Early Life Stress, Brain Development, and Obesity Risk: Is Oxytocin the Missing Link?
Abstract
:1. Obesity: Epidemiology, Etiopathophysiology, and Early Development
2. Early Life Stress
3. Impact of ELS on Metabolic Health and Eating Behavior: Evidence from Rodent Studies
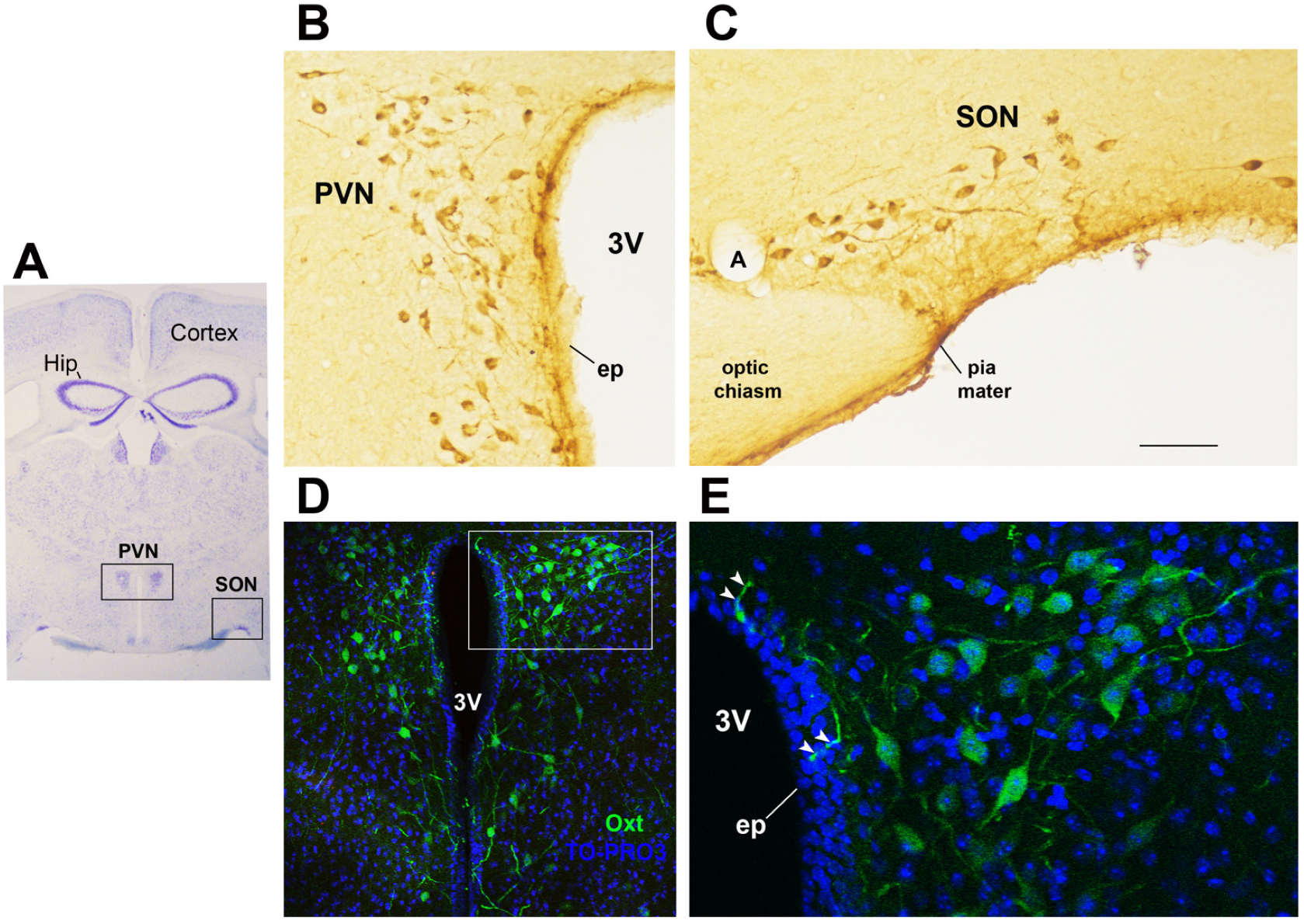
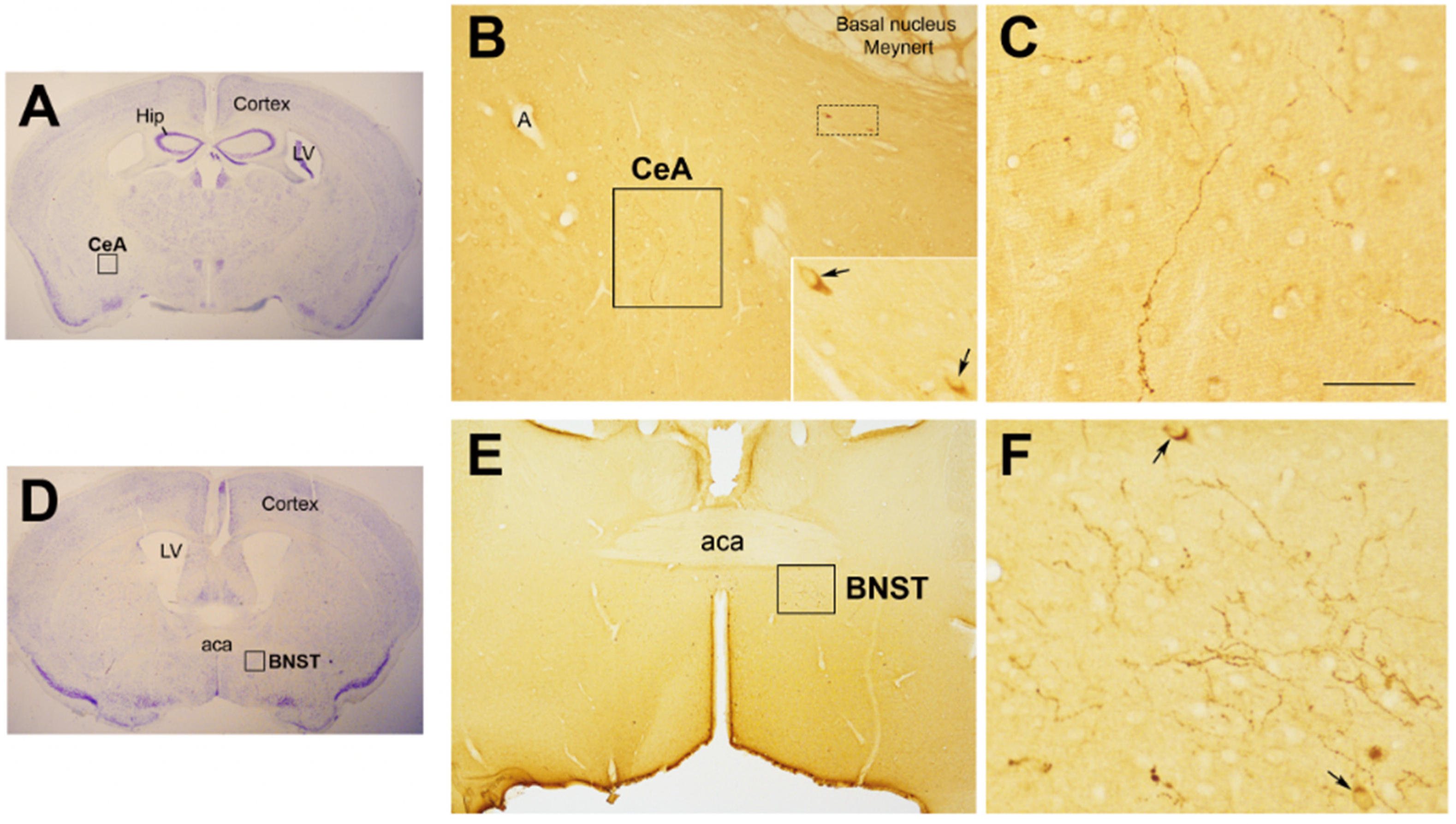
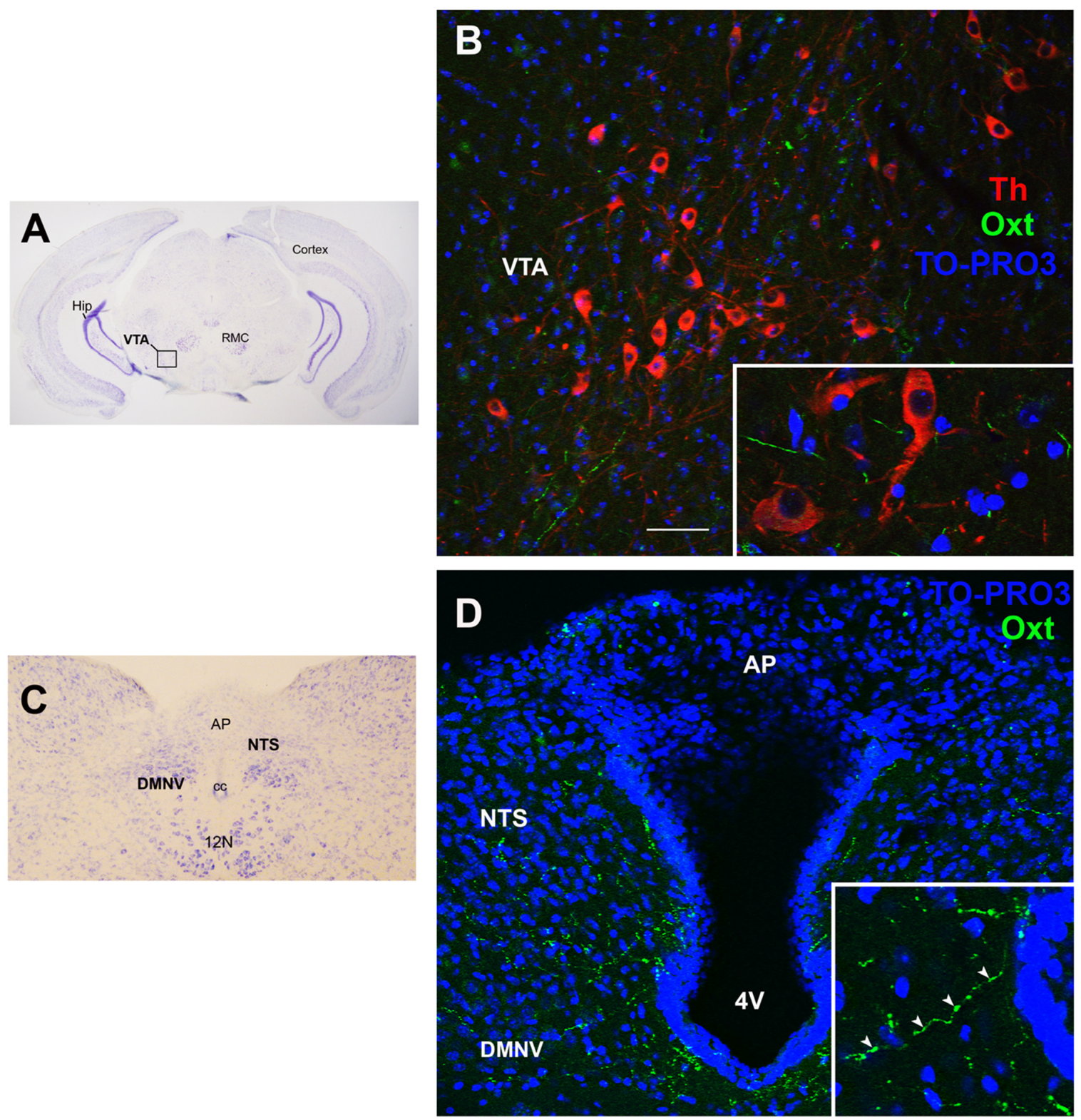
4. Oxytocin: The Neuroendocrine Hub of Social Bonding, Stress, Eating Behavior, and Metabolic Health
5. Oxytocin System: Early Development and Impact of ELS
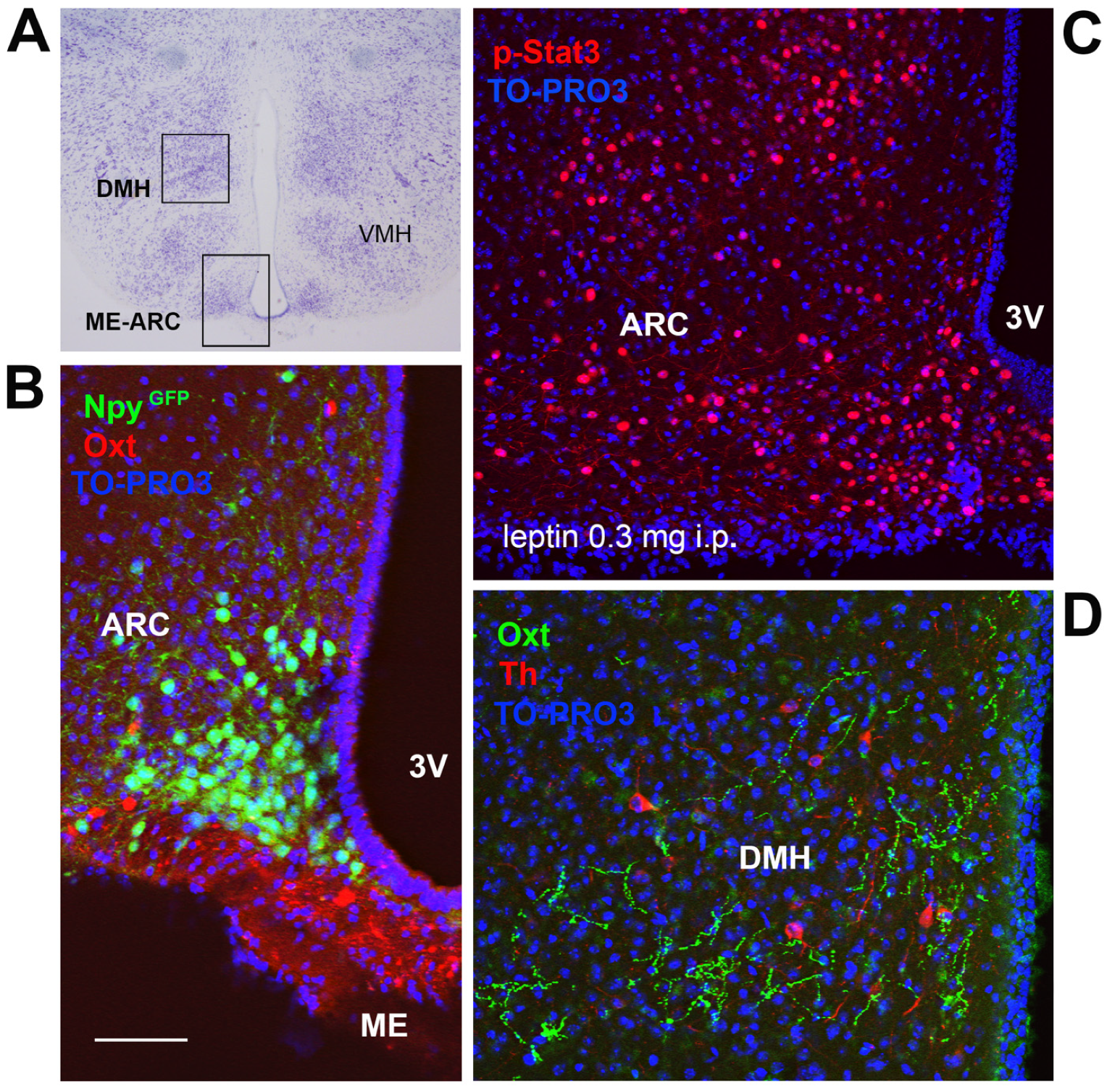
6. Leptin, Brain Development, and Early Life Stress: Beyond Energy Homeostasis Regulation
7. Oxytocin, Eating Behavior, and Metabolic Health
8. ELS, Oxytocin, Eating Behavior, and Metabolic Health: Future Research Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bluher, M. Obesity: Global epidemiology and pathogenesis. Nat. Rev. Endocrinol. 2019, 15, 288–298. [Google Scholar] [CrossRef] [PubMed]
- Ward, Z.J.; Bleich, S.N.; Cradock, A.L.; Barrett, J.L.; Giles, C.M.; Flax, C.; Long, M.W.; Gortmaker, S.L. Projected U.S. State-Level Prevalence of Adult Obesity and Severe Obesity. N. Engl. J. Med. 2019, 381, 2440–2450. [Google Scholar] [CrossRef] [PubMed]
- EASO. Eauropean Association for the Study of Obesity. Obesity Statistics. 2020. Available online: https://www.karger.com/Article/FullText/508082 (accessed on 24 January 2022).
- WHO. WHO European Childhood Obesity Surveillance Initiative (COSI). Available online: https://www.euro.who.int/en/health-topics/disease-prevention/nutrition/activities/who-european-childhood-obesity-surveillance-initiative-cosi (accessed on 24 January 2022).
- Geserick, M.; Vogel, M.; Gausche, R.; Lipek, T.; Spielau, U.; Keller, E.; Pfaffle, R.; Kiess, W.; Korner, A. Acceleration of BMI in Early Childhood and Risk of Sustained Obesity. N. Engl. J. Med. 2018, 379, 1303–1312. [Google Scholar] [CrossRef] [PubMed]
- WHO. Obesity Report. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 25 January 2021).
- Bray, G.A.; Kim, K.K.; Wilding, J.P.H.; World Obesity, F. Obesity: A chronic relapsing progressive disease process. A position statement of the World Obesity Federation. Obes. Rev. 2017, 18, 715–723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colleluori, G.; Perugini, J.; Barbatelli, G.; Cinti, S. Mammary gland adipocytes in lactation cycle, obesity and breast cancer. Rev. Endocr. Metab. Disord. 2021, 22, 241–255. [Google Scholar] [CrossRef]
- US Government Office for Science. Tackling Obesities: Future Choices—Project Report. 2007. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/287937/07-1184x-tackling-obesities-future-choices-report.pdf (accessed on 7 February 2022).
- Batsis, J.A.; Villareal, D.T. Sarcopenic obesity in older adults: Aetiology, epidemiology and treatment strategies. Nat. Rev. Endocrinol. 2018, 14, 513–537. [Google Scholar] [CrossRef]
- Severi, I.; Fosca, M.; Colleluori, G.; Marini, F.; Imperatori, L.; Senzacqua, M.; Di Vincenzo, A.; Barbatelli, G.; Fiori, F.; Rau, J.V.; et al. High-Fat Diet Impairs Mouse Median Eminence: A Study by Transmission and Scanning Electron Microscopy Coupled with Raman Spectroscopy. Int. J. Mol. Sci. 2021, 22, 8049. [Google Scholar] [CrossRef]
- Colleluori, G.; Villareal, D.T. Aging, obesity, sarcopenia and the effect of diet and exercise intervention. Exp. Gerontol. 2021, 155, 111561. [Google Scholar] [CrossRef]
- Giordano, A.; Murano, I.; Mondini, E.; Perugini, J.; Smorlesi, A.; Severi, I.; Barazzoni, R.; Scherer, P.E.; Cinti, S. Obese adipocytes show ultrastructural features of stressed cells and die of pyroptosis. J. Lipid Res. 2013, 54, 2423–2436. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Ballantyne, C.M. Skeletal muscle inflammation and insulin resistance in obesity. J. Clin. Investig. 2017, 127, 43–54. [Google Scholar] [CrossRef]
