Glycomic and Glycoproteomic Techniques in Neurodegenerative Disorders and Neurotrauma: Towards Personalized Markers
Abstract
1. Introduction
1.1. Post-Translational Modifications (PTMs)—An Overview
1.2. Glycosylation of Proteins
1.3. Dynamics of Glycosylation
1.4. Pathophysiological Aspects of Mis-Glycosylated Products
2. Glycomics and Glycoproteomics Methodology
2.1. Challenges in Glycomics and Glycoproteomics
2.2. Enrichment of Glycoproteins
2.2.1. Lectin Enrichment
2.2.2. HILIC Enrichment
2.2.3. Hydrazide Chemistry Enrichment
2.2.4. Click Chemistry Enrichment
2.2.5. Boronic Acid Enrichment
2.3. Technologies in Glycomics and Glycoproteomics
2.3.1. Lectin Microarray in Glycomics and Glycoproteomics
2.3.2. MS-based Glycomics and Glycoproteomics
2.3.3. Dissociation and Acquisition Techniques Facilitate MS-based Glycomic and Glycoproteomic Identification and Quantitation
2.3.4. Software to Facilitate Automated Data Processing
3. Glycomics and Glycoproteomics of Human Biofluid
4. Glycoproteomics and Neurodegeneration
4.1. Glycosylation and Neurodegenerative Diseases
4.1.1. Alzheimer’s Disease
4.1.2. Huntington’s Disease
4.1.3. Multiple Sclerosis Disease
4.1.4. Amyotrophic Lateral Sclerosis Disease
4.1.5. Parkinson’s Disease
4.2. Glycoproteomics and Psychiatric Disorders
4.2.1. Depressive Disorders
4.2.2. Neurodevelopmental Disorders
4.2.3. Schizophrenia and Related Psychotic Disorders
4.2.4. Sleep-Wake Disorders
4.2.5. Trauma- and Stressor-Related Disorders
5. Glycoproteomics and TBI
5.1. Post-Translational Modifications and TBI
5.2. Glycosylation in Neurotrauma
5.3. Neuronal Death following Experimental TBI
6. Glycoproteomics and Glycosylation: Role in Personalized Medicine
6.1. Glycomics and Glycoproteomics in Cancer Studies
6.2. Glycomics and Glycoproteomics in Prion Disease
6.3. Glycomics and Glycoproteomics in Neurodegenerative Diseases
7. Potential Biomarkers in Disease Diagnosis (Clinical Application)
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Aslam, B.; Basit, M.; Nisar, M.A.; Khurshid, M.; Rasool, M.H. Proteomics: Technologies and Their Applications. J. Chromatogr. Sci. 2017, 55, 182–196. [Google Scholar] [CrossRef] [PubMed]
- Ramazi, S.; Zahiri, J. Posttranslational modifications in proteins: Resources, tools and prediction methods. Database (Oxford) 2021, 2021, baab012. [Google Scholar] [CrossRef] [PubMed]
- Klionsky, D.J.; Abdelmohsen, K.; Abe, A.; Abedin, M.J.; Abeliovich, H.; Acevedo Arozena, A.; Adachi, H.; Adams, C.M.; Adams, P.D.; Adeli, K.; et al. Guidelines for the use and interpretation of assays for monitoring autophagy (3rd edition). Autophagy 2016, 12, 1–222. [Google Scholar] [CrossRef] [PubMed]
- Ryslava, H.; Doubnerova, V.; Kavan, D.; Vanek, O. Effect of posttranslational modifications on enzyme function and assembly. J. Proteom. 2013, 92, 80–109. [Google Scholar] [CrossRef]
- Dong, X.; Mondello, S.; Kobeissy, F.; Talih, F.; Ferri, R.; Mechref, Y. LC-MS/MS glycomics of idiopathic rapid eye movement sleep behavior disorder. Electrophoresis 2018, 39, 3096–3103. [Google Scholar] [CrossRef]
- Kobeissy, F.H.; Sadasivan, S.; Oli, M.W.; Robinson, G.; Larner, S.F.; Zhang, Z.; Hayes, R.L.; Wang, K.K. Neuroproteomics and systems biology-based discovery of protein biomarkers for traumatic brain injury and clinical validation. Proteom. Clin. Appl. 2008, 2, 1467–1483. [Google Scholar] [CrossRef]
- Strumillo, M.; Beltrao, P. Towards the computational design of protein post-translational regulation. Bioorg. Med. Chem. 2015, 23, 2877–2882. [Google Scholar] [CrossRef]
- Karve, T.M.; Cheema, A.K. Small changes huge impact: The role of protein posttranslational modifications in cellular homeostasis and disease. J. Amino Acids 2011, 2011, 207691. [Google Scholar] [CrossRef]
- Ohtsubo, K.; Marth, J.D. Glycosylation in Cellular Mechanisms of Health and Disease. Cell 2006, 126, 813–1004. [Google Scholar] [CrossRef]
- Wang, W.; Gopal, S.; Pocock, R.; Xiao, Z. Glycan Mimetics from Natural Products: New Therapeutic Opportunities for Neurodegenerative Disease. Molecules 2019, 24, 4604. [Google Scholar] [CrossRef]
- Vu, L.D.; Gevaert, K.; De Smet, I. Protein Language: Post-Translational Modifications Talking to Each Other. Trends Plant Sci. 2018, 23, 1068–1080. [Google Scholar] [CrossRef] [PubMed]
- Dewhurst, H.M.; Choudhury, S.; Torres, M.P. Structural Analysis of PTM Hotspots (SAPH-ire)—A Quantitative Informatics Method Enabling the Discovery of Novel Regulatory Elements in Protein Families. Mol. Cell. Proteom. MCP 2015, 14, 2285–2297. [Google Scholar] [CrossRef] [PubMed]
- Stowell, S.R.; Ju, T.; Cummings, R.D. Protein glycosylation in cancer. Annu. Rev. Pathol. 2015, 10, 473–510. [Google Scholar] [CrossRef] [PubMed]
- Feizi, T.E.; Haltiwanger, R.S. Editorial overview: Carbohydrate-protein interactions and glycosylation: Glycan synthesis and recognition: Finding the perfect partner in a sugar-coated life. Curr. Opin. Struct. Biol. 2015, 34, vii–ix. [Google Scholar] [CrossRef]
- Tzeng, S.F.; Tsai, C.H.; Chao, T.K.; Chou, Y.C.; Yang, Y.C.; Tsai, M.H.; Cha, T.L.; Hsiao, P.W. O-Glycosylation-mediated signaling circuit drives metastatic castration-resistant prostate cancer. FASEB J. 2018, 32, 6869–6882. [Google Scholar] [CrossRef]
- De Vreede, G.; Morrison, H.A.; Houser, A.M.; Boileau, R.M.; Andersen, D.; Colombani, J.; Bilder, D. A Drosophila Tumor Suppressor Gene Prevents Tonic TNF Signaling through Receptor N-Glycosylation. Dev. Cell 2018, 45, 595–605.e4. [Google Scholar] [CrossRef]
- Oyama, M.; Kariya, Y.; Kariya, Y.; Matsumoto, K.; Kanno, M.; Yamaguchi, Y.; Hashimoto, Y. Biological role of site-specific O-glycosylation in cell adhesion activity and phosphorylation of osteopontin. Biochem. J. 2018, 475, 1583–1595. [Google Scholar] [CrossRef]
- Singh, C.; Shyanti, R.K.; Singh, V.; Kale, R.K.; Mishra, J.P.N.; Singh, R.P. Integrin expression and glycosylation patterns regulate cell-matrix adhesion and alter with breast cancer progression. Biochem. Biophys. Res. Commun. 2018, 499, 374–380. [Google Scholar] [CrossRef]
- Sperandio, M.; Gleissner, C.A.; Ley, K. Glycosylation in immune cell trafficking. Immunol. Rev. 2009, 230, 97–113. [Google Scholar] [CrossRef]
- Veillon, L.; Huang, Y.; Peng, W.; Dong, X.; Cho, B.G.; Mechref, Y. Characterization of isomeric glycan structures by LC-MS/MS. Electrophoresis 2017, 38, 2100–2114. [Google Scholar] [CrossRef]
- Sola, R.J.; Griebenow, K. Effects of glycosylation on the stability of protein pharmaceuticals. J. Pharm. Sci. 2009, 98, 1223–1245. [Google Scholar] [CrossRef] [PubMed]
- Varki, A.; Cummings, R.D.; Esko, J.D.; Stanley, P.; Hart, G.W.; Aebi, M.; Darvill, A.G.; Kinoshita, T.; Packer, N.H.; Prestegard, J.H.; et al. (Eds.) Essentials of Glycobiology, 3rd ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2015. [Google Scholar]
- Bieberich, E. Synthesis, Processing, and Function of N-glycans in N-glycoproteins. Adv. Neurobiol. 2014, 9, 47–70. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.R.; Shin, J.W.; Na, J.I.; Nam, K.M.; Lee, H.S.; Park, K.C. Novel Antioxidant Tripeptide “ACQ” Can Prevent UV-Induced Cell Death and Preserve the Number of Epidermal Stem Cells. Oxid. Med. Cell Longev. 2015, 2015, 359740. [Google Scholar] [CrossRef] [PubMed]
- Kobata, A. Glycobiology in the field of aging research--introduction to glycogerontology. Biochimie 2003, 85, 13–24. [Google Scholar] [CrossRef]
- Grigorian, A.; Demetriou, M. Manipulating cell surface glycoproteins by targeting N-glycan-galectin interactions. Methods Enzymol. 2010, 480, 245–266. [Google Scholar] [CrossRef]
- Schmaltz, R.M.; Hanson, S.R.; Wong, C.H. Enzymes in the synthesis of glycoconjugates. Chem. Rev. 2011, 111, 4259–4307. [Google Scholar] [CrossRef]
- Schnaar, R.L. Glycobiology simplified: Diverse roles of glycan recognition in inflammation. J. Leukoc. Biol. 2016, 99, 825–838. [Google Scholar] [CrossRef]
- Dube, D.H.; Bertozzi, C.R. Glycans in cancer and inflammation—Potential for therapeutics and diagnostics. Nat. Rev. Drug Discov. 2005, 4, 477–488. [Google Scholar] [CrossRef]
- Yu, C.; Lv, B.; Min, S.; Ren, L.; Yu, J. Combined usage of monosaccharides with polysaccharides may decelerate tumor growth and malignance versus solely using a certain kind of saccharide. Biochem. Biophys. Res. Commun. 2020, 525, 800–805. [Google Scholar] [CrossRef]
- Wu, D.; Struwe, W.B.; Harvey, D.J.; Ferguson, M.A.J.; Robinson, C.V. N-glycan microheterogeneity regulates interactions of plasma proteins. Proc. Natl. Acad. Sci. USA 2018, 115, 8763–8768. [Google Scholar] [CrossRef]
- Wooding, K.M.; Peng, W.; Mechref, Y. Characterization of Pharmaceutical IgG and Biosimilars Using Miniaturized Platforms and LC-MS-MS. Curr. Pharm. Biotechnol. 2016, 17, 788–801. [Google Scholar] [CrossRef] [PubMed]
- Kailemia, M.J.; Xu, G.; Wong, M.; Li, Q.; Goonatilleke, E.; Leon, F.; Lebrilla, C.B. Recent Advances in the Mass Spectrometry Methods for Glycomics and Cancer. Anal. Chem. 2018, 90, 208–224. [Google Scholar] [CrossRef] [PubMed]
- Moremen, K.W.; Tiemeyer, M.; Nairn, A.V. Vertebrate protein glycosylation: Diversity, synthesis and function. Nat. Rev. Mol. Cell Biol. 2012, 13, 448–462. [Google Scholar] [CrossRef] [PubMed]
- Varki, A. Biological roles of glycans. Glycobiology 2017, 27, 3–49. [Google Scholar] [CrossRef] [PubMed]
- National Research Council (US) Committee on Assessing the Importance and Impact of Glycomics and Glycosciences. The National Academies Collection: Reports funded by National Institutes of Health. In Transforming Glycoscience: A Roadmap for the Future; National Academies Press (US) National Academy of Sciences: Washington, DC, USA, 2012. [Google Scholar]
- Trombetta, E.S. The contribution of N-glycans and their processing in the endoplasmic reticulum to glycoprotein biosynthesis. Glycobiology 2003, 13, 77R–91R. [Google Scholar] [CrossRef]
- Davids, M.; Kane, M.S.; He, M.; Wolfe, L.A.; Li, X.; Raihan, M.A.; Chao, K.R.; Bone, W.P.; Boerkoel, C.F.; Gahl, W.A.; et al. Disruption of Golgi morphology and altered protein glycosylation in PLA2G6-associated neurodegeneration. J. Med. Genet. 2016, 53, 180–189. [Google Scholar] [CrossRef]
- Ahat, E.; Xiang, Y.; Zhang, X.; Bekier, M.E., 2nd; Wang, Y. GRASP depletion-mediated Golgi destruction decreases cell adhesion and migration via the reduction of alpha5beta1 integrin. Mol. Biol. Cell 2019, 30, 766–777. [Google Scholar] [CrossRef]
- Xiang, Y.; Zhang, X.; Nix, D.B.; Katoh, T.; Aoki, K.; Tiemeyer, M.; Wang, Y. Regulation of protein glycosylation and sorting by the Golgi matrix proteins GRASP55/65. Nat. Commun. 2013, 4, 1659. [Google Scholar] [CrossRef]
- Freeze, H.H.; Chong, J.X.; Bamshad, M.J.; Ng, B.G. Solving glycosylation disorders: Fundamental approaches reveal complicated pathways. Am. J. Hum. Genet. 2014, 94, 161–175. [Google Scholar] [CrossRef]
- Van den Boogert, M.A.W.; Rader, D.J.; Holleboom, A.G. New insights into the role of glycosylation in lipoprotein metabolism. Curr. Opin. Lipidol. 2017, 28, 502–506. [Google Scholar] [CrossRef]
- Furukawa, K.; Ohkawa, Y.; Yamauchi, Y.; Hamamura, K.; Ohmi, Y.; Furukawa, K. Fine tuning of cell signals by glycosylation. J. Biochem. 2012, 151, 573–578. [Google Scholar] [CrossRef] [PubMed]
- Hansen, L.; Lind-Thomsen, A.; Joshi, H.J.; Pedersen, N.B.; Have, C.T.; Kong, Y.; Wang, S.; Sparso, T.; Grarup, N.; Vester-Christensen, M.B.; et al. A glycogene mutation map for discovery of diseases of glycosylation. Glycobiology 2015, 25, 211–224. [Google Scholar] [CrossRef]
- Khan, A.H.; Bayat, H.; Rajabibazl, M.; Sabri, S.; Rahimpour, A. Humanizing glycosylation pathways in eukaryotic expression systems. World J. Microbiol. Biotechnol. 2017, 33, 4. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, Y. Glycosylation Quality Control by the Golgi Structure. J. Mol. Biol. 2016, 428, 3183–3193. [Google Scholar] [CrossRef] [PubMed]
- Briggs, A.M.; Cross, M.J.; Hoy, D.G.; Sanchez-Riera, L.; Blyth, F.M.; Woolf, A.D.; March, L. Musculoskeletal Health Conditions Represent a Global Threat to Healthy Aging: A Report for the 2015 World Health Organization World Report on Ageing and Health. Gerontologist 2016, 56, S243–S255. [Google Scholar] [CrossRef] [PubMed]
- Rattan, S.I. Increased molecular damage and heterogeneity as the basis of aging. Biol. Chem. 2008, 389, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Blomme, B.; Van Steenkiste, C.; Callewaert, N.; Van Vlierberghe, H. Alteration of protein glycosylation in liver diseases. J. Hepatol. 2009, 50, 592–603. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.K.; Chung, T.W.; Lee, J.Y.; Lee, Y.C.; Morton, R.E.; Kim, C.H. The hepatitis B virus X protein inhibits secretion of apolipoprotein B by enhancing the expression of N-acetylglucosaminyltransferase III. J. Biol. Chem. 2004, 279, 28106–28112. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Guo, Y.; Du, H.; Zhong, Y.; Zhang, J.; Li, X.; Yu, H.; Zhang, Z.; Jia, Z.; Li, Z. Comparative Analysis for Glycopatterns and Complex-Type N-Glycans of Glycoprotein in Sera from Chronic Hepatitis B- and C-Infected Patients. Front. Physiol. 2017, 8, 596. [Google Scholar] [CrossRef]
- Miura, Y.; Endo, T. Glycomics and glycoproteomics focused on aging and age-related diseases--Glycans as a potential biomarker for physiological alterations. Biochim. Biophys. Acta 2016, 1860, 1608–1614. [Google Scholar] [CrossRef]
- Testa, R.; Vanhooren, V.; Bonfigli, A.R.; Boemi, M.; Olivieri, F.; Ceriello, A.; Genovese, S.; Spazzafumo, L.; Borelli, V.; Bacalini, M.G.; et al. N-glycomic changes in serum proteins in type 2 diabetes mellitus correlate with complications and with metabolic syndrome parameters. PLoS ONE 2015, 10, e0119983. [Google Scholar] [CrossRef] [PubMed]
- Smilowitz, J.T.; Totten, S.M.; Huang, J.; Grapov, D.; Durham, H.A.; Lammi-Keefe, C.J.; Lebrilla, C.; German, J.B. Human milk secretory immunoglobulin a and lactoferrin N-glycans are altered in women with gestational diabetes mellitus. J. Nutr. 2013, 143, 1906–1912. [Google Scholar] [CrossRef] [PubMed]
- Carney, E.F. Polycystic kidney disease: PMM2 mutation causes PKD and hyperinsulinism. Nat. Rev. Nephrol. 2017, 13, 321. [Google Scholar] [CrossRef] [PubMed]
- Caglayan, A.O.; Comu, S.; Baranoski, J.F.; Parman, Y.; Kaymakcalan, H.; Akgumus, G.T.; Caglar, C.; Dolen, D.; Erson-Omay, E.Z.; Harmanci, A.S.; et al. NGLY1 mutation causes neuromotor impairment, intellectual disability, and neuropathy. Eur. J. Med. Genet. 2015, 58, 39–43. [Google Scholar] [CrossRef] [PubMed]
- Song, E.; Mechref, Y. Defining glycoprotein cancer biomarkers by MS in conjunction with glycoprotein enrichment. Biomark. Med. 2015, 9, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Epp, A.; Sullivan, K.C.; Herr, A.B.; Strait, R.T. Immunoglobulin Glycosylation Effects in Allergy and Immunity. Curr. Allergy Asthma. Rep. 2016, 16, 79. [Google Scholar] [CrossRef]
