGFC. Int. J. Clin. Exp. Pathol. 2015, 8, 12533–12540. [Google Scholar] [PubMed]
- Du, R.; Tang, G.; Tang, Z.; Kuang, Y. Ectopic expression of CC chemokine receptor 7 promotes prostate cancer cells metastasis via Notch1 signaling. J. Cell Biochem. 2019, 120, 9639–9647. [Google Scholar] [CrossRef] [PubMed]
- Maolake, A.; Izumi, K.; Natsagdorj, A.; Iwamoto, H.; Kadomoto, S.; Makino, T.; Naito, R.; Shigehara, K.; Kadono, Y.; Hiratsuka, K.; et al. Tumor necrosis factor-α induces prostate cancer cell migration in lymphatic metastasis through CCR7 upregulation. Cancer Sci. 2018, 109, 1524–1531. [Google Scholar] [CrossRef]
- Capitano, M.L.; Jaiswal, A.; Broxmeyer, H.E.; Pride, Y.; Glover, S.; Amlashi, F.G.; Kirby, A.; Srinivasan, G.; Williamson, E.A.; Mais, D.; et al. A humanized monoclonal antibody against the endothelial chemokine CCL21 for the diagnosis and treatment of inflammatory bowel disease. PLoS ONE 2021, 16, e0252805. [Google Scholar] [CrossRef] [PubMed]
- McNamee, E.N.; Masterson, J.C.; Veny, M.; Collins, C.B.; Jedlicka, P.; Byrne, F.R.; Ng, G.Y.; Rivera-Nieves, J. Chemokine receptor CCR7 regulates the intestinal TH1/TH17/Treg balance during Crohn’s-like murine ileitis. J. Leukoc Biol. 2015, 97, 1011–1022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taquet, N.; Dumont, S.; Vonesch, J.L.; Hentsch, D.; Reimund, J.M.; Muller, C.D. Differential between protein and mRNA expression of CCR7 and SSTR5 receptors in Crohn’s disease patients. Mediat. Inflamm. 2009, 2009, 285812. [Google Scholar] [CrossRef] [Green Version]
- Shi, W.; Zou, R.; Yang, M.; Mai, L.; Ren, J.; Wen, J.; Liu, Z.; Lai, R. Analysis of Genes Involved in Ulcerative Colitis Activity and Tumorigenesis Through Systematic Mining of Gene Co-expression Networks. Front. Physiol. 2019, 10, 662. [Google Scholar] [CrossRef]
- Günther, K.; Leier, J.; Henning, G.; Dimmler, A.; Weissbach, R.; Hohenberger, W.; Förster, R. Prediction of lymph node metastasis in colorectal carcinoma by expressionof chemokine receptor CCR7. Int. J. Cancer 2005, 116, 726–733. [Google Scholar] [CrossRef]
- Yu, S.; Duan, J.; Zhou, Z.; Pang, Q.; Wuyang, J.; Liu, T.; He, X.; Xinfa, L.; Chen, Y. A critical role of CCR7 in invasiveness and metastasis of SW620 colon cancer cell in vitro and in vivo. Cancer Biol. Ther. 2008, 7, 1037–1043. [Google Scholar] [CrossRef]
- Nagasawa, S.; Tsuchida, K.; Shiozawa, M.; Hiroshima, Y.; Kimura, Y.; Hashimoto, I.; Watanabe, H.; Kano, K.; Numata, M.; Aoyama, T.; et al. Clinical Significance of Chemokine Receptor CXCR4 and CCR7 mRNA Expression in Patients with Colorectal Cancer. Anticancer Res. 2021, 41, 4489–4495. [Google Scholar] [CrossRef] [PubMed]
- Schimanski, C.C.; Schwald, S.; Simiantonaki, N.; Jayasinghe, C.; Gönner, U.; Wilsberg, V.; Junginger, T.; Berger, M.R.; Galle, P.R.; Moehler, M. Effect of chemokine receptors CXCR4 and CCR7 on the metastatic behavior of human colorectal cancer. Clin. Cancer Res. 2005, 11, 1743–1750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shannon, L.A.; McBurney, T.M.; Wells, M.A.; Roth, M.E.; Calloway, P.A.; Bill, C.A.; Islam, S.; Vines, C.M. CCR7/CCL19 controls expression of EDG-1 in T cells. J. Biol. Chem. 2012, 287, 11656–11664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Na, I.K.; Busse, A.; Scheibenbogen, C.; Ghadjar, P.; Coupland, S.E.; Letsch, A.; Loddenkemper, C.; Stroux, A.; Bauer, S.; Thiel, E.; et al. Identification of truncated chemokine receptor 7 in human colorectal cancer unable to localize to the cell surface and unreactive to external ligands. Int. J. Cancer 2008, 123, 1565–1572. [Google Scholar] [CrossRef] [PubMed]
- Mumtaz, M.; Wågsäter, D.; Löfgren, S.; Hugander, A.; Zar, N.; Dimberg, J. Decreased expression of the chemokine CCL21 in human colorectal adenocarcinomas. Oncol. Rep. 2009, 21, 153–158. [Google Scholar]
- Lu, J.; Zhao, J.; Feng, H.; Wang, P.; Zhang, Z.; Zong, Y.; Ma, J.; Zheng, M.; Lu, A. Antitumor efficacy of CC motif chemokine ligand 19 in colorectal cancer. Dig. Dis. Sci. 2014, 59, 2153–2162. [Google Scholar] [CrossRef]
- Xu, Z.; Zhu, C.; Chen, C.; Zong, Y.; Feng, H.; Liu, D.; Feng, W.; Zhao, J.; Lu, A. CCL19 suppresses angiogenesis through promoting miR-206 and inhibiting Met/ERK/Elk-1/HIF-1α/VEGF-A pathway in colorectal cancer. Cell Death Dis. 2018, 9, 974. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Sun, R.; Tao, K.; Wang, G. The CCL21/CCR7 pathway plays a key role in human colon cancer metastasis through regulation of matrix metalloproteinase-9. Dig. Liver Dis. 2011, 43, 40–47. [Google Scholar] [CrossRef]
- Yu, S.; Hou, Q.; Sun, H.; Liu, J.; Li, J. Upregulation of C-C chemokine receptor type 7 expression by membrane-associated prostaglandin E synthase-1/prostaglandin E2 requires glycogen synthase kinase 3β-mediated signal transduction in colon cancer cells. Mol. Med. Rep. 2015, 12, 7169–7175. [Google Scholar] [CrossRef]
- Soto, H.; Wang, W.; Strieter, R.M.; Copeland, N.G.; Gilbert, D.J.; Jenkins, N.A.; Hedrick, J.; Zlotnik, A. The CC chemokine 6Ckine binds the CXC chemokine receptor CXCR3. Proc. Natl. Acad. Sci. USA 1998, 95, 8205–8210. [Google Scholar] [CrossRef] [Green Version]
- Kawada, K.; Hosogi, H.; Sonoshita, M.; Sakashita, H.; Manabe, T.; Shimahara, Y.; Sakai, Y.; Takabayashi, A.; Oshima, M.; Taketo, M.M. Chemokine receptor CXCR3 promotes colon cancer metastasis to lymph nodes. Oncogene 2007, 26, 4679–4688. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Xu, J.; He, G.; Huang, J.; Xu, W.; Qin, J.; Zheng, P.; Ji, M.; Chang, W.; Ren, L.; et al. CCR7 high expression leads to cetuximab resistance by cross-talking with EGFR pathway in PI3K/AKT signals in colorectal cancer. Am. J. Cancer Res. 2019, 9, 2531–2543. [Google Scholar] [PubMed]
- Ding, Y.; Shimada, Y.; Maeda, M.; Kawabe, A.; Kaganoi, J.; Komoto, I.; Hashimoto, Y.; Miyake, M.; Hashida, H.; Imamura, M. Association of CC chemokine receptor 7 with lymph node metastasis of esophageal squamous cell carcinoma. Clin. Cancer Res. 2003, 9, 3406–3412. [Google Scholar] [PubMed]
- Ishida, K.; Iwahashi, M.; Nakamori, M.; Nakamura, M.; Yokoyama, S.; Iida, T.; Naka, T.; Nakamura, Y.; Yamaue, H. High CCR7 mRNA expression of cancer cells is associated with lymph node involvement in patients with esophageal squamous cell carcinoma. Int. J. Oncol. 2009, 34, 915–922. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Wang, Z.; Liu, X.; Jiang, W.; Shi, M. CCR7 and VEGF-C: Molecular indicator of lymphatic metastatic recurrence in pN0 esophageal squamous cell carcinoma after Ivor-Lewis esophagectomy? Ann. Surg. Oncol. 2012, 19, 3606–3612. [Google Scholar] [CrossRef]
- Shi, M.; Chen, D.; Yang, D.; Liu, X.Y. CCL21-CCR7 promotes the lymph node metastasis of esophageal squamous cell carcinoma by up-regulating MUC1. J. Exp. Clin. Cancer Res. 2015, 34, 149. [Google Scholar] [CrossRef] [Green Version]
- Irino, T.; Takeuchi, H.; Matsuda, S.; Saikawa, Y.; Kawakubo, H.; Wada, N.; Takahashi, T.; Nakamura, R.; Fukuda, K.; Omori, T.; et al. CC-Chemokine receptor CCR7: A key molecule for lymph node metastasis in esophageal squamous cell carcinoma. BMC Cancer 2014, 14, 291. [Google Scholar] [CrossRef]
- Jemal, A.; Bray, F.; Center, M.M.; Ferlay, J.; Ward, E.; Forman, D. Global cancer statistics. CA Cancer J. Clin. 2011, 61, 69–90. [Google Scholar] [CrossRef] [Green Version]
- Kwak, M.K.; Hur, K.; Park, D.J.; Lee, H.J.; Lee, H.S.; Kim, W.H.; Lee, K.U.; Choe, K.J.; Yang, H.K. Expression of chemokine receptors in human gastric cancer. Tumour. Biol. 2005, 26, 65–70. [Google Scholar] [CrossRef]
- Mashino, K.; Sadanaga, N.; Yamaguchi, H.; Tanaka, F.; Ohta, M.; Shibuta, K.; Inoue, H.; Mori, M. Expression of chemokine receptor CCR7 is associated with lymph node metastasis of gastric carcinoma. Cancer Res. 2002, 62, 2937–2941. [Google Scholar]
- Arigami, T.; Natsugoe, S.; Uenosono, Y.; Arima, H.; Mataki, Y.; Ehi, K.; Yanagida, S.; Ishigami, S.; Hokita, S.; Aikou, T. Lymphatic invasion using D2-40 monoclonal antibody and its relationship to lymph node micrometastasis in pN0 gastric cancer. Br. J. Cancer 2005, 93, 688–693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, S.; Shen, Z.; Wang, Y.; Ma, H.; Xu, S.; Qin, J.; Chen, L.; Tao, H.; Zhen, Z.; Chen, G.; et al. CCR7 expression and intratumoral FOXP3+ regulatory T cells are correlated with overall survival and lymph node metastasis in gastric cancer. PLoS ONE 2013, 8, e74430. [Google Scholar] [CrossRef] [Green Version]
