Arabinogalactan Proteins in the Digestive Glands of Dionaea muscipula J.Ellis Traps
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Histological and Immunochemical Analysis
2.3. Immunogold Labeling Distribution of AGP
2.4. Morphological Observation
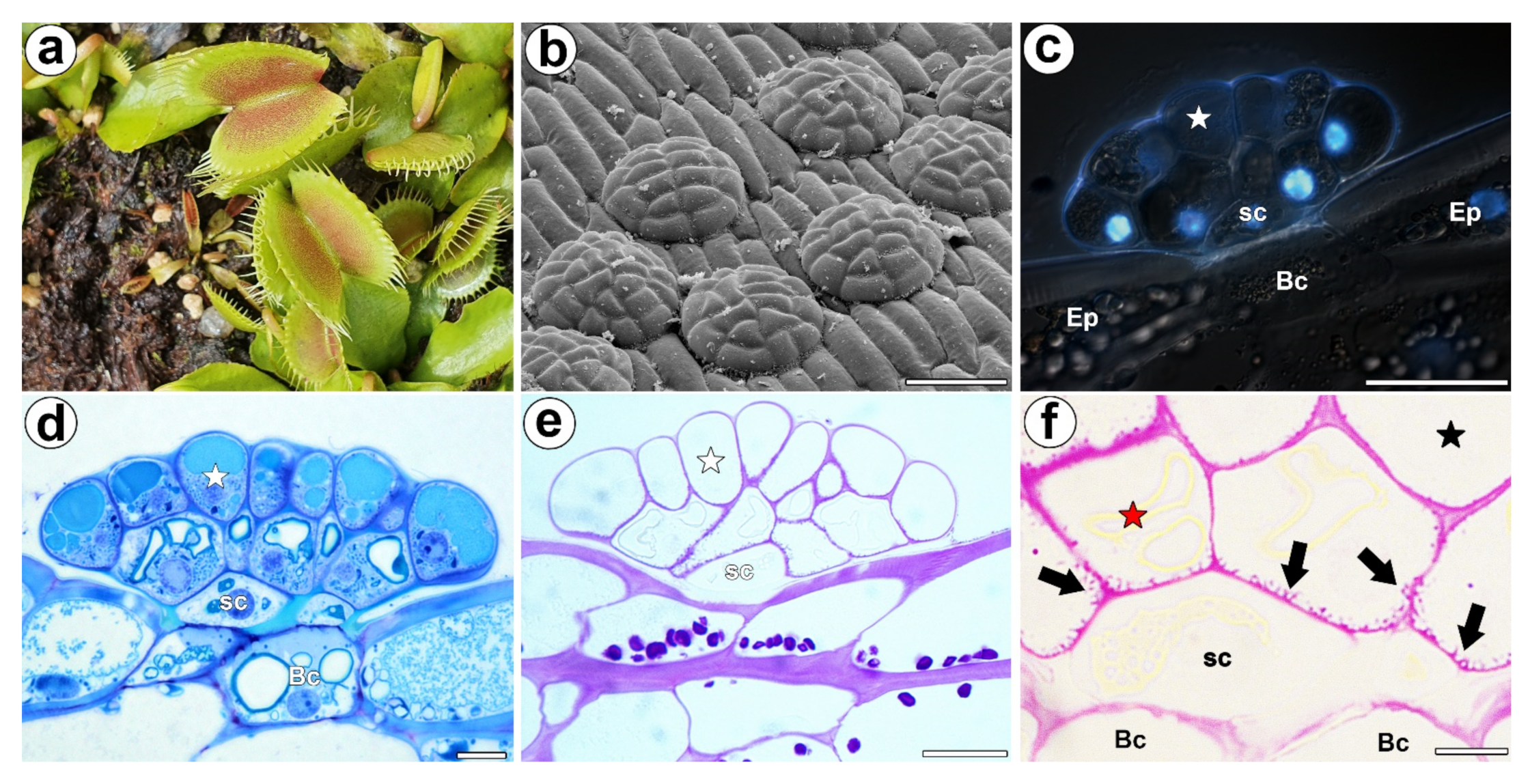
3. Results
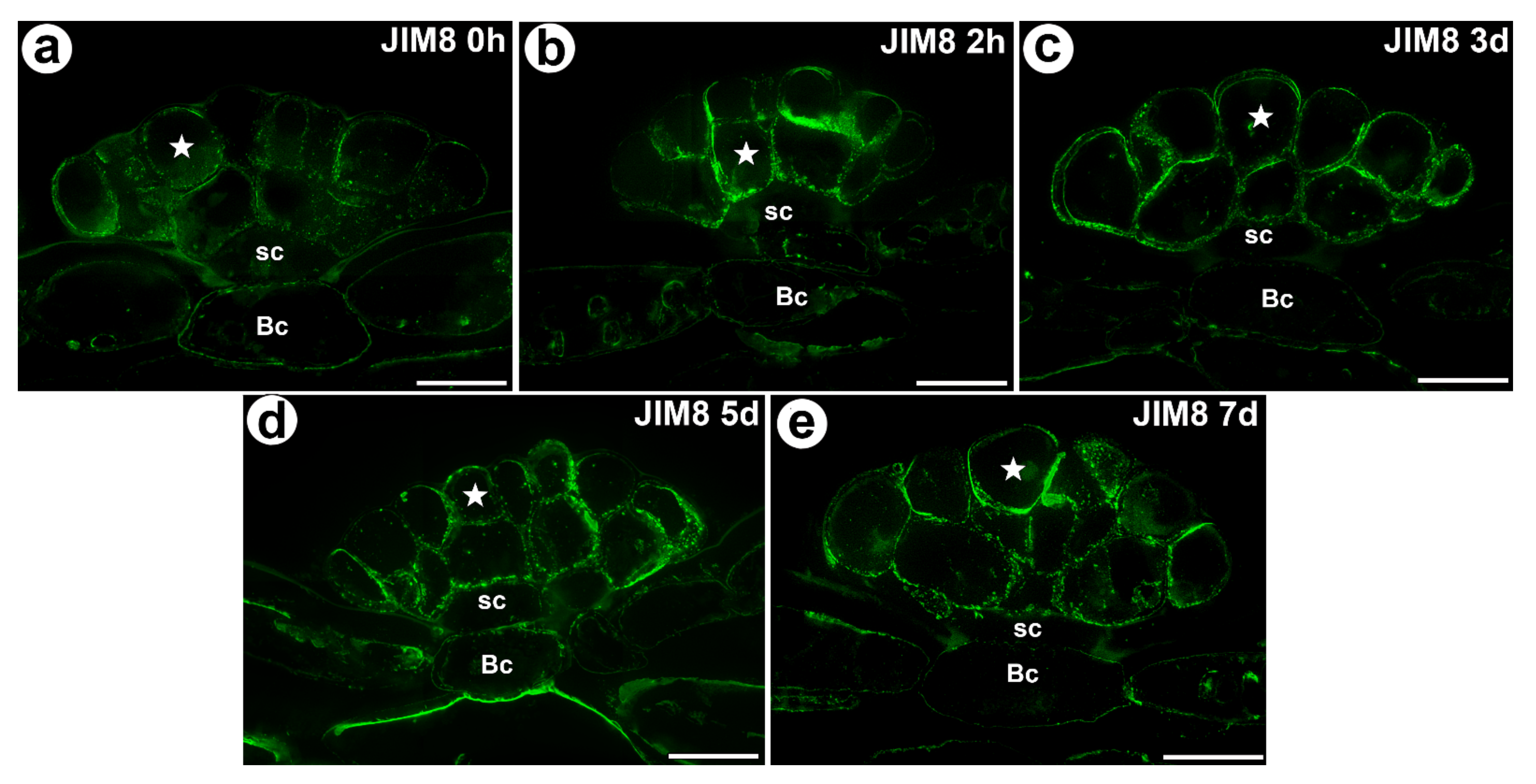
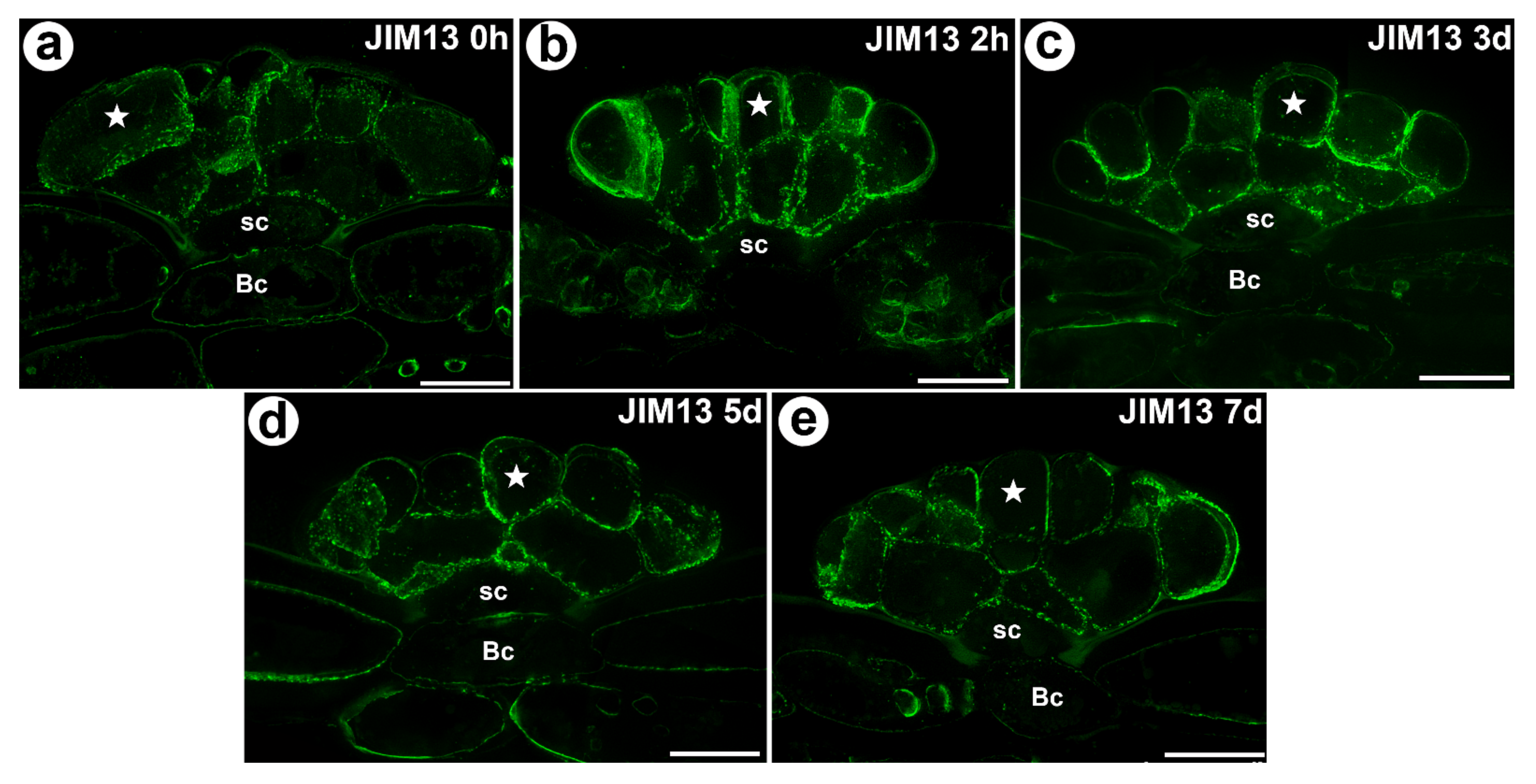
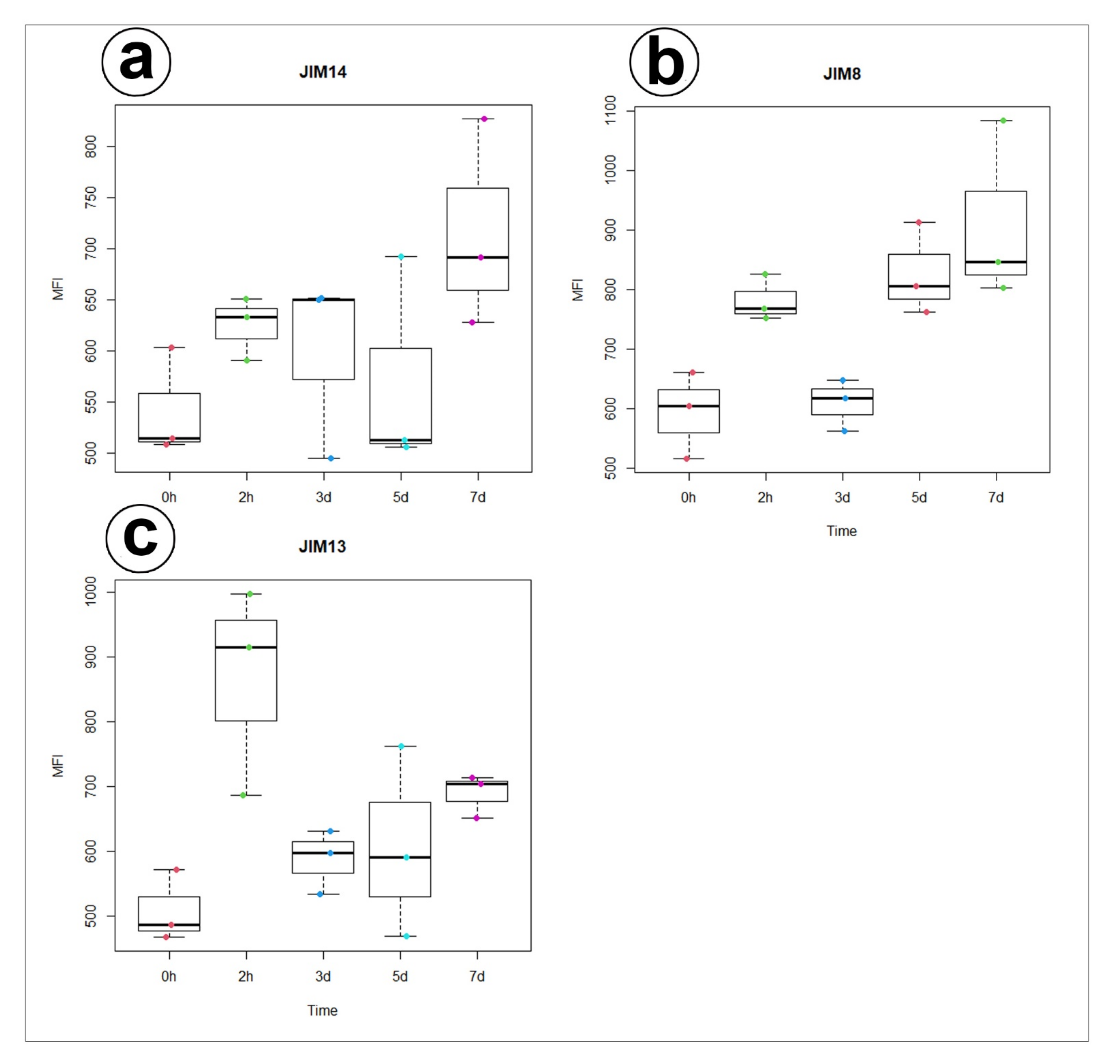
3.1. AGP Distribution
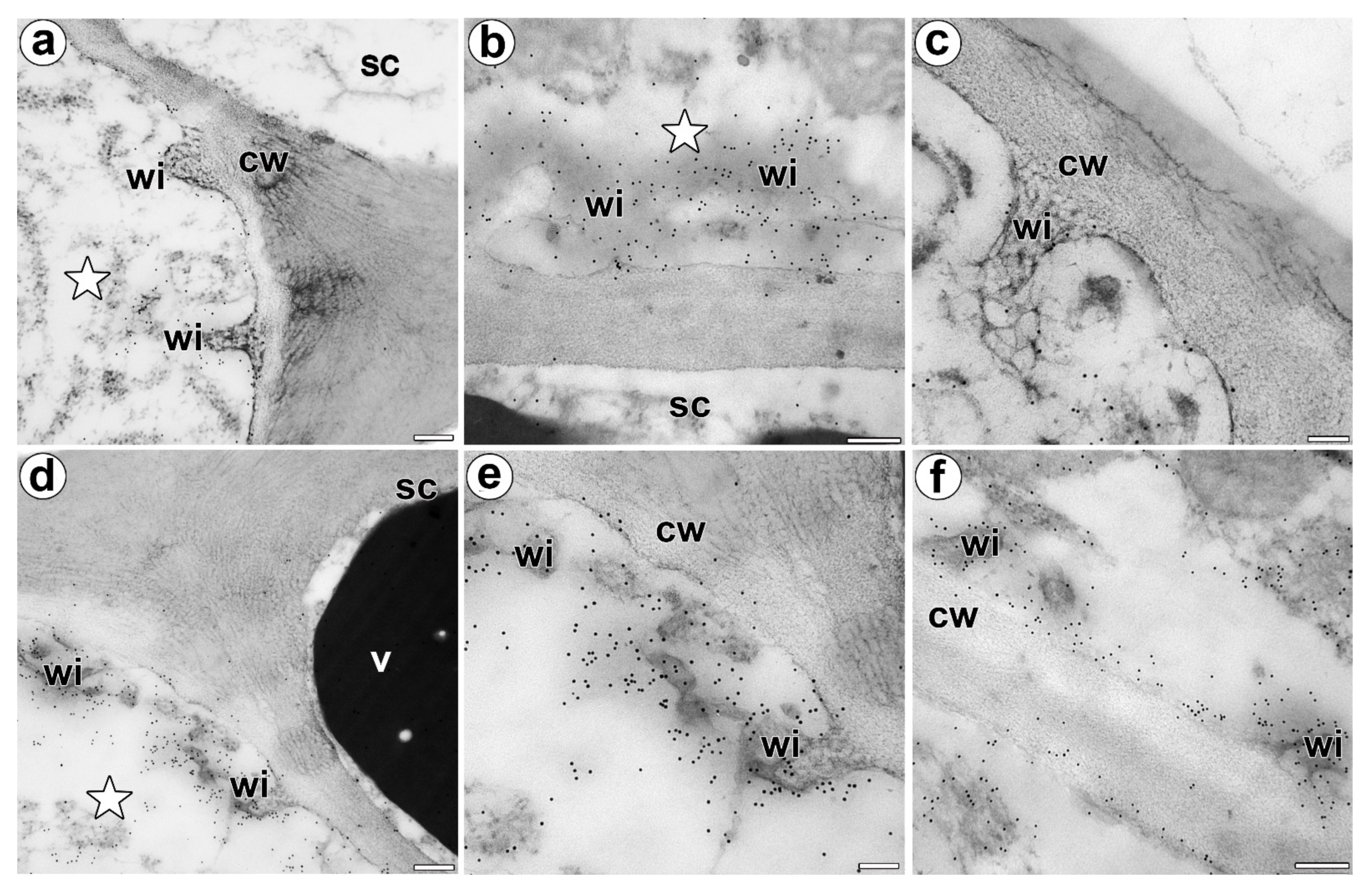
3.2. Wall Ingrowths Morphology and Immunogold Labeling Distribution of AGP in the Secretory Cells of Digestive Glands
4. Discussion
4.1. The Secretory Cells of the Dionaea as Transfer Cells
4.2. Possible Role of AGP
4.3. Changes in Digestive Glands after Stimulation
5. Conclusions
- There was an accumulation of AGP in the cell walls of the gland secretory cells;
- An accumulation of specific AGP at the site where the wall labyrinth occurred;
- The epitope that is recognized by JIM14 was a useful marker of the digestive glands;
- During experiment significant increase of mean value of fluorescence intensity for AGP detected with JIM8 was observed for unfed (0 h) and traps after 3 d and 7 d of feeding;
- For AGP epitope recognized with JIM13 antibody significant changes of mean value of fluorescence intensity were observed only at the beginning of the experiment (after 2 h of feeding);
- Future studies will have to do a comparison with related species.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Griggs, R.F. Victims of the Venus flytrap. Science 1935, 81, 7–8. [Google Scholar]
- Lloyd, F.E. The Carnivorous Plants; Chronica Botanica Company: Waltham, MA, USA, 1942. [Google Scholar]
