Anti-microRNA-21 Therapy on Top of ACE Inhibition Delays Renal Failure in Alport Syndrome Mouse Models
Abstract
1. Introduction
2. Materials and Methods
2.1. Mouse Models and Treatments
- (1)
- Treated with Vehicle (Veh).
- (2)
- Treated with anti-miR-21 (Lademirsen).
- (3)
- Treated with Ramipril plus vehicle (ACEi).
- (4)
- Treated with Ramipril plus Lademirsen (ACEi-anti-miR-21).
2.2. Renal Function Evaluation
2.3. Histology, Immunostaining and Quantitative Analysis
2.4. Target Engagement and RNA-Seq Analyses
2.5. Statistics
3. Results
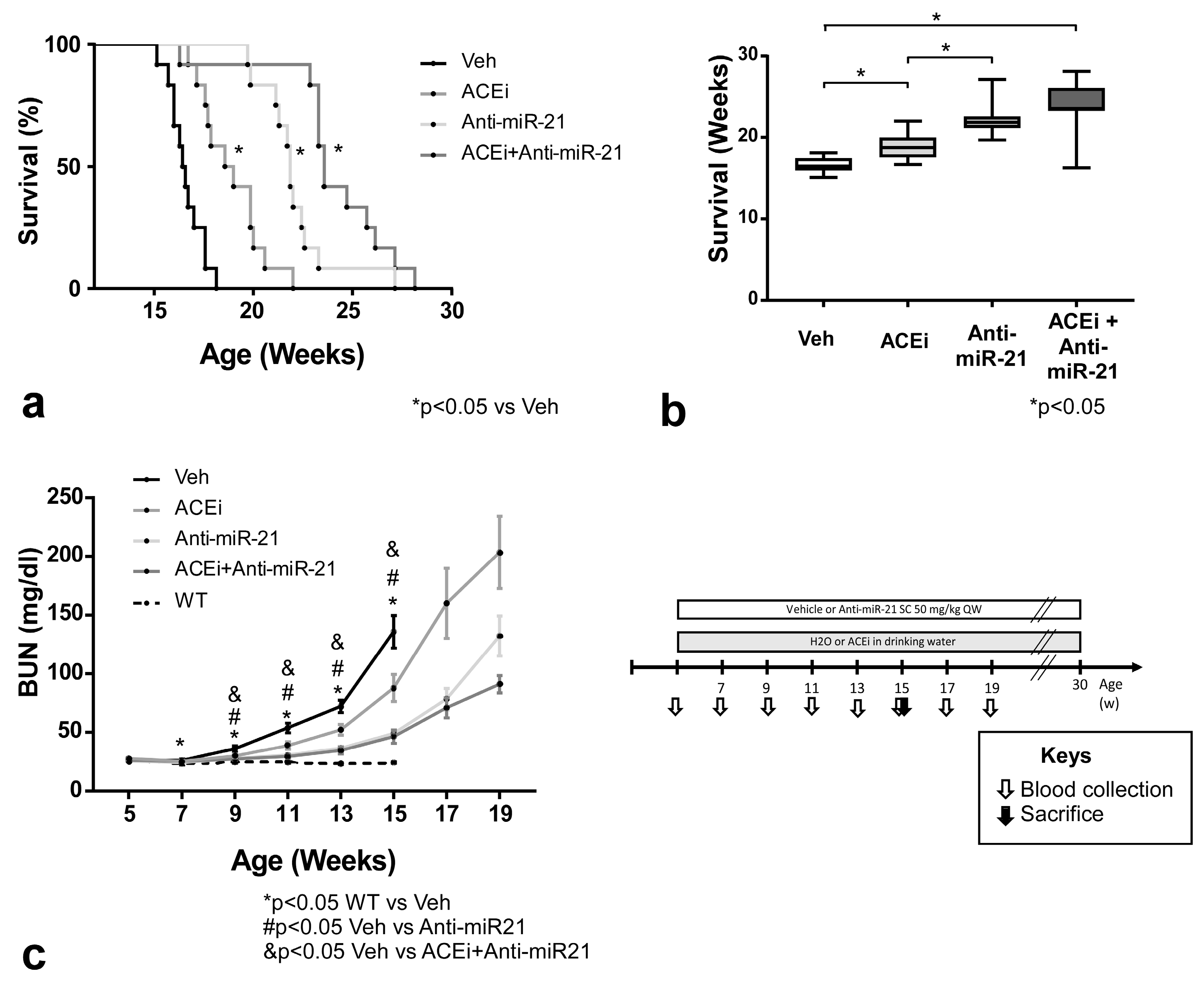
3.1. Effect of ACEi, Anti-miR-21 or the Combination of Early ACEi and Anti-miR-21 on Kidney Failure and Survival of AS Mice in 129/SvJ Background
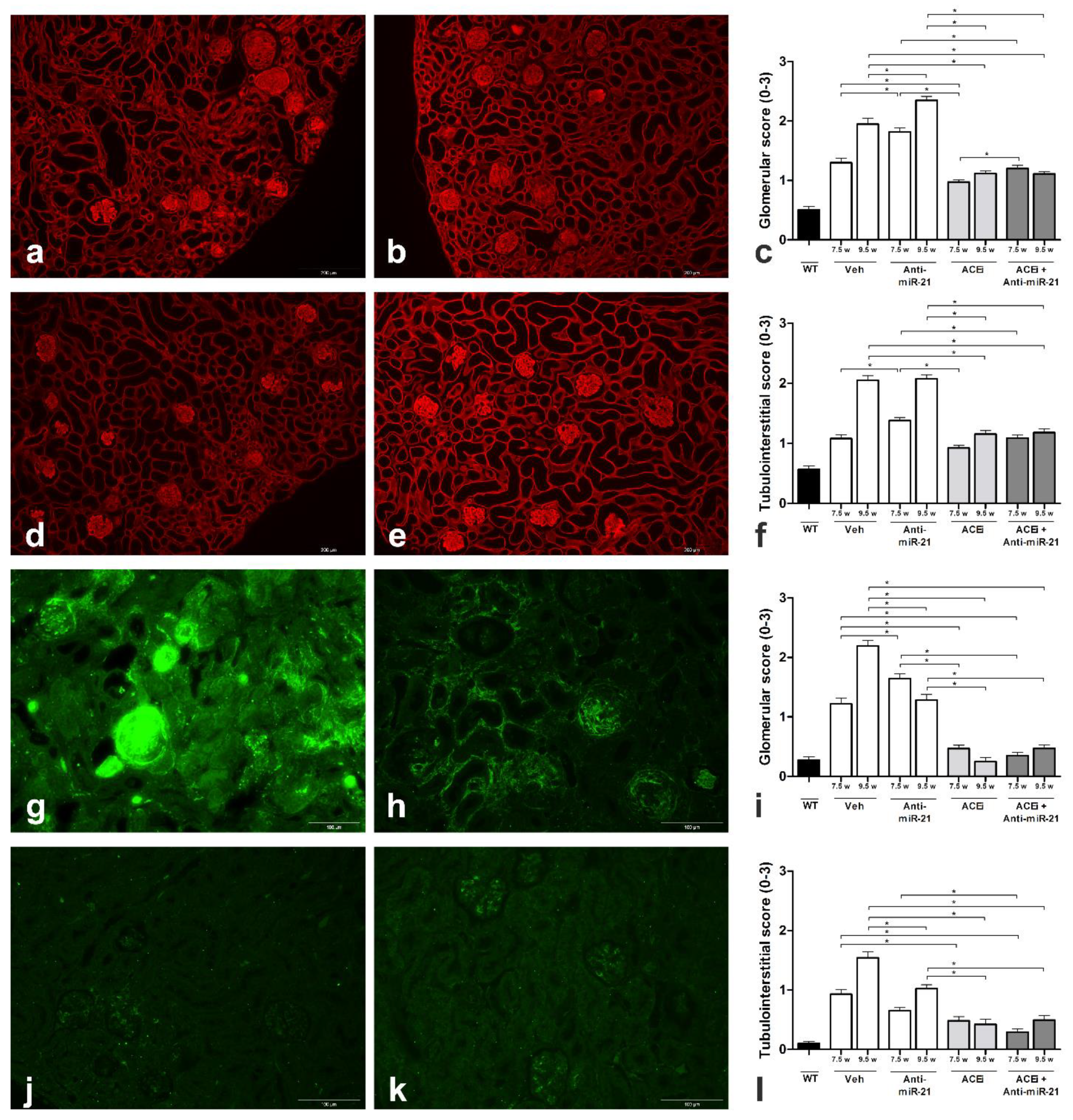
3.2. Effect of ACEi, Anti-miR-21 or the Combination of Early ACEi and Anti-miR-21 on Kidney Pathology in AS Mice with a 129/SvJ Background
3.3. Effect of ACEi, Anti-miR-21 or the Combination of ACEi and Anti-miR-21 on Survival and Renal Function in Moderately Slow-Progressing Alport Mice
3.4. Effect of Anti-miR-21 or ACEi on Kidney Pathology in F1-Col4a3−/− Mice
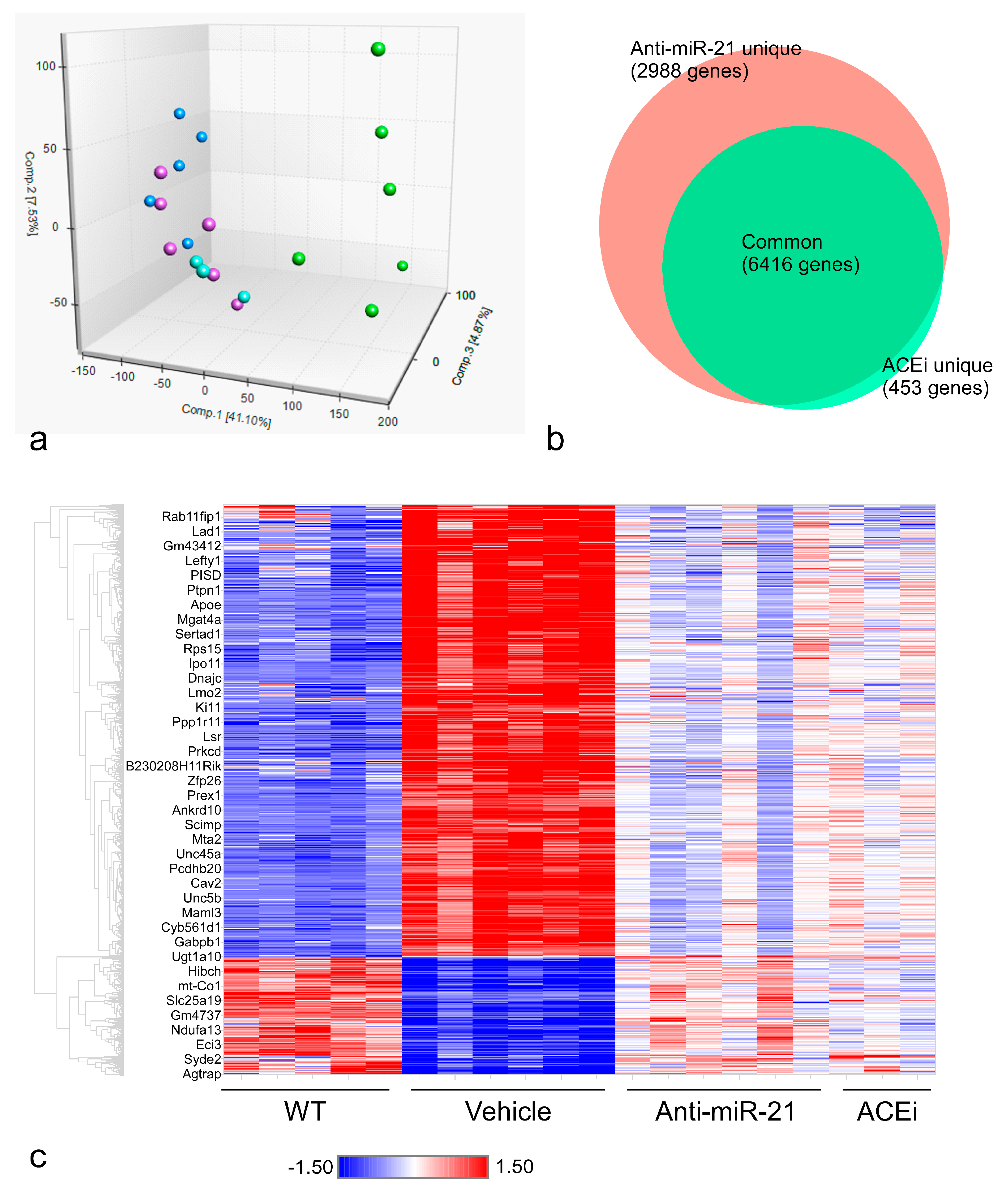
3.5. Anti-miR21 Treatment Led to Larger Magnitudes of Expression Normalization of Differentially Expressed Genes than Ramipril Treatment
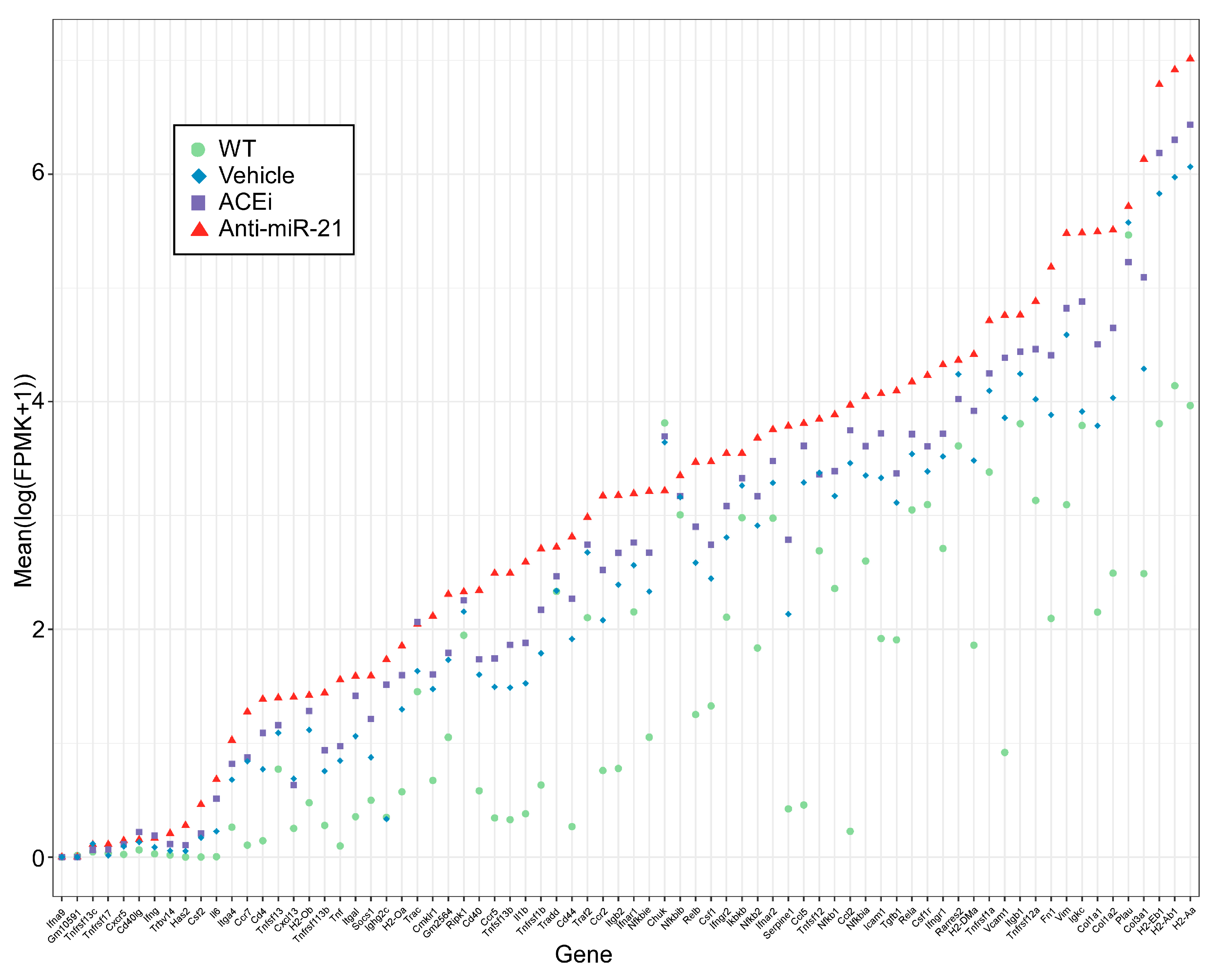
3.6. Anti-miR-21, but Not ACEi, Significantly Normalized Gene Expression Patterns in Renal Tubulointerstitial Fibrosis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hudson, B.G.; Tryggvason, K.; Sundaramoorthy, M.; Neilson, E.G. Alport’s syndrome, Goodpasture’s syndrome, and type IV collagen. N. Engl. J. Med. 2003, 348, 2543–2556. [Google Scholar] [CrossRef]