- Uemura, N.; Okamoto, S.; Yamamoto, S.; Matsumura, N.; Yamaguchi, S.; Yamakido, M.; Taniyama, K.; Sasaki, N.; Schlemper, R.J. Helicobacter pylori infection and the development of gastric cancer. N. Engl. J. Med. 2001, 345, 784–789. [Google Scholar] [CrossRef] [PubMed]
- Schmausser, B.; Endrich, S.; Brändlein, S.; Schär, J.; Beier, D.; Müller-Hermelink, H.K.; Eck, M. The chemokine receptor CCR7 is expressed on epithelium of non-inflamed gastric mucosa, Helicobacter pylori gastritis, gastric carcinoma and its precursor lesions and up-regulated by H. pylori. Clin. Exp. Immunol. 2005, 139, 323–327. [Google Scholar] [CrossRef]
- Deutsch, A.J.; Steinbauer, E.; Hofmann, N.A.; Strunk, D.; Gerlza, T.; Beham-Schmid, C.; Schaider, H.; Neumeister, P. Chemokine receptors in gastric MALT lymphoma: Loss of CXCR4 and upregulation of CXCR7 is associated with progression to diffuse large B-cell lymphoma. Mod. Pathol. 2013, 26, 182–194. [Google Scholar] [CrossRef]
- Yan, C.; Zhu, Z.G.; Yu, Y.Y.; Ji, J.; Zhang, Y.; Ji, Y.B.; Yan, M.; Chen, J.; Liu, B.Y.; Yin, H.R.; et al. Expression of vascular endothelial growth factor C and chemokine receptor CCR7 in gastric carcinoma and their values in predicting lymph node metastasis. World J. Gastroenterol. 2004, 10, 783–790. [Google Scholar] [CrossRef]
- Deguchi, K.; Ichikawa, D.; Soga, K.; Watanabe, K.; Kosuga, T.; Takeshita, H.; Konishi, H.; Morimura, R.; Tsujiura, M.; Komatsu, S.; et al. Clinical significance of vascular endothelial growth factors C and D and chemokine receptor CCR7 in gastric cancer. Anticancer Res. 2010, 30, 2361–2366. [Google Scholar]
- Wang, W.N.; Chen, Y.; Zhang, Y.D.; Hu, T.H. The regulatory mechanism of CCR7 gene expression and its involvement in the metastasis and progression of gastric cancer. Tumour. Biol. 2013, 34, 1865–1871. [Google Scholar] [CrossRef]
- Chang, W.J.; Du, Y.; Zhao, X.; Ma, L.Y.; Cao, G.W. Inflammation-related factors predicting prognosis of gastric cancer. World J. Gastroenterol. 2014, 20, 4586–4596. [Google Scholar] [CrossRef]
- Ma, H.; Gao, L.; Li, S.; Qin, J.; Chen, L.; Liu, X.; Xu, P.; Wang, F.; Xiao, H.; Zhou, S.; et al. CCR7 enhances TGF-β1-induced epithelial-mesenchymal transition and is associated with lymph node metastasis and poor overall survival in gastric cancer. Oncotarget 2015, 6, 24348–24360. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhou, Y.; Yang, Y. CCR7 pathway induces epithelial-mesenchymal transition through up-regulation of Snail signaling in gastric cancer. Med. Oncol. 2015, 32, 467. [Google Scholar] [CrossRef] [PubMed]
- Du, P.; Liu, Y.; Ren, H.; Zhao, J.; Zhang, X.; Patel, R.; Hu, C.; Gan, J.; Huang, G. Expression of chemokine receptor CCR7 is a negative prognostic factor for patients with gastric cancer: A meta-analysis. Gastric Cancer 2017, 20, 235–245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yadav, D.; Lowenfels, A.B. The epidemiology of pancreatitis and pancreatic cancer. Gastroenterology 2013, 144, 1252–1261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, B.; Cui, K.; Wang, C.L.; Wang, A.L.; Zhang, B.; Zhou, W.Y.; Zhao, W.H.; Li, S. The chemotactic interaction between CCL21 and its receptor, CCR7, facilitates the progression of pancreatic cancer via induction of angiogenesis and lymphangiogenesis. J. Hepatobiliary Pancreat. Sci. 2011, 18, 821–828. [Google Scholar] [CrossRef]
- Sperveslage, J.; Frank, S.; Heneweer, C.; Egberts, J.; Schniewind, B.; Buchholz, M.; Bergmann, F.; Giese, N.; Munding, J.; Hahn, S.A.; et al. Lack of CCR7 expression is rate limiting for lymphatic spread of pancreatic ductal adenocarcinoma. Int. J. Cancer 2012, 131, E371–E381. [Google Scholar] [CrossRef]
- Guo, J.; Lou, W.; Ji, Y.; Zhang, S. Effect of CCR7, CXCR4 and VEGF-C on the lymph node metastasis of human pancreatic ductal adenocarcinoma. Oncol. Lett. 2013, 5, 1572–1578. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Xu, B.; Xu, G.; Liu, R. CCR7 regulates Twist to induce the epithelial-mesenchymal transition in pancreatic ductal adenocarcinoma. Tumour. Biol. 2016, 37, 419–424. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, D.; Li, Y.; Liu, Y.; Xie, X.; Wu, Y.; Zhou, Y.; Ren, J.; Zhang, J.; Zhu, H.; et al. CCL21/CCR7 Axis Contributed to CD133+ Pancreatic Cancer Stem-Like Cell Metastasis via EMT and Erk/NF-κB Pathway. PLoS ONE 2016, 11, e0158529. [Google Scholar] [CrossRef] [Green Version]
- Cui, K.; Zou, H.; Shi, M.; Ou, Y.; Han, L.; Zhang, B.; Hu, D.; Li, S. Gene Expression Profiles in Chemokine (C-C Motif) Ligand 21-Overexpressing Pancreatic Cancer Cells. Pathol. Oncol. Res. 2020, 26, 201–208. [Google Scholar] [CrossRef]
- Hirth, M.; Gandla, J.; Höper, C.; Gaida, M.M.; Agarwal, N.; Simonetti, M.; Demir, A.; Xie, Y.; Weiss, C.; Michalski, C.W.; et al. CXCL10 and CCL21 Promote Migration of Pancreatic Cancer Cells Toward Sensory Neurons and Neural Remodeling in Tumors in Mice, Associated With Pain in Patients. Gastroenterology 2020, 159, 665–681.e13. [Google Scholar] [CrossRef]
- Lee, J.J.; Yeh, C.Y.; Jung, C.J.; Chen, C.W.; Du, M.K.; Yu, H.M.; Yang, C.J.; Lin, H.Y.; Sun, A.; Ko, J.Y.; et al. Skewed distribution of IL-7 receptor-α-expressing effector memory CD8+ T cells with distinct functional characteristics in oral squamous cell carcinoma. PLoS ONE 2014, 9, e85521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uchida, D.; Begum, N.M.; Almofti, A.; Nakashiro, K.; Kawamata, H.; Tateishi, Y.; Hamakawa, H.; Yoshida, H.; Sato, M. Possible role of stromal-cell-derived factor-1/CXCR4 signaling on lymph node metastasis of oral squamous cell carcinoma. Exp. Cell Res. 2003, 290, 289–302. [Google Scholar] [CrossRef]
- Tsuzuki, H.; Takahashi, N.; Kojima, A.; Narita, N.; Sunaga, H.; Takabayashi, T.; Fujieda, S. Oral and oropharyngeal squamous cell carcinomas expressing CCR7 have poor prognoses. Auris Nasus Larynx 2006, 33, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Shang, Z.J.; Liu, K.; Shao, Z. Expression of chemokine receptor CCR7 is associated with cervical lymph node metastasis of oral squamous cell carcinoma. Oral Oncol. 2009, 45, 480–485. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; Nakano, H.; Aritomi, K.; Wang, C.R.; Gunn, M.D.; Kakiuchi, T. Mice lacking expression of the chemokines CCL21-ser and CCL19 (plt mice) demonstrate delayed but enhanced T cell immune responses. J. Exp. Med. 2001, 193, 207–218. [Google Scholar] [CrossRef]
- Mburu, Y.K.; Wang, J.; Wood, M.A.; Walker, W.H.; Ferris, R.L. CCR7 mediates inflammation-associated tumor progression. Immunol. Res. 2006, 36, 61–72. [Google Scholar] [CrossRef]
- Oliveira-Neto, H.H.; de Souza, P.P.; da Silva, M.R.; Mendonça, E.F.; Silva, T.A.; Batista, A.C. The expression of chemokines CCL19, CCL21 and their receptor CCR7 in oral squamous cell carcinoma and its relevance to cervical lymph node metastasis. Tumour. Biol. 2013, 34, 65–70. [Google Scholar] [CrossRef]
- Xia, X.; Liu, K.; Zhang, H.; Shang, Z. Correlation between CCR7 expression and lymph node metastatic potential of human tongue carcinoma. Oral Dis. 2015, 21, 123–131. [Google Scholar] [CrossRef]
- Al-Shareef, H.; Hiraoka, S.I.; Tanaka, N.; Shogen, Y.; Lee, A.D.; Bakhshishayan, S.; Kogo, M. Use of NRP1, a novel biomarker, along with VEGF-C, VEGFR-3, CCR7 and SEMA3E, to predict lymph node metastasis in squamous cell carcinoma of the tongue. Oncol. Rep. 2016, 36, 2444–2454. [Google Scholar] [CrossRef] [Green Version]
- Domingueti, C.B.; Janini, J.B.; Paranaíba, L.M.; Lozano-Burgos, C.; Olivero, P.; González-Arriagada, W.A. Prognostic value of immunoexpression of CCR4, CCR5, CCR7 and CXCR4 in squamous cell carcinoma of tongue and floor of the mouth. Med. Oral Patol. Oral Cir. Bucal 2019, 24, e354–e363. [Google Scholar] [CrossRef]
- Yang, H.; Fu, G.; Liu, F.; Hu, C.; Lin, J.; Tan, Z.; Fu, Y.; Ji, F.; Cao, M. LncRNA THOR promotes tongue squamous cell carcinomas by stabilizing IGF2BP1 downstream targets. Biochimie 2019, 165, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Shi, T.T.; Li, R.; Zhao, L. Long noncoding RNA UCA1 regulates CCR7 expression to promote tongue squamous cell carcinoma progression by sponging miR-138-5p. Neoplasma 2020, 67, 1256–1265. [Google Scholar] [CrossRef] [PubMed]