- Schulze, W.; Schulze, E.D.; Schulze, I.; Oren, R. Quantification of insect nitrogen utilization by the Venus fly trap Dionaea muscipula catching prey with highly variable isotope signatures. J. Exp. Bot. 2001, 52, 1041–1049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darwin, C. Insectivorous Plants; John Murray: London, UK, 1875. [Google Scholar]
- Forterre, Y.; Skotheim, J.M.; Dumais, J.; Mahadevan, L. How the Venus flytrap snaps. Nature 2005, 433, 421–425. [Google Scholar] [CrossRef] [PubMed]
- Scherzer, S.; Federle, W.; Al-Rasheid, K.A.S.; Hedrich, R. Venus flytrap trigger hairs are micronewton mechano-sensors that can detect small insect prey. Nat. Plants 2019, 7, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Volkov, A.G.; Carrell, H.; Markin, V.S. Biologically Closed Electrical Circuits in Venus Flytrap. Plant Physiol. 2009, 149, 1661–1667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Böhm, J.; Scherzer, S.; Król, E.; Kreuzer, I.; von Meyer, K.; Lorey, C.; Mueller, T.D.; Shabala, L.; Monte, I.; Solano, R.; et al. The Venus flytrap Dionaea muscipula counts prey-induced action potentials to induce sodium uptake. Curr. Biol. 2016, 26, 286–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bemm, F.; Becker, D.; Larisch, C.; Kreuzer, I.; Escalante-Perez, M.; Schulze, W.X.; Ankenbrand, M.; Van de Weyer, A.L.; Krol, E.; Al-Rasheid, K.A.; et al. Venus flytrap carnivorous lifestyle builds on herbivore defense strategies. Genome Res. 2016, 26, 812–825. [Google Scholar] [CrossRef] [Green Version]
- Scherzer, S.; Shabala, L.; Hedrich, B.; Fromm, J.; Bauer, H.; Munz, E.; Jakob, P.; Al-Rascheid, K.A.S.; Kreuzer, I.; Becker, D.; et al. Insect haptoelectrical stimulation of Venus flytrap triggers exocytosis in gland cells. Proc. Natl. Acad. Sci. USA 2017, 114, 4822–4827. [Google Scholar] [CrossRef] [Green Version]
- Hedrich, R.; Neher, E. Venus flytrap: How an excitable, carnivorous plant works. Trends Plant Sci. 2018, 23, 220–234. [Google Scholar] [CrossRef] [PubMed]