- Lee, J.S.; Kim, S.H.; Jun, D.W.; Han, J.H.; Jang, E.C.; Park, J.Y.; Son, B.K.; Kim, S.H.; Jo, Y.J.; Park, Y.S.; et al. Clinical implications of fatty pancreas: Correlations between fatty pancreas and metabolic syndrome. World J. Gastroenterol. 2009, 15, 1869–1875. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, G.; Zhang, H.; Karin, M.; Bai, H.; Cai, D. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell 2008, 135, 61–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giordano, A.; Nisoli, E. Neuroendocrinology of Energy Balance. Obesity, Endocrinology. 2018. Available online: https://link.springer.com/referenceworkentry/10.1007/978-3-319-47685-8_4-1?noAccess=true (accessed on 10 February 2022). [CrossRef]
- Leng, G.; Adan, R.A.H.; Belot, M.; Brunstrom, J.M.; de Graaf, K.; Dickson, S.L.; Hare, T.; Maier, S.; Menzies, J.; Preissl, H.; et al. The determinants of food choice. Proc. Nutr. Soc. 2017, 76, 316–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andermann, M.L.; Lowell, B.B. Toward a Wiring Diagram Understanding of Appetite Control. Neuron 2017, 95, 757–778. [Google Scholar] [CrossRef] [PubMed]
- Myers, M.G., Jr.; Affinati, A.H.; Richardson, N.; Schwartz, M.W. Central nervous system regulation of organismal energy and glucose homeostasis. Nat. Metab. 2021, 3, 737–750. [Google Scholar] [CrossRef]
- Leng, G. The Heart of the Brain: The Hypothalamus and Its Hormones; The MIT Press: Cambridge, MA, USA, 2018. [Google Scholar]
- Waterson, M.J.; Horvath, T.L. Neuronal Regulation of Energy Homeostasis: Beyond the Hypothalamus and Feeding. Cell Metab. 2015, 22, 962–970. [Google Scholar] [CrossRef] [Green Version]
- Locke, A.E.; Kahali, B.; Berndt, S.I.; Justice, A.E.; Pers, T.H.; Day, F.R.; Powell, C.; Vedantam, S.; Buchkovich, M.L.; Yang, J.; et al. Genetic studies of body mass index yield new insights for obesity biology. Nature 2015, 518, 197–206. [Google Scholar] [CrossRef] [Green Version]
- Akbari, P.; Gilani, A.; Sosina, O.; Kosmicki, J.A.; Khrimian, L.; Fang, Y.Y.; Persaud, T.; Garcia, V.; Sun, D.; Li, A.; et al. Sequencing of 640,000 exomes identifies GPR75 variants associated with protection from obesity. Science 2021, 373, 8683. [Google Scholar] [CrossRef]
- Moreno-Mendez, E.; Quintero-Fabian, S.; Fernandez-Mejia, C.; Lazo-de-la-Vega-Monroy, M. Early-life programming of adipose tissue. Nutr. Res. Rev. 2020, 33, 244–259. [Google Scholar] [CrossRef]
- Miller, G.E.; Chen, E.; Parker, K.J. Psychological stress in childhood and susceptibility to the chronic diseases of aging: Moving toward a model of behavioral and biological mechanisms. Psychol. Bull. 2011, 137, 959–997. [Google Scholar] [CrossRef]
- Miller, A.L.; Lumeng, J.C. Pathways of Association from Stress to Obesity in Early Childhood. Obesity 2018, 26, 1117–1124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carter, C.S. The Role of Oxytocin and Vasopressin in Attachment. Psychodyn. Psychiatry 2017, 45, 499–517. [Google Scholar] [CrossRef] [PubMed]
- Lawson, E.A.; Olszewski, P.K.; Weller, A.; Blevins, J.E. The role of oxytocin in regulation of appetitive behaviour, body weight and glucose homeostasis. J. Neuroendocrinol. 2020, 32, e12805. [Google Scholar] [CrossRef] [PubMed]
- Baracz, S.J.; Everett, N.A.; Cornish, J.L. The impact of early life stress on the central oxytocin system and susceptibility for drug addiction: Applicability of oxytocin as a pharmacotherapy. Neurosci. Biobehav. Rev. 2020, 110, 114–132. [Google Scholar] [CrossRef]
- Bowlby, J. Attachment and Loss, 2nd ed.; Basic Books: New York, NY, USA, 1969. [Google Scholar]
- Winnicott, D.W. Mother and Child: A Primer of First Relationships; Basic Books: New York, NY, USA, 1957. [Google Scholar]
- Nelson, C.A., 3rd; Gabard-Durnam, L.J. Early Adversity and Critical Periods: Neurodevelopmental Consequences of Violating the Expectable Environment. Trends Neurosci. 2020, 43, 133–143. [Google Scholar] [CrossRef]