- Kruegel, J.; Rubel, D.; Gross, O. Alport syndrome—Insights from basic and clinical research. Nat. Rev. Nephrol. 2013, 9, 170–178. [Google Scholar] [CrossRef]
- Cosgrove, D.; Meehan, D.T.; Grunkemeyer, J.A.; Kornak, J.M.; Sayers, R.; Hunter, W.J.; Samuelson, G.C. Collagen COL4A3 knockout: A mouse model for autosomal Alport syndrome. Genes Dev. 1996, 10, 2981–2992. [Google Scholar] [CrossRef]
- Gross, O.; Beirowski, B.; Koepke, M.L.; Kuck, J.; Reiner, M.; Addicks, K.; Smyth, N.; Schulze-Lohoff, E.; Weber, M. Preemptive ramipril therapy delays renal failure and reduces renal fibrosis in COL4A3-knockout mice with Alport syndrome. Kidney Int. 2003, 63, 438–446. [Google Scholar] [CrossRef]
- Koepke, M.L.; Weber, M.; Schulze-Lohoff, E.; Beirowski, B.; Segerer, S.; Gross, O. Nephroprotective effect of the HMG-CoA-reductase inhibitor cerivastatin in a mouse model of progressive renal fibrosis in Alport syndrome. Nephrol. Dial. Transplant. 2007, 22, 1062–1069. [Google Scholar] [CrossRef][Green Version]
- Ninichuk, V.; Gross, O.; Segerer, S.; Hoffmann, R.; Radomska, E.; Buchstaller, A.; Huss, R.; Akis, N.; Schlöndorff, D.; Anders, H.J. Multipotent mesenchymal stem cells reduce interstitial fibrosis but do not delay progression of chronic kidney disease in collagen4A3-deficient mice. Kidney Int. 2006, 70, 121–129. [Google Scholar] [CrossRef]
- Cosgrove, D.; Rodgers, K.; Meehan, D.; Miller, C.; Bovard, K.; Gilroy, A.; Gardner, H.; Kotelianski, V.; Gotwals, P.; Amatucci, A.; et al. Integrin alpha1beta1 and transforming growth factor-beta1 play distinct roles in alport glomerular pathogenesis and serve as dual targets for metabolic therapy. Am. J. Pathol. 2000, 157, 1649–1659. [Google Scholar] [CrossRef]