- Potapenko, I.O.; Luders, T.; Russnes, H.G.; Helland, A.; Sorlie, T.; Kristensen, V.N.; Nord, S.; Lingjaerde, O.C.; Borresen-Dale, A.L.; Haakensen, V.D. Glycan-related gene expression signatures in breast cancer subtypes; relation to survival. Mol. Oncol. 2015, 9, 861–876. [Google Scholar] [CrossRef]
- Vajaria, B.N.; Patel, P.S. Glycosylation: A hallmark of cancer? Glycoconj. J. 2017, 34, 147–156. [Google Scholar] [CrossRef]
- Huang, Y.; Zhou, S.; Zhu, J.; Lubman, D.M.; Mechref, Y. LC-MS/MS isomeric profiling of permethylated N-glycans derived from serum haptoglobin of hepatocellular carcinoma (HCC) and cirrhotic patients. Electrophoresis 2017, 38, 2160–2167. [Google Scholar] [CrossRef]
- Zhu, R.; Zacharias, L.; Wooding, K.M.; Peng, W.; Mechref, Y. Glycoprotein Enrichment Analytical Techniques: Advantages and Disadvantages. Methods Enzymol. 2017, 585, 397–429. [Google Scholar] [CrossRef]
- Jung, K.; Cho, W. Serial affinity chromatography as a selection tool in glycoproteomics. Anal. Chem. 2013, 85, 7125–7132. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Hancock, W.S. Approach to the comprehensive analysis of glycoproteins isolated from human serum using a multi-lectin affinity column. J. Chromatogr. A 2004, 1053, 79–88. [Google Scholar] [CrossRef]
- Zauner, G.; Deelder, A.M.; Wuhrer, M. Recent advances in hydrophilic interaction liquid chromatography (HILIC) for structural glycomics. Electrophoresis 2011, 32, 3456–3466. [Google Scholar] [CrossRef] [PubMed]
- Selman, M.H.; Hemayatkar, M.; Deelder, A.M.; Wuhrer, M. Cotton HILIC SPE microtips for microscale purification and enrichment of glycans and glycopeptides. Anal. Chem. 2011, 83, 2492–2499. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.P.; Ruan, C.P.; Wang, H.; Hu, Z.Q.; Fang, M.; Gu, X.; Ji, J.; Zhao, J.Y.; Gao, C.F. Identification and assessment of new biomarkers for colorectal cancer with serum N-glycan profiling. Cancer 2012, 118, 639–650. [Google Scholar] [CrossRef]
- Zhao, J.; Song, E.; Zhu, R.; Mechref, Y. Parallel data acquisition of in-source fragmented glycopeptides to sequence the glycosylation sites of proteins. Electrophoresis 2016, 37, 1420–1430. [Google Scholar] [CrossRef]
- Wang, J.; Li, J.; Wang, Y.; Gao, M.; Zhang, X.; Yang, P. Development of Versatile Metal-Organic Framework Functionalized Magnetic Graphene Core-Shell Biocomposite for Highly Specific Recognition of Glycopeptides. ACS Appl. Mater. Interfaces 2016, 8, 27482–27489. [Google Scholar] [CrossRef]
- Ma, W.; Xu, L.; Li, X.; Shen, S.; Wu, M.; Bai, Y.; Liu, H. Cysteine-Functionalized Metal-Organic Framework: Facile Synthesis and High Efficient Enrichment of N-Linked Glycopeptides in Cell Lysate. ACS Appl. Mater. Interfaces 2017, 9, 19562–19568. [Google Scholar] [CrossRef]
- Liu, J.; Yang, K.; Shao, W.; Li, S.; Wu, Q.; Zhang, S.; Qu, Y.; Zhang, L.; Zhang, Y. Synthesis of Zwitterionic Polymer Particles via Combined Distillation Precipitation Polymerization and Click Chemistry for Highly Efficient Enrichment of Glycopeptide. ACS Appl. Mater. Interfaces 2016, 8, 22018–22024. [Google Scholar] [CrossRef]
- Cao, W.; Huang, J.; Jiang, B.; Gao, X.; Yang, P. Highly Selective Enrichment of Glycopeptides Based on Zwitterionically Functionalized Soluble Nanopolymers. Sci. Rep. 2016, 6, 29776. [Google Scholar] [CrossRef]
- Wan, H.; Huang, J.; Liu, Z.; Li, J.; Zhang, W.; Zou, H. A dendrimer-assisted magnetic graphene–silica hydrophilic composite for efficient and selective enrichment of glycopeptides from the complex sample. Chem. Commun. 2015, 51, 9391–9394. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Wang, J.; Yao, J.; Deng, C. Hydrophilic Mesoporous Silica Materials for Highly Specific Enrichment of N-Linked Glycopeptide. Anal. Chem. 2017, 89, 1764–1771. [Google Scholar] [CrossRef] [PubMed]
- De Jong, E.P.; Griffin, T.J. Online nanoscale ERLIC-MS outperforms RPLC-MS for shotgun proteomics in complex mixtures. J. Proteome Res. 2012, 11, 5059–5064. [Google Scholar] [CrossRef] [PubMed]
- Zacharias, L.G.; Hartmann, A.K.; Song, E.; Zhao, J.; Zhu, R.; Mirzaei, P.; Mechref, Y. HILIC and ERLIC Enrichment of Glycopeptides Derived from Breast and Brain Cancer Cells. J. Proteome Res. 2016, 15, 3624–3634. [Google Scholar] [CrossRef]
- Zhang, H.; Li, X.J.; Martin, D.B.; Aebersold, R. Identification and quantification of N-linked glycoproteins using hydrazide chemistry, stable isotope labeling and mass spectrometry. Nat. Biotechnol. 2003, 21, 660–666. [Google Scholar] [CrossRef]
- Chen, R.; Tan, Y.; Wang, M.; Wang, F.; Yao, Z.; Dong, L.; Ye, M.; Wang, H.; Zou, H. Development of glycoprotein capture-based label-free method for the high-throughput screening of differential glycoproteins in hepatocellular carcinoma. Mol. Cell Proteom. 2011, 10, M110.006445. [Google Scholar] [CrossRef]
- Zeng, X.; Hood, B.L.; Sun, M.; Conrads, T.P.; Day, R.S.; Weissfeld, J.L.; Siegfried, J.M.; Bigbee, W.L. Lung cancer serum biomarker discovery using glycoprotein capture and liquid chromatography mass spectrometry. J. Proteome Res. 2010, 9, 6440–6449. [Google Scholar] [CrossRef]
- Whelan, S.A.; Lu, M.; He, J.; Yan, W.; Saxton, R.E.; Faull, K.F.; Whitelegge, J.P.; Chang, H.R. Mass spectrometry (LC-MS/MS) site-mapping of N-glycosylated membrane proteins for breast cancer biomarkers. J. Proteome Res. 2009, 8, 4151–4160. [Google Scholar] [CrossRef]
- Wang, Q.; Chan, T.R.; Hilgraf, R.; Fokin, V.V.; Sharpless, K.B.; Finn, M.G. Bioconjugation by copper(I)-catalyzed azide-alkyne [3 + 2] cycloaddition. J. Am. Chem. Soc. 2003, 125, 3192–3193. [Google Scholar] [CrossRef]
- Agard, N.J.; Prescher, J.A.; Bertozzi, C.R. A strain-promoted [3 + 2] azide-alkyne cycloaddition for covalent modification of biomolecules in living systems. J. Am. Chem. Soc. 2004, 126, 15046–15047. [Google Scholar] [CrossRef]
- Ma, R.; Hu, J.; Cai, Z.; Ju, H. Facile synthesis of boronic acid-functionalized magnetic carbon nanotubes for highly specific enrichment of glycopeptides. Nanoscale 2014, 6, 3150–3156. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhao, W.; Zhao, Y.; Mao, Y.; Su, T.; Zhong, Y.; Wang, S.; Zhai, R.; Cheng, J.; Fang, X.; et al. Comparative Glycoproteomic Profiling of Human Body Fluid between Healthy Controls and Patients with Papillary Thyroid Carcinoma. J. Proteome Res. 2019, 19, 2539–2552. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Liu, Q.; Li, Y.; Deng, C. Core-shell structured magnetic metal-organic framework composites for highly selective detection of N-glycopeptides based on boronic acid affinity chromatography. J. Chromatogr. A 2018, 1540, 87–93. [Google Scholar] [CrossRef] [PubMed]
- Xiao, H.; Chen, W.; Smeekens, J.M.; Wu, R. An enrichment method based on synergistic and reversible covalent interactions for large-scale analysis of glycoproteins. Nat. Commun. 2018, 9, 1692. [Google Scholar] [CrossRef]
- Dell, A.; Morris, H.R. Glycoprotein structure determination by mass spectrometry. Science 2001, 291, 2351–2356. [Google Scholar] [CrossRef]
- Mechref, Y.; Novotny, M.V. Structural investigations of glycoconjugates at high sensitivity. Chem. Rev. 2002, 102, 321–369. [Google Scholar] [CrossRef]
- Banazadeh, A.; Peng, W.; Veillon, L.; Mechref, Y. Carbon Nanoparticles and Graphene Nanosheets as MALDI Matrices in Glycomics: A New Approach to Improve Glycan Profiling in Biological Samples. J. Am. Soc. Mass Spectrom. 2018, 29, 1892–1900. [Google Scholar] [CrossRef]
- Novotny, M.V.; Mechref, Y. New hyphenated methodologies in high-sensitivity glycoprotein analysis. J. Sep. Sci. 2005, 28, 1956–1968. [Google Scholar] [CrossRef]
- Zheng, T.; Peelen, D.; Smith, L.M. Lectin arrays for profiling cell surface carbohydrate expression. J. Am. Chem. Soc. 2005, 127, 9982–9983. [Google Scholar] [CrossRef]
- Hiono, T.; Matsuda, A.; Wagatsuma, T.; Okamatsu, M.; Sakoda, Y.; Kuno, A. Lectin microarray analyses reveal host cell-specific glycan profiles of the hemagglutinins of influenza A viruses. Virology 2019, 527, 132–140. [Google Scholar] [CrossRef]
- Fukuyama, Y.; Nakaya, S.; Yamazaki, Y.; Tanaka, K. Ionic liquid matrixes optimized for MALDI-MS of sulfated/sialylated/neutral oligosaccharides and glycopeptides. Anal. Chem. 2008, 80, 2171–2179. [Google Scholar] [CrossRef] [PubMed]
- Fukuyama, Y.; Funakoshi, N.; Takeyama, K.; Hioki, Y.; Nishikaze, T.; Kaneshiro, K.; Kawabata, S.; Iwamoto, S.; Tanaka, K. 3-Aminoquinoline/p-coumaric acid as a MALDI matrix for glycopeptides, carbohydrates, and phosphopeptides. Anal. Chem. 2014, 86, 1937–1942. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, P.B.; Mirgorodskaya, E.; Costello, C.E. High pressure matrix-assisted laser desorption/ionization Fourier transform mass spectrometry for minimization of ganglioside fragmentation. J. Am. Soc. Mass Spectrom. 2002, 13, 402–407. [Google Scholar] [CrossRef]
- Reiding, K.R.; Blank, D.; Kuijper, D.M.; Deelder, A.M.; Wuhrer, M. High-throughput profiling of protein N-glycosylation by MALDI-TOF-MS employing linkage-specific sialic acid esterification. Anal. Chem. 2014, 86, 5784–5793. [Google Scholar] [CrossRef]
- Banazadeh, A.; Nieman, R.; Goli, M.; Peng, W.; Hussein, A.; Bursal, E.; Lischka, H.; Mechref, Y. Characterization of glycan isomers using magnetic carbon nanoparticles as a MALDI co-matrix. RSC Adv. 2019, 9, 20137–20148. [Google Scholar] [CrossRef]
- Toghi Eshghi, S.; Yang, S.; Wang, X.; Shah, P.; Li, X.; Zhang, H. Imaging of N-linked glycans from formalin-fixed paraffin-embedded tissue sections using MALDI mass spectrometry. ACS Chem. Biol. 2014, 9, 2149–2156. [Google Scholar] [CrossRef]
- Powers, T.W.; Jones, E.E.; Betesh, L.R.; Romano, P.R.; Gao, P.; Copland, J.A.; Mehta, A.S.; Drake, R.R. Matrix assisted laser desorption ionization imaging mass spectrometry workflow for spatial profiling analysis of N-linked glycan expression in tissues. Anal. Chem. 2013, 85, 9799–9806. [Google Scholar] [CrossRef]
- Holst, S.; Heijs, B.; de Haan, N.; van Zeijl, R.J.; Briaire-de Bruijn, I.H.; van Pelt, G.W.; Mehta, A.S.; Angel, P.M.; Mesker, W.E.; Tollenaar, R.A.; et al. Linkage-Specific in Situ Sialic Acid Derivatization for N-Glycan Mass Spectrometry Imaging of Formalin-Fixed Paraffin-Embedded Tissues. Anal. Chem. 2016, 88, 5904–5913. [Google Scholar] [CrossRef]
- Nishikaze, T.; Kawabata, S.-i.; Tanaka, K. In-depth structural characterization of N-linked glycopeptides using complete derivatization for carboxyl groups followed by positive-and negative-ion tandem mass spectrometry. Anal. Chem. 2014, 86, 5360–5369. [Google Scholar] [CrossRef]
- Yang, S.; Wu, W.W.; Shen, R.-F.; Bern, M.; Cipollo, J. Identification of Sialic Acid Linkages on Intact Glycopeptides via Differential Chemical Modification Using IntactGIG-HILIC. J. Am. Soc. Mass Spectrom. 2018, 29, 1273–1283. [Google Scholar] [CrossRef]
- Shajahan, A.; Supekar, N.T.; Heiss, C.; Ishihara, M.; Azadi, P. Tool for Rapid Analysis of Glycopeptide by Permethylation via One-Pot Site Mapping and Glycan Analysis. Anal. Chem. 2017, 89, 10734–10743. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Veillon, L.; Dong, X.; Huang, Y.; Mechref, Y. Direct comparison of derivatization strategies for LC-MS/MS analysis of N-glycans. Analyst 2017, 142, 4446–4455. [Google Scholar] [CrossRef]
- Zhou, S.; Hu, Y.; Mechref, Y. High-temperature LC-MS/MS of permethylated glycans derived from glycoproteins. Electrophoresis 2016, 37, 1506–1513. [Google Scholar] [CrossRef] [PubMed]
- Wuhrer, M.; de Boer, A.R.; Deelder, A.M. Structural glycomics using hydrophilic interaction chromatography (HILIC) with mass spectrometry. Mass Spectrom. Rev. 2009, 28, 192–206. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T. Peptide separation by Hydrophilic-Interaction Chromatography: A review. J. Biochem. Biophys. Methods 2004, 60, 265–280. [Google Scholar] [CrossRef] [PubMed]
- Mauko, L.; Lacher, N.A.; Pelzing, M.; Nordborg, A.; Haddad, P.R.; Hilder, E.F. Comparison of ZIC-HILIC and graphitized carbon-based analytical approaches combined with exoglycosidase digestions for analysis of glycans from monoclonal antibodies. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2012, 911, 93–104. [Google Scholar] [CrossRef]
- Jiang, K.; Wang, C.; Sun, Y.; Liu, Y.; Zhang, Y.; Huang, L.; Wang, Z. Comparison of chicken and pheasant ovotransferrin N-glycoforms via electrospray ionization mass spectrometry and liquid chromatography coupled with mass spectrometry. J. Agric. Food Chem. 2014, 62, 7245–7254. [Google Scholar] [CrossRef]
- Mancera-Arteu, M.; Gimenez, E.; Barbosa, J.; Sanz-Nebot, V. Identification and characterization of isomeric N-glycans of human alfa-acid-glycoprotein by stable isotope labelling and ZIC-HILIC-MS in combination with exoglycosidase digestion. Anal. Chim. Acta 2016, 940, 92–103. [Google Scholar] [CrossRef]
- Zhao, J.; Li, S.; Li, C.; Wu, S.L.; Xu, W.; Chen, Y.; Shameem, M.; Richardson, D.; Li, H. Identification of Low Abundant Isomeric N-Glycan Structures in Biological Therapeutics by LC/MS. Anal. Chem. 2016, 88, 7049–7059. [Google Scholar] [CrossRef]
- Tousi, F.; Bones, J.; Hancock, W.S.; Hincapie, M. Differential chemical derivatization integrated with chromatographic separation for analysis of isomeric sialylated N-glycans: A nano-hydrophilic interaction liquid chromatography-MS platform. Anal. Chem. 2013, 85, 8421–8428. [Google Scholar] [CrossRef]
- Tao, S.; Huang, Y.; Boyes, B.E.; Orlando, R. Liquid chromatography-selected reaction monitoring (LC-SRM) approach for the separation and quantitation of sialylated N-glycans linkage isomers. Anal. Chem. 2014, 86, 10584–10590. [Google Scholar] [CrossRef]
- Ahn, J.; Bones, J.; Yu, Y.Q.; Rudd, P.M.; Gilar, M. Separation of 2-aminobenzamide labeled glycans using hydrophilic interaction chromatography columns packed with 1.7 microm sorbent. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2010, 878, 403–408. [Google Scholar] [CrossRef]
- Mancera-Arteu, M.; Giménez, E.; Barbosa, J.; Peracaula, R.; Sanz-Nebot, V. Zwitterionic-hydrophilic interaction capillary liquid chromatography coupled to tandem mass spectrometry for the characterization of human alpha-acid-glycoprotein N-glycan isomers. Anal. Chim. Acta 2017, 991, 76–88. [Google Scholar] [CrossRef] [PubMed]
- West, C.; Elfakir, C.; Lafosse, M. Porous graphitic carbon: A versatile stationary phase for liquid chromatography. J. Chromatogr. A 2010, 1217, 3201–3216. [Google Scholar] [CrossRef]
- Pabst, M.; Altmann, F. Influence of electrosorption, solvent, temperature, and ion polarity on the performance of LC-ESI-MS using graphitic carbon for acidic oligosaccharides. Anal. Chem. 2008, 80, 7534–7542. [Google Scholar] [CrossRef] [PubMed]