- Rice, C.J.; Sandman, C.A.; Lenjavi, M.R.; Baram, T.Z. A novel mouse model for acute and long-lasting consequences of early life stress. Endocrinology 2008, 149, 4892–4900. [Google Scholar] [CrossRef] [Green Version]
- Molet, J.; Maras, P.M.; Avishai-Eliner, S.; Baram, T.Z. Naturalistic rodent models of chronic early-life stress. Dev. Psychobiol. 2014, 56, 1675–1688. [Google Scholar] [CrossRef] [Green Version]
- Curley, J.P.; Champagne, F.A. Influence of maternal care on the developing brain: Mechanisms, temporal dynamics and sensitive periods. Front. Neuroendocrinol. 2016, 40, 52–66. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Caceres, C.; Balland, E.; Prevot, V.; Luquet, S.; Woods, S.C.; Koch, M.; Horvath, T.L.; Yi, C.X.; Chowen, J.A.; Verkhratsky, A.; et al. Role of astrocytes, microglia, and tanycytes in brain control of systemic metabolism. Nat. Neurosci. 2019, 22, 7–14. [Google Scholar] [CrossRef] [Green Version]
- Abbink, M.R.; van Deijk, A.F.; Heine, V.M.; Verheijen, M.H.; Korosi, A. The involvement of astrocytes in early-life adversity induced programming of the brain. Glia 2019, 67, 1637–1653. [Google Scholar] [CrossRef]
- Ruiz, R.; Roque, A.; Pineda, E.; Licona-Limon, P.; Jose Valdez-Alarcon, J.; Lajud, N. Early life stress accelerates age-induced effects on neurogenesis, depression, and metabolic risk. Psychoneuroendocrinology 2018, 96, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Goodwill, H.L.; Manzano-Nieves, G.; Gallo, M.; Lee, H.I.; Oyerinde, E.; Serre, T.; Bath, K.G. Early life stress leads to sex differences in development of depressive-like outcomes in a mouse model. Neuropsychopharmacology 2019, 44, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Novick, A.M.; Levandowski, M.L.; Laumann, L.E.; Philip, N.S.; Price, L.H.; Tyrka, A.R. The effects of early life stress on reward processing. J. Psychiatr. Res. 2018, 101, 80–103. [Google Scholar] [CrossRef] [PubMed]
- Paternain, L.; Martisova, E.; Milagro, F.I.; Ramirez, M.J.; Martinez, J.A.; Campion, J. Postnatal maternal separation modifies the response to an obesogenic diet in adulthood in rats. Dis. Model Mech. 2012, 5, 691–697. [Google Scholar] [CrossRef] [Green Version]
- Maniam, J.; Antoniadis, C.P.; Wang, K.W.; Morris, M.J. Early Life Stress Induced by Limited Nesting Material Produces Metabolic Resilience in Response to a High-Fat and High-Sugar Diet in Male Rats. Front. Endocrinol. 2015, 6, 138. [Google Scholar] [CrossRef] [Green Version]
- Yam, K.Y.; Naninck, E.F.G.; Abbink, M.R.; la Fleur, S.E.; Schipper, L.; van den Beukel, J.C.; Grefhorst, A.; Oosting, A.; van der Beek, E.M.; Lucassen, P.J.; et al. Exposure to chronic early-life stress lastingly alters the adipose tissue, the leptin system and changes the vulnerability to western-style diet later in life in mice. Psychoneuroendocrinology 2017, 77, 186–195. [Google Scholar] [CrossRef]
- Ryu, V.; Yoo, S.B.; Kang, D.W.; Lee, J.H.; Jahng, J.W. Post-weaning isolation promotes food intake and body weight gain in rats that experienced neonatal maternal separation. Brain Res. 2009, 1295, 127–134. [Google Scholar] [CrossRef]
- Eller, O.C.; Morris, E.M.; Thyfault, J.P.; Christianson, J.A. Early life stress reduces voluntary exercise and its prevention of diet-induced obesity and metabolic dysfunction in mice. Physiol. Behav. 2020, 223, 113000. [Google Scholar] [CrossRef]
- de Lima, R.M.S.; Dos Santos Bento, L.V.; di Marcello Valladao Lugon, M.; Barauna, V.G.; Bittencourt, A.S.; Dalmaz, C.; de Vasconcellos Bittencourt, A.P.S. Early life stress and the programming of eating behavior and anxiety: Sex-specific relationships with serotonergic activity and hypothalamic neuropeptides. Behav. Brain Res. 2020, 379, 112399. [Google Scholar] [CrossRef]