- Sedrakyan, S.; Da Sacco, S.; Milanesi, A.; Shiri, L.; Petrosyan, A.; Varimezova, R.; Warburton, D.; Lemley, K.V.; De Filippo, R.E.; Perin, L. Injection of amniotic fluid stem cells delays progression of renal fibrosis. J. Am. Soc. Nephrol. 2012, 23, 661–673. [Google Scholar] [CrossRef]
- Rubel, D.; Stock, J.; Ciner, A.; Hiller, H.; Girgert, R.; Müller, G.A.; Gross, O. Antifibrotic, nephroprotective effects of paricalcitol versus calcitriol on top of ACE-inhibitor therapy in the COL4A3 knockout mouse model for progressive renal fibrosis. Nephrol. Dial. Transplant. 2014, 29, 1012–1019. [Google Scholar] [CrossRef][Green Version]
- Gross, O.; Licht, C.; Anders, H.J.; Hoppe, B.; Beck, B.; Tönshoff, B.; Höcker, B.; Wygoda, S.; Ehrich, J.H.; Pape, L.; et al. Early angiotensin-converting enzyme inhibition in Alport syndrome delays renal failure and improves life expectancy. Kidney Int. 2012, 81, 494–501. [Google Scholar] [CrossRef]
- Savige, J.; Gregory, M.; Gross, O.; Kashtan, C.; Ding, J.; Flinter, F. Expert guidelines for the management of Alport syndrome and thin basement membrane nephropathy. J. Am. Soc. Nephrol. 2013, 24, 364–375. [Google Scholar] [CrossRef]
- Kashtan, C.E.; Ding, J.; Gregory, M.; Gross, O.; Heidet, L.; Knebelmann, B.; Rheault, M.; Licht, C. Alport Syndrome Research Collaborative. Clinical practice recommendations for the treatment of Alport syndrome: A statement of the Alport Syndrome Research Collaborative. Pediatr. Nephrol. 2013, 28, 5–11. [Google Scholar] [CrossRef]
- Gross, O.; Perin, L.; Deltas, C. Alport syndrome from bench to bedside: The potential of current treatment beyond RAAS blockade and the horizon of future therapies. Nephrol. Dial. Transplant. 2014, 29 (Suppl. S4), iv124–iv130. [Google Scholar] [CrossRef]