- Pitkin, L.; Luangdilok, S.; Corbishley, C.; Wilson, P.O.; Dalton, P.; Bray, D.; Mady, S.; Williamson, P.; Odutoye, T.; Rhys Evans, P.; et al. Expression of CC chemokine receptor 7 in tonsillar cancer predicts cervical nodal metastasis, systemic relapse and survival. Br. J. Cancer 2007, 97, 670–677. [Google Scholar] [CrossRef] [Green Version]
- Warnakulasuriya, S. Significant oral cancer risk associated with low socioeconomic status. Evid. Based Dent. 2009, 10, 4–5. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Zhao, Z.J.; Liu, F.Y.; Sun, L.Y.; Ding, X.; Zhang, W.Z.; Shang, D.H.; Sun, C.F. The chemokine receptor 7 regulates cell adhesion and migration via beta1 integrin in metastatic squamous cell carcinoma of the head and neck. Oncol. Rep. 2010, 24, 989–995. [Google Scholar] [CrossRef] [PubMed]
- Ueda, M.; Shimada, T.; Goto, Y.; Tei, K.; Nakai, S.; Hisa, Y.; Kannagi, R. Expression of CC-chemokine receptor 7 (CCR7) and CXC-chemokine receptor 4 (CXCR4) in head and neck squamous cell carcinoma. Auris Nasus Larynx 2010, 37, 488–495. [Google Scholar] [CrossRef]
- González-Arriagada, W.A.; Lozano-Burgos, C.; Zúñiga-Moreta, R.; González-Díaz, P.; Coletta, R.D. Clinicopathological significance of chemokine receptor (CCR1, CCR3, CCR4, CCR5, CCR7 and CXCR4) expression in head and neck squamous cell carcinomas. J. Oral Pathol. Med. 2018, 47, 755–763. [Google Scholar] [CrossRef]
- Wang, J.; Xi, L.; Hunt, J.L.; Gooding, W.; Whiteside, T.L.; Chen, Z.; Godfrey, T.E.; Ferris, R.L. Expression pattern of chemokine receptor 6 (CCR6) and CCR7 in squamous cell carcinoma of the head and neck identifies a novel metastatic phenotype. Cancer Res. 2004, 64, 1861–1866. [Google Scholar] [CrossRef] [Green Version]
- Ou, D.L.; Chien, H.F.; Chen, C.L.; Lin, T.C.; Lin, L.I. Role of Twist in head and neck carcinoma with lymph node metastasis. Anticancer Res. 2008, 28, 1355–1359. [Google Scholar]
- Fandi, A.; Altun, M.; Azli, N.; Armand, J.P.; Cvitkovic, E. Nasopharyngeal cancer: Epidemiology, staging, and treatment. Semin. Oncol. 1994, 21, 382–397. [Google Scholar]
- Ou, D.L.; Chen, C.L.; Lin, S.B.; Hsu, C.H.; Lin, L.I. Chemokine receptor expression profiles in nasopharyngeal carcinoma and their association with metastasis and radiotherapy. J. Pathol. 2006, 210, 363–373. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Yi, L.; Jiang, Y. Highly expressed CCR7 predicts poor prognosis in locally advanced nasopharyngeal carcinoma. Ir. J. Med. Sci. 2020, 189, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Mays, A.C.; Feng, X.; Browne, J.D.; Sullivan, C.A. Chemokine and Chemokine Receptor Profiles in Metastatic Salivary Adenoid Cystic Carcinoma. Anticancer Res. 2016, 36, 4013–4018. [Google Scholar] [PubMed]
- Li, P.; Liu, F.; Sun, L.; Zhao, Z.; Ding, X.; Shang, D.; Xu, Z.; Sun, C. Chemokine receptor 7 promotes cell migration and adhesion in metastatic squamous cell carcinoma of the head and neck by activating integrin αvβ3. Int. J. Mol. Med. 2011, 27, 679–687. [Google Scholar] [CrossRef]
- Playford, M.P.; Schaller, M.D. The interplay between Src and integrins in normal and tumor biology. Oncogene 2004, 23, 7928–7946. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Yang, X.; Zhang, Q.; Chen, L. Effects of CCR7 and Src on invasion and migration of salivary gland tumor. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 3813–3820. [Google Scholar] [CrossRef]
- Constantin, G.; Majeed, M.; Giagulli, C.; Piccio, L.; Kim, J.Y.; Butcher, E.C.; Laudanna, C. Chemokines trigger immediate beta2 integrin affinity and mobility changes: Differential regulation and roles in lymphocyte arrest under flow. Immunity 2000, 13, 759–769. [Google Scholar] [CrossRef] [Green Version]
- Tilton, B.; Ho, L.; Oberlin, E.; Loetscher, P.; Baleux, F.; Clark-Lewis, I.; Thelen, M. Signal transduction by CXC chemokine receptor 4. Stromal cell-derived factor 1 stimulates prolonged protein kinase B and extracellular signal-regulated kinase 2 activation in T lymphocytes. J. Exp. Med. 2000, 192, 313–324. [Google Scholar] [CrossRef]
- Liu, F.Y.; Zhao, Z.J.; Li, P.; Ding, X.; Zong, Z.H.; Sun, C.F. Mammalian target of rapamycin (mTOR) is involved in the survival of cells mediated by chemokine receptor 7 through PI3K/Akt in metastatic squamous cell carcinoma of the head and neck. Br. J. Oral Maxillofac. Surg. 2010, 48, 291–296. [Google Scholar] [CrossRef]
- Liu, F.Y.; Safdar, J.; Li, Z.N.; Fang, Q.G.; Zhang, X.; Xu, Z.F.; Sun, C.F. CCR7 regulates cell migration and invasion through JAK2/STAT3 in metastatic squamous cell carcinoma of the head and neck. Biomed. Res. Int. 2014, 2014, 415375. [Google Scholar] [CrossRef]
- Liu, F.Y.; Safdar, J.; Li, Z.N.; Fang, Q.G.; Zhang, X.; Xu, Z.F.; Sun, C.F. CCR7 regulates cell migration and invasion through MAPKs in metastatic squamous cell carcinoma of head and neck. Int. J. Oncol. 2014, 45, 2502–2510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Z.J.; Liu, F.Y.; Li, P.; Ding, X.; Zong, Z.H.; Sun, C.F. CCL19-induced chemokine receptor 7 activates the phosphoinositide-3 kinase-mediated invasive pathway through Cdc42 in metastatic squamous cell carcinoma of the head and neck. Oncol. Rep. 2011, 25, 729–737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mburu, Y.K.; Abe, K.; Ferris, L.K.; Sarkar, S.N.; Ferris, R.L. Human β-defensin 3 promotes NF-κB-mediated CCR7 expression and anti-apoptotic signals in squamous cell carcinoma of the head and neck. Carcinogenesis 2011, 32, 168–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mburu, Y.K.; Egloff, A.M.; Walker, W.H.; Wang, L.; Seethala, R.R.; van Waes, C.; Ferris, R.L. Chemokine receptor 7 (CCR7) gene expression is regulated by NF-κB and activator protein 1 (AP1) in metastatic squamous cell carcinoma of head and neck (SCCHN). J. Biol. Chem. 2012, 287, 3581–3590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhen-jin, Z.; Peng, L.; Fa-yu, L.; Liyan, S.; Chang-fu, S. PKCα take part in CCR7/NF-κB autocrine signaling loop in CCR7-positive squamous cell carcinoma of head and neck. Mol. Cell Biochem. 2011, 357, 181–187. [Google Scholar] [CrossRef]
- Guo, N.; Liu, F.; Yang, L.; Huang, J.; Ding, X.; Sun, C. Chemokine receptor 7 enhances cell chemotaxis and migration of metastatic squamous cell carcinoma of head and neck through activation of matrix metalloproteinase-9. Oncol. Rep. 2014, 32, 794–800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.; Zheng, X.; Yang, L.; Liu, F.; Zhang, E.; Duan, W.; Bai, S.; Safdar, J.; Li, Z.; Sun, C. Chemokine receptor 7 promotes tumor migration and invasiveness via the RhoA/ROCK pathway in metastatic squamous cell carcinoma of the head and neck. Oncol. Rep. 2015, 33, 849–855. [Google Scholar] [CrossRef] [Green Version]
- Yue, Y.; Li, Z.N.; Fang, Q.G.; Zhang, X.; Yang, L.L.; Sun, C.F.; Liu, F.Y. The role of Pyk2 in the CCR7-mediated regulation of metastasis and viability in squamous cell carcinoma of the head and neck cells in vivo and in vitro. Oncol. Rep. 2015, 34, 3280–3287. [Google Scholar] [CrossRef]
- Nijkamp, M.M.; Span, P.N.; Hoogsteen, I.J.; van der Kogel, A.J.; Kaanders, J.H.; Bussink, J. Expression of E-cadherin and vimentin correlates with metastasis formation in head and neck squamous cell carcinoma patients. Radiother. Oncol. 2011, 99, 344–348. [Google Scholar] [CrossRef]
- Curry, J.M.; Sprandio, J.; Cognetti, D.; Luginbuhl, A.; Bar-ad, V.; Pribitkin, E.; Tuluc, M. Tumor microenvironment in head and neck squamous cell carcinoma. Semin. Oncol. 2014, 41, 217–234. [Google Scholar] [CrossRef] [Green Version]
- Swartz, J.E.; Pothen, A.J.; Stegeman, I.; Willems, S.M.; Grolman, W. Clinical implications of hypoxia biomarker expression in head and neck squamous cell carcinoma: A systematic review. Cancer Med. 2015, 4, 1101–1116. [Google Scholar] [CrossRef] [PubMed]
- Basheer, H.A.; Pakanavicius, E.; Cooper, P.A.; Shnyder, S.D.; Martin, L.; Hunter, K.D.; Vinader, V.; Afarinkia, K. Hypoxia modulates CCR7 expression in head and neck cancers. Oral Oncol. 2018, 80, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.; Liu, M.D.; Wu, H.; Pang, P.; Wang, S.; Li, Z.N.; Sun, C.F.; Liu, F.Y. Overexpression of hsa-miR-125a-5p enhances proliferation, migration and invasion of head and neck squamous cell carcinoma cell lines by upregulating C-C chemokine receptor type 7. Oncol. Lett. 2018, 15, 9703–9710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, H.; Pang, P.; Liu, M.D.; Wang, S.; Jin, S.; Liu, F.Y.; Sun, C.F. Upregulated miR-20a-5p expression promotes proliferation and invasion of head and neck squamous cell carcinoma cells by targeting of TNFRSF21. Oncol. Rep. 2018, 40, 1138–1146. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.D.; Wu, H.; Wang, S.; Pang, P.; Jin, S.; Sun, C.F.; Liu, F.Y. MiR-1275 promotes cell migration, invasion and proliferation in squamous cell carcinoma of head and neck via up-regulating IGF-1R and CCR7. Gene 2018, 646, 1–7. [Google Scholar] [CrossRef]