- Yam, K.Y.; Naninck, E.F.; Schmidt, M.V.; Lucassen, P.J.; Korosi, A. Early-life adversity programs emotional functions and the neuroendocrine stress system: The contribution of nutrition, metabolic hormones and epigenetic mechanisms. Stress 2015, 18, 328–342. [Google Scholar] [CrossRef]
- Thomas, A.W.; Delevich, K.; Chang, I.; Wilbrecht, L. Variation in early life maternal care predicts later long range frontal cortex synapse development in mice. Dev. Cogn. Neurosci. 2020, 41, 100737. [Google Scholar] [CrossRef] [PubMed]
- Pena, C.J.; Kronman, H.G.; Walker, D.M.; Cates, H.M.; Bagot, R.C.; Purushothaman, I.; Issler, O.; Loh, Y.E.; Leong, T.; Kiraly, D.D.; et al. Early life stress confers lifelong stress susceptibility in mice via ventral tegmental area OTX2. Science 2017, 356, 1185–1188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pena, C.J.; Smith, M.; Ramakrishnan, A.; Cates, H.M.; Bagot, R.C.; Kronman, H.G.; Patel, B.; Chang, A.B.; Purushothaman, I.; Dudley, J.; et al. Early life stress alters transcriptomic patterning across reward circuitry in male and female mice. Nat. Commun. 2019, 10, 5098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, O.H.; Herald, J.B.; Leachman, J.; Tezanos, A.V.; Cohn, D.M.; Loria, A.S. A model of neglect during postnatal life heightens obesity-induced hypertension and is linked to a greater metabolic compromise in female mice. Int. J. Obes. 2018, 42, 1354–1365. [Google Scholar] [CrossRef] [PubMed]
- Parel, S.T.; Pena, C.J. Genome-wide Signatures of Early-Life Stress: Influence of Sex. Biol. Psychiatry 2022, 91, 36–42. [Google Scholar] [CrossRef]
- Demaestri, C.; Pan, T.; Critz, M.; Ofray, D.; Gallo, M.; Bath, K.G. Type of early life adversity confers differential, sex-dependent effects on early maturational milestones in mice. Horm. Behav. 2020, 124, 104763. [Google Scholar] [CrossRef]
- Ross, R.; Neeland, I.J.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; Cuevas, A.; Hu, F.B.; et al. Waist circumference as a vital sign in clinical practice: A Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat. Rev. Endocrinol. 2020, 16, 177–189. [Google Scholar] [CrossRef]
- Aguirre, L.E.; Colleluori, G.; Fowler, K.E.; Jan, I.Z.; Villareal, K.; Qualls, C.; Robbins, D.; Villareal, D.T.; Armamento-Villareal, R. High aromatase activity in hypogonadal men is associated with higher spine bone mineral density, increased truncal fat and reduced lean mass. Eur. J. Endocrinol. 2015, 173, 167–174. [Google Scholar] [CrossRef] [Green Version]
- Colleluori, G.; Aguirre, L.E.; Qualls, C.; Chen, R.; Napoli, N.; Villareal, D.T.; Armamento-Villareal, R. Adipocytes ESR1 Expression, Body Fat and Response to Testosterone Therapy in Hypogonadal Men Vary According to Estradiol Levels. Nutrients 2018, 10, 1226. [Google Scholar] [CrossRef] [Green Version]
- Colleluori, G.; Chen, R.; Napoli, N.; Aguirre, L.E.; Qualls, C.; Villareal, D.T.; Armamento-Villareal, R. Fat Mass Follows a U-Shaped Distribution Based on Estradiol Levels in Postmenopausal Women. Front. Endocrinol. 2018, 9, 315. [Google Scholar] [CrossRef]
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020, 584, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Jurek, B.; Neumann, I.D. The Oxytocin Receptor: From Intracellular Signaling to Behavior. Physiol. Rev. 2018, 98, 1805–1908. [Google Scholar] [CrossRef] [PubMed]
- Ohlsson, B.; Truedsson, M.; Djerf, P.; Sundler, F. Oxytocin is expressed throughout the human gastrointestinal tract. Regul. Pept. 2006, 135, 7–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monstein, H.J.; Grahn, N.; Truedsson, M.; Ohlsson, B. Oxytocin and oxytocin-receptor mRNA expression in the human gastrointestinal tract: A polymerase chain reaction study. Regul. Pept. 2004, 119, 39–44. [Google Scholar] [CrossRef]