- Pabst, M.; Bondili, J.S.; Stadlmann, J.; Mach, L.; Altmann, F. Mass + retention time = structure: A strategy for the analysis of N-glycans by carbon LC-ESI-MS and its application to fibrin N-glycans. Anal. Chem. 2007, 79, 5051–5057. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lindsay, L.L.; Hedrick, J.L.; Lebrilla, C.B. Strategy for profiling and structure elucidation of mucin-type oligosaccharides by mass spectrometry. Anal. Chem. 2004, 76, 5990–6001. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.; An, H.J.; Ozcan, S.; Ro, G.S.; Soares, S.; DeVere-White, R.; Lebrilla, C.B. Comprehensive native glycan profiling with isomer separation and quantitation for the discovery of cancer biomarkers. Analyst 2011, 136, 3663–3671. [Google Scholar] [CrossRef]
- Zhou, S.; Huang, Y.; Dong, X.; Peng, W.; Veillon, L.; Kitagawa, D.A.S.; Aquino, A.J.A.; Mechref, Y. Isomeric Separation of Permethylated Glycans by Porous Graphitic Carbon (PGC)-LC-MS/MS at High Temperatures. Anal. Chem. 2017, 89, 6590–6597. [Google Scholar] [CrossRef]
- Peng, W.; Goli, M.; Mirzaei, P.; Mechref, Y. Revealing the Biological Attributes of N-Glycan Isomers in Breast Cancer Brain Metastasis Using Porous Graphitic Carbon (PGC) Liquid Chromatography-Tandem Mass Spectrometry (LC-MS/MS). J. Proteome Res. 2019, 18, 3731–3740. [Google Scholar] [CrossRef]
- Dong, X.; Zhou, S.; Mechref, Y. LC-MS/MS analysis of permethylated free oligosaccharides and N-glycans derived from human, bovine, and goat milk samples. Electrophoresis 2016, 37, 1532–1548. [Google Scholar] [CrossRef] [PubMed]
- Grey, C.; Edebrink, P.; Krook, M.; Jacobsson, S.P. Development of a high performance anion exchange chromatography analysis for mapping of oligosaccharides. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2009, 877, 1827–1832. [Google Scholar] [CrossRef] [PubMed]
- Kandzia, S.; Costa, J. N-glycosylation analysis by HPAEC-PAD and mass spectrometry. In Ovarian Cancer: Methods and Protocols; Malek, A., Tchernitsa, O., Walker, J.M., Eds.; Methods in Molecular Biology; Springer Science Humana Press: Totowa, NJ, USA, 2013; Volume 1049, pp. 301–312. [Google Scholar]
- Behan, J.L.; Smith, K.D. The analysis of glycosylation: A continued need for high pH anion exchange chromatography. Biomed. Chromatogr. 2011, 25, 39–46. [Google Scholar] [CrossRef]
- Bones, J.; McLoughlin, N.; Hilliard, M.; Wynne, K.; Karger, B.L.; Rudd, P.M. 2D-LC analysis of BRP 3 erythropoietin N-glycosylation using anion exchange fractionation and hydrophilic interaction UPLC reveals long poly-N-acetyl lactosamine extensions. Anal. Chem. 2011, 83, 4154–4162. [Google Scholar] [CrossRef] [PubMed]
- Rohrer, J.S.; Basumallick, L.; Hurum, D.C. Profiling N-linked oligosaccharides from IgG by high-performance anion-exchange chromatography with pulsed amperometric detection. Glycobiology 2016, 26, 582–591. [Google Scholar] [CrossRef] [PubMed]
- Maier, M.; Reusch, D.; Bruggink, C.; Bulau, P.; Wuhrer, M.; Molhoj, M. Applying mini-bore HPAEC-MS/MS for the characterization and quantification of Fc N-glycans from heterogeneously glycosylated IgGs. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2016, 1033–1034, 342–352. [Google Scholar] [CrossRef]
- Gimenez, E.; Ramos-Hernan, R.; Benavente, F.; Barbosa, J.; Sanz-Nebot, V. Capillary electrophoresis time-of-flight mass spectrometry for a confident elucidation of a glycopeptide map of recombinant human erythropoietin. Rapid Commun. Mass Spectrom. 2011, 25, 2307–2316. [Google Scholar] [CrossRef]
- Imami, K.; Ishihama, Y.; Terabe, S. On-line selective enrichment and ion-pair reaction for structural determination of sulfated glycopeptides by capillary electrophoresis-mass spectrometry. J. Chromatogr. A 2008, 1194, 237–242. [Google Scholar] [CrossRef]
- Thakur, D.; Rejtar, T.; Karger, B.L.; Washburn, N.J.; Bosques, C.J.; Gunay, N.S.; Shriver, Z.; Venkataraman, G. Profiling the glycoforms of the intact alpha subunit of recombinant human chorionic gonadotropin by high-resolution capillary electrophoresis-mass spectrometry. Anal. Chem. 2009, 81, 8900–8907. [Google Scholar] [CrossRef]
- Ongay, S.; Neusüss, C. Isoform differentiation of intact AGP from human serum by capillary electrophoresis-mass spectrometry. Anal. Bioanal. Chem. 2010, 398, 845–855. [Google Scholar] [CrossRef]
- Neusüss, C.; Pelzing, M. Capillary zone electrophoresis-mass spectrometry for the characterization of isoforms of intact glycoproteins. Methods Mol. Biol. 2009, 492, 201–213. [Google Scholar] [CrossRef]
- Reusch, D.; Haberger, M.; Kailich, T.; Heidenreich, A.K.; Kampe, M.; Bulau, P.; Wuhrer, M. High-throughput glycosylation analysis of therapeutic immunoglobulin G by capillary gel electrophoresis using a DNA analyzer. MAbs 2014, 6, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Adamczyk, B.; Tharmalingam-Jaikaran, T.; Schomberg, M.; Szekrenyes, A.; Kelly, R.M.; Karlsson, N.G.; Guttman, A.; Rudd, P.M. Comparison of separation techniques for the elucidation of IgG N-glycans pooled from healthy mammalian species. Carbohydr. Res. 2014, 389, 174–185. [Google Scholar] [CrossRef]
- Zhong, X.; Chen, Z.; Snovida, S.; Liu, Y.; Rogers, J.C.; Li, L. Capillary Electrophoresis-Electrospray Ionization-Mass Spectrometry for Quantitative Analysis of Glycans Labeled with Multiplex Carbonyl-Reactive Tandem Mass Tags. Anal. Chem. 2015, 87, 6527–6534. [Google Scholar] [CrossRef]
- Varadi, C.; Mittermayr, S.; Millan-Martin, S.; Bones, J. Quantitative twoplex glycan analysis using (12)C6 and (13)C6 stable isotope 2-aminobenzoic acid labelling and capillary electrophoresis mass spectrometry. Anal. Bioanal. Chem. 2016, 408, 8691–8700. [Google Scholar] [CrossRef]
- Iwatsuka, K.; Iwamoto, H.; Kinoshita, M.; Inada, K.; Yasueda, S.; Kakehi, K. Comparative studies of N-glycans and glycosaminoglycans present in SIRC (Statens Seruminstitut rabbit cornea) cells and corneal epithelial cells from rabbit eyes. Curr. Eye Res. 2014, 39, 686–694. [Google Scholar] [CrossRef] [PubMed]
- Lu, G.; Crihfield, C.L.; Gattu, S.; Veltri, L.M.; Holland, L.A. Capillary Electrophoresis Separations of Glycans. Chem. Rev. 2018, 118, 7867–7885. [Google Scholar] [CrossRef]
- Feng, H.T.; Li, P.; Rui, G.; Stray, J.; Khan, S.; Chen, S.M.; Li, S.F.Y. Multiplexing N-glycan analysis by DNA analyzer. Electrophoresis 2017, 38, 1788–1799. [Google Scholar] [CrossRef] [PubMed]
- Gabelica, V.; Marklund, E. Fundamentals of ion mobility spectrometry. Curr. Opin. Chem. Biol. 2018, 42, 51–59. [Google Scholar] [CrossRef]
- Snyder, C.M.; Zhou, X.; Karty, J.A.; Fonslow, B.R.; Novotny, M.V.; Jacobson, S.C. Capillary electrophoresis-mass spectrometry for direct structural identification of serum N-glycans. J. Chromatogr. A 2017, 1523, 127–139. [Google Scholar] [CrossRef]
- Ewing, M.A.; Glover, M.S.; Clemmer, D.E. Hybrid ion mobility and mass spectrometry as a separation tool. J. Chromatogr. A 2016, 1439, 3–25. [Google Scholar] [CrossRef]
- Zhu, F.F.; Trinidad, J.C.; Clemmer, D.E. Glycopeptide Site Heterogeneity and Structural Diversity Determined by Combined Lectin Affinity Chromatography/IMS/CID/MS Techniques. J. Am. Soc. Mass Spectrom. 2015, 26, 1092–1102. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, J.; Pagel, K. Glycan Analysis by Ion Mobility-Mass Spectrometry. Angew. Chem. Int. Ed. Engl. 2017, 56, 8342–8349. [Google Scholar] [CrossRef] [PubMed]
- Manz, C.; Pagel, K. Glycan analysis by ion mobility-mass spectrometry and gas-phase spectroscopy. Curr. Opin. Chem. Biol. 2018, 42, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Kizuka, Y.; Kitazume, S.; Fujinawa, R.; Saito, T.; Iwata, N.; Saido, T.C.; Nakano, M.; Yamaguchi, Y.; Hashimoto, Y.; Staufenbiel, M.; et al. An aberrant sugar modification of BACE1 blocks its lysosomal targeting in Alzheimer’s disease. EMBO Mol. Med. 2015, 7, 175–189. [Google Scholar] [CrossRef]
- Lee, J.; Ha, S.; Kim, M.; Kim, S.W.; Yun, J.; Ozcan, S.; Hwang, H.; Ji, I.J.; Yin, D.; Webster, M.J.; et al. Spatial and temporal diversity of glycome expression in mammalian brain. Proc. Natl. Acad. Sci. USA 2020, 117, 28743–28753. [Google Scholar] [CrossRef]
- Cho, B.G.; Veillon, L.; Mechref, Y. N-Glycan Profile of Cerebrospinal Fluids from Alzheimer’s Disease Patients Using Liquid Chromatography with Mass Spectrometry. J. Proteome Res. 2019, 18, 3770–3779. [Google Scholar] [CrossRef]
- Hu, Y.; Mayampurath, A.; Khan, S.; Cohen, J.K.; Mechref, Y.; Volchenboum, S.L. N-linked glycan profiling in neuroblastoma cell lines. J. Proteome Res. 2015, 14, 2074–2081. [Google Scholar] [CrossRef]
- Wildburger, N.C.; Zhou, S.; Zacharias, L.G.; Kroes, R.A.; Moskal, J.R.; Schmidt, M.; Mirzaei, P.; Gumin, J.; Lang, F.F.; Mechref, Y.; et al. Integrated Transcriptomic and Glycomic Profiling of Glioma Stem Cell Xenografts. J. Proteome Res. 2015, 14, 3932–3939. [Google Scholar] [CrossRef]
- Kizuka, Y.; Nakano, M.; Kitazume, S.; Saito, T.; Saido, T.C.; Taniguchi, N. Bisecting GlcNAc modification stabilizes BACE1 protein under oxidative stress conditions. Biochem. J. 2016, 473, 21–30. [Google Scholar] [CrossRef]
- Lin, Y.; Zhu, J.; Pan, L.; Zhang, J.; Tan, Z.; Olivares, J.; Singal, A.G.; Parikh, N.D.; Lubman, D.M. A Panel of Glycopeptides as Candidate Biomarkers for Early Diagnosis of NASH Hepatocellular Carcinoma Using a Stepped HCD Method and PRM Evaluation. J. Proteome Res. 2021, 20, 3278–3289. [Google Scholar] [CrossRef]
- Lee, J.Y.; Lee, H.K.; Park, G.W.; Hwang, H.; Jeong, H.K.; Yun, K.N.; Ji, E.S.; Kim, K.H.; Kim, J.S.; Kim, J.W.; et al. Characterization of Site-Specific N-Glycopeptide Isoforms of α-1-Acid Glycoprotein from an Interlaboratory Study Using LC–MS/MS. J. Proteome Res. 2016, 15, 4146–4164. [Google Scholar] [CrossRef] [PubMed]
- Frese, C.K.; Altelaar, A.F.; Hennrich, M.L.; Nolting, D.; Zeller, M.; Griep-Raming, J.; Heck, A.J.; Mohammed, S. Improved peptide identification by targeted fragmentation using CID, HCD and ETD on an LTQ-Orbitrap Velos. J. Proteome Res. 2011, 10, 2377–2388. [Google Scholar] [CrossRef] [PubMed]
- Segu, Z.M.; Mechref, Y. Characterizing protein glycosylation sites through higher-energy C-trap dissociation. Rapid Commun. Mass Spectrom. 2010, 24, 1217–1225. [Google Scholar] [CrossRef] [PubMed]
- Singh, C.; Zampronio, C.G.; Creese, A.J.; Cooper, H.J. Higher energy collision dissociation (HCD) product ion-triggered electron transfer dissociation (ETD) mass spectrometry for the analysis of N-linked glycoproteins. J. Proteome Res. 2012, 11, 4517–4525. [Google Scholar] [CrossRef] [PubMed]
- Saba, J.; Dutta, S.; Hemenway, E.; Viner, R. Increasing the productivity of glycopeptides analysis by using higher-energy collision dissociation-accurate mass-product-dependent electron transfer dissociation. Int. J. Proteom. 2012, 2012, 560391. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.K.; Desaire, H. Carbohydrates on Proteins: Site-Specific Glycosylation Analysis by Mass Spectrometry. Annu. Rev. Anal. Chem. 2015, 8, 463–483. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Khatri, K.; Zaia, J. Algorithms and Design Strategies Towards Automated Glycoproteomics Analysis. Mass Spectrom. Rev. 2017, 36, 475–498. [Google Scholar] [CrossRef]
- Schindler, B.; Laloy-Borgna, G.; Barnes, L.; Allouche, A.R.; Bouju, E.; Dugas, V.; Demesmay, C.; Compagnon, I. Online Separation and Identification of Isomers Using Infrared Multiple Photon Dissociation Ion Spectroscopy Coupled to Liquid Chromatography: Application to the Analysis of Disaccharides Regio-Isomers and Monosaccharide Anomers. Anal. Chem. 2018, 90, 11741–11745. [Google Scholar] [CrossRef]
- Renois-Predelus, G.; Schindler, B.; Compagnon, I. Analysis of Sulfate Patterns in Glycosaminoglycan Oligosaccharides by MS(n) Coupled to Infrared Ion Spectroscopy: The Case of GalNAc4S and GalNAc6S. J. Am. Soc. Mass Spectrom. 2018, 29, 1242–1249. [Google Scholar] [CrossRef] [PubMed]
- Riggs, D.L.; Hofmann, J.; Hahm, H.S.; Seeberger, P.H.; Pagel, K.; Julian, R.R. Glycan Isomer Identification Using Ultraviolet Photodissociation Initiated Radical Chemistry. Anal. Chem. 2018, 90, 11581–11588. [Google Scholar] [CrossRef] [PubMed]
- Madsen, J.A.; Ko, B.J.; Xu, H.; Iwashkiw, J.A.; Robotham, S.A.; Shaw, J.B.; Feldman, M.F.; Brodbelt, J.S. Concurrent automated sequencing of the glycan and peptide portions of O-linked glycopeptide anions by ultraviolet photodissociation mass spectrometry. Anal. Chem. 2013, 85, 9253–9261. [Google Scholar] [CrossRef] [PubMed]
- Cotham, V.C.; Brodbelt, J.S. Characterization of Therapeutic Monoclonal Antibodies at the Subunit-Level using Middle-Down 193 nm Ultraviolet Photodissociation. Anal. Chem. 2016, 88, 4004–4013. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, W.D.; Jackson, G.P. Charge transfer dissociation (CTD) mass spectrometry of peptide cations using kiloelectronvolt helium cations. J. Am. Soc. Mass Spectrom. 2014, 25, 1939–1943. [Google Scholar] [CrossRef] [PubMed]
- Mendis, P.M.; Sasiene, Z.J.; Ropartz, D.; Rogniaux, H.; Jackson, G.P. Ultra-high-performance liquid chromatography charge transfer dissociation mass spectrometry (UHPLC-CTD-MS) as a tool for analyzing the structural heterogeneity in carrageenan oligosaccharides. Anal. Bioanal Chem. 2022, 414, 303–318. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, F.; Franc, V.; Halim, L.A.; Schellekens, H.; Heck, A.J. Hybrid mass spectrometry approaches in glycoprotein analysis and their usage in scoring biosimilarity. Nat. Commun. 2016, 7, 13397. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Wang, B.; Chen, Z.; Urabe, G.; Glover, M.S.; Shi, X.; Guo, L.W.; Kent, K.C.; Li, L. Electron-Transfer/Higher-Energy Collision Dissociation (EThcD)-Enabled Intact Glycopeptide/Glycoproteome Characterization. J. Am. Soc. Mass Spectrom. 2017, 28, 1751–1764. [Google Scholar] [CrossRef]
- Crittenden, C.M.; Escobar, E.E.; Williams, P.E.; Sanders, J.D.; Brodbelt, J.S. Characterization of Antigenic Oligosaccharides from Gram-Negative Bacteria via Activated Electron Photodetachment Mass Spectrometry. Anal. Chem. 2019, 91, 4672–4679. [Google Scholar] [CrossRef]
- Klein, D.R.; Powers, M.J.; Trent, M.S.; Brodbelt, J.S. Top-Down Characterization of Lipooligosaccharides from Antibiotic-Resistant Bacteria. Anal. Chem. 2019, 91, 9608–9615. [Google Scholar] [CrossRef]
- Kurz, S.; Sheikh, M.O.; Lu, S.; Wells, L.; Tiemeyer, M. Separation and Identification of Permethylated Glycan Isomers by Reversed Phase NanoLC-NSI-MS(n). Mol. Cell Proteom. 2021, 20, 100045. [Google Scholar] [CrossRef]
- Ashline, D.J.; Yu, Y.; Lasanajak, Y.; Song, X.; Hu, L.; Ramani, S.; Prasad, V.; Estes, M.K.; Cummings, R.D.; Smith, D.F.; et al. Structural characterization by multistage mass spectrometry (MSn) of human milk glycans recognized by human rotaviruses. Mol. Cell Proteom. 2014, 13, 2961–2974. [Google Scholar] [CrossRef]
