Dynamic Changes in miR-21 Regulate Right Ventricular Dysfunction in Congenital Heart Disease-Related Pulmonary Arterial Hypertension
Abstract
:1. Introduction
2. Methods
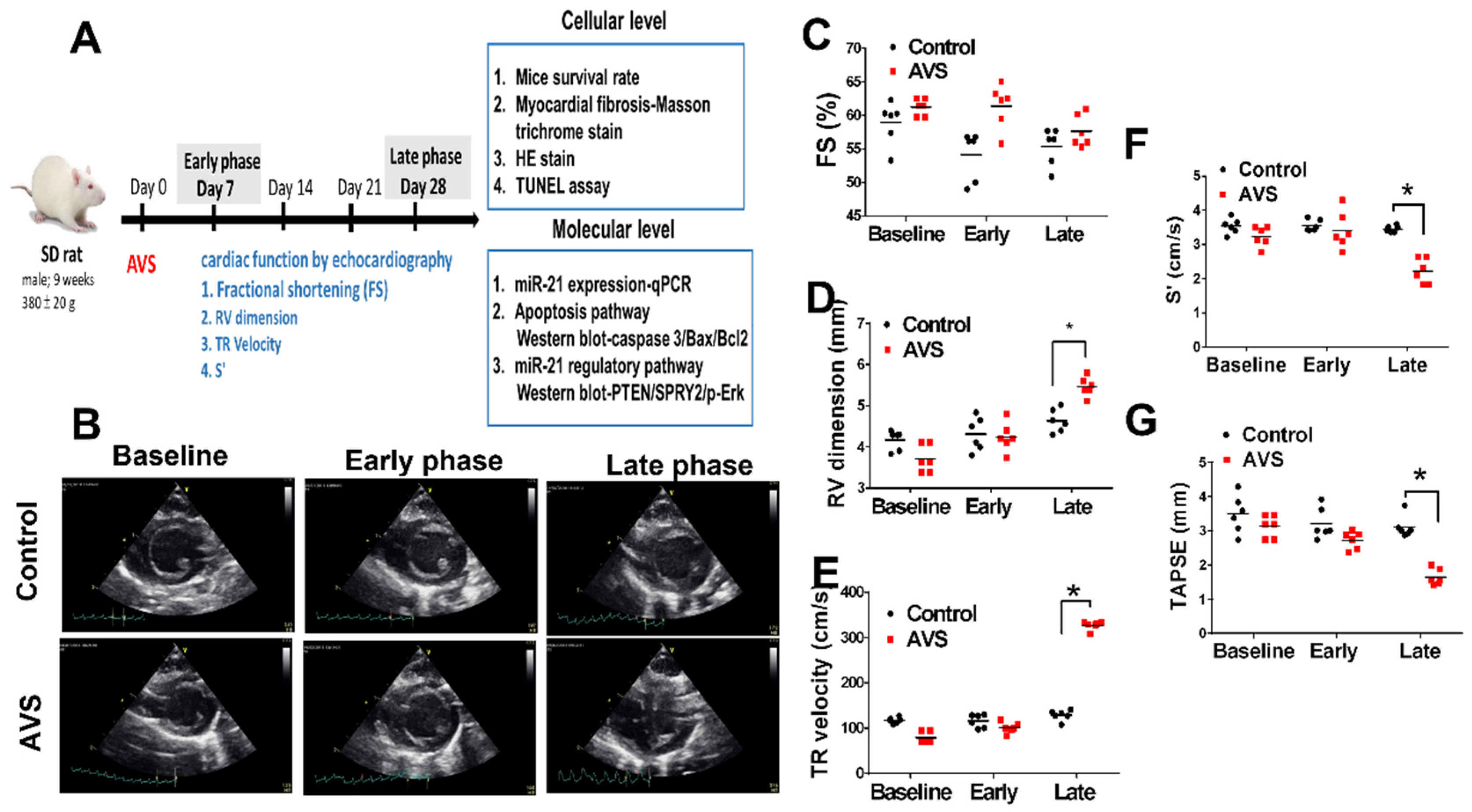
2.1. Study Designs of Animals
2.2. Microflow-Mediated Shear Stress System
2.3. Patients and Study Design
3. Results
3.1. miR-21 Mediayed Compensation and Decompensation of RV Function in Rats with PAH
3.2. The Upregulation of miR-21 in Rats of AVS mainly in RV Cardiomyocytes instead of Fibroblast
3.3. Mir-21 Regulated RV Hypertrophy and Apoptosis in Rats with PAH through the Spry2 and PTEN Pathways
3.4. Overexpression of miR-21 Mitigates Flow Shear-Induced Apoptosis in Cardiomyocytes
3.5. Demographic Characteristics of the Enrolled PAH Patients
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations and Acronyms
| RV | right ventricular |
| PAH | pulmonary arterial hypertension |
| miR-21 | microRNA-21 |
| HF | heart failure |
| AVS | Aorto-venous fistula |
| IPAH | idiopathic PAH |
| CTD | connective tissue disease |
| CHD | congenital heart disease |
| RHC | right heart catheterization |
| PCWP | pulmonary capillary wedge pressure |
| PVR | pulmonary vascular resistance |
| PAP | pulmonary arterial pressure |
| PCWP | pulmonary capillary wedge pressure |
| CO | cardiac output |
| EF | ejection fraction |
| FS | fractional shortening |
References
- Galie, N.; Humbert, M.; Vachiery, J.L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Noordegraaf, A.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur. Heart. J. 2016, 37, 67–119. [Google Scholar] [CrossRef] [PubMed]
- George, M.G.; Schieb, L.J.; Ayala, C.; Talwalkar, A.; Levant, S. Pulmonary hypertension surveillance: United States, 2001 to 2010. Chest 2014, 146, 476–495. [Google Scholar] [CrossRef] [Green Version]
- Chakinala, M.M. Changing the prognosis of pulmonary arterial hypertension: Impact of medical therapy. Semin. Respir. Crit. Care Med. 2005, 26, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Thenappan, T.; Ormiston, M.L.; Ryan, J.J.; Archer, S.L. Pulmonary arterial hypertension: Pathogenesis and clinical management. BMJ 2018, 360, j5492. [Google Scholar] [CrossRef] [PubMed]
- Schafer, M.; Kheyfets, V.O.; Schroeder, J.D.; Dunning, J.; Shandas, R.; Buckner, J.K.; Browning, J.; Hertzberg, J.; Hunter, K.S.; Fenster, B.E. Main pulmonary arterial wall shear stress correlates with invasive hemodynamics and stiffness in pulmonary hypertension. Pulm. Circ. 2016, 6, 37–45. [Google Scholar] [CrossRef] [Green Version]