- Gergely, Z.R.; Martinez, D.E.; Donohoe, B.S.; Mogelsvang, S.; Herder, R.; Staehelin, L.A. 3D electron tomographic and biochemical analysis of ER, Golgi and trans Golgi network membrane systems in stimulated Venus flytrap (Dionaea muscipula) glandular cells. J. Biol. Res.-Thessalon. 2018, 25, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boulogne, C.; Gillet, C.; Hughes, L.; LE Bars, R.; Canette, A.; Hawes, C.R.; Satiat-Jeunemaitre, B. Functional organisation of the endomembrane network in the digestive gland of the Venus flytrap: Revisiting an old story with a new microscopy toolbox. J. Microsc. 2020, 28, 86–103. [Google Scholar] [CrossRef]
- Scala, J.; Schwab, D.; Simmons, E. The fine structure of the digestive gland of Venus’s-flytrap. Am. J. Bot. 1968, l55, 649–657. [Google Scholar] [CrossRef]
- Robins, R.J.; Juniper, B.E. The secretory cycle of Dionaea muscipula Ellis I. The fine structure and the effect of stimulation on the fine structure of the digestive gland cells. New Phytol. 1980, 86, 279–296. [Google Scholar] [CrossRef]
- Juniper, B.E.; Robbins, R.J.; Joel, D.M. The Carnivorous Plants; Academic Press: London, UK, 1989. [Google Scholar]
- Escalante-Perez, M.; Krol, E.; Stange, A.; Geiger, D.; Al-Rasheid, K.A.; Hause, B.; Neher, E.; Hedrich, R. A special pair of phytohormones controls excitability, slow closure, and external stomach formation in the Venus flytrap. Proc. Natl. Acad. Sci. USA 2011, 108, 15492–15497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pavlovič, A.; Mithöfer, A. Jasmonate signalling in carnivorous plants: Copycat of plant defence mechanisms. J. Exp. Bot. 2019, 70, 3379–3389. [Google Scholar] [CrossRef]