- Welch, M.G.; Margolis, K.G.; Li, Z.; Gershon, M.D. Oxytocin regulates gastrointestinal motility, inflammation, macromolecular permeability, and mucosal maintenance in mice. Am. J. Physiol. Gastrointest. Liver Physiol. 2014, 307, G848–G862. [Google Scholar] [CrossRef] [Green Version]
- Lawson, E.A. The effects of oxytocin on eating behaviour and metabolism in humans. Nat. Rev. Endocrinol. 2017, 13, 700–709. [Google Scholar] [CrossRef]
- Chini, B.; Verhage, M.; Grinevich, V. The Action Radius of Oxytocin Release in the Mammalian CNS: From Single Vesicles to Behavior. Trends Pharmacol. Sci. 2017, 38, 982–991. [Google Scholar] [CrossRef]
- Leng, G.; Caquineau, C.; Sabatier, N. Regulation of oxytocin secretion. Vitam. Horm. 2005, 71, 27–58. [Google Scholar] [CrossRef]
- Haussler, H.U.; Jirikowski, G.F.; Caldwell, J.D. Sex differences among oxytocin-immunoreactive neuronal systems in the mouse hypothalamus. J. Chem. Neuroanat. 1990, 3, 271–276. [Google Scholar]
- Liao, P.Y.; Chiu, Y.M.; Yu, J.H.; Chen, S.K. Mapping Central Projection of Oxytocin Neurons in Unmated Mice Using Cre and Alkaline Phosphatase Reporter. Front. Neuroanat. 2020, 14, 559402. [Google Scholar] [CrossRef]
- Francis, D.D.; Young, L.J.; Meaney, M.J.; Insel, T.R. Naturally occurring differences in maternal care are associated with the expression of oxytocin and vasopressin (V1a) receptors: Gender differences. J. Neuroendocrinol. 2002, 14, 349–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamamoto, Y.; Cushing, B.S.; Kramer, K.M.; Epperson, P.D.; Hoffman, G.E.; Carter, C.S. Neonatal manipulations of oxytocin alter expression of oxytocin and vasopressin immunoreactive cells in the paraventricular nucleus of the hypothalamus in a gender-specific manner. Neuroscience 2004, 125, 947–955. [Google Scholar] [CrossRef] [PubMed]
- Veenema, A.H.; Bredewold, R.; Neumann, I.D. Opposite effects of maternal separation on intermale and maternal aggression in C57BL/6 mice: Link to hypothalamic vasopressin and oxytocin immunoreactivity. Psychoneuroendocrinology 2007, 32, 437–450. [Google Scholar] [CrossRef] [PubMed]
- Feldman, R. The Neurobiology of Human Attachments. Trends Cogn. Sci. 2017, 21, 80–99. [Google Scholar] [CrossRef]
- Kim, S.; Kwok, S.; Mayes, L.C.; Potenza, M.N.; Rutherford, H.J.V.; Strathearn, L. Early adverse experience and substance addiction: Dopamine, oxytocin, and glucocorticoid pathways. Ann. N. Y. Acad. Sci. 2017, 1394, 74–91. [Google Scholar] [CrossRef] [Green Version]
- Ding, C.; Leow, M.K.; Magkos, F. Oxytocin in metabolic homeostasis: Implications for obesity and diabetes management. Obes. Rev. 2019, 20, 22–40. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Wu, C.; Chen, Q.; Chen, X.; Xu, Z.; Wu, J.; Cai, D. Treatment of obesity and diabetes using oxytocin or analogs in patients and mouse models. PLoS ONE 2013, 8, e61477. [Google Scholar] [CrossRef] [Green Version]
- McCormack, S. Intranasal Oxytocin to Promote Weight Loss in Children, Adolescents, and Adults with Brain Tumors and Hypothalamic Obesity Syndrome. Recruiting. Available online: https://clinicaltrials.gov/ct2/show/NCT02849743 (accessed on 24 January 2022).
- Lawson, E.A. A Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Effects of Repeat Doses of Intranasal Oxytocin in Obese Adults. Completed. Available online: https://clinicaltrials.gov/ct2/show/NCT03043053 (accessed on 24 January 2022).
- Espinoza, S. The Physiologic Effects of Intranasal Oxytocin on Sarcopenic Obesity (INOSO). Completed. Available online: https://clinicaltrials.gov/ct2/show/NCT03119610 (accessed on 24 January 2022).
- Francis, D.D.; Champagne, F.C.; Meaney, M.J. Variations in maternal behaviour are associated with differences in oxytocin receptor levels in the rat. J. Neuroendocrinol. 2000, 12, 1145–1148. [Google Scholar] [CrossRef]
- Tsuda, M.C.; Yamaguchi, N.; Ogawa, S. Early life stress disrupts peripubertal development of aggression in male mice. Neuroreport 2011, 22, 259–263. [Google Scholar] [CrossRef]
