Decrease in the Size of Fat-Enlarged Axillary Lymph Nodes and Serum Lipids after Bariatric Surgery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Methodology
2.3. Clinical Definitions
2.4. Statistical Analysis
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hruby, A.; Hu, F.B. The Epidemiology of Obesity: A Big Picture. Pharmacoeconomics 2015, 33, 673–689. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, I.; Lohmann, A.E.; Goodwin, P.J. Normal Weight Adiposity and Postmenopausal Breast Cancer Risk. JAMA Oncol. 2019, 5, 150–151. [Google Scholar] [CrossRef] [PubMed]
- Shimabukuro, M.; Kozuka, C.; Taira, S.-I.; Yabiku, K.; Dagvasumberel, M.; Ishida, M.; Matsumoto, S.; Yagi, S.; Fukuda, D.; Yamakawa, K.; et al. Ectopic fat deposition and global cardiometabolic risk: New paradigm in cardiovascular medicine. J. Med. Investig. 2013, 60, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neeland, I.J.; Turer, A.T.; Ayers, C.R.; Powell-Wiley, T.M.; Vega, G.L.; Farzaneh-Far, R.; Grundy, S.M.; Khera, A.; McGuire, D.K.; de Lemos, J.A. Dysfunctional adiposity and the risk of prediabetes and type 2 diabetes in obese adults. JAMA J. Am. Med. Assoc. 2012, 308, 1150–1159. [Google Scholar] [CrossRef] [Green Version]
- Neeland, I.J.; Poirier, P.; Després, J.P. Cardiovascular and Metabolic Heterogeneity of Obesity: Clinical Challenges and Implications for Management. Circulation 2018, 137, 1391–1406. [Google Scholar] [CrossRef]
- Snel, M.; Jonker, J.T.; Schoones, J.W.; Lamb, H.; de Roos, A.; Pijl, H.; Smit, J.; Meinders, A.E.; Jazet, I. Ectopic Fat and Insulin Resistance: Pathophysiology and Effect of Diet and Lifestyle Interventions. Int. J. Endocrinol. 2012, 2012, 983814. [Google Scholar] [CrossRef]
- diFlorio-Alexander, R.M.; Haider, S.J.; MacKenzie, T.; Goodrich, M.E.; Weiss, J.; Onega, T. Correlation between obesity and fat-infiltrated axillary lymph nodes visualized on mammography. Br. J. Radiol. 2018, 91, 20170110. [Google Scholar] [CrossRef]
- Keshavarz, E.; Ahangaran, A.; Pouya, E.K.; Maheronnaghsh, R.; Chavoshi, M.; Rouzrokh, P. Effects of Obesity on Axillary Lymph Node Structure: Association of Hilar Fat Deposition and Alterations in Cortex Width. Maedica 2020, 15, 99–104. [Google Scholar]
- Ring, N.Y.; Patel, K.J.; Suriawinata, A.; Cervantes, E.; diFlorio-Alexander, R. Correlation of mammographic fat-infiltrated lymph nodes with NAFLD. In Proceedings of the Liver Meeting, Washington, DC, USA, 23 October 2017. [Google Scholar]
- diFlorio-Alexander, R.M.; Song, Q.; Dwan, D.; Austin-Strohbehn, J.A.; Muller, K.E.; Kinlaw, W.B.; MacKenzie, T.A.; Karagas, M.R.; Hassanpour, S. Fat-enlarged axillary lymph nodes are associated with node-positive breast cancer in obese patients. Breast Cancer Res. Treat. 2021, 189, 257–267. [Google Scholar] [CrossRef]