- Boucherat, O.; Potus, F.; Bonnet, S. microRNA and Pulmonary Hypertension. Adv. Exp. Med. Biol. 2015, 888, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.T.; Hsu, C.H.; Huang, T.L.; Tsai, Y.C.; Chiang, C.Y.; Chen, Z.C.; Shih, J.Y. MicroRNA-21 is Associated with the Severity of Right Ventricular Dysfunction in Patients with Hypoxia-Induced Pulmonary Hypertension. Acta Cardiol. Sin. 2018, 34, 511–517. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, C. MicroRNA-21 in cardiovascular disease. J. Cardiovasc. Transl. Res. 2010, 3, 251–255. [Google Scholar] [CrossRef] [Green Version]
- Zhou, G.; Chen, T.; Raj, J.U. MicroRNAs in pulmonary arterial hypertension. Am. J. Respir. Cell. Mol. Biol. 2015, 52, 139–151. [Google Scholar] [CrossRef] [Green Version]
- Bonci, D. MicroRNA-21 as therapeutic target in cancer and cardiovascular disease. Recent Pat. Cardiovasc. Drug Discov. 2010, 5, 156–161. [Google Scholar] [CrossRef]
- Parikh, V.N.; Jin, R.C.; Rabello, S.; Gulbahce, N.; White, K.; Hale, A.; Cottrill, K.A.; Shaik, R.S.; Waxman, A.B.; Zhang, Y.Y.; et al. MicroRNA-21 integrates pathogenic signaling to control pulmonary hypertension: Results of a network bioinformatics approach. Circulation 2012, 125, 1520–1532. [Google Scholar] [CrossRef]
- Chang, W.T.; Fisch, S.; Dangwal, S.; Mohebali, J.; Fiedler, A.G.; Chen, M.; Hsu, C.H.; Yang, Y.; Qiu, Y.; Alexander, K.M.; et al. MicroRNA-21 regulates right ventricular remodeling secondary to pulmonary arterial pressure overload. J. Mol. Cell. Cardiol. 2021, 154, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.C.; Li, Y.S.; Haga, J.H.; Kaunas, R.; Chiu, J.J.; Su, F.C.; Usami, S.; Chien, S. Directional shear flow and Rho activation prevent the endothelial cell apoptosis induced by micropatterned anisotropic geometry. Proc. Natl. Acad. Sci. USA 2007, 104, 1254–1259. [Google Scholar] [CrossRef] [Green Version]
- Galie, N.; Humbert, M.; Vachiery, J.L.; Gibbs, S.; Lang, I.; Torbicki, A.; Simonneau, G.; Peacock, A.; Vonk Noordegraaf, A.; Beghetti, M.; et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur. Respir. J. 2015, 46, 903–975. [Google Scholar] [CrossRef]
- Sharifi Kia, D.; Kim, K.; Simon, M.A. Current Understanding of the Right Ventricle Structure and Function in Pulmonary Arterial Hypertension. Front. Physiol. 2021, 12, 641310. [Google Scholar] [CrossRef]