- Schulze, W.X.; Sanggaard, K.W.; Kreuzer, I.; Knudsen, A.D.; Bemm, F.; Thøgersen, I.B.; Bräutigam, A.; Thomsen, L.R.; Schliesky, S.; Dyrlund, T.F.; et al. The Protein Composition of the Digestive Fluid from the Venus Flytrap Sheds Light on Prey Digestion Mechanisms. Mol. Cell. Proteom. 2012, 11, 1306–1319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palfalvi, G.; Hackl, T.; Terhoeven, N.; Shibata, T.F.; Nishiyama, T.; Ankenbrand, M.; Becker, D.; Förster, F.; Freund, M.; Iosip, A.; et al. Genomes of the Venus Flytrap and Close Relatives Unveil the Roots of Plant Carnivory. Curr. Biol. 2020, 30, 2312–2320. [Google Scholar] [CrossRef] [PubMed]
- Schwab, D.W.; Simmons, E.; Scala, J. Fine structure changes during function of the digestive gland of Venus’s-flytrap. Am. J. Bot. 1969, 56, 88–100. [Google Scholar] [CrossRef]
- Robins, R.J.; Juniper, B.E. The secretory cycle of Dionaea muscipula Ellis. II. Storage and synthesis of the secretory proteins. New Phytol. 1980, 86, 297–311. [Google Scholar] [CrossRef]
- Robins, R.J.; Juniper, B.E. The secretory cycle of Dionaea muscipula Ellis: III. The mechanism of release of digestive secretion. New Phytol. 1980, 86, 313–327. [Google Scholar] [CrossRef]
- Showalter, A.M. Structure and function of plant cell wall proteins. Plant Cell 1993, 5, 9–23. [Google Scholar]
- Showalter, A.M.; Keppler, B.; Lichtenberg, J.; Gu, D.; Welch, L.R. A bioinformatics approach to the identification, classification, and analysis of hydroxyproline-rich glycoproteins. Plant Physiol. 2010, 153, 485–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamport, D.T.A.; Várnai, P. Periplasmic arabinogalactan glycoproteins act as a calcium capacitor that regulates plant growth and development. New Phytol. 2013, 197, 58–64. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Takáč, T.; Li, X.; Chen, H.; Wang, Y.; Xu, E.; Xie, L.; Su, Z.; Šamaj, J.; Xu, C. Variable content and distribution of arabinogalactan proteins in banana (Musa spp.) under low temperature stress. Front. Plant Sci. 2015, 6, 