- Armstrong, C. JNC8 guidelines for the management of hypertension in adults. Am. Fam. Physician 2014, 90, 503–504. [Google Scholar]
- PREVENTION, I. Standards of medical care in diabetes—2011. Diabetes Care 2011, 34 (Suppl. 1), S11. [Google Scholar]
- Rosenson, R.S. High Cholesterol and Lipids (Beyond the Basics); Freeman, M.W., Ed.; UpToDate: Waltham, MA, USA, 2021. [Google Scholar]
- Goff, D.C., Jr.; Lloyd-Jones, D.M.; Bennett, G.; Coady, S.; D’Agostino, R.B.; Gibbons, R.; Greenland, P.; Lackland, D.T.; Levy, D.; O’Donnell, C.J.; et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2014, 129 (Suppl. 2), S49–S73. [Google Scholar] [PubMed] [Green Version]
- Petersen, K.F.; Dufour, S.; Befroy, D.; Lehrke, M.; Hendler, R.E.; Shulman, G.I. Reversal of Nonalcoholic Hepatic Steatosis, Hepatic Insulin Resistance, and Hyperglycemia by Moderate Weight Reduction in Patients with Type 2 Diabetes. Diabetes 2005, 54, 603–608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdullah, S.M.; Defina, L.F.; Leonard, D.; Barlow, C.E.; Radford, N.B.; Willis, B.L.; Rohatgi, A.; McGuire, D.K.; de Lemos, J.A.; Grundy, S.M.; et al. Long-term association of low-density lipoprotein cholesterol with cardiovascular mortality in individuals at low 10-year risk of atherosclerotic cardiovascular disease: Results from the Cooper Center Longitudinal Study. Circulation 2018, 138, 2315–2325. [Google Scholar] [CrossRef] [PubMed]
- Ballantyne, C.M.; Grundy, S.M.; Oberman, A.; Kreisberg, R.A.; Havel, R.J.; Frost, P.H.; Haffner, S.M. Hyperlipidemia: Diagnostic and therapeutic perspectives. J. Clin. Endocrinol. Metab. 2000, 85, 2089–2112. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Danielson, E.; Fonseca, F.A.; Genest, J.; Koenig, W.; Libby, P.; Lorenzatti, A.J.; MacFadyen, J.G.; Nordestgaard, B.G.; Shepherd, J.; et al. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N. Engl. J. Med. 2008, 359, 2195–2207. [Google Scholar] [CrossRef] [Green Version]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; De Ferranti, S.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 139, e1082–e1143. [Google Scholar]
- Chang, J.M.; Leung, J.W.T.; Moy, L.; Ha, S.M.; Moon, W.K. Axillary Nodal Evaluation in Breast Cancer: State of the Art. Radiology 2020, 295, 500–515. [Google Scholar] [CrossRef] [Green Version]
- Pieńkowska, J.; Brzeska, B.; Kaszubowski, M.; Kozak, O.; Jankowska, A.; Szurowska, E. The correlation between the MRI-evaluated ectopic fat accumulation and the incidence of diabetes mellitus and hypertension depends on body mass index and waist circumference ratio. PLoS ONE 2020, 15, e0226889. [Google Scholar] [CrossRef] [Green Version]
- Trouwborst, I.; Bowser, S.M.; Goossens, G.; Blaak, E.E. Ectopic Fat Accumulation in Distinct Insulin Resistant Phenotypes; Targets for Personalized Nutritional Interventions. Front. Nutr. 2018, 5, 77. [Google Scholar] [CrossRef]