- Wang, S.; Jin, S.; Liu, M.D.; Pang, P.; Wu, H.; Qi, Z.Z.; Liu, F.Y.; Sun, C.F. Hsa-let-7e-5p Inhibits the Proliferation and Metastasis of Head and Neck Squamous Cell Carcinoma Cells by Targeting Chemokine Receptor 7. J. Cancer 2019, 10, 1941–1948. [Google Scholar] [CrossRef]
- Nikiforov, Y.E. Thyroid carcinoma: Molecular pathways and therapeutic targets. Mod. Pathol. 2008, 21 (Suppl. 2), S37–S43. [Google Scholar] [CrossRef] [Green Version]
- Sancho, M.; Vieira, J.M.; Casalou, C.; Mesquita, M.; Pereira, T.; Cavaco, B.M.; Dias, S.; Leite, V. Expression and function of the chemokine receptor CCR7 in thyroid carcinomas. J. Endocrinol. 2006, 191, 229–238. [Google Scholar] [CrossRef]
- Wagner, P.L.; Moo, T.A.; Arora, N.; Liu, Y.F.; Zarnegar, R.; Scognamiglio, T.; Fahey, T.J. The chemokine receptors CXCR4 and CCR7 are associated with tumor size and pathologic indicators of tumor aggressiveness in papillary thyroid carcinoma. Ann. Surg. Oncol. 2008, 15, 2833–2841. [Google Scholar] [CrossRef]
- González, H.E.; Leiva, A.; Tobar, H.; Böhmwald, K.; Tapia, G.; Torres, J.; Mosso, L.M.; Bueno, S.M.; Gonzalez, P.; Kalergis, A.M.; et al. Altered chemokine receptor expression in papillary thyroid cancer. Thyroid 2009, 19, 957–965. [Google Scholar] [CrossRef]
- Howard, B.A.; Furumai, R.; Campa, M.J.; Rabbani, Z.N.; Vujaskovic, Z.; Wang, X.F.; Patz, E.F. Stable RNA interference-mediated suppression of cyclophilin A diminishes non-small-cell lung tumor growth in vivo. Cancer Res. 2005, 65, 8853–8860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arenberg, D.A.; Zlotnick, A.; Strom, S.R.; Burdick, M.D.; Strieter, R.M. The murine CC chemokine, 6C-kine, inhibits tumor growth and angiogenesis in a human lung cancer SCID mouse model. Cancer Immunol. Immunother. 2001, 49, 587–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koizumi, K.; Kozawa, Y.; Ohashi, Y.; Nakamura, E.S.; Aozuka, Y.; Sakurai, H.; Ichiki, K.; Doki, Y.; Misaki, T.; Saiki, I. CCL21 promotes the migration and adhesion of highly lymph node metastatic human non-small cell lung cancer Lu-99 in vitro. Oncol. Rep. 2007, 17, 1511–1516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Liu, L.; Qiu, X.; Jiang, L.; Huang, B.; Li, H.; Li, Z.; Luo, W.; Wang, E. CCL21/CCR7 promotes G2/M phase progression via the ERK pathway in human non-small cell lung cancer cells. PLoS ONE 2011, 6, e21119. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Liu, L.; Qiu, X.; Liu, Z.; Li, H.; Li, Z.; Luo, W.; Wang, E. CCL21/CCR7 prevents apoptosis via the ERK pathway in human non-small cell lung cancer cells. PLoS ONE 2012, 7, e33262. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Xiao, X.; An, H.; Wang, J.; Ma, Y.; Qian, Y.H. Inhibition of CCR7 promotes NF-κB-dependent apoptosis and suppresses epithelial-mesenchymal transition in non-small cell lung cancer. Oncol. Rep. 2017, 37, 2913–2919. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Sun, L.; Yin, L.; Ming, J.; Zhang, S.; Luo, W.; Qiu, X. CCL19/CCR7 upregulates heparanase via specificity protein-1 (Sp1) to promote invasion of cell in lung cancer. Tumour. Biol. 2013, 34, 2703–2708. [Google Scholar] [CrossRef]
- Zhong, G.; Chen, L.; Yin, R.; Qu, Y.; Bao, Y.; Xiao, Q.; Zhang, Z.; Shen, Y.; Li, C.; Xu, Y.; et al. Chemokine (C-C motif) ligand 21/C-C chemokine receptor type 7 triggers migration and invasion of human lung cancer cells by epithelial-mesenchymal transition via the extracellular signal-regulated kinase signaling pathway. Mol. Med. Rep. 2017, 15, 4100–4108. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; Hu, J.; Ma, J.; Feng, K.; Zhang, X.; Yang, S.; Wang, W.; Zhang, J.; Zhang, Y. RNAi-mediated silencing of VEGF-C inhibits non-small cell lung cancer progression by simultaneously down-regulating the CXCR4, CCR7, VEGFR-2 and VEGFR-3-dependent axes-induced ERK, p38 and AKT signalling pathways. Eur. J. Cancer 2011, 47, 2353–2363. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, Q.; Li, Y.; Tang, N.; Qiu, X. CCL21/CCR7 up-regulate vascular endothelial growth factor-D expression via ERK pathway in human non-small cell lung cancer cells. Int. J. Clin. Exp. Pathol. 2015, 8, 15729–15738. [Google Scholar]
- Yu, J.; Tao, S.; Hu, P.; Wang, R.; Fang, C.; Xu, Y.; Qi, D.; Wei, Z.; Zhang, J.; Tan, Q. CCR7 promote lymph node metastasis via regulating VEGF-C/D-R3 pathway in lung adenocarcinoma. J. Cancer 2017, 8, 2060–2068. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Qiu, X.; Zhang, S.; Zhang, Q.; Wang, E. Hypoxia induced CCR7 expression via HIF-1alpha and HIF-2alpha correlates with migration and invasion in lung cancer cells. Cancer Biol. Ther. 2009, 8, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Kawada, K.; Sonoshita, M.; Sakashita, H.; Takabayashi, A.; Yamaoka, Y.; Manabe, T.; Inaba, K.; Minato, N.; Oshima, M.; Taketo, M.M. Pivotal role of CXCR3 in melanoma cell metastasis to lymph nodes. Cancer Res. 2004, 64, 4010–4017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maekawa, S.; Iwasaki, A.; Shirakusa, T.; Kawakami, T.; Yanagisawa, J.; Tanaka, T.; Shibaguchi, H.; Kinugasa, T.; Kuroki, M. Association between the expression of chemokine receptors CCR7 and CXCR3, and lymph node metastatic potential in lung adenocarcinoma. Oncol. Rep. 2008, 19, 1461–1468. [Google Scholar]
- Baran, K.; Kiszałkiewicz, J.; Migdalska-Sęk, M.; Jabłoński, S.; Kordiak, J.; Antczak, A.; Góralska, K.; Brzeziańska-Lasota, E. An assessment of the relationship between the expression of CCR7/CCL19 axis and selected regulatory miRNAs in non-small cell lung cancer. Mol. Biol. Rep. 2019, 46, 5389–5396. [Google Scholar] [CrossRef] [Green Version]
- Lau, S.K.; Boutros, P.C.; Pintilie, M.; Blackhall, F.H.; Zhu, C.Q.; Strumpf, D.; Johnston, M.R.; Darling, G.; Keshavjee, S.; Waddell, T.K.; et al. Three-gene prognostic classifier for early-stage non small-cell lung cancer. J. Clin. Oncol. 2007, 25, 5562–5569. [Google Scholar] [CrossRef]
- Ma, Y.; Zhou, Y.; Zhang, H.; Su, X. Immune Response-Related Genes. Pharm. Pers. Med. 2020, 13, 511–519. [Google Scholar] [CrossRef]
- Itakura, M.; Terashima, Y.; Shingyoji, M.; Yokoi, S.; Ohira, M.; Kageyama, H.; Matui, Y.; Yoshida, Y.; Ashinuma, H.; Moriya, Y.; et al. High CC chemokine receptor 7 expression improves postoperative prognosis of lung adenocarcinoma patients. Br. J. Cancer 2013, 109, 1100–1108. [Google Scholar] [CrossRef] [Green Version]
- Yue, Z.; Ningning, D.; Lin, Y.; Jianming, Y.; Hongtu, Z.; Ligong, Y.; Feng, L.; Shuaibo, W.; Yousheng, M. Correlation between CXCR4, CXCR5 and CCR7 expression and survival outcomes in patients with clinical T1N0M0 non-small cell lung cancer. Thorac Cancer 2020, 11, 2955–2965. [Google Scholar] [CrossRef]
- Liu, F.; Wu, H. CC Chemokine Receptors in Lung Adenocarcinoma: The Inflammation-Related Prognostic Biomarkers and Immunotherapeutic Targets. J. Inflamm. Res. 2021, 14, 267–285. [Google Scholar] [CrossRef]
- Basile, J.; Thiers, B.; Maize, J.; Lathers, D.M. Chemokine receptor expression in non-melanoma skin cancer. J. Cutan. Pathol. 2008, 35, 623–629. [Google Scholar] [CrossRef] [PubMed]
- Wiley, H.E.; Gonzalez, E.B.; Maki, W.; Wu, M.T.; Hwang, S.T. Expression of CC chemokine receptor-7 and regional lymph node metastasis of B16 murine melanoma. J. Natl. Cancer Inst. 2001, 93, 1638–1643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fang, L.; Lee, V.C.; Cha, E.; Zhang, H.; Hwang, S.T. CCR7 regulates B16 murine melanoma cell tumorigenesis in skin. J. Leukoc. Biol. 2008, 84, 965–972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emmett, M.S.; Lanati, S.; Dunn, D.B.; Stone, O.A.; Bates, D.O. CCR7 mediates directed growth of melanomas towards lymphatics. Microcirculation 2011, 18, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Shields, J.D.; Emmett, M.S.; Dunn, D.B.; Joory, K.D.; Sage, L.M.; Rigby, H.; Mortimer, P.S.; Orlando, A.; Levick, J.R.; Bates, D.O. Chemokine-mediated migration of melanoma cells towards lymphatics--a mechanism contributing to metastasis. Oncogene 2007, 26, 2997–3005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shields, J.D.; Kourtis, I.C.; Tomei, A.A.; Roberts, J.M.; Swartz, M.A. Induction of lymphoidlike stroma and immune escape by tumors that express the chemokine CCL21. Science 2010, 328, 749–752. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, H.; Fujimoto, A.; Tanaka, M.; Yamano, T.; Hsueh, E.; Hoon, D.S. CCL21 chemokine regulates chemokine receptor CCR7 bearing malignant melanoma cells. Clin. Cancer Res. 2004, 10, 2351–2358. [Google Scholar] [CrossRef] [Green Version]