353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Płachno, B.J.; Kapusta, M.; Świątek, P.; Banaś, K.; Miranda, V.F.O.; Bogucka-Kocka, A. Spatio-Temporal Distribution of Cell Wall Components in the Placentas, Ovules and Female Gametophytes of Utricularia during Pollination. Int. J. Mol. Sci. 2021, 22, 5622. [Google Scholar] [CrossRef] [PubMed]
- Płachno, B.J.; Kapusta, M.; Świątek, P.; Stolarczyk, P.; Kocki, J. Immunodetection of Pectic Epitopes, Arabinogalactan Proteins, and Extensins in Mucilage Cells from the Ovules of Pilosella officinarum Vaill. and Taraxacum officinale Agg. (Asteraceae). Int. J. Mol. Sci. 2020, 21, 9642. [Google Scholar] [CrossRef]
- Knox, J.P.; Day, S.; Roberts, K. A set of cell surface glycoproteins forms an early marker of cell position, but not cell type, in the root apical meristem of Daucus carota L. Development 1989, 106, 47–56. [Google Scholar] [CrossRef]
- Pennell, R.I.; Janniche, L.; Kjellbom, P.; Scofield, G.N.; Peart, J.M.; Roberts, K. Developmental regulation of a plasma membrane arabinogalactan protein epitope in oilseed rape flowers. Plant. Cell 1991, 3, 1317–1326. [Google Scholar] [CrossRef]
- Knox, J.P.; Linstead, P.J.; Cooper, J.P.C.; Roberts, K. Developmentally regulated epitopes of cell surface arabinogalactan proteins and their relation to root tissue pattern formation. Plant J. 1991, 1, 317–326. [Google Scholar] [CrossRef] [PubMed]
- Pennell, R.I.; Knox, P.J.; Scofield, G.N.; Selvendran, R.R.; Roberts, K.A. family of abundant plasma membrane-associated glycoproteins related to the arabinogalactan proteins is unique to flowering plants. J. Cell Biol. 1989, 108, 1967–1977. [Google Scholar] [CrossRef]
- Wędzony, M. Fluorescence Microscopy for Botanists; Department of Plant Physiology Monographs 5: Kraków, Poland, 1996. (In Polish) [Google Scholar]