- Happe, C.M.; Szulcek, R.; Voelkel, N.F.; Bogaard, H.J. Reconciling paradigms of abnormal pulmonary blood flow and quasi-malignant cellular alterations in pulmonary arterial hypertension. Vascul. Pharmacol. 2016, 83, 17–25. [Google Scholar] [CrossRef]
- Nour, S.; Dai, G.; Carbognani, D.; Feng, M.; Yang, D.; Lila, N.; Chachques, J.C.; Wu, G. Intrapulmonary shear stress enhancement: A new therapeutic approach in pulmonary arterial hypertension. Pediatr Cardiol 2012, 33, 1332–1342. [Google Scholar] [CrossRef]
- Green, D.E.; Murphy, T.C.; Kang, B.Y.; Searles, C.D.; Hart, C.M. PPARgamma Ligands Attenuate Hypoxia-Induced Proliferation in Human Pulmonary Artery Smooth Muscle Cells through Modulation of MicroRNA-21. PLoS ONE 2015, 10, e0133391. [Google Scholar] [CrossRef] [PubMed]
- Marin, T.; Gongol, B.; Chen, Z.; Woo, B.; Subramaniam, S.; Chien, S.; Shyy, J.Y. Mechanosensitive microRNAs-role in endothelial responses to shear stress and redox state. Free Radic. Biol. Med. 2013, 64, 61–68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, D.; Deuse, T.; Stubbendorff, M.; Chernogubova, E.; Erben, R.G.; Eken, S.M.; Jin, H.; Li, Y.; Busch, A.; Heeger, C.H.; et al. Local MicroRNA Modulation Using a Novel Anti-miR-21-Eluting Stent Effectively Prevents Experimental In-Stent Restenosis. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1945–1953. [Google Scholar] [CrossRef] [Green Version]
- Bronnum, H.; Andersen, D.C.; Schneider, M.; Sandberg, M.B.; Eskildsen, T.; Nielsen, S.B.; Kalluri, R.; Sheikh, S.P. miR-21 promotes fibrogenic epithelial-to-mesenchymal transition of epicardial mesothelial cells involving Programmed Cell Death 4 and Sprouty-1. PLoS ONE 2013, 8, e56280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, D.; Wen, Z.; Han, W.; Li, W.; Pan, L.; Zhang, R. Curcumin protects against inflammation and lung injury in rats with acute pulmonary embolism with the involvement of microRNA-21/PTEN/NF-kappaB axis. Mol. Cell. Biochem. 2021, 476, 2823–2835. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Wan, L.; Fan, Y.; Wang, K.; Bu, L.; Huang, T.; Cheng, Z.; Shen, B. Ischemic postconditioning-mediated miRNA-21 protects against cardiac ischemia/reperfusion injury via PTEN/Akt pathway. PLoS ONE 2013, 8, e75872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sayed, D.; He, M.; Hong, C.; Gao, S.; Rane, S.; Yang, Z.; Abdellatif, M. MicroRNA-21 is a downstream effector of AKT that mediates its antiapoptotic effects via suppression of Fas