- Wei, F.; Li, W.; Ma, B.; Deng, X.; Zhang, L.; Zhao, L.; Zheng, T.; Jing, Y. Experiences affect social behaviors via altering neuronal morphology and oxytocin system. Psychoneuroendocrinology 2021, 129, 105247. [Google Scholar] [CrossRef]
- Ellis, B.J.; Horn, A.J.; Carter, C.S.; van IJzendoorn, M.H.; Bakermans-Kranenburg, M.J. Developmental programming of oxytocin through variation in early-life stress: Four meta-analyses and a theoretical reinterpretation. Clin. Psychol. Rev. 2021, 86, 101985. [Google Scholar] [CrossRef] [PubMed]
- Krause, S.; Boeck, C.; Gumpp, A.M.; Rottler, E.; Schury, K.; Karabatsiakis, A.; Buchheim, A.; Gundel, H.; Kolassa, I.T.; Waller, C. Child Maltreatment Is Associated with a Reduction of the Oxytocin Receptor in Peripheral Blood Mononuclear Cells. Front. Psychol. 2018, 9, 173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frontini, A.B.P.; Tonello, C.; Valerio, A.; Nisoli, E.; Cinti, A.; Giordano, A. Leptin-dependent STAT3 phosphorylation in postnatal mouse hypothalamus. Brain Res. 2008, 1215, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Proenca, R.; Maffei, M.; Barone, M.; Leopold, L.; Friedman, J.M. Positional cloning of the mouse obese gene and its human homologue. Nature 1994, 372, 425–432. [Google Scholar] [CrossRef]
- Maffei, M.; Giordano, A. Leptin, the brain and energy homeostasis: From an apparently simple to a highly complex neuronal system. Rev. Endocr. Metab. Disord. 2021. Online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Caron, A.; Lee, S.; Elmquist, J.K.; Gautron, L. Leptin and brain-adipose crosstalks. Nat. Rev. Neurosci. 2018, 19, 153–165. [Google Scholar] [CrossRef]
- Bouret, S.G. Neurodevelopmental actions of leptin. Brain Res. 2010, 1350, 2–9. [Google Scholar] [CrossRef] [Green Version]
- Bereiter, D.A.; Jeanrenaud, B. Altered neuroanatomical organization in the central nervous system of the genetically obese (ob/ob) mouse. Brain Res. 1979, 165, 249–260. [Google Scholar] [CrossRef]
- Pinto, S.; Roseberry, A.G.; Liu, H.; Diano, S.; Shanabrough, M.; Cai, X.; Friedman, J.M.; Horvath, T.L. Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science 2004, 304, 110–115. [Google Scholar] [CrossRef]
- Steppan, C.M.; Swick, A.G. A role for leptin in brain development. Biochem. Biophys. Res. Commun. 1999, 256, 600–602. [Google Scholar] [CrossRef]
- Ahima, R.S.; Bjorbaek, C.; Osei, S.; Flier, J.S. Regulation of neuronal and glial proteins by leptin: Implications for brain development. Endocrinology 1999, 140, 2755–2762. [Google Scholar] [CrossRef] [PubMed]
- Bouret, S.G.; Draper, S.J.; Simerly, R.B. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science 2004, 304, 108–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramos-Lobo, A.M.; Teixeira, P.D.; Furigo, I.C.; Melo, H.M.; e Silva, N.D.M.L.; De Felice, F.G.; Donato, J., Jr. Long-term consequences of the absence of leptin signaling in early life. Elife 2019, 8, e40970. [Google Scholar] [CrossRef] [PubMed]
- Kamitakahara, A.; Bouyer, K.; Wang, C.H.; Simerly, R. A critical period for the trophic actions of leptin on AgRP neurons in the arcuate nucleus of the hypothalamus. J. Comp. Neurol. 2018, 526, 133–145. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Yoo, S.B.; Kim, J.Y.; Lee, J.Y.; Kim, B.T.; Park, K.; Jahng, J.W. Early life stress experience may blunt hypothalamic leptin signalling. J. Biosci. 2017, 42, 131–138. [Google Scholar] [CrossRef]
- Leachman, J.R.; Rea, M.D.; Cohn, D.M.; Xu, X.; Fondufe-Mittendorf, Y.N.; Loria, A.S. Exacerbated obesogenic response in female mice exposed to early life stress is linked to fat depot-specific upregulation of leptin protein expression. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E852–E862. [Google Scholar] [CrossRef]