- Lustofin, K.; Świątek, P.; Stolarczyk, P.; Miranda, V.F.O.; Płachno, B.J. Do food trichomes occur in Pinguicula (Lentibulariaceae) flowers? Ann. Bot. 2020, 126, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Showalter, A.M. Arabinogalactan-proteins: Structure, expression and function. Cell. Mol. Life Sci. 2001, 58, 1399–1417. [Google Scholar] [CrossRef]
- Boughanmi, N.; Thibault, F.; Decou, R.; Fleurat-Lessard, P.; Béré, E.; Costa, G.; Lhernould, S. NaCl effect on the distribution of wall ingrowth polymers and arabinogalactan proteins in type A transfer cells of Medicago sativa Gabès leaves. Protoplasma 2010, 242, 69–80. [Google Scholar] [CrossRef]
- Coimbra, S.; Duarte, C. Arabinogalactan proteins may facilitate the movement of pollen tubes from the stigma to the ovules in Actinidia deliciosa and Amaranthus hypochondriacus. Euphytica 2003, 133, 171–178. [Google Scholar] [CrossRef]
- Coimbra, S.; Almeida, J.; Junqueira, V.; Costa, M.L.; Pereira, L.G. Arabinogalactan proteins as molecular markers in Arabidopsis thaliana sexual reproduction. J. Exp. Bot. 2007, 58, 4027–4035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leszczuk, A.; Szczuka, E.; Zdunek, A. Arabinogalactan proteins: Distribution during the development of male and female gametophytes. Plant. Physiol. Biochem. 2019, 135, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Offler, C.E.; Patrick, J.W. Transfer cells: What regulates the development of their intricate wall labyrinths? New Phytol. 2020, 228, 427–444. [Google Scholar] [CrossRef]
- Jauh, G.Y.; Lord, E.M. Localization of pectins and arabinogalactan-proteins in lily (Lilium longiflorum L.) pollen tube and style, and their possible roles in pollination. Planta 1996, 199, 251–261. [Google Scholar] [CrossRef]