- Godwin, J.G.; Ge, X.; Stephan, K.; Jurisch, A.; Tullius, S.G.; Iacomini, J. Identification of a microRNA signature of renal ischemia reperfusion injury. Proc. Natl. Acad. Sci. USA 2010, 107, 14339–14344. [Google Scholar] [CrossRef]
- Chau, B.N.; Xin, C.; Hartner, J.; Ren, S.; Castano, A.P.; Linn, G.; Li, J.; Tran, P.T.; Kaimal, V.; Huang, X.; et al. MicroRNA-21 promotes fibrosis of the kidney by silencing metabolic pathways. Sci. Transl. Med. 2012, 4, 121ra18. [Google Scholar] [CrossRef]
- Denby, L.; Ramdas, V.; McBride, M.W.; Wang, J.; Robinson, H.; McClure, J.; Crawford, W.; Lu, R.; Hillyard, D.Z.; Khanin, R.; et al. miR-21 and miR-214 are consistently modulated during renal injury in rodent models. Am. J. Pathol. 2011, 179, 661–672. [Google Scholar] [CrossRef]
- Zhong, X.; Chung, A.C.K.; Chen, H.Y.; Dong, Y.; Meng, X.M.; Li, R.; Yang, W.; Hou, F.F.; Lan, H.Y. miR-21 is a key therapeutic target for renal injury in a mouse model of type 2 diabetes. Diabetologia 2013, 56, 663–674. [Google Scholar] [CrossRef]
- Gomez, I.G.; MacKenna, D.A.; Johnson, B.G.; Kaimal, V.; Roach, A.M.; Ren, S.; Nakagawa, N.; Xin, C.; Newitt, R.; Pandya, S.; et al. Anti-microRNA-21 oligonucleotides prevent Alport nephropathy progression by stimulating metabolic pathways. J. Clin. Investig. 2015, 125, 141–156. [Google Scholar] [CrossRef]
- Gross, O.; Tönshoff, B.; Weber, L.T.; Pape, L.; Latta, K.; Fehrenbach, H.; Lange-Sperandio, B.; Zappel, H.; Hoyer, P.; Staude, H.; et al. German Pediatric Nephrology (GPN) Study Group and EARLY PRO-TECT Alport Investigators. A multicenter, randomized, placebo-controlled, double-blind phase 3 trial with open-arm comparison indicates safety and efficacy of nephroprotective therapy with ramipril in children with Alport’s syndrome. Kidney Int. 2020, 97, 1275–1286. [Google Scholar]