- Ashline, D.J.; Zhang, H.; Reinhold, V.N. Isomeric complexity of glycosylation documented by MSn. Anal. Bioanal. Chem. 2017, 409, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Deguchi, K.; Ito, H.; Takegawa, Y.; Shinji, N.; Nakagawa, H.; Nishimura, S. Complementary structural information of positive- and negative-ion MSn spectra of glycopeptides with neutral and sialylated N-glycans. Rapid Commun. Mass Spectrom. 2006, 20, 741–746. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, N.; Itoh, S.; Yamaguchi, T. LC/MSn for glycoprotein analysis: N-linked glycosylation analysis and peptide sequencing of glycopeptides. Methods Mol. Biol. 2009, 534, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Hu, Y.; DeSantos-Garcia, J.L.; Mechref, Y. Quantitation of permethylated N-glycans through multiple-reaction monitoring (MRM) LC-MS/MS. J. Am. Soc. Mass Spectrom. 2015, 26, 596–603. [Google Scholar] [CrossRef] [PubMed]
- Song, E.; Pyreddy, S.; Mechref, Y. Quantification of glycopeptides by multiple reaction monitoring liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. RCM 2012, 26, 1941–1954. [Google Scholar] [CrossRef]
- Yin, H.; Zhu, J.; Wang, M.; Yao, Z.P.; Lubman, D.M. Quantitative Analysis of α-1-Antitrypsin Glycosylation Isoforms in HCC Patients Using LC-HCD-PRM-MS. Anal. Chem. 2020, 92, 8201–8208. [Google Scholar] [CrossRef]
- Gutierrez Reyes, C.D.; Huang, Y.; Atashi, M.; Zhang, J.; Zhu, J.; Liu, S.; Parikh, N.D.; Singal, A.G.; Dai, J.; Lubman, D.M.; et al. PRM-MS Quantitative Analysis of Isomeric N-Glycopeptides Derived from Human Serum Haptoglobin of Patients with Cirrhosis and Hepatocellular Carcinoma. Metabolites 2021, 11, 563. [Google Scholar] [CrossRef]
- Cooper, C.A.; Gasteiger, E.; Packer, N.H. GlycoMod--a software tool for determining glycosylation compositions from mass spectrometric data. Proteomics 2001, 1, 340–349. [Google Scholar] [CrossRef]
- Maxwell, E.; Tan, Y.; Tan, Y.; Hu, H.; Benson, G.; Aizikov, K.; Conley, S.; Staples, G.O.; Slysz, G.W.; Smith, R.D.; et al. GlycReSoft: A software package for automated recognition of glycans from LC/MS data. PLoS ONE 2012, 7, e45474. [Google Scholar] [CrossRef]
- Wang, M.; Shajahan, A.; Pepi, L.E.; Azadi, P.; Zaia, J. Glycoproteomic Sample Processing, LC-MS, and Data Analysis Using GlycReSoft. Curr. Protoc. 2021, 1, e84. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.Y. Generation of asparagine-linked glycan structure databases and their use. J. Am. Soc. Mass Spectrom. 2009, 20, 1739–1742. [Google Scholar] [CrossRef] [PubMed]
- Vakhrushev, S.Y.; Dadimov, D.; Peter-Katalinić, J. Software platform for high-throughput glycomics. Anal. Chem. 2009, 81, 3252–3260. [Google Scholar] [CrossRef] [PubMed]
- Apte, A.; Meitei, N.S. Bioinformatics in glycomics: Glycan characterization with mass spectrometric data using SimGlycan. Methods Mol. Biol. 2010, 600, 269–281. [Google Scholar] [CrossRef]
- Hu, Y.; Zhou, S.; Yu, C.Y.; Tang, H.; Mechref, Y. Automated annotation and quantitation of glycans by liquid chromatography/electrospray ionization mass spectrometric analysis using the MultiGlycan-ESI computational tool. Rapid Commun. Mass Spectrom. 2015, 29, 135–142. [Google Scholar] [CrossRef]
- Hong, P.; Sun, H.; Sha, L.; Pu, Y.; Khatri, K.; Yu, X.; Tang, Y.; Lin, C. GlycoDeNovo—An Efficient Algorithm for Accurate de novo Glycan Topology Reconstruction from Tandem Mass Spectra. J. Am. Soc. Mass Spectrom. 2017, 28, 2288–2301. [Google Scholar] [CrossRef]
- Maass, K.; Ranzinger, R.; Geyer, H.; von der Lieth, C.W.; Geyer, R. “Glyco-peakfinder”—de novo composition analysis of glycoconjugates. Proteomics 2007, 7, 4435–4444. [Google Scholar] [CrossRef]
- Horlacher, O.; Jin, C.; Alocci, D.; Mariethoz, J.; Müller, M.; Karlsson, N.G.; Lisacek, F. Glycoforest 1.0. Anal. Chem. 2017, 89, 10932–10940. [Google Scholar] [CrossRef]
- Ceroni, A.; Maass, K.; Geyer, H.; Geyer, R.; Dell, A.; Haslam, S.M. GlycoWorkbench: A tool for the computer-assisted annotation of mass spectra of glycans. J. Proteome Res. 2008, 7, 1650–1659. [Google Scholar] [CrossRef]
- MacLean, B.; Tomazela, D.M.; Shulman, N.; Chambers, M.; Finney, G.L.; Frewen, B.; Kern, R.; Tabb, D.L.; Liebler, D.C.; MacCoss, M.J. Skyline: An open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics 2010, 26, 966–968. [Google Scholar] [CrossRef]
- Irungu, J.; Go, E.P.; Dalpathado, D.S.; Desaire, H. Simplification of mass spectral analysis of acidic glycopeptides using GlycoPep ID. Anal. Chem. 2007, 79, 3065–3074. [Google Scholar] [CrossRef] [PubMed]
- Ozohanics, O.; Krenyacz, J.; Ludányi, K.; Pollreisz, F.; Vékey, K.; Drahos, L. GlycoMiner: A new software tool to elucidate glycopeptide composition. Rapid Commun. Mass Spectrom. 2008, 22, 3245–3254. [Google Scholar] [CrossRef] [PubMed]
- Pompach, P.; Chandler, K.B.; Lan, R.; Edwards, N.; Goldman, R. Semi-automated identification of N-Glycopeptides by hydrophilic interaction chromatography, nano-reverse-phase LC-MS/MS, and glycan database search. J. Proteome Res. 2012, 11, 1728–1740. [Google Scholar] [CrossRef] [PubMed]
- Mayampurath, A.M.; Wu, Y.; Segu, Z.M.; Mechref, Y.; Tang, H. Improving confidence in detection and characterization of protein N-glycosylation sites and microheterogeneity. Rapid Commun. Mass Spectrom. 2011, 25, 2007–2019. [Google Scholar] [CrossRef]
- Woodin, C.L.; Hua, D.; Maxon, M.; Rebecchi, K.R.; Go, E.P.; Desaire, H. GlycoPep grader: A web-based utility for assigning the composition of N-linked glycopeptides. Anal. Chem. 2012, 84, 4821–4829. [Google Scholar] [CrossRef]
- Polasky, D.A.; Yu, F.; Teo, G.C.; Nesvizhskii, A.I. Fast and comprehensive N- and O-glycoproteomics analysis with MSFragger-Glyco. Nat. Methods 2020, 17, 1125–1132. [Google Scholar] [CrossRef]
- Zeng, W.F.; Liu, M.Q.; Zhang, Y.; Wu, J.Q.; Fang, P.; Peng, C.; Nie, A.; Yan, G.; Cao, W.; Liu, C.; et al. pGlyco: A pipeline for the identification of intact N-glycopeptides by using HCD- and CID-MS/MS and MS3. Sci. Rep. 2016, 6, 25102. [Google Scholar] [CrossRef]
- Bern, M.; Kil, Y.J.; Becker, C. Byonic: Advanced peptide and protein identification software. Curr. Protoc. Bioinform. 2012, 40, 13–20. [Google Scholar] [CrossRef]
- Park, G.W.; Kim, J.Y.; Hwang, H.; Lee, J.Y.; Ahn, Y.H.; Lee, H.K.; Ji, E.S.; Kim, K.H.; Jeong, H.K.; Yun, K.N.; et al. Integrated GlycoProteome Analyzer (I-GPA) for Automated Identification and Quantitation of Site-Specific N-Glycosylation. Sci. Rep. 2016, 6, 21175. [Google Scholar] [CrossRef]
- Nasir, W.; Toledo, A.G.; Noborn, F.; Nilsson, J.; Wang, M.; Bandeira, N.; Larson, G. SweetNET: A Bioinformatics Workflow for Glycopeptide MS/MS Spectral Analysis. J. Proteome Res. 2016, 15, 2826–2840. [Google Scholar] [CrossRef]
- Ye, Z.; Mao, Y.; Clausen, H.; Vakhrushev, S.Y. Glyco-DIA: A method for quantitative O-glycoproteomics with in silico-boosted glycopeptide libraries. Nat. Methods 2019, 16, 902–910. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.M.; Rejtar, T.; Li, L.; Karger, B.L. N-Glycan structure annotation of glycopeptides using a linearized glycan structure database (GlyDB). J. Proteome Res. 2007, 6, 3162–3173. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Xin, L.; Shan, B.; Lajoie, G.A.; Ma, B. GlycoMaster DB: Software to assist the automated identification of N-linked glycopeptides by tandem mass spectrometry. J. Proteome Res. 2014, 13, 3881–3895. [Google Scholar] [CrossRef] [PubMed]
- Chandler, K.B.; Pompach, P.; Goldman, R.; Edwards, N. Exploring site-specific N-glycosylation microheterogeneity of haptoglobin using glycopeptide CID tandem mass spectra and glycan database search. J. Proteome Res. 2013, 12, 3652–3666. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Hua, D.; Clark, D.F.; Go, E.P.; Desaire, H. GlycoPep Detector: A tool for assigning mass spectrometry data of N-linked glycopeptides on the basis of their electron transfer dissociation spectra. Anal. Chem. 2013, 85, 5023–5032. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.W.; Liang, S.Y.; Pu, T.H.; Chang, F.Y.; Khoo, K.H. Sweet-Heart - An integrated suite of enabling computational tools for automated MS2/MS3 sequencing and identification of glycopeptides. J. Proteom. 2013, 84, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Veenstra, T.D. Analysis of biofluids for biomarker research. Proteom. Clin. Appl. 2008, 2, 1403–1412. [Google Scholar] [CrossRef]
- Adamczyk, B.; Tharmalingam, T.; Rudd, P.M. Glycans as cancer biomarkers. Biochim. Biophys. Acta 2012, 1820, 1347–1353. [Google Scholar] [CrossRef]
- Patil, K.; Yelamanchi, S.; Kumar, M.; Hinduja, I.; Prasad, T.S.K.; Gowda, H.; Mukherjee, S. Quantitative mass spectrometric analysis to unravel glycoproteomic signature of follicular fluid in women with polycystic ovary syndrome. PLoS ONE 2019, 14, e0214742. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, M.; Sun, B.; Li, Y.; Xu, P.; Liu, C.; Liu, L.; Liu, X. Hyperammonemia enhances the function and expression of P-glycoprotein and Mrp2 at the blood-brain barrier through NF-kappaB. J. Neurochem. 2014, 131, 791–802. [Google Scholar] [CrossRef]
- Suttapitugsakul, S.; Sun, F.; Wu, R. Recent Advances in Glycoproteomic Analysis by Mass Spectrometry. Anal. Chem. 2020, 92, 267–291. [Google Scholar] [CrossRef] [PubMed]
- Doherty, M.; Theodoratou, E.; Walsh, I.; Adamczyk, B.; Stockmann, H.; Agakov, F.; Timofeeva, M.; Trbojevic-Akmacic, I.; Vuckovic, F.; Duffy, F.; et al. Plasma N-glycans in colorectal cancer risk. Sci. Rep. 2018, 8, 8655. [Google Scholar] [CrossRef]
- Kamiyama, T.; Yokoo, H.; Furukawa, J.; Kurogochi, M.; Togashi, T.; Miura, N.; Nakanishi, K.; Kamachi, H.; Kakisaka, T.; Tsuruga, Y.; et al. Identification of novel serum biomarkers of hepatocellular carcinoma using glycomic analysis. Hepatology 2013, 57, 2314–2325. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Xie, Q.; Wang, Y.; Liang, Y.; Xu, X.; Li, Y.; Miao, J.; Chen, Z.; Li, Y. Liquid chromatography mass spectrometry-based O-glycomics to evaluate glycosylation alterations in gastric cancer. Proteom. Clin. Appl. 2016, 10, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.; Saunders, M.; Dimapasoc, L.M.; Jeong, S.H.; Kim, B.J.; Kim, S.; So, M.; Lee, K.S.; Kim, J.H.; Lam, K.S.; et al. Differentiation of cancer cell origin and molecular subtype by plasma membrane N-glycan profiling. J. Proteome Res. 2014, 13, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.; Williams, C.C.; Dimapasoc, L.M.; Ro, G.S.; Ozcan, S.; Miyamoto, S.; Lebrilla, C.B.; An, H.J.; Leiserowitz, G.S. Isomer-specific chromatographic profiling yields highly sensitive and specific potential N-glycan biomarkers for epithelial ovarian cancer. J. Chromatogr. A 2013, 1279, 58–67. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, C.; Wang, R.; Wu, Y.; Zhang, L.; Liu, B.-F.; Cheng, L.; Liu, X. Isomer-specific profiling of N-glycans derived from human serum for potential biomarker discovery in pancreatic cancer. J. Proteom. 2018, 181, 160–169. [Google Scholar] [CrossRef]
- Muronetz, V.I.; Barinova, K.V.; Stroylova, Y.Y.; Semenyuk, P.I.; Schmalhausen, E.V. Glyceraldehyde-3-phosphate dehydrogenase: Aggregation mechanisms and impact on amyloid neurodegenerative diseases. Int. J. Biol. Macromol. 2017, 100, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Marijanovic, Z.; Caputo, A.; Campana, V.; Zurzolo, C. Identification of an intracellular site of prion conversion. PLoS Pathog. 2009, 5, e1000426. [Google Scholar] [CrossRef]
- Yi, J.H.; Katagiri, Y.; Susarla, B.; Figge, D.; Symes, A.J.; Geller, H.M. Alterations in sulfated chondroitin glycosaminoglycans following controlled cortical impact injury in mice. J. Comp. Neurol. 2012, 520, 3295–3313. [Google Scholar] [CrossRef]
- Yuzwa, S.A.; Shan, X.; Macauley, M.S.; Clark, T.; Skorobogatko, Y.; Vosseller, K.; Vocadlo, D.J. Increasing O-GlcNAc slows neurodegeneration and stabilizes tau against aggregation. Nat. Chem. Biol. 2012, 8, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Harlalka, G.V.; Lehman, A.; Chioza, B.; Baple, E.L.; Maroofian, R.; Cross, H.; Sreekantan-Nair, A.; Priestman, D.A.; Al-Turki, S.; McEntagart, M.E.; et al. Mutations in B4GALNT1 (GM2 synthase) underlie a new disorder of ganglioside biosynthesis. Brain 2013, 136, 3618–3624. [Google Scholar] [CrossRef] [PubMed]
- Li, J.J.; Dickson, D.; Hof, P.R.; Vlassara, H. Receptors for advanced glycosylation endproducts in human brain: Role in brain homeostasis. Mol. Med. 1998, 4, 46–60. [Google Scholar] [CrossRef] [PubMed]
- Moniruzzaman, M.; Ishihara, S.; Nobuhara, M.; Higashide, H.; Funamoto, S. Glycosylation status of nicastrin influences catalytic activity and substrate preference of gamma-secretase. Biochem. Biophys. Res. Commun. 2018, 502, 98–103. [Google Scholar] [CrossRef] [PubMed]
- Yi, C.W.; Wang, L.Q.; Huang, J.J.; Pan, K.; Chen, J.; Liang, Y. Glycosylation Significantly Inhibits the Aggregation of Human Prion Protein and Decreases Its Cytotoxicity. Sci. Rep. 2018, 8, 12603. [Google Scholar] [CrossRef]
- Nakagawa, K.; Kitazume, S.; Oka, R.; Maruyama, K.; Saido, T.C.; Sato, Y.; Endo, T.; Hashimoto, Y. Sialylation enhances the secretion of neurotoxic amyloid-beta peptides. J. Neurochem. 2006, 96, 924–933. [Google Scholar] [CrossRef]
- Wang, A.C.; Jensen, E.H.; Rexach, J.E.; Vinters, H.V.; Hsieh-Wilson, L.C. Loss of O-GlcNAc glycosylation in forebrain excitatory neurons induces neurodegeneration. Proc. Natl. Acad. Sci. USA 2016, 113, 15120–15125. [Google Scholar] [CrossRef]
- Demirev, A.V.; Song, H.L.; Cho, M.H.; Cho, K.; Peak, J.J.; Yoo, H.J.; Kim, D.H.; Yoon, S.Y. V232M substitution restricts a distinct O-glycosylation of PLD3 and its neuroprotective function. Neurobiol. Dis. 2019, 129, 182–194. [Google Scholar] [CrossRef]
- Vicente Miranda, H.; Szego, E.M.; Oliveira, L.M.A.; Breda, C.; Darendelioglu, E.; de Oliveira, R.M.; Ferreira, D.G.; Gomes, M.A.; Rott, R.; Oliveira, M.; et al. Glycation potentiates alpha-synuclein-associated neurodegeneration in synucleinopathies. Brain 2017, 140, 1399–1419. [Google Scholar] [CrossRef]
- Gilch, S.; Kehler, C.; Schätzl, H.M. The prion protein requires cholesterol for cell surface localization. Mol. Cell Neurosci. 2006, 31, 346–353. [Google Scholar] [CrossRef]
- Charlwood, J.; Dingwall, C.; Matico, R.; Hussain, I.; Johanson, K.; Moore, S.; Powell, D.J.; Skehel, J.M.; Ratcliffe, S.; Clarke, B.; et al. Characterization of the glycosylation profiles of Alzheimer’s beta -secretase protein Asp-2 expressed in a variety of cell lines. J. Biol. Chem. 2001, 276, 16739–16748. [Google Scholar] [CrossRef] [PubMed]
- Frenkel-Pinter, M.; Stempler, S.; Tal-Mazaki, S.; Losev, Y.; Singh-Anand, A.; Escobar-Alvarez, D.; Lezmy, J.; Gazit, E.; Ruppin, E.; Segal, D. Altered protein glycosylation predicts Alzheimer’s disease and modulates its pathology in disease model Drosophila. Neurobiol. Aging 2017, 56, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Gizaw, S.T.; Koda, T.; Amano, M.; Kamimura, K.; Ohashi, T.; Hinou, H.; Nishimura, S. A comprehensive glycome profiling of Huntington’s disease transgenic mice. Biochim. Biophys. Acta 2015, 1850, 1704–1718. [Google Scholar] [CrossRef] [PubMed]