- Vaughn, K.C.; Talbot, M.J.; Offler, C.E.; Mc Curdy, D.W. Wall ingrowths in epidermal transfer cells of Vicia faba cotyledons are modified primary walls marked by localized accumulations of arabinogalactan proteins. Plant Cell Physiol. 2007, 48, 159–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henry, J.S.; Lopez, R.A.; Renzaglia, K.S. Differential localization of cell wall polymers across generations in the placenta of Marchantia polymorpha. J. Plant Res. 2020, 133, 911–924. [Google Scholar] [CrossRef]
- Henry, J.S.; Ligrone, R.; Vaughn, K.C.; Lopez, R.A.; Renzaglia, K.S. Cell wall polymers in the Phaeoceros placenta reflect developmental and functional differences across generations. Bryophyt. Divers. Evol. 2021, 43, 265–283. [Google Scholar] [CrossRef]
- Seifert, G.J.; Roberts, K. The biology of arabinogalactan proteins. Ann. Rev. Plant Biol. 2007, 58, 137–161. [Google Scholar] [CrossRef] [PubMed]
- Lamport, D.T.A.; Kieliszewski, M.J.; Showalter, A.M. Salt stress upregulates periplasmic arabinogalactan proteins: Using salt stress to analyse AGP function. New Phytol. 2006, 169, 479–492. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Fan, W.; Li, X.; Chen, H.; Takáč, T.; Šamajová, O.; Fabrice, M.R.; Xie, L.; Ma, J.; Šamaj, J.; et al. Expression and distribution of extensins and AGPs in susceptible and resistant banana cultivars in response to wounding and Fusarium oxysporum. Sci. Rep. 2017, 7, 42400. [Google Scholar] [CrossRef] [PubMed]
- Leszczuk, A.; Pieczywek, P.M.; Gryta, A.; Frąc, M.; Zdunek, A. Immunocytochemical studies on the distribution of arabinogalactan proteins (AGPs) as a response to fungal infection in Malus x domestica fruit. Sci. Rep. 2019, 9, 17428. [Google Scholar] [CrossRef] [PubMed]
- Kacprzyk, J.; Daly, C.T.; McCabe, P.F. The botanical dance of death: Programmed cell death in plants. Adv. Bot. Res. 2011, 60, 169–261. [Google Scholar]
- Lopez-Hernandez, F.; Tryfona, T.; Rizza, A.; Yu, X.; Harris, M.O.; Webb, A.A.; Kotake, T.; Dupree, P. Calcium binding by arabinogalactan polysaccharides is important for normal plant development. Plant Cell 2020, 32, 3346–3369. [Google Scholar] [CrossRef]