- Guo, J.; Song, W.; Boulanger, J.; Xu, E.Y.; Wang, F.; Zhang, Y.; He, Q.; Wang, S.; Yang, L.; Pryce, C.; et al. Dysregulated Expression of microRNA-21 and Disease-Related Genes in Human Patients and in a Mouse Model of Alport Syndrome. Hum. Gene Ther. 2019, 30, 865–881. [Google Scholar] [CrossRef]
- Remuzzi, G.; Grinyo, J.; Ruggenenti, P.; Beatini, M.; Cole, E.H.; Milford, E.L.; Brenner, B.M. Early Experience with Dual Kidney Transplantation in Adults using Expanded Donor Criteria. J. Am. Soc. Nephrol. 1999, 10, 2591–2598. [Google Scholar] [CrossRef]
- Cabrera-Salazar, M.A.; Deriso, M.; Bercury, S.D.; Li, L.; Lydon, J.T.; Weber, W.; Pande, N.; Cromwell, M.A.; Copeland, D.; Leonard, J.; et al. Systemic delivery of a glucosylceramide synthase inhibitor reduces CNS substrates and increases lifespan in a mouse model of type 2 Gaucher disease. PLoS ONE 2012, 7, e43310. [Google Scholar] [CrossRef]
- Hu, J.; Ge, H.; Newman, M.; Liu, K. OSA: A fast and accurate alignment tool for RNA-Seq. Bioinformatics 2012, 28, 1933–1934. [Google Scholar] [CrossRef]
- Mortazavi, A.; Williams, B.A.; McCue, K.; Schaeffer, L.; Wold, B. Mapping and quantifying mammalian transcriptomes by RNA-Seq. Nat. Methods 2008, 5, 621–628. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Birger, C. Gene Set Enrichment Analysis against a User-Supplied Ranked List of Genes. 2017. Available online: https://software.broadinstitute.org/cancer/software/genepattern/modules/docs/GSEAPreranked/5 (accessed on 21 August 2018).