- Mori, T.; Kim, J.; Yamano, T.; Takeuchi, H.; Huang, S.; Umetani, N.; Koyanagi, K.; Hoon, D.S. Epigenetic up-regulation of C-C chemokine receptor 7 and C-X-C chemokine receptor 4 expression in melanoma cells. Cancer Res. 2005, 65, 1800–1807. [Google Scholar] [CrossRef] [Green Version]
- Cianfarani, F.; Mastroeni, S.; Odorisio, T.; Passarelli, F.; Cattani, C.; Mannooranparampil, T.J.; Fortes, C.; Failla, C.M. Expression of vascular endothelial growth factor-C in primary cutaneous melanoma predicts sentinel lymph node positivity. J. Cutan. Pathol. 2012, 39, 826–834. [Google Scholar] [CrossRef]
- Takekoshi, T.; Fang, L.; Paragh, G.; Hwang, S.T. CCR7-expressing B16 melanoma cells downregulate interferon-γ-mediated inflammation and increase lymphangiogenesis in the tumor microenvironment. Oncogenesis 2012, 1, e9. [Google Scholar] [CrossRef] [Green Version]
- Fankhauser, M.; Broggi, M.A.S.; Potin, L.; Bordry, N.; Jeanbart, L.; Lund, A.W.; Da Costa, E.; Hauert, S.; Rincon-Restrepo, M.; Tremblay, C.; et al. Tumor lymphangiogenesis promotes T cell infiltration and potentiates immunotherapy in melanoma. Sci. Transl. Med. 2017, 9, eaal4712. [Google Scholar] [CrossRef] [Green Version]
- Kühnelt-Leddihn, L.; Müller, H.; Eisendle, K.; Zelger, B.; Weinlich, G. Overexpression of the chemokine receptors CXCR4, CCR7, CCR9, and CCR10 in human primary cutaneous melanoma: A potential prognostic value for CCR7 and CCR10? Arch. Dermatol. Res. 2012, 304, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Weinlich, G.; Eisendle, K.; Hassler, E.; Baltaci, M.; Fritsch, P.O.; Zelger, B. Metallothionein―Overexpression as a highly significant prognostic factor in melanoma: A prospective study on 1270 patients. Br. J. Cancer 2006, 94, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Pinto, S.; Martínez-Romero, A.; O’Connor, J.E.; Gil-Benso, R.; San-Miguel, T.; Terrádez, L.; Monteagudo, C.; Callaghan, R.C. Intracellular coexpression of CXC- and CC- chemokine receptors and their ligands in human melanoma cell lines and dynamic variations after xenotransplantation. BMC Cancer 2014, 14, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cristiani, C.M.; Turdo, A.; Ventura, V.; Apuzzo, T.; Capone, M.; Madonna, G.; Mallardo, D.; Garofalo, C.; Giovannone, E.D.; Grimaldi, A.M.; et al. Accumulation of Circulating CCR7. Cancer Immunol. Res. 2019, 7, 841–852. [Google Scholar] [CrossRef]
- Li, H.; Alizadeh, H.; Niederkorn, J.Y. Differential expression of chemokine receptors on uveal melanoma cells and their metastases. Investig. Ophthalmol. Vis. Sci. 2008, 49, 636–643. [Google Scholar] [CrossRef]
- Dobner, B.C.; Riechardt, A.I.; Joussen, A.M.; Englert, S.; Bechrakis, N.E. Expression of haematogenous and lymphogenous chemokine receptors and their ligands on uveal melanoma in association with liver metastasis. Acta Ophthalmol. 2012, 90, e638–e644. [Google Scholar] [CrossRef]
- Virgili, G.; Gatta, G.; Ciccolallo, L.; Capocaccia, R.; Biggeri, A.; Crocetti, E.; Lutz, J.M.; Paci, E.; Group, E.W. Incidence of uveal melanoma in Europe. Ophthalmology 2007, 114, 2309–2315. [Google Scholar] [CrossRef]
- Scala, S.; Ieranò, C.; Ottaiano, A.; Franco, R.; La Mura, A.; Liguori, G.; Mascolo, M.; Staibano, S.; Ascierto, P.A.; Botti, G.; et al. CXC chemokine receptor 4 is expressed in uveal malignant melanoma and correlates with the epithelioid-mixed cell type. Cancer Immunol. Immunother. 2007, 56, 1589–1595. [Google Scholar] [CrossRef]
- van den Bosch, T.; Koopmans, A.E.; Vaarwater, J.; van den Berg, M.; de Klein, A.; Verdijk, R.M. Chemokine receptor CCR7 expression predicts poor outcome in uveal melanoma and relates to liver metastasis whereas expression of CXCR4 is not of clinical relevance. Invest. Ophthalmol. Vis. Sci. 2013, 54, 7354–7361. [Google Scholar] [CrossRef] [Green Version]
- Laverdiere, C.; Hoang, B.H.; Yang, R.; Sowers, R.; Qin, J.; Meyers, P.A.; Huvos, A.G.; Healey, J.H.; Gorlick, R. Messenger RNA expression levels of CXCR4 correlate with metastatic behavior and outcome in patients with osteosarcoma. Clin. Cancer Res. 2005, 11, 2561–2567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- von Luettichau, I.; Segerer, S.; Wechselberger, A.; Notohamiprodjo, M.; Nathrath, M.; Kremer, M.; Henger, A.; Djafarzadeh, R.; Burdach, S.; Huss, R.; et al. A complex pattern of chemokine receptor expression is seen in osteosarcoma. BMC Cancer 2008, 8, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sand, L.G.; Berghuis, D.; Szuhai, K.; Hogendoorn, P.C. Expression of CCL21 in Ewing sarcoma shows an inverse correlation with metastases and is a candidate target for immunotherapy. Cancer Immunol. Immunother. 2016, 65, 995–1002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, G.; Yang, Y.; Xu, S.; Ma, L.; He, M.; Zhang, Z. Slug signaling is up-regulated by CCL21/CCR7 [corrected] to induce EMT in human chondrosarcoma. Med. Oncol. 2015, 32, 478. [Google Scholar] [CrossRef] [PubMed]
- Kohno, T.; Moriuchi, R.; Katamine, S.; Yamada, Y.; Tomonaga, M.; Matsuyama, T. Identification of genes associated with the progression of adult T cell leukemia (ATL). Jpn. J. Cancer Res. 2000, 91, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Harasawa, H.; Yamada, Y.; Hieshima, K.; Jin, Z.; Nakayama, T.; Yoshie, O.; Shimizu, K.; Hasegawa, H.; Hayashi, T.; Imaizumi, Y.; et al. Survey of chemokine receptor expression reveals frequent co-expression of skin-homing CCR4 and CCR10 in adult T-cell leukemia/lymphoma. Leuk. Lymphoma 2006, 47, 2163–2173. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, H.; Nomura, T.; Kohno, M.; Tateishi, N.; Suzuki, Y.; Maeda, N.; Fujisawa, R.; Yoshie, O.; Fujita, S. Increased chemokine receptor CCR7/EBI1 expression enhances the infiltration of lymphoid organs by adult T-cell leukemia cells. Blood 2000, 95, 30–38. [Google Scholar] [CrossRef]
- Sakihama, S.; Morichika, K.; Saito, R.; Miyara, M.; Miyagi, T.; Hayashi, M.; Uchihara, J.; Tomoyose, T.; Ohshiro, K.; Nakayama, S.; et al. Genetic profile of adult T-cell leukemia/lymphoma in Okinawa: Association with prognosis, ethnicity, and HTLV-1 strains. Cancer Sci. 2021, 112, 1300–1309. [Google Scholar] [CrossRef]
- Kataoka, K.; Nagata, Y.; Kitanaka, A.; Shiraishi, Y.; Shimamura, T.; Yasunaga, J.; Totoki, Y.; Chiba, K.; Sato-Otsubo, A.; Nagae, G.; et al. Integrated molecular analysis of adult T cell leukemia/lymphoma. Nat. Genet. 2015, 47, 1304–1315. [Google Scholar] [CrossRef]
- Kagdi, H.H.; Demontis, M.A.; Fields, P.A.; Ramos, J.C.; Bangham, C.R.; Taylor, G.P. Risk stratification of adult T-cell leukemia/lymphoma using immunophenotyping. Cancer Med. 2017, 6, 298–309. [Google Scholar] [CrossRef]
- Chunsong, H.; Yuling, H.; Li, W.; Jie, X.; Gang, Z.; Qiuping, Z.; Qingping, G.; Kejian, Z.; Li, Q.; Chang, A.E.; et al. CXC chemokine ligand 13 and CC chemokine ligand 19 cooperatively render resistance to apoptosis in B cell lineage acute and chronic lymphocytic leukemia CD23+CD5+ B cells. J. Immunol. 2006, 177, 6713–6722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corcione, A.; Arduino, N.; Ferretti, E.; Pistorio, A.; Spinelli, M.; Ottonello, L.; Dallegri, F.; Basso, G.; Pistoia, V. Chemokine receptor expression and function in childhood acute lymphoblastic leukemia of B-lineage. Leuk. Res. 2006, 30, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Weng, A.P.; Ferrando, A.A.; Lee, W.; Morris, J.P.; Silverman, L.B.; Sanchez-Irizarry, C.; Blacklow, S.C.; Look, A.T.; Aster, J.C. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science 2004, 306, 269–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Au-Yeung, B.B.; Deindl, S.; Hsu, L.Y.; Palacios, E.H.; Levin, S.E.; Kuriyan, J.; Weiss, A. The structure, regulation, and function of ZAP-70. Immunol. Rev. 2009, 228, 41–57. [Google Scholar] [CrossRef] [PubMed]