- Suda, H.; Mano, H.; Toyota, M.; Fukushima, K.; Mimura, T.; Tsutsui, I.; Hedrich, R.; Tamada, Y.; Hasebe, M. Calcium dynamics during trap closure visualized in transgenic Venus flytrap. Nat. Plants 2020, 6, 1219–1224. [Google Scholar] [CrossRef]
- Adlassnig, W.; Koller-Peroutka, M.; Bauer, S.; Koshkin, E.; Lendl, T.; Lichtscheidl, I.K. Endocytotic uptake of nutrients in carnivorous plants. Plant J. 2012, 71, 303–313. [Google Scholar] [CrossRef]
- Schnepf, E. Zur Cytologie und Physiologie pflanzlicher Drüsen. 4. Teil, Licht- und elektronenmikroskopische Untersuchungen an Septalnektarien. Protoplasma 1964, 58, 137–171. [Google Scholar] [CrossRef]
- Samaj, J.; Samajova, O.; Peters, M.; Baluska, F.; Lichtscheidl, I.; Knox, J.P.; Volkmann, D. Immunolocalization of LM2 arabinogalactan protein epitope associated with endomembranes of plant cells. Protoplasma 2000, 212, 186–196. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Płachno, B.J.; Kapusta, M.; Stolarczyk, P.; Świątek, P. Arabinogalactan Proteins in the Digestive Glands of Dionaea muscipula J.Ellis Traps. Cells 2022, 11, 586. https://doi.org/10.3390/cells11030586
Płachno BJ, Kapusta M, Stolarczyk P, Świątek P. Arabinogalactan Proteins in the Digestive Glands of Dionaea muscipula J.Ellis Traps. Cells. 2022; 11(3):586. https://doi.org/10.3390/cells11030586
Chicago/Turabian StylePłachno, Bartosz J., Małgorzata Kapusta, Piotr Stolarczyk, and Piotr Świątek. 2022. "Arabinogalactan Proteins in the Digestive Glands of Dionaea muscipula J.Ellis Traps" Cells 11, no. 3: 586. https://doi.org/10.3390/cells11030586
APA StylePłachno, B. J., Kapusta, M., Stolarczyk, P., & Świątek, P. (2022). Arabinogalactan Proteins in the Digestive Glands of Dionaea muscipula J.Ellis Traps. Cells, 11(3), 586. https://doi.org/10.3390/cells11030586