- Khatri, P.; Sirota, M.; Butte, A.J. Ten Years of Pathway Analysis: Current Approaches and Outstanding Challenges. PLoS Comput. Biol. 2012, 8, e1002375. [Google Scholar] [CrossRef]
- Gross, O.; Girgert, R.; Beirowski, B.; Kretzler, M.; Kang, H.G.; Kruegel, J.; Miosge, N.; Busse, A.C.; Segerer, S.; Vogel, W.F.; et al. Loss of collagen-receptor DDR1 delays renal fibrosis in hereditary type IV collagen disease. Matrix Biol. 2010, 29, 346–356. [Google Scholar] [CrossRef]
- Sawai, K.; Mahato, R.I.; Oka, Y.; Takakura, Y.; Hashida, M. Disposition of oligonucleotides in isolated perfused rat kidney: Involvement of scavenger receptors in their renal uptake. J. Pharmacol. Exp. Ther. 1996, 279, 284–290. [Google Scholar]
- Gross, O.; Kashtan, C.E.; Rheault, M.N.; Flinter, F.; Savige, J.; Miner, J.H.; Torra, R.; Ars, E.; Deltas, C.; Savva, I.; et al. Advances and unmet needs in genetic, basic and clinical science in Alport syndrome: Report from the 2015 International Workshop on Alport Syndrome. Nephrol. Dial. Transplant. 2017, 32, 916–924. [Google Scholar] [CrossRef]



| Top 5 Upregulated Pathways in Alport Mice | Size | ES | NES |
|---|---|---|---|
| Immune response: T-cell co-signaling receptors | 62 | 0.81 | 2.28 |
| Chemokines in inflammation in adipose tissue and liver | 50 | 0.82 | 2.28 |
| Renal tubulointertitial injury in lupus nephritis | 74 | 0.78 | 2.24 |
| Breakdown of CD4 and T cell peripheral tolerance in type 1 diabetes mellitus | 70 | 0.77 | 2.22 |
| Cytokine-induced fibroblast/myofibroblast migration and extracellular matrix production | 42 | 0.82 | 2.21 |
| Top 5 Downregulated Pathways in Alport Mice | Size | ES | NES |
| Oxidative phosphorylation | 77 | −0.84 | −3.35 |
| Ubiquinone metabolism | 38 | −0.84 | −2.87 |