- Alsadeq, A.; Fedders, H.; Vokuhl, C.; Belau, N.M.; Zimmermann, M.; Wirbelauer, T.; Spielberg, S.; Vossen-Gajcy, M.; Cario, G.; Schrappe, M.; et al. The role of ZAP70 kinase in acute lymphoblastic leukemia infiltration into the central nervous system. Haematologica 2017, 102, 346–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.; Nam, K.T.; Cho, S.H.; Gudapati, P.; Hwang, Y.; Park, D.S.; Potter, R.; Chen, J.; Volanakis, E.; Boothby, M. Vital roles of mTOR complex 2 in Notch-driven thymocyte differentiation and leukemia. J. Exp. Med. 2012, 209, 713–728. [Google Scholar] [CrossRef] [PubMed]
- Herling, M.; Khoury, J.D.; Washington, L.T.; Duvic, M.; Keating, M.J.; Jones, D. A systematic approach to diagnosis of mature T-cell leukemias reveals heterogeneity among WHO categories. Blood 2004, 104, 328–335. [Google Scholar] [CrossRef] [Green Version]
- Matutes, E.; Brito-Babapulle, V.; Swansbury, J.; Ellis, J.; Morilla, R.; Dearden, C.; Sempere, A.; Catovsky, D. Clinical and laboratory features of 78 cases of T-prolymphocytic leukemia. Blood 1991, 78, 3269–3274. [Google Scholar] [CrossRef] [Green Version]
- Cuesta-Mateos, C.; Fuentes, P.; Schrader, A.; Juárez-Sánchez, R.; Loscertales, J.; Mateu-Albero, T.; Vega-Piris, L.; Espartero-Santos, M.; Marcos-Jimenez, A.; Sánchez-López, B.A.; et al. CCR7 as a novel therapeutic target in t-cell PROLYMPHOCYTIC leukemia. Biomark. Res. 2020, 8, 54. [Google Scholar] [CrossRef]
- Alfonso-Pérez, M.; López-Giral, S.; Quintana, N.E.; Loscertales, J.; Martín-Jiménez, P.; Muñoz, C. Anti-CCR7 monoclonal antibodies as a novel tool for the treatment of chronic lymphocyte leukemia. J. Leukoc. Biol. 2006, 79, 1157–1165. [Google Scholar] [CrossRef]
- López-Giral, S.; Quintana, N.E.; Cabrerizo, M.; Alfonso-Pérez, M.; Sala-Valdés, M.; De Soria, V.G.; Fernández-Rañada, J.M.; Fernández-Ruiz, E.; Muñoz, C. Chemokine receptors that mediate B cell homing to secondary lymphoid tissues are highly expressed in B cell chronic lymphocytic leukemia and non-Hodgkin lymphomas with widespread nodular dissemination. J. Leukoc. Biol. 2004, 76, 462–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, S.; Fulcher, D. Chemokine receptor expression in B-cell lymphoproliferative disorders. Leuk. Lymphoma 2004, 45, 2491–2496. [Google Scholar] [CrossRef] [PubMed]
- Cuesta-Mateos, C.; López-Giral, S.; Alfonso-Pérez, M.; de Soria, V.G.; Loscertales, J.; Guasch-Vidal, S.; Beltrán, A.E.; Zapata, J.M.; Muñoz-Calleja, C. Analysis of migratory and prosurvival pathways induced by the homeostatic chemokines CCL19 and CCL21 in B-cell chronic lymphocytic leukemia. Exp. Hematol. 2010, 38, 756–764.e1. [Google Scholar] [CrossRef] [PubMed]
- Malet-Engra, G.; Viaud, J.; Ysebaert, L.; Farcé, M.; Lafouresse, F.; Laurent, G.; Gaits-Iacovoni, F.; Scita, G.; Dupré, L. CIP4 controls CCL19-driven cell steering and chemotaxis in chronic lymphocytic leukemia. Cancer Res. 2013, 73, 3412–3424. [Google Scholar] [CrossRef] [Green Version]
- Liso, V.; Capalbo, S.; Lapietra, A.; Pavone, V.; Guarini, A.; Specchia, G. Evaluation of trisomy 12 by fluorescence in situ hybridization in peripheral blood, bone marrow and lymph nodes of patients with B-cell chronic lymphocytic leukemia. Haematologica 1999, 84, 212–217. [Google Scholar]
- Ganghammer, S.; Hutterer, E.; Hinterseer, E.; Brachtl, G.; Asslaber, D.; Krenn, P.W.; Girbl, T.; Berghammer, P.; Geisberger, R.; Egle, A.; et al. CXCL12-induced VLA-4 activation is impaired in trisomy 12 chronic lymphocytic leukemia cells: A role for CCL21. Oncotarget 2015, 6, 12048–12060. [Google Scholar] [CrossRef] [Green Version]
- Kamiguti, A.S.; Lee, E.S.; Till, K.J.; Harris, R.J.; Glenn, M.A.; Lin, K.; Chen, H.J.; Zuzel, M.; Cawley, J.C. The role of matrix metalloproteinase 9 in the pathogenesis of chronic lymphocytic leukaemia. Br. J. Haematol. 2004, 125, 128–140. [Google Scholar] [CrossRef]
- Redondo-Muñoz, J.; José Terol, M.; García-Marco, J.A.; García-Pardo, A. Matrix metalloproteinase-9 is up-regulated by CCL21/CCR7 interaction via extracellular signal-regulated kinase-1/2 signaling and is involved in CCL21-driven B-cell chronic lymphocytic leukemia cell invasion and migration. Blood 2008, 111, 383–386. [Google Scholar] [CrossRef] [Green Version]
- Catusse, J.; Leick, M.; Groch, M.; Clark, D.J.; Buchner, M.V.; Zirlik, K.; Burger, M. Role of the atypical chemoattractant receptor CRAM in regulating CCL19 induced CCR7 responses in B-cell chronic lymphocytic leukemia. Mol. Cancer 2010, 9, 297. [Google Scholar] [CrossRef] [Green Version]
- Laufer, J.M.; Kindinger, I.; Artinger, M.; Pauli, A.; Legler, D.F. CCR7 Is Recruited to the Immunological Synapse, Acts as Co-stimulatory Molecule and Drives LFA-1 Clustering for Efficient T Cell Adhesion Through ZAP70. Front. Immunol. 2018, 9, 3115. [Google Scholar] [CrossRef] [Green Version]
- Laufer, J.M.; Lyck, R.; Legler, D.F. ZAP70 expression enhances chemokine-driven chronic lymphocytic leukemia cell migration and arrest by valency regulation of integrins. FASEB J. 2018, 32, 4824–4835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richardson, R.B. Promotional etiology for common childhood acute lymphoblastic leukemia: The infective lymphoid recovery hypothesis. Leuk. Res. 2011, 35, 1425–1431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matloubian, M.; Lo, C.G.; Cinamon, G.; Lesneski, M.J.; Xu, Y.; Brinkmann, V.; Allende, M.L.; Proia, R.L.; Cyster, J.G. Lymphocyte egress from thymus and peripheral lymphoid organs is dependent on S1P receptor 1. Nature 2004, 427, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Sic, H.; Kraus, H.; Madl, J.; Flittner, K.A.; von Münchow, A.L.; Pieper, K.; Rizzi, M.; Kienzler, A.K.; Ayata, K.; Rauer, S.; et al. Sphingosine-1-phosphate receptors control B-cell migration through signaling components associated with primary immunodeficiencies, chronic lymphocytic leukemia, and multiple sclerosis. J. Allergy Clin. Immunol. 2014, 134, 420–428. [Google Scholar] [CrossRef]
- Capitani, N.; Patrussi, L.; Trentin, L.; Lucherini, O.M.; Cannizzaro, E.; Migliaccio, E.; Frezzato, F.; Gattazzo, C.; Forconi, F.; Pelicci, P.; et al. S1P1 expression is controlled by the pro-oxidant activity of p66Shc and is impaired in B-CLL patients with unfavorable prognosis. Blood 2012, 120, 4391–4399. [Google Scholar] [CrossRef] [Green Version]
- Patrussi, L.; Capitani, N.; Martini, V.; Pizzi, M.; Trimarco, V.; Frezzato, F.; Marino, F.; Semenzato, G.; Trentin, L.; Baldari, C.T. Enhanced Chemokine Receptor Recycling and Impaired S1P1 Expression Promote Leukemic Cell Infiltration of Lymph Nodes in Chronic Lymphocytic Leukemia. Cancer Res. 2015, 75, 4153–4163. [Google Scholar] [CrossRef] [Green Version]
- NOWELL, P.C.; HUNGERFORD, D.A. Chromosome studies on normal and leukemic human leukocytes. J. Natl. Cancer Inst. 1960, 25, 85–109. [Google Scholar]
- Gordon, M.Y.; Dowding, C.R.; Riley, G.P.; Goldman, J.M.; Greaves, M.F. Altered adhesive interactions with marrow stroma of haematopoietic progenitor cells in chronic myeloid leukaemia. Nature 1987, 328, 342–344. [Google Scholar] [CrossRef]
- Jongen-Lavrencic, M.; Salesse, S.; Delwel, R.; Verfaillie, C.M. BCR/ABL-mediated downregulation of genes implicated in cell adhesion and motility leads to impaired migration toward CCR7 ligands CCL19 and CCL21 in primary BCR/ABL-positive cells. Leukemia 2005, 19, 373–380. [Google Scholar] [CrossRef] [Green Version]
- Kubo, K.; Iwakami, M.; Muromoto, R.; Inagaki, T.; Kitai, Y.; Kon, S.; Sekine, Y.; Oritani, K.; Matsuda, T. CCR7 is involved in BCR-ABL/STAP-2-mediated cell growth in hematopoietic Ba/F3 cells. Biochem. Biophys. Res. Commun. 2015, 463, 825–831. [Google Scholar] [CrossRef] [Green Version]
- Kaufman, D.; Longo, D.L. Hodgkin’s disease. Crit. Rev. Oncol. Hematol. 1992, 13, 135–187. [Google Scholar] [CrossRef]
- Höpken, U.E.; Foss, H.D.; Meyer, D.; Hinz, M.; Leder, K.; Stein, H.; Lipp, M. Up-regulation of the chemokine receptor CCR7 in classical but not in lymphocyte-predominant Hodgkin disease correlates with distinct dissemination of neoplastic cells in lymphoid organs. Blood 2002, 99, 1109–1116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Wang, S.; Zhao, G.; Sun, B. Effect of chemokine receptors CCR7 on disseminated behavior of human T cell lymphoma: Clinical and experimental study. J. Exp. Clin. Cancer Res. 2011, 30, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barista, I.; Romaguera, J.E.; Cabanillas, F. Mantle-cell lymphoma. Lancet Oncol. 2001, 2, 141–148. [Google Scholar] [CrossRef]