- Frenkel-Pinter, M.; Shmueli, M.D.; Raz, C.; Yanku, M.; Zilberzwige, S.; Gazit, E.; Segal, D. Interplay between protein glycosylation pathways in Alzheimer’s disease. Sci. Adv. 2017, 3, e1601576. [Google Scholar] [CrossRef]
- Huttenrauch, M.; Ogorek, I.; Klafki, H.; Otto, M.; Stadelmann, C.; Weggen, S.; Wiltfang, J.; Wirths, O. Glycoprotein NMB: A novel Alzheimer’s disease associated marker expressed in a subset of activated microglia. Acta Neuropathol. Commun. 2018, 6, 108. [Google Scholar] [CrossRef]
- Ilic, K.; Mlinac-Jerkovic, K.; Jovanov-Milosevic, N.; Simic, G.; Habek, N.; Bogdanovic, N.; Kalanj-Bognar, S. Hippocampal expression of cell-adhesion glycoprotein neuroplastin is altered in Alzheimer’s disease. J. Cell Mol. Med. 2019, 23, 1602–1607. [Google Scholar] [CrossRef]
- Garcia-Ayllon, M.S.; Botella-Lopez, A.; Cuchillo-Ibanez, I.; Rabano, A.; Andreasen, N.; Blennow, K.; Avila, J.; Saez-Valero, J. HNK-1 Carrier Glycoproteins Are Decreased in the Alzheimer’s Disease Brain. Mol. Neurobiol. 2017, 54, 188–199. [Google Scholar] [CrossRef]
- Ribaudo, G.; Coghi, P.; Zanforlin, E.; Law, B.Y.K.; Wu, Y.Y.J.; Han, Y.; Qiu, A.C.; Qu, Y.Q.; Zagotto, G.; Wong, V.K.W. Semi-synthetic isoflavones as BACE-1 inhibitors against Alzheimer’s disease. Bioorg. Chem. 2019, 87, 474–483. [Google Scholar] [CrossRef]
- Hartz, A.M.; Zhong, Y.; Wolf, A.; LeVine, H., 3rd; Miller, D.S.; Bauer, B. Abeta40 Reduces P-Glycoprotein at the Blood-Brain Barrier through the Ubiquitin-Proteasome Pathway. J. Neurosci. 2016, 36, 1930–1941. [Google Scholar] [CrossRef]
- Chai, A.B.; Leung, G.K.F.; Callaghan, R.; Gelissen, I.C. P-glycoprotein: A role in the export of amyloid-beta in Alzheimer’s disease? FEBS J. 2020, 287, 612–625. [Google Scholar] [CrossRef]
- Mohamed, L.A.; Keller, J.N.; Kaddoumi, A. Role of P-glycoprotein in mediating rivastigmine effect on amyloid-beta brain load and related pathology in Alzheimer’s disease mouse model. Biochim. Biophys. Acta 2016, 1862, 778–787. [Google Scholar] [CrossRef]
- Kao, Y.H.; Chern, Y.; Yang, H.T.; Chen, H.M.; Lin, C.J. Regulation of P-glycoprotein expression in brain capillaries in Huntington’s disease and its impact on brain availability of antipsychotic agents risperidone and paliperidone. J. Cereb. Blood Flow Metab. 2016, 36, 1412–1423. [Google Scholar] [CrossRef] [PubMed]
- Ban, J.J.; Chung, J.Y.; Lee, M.; Im, W.; Kim, M. MicroRNA-27a reduces mutant hutingtin aggregation in an in vitro model of Huntington’s disease. Biochem. Biophys. Res. Commun. 2017, 488, 316–321. [Google Scholar] [CrossRef]
- Bras, I.C.; Konig, A.; Outeiro, T.F. Glycation in Huntington’s Disease: A Possible Modifier and Target for Intervention. J. Huntingtons Dis. 2019, 8, 245–256. [Google Scholar] [CrossRef] [PubMed]
- Vicente Miranda, H.; Gomes, M.A.; Branco-Santos, J.; Breda, C.; Lazaro, D.F.; Lopes, L.V.; Herrera, F.; Giorgini, F.; Outeiro, T.F. Glycation potentiates neurodegeneration in models of Huntington’s disease. Sci. Rep. 2016, 6, 36798. [Google Scholar] [CrossRef] [PubMed]
- McGeer, P.L.; Itagaki, S.; McGeer, E.G. Expression of the histocompatibility glycoprotein HLA-DR in neurological disease. Acta Neuropathol. 1988, 76, 550–557. [Google Scholar] [CrossRef] [PubMed]
- Steffan, J.S.; Kazantsev, A.; Spasic-Boskovic, O.; Greenwald, M.; Zhu, Y.Z.; Gohler, H.; Wanker, E.E.; Bates, G.P.; Housman, D.E.; Thompson, L.M. The Huntington’s disease protein interacts with p53 and CREB-binding protein and represses transcription. Proc. Natl. Acad. Sci. USA 2000, 97, 6763–6768. [Google Scholar] [CrossRef]
- Pacini, G.; Ieronymaki, M.; Nuti, F.; Sabatino, G.; Larregola, M.; Aharoni, R.; Papini, A.M.; Rovero, P. Epitope mapping of anti-myelin oligodendrocyte glycoprotein (MOG) antibodies in a mouse model of multiple sclerosis: Microwave-assisted synthesis of the peptide antigens and ELISA screening. J. Pept. Sci. 2016, 22, 52–58. [Google Scholar] [CrossRef]
- Androutsou, M.E.; Tapeinou, A.; Vlamis-Gardikas, A.; Tselios, T. Myelin Oligodendrocyte Glycoprotein and Multiple Sclerosis. Med. Chem. 2018, 14, 120–128. [Google Scholar] [CrossRef]
- Khare, P.; Challa, D.K.; Devanaboyina, S.C.; Velmurugan, R.; Hughes, S.; Greenberg, B.M.; Ober, R.J.; Ward, E.S. Myelin oligodendrocyte glycoprotein-specific antibodies from multiple sclerosis patients exacerbate disease in a humanized mouse model. J. Autoimmun. 2018, 86, 104–115. [Google Scholar] [CrossRef]
- Bronge, M.; Ruhrmann, S.; Carvalho-Queiroz, C.; Nilsson, O.B.; Kaiser, A.; Holmgren, E.; Macrini, C.; Winklmeier, S.; Meinl, E.; Brundin, L.; et al. Myelin oligodendrocyte glycoprotein revisited-sensitive detection of MOG-specific T-cells in multiple sclerosis. J. Autoimmun. 2019, 102, 38–49. [Google Scholar] [CrossRef]
- Budge, K.M.; Neal, M.L.; Richardson, J.R.; Safadi, F.F. Glycoprotein NMB: An Emerging Role in Neurodegenerative Disease. Mol. Neurobiol. 2018, 55, 5167–5176. [Google Scholar] [CrossRef] [PubMed]
- Chan, G.N.; Evans, R.A.; Banks, D.B.; Mesev, E.V.; Miller, D.S.; Cannon, R.E. Selective induction of P-glycoprotein at the CNS barriers during symptomatic stage of an ALS animal model. Neurosci. Lett. 2017, 639, 103–113. [Google Scholar] [CrossRef]
- Mohamed, L.A.; Markandaiah, S.S.; Bonanno, S.; Pasinelli, P.; Trotti, D. Excess glutamate secreted from astrocytes drives upregulation of P-glycoprotein in endothelial cells in amyotrophic lateral sclerosis. Exp. Neurol. 2019, 316, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Dunn, A.R.; Stout, K.A.; Ozawa, M.; Lohr, K.M.; Hoffman, C.A.; Bernstein, A.I.; Li, Y.; Wang, M.; Sgobio, C.; Sastry, N.; et al. Synaptic vesicle glycoprotein 2C (SV2C) modulates dopamine release and is disrupted in Parkinson disease. Proc. Natl. Acad. Sci. USA 2017, 114, E2253–E2262. [Google Scholar] [CrossRef] [PubMed]
- Moloney, E.B.; Moskites, A.; Ferrari, E.J.; Isacson, O.; Hallett, P.J. The glycoprotein GPNMB is selectively elevated in the substantia nigra of Parkinson’s disease patients and increases after lysosomal stress. Neurobiol. Dis. 2018, 120, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gan, L.; Li, Z.; Lv, Q.; Huang, W. Rabies virus glycoprotein (RVG29)-linked microRNA-124-loaded polymeric nanoparticles inhibit neuroinflammation in a Parkinson’s disease model. Int. J. Pharm. 2019, 567, 118449. [Google Scholar] [CrossRef]
- Ma, L.; Song, J.; Sun, X.; Ding, W.; Fan, K.; Qi, M.; Xu, Y.; Zhang, W. Role of microtubule-associated protein 6 glycosylated with Gal-(beta-1,3)-GalNAc in Parkinson’s disease. Aging 2019, 11, 4597–4610. [Google Scholar] [CrossRef]
- Papuc, E.; Wilczynska, B.; Rejdak, K. Humoral response against myelin associated glycoprotein reflects oligodendroglial degeneration in Parkinson’s disease. Ann. Agric. Environ. Med. 2016, 23, 390–393. [Google Scholar] [CrossRef]
- Yamagata, H.; Uchida, S.; Matsuo, K.; Harada, K.; Kobayashi, A.; Nakashima, M.; Higuchi, F.; Watanuki, T.; Matsubara, T.; Watanabe, Y. Altered plasma protein glycosylation in a mouse model of depression and in patients with major depression. J. Affect. Disord. 2018, 233, 79–85. [Google Scholar] [CrossRef]
- Yoo, S.-W.; Motari, M.G.; Susuki, K.; Prendergast, J.; Mountney, A.; Hurtado, A.; Schnaar, R.L. Sialylation regulates brain structure and function. FASEB J. 2015, 29, 3040–3053. [Google Scholar] [CrossRef] [PubMed]
- Park, D.I.; Štambuk, J.; Razdorov, G.; Pučić-Baković, M.; Martins-de-Souza, D.; Lauc, G.; Turck, C.W. Blood plasma/IgG N-glycome biosignatures associated with major depressive disorder symptom severity and the antidepressant response. Sci. Rep. 2018, 8, 179. [Google Scholar] [CrossRef] [PubMed]
- Boeck, C.; Pfister, S.; Bürkle, A.; Vanhooren, V.; Libert, C.; Salinas-Manrique, J.; Dietrich, D.E.; Kolassa, I.T.; Karabatsiakis, A. Alterations of the serum N-glycan profile in female patients with Major Depressive Disorder. J. Affect. Disord. 2018, 234, 139–147. [Google Scholar] [CrossRef] [PubMed]
- Van der Zwaag, B.; Franke, L.; Poot, M.; Hochstenbach, R.; Spierenburg, H.A.; Vorstman, J.A.; van Daalen, E.; de Jonge, M.V.; Verbeek, N.E.; Brilstra, E.H.; et al. Gene-network analysis identifies susceptibility genes related to glycobiology in autism. PLoS ONE 2009, 4, e5324. [Google Scholar] [CrossRef]
- Pivac, N.; Knezević, A.; Gornik, O.; Pucić, M.; Igl, W.; Peeters, H.; Crepel, A.; Steyaert, J.; Novokmet, M.; Redzić, I.; et al. Human plasma glycome in attention-deficit hyperactivity disorder and autism spectrum disorders. Mol. Cell Proteom. 2011, 10, M110.004200. [Google Scholar] [CrossRef]
- Mueller, T.M.; Meador-Woodruff, J.H. Post-translational protein modifications in schizophrenia. NPJ Schizophr. 2020, 6, 5. [Google Scholar] [CrossRef]
- Kippe, J.M.; Mueller, T.M.; Haroutunian, V.; Meador-Woodruff, J.H. Abnormal N-acetylglucosaminyltransferase expression in prefrontal cortex in schizophrenia. Schizophr. Res. 2015, 166, 219–224. [Google Scholar] [CrossRef]
- Tucholski, J.; Simmons, M.S.; Pinner, A.L.; McMillan, L.D.; Haroutunian, V.; Meador-Woodruff, J.H. N-linked glycosylation of cortical N-methyl-D-aspartate and kainate receptor subunits in schizophrenia. Neuroreport 2013, 24, 688–691. [Google Scholar] [CrossRef]
- Tucholski, J.; Simmons, M.S.; Pinner, A.L.; Haroutunian, V.; McCullumsmith, R.E.; Meador-Woodruff, J.H. Abnormal N-linked glycosylation of cortical AMPA receptor subunits in schizophrenia. Schizophr. Res. 2013, 146, 177–183. [Google Scholar] [CrossRef]
- Mueller, T.M.; Haroutunian, V.; Meador-Woodruff, J.H. N-Glycosylation of GABAA receptor subunits is altered in Schizophrenia. Neuropsychopharmacology 2014, 39, 528–537. [Google Scholar] [CrossRef]
- Telford, J.E.; Bones, J.; McManus, C.; Saldova, R.; Manning, G.; Doherty, M.; Leweke, F.M.; Rothermundt, M.; Guest, P.C.; Rahmoune, H.; et al. Antipsychotic treatment of acute paranoid schizophrenia patients with olanzapine results in altered glycosylation of serum glycoproteins. J. Proteome Res. 2012, 11, 3743–3752. [Google Scholar] [CrossRef] [PubMed]
- Stanta, J.L.; Saldova, R.; Struwe, W.B.; Byrne, J.C.; Leweke, F.M.; Rothermund, M.; Rahmoune, H.; Levin, Y.; Guest, P.C.; Bahn, S.; et al. Identification of N-glycosylation changes in the CSF and serum in patients with schizophrenia. J. Proteome Res. 2010, 9, 4476–4489. [Google Scholar] [CrossRef] [PubMed]
- Bauer, D.; Haroutunian, V.; Meador-Woodruff, J.H.; McCullumsmith, R.E. Abnormal glycosylation of EAAT1 and EAAT2 in prefrontal cortex of elderly patients with schizophrenia. Schizophr. Res. 2010, 117, 92–98. [Google Scholar] [CrossRef] [PubMed]
- Narayan, S.; Head, S.R.; Gilmartin, T.J.; Dean, B.; Thomas, E.A. Evidence for disruption of sphingolipid metabolism in schizophrenia. J. Neurosci. Res. 2009, 87, 278–288. [Google Scholar] [CrossRef] [PubMed]
- Varma, R.; Michos, G.A.; Gordon, B.J.; Varma, R.S.; Shirey, R.E. Serum glycoconjugates in children with schizophrenia and conduct and adjustment disorders. Biochem. Med. 1983, 30, 206–214. [Google Scholar] [CrossRef]
- Varma, R.; Hoshino, A.Y.; Vercellotti, J.R. Serum glycoproteins in schizophrenia. Carbohydr. Res. 1980, 82, 343–351. [Google Scholar] [CrossRef]
- Moreno-Villanueva, M.; Morath, J.; Vanhooren, V.; Elbert, T.; Kolassa, S.; Libert, C.; Bürkle, A.; Kolassa, I.T. N-glycosylation profiling of plasma provides evidence for accelerated physiological aging in post-traumatic stress disorder. Transl. Psychiatry 2013, 3, e320. [Google Scholar] [CrossRef]
- Tudor, L.; Nedic Erjavec, G.; Nikolac Perkovic, M.; Konjevod, M.; Svob Strac, D.; Uzun, S.; Kozumplik, O.; Jovanovic, T.; Lauc, G.; Pivac, N. N-glycomic Profile in Combat Related Post-Traumatic Stress Disorder. Biomolecules 2019, 9, 834. [Google Scholar] [CrossRef]
- Barisic, K.; Lauc, G.; Dumic, J.; Pavlovic, M.; Flogel, M. Changes of glycoprotein patterns in sera of humans under stress. Eur. J. Clin. Chem. Clin. Biochem. 1996, 34, 97–101. [Google Scholar]
- Floegel, M.; Lauc, G.; Žanić-Grubišić, T.; Dumić, J.; Barišić, K. Novel 57 kd glycoprotein in sera of humans under stress. Croat. Chem. Acta 1996, 69, 371–378. [Google Scholar]
- Lauc, G.; Dabelić, S.; Dumić, J.; Flögel, M. Stressin and natural killer cell activity in professional soldiers. Ann. N. Y. Acad. Sci. 1998, 851, 526–530. [Google Scholar] [CrossRef] [PubMed]
- Konjevod, M.; Tudor, L.; Svob Strac, D.; Nedic Erjavec, G.; Barbas, C.; Zarkovic, N.; Nikolac Perkovic, M.; Uzun, S.; Kozumplik, O.; Lauc, G.; et al. Metabolomic and glycomic findings in posttraumatic stress disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2019, 88, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Reimand, J.; Wagih, O.; Bader, G.D. Evolutionary constraint and disease associations of post-translational modification sites in human genomes. PLoS Genet. 2015, 11, e1004919. [Google Scholar] [CrossRef]
- Chauhan, N.B. Chronic neurodegenerative consequences of traumatic brain injury. Restor. Neurol. Neurosci. 2014, 32, 337–365. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.; Rhim, H. Functional significance of O-GlcNAc modification in regulating neuronal properties. Pharmacol. Res. 2018, 129, 295–307. [Google Scholar] [CrossRef]
- Endo, T. Structure, function and pathology of O-mannosyl glycans. Glycoconj. J. 2004, 21, 3–7. [Google Scholar] [CrossRef]
- Yang, W.J.; Chen, W.; Chen, L.; Guo, Y.J.; Zeng, J.S.; Li, G.Y.; Tong, W.S. Involvement of tau phosphorylation in traumatic brain injury patients. Acta Neurol. Scand. 2017, 135, 622–627. [Google Scholar] [CrossRef]
- Lazarus, R.C.; Buonora, J.E.; Jacobowitz, D.M.; Mueller, G.P. Protein carbonylation after traumatic brain injury: Cell specificity, regional susceptibility, and gender differences. Free Radic. Biol. Med. 2015, 78, 89–100. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Z.; Zhang, Q.; Chen, L.; Huang, X.; Zhang, Y.; Liu, X.; Liu, W.; Li, W. Mechanisms Underlying H2O2-Evoked Carbonyl Modification of Cytoskeletal Protein and Axon Injury in PC-12 Cells. Cell Physiol. Biochem. 2018, 48, 1088–1098. [Google Scholar] [CrossRef]
- Peng, W.; Zhao, J.; Dong, X.; Banazadeh, A.; Huang, Y.; Hussien, A.; Mechref, Y. Clinical application of quantitative glycomics. Expert Rev. Proteom. 2018, 15, 1007–1031. [Google Scholar] [CrossRef]
- Freeze, H.H.; Eklund, E.A.; Ng, B.G.; Patterson, M.C. Neurological aspects of human glycosylation disorders. Annu. Rev. Neurosci. 2015, 38, 105–125. [Google Scholar] [CrossRef] [PubMed]