ligand. J. Biol. Chem. 2010, 285, 20281–20290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lhamyani, S.; Gentile, A.M.; Giraldez-Perez, R.M.; Feijóo-Cuaresma, M.; Romero-Zerbo, S.Y.; Clemente-Postigo, M.; Zayed, H.; Olivera, W.; Bermúdez-Silva, F.; Salas, J.; et al. miR-21 mimic blocks obesity in mice: A novel therapeutic option. Mol. Ther. Nucleic Acids 2021, 26, 401–416. [Google Scholar] [CrossRef]
- Wang, S.Y.; Kim, H.; Kwak, G.; Jo, S.D.; Cho, D.; Yang, Y.; Kwon, I.C.; Jeong, J.H.; Kim, S.H. Development of microRNA-21 mimic nanocarriers for the treatment of cutaneous wounds. Theranostics 2020, 10, 3240–3253. [Google Scholar] [CrossRef]
- Ryoo, S.R.; Yim, Y.; Kim, Y.K.; Park, I.S.; Na, H.Y.; Lee, J.; Jang, H.; Won, C.; Hong, S.; Kim, S.Y.; et al. High-throughput chemical screening to discover new modulators of microRNA expression in living cells by using graphene-based biosensor. Sci. Rep. 2018, 8, 11413. [Google Scholar] [CrossRef] [Green Version]
- Connelly, C.M.; Boer, R.E.; Moon, M.H.; Gareiss, P.; Schneekloth, J.S., Jr. Discovery of Inhibitors of MicroRNA-21 Processing Using Small Molecule Microarrays. ACS Chem. Biol. 2017, 12, 435–443. [Google Scholar] [CrossRef]
- Zhu, Z.; Godana, D.; Li, A.; Rodriguez, B.; Gu, C.; Tang, H.; Minshall, R.D.; Huang, W.; Chen, J. Echocardiographic assessment of right ventricular function in experimental pulmonary hypertension. Pulm. Circ. 2019, 9, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Yamamichi, N.; Shimomura, R.; Inada, K.-I.; Sakurai, K.; Haraguchi, T.; Ozaki, Y.; Fujita, S.; Mizutani, T.; Furukawa, C.; Fujishiro, M.; et al. Locked Nucleic Acid In situ Hybridization Analysis of miR-21 Expression during Colorectal Cancer Development. Clin. Cancer Res. 2009, 15, 4009–4016. [Google Scholar] [CrossRef] [Green Version]
- Hsu, C.-Y.; Hsieh, T.-H.; Tsai, C.-F.; Tsai, H.-P.; Chen, H.-S.; Chang, Y.; Chuang, H.-Y.; Lee, J.-N.; Hsu, Y.-L.; Tsai, E.-M. miRNA-199a-5p regulates VEGFA in endometrial mesenchymal stem cells and contributes to the pathogenesis of endometriosis. J. Pathol. 2013, 232, 330–343. [Google Scholar] [CrossRef] [PubMed]
- Rudski, L.G.; Lai, W.W.; Afilalo, J.; Hua, L.; Handschumacher, M.D.; Chandrasekaran, K.; Solomon, S.D.; Louie, E.K.; Schiller, N.B. Guidelines for the echocardiographic assessment of the right heart in adults: A report from the American Society of Echocardiography endorsed by the European Association of Echocardiography, a registered branch of the European Society of Cardiology, and the Canadian Society of Echocardiography. J. Am. Soc. Echocardiogr. 2010, 23, 685–713, quiz 786–788. [Google Scholar] [PubMed]