- Corcione, A.; Arduino, N.; Ferretti, E.; Raffaghello, L.; Roncella, S.; Rossi, D.; Fedeli, F.; Ottonello, L.; Trentin, L.; Dallegri, F.; et al. CCL19 and CXCL12 trigger in vitro chemotaxis of human mantle cell lymphoma B cells. Clin. Cancer Res. 2004, 10, 964–971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oyama, T.; Ichimura, K.; Suzuki, R.; Suzumiya, J.; Ohshima, K.; Yatabe, Y.; Yokoi, T.; Kojima, M.; Kamiya, Y.; Taji, H.; et al. Senile EBV+ B-cell lymphoproliferative disorders: A clinicopathologic study of 22 patients. Am. J. Surg. Pathol. 2003, 27, 16–26. [Google Scholar] [CrossRef]
- Gebauer, N.; Künstner, A.; Ketzer, J.; Witte, H.M.; Rausch, T.; Benes, V.; Zimmermann, J.; Gebauer, J.; Merz, H.; Bernard, V.; et al. Genomic insights into the pathogenesis of Epstein-Barr virus-associated diffuse large B-cell lymphoma by whole-genome and targeted amplicon sequencing. Blood Cancer J. 2021, 11, 102. [Google Scholar] [CrossRef]
- Kocks, J.R.; Adler, H.; Danzer, H.; Hoffmann, K.; Jonigk, D.; Lehmann, U.; Förster, R. Chemokine receptor CCR7 contributes to a rapid and efficient clearance of lytic murine gamma-herpes virus 68 from the lung, whereas bronchus-associated lymphoid tissue harbors virus during latency. J. Immunol. 2009, 182, 6861–6869. [Google Scholar] [CrossRef] [Green Version]
- Deangelis, L.M.; Hormigo, A. Treatment of primary central nervous system lymphoma. Semin. Oncol. 2004, 31, 684–692. [Google Scholar] [CrossRef]
- Jahnke, K.; Coupland, S.E.; Na, I.K.; Loddenkemper, C.; Keilholz, U.; Korfel, A.; Stein, H.; Thiel, E.; Scheibenbogen, C. Expression of the chemokine receptors CXCR4, CXCR5, and CCR7 in primary central nervous system lymphoma. Blood 2005, 106, 384–385. [Google Scholar] [CrossRef]
- O’Connor, T.; Zhou, X.; Kosla, J.; Adili, A.; Garcia Beccaria, M.; Kotsiliti, E.; Pfister, D.; Johlke, A.L.; Sinha, A.; Sankowski, R.; et al. Age-Related Gliosis Promotes Central Nervous System Lymphoma through CCL19-Mediated Tumor Cell Retention. Cancer Cell 2019, 36, 250–267.e259. [Google Scholar] [CrossRef] [PubMed]
- Brown, E.E.; Lan, Q.; Zheng, T.; Zhang, Y.; Wang, S.S.; Hoar-Zahm, S.; Chanock, S.J.; Rothman, N.; Baris, D. Common variants in genes that mediate immunity and risk of multiple myeloma. Int. J. Cancer 2007, 120, 2715–2722. [Google Scholar] [CrossRef] [PubMed]
- Purdue, M.P.; Lan, Q.; Menashe, I.; Zheng, T.; Zhang, Y.; Yeager, M.; Hosgood, H.D.; Zahm, S.H.; Chanock, S.J.; Rothman, N.; et al. Variation in innate immunity genes and risk of multiple myeloma. Hematol. Oncol. 2011, 29, 42–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capriotti, E.; Vonderheid, E.C.; Thoburn, C.J.; Bright, E.C.; Hess, A.D. Chemokine receptor expression by leukemic T cells of cutaneous T-cell lymphoma: Clinical and histopathological correlations. J. Investig. Dermatol. 2007, 127, 2882–2892. [Google Scholar] [CrossRef] [Green Version]
- Wilcox, R.A. Cutaneous T-cell lymphoma: 2017 update on diagnosis, risk-stratification, and management. Am. J. Hematol. 2017, 92, 1085–1102. [Google Scholar] [CrossRef] [Green Version]
- Hu, S.C.; Lin, C.L.; Hong, C.H.; Yu, H.S.; Chen, G.S.; Lee, C.H. CCR7 expression correlates with subcutaneous involvement in mycosis fungoides skin lesions and promotes migration of mycosis fungoides cells (MyLa) through mTOR activation. J. Dermatol. Sci. 2014, 74, 31–38. [Google Scholar] [CrossRef]
- Hong, C.H.; Lin, S.H.; Lee, C.H. CCL21 Induces mTOR-dependent MALAT1 Expression, Leading to Cell Migration in Cutaneous T-Cell Lymphoma. In Vivo 2019, 33, 793–800. [Google Scholar] [CrossRef] [Green Version]
- Rehm, A.; Anagnostopoulos, I.; Gerlach, K.; Broemer, M.; Scheidereit, C.; Jöhrens, K.; Hübler, M.; Hetzer, R.; Stein, H.; Lipp, M.; et al. Identification of a chemokine receptor profile characteristic for mediastinal large B-cell lymphoma. Int. J. Cancer 2009, 125, 2367–2374. [Google Scholar] [CrossRef]
- Sun, C.C.; Zhang, Y.S.; Xue, X.; Cheng, Y.N.; Liu, H.P.; Zhao, C.R.; Lou, H.X.; Qu, X.J. Inhibition of angiogenesis involves in anticancer activity of riccardin D, a macrocyclic bisbibenzyl, in human lung carcinoma. Eur. J. Pharmacol. 2011, 667, 136–143. [Google Scholar] [CrossRef]
- Agostini, M.L.; Andres, E.L.; Sims, A.C.; Graham, R.L.; Sheahan, T.P.; Lu, X.; Smith, E.C.; Case, J.B.; Feng, J.Y.; Jordan, R.; et al. Coronavirus Susceptibility to the Antiviral Remdesivir (GS-5734) Is Mediated by the Viral Polymerase and the Proofreading Exoribonuclease. mBio 2018, 9, e00221-18. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Hawk, N.; Yue, P.; Kauh, J.; Ramalingam, S.S.; Fu, H.; Khuri, F.R.; Sun, S.Y. Overcoming mTOR inhibition-induced paradoxical activation of survival signaling pathways enhances mTOR inhibitors’ anticancer efficacy. Cancer Biol. Ther. 2008, 7, 1952–1958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pang, M.F.; Georgoudaki, A.M.; Lambut, L.; Johansson, J.; Tabor, V.; Hagikura, K.; Jin, Y.; Jansson, M.; Alexander, J.S.; Nelson, C.M.; et al. TGF-β1-induced EMT promotes targeted migration of breast cancer cells through the lymphatic system by the activation of CCR7/CCL21-mediated chemotaxis. Oncogene 2016, 35, 748–760. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, C.; Nie, L.; Gu, M.; Wu, A.; Han, X.; Wang, X.; Shao, J.; Xia, Z. Transforming growth factor (TGF)-β-activated kinase 1 (TAK1) activation requires phosphorylation of serine 412 by protein kinase A catalytic subunit α (PKACα) and X-linked protein kinase (PRKX). J. Biol. Chem. 2014, 289, 24226–24237. [Google Scholar] [CrossRef] [Green Version]
- Birge, R.B.; Kalodimos, C.; Inagaki, F.; Tanaka, S. Crk and CrkL adaptor proteins: Networks for physiological and pathological signaling. Cell Commun. Signal. 2009, 7, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Q.; Yi, F.; Zhang, Y.; Jun Li, D.K.; Wei, Y.; Yu, H. CRKL regulates alternative splicing of cancer-related genes in cervical cancer samples and HeLa cell. BMC Cancer 2019, 19, 499. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Croce, C.M. The role of MicroRNAs in human cancer. Signal Transduct. Target. Ther. 2016, 1, 15004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cunningham, H.D.; Kim, E.; August, K.; Vines, C.M. Novel Single Chain Antibodies to Inhibit CCR7 Mediated-entry of pediatric T-cell acute Lymphoblastic Leukemia into the CNS. Ann. Oncol. 2014, 25, 327–329. [Google Scholar] [CrossRef]
- Cuesta-Mateos, C.; Brown, J.R.; Terrón, F.; Muñoz-Calleja, C. Of Lymph Nodes and CLL Cells: Deciphering the Role of CCR7 in the Pathogenesis of CLL and Understanding Its Potential as Therapeutic Target. Front. Immunol. 2021, 12, 662866. [Google Scholar] [CrossRef]
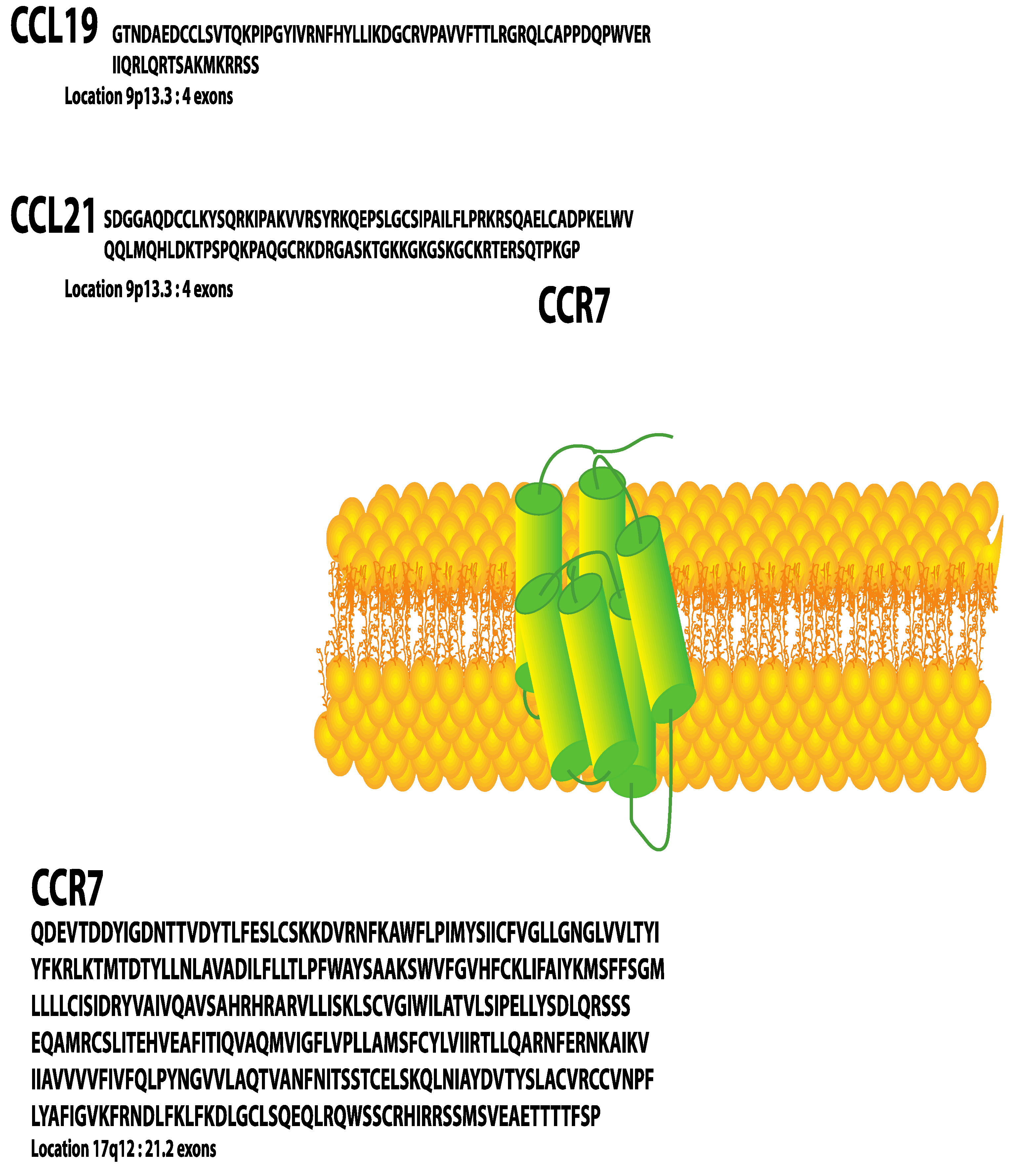
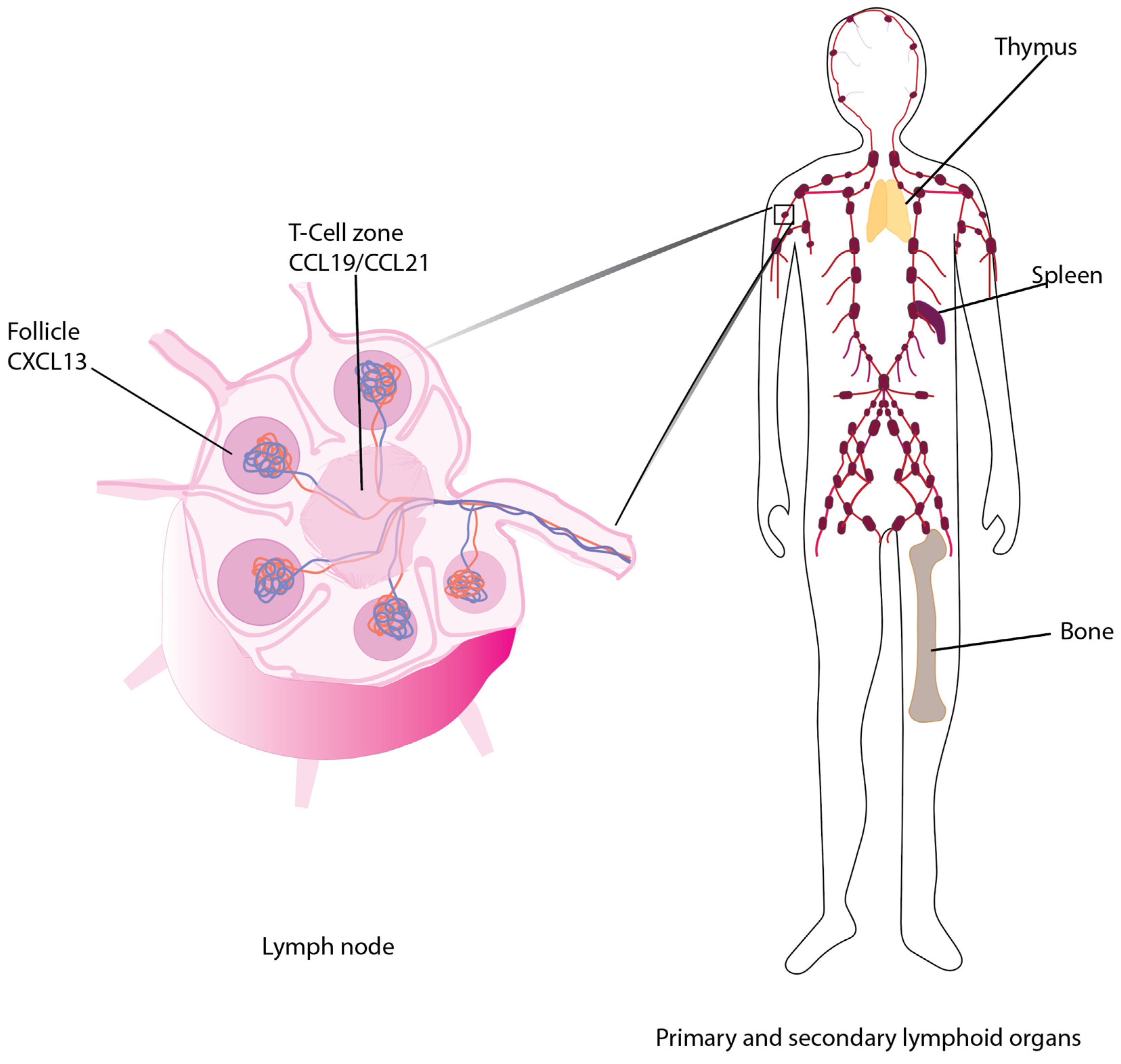
| Signaling Event | Observation | Reference |
|---|---|---|
| CCR7/CCL21 | Promotes migration/invasion via activation of actin | [32] |
| CCR7/EGFR | Shortened patient survival time, high local recurrence; no difference in 5 year survival. | [60,61] |
| CCR7/HER2-neu | Correlates with LN metastases | [65] |
| CCR7 in inflammatory BCA | Decreased 5 year survival | [55] |
| CCR7 Mutations in basal-like breast cancer | Site-dependent reduction or promotion of disease progression or survival | [85] |
| CCR7 targeting | Promoted bone or skin metastases | [56,57] |
| Cell death | Anoikis inhibitor, TAK1 promotes CCR7 expression/tumor growth | [70,71,72] |
| EMT | TGF-β1 induced EMT | [75,76,77] |
| Endothelin (ET-1 activation) | May upregulate CCR7 expression via HIF-1; may promote invasion | [36] |
| Ets-1 | Promotes CCR7 expression in TBNC | [50] |
| CCR7 expressed by spindle-shaped stromal cells | Not associated with patient survival | [59] |
| Luminal A Breast cancer | Does not promote CCL21 chemotaxis | [51] |
| Luminal B and TNBC breast cancer | High levels of CCR7 promotes Notch-linked tumor formation | [33,52,53,78] |
| miR-let-7a | In patient cells and cell lines prevents chemotaxis and invasion | [83] |