- Bouillon-Minois, J.B.; Trousselard, M.; Thivel, D.; Benson, A.C.; Schmidt, J.; Moustafa, F.; Bouvier, D.; Dutheil, F. Leptin as a Biomarker of Stress: A Systematic Review and Meta-Analysis. Nutrients 2021, 13, 3350. [Google Scholar] [CrossRef]
- Perello, M.; Raingo, J. Leptin activates oxytocin neurons of the hypothalamic paraventricular nucleus in both control and diet-induced obese rodents. PLoS ONE 2013, 8, e59625. [Google Scholar] [CrossRef] [Green Version]
- Sun, L.; Lizneva, D.; Ji, Y.; Colaianni, G.; Hadelia, E.; Gumerova, A.; Ievleva, K.; Kuo, T.C.; Korkmaz, F.; Ryu, V.; et al. Oxytocin regulates body composition. Proc. Natl. Acad. Sci. USA 2019, 116, 26808–26815. [Google Scholar] [CrossRef]
- Takayanagi, Y.; Kasahara, Y.; Onaka, T.; Takahashi, N.; Kawada, T.; Nishimori, K. Oxytocin receptor-deficient mice developed late-onset obesity. Neuroreport 2008, 19, 951–955. [Google Scholar] [CrossRef]
- Kasahara, Y.; Takayanagi, Y.; Kawada, T.; Itoi, K.; Nishimori, K. Impaired thermoregulatory ability of oxytocin-deficient mice during cold-exposure. Biosci. Biotechnol. Biochem. 2007, 71, 3122–3126. [Google Scholar] [CrossRef] [PubMed]
- Billings, L.B.; Spero, J.A.; Vollmer, R.R.; Amico, J.A. Oxytocin null mice ingest enhanced amounts of sweet solutions during light and dark cycles and during repeated shaker stress. Behav. Brain Res. 2006, 171, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.M.; Hsu, T.M.; Suarez, A.N.; Subramanian, K.S.; Fatemi, R.A.; Cortella, A.M.; Noble, E.E.; Roitman, M.F.; Kanoski, S.E. Central oxytocin signaling inhibits food reward-motivated behaviors and VTA dopamine responses to food-predictive cues in male rats. Horm. Behav. 2020, 126, 104855. [Google Scholar] [CrossRef] [PubMed]
- Gajdosechova, L.; Krskova, K.; Segarra, A.B.; Spolcova, A.; Suski, M.; Olszanecki, R.; Zorad, S. Hypooxytocinaemia in obese Zucker rats relates to oxytocin degradation in liver and adipose tissue. J. Endocrinol. 2014, 220, 333–343. [Google Scholar] [CrossRef] [Green Version]
- Yi, K.J.; So, K.H.; Hata, Y.; Suzuki, Y.; Kato, D.; Watanabe, K.; Aso, H.; Kasahara, Y.; Nishimori, K.; Chen, C.; et al. The regulation of oxytocin receptor gene expression during adipogenesis. J. Neuroendocrinol. 2015, 27, 335–342. [Google Scholar] [CrossRef]
- Assinder, S.J.; Boumelhem, B.B. Oxytocin stimulates lipolysis, prostaglandin E2 synthesis, and leptin secretion in 3T3-L1 adipocytes. Mol. Cell Endocrinol. 2021, 534, 111381. [Google Scholar] [CrossRef]
- Maejima, Y.; Iwasaki, Y.; Yamahara, Y.; Kodaira, M.; Sedbazar, U.; Yada, T. Peripheral oxytocin treatment ameliorates obesity by reducing food intake and visceral fat mass. Aging 2011, 3, 1169–1177. [Google Scholar] [CrossRef]
- Labyb, M.; Chretien, C.; Caillon, A.; Rohner-Jeanrenaud, F.; Altirriba, J. Oxytocin Administration Alleviates Acute but Not Chronic Leptin Resistance of Diet-Induced Obese Mice. Int. J. Mol. Sci. 2018, 20, 88. [Google Scholar] [CrossRef] [Green Version]
- Beranger, G.E.; Pisani, D.F.; Castel, J.; Djedaini, M.; Battaglia, S.; Amiaud, J.; Boukhechba, F.; Ailhaud, G.; Michiels, J.F.; Heymann, D.; et al. Oxytocin reverses ovariectomy-induced osteopenia and body fat gain. Endocrinology 2014, 155, 1340–1352. [Google Scholar] [CrossRef] [Green Version]
- Elabd, C.; Basillais, A.; Beaupied, H.; Breuil, V.; Wagner, N.; Scheideler, M.; Zaragosi, L.E.; Massiera, F.; Lemichez, E.; Trajanoski, Z.; et al. Oxytocin controls differentiation of human mesenchymal stem cells and reverses osteoporosis. Stem Cells 2008, 26, 2399–2407. [Google Scholar] [CrossRef] [Green Version]