- Bossone, E.; D’Andrea, A.; DʹAlto, M.; Citro, R.; Argiento, P.; Ferrara, F.; Cittadini, A.; Rubenfire, M.; Naeije, R. Echocardiography in Pulmonary Arterial Hypertension: From Diagnosis to Prognosis. J. Am. Soc. Echocardiogr. 2013, 26, 1–14. [Google Scholar] [CrossRef] [PubMed]







| Normal Control (N = 10) | HF Hospitalization (-) N = 57 | HF Hospitalization (+) N = 19 | p Value | |
|---|---|---|---|---|
| Clinical parameters | ||||
| Age (y/o) | 50.2 ± 8.5 | 52.1 ± 22.2 | 50.4 ± 23.7 | 0.64 |
| Male gender, N (%) | 4 (40) | 19 (33.3) | 7 (36.8) | |
| Body height (cm) | 163.8 ± 17.4 | 156 ± 25.4 | 161.6 ± 24.9 | 0.81 |
| Body weight (kg) | 68.6 ± 7.1 | 60.6 ± 15.7 | 49.5 ± 9.9 | 0.08 |
| Diabetes, N (%) | 0 | 4 (7) | 1 (5.2) | 0.2 |
| Systemic HTN, N (%) | 0 | 9 (15.7) | 1 (5.2) | 0.61 |
| Smoking, N (%) | 0 | 0 (0) | 1(5.2) | 0.28 |
| Cancer, N (%) | 0 | 4 (7) | 3 (15.7) | 0.36 |
| Etiologies | ||||
| ASD, N (%) | 49 (85.9) | 14 (73.6) | 0.12 | |
| VSD, N (%) | 8 (14) | 4 (21.1) | 0.43 | |
| Surgical closure, N (%) | 26 (45.6) | 10 (52.6) | 0.72 | |
| Percutaneous occluder, N (%) | 6 (10.5) | 3 (15.8) | 0.81 | |
| Functional capacity | ||||
| NYFc I, N (%) | 10 (100) | 18 (31.6) | 4 (21) | 0.24 |
| NYFc II, N (%) | - | 26 (45.6) | 9 (47.3) | |
| NYFc III, N (%) | - | 13 (22.8) | 5 (26.3) | |
| NYFc IV, N (%) | - | 0 (0) | 1 (5.2) | |
| 6MWD (m) | - | 404.4 ± 51.1 | 389.6 ± 82.1 | 0.68 |
| Serologic markers | ||||
| Hemoglobin (mg/dl) | 13.1 ± 2.1 | 15.5 ± 21.9 | 12.4 ± 4 | 0.2 |
| eGFR (mL/min/1.73m2) | 90.7 ± 38 | 88.5 ± 44.7 | 83.9 ± 49.3 | 0.78 |
| ALT (IU/l) | 18.9 ± 8.4 | 24.8 ± 16 | 25.5 ± 12.1 | 0.9 |
| Bilirubin (mg/dl) | 0.9 ± 1.4 | 0.8 ± 0.3 | 1.05 ± 0.5 | 0.39 |
| NT-proBNP | 12.3 ± 3.8 | 458.6 ±87.5 | 613.8 ±61.2 | 0.01 |
| Circulating miR-21 | 15.25 ± 6.23 | 29.83 ± 37.93 | 9.68 ± 21.25 | 0.008 |
| Echocardiographic parameters | ||||
| LVEF (%) | 70.5 ± 6.4 | 69.5 ± 7.2 | 72 ± 4.4 | 0.86 |
| RA area (cm2) | 12.8 ± 4.6 | 14.9 ± 7.9 | 15.3 ± 9.7 | 0.73 |
| TAPSE (cm) | 2.1 ± 0.5 | 1.8 ± 0.4 | 1.1 ± 0.6 | 0.02 |
| S’ (cm/s) | 15.6 ± 4.6 | 11.2 ± 6.7 | 7.6 ± 5.3 | 0.04 |
| PAP (mmHg) | 15.7 ± 2.5 | 59.6 ± 24.3 | 72.1 ± 39.3 | 0.18 |
| Pericardial effusion, N (%) | 0 (0) | 8 (14) | 2 (10.5) | 0.12 |