| Mitochondrial dysfunction in neurodegenerative diseases | 91 | −0.67 | −2.74 |
| Mechanism of Pioglitazone/Rosiglitazone/Metformin cooperative action in type 2 diabetes mellitus | 46 | −0.75 | −2.73 |
| Glycine, serine, cysteine and threonine metabolism | 39 | −0.77 | −2.70 |
| Top 5 Pathways Downregulated by ACEi | Size | ES | NES |
|---|---|---|---|
| Wnt signaling in gastric cancer | 59 | −0.74 | −2.33 |
| Stem cells: embryonal epaxial myogenesis | 45 | −0.77 | −2.32 |
| Development: regulation of lung epithelial progenitor cell differentiation | 61 | −0.72 | −2.32 |
| Inhibition of oligodendrocyte precursor cell differentiation by wnt signaling | 44 | −0.77 | −2.32 |
| Deregulation of canonical wnt signaling in major depressive disorder | 53 | −0.74 | −2.31 |
| Top 5 Pathways Upregulated by ACEi | Size | ES | NES |
| Oxidative phosphorylation | 77 | 0.84 | 2.84 |
| Ubiquinone metabolism | 38 | 0.84 | 2.56 |
| Butanoate metabolism | 24 | 0.90 | 2.48 |
| Mitochondrial dysfunction in neurodegenerative diseases | 91 | 0.70 | 2.42 |
| Mechanism of Pioglitazone/Rosiglitazone/Metformin cooperative action in type 2 diabetes mellitus | 46 | 0.78 | 2.40 |
| Top 5 Pathways Downregulated by Anti-miR-21 | Size | ES | NES |
|---|---|---|---|
| Renal tubulointertitial injury in lupus nephritis | 74 | −0.71 | −2.24 |
| Development: regulation of epithelial-to-mesenchymal transition (EMT) | 88 | −0.67 | −2.24 |
| TH2 cytokine- and TNF-alpha-induced profibrotic response in asthmatic airway fibroblasts/myofibroblasts | 63 | −0.7 | −2.21 |
| Immune response: T-cell co-signaling receptors | 62 | −0.7 | −2.21 |
| WNT signaling in proliferative-type melanoma cells | 60 | −0.71 | −2.21 |
| Top 5 Pathways Upregulated by Anti-miR-21 | Size | ES | NES |