- Deblon, N.; Veyrat-Durebex, C.; Bourgoin, L.; Caillon, A.; Bussier, A.L.; Petrosino, S.; Piscitelli, F.; Legros, J.J.; Geenen, V.; Foti, M.; et al. Mechanisms of the anti-obesity effects of oxytocin in diet-induced obese rats. PLoS ONE 2011, 6, e25565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altirriba, J.; Poher, A.L.; Caillon, A.; Arsenijevic, D.; Veyrat-Durebex, C.; Lyautey, J.; Dulloo, A.; Rohner-Jeanrenaud, F. Divergent effects of oxytocin treatment of obese diabetic mice on adiposity and diabetes. Endocrinology 2014, 155, 4189–4201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blevins, J.E.; Schwartz, M.W.; Baskin, D.G. Evidence that paraventricular nucleus oxytocin neurons link hypothalamic leptin action to caudal brain stem nuclei controlling meal size. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 287, R87–R96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matarazzo, V.; Schaller, F.; Nedelec, E.; Benani, A.; Penicaud, L.; Muscatelli, F.; Moyse, E.; Bauer, S. Inactivation of Socs3 in the hypothalamus enhances the hindbrain response to endogenous satiety signals via oxytocin signaling. J. Neurosci. 2012, 32, 17097–17107. [Google Scholar] [CrossRef]
- Yuan, J.; Zhang, R.; Wu, R.; Gu, Y.; Lu, Y. The effects of oxytocin to rectify metabolic dysfunction in obese mice are associated with increased thermogenesis. Mol. Cell Endocrinol. 2020, 514, 110903. [Google Scholar] [CrossRef]
- Wu, Z.; Xu, Y.; Zhu, Y.; Sutton, A.K.; Zhao, R.; Lowell, B.B.; Olson, D.P.; Tong, Q. An obligate role of oxytocin neurons in diet induced energy expenditure. PLoS ONE 2012, 7, e45167. [Google Scholar] [CrossRef]
- Giordano, A.; Frontini, A.; Cinti, S. Convertible visceral fat as a therapeutic target to curb obesity. Nat. Rev. Drug Discov. 2016, 15, 405–424. [Google Scholar] [CrossRef]
- Wigger, D.C.; Groger, N.; Lesse, A.; Krause, S.; Merz, T.; Gundel, H.; Braun, K.; McCook, O.; Radermacher, P.; Bock, J.; et al. Maternal Separation Induces Long-Term Alterations in the Cardiac Oxytocin Receptor and Cystathionine gamma-Lyase Expression in Mice. Oxid. Med. Cell Longev. 2020, 2020, 4309605. [Google Scholar] [CrossRef] [Green Version]
- McCook, O.; Denoix, N.; Radermacher, P.; Waller, C.; Merz, T. H2S and Oxytocin Systems in Early Life Stress and Cardiovascular Disease. J. Clin. Med. 2021, 10, 3484. [Google Scholar] [CrossRef]
- Szczepanska-Sadowska, E.; Wsol, A.; Cudnoch-Jedrzejewska, A.; Zera, T. Complementary Role of Oxytocin and Vasopressin in Cardiovascular Regulation. Int. J. Mol. Sci. 2021, 22, 11465. [Google Scholar] [CrossRef]
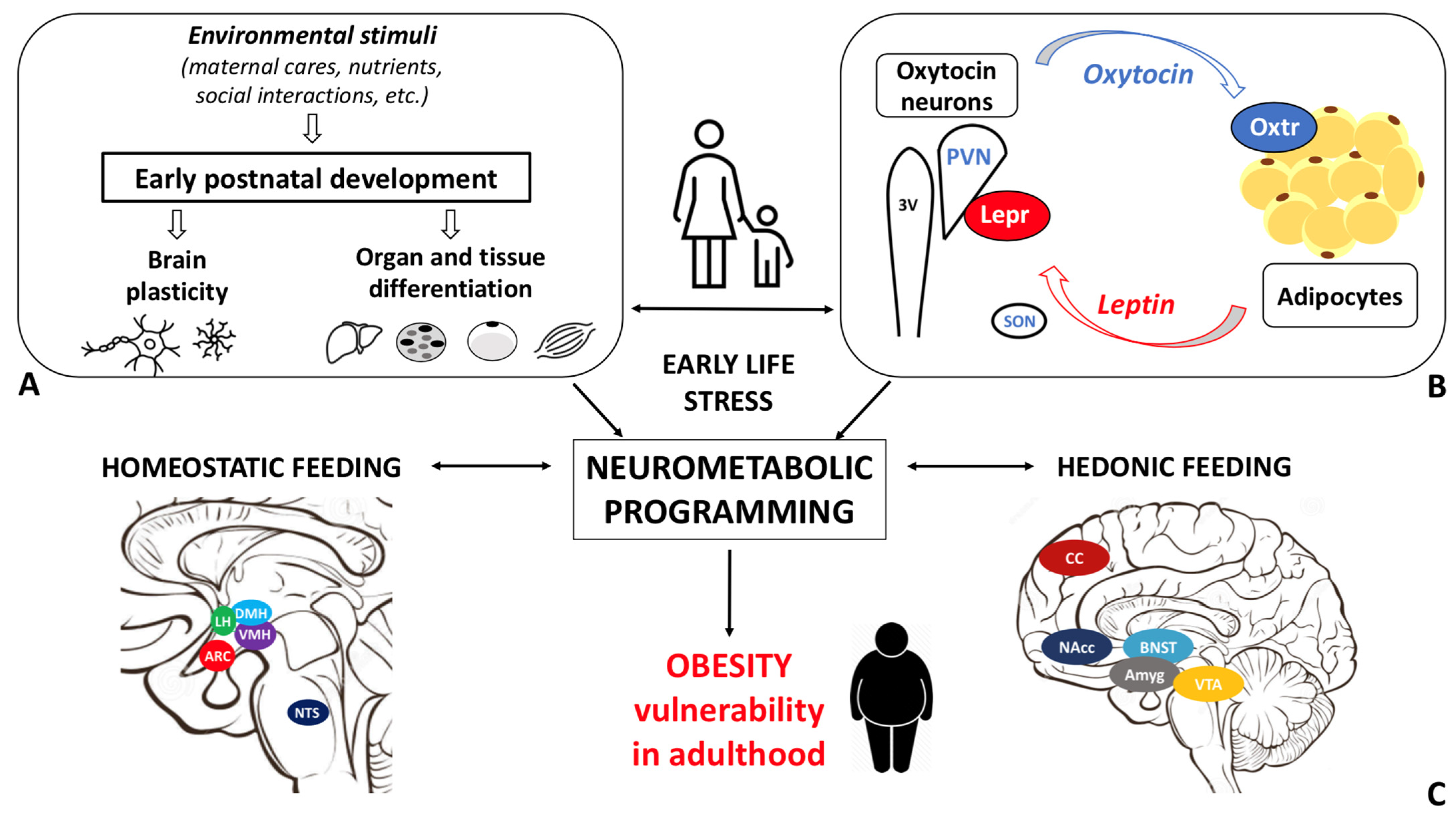
| 1 | Do the Oxt and Leptin systems influence each other’s development? |
| 2 | Is the Oxt–Lep systems interaction impacted by obesity? |
| 3 | Is Oxt the mediator of ELS-induced changes in metabolic health? |
| 4 | What are the consequences of ELS on short- and long-term eating behaviors, such as chow vs. palatable food consumption? |
| 5 | What are the consequences of ELS on total weight changes and metabolic health, such as adipose tissue development and resting energy expenditure? |
| 6 | What are the consequences of ELS on the vulnerability to an obesogenic environment in adulthood, such as adipose tissue dysfunction and metabolic abnormalities? |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colleluori, G.; Galli, C.; Severi, I.; Perugini, J.; Giordano, A. Early Life Stress, Brain Development, and Obesity Risk: Is Oxytocin the Missing Link? Cells 2022, 11, 623. https://doi.org/10.3390/cells11040623
Colleluori G, Galli C, Severi I, Perugini J, Giordano A. Early Life Stress, Brain Development, and Obesity Risk: Is Oxytocin the Missing Link? Cells. 2022; 11(4):623. https://doi.org/10.3390/cells11040623
Chicago/Turabian StyleColleluori, Georgia, Chiara Galli, Ilenia Severi, Jessica Perugini, and Antonio Giordano. 2022. "Early Life Stress, Brain Development, and Obesity Risk: Is Oxytocin the Missing Link?" Cells 11, no. 4: 623. https://doi.org/10.3390/cells11040623
APA StyleColleluori, G., Galli, C., Severi, I., Perugini, J., & Giordano, A. (2022). Early Life Stress, Brain Development, and Obesity Risk: Is Oxytocin the Missing Link? Cells, 11(4), 623. https://doi.org/10.3390/cells11040623