| Right heart catheterization | ||||
| Heart rate (bpm) | - | 84.7 ± 11.8 | 86.7 ± 11.1 | 0.55 |
| SBP (mmHg) | - | 120 ± 14.7 | 114.8 ± 9.7 | 0.18 |
| DBP (mmHg) | - | 72.1 ± 9.2 | 70.2 ± 7.3 | 0.46 |
| SaO2 (%) | - | 97.2 ± 2.6 | 98.2 ± 2.2 | 0.58 |
| RA pressure (mmHg) | - | 9.2 ± 3.2 | 11.5 ± 4.4 | 0.15 |
| mRV pressure(mmHg) | - | 29.6 ± 10.9 | 36.2 ± 10.1 | 0.14 |
| mPA pressure (mmHg) | - | 39.1 ± 17.1 | 50.6 ± 13.4 | 0.08 |
| Wedge (mmHg) | - | 11.7 ± 3.1 | 13.7 ± 2.3 | 0.31 |
| Cardiac index (l/m2) | - | 3.5 ± 0.9 | 2.6 ± 1.2 | 0.1 |
| PVR (woods) | - | 6.1 ± 5.2 | 8.1 ± 4.5 | 0.32 |
| Univariate | Multivariable | |||||
|---|---|---|---|---|---|---|
| Model 1 | Model 2 | |||||
| HR (95% CI) | p | HR (95% CI) | p | HR (95% CI) | p | |
| Age | 0.99 (0.95–1) | 0.94 | ||||
| Male gender | 0.57 (0.14–2.1) | 0.42 | ||||
| mPAP (RHC) | 1.02 (0.96–1.08) | 0.52 | ||||
| NT-proBNP | 1.12 (1.01–1.28) | 0.05 | 1.001 (1–1.02) | 0.05 | 1.001 (1–1.12) | 0.08 |
| TAPSE | 1.09 (0.24–4.96) | 0.9 | ||||
| RV S’ | 0.88 (0.61–1.21) | 0.52 | ||||
| Circulating miR-21 | 0.92 (0.84–0.99) | 0.02 | 0.9 (0.8–0.92) | 0.04 | ||
| Circulating miR-21< 12 | 16 (1.92–13.01) | 0.01 | 9.62 (1.05–12.16) | 0.01 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, W.-T.; Wu, C.-C.; Lin, Y.-W.; Shih, J.-Y.; Chen, Z.-C.; Wu, S.-N.; Wu, C.-C.; Hsu, C.-H. Dynamic Changes in miR-21 Regulate Right Ventricular Dysfunction in Congenital Heart Disease-Related Pulmonary Arterial Hypertension. Cells 2022, 11, 564. https://doi.org/10.3390/cells11030564
Chang W-T, Wu C-C, Lin Y-W, Shih J-Y, Chen Z-C, Wu S-N, Wu C-C, Hsu C-H. Dynamic Changes in miR-21 Regulate Right Ventricular Dysfunction in Congenital Heart Disease-Related Pulmonary Arterial Hypertension. Cells. 2022; 11(3):564. https://doi.org/10.3390/cells11030564
Chicago/Turabian StyleChang, Wei-Ting, Chia-Chun Wu, Yu-Wen Lin, Jhih-Yuan Shih, Zhih-Cherng Chen, Sheng-Nan Wu, Chia-Ching Wu, and Chih-Hsin Hsu. 2022. "Dynamic Changes in miR-21 Regulate Right Ventricular Dysfunction in Congenital Heart Disease-Related Pulmonary Arterial Hypertension" Cells 11, no. 3: 564. https://doi.org/10.3390/cells11030564
APA StyleChang, W.-T., Wu, C.-C., Lin, Y.-W., Shih, J.-Y., Chen, Z.-C., Wu, S.-N., Wu, C.-C., & Hsu, C.-H. (2022). Dynamic Changes in miR-21 Regulate Right Ventricular Dysfunction in Congenital Heart Disease-Related Pulmonary Arterial Hypertension. Cells, 11(3), 564. https://doi.org/10.3390/cells11030564