- Gill, A.C.; Castle, A.R. The cellular and pathologic prion protein. Handb. Clin. Neurol. 2018, 153, 21–44. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Zhang, D.; Tao, T.; Liu, X.; Sun, X.; Wang, Y.; Shen, A. O-GlcNAc glycosylation of p27(kip1) promotes astrocyte migration and functional recovery after spinal cord contusion. Exp. Cell Res. 2015, 339, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Li, C.X.; Ma, G.Y.; Guo, M.F.; Liu, Y. Sialic acid accelerates the electrophoretic velocity of injured dorsal root ganglion neurons. Neural Regen. Res. 2015, 10, 972–975. [Google Scholar] [CrossRef]
- Karve, I.P.; Taylor, J.M.; Crack, P.J. The contribution of astrocytes and microglia to traumatic brain injury. Br. J. Pharmacol. 2016, 173, 692–702. [Google Scholar] [CrossRef]
- Mortezaee, K.; Khanlarkhani, N.; Beyer, C.; Zendedel, A. Inflammasome: Its role in traumatic brain and spinal cord injury. J. Cell Physiol. 2018, 233, 5160–5169. [Google Scholar] [CrossRef]
- Loane, D.J.; Kumar, A. Microglia in the TBI brain: The good, the bad, and the dysregulated. Exp. Neurol. 2016, 275 Pt 3, 316–327. [Google Scholar] [CrossRef]
- McIntosh, T.K.; Smith, D.H.; Meaney, D.F.; Kotapka, M.J.; Gennarelli, T.A.; Graham, D.I. Neuropathological sequelae of traumatic brain injury: Relationship to neurochemical and biomechanical mechanisms. Lab. Investig. 1996, 74, 315–342. [Google Scholar]
- Roberts, P.J.; Davies, S.W. Excitatory receptors and their role in excitotoxicity. Biochem. Soc. Trans. 1987, 15, 218–219. [Google Scholar]
- Katayama, Y.; Becker, D.P.; Tamura, T.; Hovda, D.A. Massive increases in extracellular potassium and the indiscriminate release of glutamate following concussive brain injury. J. Neurosurg. 1990, 73, 889–900. [Google Scholar] [CrossRef]
- Vink, R.; Van Den Heuvel, C. Recent advances in the development of multifactorial therapies for the treatment of traumatic brain injury. Expert Opin. Investig. Drugs 2004, 13, 1263–1274. [Google Scholar] [CrossRef]
- Hayes, R.L.; Dixon, C.E. Neurochemical changes in mild head injury. Semin. Neurol. 1994, 14, 25–31. [Google Scholar] [CrossRef]
- Barkhoudarian, G.; Hovda, D.A.; Giza, C.C. The Molecular Pathophysiology of Concussive Brain Injury - an Update. Phys. Med. Rehabil. Clin. N. Am. 2016, 27, 373–393. [Google Scholar] [CrossRef] [PubMed]
- Higgins, G.C.; Beart, P.M.; Shin, Y.S.; Chen, M.J.; Cheung, N.S.; Nagley, P. Oxidative stress: Emerging mitochondrial and cellular themes and variations in neuronal injury. J. Alzheimers Dis. 2010, 20, S453–S473. [Google Scholar] [CrossRef] [PubMed]
- Cernak, I.; Savic, V.J.; Kotur, J.; Prokic, V.; Veljovic, M.; Grbovic, D. Characterization of plasma magnesium concentration and oxidative stress following graded traumatic brain injury in humans. J. Neurotrauma 2000, 17, 53–68. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Hall, E.D.; Yonkers, P.A.; Andrus, P.K.; Cox, J.W.; Anderson, D.K. Biochemistry and pharmacology of lipid antioxidants in acute brain and spinal cord injury. J. Neurotrauma 1992, 9, S425–S442. [Google Scholar]
- Shohami, E.; Beit-Yannai, E.; Horowitz, M.; Kohen, R. Oxidative stress in closed-head injury: Brain antioxidant capacity as an indicator of functional outcome. J. Cereb. Blood Flow. Metab. 1997, 17, 1007–1019. [Google Scholar] [CrossRef]
- Marklund, N.; Clausen, F.; Lewander, T.; Hillered, L. Monitoring of reactive oxygen species production after traumatic brain injury in rats with microdialysis and the 4-hydroxybenzoic acid trapping method. J. Neurotrauma 2001, 18, 1217–1227. [Google Scholar] [CrossRef]
- Lewen, A.; Matz, P.; Chan, P.H. Free radical pathways in CNS injury. J. Neurotrauma 2000, 17, 871–890. [Google Scholar]
- Kim, J.S.; He, L.; Lemasters, J.J. Mitochondrial permeability transition: A common pathway to necrosis and apoptosis. Biochem. Biophys. Res. Commun. 2003, 304, 463–470. [Google Scholar] [PubMed]
- Tavazzi, B.; Signoretti, S.; Lazzarino, G.; Amorini, A.M.; Delfini, R.; Cimatti, M.; Marmarou, A.; Vagnozzi, R. Cerebral oxidative stress and depression of energy metabolism correlate with severity of diffuse brain injury in rats. Neurosurgery 2005, 56, 582–589, discussion 582–589. [Google Scholar] [CrossRef] [PubMed]
- Chan, I.S.; Ginsburg, G.S. Personalized medicine: Progress and promise. Annu. Rev. Genom. Hum. Genet. 2011, 12, 217–244. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, A.; Gomes, J.; Ismail, M.N.; Haslam, S.M.; Mendes, N.; Osório, H.; David, L.; Le Pendu, J.; Haas, R.; Dell, A.; et al. Fut2-null mice display an altered glycosylation profile and impaired BabA-mediated Helicobacter pylori adhesion to gastric mucosa. Glycobiology 2009, 19, 1525–1536. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, M.; Eriksson, S.; Mendes, N.; Serpa, J.; Figueiredo, C.; Resende, L.P.; Ruvoën-Clouet, N.; Haas, R.; Borén, T.; Le Pendu, J.; et al. Infection by Helicobacter pylori expressing the BabA adhesin is influenced by the secretor phenotype. J. Pathol. 2008, 215, 308–316. [Google Scholar] [CrossRef]
- Hakomori, S. Tumor malignancy defined by aberrant glycosylation and sphingo(glyco)lipid metabolism. Cancer Res. 1996, 56, 5309–5318. [Google Scholar]
- Hakomori, S. Aberrant glycosylation in tumors and tumor-associated carbohydrate antigens. Adv. Cancer Res. 1989, 52, 257–331. [Google Scholar] [CrossRef]
- Almeida, A.; Kolarich, D. The promise of protein glycosylation for personalised medicine. Biochim. Biophys. Acta 2016, 1860, 1583–1595. [Google Scholar] [CrossRef]
- Morris, B. The components of the Wired Spanning Forest are recurrent. Probab. Theory Relat. Fields 2003, 125, 259–265. [Google Scholar] [CrossRef]
- Alley, W.R., Jr.; Vasseur, J.A.; Goetz, J.A.; Svoboda, M.; Mann, B.F.; Matei, D.E.; Menning, N.; Hussein, A.; Mechref, Y.; Novotny, M.V. N-linked glycan structures and their expressions change in the blood sera of ovarian cancer patients. J. Proteome Res. 2012, 11, 2282–2300. [Google Scholar] [CrossRef]
- Abd Hamid, U.M.; Royle, L.; Saldova, R.; Radcliffe, C.M.; Harvey, D.J.; Storr, S.J.; Pardo, M.; Antrobus, R.; Chapman, C.J.; Zitzmann, N.; et al. A strategy to reveal potential glycan markers from serum glycoproteins associated with breast cancer progression. Glycobiology 2008, 18, 1105–1118. [Google Scholar] [CrossRef]
- Hammarström, S. The carcinoembryonic antigen (CEA) family: Structures, suggested functions and expression in normal and malignant tissues. Semin. Cancer Biol. 1999, 9, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, M.J.; Mitchell, E.P. Carcinoembryonic antigen in the staging and follow-up of patients with colorectal cancer. Cancer Investig. 2005, 23, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, J.; Tewari, H.B.; Bhatnagar, M.; Austin, G.E. Comparison of carcinoembryonic antigen in tissue and serum with grade and stage of colon cancer. Anticancer Res. 1999, 19, 2181–2187. [Google Scholar] [PubMed]
- Amri, R.; Bordeianou, L.G.; Sylla, P.; Berger, D.L. Preoperative carcinoembryonic antigen as an outcome predictor in colon cancer. J. Surg. Oncol. 2013, 108, 14–18. [Google Scholar] [CrossRef]
- Hiraizumi, S.; Takasaki, S.; Ohuchi, N.; Harada, Y.; Nose, M.; Mori, S.; Kobata, A. Altered glycosylation of membrane glycoproteins associated with human mammary carcinoma. Jpn. J. Cancer Res. 1992, 83, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- Brooks, S.A.; Leathem, A.J. Prediction of lymph node involvement in breast cancer by detection of altered glycosylation in the primary tumour. Lancet 1991, 338, 71–74. [Google Scholar] [CrossRef]
- Büll, C.; Stoel, M.A.; den Brok, M.H.; Adema, G.J. Sialic acids sweeten a tumor’s life. Cancer Res. 2014, 74, 3199–3204. [Google Scholar] [CrossRef]
- Arnal-Estapé, A.; Nguyen, D.X. Sweets for a bitter end: Lung cancer cell-surface protein glycosylation mediates metastatic colonization. Cancer Discov. 2015, 5, 109–111. [Google Scholar] [CrossRef]
- Thompson, L. World Health Organization classification of tumours: Pathology and genetics of head and neck tumours. Ear Nose Throat J. 2006, 85, 74. [Google Scholar] [CrossRef]
- Goldbrunner, R.H.; Bernstein, J.J.; Tonn, J.C. Cell-extracellular matrix interaction in glioma invasion. Acta Neurochir. 1999, 141, 295–305. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, Y.; Jung, S.; Salhia, B.; Lee, S.; Hubbard, S.; Taylor, M.; Mainprize, T.; Akaishi, K.; van Furth, W.; Rutka, J.T. Hyaluronate receptors mediating glioma cell migration and proliferation. J. Neurooncol. 2001, 53, 115–127. [Google Scholar] [CrossRef]
- Yamaguchi, Y. Lecticans: Organizers of the brain extracellular matrix. Cell Mol. Life Sci. 2000, 57, 276–289. [Google Scholar] [CrossRef] [PubMed]
- Gary, S.C.; Kelly, G.M.; Hockfield, S. BEHAB/brevican: A brain-specific lectican implicated in gliomas and glial cell motility. Curr. Opin. Neurobiol. 1998, 8, 576–581. [Google Scholar] [CrossRef]
- Viapiano, M.S.; Bi, W.L.; Piepmeier, J.; Hockfield, S.; Matthews, R.T. Novel tumor-specific isoforms of BEHAB/brevican identified in human malignant gliomas. Cancer Res. 2005, 65, 6726–6733. [Google Scholar] [CrossRef] [PubMed]
- Lemjabbar-Alaoui, H.; McKinney, A.; Yang, Y.W.; Tran, V.M.; Phillips, J.J. Glycosylation alterations in lung and brain cancer. Adv. Cancer Res. 2015, 126, 305–344. [Google Scholar] [CrossRef]
- Yamamoto, H.; Swoger, J.; Greene, S.; Saito, T.; Hurh, J.; Sweeley, C.; Leestma, J.; Mkrdichian, E.; Cerullo, L.; Nishikawa, A.; et al. Beta1,6-N-acetylglucosamine-bearing N-glycans in human gliomas: Implications for a role in regulating invasivity. Cancer Res. 2000, 60, 134–142. [Google Scholar]
- Seberger, P.J.; Chaney, W.G. Control of metastasis by Asn-linked, beta1-6 branched oligosaccharides in mouse mammary cancer cells. Glycobiology 1999, 9, 235–241. [Google Scholar] [CrossRef][Green Version]
- Schauer, R. Sialic acids and their role as biological masks. Trends Biochem. Sci. 1985, 10, 357–360. [Google Scholar] [CrossRef]
- Hudak, J.E.; Canham, S.M.; Bertozzi, C.R. Glycocalyx engineering reveals a Siglec-based mechanism for NK cell immunoevasion. Nat. Chem. Biol. 2014, 10, 69–75. [Google Scholar] [CrossRef]
- Prusiner, S.B. Novel proteinaceous infectious particles cause scrapie. Science 1982, 216, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Katorcha, E.; Makarava, N.; Savtchenko, R.; Baskakov, I.V. Sialylation of the prion protein glycans controls prion replication rate and glycoform ratio. Sci. Rep. 2015, 5, 16912. [Google Scholar] [CrossRef] [PubMed]
- Schachter, H. Congenital disorders involving defective N-glycosylation of proteins. Cell Mol. Life Sci. 2001, 58, 1085–1104. [Google Scholar] [CrossRef]
- Ma, J.; Lindquist, S. De novo generation of a PrPSc-like conformation in living cells. Nat. Cell Biol. 1999, 1, 358–361. [Google Scholar] [CrossRef] [PubMed]
- Winklhofer, K.F.; Heller, U.; Reintjes, A.; Tatzelt, J. Inhibition of complex glycosylation increases the formation of PrPsc. Traffic 2003, 4, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Rudd, P.M.; Endo, T.; Colominas, C.; Groth, D.; Wheeler, S.F.; Harvey, D.J.; Wormald, M.R.; Serban, H.; Prusiner, S.B.; Kobata, A.; et al. Glycosylation differences between the normal and pathogenic prion protein isoforms. Proc. Natl. Acad. Sci. USA 1999, 96, 13044–13049. [Google Scholar] [CrossRef] [PubMed]
- Wille, H.; Michelitsch, M.D.; Guenebaut, V.; Supattapone, S.; Serban, A.; Cohen, F.E.; Agard, D.A.; Prusiner, S.B. Structural studies of the scrapie prion protein by electron crystallography. Proc. Natl. Acad. Sci. USA 2002, 99, 3563–3568. [Google Scholar] [CrossRef]
- Requena, J.R.; Wille, H. The structure of the infectious prion protein: Experimental data and molecular models. Prion 2014, 8, 60–66. [Google Scholar] [CrossRef]
- Xiao, X.; Yuan, J.; Haik, S.; Cali, I.; Zhan, Y.; Moudjou, M.; Li, B.; Laplanche, J.L.; Laude, H.; Langeveld, J.; et al. Glycoform-selective prion formation in sporadic and familial forms of prion disease. PLoS ONE 2013, 8, e58786. [Google Scholar] [CrossRef]
- Somerville, R.A. Host and transmissible spongiform encephalopathy agent strain control glycosylation of PrP. J. Gen. Virol. 1999, 80, 1865–1872. [Google Scholar] [CrossRef]
- Collinge, J.; Sidle, K.C.; Meads, J.; Ironside, J.; Hill, A.F. Molecular analysis of prion strain variation and the aetiology of ‘new variant’ CJD. Nature 1996, 383, 685–690. [Google Scholar] [CrossRef] [PubMed]
- Lüdemann, N.; Clement, A.; Hans, V.H.; Leschik, J.; Behl, C.; Brandt, R. O-glycosylation of the tail domain of neurofilament protein M in human neurons and in spinal cord tissue of a rat model of amyotrophic lateral sclerosis (ALS). J. Biol. Chem. 2005, 280, 31648–31658. [Google Scholar] [CrossRef] [PubMed]
- Moll, T.; Shaw, P.J.; Cooper-Knock, J. Disrupted glycosylation of lipids and proteins is a cause of neurodegeneration. Brain 2019, 143, 1332–1340. [Google Scholar] [CrossRef] [PubMed]
- Vajn, K.; Viljetić, B.; Degmečić, I.V.; Schnaar, R.L.; Heffer, M. Differential distribution of major brain gangliosides in the adult mouse central nervous system. PLoS ONE 2013, 8, e75720. [Google Scholar] [CrossRef] [PubMed]
- Ariga, T. Pathogenic role of ganglioside metabolism in neurodegenerative diseases. J. Neurosci. Res. 2014, 92, 1227–1242. [Google Scholar] [CrossRef]
- Wu, G.; Lu, Z.H.; Kulkarni, N.; Ledeen, R.W. Deficiency of ganglioside GM1 correlates with Parkinson’s disease in mice and humans. J. Neurosci. Res. 2012, 90, 1997–2008. [Google Scholar] [CrossRef]
- Gylys, K.H.; Fein, J.A.; Yang, F.; Miller, C.A.; Cole, G.M. Increased cholesterol in Abeta-positive nerve terminals from Alzheimer’s disease cortex. Neurobiol. Aging 2007, 28, 8–17. [Google Scholar] [CrossRef]
- Cooper-Knock, J.; Moll, T.; Ramesh, T.; Castelli, L.; Beer, A.; Robins, H.; Fox, I.; Niedermoser, I.; Van Damme, P.; Moisse, M.; et al. Mutations in the Glycosyltransferase Domain of GLT8D1 Are Associated with Familial Amyotrophic Lateral Sclerosis. Cell Rep. 2019, 26, 2298–2306.e5. [Google Scholar] [CrossRef]
- Schneider, J.S. Altered expression of genes involved in ganglioside biosynthesis in substantia nigra neurons in Parkinson’s disease. PLoS ONE 2018, 13, e0199189. [Google Scholar] [CrossRef]
- Seyfried, T.N.; Choi, H.; Chevalier, A.; Hogan, D.; Akgoc, Z.; Schneider, J.S. Sex-Related Abnormalities in Substantia Nigra Lipids in Parkinson’s Disease. ASN Neuro 2018, 10, 1759091418781889. [Google Scholar] [CrossRef]
- Desplats, P.A.; Denny, C.A.; Kass, K.E.; Gilmartin, T.; Head, S.R.; Sutcliffe, J.G.; Seyfried, T.N.; Thomas, E.A. Glycolipid and ganglioside metabolism imbalances in Huntington’s disease. Neurobiol. Dis. 2007, 27, 265–277. [Google Scholar] [CrossRef]
- Kracun, I.; Kalanj, S.; Talan-Hranilovic, J.; Cosovic, C. Cortical distribution of gangliosides in Alzheimer’s disease. Neurochem. Int. 1992, 20, 433–438. [Google Scholar] [CrossRef]
- Yanagisawa, K.; Ihara, Y. GM1 ganglioside-bound amyloid beta-protein in Alzheimer’s disease brain. Neurobiol. Aging 1998, 19, S65–S67. [Google Scholar] [CrossRef]