| Prostaglandin E2 (PGE2)/EP2 and EP4 | PGE2 via COX-2 leads to AKT-mediated phosphorylation of SP1; SP1 binds CCR7 promoter to increase levels. | [44,48] |
| Cancer | Observation | Reference |
|---|---|---|
| Bladder | CCR7 not associated with aggressive form | [86] |
| miR-199a-5p represses CCR7 expression in normal tissues and it is reduced in bladder cancer; reduction correlates with increased MMP-9 | [88] | |
| RRBP1 knockdown increased CCR7 | [89] | |
| Gynecologic | Cervical squamous cell | [93] |
| Cervical squamous cell—elevated CCL19 blocked apoptosis | [94] | |
| Ovarian cancer—CCR7 reduced survival (cytoplasmic or nuclear) | [93] | |
| Ovarian cancer epithelial cells, SKOV-3, revealed elevated CCR7 in hypoxia; CCL21 promoted EMT/invasion | [95] | |
| CrkL induced by CCL19/CCR7 correlates with higher-stage, lymph node metastasis and reduced overall survival; CrkL knockdown attenuates EMT in SKOV-3 cells | [100] | |
| Differential gene analysis of squamous cell carcinoma demonstrated CCR7 in combination with PD-1, ZAP-70 and CD28 led to improved immune-mediated 5 year overall survival | [97,98,99] | |
| Prostate | Strong lymph node staining for CCR7 may correlate with LN metastases | [101,102] |
| CCR7 siRNA in PC-3 cells inhibits VEGF and MMP along with tumor size in xenograft | [103] | |
| Exogenous expression of CCR7 in PC-3 increased Notch1, pMAPK, pp65, MMP-9, N-cadherin and Snail (EMT) to enhance migration/invasion | [104] | |
| Pro-inflammatory cytokine TNF-α induced CCR7 in cell lines, which induced p38 MAPK phosphorylation. | [105] |
| Cancer | Observation | Reference |
|---|---|---|
| Colorectal Cancer (CC) | CCR7 expression elevated in ulcerative colitis (UC). 5 year survival reduced by CCR7. siRNA knockdown of CCR7 in SW620 human CC cells injected into athymic nude Balb/c mice reduced invasion and LN metastases. | [109] |
| Variable CCR7 expression in colorectal cancer. | [112] | |
| CCR7 elevated in tumors of the rectum. | [112] | |
| Truncated/nonfunctional mutants of CCR7. | [113] | |
| Truncated CCR7/reduced CCL21. | [115,116] | |
| Increased CCL19 correlated with increased survival. | [117] | |
| CCR7 correlates with increased MMP-9 in human SW480 cells. shRNA knockdown of CCR7 reduced metastasis and increased survival. | [119] | |
| CCR7 upregulated by COX-2. | [120] | |
| CCR7 had no effect on patient survival; however, co-expression of CCL21 receptor, CXCR3 correlated with metastases. | [122] | |
| Co-expression of CCR7 with EGFR generated cetuximab-resistant EGFR; this was reduced by expression of CCL21. | [123] | |
| Esophageal | Expressed in 45% of esophageal squamous cell carcinoma; activated by CCL21. Expression correlates with decreased survival. | [124] |
| LN metastasis, but not primary tumor expressed high levels of CCR7. | [125] | |
| Co-expression of CCR7/VEGF-C mRNAs worsened prognosis/survival compared to non-expressors. | [126] | |
| Co-expression of CCR7/MUC1 correlates with poor prognosis. MUC1 inhibition blocks CCL21-induced invasion. | [127] | |
| Murine model CCL21/CCR7 led to metastases. | [128] | |
| Gastric | ~84% or patient samples express CCR7 (RT-PCR) but only 23% stain CCR7 positive with anti-CCR7. | [130] |
| ~65% of patient samples expressed CCR7 which correlated with LN metastases (RT-PCR and IHC). In vitro gastric carcinoma cell lines (66%) migrated to CCL21. | [131] | |
| ~30% of patient samples expressed CCR7. | [132] | |
| ~70% of patient samples expressed CCR7, which correlated with LN metastases. | [133] | |
| H. pylori upregulated CCR7 in gastric epithelium. | [135] | |
| Transformation of H. pylori-linked gastritis to MALT lymphoma or B-cell lymphoma which expressed CCR7. | [136] | |
| VEGF-C/CCR7 co-expression in ~50% of cancers. | [137] | |
| VEGF-C, VEGF-D or CCR7 predicted lymphatic invasion of gastric tumor. | [138] | |
| HIF-1α upregulates CCR7/increases COX-2 and MMPs. | [140] | |
| Gastric cancer mediated by CCR7 by inducing EMT via TGF-β1. | [141] | |
| CCR7 correlates with Snail, which represses E-cadherin to promote EMT. | [142] | |
| Meta-analysis indicates that CCR7 significantly increases risk of lower 5 year overall survival rate for CCR7+ vs. CCR7- gastric cancers (HR = 0.46, 95% CI 0.31–0.70. p < 0.001). | [143] | |
| Pancreatic | CCR7 regulates Twist to promote EMT. | [148] |
| CCR7 levels are high, and ligand CCL21 is low in pancreatic cancer tissues compared to normal pancreas. | [145] | |
| CCR7 induced Twist in 72% of patient samples. May mediate EMT, tumor progression and LN metastasis. | [148] | |
| CCR7/CCL19 in PANC1 cells induced pERK, pAKT, N-caherin and MMP-9 (EMT). | [148] | |
| CCR7 elevated in CD133+ pancreatic cells via the ERK/NF-κB pathway. | [149] | |
| PANC1 cells induced MMP-9, ATM, and BRCA1 but downregulated CASP8 when stimulated with CCL21. | [150] | |
| CCL21 downregulates AKT1, FOS and JUN. | [150] | |
| CCL21 promoted sensitivity to pain. | [151] |
| Cancer | Observation | Reference |
|---|---|---|
| Oral | ~65% were positive for CCR7/correlated with tumor progression, large lymph node metastases and reduced survival. | [154,155] |
| B7E3 oral squamous cell carcinoma in mice had higher growth rate + CCL19/CCL21-ser than in plt mice lacking these ligands. | [157] | |
| No effect of CCR7, CCL19 and CCL21 mRNAs which are expressed in squamous cell carcinoma and normal oral mucosa. | [158] | |
| CCR7 activation in tongue squamous cell carcinoma cell line SCC4 led to more aggressive cancer, while inhibition reduced migration/invasion. No effect on cell growth. | [159] | |
| CCR7,VEGF-C and VEGFR-3 correlated with lymph node metastases in tongue cancer. | [160] | |
| CCR7 expressed at higher levels in male tongue cancer patients than females/no effect on prognosis. | [160] | |
| CCR7 is a biomarker of poor prognosis and shorter disease-free survival in tongue cancer. | [161] | |
| UCA long non-coding RNA is co-upregulated in conjunction with CCR7 in tongue squamous carcinoma cells—Correlates with increased proliferation, migration and glycolysis. | [162,163] | |
| Upregulation of CCR7 in squamous carcinoma of the tonsils led to poor prognosis. | [164] | |
| Non-oral | High levels of CCR7 mRNA correlate with poorly differentiated tumors and lymph node metastases. | [167] |
| CCR7 promotes progression. | [168,169] | |
| Twist expression leading to EMT is linked to CCR7 expression. | [170] | |
| Epithelial nasopharyngeal patient samples had heterogenous expression of CCR7 (CXCR4,CXCR6) in liver metastases. | [172] | |
| Nasopharyngeal cancer-high levels of CCR7 correlate with poor prognosis. | [173] | |
| CCR7 does not appear to contribute to lung metastases. | [174] | |
| CCR7 significantly increases migration in PCI-37B cell line (HNCC) and lymph node metastasis via integrin αvβ3 (p < 0.05). Effect mediated by Src. | [166,175,177] | |
| β-defensins induce CCR7 expression via NF-kB promote cell survival and migration. | [184,185] | |
| Autologous CCR7 stimulation of HNCC by CCL19 induced mTOR activation, via PI3K and JAK2/STAT3-mediated migration via activation of Cdc42. | [180,181,183] | |
| Autologous CCR7 stimulation of HNCC activates ERK1/2 and JNK phosphorylation and EMT. | [182] | |
| Thyroid | CCR7 expression is 9-fold higher in papillary thyroid cancer and medullary thyroid cancer compared to follicular and poorly differentiated tumors. | [199] |
| CCL21 activation of CCR7 in TPC-1 induced proliferation, migration, MMP-2, MMP-9 and increased β1-integrin expression. | [199] | |
| IHC staining of 65 patients—angiolymphatic invasive or tumors with LN metastasis had elevated CCR7 when compared to non-invasive tumors. | [200] | |
| Only 5–10% of cells express CCR7 in 30 patient samples. | [201] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bill, C.A.; Allen, C.M.; Vines, C.M. C-C Chemokine Receptor 7 in Cancer. Cells 2022, 11, 656. https://doi.org/10.3390/cells11040656
Bill CA, Allen CM, Vines CM. C-C Chemokine Receptor 7 in Cancer. Cells. 2022; 11(4):656. https://doi.org/10.3390/cells11040656
Chicago/Turabian StyleBill, Colin A., Christopher M. Allen, and Charlotte M. Vines. 2022. "C-C Chemokine Receptor 7 in Cancer" Cells 11, no. 4: 656. https://doi.org/10.3390/cells11040656
APA StyleBill, C. A., Allen, C. M., & Vines, C. M. (2022). C-C Chemokine Receptor 7 in Cancer. Cells, 11(4), 656. https://doi.org/10.3390/cells11040656