| Oxidative phosphorylation | 77 | 0.86 | 3.16 |
| Mechanism of Pioglitazone/Rosiglitazone/Metformin cooperative action in type 2 diabetes mellitus | 46 | 0.83 | 2.81 |
| Ubiquinone metabolism | 38 | 0.87 | 2.77 |
| Mitochondrial dysfunction in neurodegenerative diseases | 91 | 0.73 | 2.74 |
| Butanoate metabolism | 24 | 0.86 | 2.51 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rubel, D.; Boulanger, J.; Craciun, F.; Xu, E.Y.; Zhang, Y.; Phillips, L.; Callahan, M.; Weber, W.; Song, W.; Ngai, N.; et al. Anti-microRNA-21 Therapy on Top of ACE Inhibition Delays Renal Failure in Alport Syndrome Mouse Models. Cells 2022, 11, 594. https://doi.org/10.3390/cells11040594
Rubel D, Boulanger J, Craciun F, Xu EY, Zhang Y, Phillips L, Callahan M, Weber W, Song W, Ngai N, et al. Anti-microRNA-21 Therapy on Top of ACE Inhibition Delays Renal Failure in Alport Syndrome Mouse Models. Cells. 2022; 11(4):594. https://doi.org/10.3390/cells11040594
Chicago/Turabian StyleRubel, Diana, Joseph Boulanger, Florin Craciun, Ethan Y. Xu, Yanqin Zhang, Lucy Phillips, Michelle Callahan, William Weber, Wenping Song, Nicholas Ngai, and et al. 2022. "Anti-microRNA-21 Therapy on Top of ACE Inhibition Delays Renal Failure in Alport Syndrome Mouse Models" Cells 11, no. 4: 594. https://doi.org/10.3390/cells11040594
APA StyleRubel, D., Boulanger, J., Craciun, F., Xu, E. Y., Zhang, Y., Phillips, L., Callahan, M., Weber, W., Song, W., Ngai, N., Bukanov, N. O., Shi, X., Hariri, A., Husson, H., Ibraghimov-Beskrovnaya, O., Liu, S., & Gross, O. (2022). Anti-microRNA-21 Therapy on Top of ACE Inhibition Delays Renal Failure in Alport Syndrome Mouse Models. Cells, 11(4), 594. https://doi.org/10.3390/cells11040594