- Hayashi, H.; Kimura, N.; Yamaguchi, H.; Hasegawa, K.; Yokoseki, T.; Shibata, M.; Yamamoto, N.; Michikawa, M.; Yoshikawa, Y.; Terao, K.; et al. A seed for Alzheimer amyloid in the brain. J. Neurosci. 2004, 24, 4894–4902. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Yamauchi, Y.; Furukawa, K.; Ohmi, Y.; Ohkawa, Y.; Zhang, Q.; Okajima, T.; Furukawa, K. Expression of B4GALNT1, an essential glycosyltransferase for the synthesis of complex gangliosides, suppresses BACE1 degradation and modulates APP processing. Sci. Rep. 2016, 6, 34505. [Google Scholar] [CrossRef]
- Abu Bakar, N.; Lefeber, D.J.; van Scherpenzeel, M. Clinical glycomics for the diagnosis of congenital disorders of glycosylation. J. Inherit. Metab. Dis 2018, 41, 499–513. [Google Scholar] [CrossRef]
- Abbott, K.L.; Pierce, J.M. Lectin-based glycoproteomic techniques for the enrichment and identification of potential biomarkers. Methods Enzymol. 2010, 480, 461–476. [Google Scholar] [CrossRef]
- Van Scherpenzeel, M.; Willems, E.; Lefeber, D.J. Clinical diagnostics and therapy monitoring in the congenital disorders of glycosylation. Glycoconj. J. 2016, 33, 345–358. [Google Scholar] [CrossRef]
- Chong, P.F.; Sakai, Y.; Torisu, H.; Tanaka, T.; Furuno, K.; Mizuno, Y.; Ohga, S.; Hara, T.; Kira, R. Leucine-rich alpha-2 glycoprotein in the cerebrospinal fluid is a potential inflammatory biomarker for meningitis. J. Neurol. Sci. 2018, 392, 51–55. [Google Scholar] [CrossRef]
- Adav, S.S.; Sze, S.K. Simultaneous Enrichment of Plasma Extracellular Vesicles and Glycoproteome for Studying Disease Biomarkers. Methods Mol. Biol. 2017, 1619, 193–201. [Google Scholar] [CrossRef]
- Moseley, R.; Stewart, J.E.; Stephens, P.; Waddington, R.J.; Thomas, D.W. Extracellular matrix metabolites as potential biomarkers of disease activity in wound fluid: Lessons learned from other inflammatory diseases? Br. J. Dermatol. 2004, 150, 401–413. [Google Scholar] [CrossRef] [PubMed]
- Gontika, M.P.; Anagnostouli, M.C. Anti-Myelin Oligodendrocyte Glycoprotein and Human Leukocyte Antigens as Markers in Pediatric and Adolescent Multiple Sclerosis: On Diagnosis, Clinical Phenotypes, and Therapeutic Responses. Mult. Scler. Int. 2018, 2018, 8487471. [Google Scholar] [CrossRef]
- Kailemia, M.J.; Park, D.; Lebrilla, C.B. Glycans and glycoproteins as specific biomarkers for cancer. Anal. Bioanal. Chem. 2017, 409, 395–410. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, V.; Liu, J.; Yang, R.; Lin, H.; Lischuk, A.; Pastores, G.; Zhang, X.; Chuang, W.L.; Mistry, P.K. Validating glycoprotein non-metastatic melanoma B (gpNMB, osteoactivin), a new biomarker of Gaucher disease. Blood Cells Mol. Dis. 2018, 68, 47–53. [Google Scholar] [CrossRef] [PubMed]
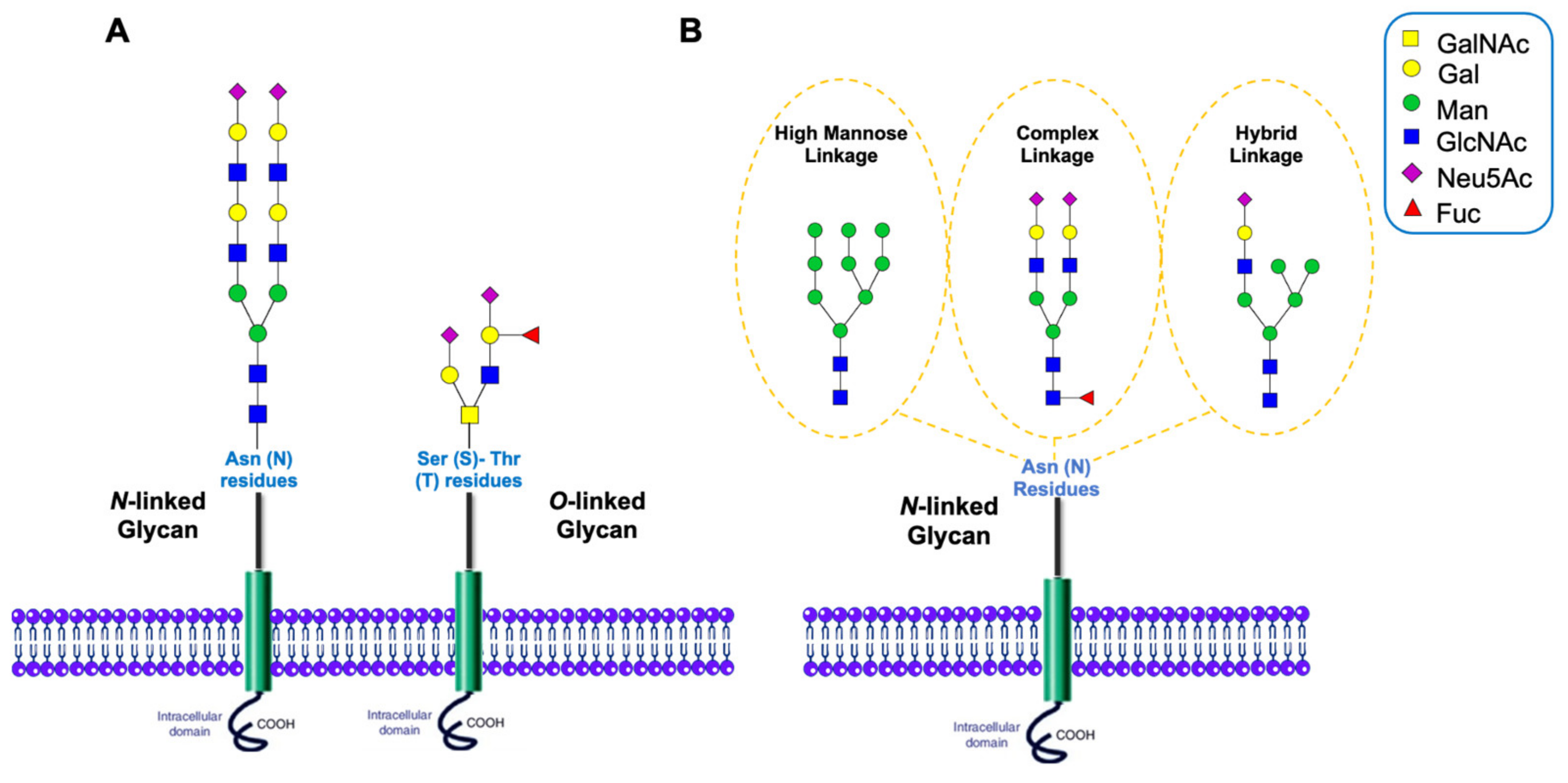
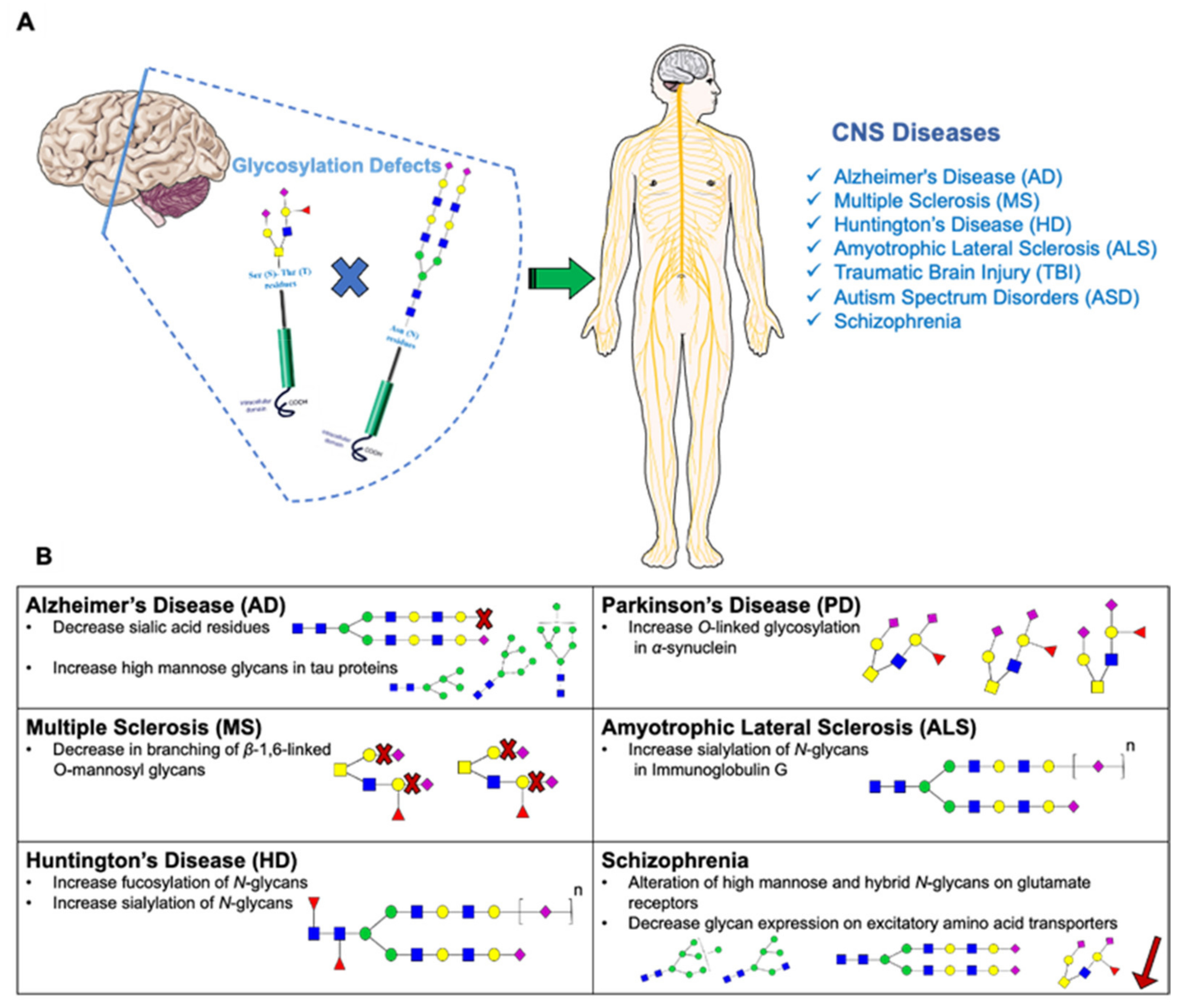
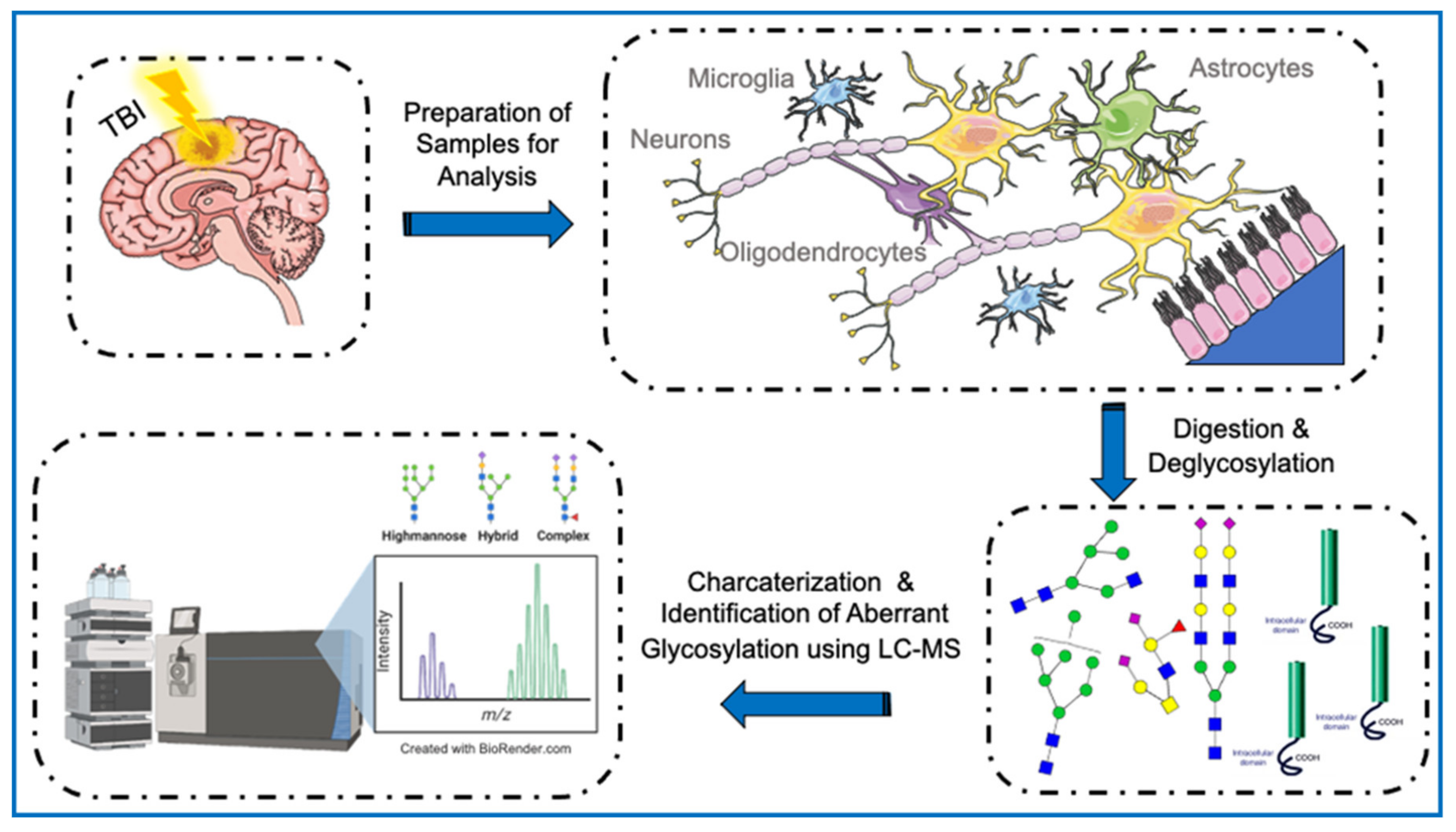
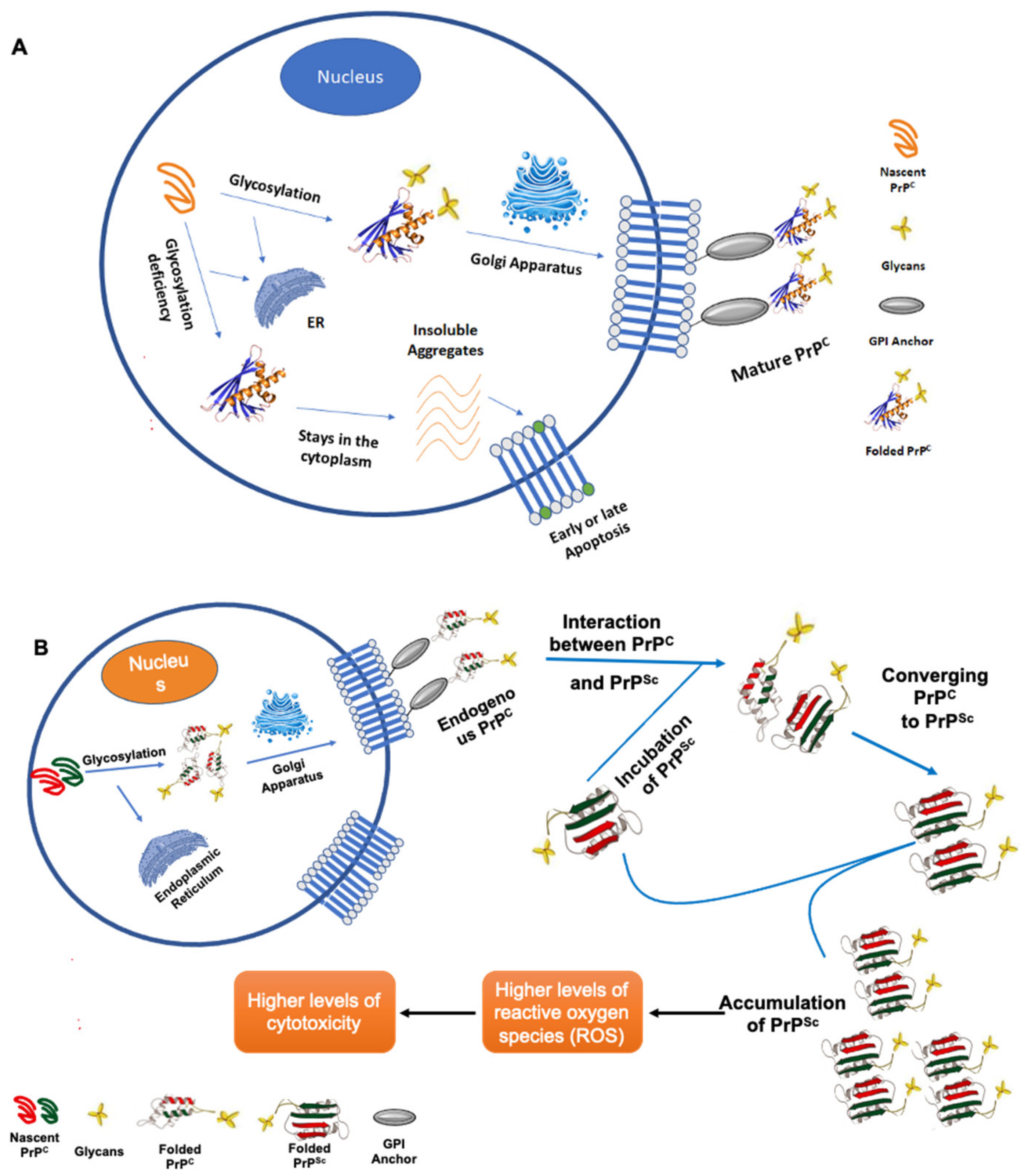
| PTM | Process | Localization | Common Amino Acids/Sites Targeted | Cellular Processes Affected |
|---|---|---|---|---|
| Phosphorylation | The addition of one or more phosphate groups to the protein by kinases | Phosphorylation occurs in the nucleus or cytosol | In animal cells: serine, tyrosine, and threonine | DNA replication and transcription, cell movement, cell metabolism, apoptosis, environmental stress responses |
| Glycosylation | The addition of carbohydrate molecules to the polypeptide chain by glycoyltransferases | Glycosylation occurs in the endoplasmic reticulum (ER), Golgi apparatus or cytosol | Serine (Ser), threonine (Thr), asparagine (Asn), and tryptophan (Trp) residues | Cell adhesion, cell-cell, and cell-matrix interactions, receptor activation and signal transduction, protein secretion and trafficking |
| Acetylation | The addition of an acetyl group by acetyltransferase (KAT) and histone acetlytransferases (HAT) | Acetylation takes place mainly in the nucleus | Lysine (Lys) residues | Transcription regulation, protein-protein interaction, cell metabolism, nuclear transport |
| Sulfation | The addition of sulfate molecules by tyrosylprotein transferases (TPST) | Sulfation takes place in the trans-Golgi network | Tyrosine (Tyr) residues | Protein-protein interactions and leukocyte rolling |
| Hydroxylation | The addition of a hydroxy (OH) group to a protein amino acid by hydroxylases | Hydroxylation occurs in the cytosol | Lysine (Lys) and proline (Pro) residues | Transcription factor regulation |
| SUMOylation | The addition of SUMO protein via three enzymes (E1, E2 and E3) | SUMOylation occurs in the cytoplasm and nucleus | Lysine (Lys) residues | Transcription regulation and signal transduction |
| Ubiquitylation | The attachment of ubiquitin to a target protein by ubiquitin ligase and ubiquitin-conjugating enzyme | Ubiquitylation takes place in the cytosol | Lysine (Lys) residues | Protein degradation, transcription regulation, apoptosis and autophagy |
| Methylation | The transfer of a methyl group or more to amino acid side chains by methyltrasnferaes | Methylation usually occurs in the nucleus | Lysine (Lys) and arginine (Arg) residues | Histone modification, transcription regulation and epigenetic silencing |
| Types of Glycosylation | |
|---|---|
| N-linked | Glycans bind to the amino group of asparagine in the ER |
| O-linked | Monosaccharides bind to the hydroxyl group of serine or threonine in the ER, Golgi, cytosol, and nucleus |
| C-linked | Mannose binds to the indole ring of tryptophan |
| Phospho-glycosylation | Glycan binds to serine via a phosphodiester bond |
| Title | Neurodegenerative
Disease | Glycosylation Aspect | Results | Analytical Methods | Ref. |
|---|---|---|---|---|---|
| Glyceraldehyde-3-phosphate dehydrogenase: Aggregation Mechanisms and Impact on Amyloid Neurodegenerative Diseases. | Amyloid neurodegenerative diseases | Glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase (GAPDH) has the ability to change the concentration of carbonyl compounds like glyceraldehyde-3-phosphate and methylglyoxal. | • Inhibition of glycolysis is due to the decreased activity of modified GAPDH. • Dysregulation of the cell metabo-lism is due to the compartmentalization of phosphorylated and glycated GAPDH and the replacement of active GAPDH in supramolecular complexes by its dena-tured form. • Blocking of the chaperone system by misfolded forms of modified GAPDH leads to the formation of amyloid struc-tures. • Denatured and modified GAPDH could mediate amyloid-like transition of susceptible proteins and peptides (amy-loid beta peptide, tau protein, al-pha-synuclein, prion, etc) | ELISA | [221] |
| Identification of an Intracellular Site of Prion Conversion. | Prion diseases | Cellular prion protein (PrPC) is a glycosyl-phosphatidyl-inositol (GPI) anchored glycoprotein that is able to misfold to a pathogenic isoform PrPSc. PrPSc acts as the causative agent of prion diseases. | • Mis-folding PrPC to PrPSc is a causa-tive agent of prion diseases. • Understanding where the conver-sion of PrPC to PrPSc occurs in cells can help to clarify the cellular mechanism of the disease and it opens the door to new therapeutic strategies aimed at the con-version compartment. • It has been found that the prion conversion occurs in the endosomal recy-cling compartment (ERC), where it trans-its after being internalized from the cell surface. | Immunofluorescence | [222] |
| Alterations in Sulfated Chondroitin Glycosaminoglycans Following Controlled Cortical Impact Injury in Mice | Traumatic Brain Injury (TBI) | Many actions of chondroitin sulfate proteoglycans (CSPGs) in the central nervous system (CNS) are governed by the specific sulfation pattern on the glycosaminoglycan (GAG) chains attached to CSPG core proteins. | • It has been found that there are specific changes in the level and localization of CSPGs and CS-GAGs in response to TBI, with the predominant elevation in 4-sulfated GAG chain surrounding the injury core. | Immunoblotting Immunostaining | [223] |
| Increasing O-GlcNAc Slows Neurodegeneration and Stabilizes Tau against Aggregation. | Alzheimer’s disease (AD) | Oligomerization of tau is a key process contributing to the progressive death of neurons in AD. Tau is modified by O-linked N-acetylglucosamine (O-GlcNAc), and in some cases, O-GlcNAc can influence tau phosphorylation. | • It has been found that the treatment of hemizygous JNPL3 tau transgenic mice with and O-GlcNAcase inhibitor elevated tau O-GlcNAc, hindered the tau aggre-gates formation and diminished neuronal cell lost. • Based on the in vitro biochemical aggregation studies, it has been suggested that the O-GlcNAc may be to prevent pro-tein aggregation. • It is also suggested that O-GlcNacase can be considered as a po-tential therapeutic target that could hin-der progression of AD. | SDS-PAGE Western blot Fluorescence immunohistochemistry (IHC) | [224] |
| Mutation in B4GALNT1 (GM2 Synthase) Underlie a New Disorder of Ganglioside Biosynthesis. | Diseases of ganglioside biosynthesis | A mutation in the B4GALNT1 gene, encoding GM2 synthase, catalyzes the second step in complex ganglioside biosynthesis, as the cause of this neurodegenerative phenotype. | • Biochemical profiling of the glycosphingolipid biosynthesis confirmed that a lack of GM2 in affected subjects is associated with a predictable elevation in its precursor levels (GM3), which can significantly facilitate the diagnosis of this disease. | MALDI mass spectrometry Gas chromatography | [225] |
| Receptors for Advanced Glycosylation Endproducts in Human Brain: Role in Brain Homeostasis. | Alzheimer’s disease (AD) and other neurodegenerative diseases | Advanced glycation end products (AGEs) are the reactive of nonenzymatic glucose macromolecule condensation products, which play role in neuroinflammation. | • Non-enzymatic glycosylation is implicated in the theory of aging. This suggests the central role of advanced glycation end products in age-relation cognitive features. | Immunohistochemistry RT-PCR Western blot Ligand blot | [226] |
| Glycosylation Status of Nicastrin Influences Catalytic Activity and Substrate Preference of γ-Secretase. | Alzheimer’s disease | The assembly of nicastrin (NCT) and its maturation occurs through complex N-glycosylation including the terminal sialic acid residues on NCT glycan, affecting γ-Secretase complex. | • γ-secretase complex catalyzes the cleavage of amyloid precursor protein to generate amyloid-β pro-tein (Aβ), the main cause of Alz-heimer’s disease. • Complex glycosylation of NCT including terminal sialylation is critical for γ-secretase activity. • Immature NCT preferentially reduced Aβ generation in both cell-based and biochemical assays. • Thorough glycosylation of NCT is critical for enzymatic activi-ty and substrate preference of γ-secretase. | Gel electrophoresis Western blot | [227] |
| Glycosylation Significantly Inhibits the Aggregation of Human Prion Protein and Decreases Its Cytotoxicity. | Prion diseases | Wild-type PrP and its monoglycosylated mutants N181D, N197D, and T199N/N181D/N197D are primarily attached to the plasma membrane via a glycosylphosphatidylinositol (GPI) anchor. This glycosylation occurs at 2 sites being Asn-181 and Asn-197 at the C-terminal through sialylation. | • Glycosylation deficiency enhances human prion protein (PrP) cytotoxicity induced by MG132 or the toxic prion peptide PrP 106–126. • Glycosylation acts as a necessary cofactor in determining PrP localization on the plasma membrane and that it significantly inhibits the aggregation of human PrP and decreases its cytotoxicity. | Western blotting Flow cytometry Circular dichroism spectroscopy Laser scanning confocal analysis | [228] |
| Disruption of Golgi Morphology and Altered Protein Glycosylation in PLA2G6-associated Neurodegeneration. | PLA2G6-associated neurodegeneration (PLAN) | N-linked and O-linked glycosylation in cerebrospinal fluid, plasma, urine, and cultured skin fibroblasts were assessed, along with sialylation and Golgi morphology in cultured fibroblasts. | • Golgi morphology, O-linked glycosylation and sialylation may play a role in the pathogenesis of PLAN and perhaps other neurodegenerative disorders. • Alteration in Golgi morphology and abnormalities of protein O-linked glycosylation and sialylation in cultured fibroblasts were rescued by lentiviral overexpression of wild type PLA2G6. | HPLC MALDI-TOF/MS Immunofluorescence Lentiviral vector | [38] |
| Sialylation Enhances the Secretion of Neurotoxic Amyloid-β Peptides. | Alzheimer’s disease | Overexpression of the β-galactoside α2,6-sialyltransferase (ST6Gal-I) in Neuro2a cells enhances α2,6-sialylation of endogenous APP and increases the extracellular levels of its metabolites. | • In the mouse model, the amount of α2,6-sialylated amyloid precursor protein (APP) appeared to be correlated with the soluble APPβ (sAPPβ) level. • It is suggested that the sialylation of APP promotes its metabolic turnover and could affect the AD pathology. | Western blot | [229] |
| Loss of O-GlcNAc Glycosylation in Forebrain Excitatory Neurons Induces Neurodegeneration. | Alzheimer’s disease | Problems in O-GlcNAc glycosylation (or O-GlcNAcylation) of proteins like α-synuclein, amyloid precursor protein (APP), and tau in forebrain excitatory neurons may induce neurodegeneration diseases. | • O-GlcNAc modification plays a central role in regulating both APP and tau and that dysfunctional O-GlcNAc signaling may contribute to improper APP processing and tau pathology. • O-GlcNAcylation levels can enhance nonamyloidogenic processing of APP by raising α-secretase activity and lowering γ-secretase activity. • O-GlcNAcylation regulates pathways critical for the maintenance of neuronal health and suggest that dysfunctional O-GlcNAc signaling may be an important contributor to neurodegenerative diseases. | Immunohistochemistry (IHC) ELISA Gene expression microarray qRT-PCR | [230] |
| V232M Substitution Restricts a Distinct O-glycosylation of PLD3 and its Neuroprotective Function. | Alzheimer’s disease | O-glycosylation at a specific site pT271 in phospholipase D3 (PLD3) is crucial for the wild-type’s normal trafficking and cellular localization. The Val232Met variant substitution impairs this O-glycosylation. | • Mutation of Val232Met variant of phospholipase D3 (PLD3) may affect AD pathogenesis by impairing this O-glycosylation, subsequently leading to enlarged lysosomes and possible aberrant protein recycling. • PLD3VM had a less neuroprotective function, while PLD3WT expression enhanced lysosomal functions, V232M weakened PLD3’s trafficking to the lysosomes. | Quantitative PCR Flow cytometry Cell immunocytochemistry | [231] |
| Glycation Potentiates α-Synuclein-associated Neurodegeneration in Synucleinopathies. | Parkinson’s disease and other neurodegenerative diseases | Glycation of the N-terminal region of α-synuclein by glucose is considered an age-associated post-translational modification. This PTM enhances α-synuclein toxicity in vitro and in vivo, in Drosophila and in mice. | • A hallmark present in Parkinson’s disease as well as other neurodegenerative diseases is α-synuclein misfolding and aggregation. • Glycation leads to reducing membrane binding of α-synuclein, impairing the clearance, and supporting the accumulation of toxic oligomers, that in turn impair neuronal synaptic transmission. • The use of glycation inhibitors allowed normal clearance of α-synuclein to be re-established, where the aggregations were reduced, alleviating the motor phenotypes in Drosophila. | Flow cytometry Immunoblotting Reverse phase HPLC Mass spectrometry Size exclusion chromatography Nuclear magnetic resonance spectrometry | [232] |
| The Prion Protein Requires Cholesterol for Cell Surface Localization. | Prion diseases and neurodegenerative disorders like Alzheimer’s disease | PrPC is a cell surface glycoprotein linked to the outer leaflet of the plasma membrane by a glycosyl-phosphatidyl-inositol (GPI) anchor. Prion conversion from PrPC to PrPSc occurs in the presence of cholesterol allowing prion propagation. | • Levels of cholesterol in the brains of affected individuals increase during the clinical course of both prion diseases and Alzheimer’s disease. • Imbalance in cholesterol homeostasis may lead to synaptic dysfunction and neurodegeneration in prion diseases and AD. | Immunoblot | [233] |
| Characterization of the Glycosylation Profiles of Alzheimer’s β-Secretase Protein Asp-2 Expressed in a Variety of Cell Lines. | Alzheimer’s disease | Asp-2 is a transmembrane aspartic protease expressed in the brain, shown to have β-secretase activity. Mature Asp-2 has four N-glycosylation sites. | • Carbohydrate structure characterization of Asp-2 expressed in Chinese hamster ovary, CV-1 origin of SV40, and baculovirus-infected SF9 cells were reported. • It has been reported that biantennary and triantennary oligosaccharides of the complex type were released from glycoproteins expressed in the mammalian cells, while high mannose glycan types were identified from glycoprotein synthesized in the baculovirus-infected cells. • Protease activity of Asp-2 is depended on its glycosylation. | Gel electrophoresis HILIC MALDI-TOF-MS | [234] |
| Altered Protein Glycosylation Predicts Alzheimer’s Disease and Modulates its Pathology in Disease Model Drosophila. | Alzheimer’s disease | The process of capping N- and O-linked glycans by a terminal sialic acid (sialylation) was reported to be altered in AD. Inhibiting the MGEA5 gene, encoding the enzyme that dynamically removes O-GlcNAc from proteins, OGA, reduces the extent of O-GlcNAc removal from tau. | • Many glycosylation-related genes are differentially expressed in brains of AD patients compared with healthy controls. • The result from the in vivo study in AD model indicates that certain alterations in expression levels of glycosylation-related genes are casually related to disease severity, whereas others are circumstantial. | Western blot | [235] |
| A Comprehensive Glycome Profiling of Huntington’s Disease Transgenic Mice. | Huntington’s disease (HD) | Total glycomics, namely, N-glycomics, O-glycomics and glycosphingolipidomics of HD transgenic mice can be a hallmark for the central nervous system disorders to discover disease biomarkers. | • Core-fucosylated and bisecting-GlcNAc types of N-glycans were found to be over expressed in the brain tissue HD mice. • Core-fucosylated and sialic acid for biantennary type glycans were found to be elevated in the sera of HD transgenic mice compared to the control mice. • Core 3 type O-glycans were increase in male and decrease in both striatum and cortexes of female HD transgenic mice. • Serum levels of core 1 type O-glycans decreased and core 2 type o-glycans were undetected for HD transgenic mice. • In glycosphingolipids, GD1 increased in brain tissue, and GM2-NeuGc decreased in serum. | Glycoblotting MALDI-TOF/MS | [236] |
| Interplay between Protein Glycosylation Pathways in Alzheimer’s Disease. | Alzheimer’s disease | Serum samples of 10 AD patients, MCI patients, and controls were studied. | • Differences in levels of glycan involved in both protein O-GlcNAcylation and N-/O-glycosylation between patients and healthy individuals can be seen, revealing brain region–specific glycosylation-related pathology in patients. • Robust decrease in protein O-GlcNAcylation and elevation in PAS staining of the soluble fraction of frontal cortex tissue of AD patients can be observed when compared to that in healthy controls. • Glycosylation alterations identified by PAS staining in the soluble membrane fractions of AD patients could be partially attributed to alterations in glycosylation of molecules other than glycoproteins, such as glycolipids. • The alterations in the AD glycome in the serum could potentially lead to novel glyco-based biomarkers for AD progression. | SDS-polyacrylamide gel electrophoresis, Western blot ELISA Lectin chip microarray | [237] |
| Title | Neurodegenerative
Disease | Glycosylation Aspect | Results | Analytical Methods | Ref. |
|---|---|---|---|---|---|
| Abnormal N-acetylglucosaminyltransferase Expression in Prefrontal Cortex in Schizophrenia. | Schizophrenia | N-linked and O-linked glycosylation in cerebrospinal fluid (CSF) and plasma along with glycosyltransferase transcripts in frontal cortex were studied. Comparison of protein expression of nine N-acetylglucosaminyltransferases (GlcNAcTs) glycosylating enzymes in postmortem tissue from the dorsolateral prefrontal cortex of 12 elderly patients with schizophrenia and 12 healthy controls was done. | • There was a decrease in protein expression of UDP-GlcNAc: BetaGal Beta-1, 3 GlcNAcT 8 (B3GNT8) and mannosyl (alpha-1, 3-)-glycoprotein beta-1, 4 GlcNAcT (MGAT4A) expression in patients with schizophrenia compared to controls, providing evidence for dysregulated glycosylation in schizophrenia. | Western blot | [270] |
| N-linked Glycosylation of Cortical N-methyl-D-aspartate and Kainate Receptor Subunits in Schizophrenia. | Schizophrenia | N-glycosylation of ionotropic glutamate receptors (iGluRs) and N-glycosylation of N-methyl-D-aspartate (NMDA) and kainate (KA) receptor subunits in the dorsolateral prefrontal cortex was studied. Comparison of NMDA and kainate receptor subunits N-glycosylation in postmortem tissue from the dorsolateral prefrontal cortex of 35 patients with schizophrenia and 31 healthy controls was performed. | • The levels of NMDA and kainite receptor subunits were unchanged between patients with schizophrenia and healthy controls. • NR1, NR2A, and NR2B NMDA receptor subunits, and GluR6 and KA2 kainate receptor subunits were N-glycosylated. • GluR6 was significantly more sensitive to endoglycosidase H in patients with schizophrenia, reflecting a large molecular mass of N-linked high mannose and/or hybrid sugars on the GluR6 protein subunit in patients with schizophrenia | SDS-polyacrylamide gel electrophoresis | [271] |
| Abnormal N-linked Glycosylation of Cortical AMPA Receptor Subunits in Schizophrenia. | Schizophrenia | N-linked glycosylation occurs in the ER and the Golgi apparatus before the assembled receptors are transported to the plasma membrane. Comparison of AMPA receptor subunit N-glycosylation in postmortem tissue from the dorsolateral prefrontal cortex of 35 schizophrenia patients and 31 healthy controls was done. | • The absolute level of AMPA receptors may not be critical, but rather changes in trafficking and activity of these receptors may contribute to schizophrenia. | Western blot Lectin-binding assays Immunoisolation | [272] |
| N-Glycosylation of GABAA Receptor Subunits is Altered in Schizophrenia. | Schizophrenia | N-glycosylation of molecules associated with glutamatergic neurotransmission were checked. Comparison of γ-aminobutyric type A receptor (GABAAR) subunit N-glycosylation in postmortem tissue from the superior temporal gyrus of 14 adult patients with schizophrenia and 14 healthy controls was performed. | • There was evidence for N-glycosylation of the α1, β1, and β2 GABAAR subunits in patients with schizophrenia, with characteristic glycan attachment on the α1, α4, and β1–3 GABAAR subunits. • Although the N-glycosylation of α1, β1, and β2 were all changed in patients with schizophrenia, the concentrations of GABAAR subunits themselves were unchanged. | Western blot Lectin Affinity Isolation | [273] |
| Antipsychotic Treatment of Acute Paranoid Schizophrenia Patients with Olanzapine Results in Altered Glycosylation of Serum Glycoproteins. | Schizophrenia | Disialylated bi- and triantennary glycans were checked. Identification of the glycosylation profile of serum proteins in 23 antipsychotic-naïve adult patients diagnosed with acute paranoid schizophrenia before and after 6 weeks of treatment with Olanzapine was performed. | • It has been shown that olanzapine treatment of schizophrenia patients resulted in changes in the glycosylation machinery associated with the biosynthesis of abundant serum proteins. • Olanzapine appeared to affect the extent of digalactosylation and disialylation of serum proteins. • As glycosylation impacts on many important cellular processes, olanzaoine-induced glycosylation changes may induce a number of downstream effects | HILIC fluorescence-based glycoanalytical technology Two-dimensional gel electrophoresis SDS-PAGE gel electrophoresis MALDI-TOF Mass Spectrometry ELISA | [274] |
| Identification of N-glycosylation Changes in the CSF and Serum in Patients with Schizophrenia. | Schizophrenia | N-glycans and sialylated glycans in the cerebrospinal fluid (CSF) appear altered in schizophrenia patients. Comparison of serum and CSF glycans of adult patients with first onset unmedicated schizophrenia (19 for serum and 14 for CSF) and healthy controls (19 for serum and 18 for CSF) was done. | • Changes in protein glycosylation are associated with disease physiopathology, with some of the alterations being gender specific, and can be hold potential as diagnostic tools for schizophrenia. | NP-HPLC | [275] |
| Abnormal Glycosylation of EAAT1 and EAAT2 in Prefrontal Cortex of Elderly Patients with Schizophrenia. | Schizophrenia | N-glycosylation can regulate excitatory amino acid transporters (EAATs). Comparison of the glycosylation pattern of EAATs in postmortem tissue from the dorsolateral prefrontal and anterior cingulate cortices of 35 adult patients with schizophrenia and 33 healthy controls was performed. | • There is significantly less glycosylation of both EAAT1 and EAAT2 (glial transporters) in neuronal postmortem tissues of patients with schizophrenia. • There was no evidence for N-linked glycosylation of EAAT3 (neuronal transporter) in postmortem tissues of either patients with schizophrenia or healthy controls. • Deficits in glycosylation that are glia-specific may have a role in the pathophysiology of schizophrenia. | Gel Electrophoresis Western blot | [276] |
| Evidence for Disruption of Sphingolipid Metabolism in Schizophrenia. | Schizophrenia | This study compares the expression of genes encoding proteins related to glycobiology in the prefrontal cortex, related to N- and O-linked glycan biosynthesis of 30 adult patients with schizophrenia and 30 healthy controls. | • There was a statistically significant decrease in the expression of seven genes encoding for glycan transferases in the N- and O-linked glycan biosynthetic pathways and glycosphingolipid metabolism in patients with short-term illness, and one gene in those with chronic illness. | Spectrophotometer Microarray Analysis PCR | [277] |
| Serum Glycoconjugates in Children with Schizophrenia and Conduct and Adjustment Disorders. | Schizophrenia | Glycoproteins and glycosaminoglycans are altered in the sera of children. Comparison of serum glycoproteins in 8 children with schizophrenia, 11 with conduct disorder, 6 with adjustment disorder and 20 13–17 years of age healthy controls was conducted. | • The serum glycosaminoglycans were significantly elevated only in children with schizophrenia (versus normal range in the three other groups). • The protein-bound carbohydrates were all significantly elevated in children with schizophrenia (versus only arabinose and galactosamine in children with conduct disorder, and only galactosamine in children with adjustment disorder). | Chemical ionization-mass spectrometry | [278] |
| Serum Glycoproteins in Schizophrenia. | Schizophrenia | Serum glycoproteins containing glucose and L-arabinose, in addition to mannose, galactose, fucose, sialic acid, and a trace of xylose are examined. Comparison of serum glycoproteins and their carbohydrate component in 30 adult patients with schizophrenia and 20 healthy controls was performed. | • The mean concentration of each of the protein-bound carbohydrate components was significantly elevated in patients with schizophrenia • The electrophoretic patterns for serum glycoprotein showed increases in alpha-2 and beta globulins in patients with schizophrenia. • The contents of glucose and arabinose were higher in serum glycoproteins from patients with schizophrenia. | GLC-electron-impact mass spectrometry | [279] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobeissy, F.; Kobaisi, A.; Peng, W.; Barsa, C.; Goli, M.; Sibahi, A.; El Hayek, S.; Abdelhady, S.; Ali Haidar, M.; Sabra, M.; et al. Glycomic and Glycoproteomic Techniques in Neurodegenerative Disorders and Neurotrauma: Towards Personalized Markers. Cells 2022, 11, 581. https://doi.org/10.3390/cells11030581
Kobeissy F, Kobaisi A, Peng W, Barsa C, Goli M, Sibahi A, El Hayek S, Abdelhady S, Ali Haidar M, Sabra M, et al. Glycomic and Glycoproteomic Techniques in Neurodegenerative Disorders and Neurotrauma: Towards Personalized Markers. Cells. 2022; 11(3):581. https://doi.org/10.3390/cells11030581
Chicago/Turabian StyleKobeissy, Firas, Abir Kobaisi, Wenjing Peng, Chloe Barsa, Mona Goli, Ahmad Sibahi, Samer El Hayek, Samar Abdelhady, Muhammad Ali Haidar, Mirna Sabra, and et al. 2022. "Glycomic and Glycoproteomic Techniques in Neurodegenerative Disorders and Neurotrauma: Towards Personalized Markers" Cells 11, no. 3: 581. https://doi.org/10.3390/cells11030581
APA StyleKobeissy, F., Kobaisi, A., Peng, W., Barsa, C., Goli, M., Sibahi, A., El Hayek, S., Abdelhady, S., Ali Haidar, M., Sabra, M., Orešič, M., Logroscino, G., Mondello, S., Eid, A. H., & Mechref, Y. (2022). Glycomic and Glycoproteomic Techniques in Neurodegenerative Disorders and Neurotrauma: Towards Personalized Markers. Cells, 11(3), 581. https://doi.org/10.3390/cells11030581