- Britton, K.A.; Fox, C.S. Ectopic Fat Depots and Cardiovascular Disease. Circulation 2011, 124, e837–e841. [Google Scholar] [CrossRef] [PubMed]
- Frydrych, L.M.; Bian, G.; O’Lone, D.E.; Ward, P.A.; Delano, M.J. Obesity and type 2 diabetes mellitus drive immune dysfunction, infection development, and sepsis mortality. J. Leukoc. Biol. 2018, 104, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Lee, S.; Kim, Y.; Kim, B.; Choi, H.-S.; Kawada, T.; Kwon, B.; Yu, R. Visceral Fat Accumulation Induced by a High-fat Diet Causes the Atrophy of Mesenteric Lymph Nodes in Obese Mice. Obesity 2008, 16, 1261–1269. [Google Scholar] [CrossRef] [PubMed]
- Nitti, M.D.; Hespe, G.E.; Kataru, R.P.; García Nores, G.D.; Savetsky, I.L.; Torrisi, J.S.; Gardenier, J.C.; Dannenberg, A.J.; Mehrara, B.J. Obesity-induced lymphatic dysfunction is reversible with weight loss. J. Physiol. 2016, 594, 7073–7087. [Google Scholar] [CrossRef] [PubMed]
- Magnuson, A.M.; Regan, D.; Booth, A.D.; Fouts, J.K.; Solt, C.M.; Hill, J.L.; Dow, S.W.; Foster, M.T. High-fat diet induced central adiposity (visceral fat) is associated with increased fibrosis and decreased immune cellularity of the mesenteric lymph node in mice. Eur. J. Nutr. 2020, 59, 1641–1654. [Google Scholar] [CrossRef]
- Dixon, J.B. Lymphatic lipid transport: Sewer or subway? Trends Endocrinol. Metab. 2010, 21, 480–487. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.-H.; Elvington, A.; Randolph, G.J. The role of the lymphatic system in cholesterol transport. Front. Pharm. 2015, 6, 182. [Google Scholar] [CrossRef] [Green Version]
- Krievina, G.; Tretjakovs, P.; Skuja, I.; Silina, V.; Keisa, L.; Krievina, D.; Bahs, G. Ectopic Adipose Tissue Storage in the Left and the Right Renal Sinus is Asymmetric and Associated With Serum Kidney Injury Molecule-1 and Fibroblast Growth Factor-21 Levels Increase. EBioMedicine 2016, 13, 274–283. [Google Scholar] [CrossRef] [Green Version]
- Hall, M.E.; Do Carmo, J.M.; Da Silva, A.A.; Juncos, L.A.; Wang, Z.; Hall, J.E. Obesity, hypertension, and chronic kidney disease. Int. J. Nephrol. Renov. Dis. 2014, 7, 75–88. [Google Scholar] [CrossRef] [Green Version]
- Gastaldelli, A.; Gaggini, M. Ectopic fat: A target for cardiometabolic risk management. Expert Rev. Cardiovasc. Ther. 2016, 14, 1301–1303. [Google Scholar] [CrossRef] [Green Version]
- Ross, R.; Soni, S.; Houle, S.A. Negative Energy Balance Induced by Exercise or Diet: Effects on Visceral Adipose Tissue and Liver Fat. Nutrients 2020, 12, 891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Margolies, L.; Salvatore, M.; Hecht, H.S.; Kotkin, S.; Yip, R.; Baber, U.; Bishay, V.; Narula, J.; Yankelevitz, D.; Henschke, C. Digital Mammography and Screening for Coronary Artery Disease. JACC Cardiovasc. Imaging 2016, 9, 350–360. [Google Scholar] [CrossRef] [PubMed]
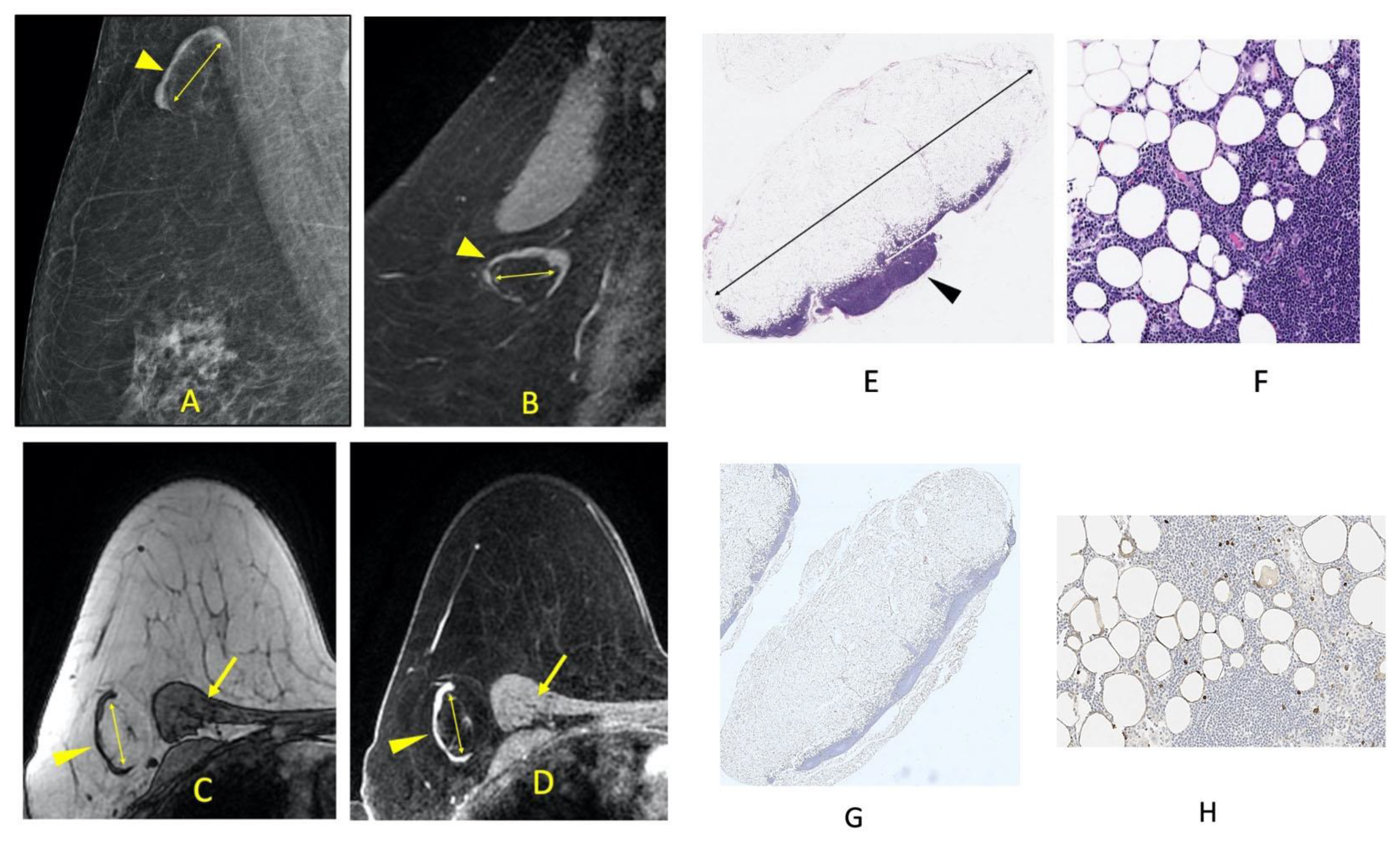
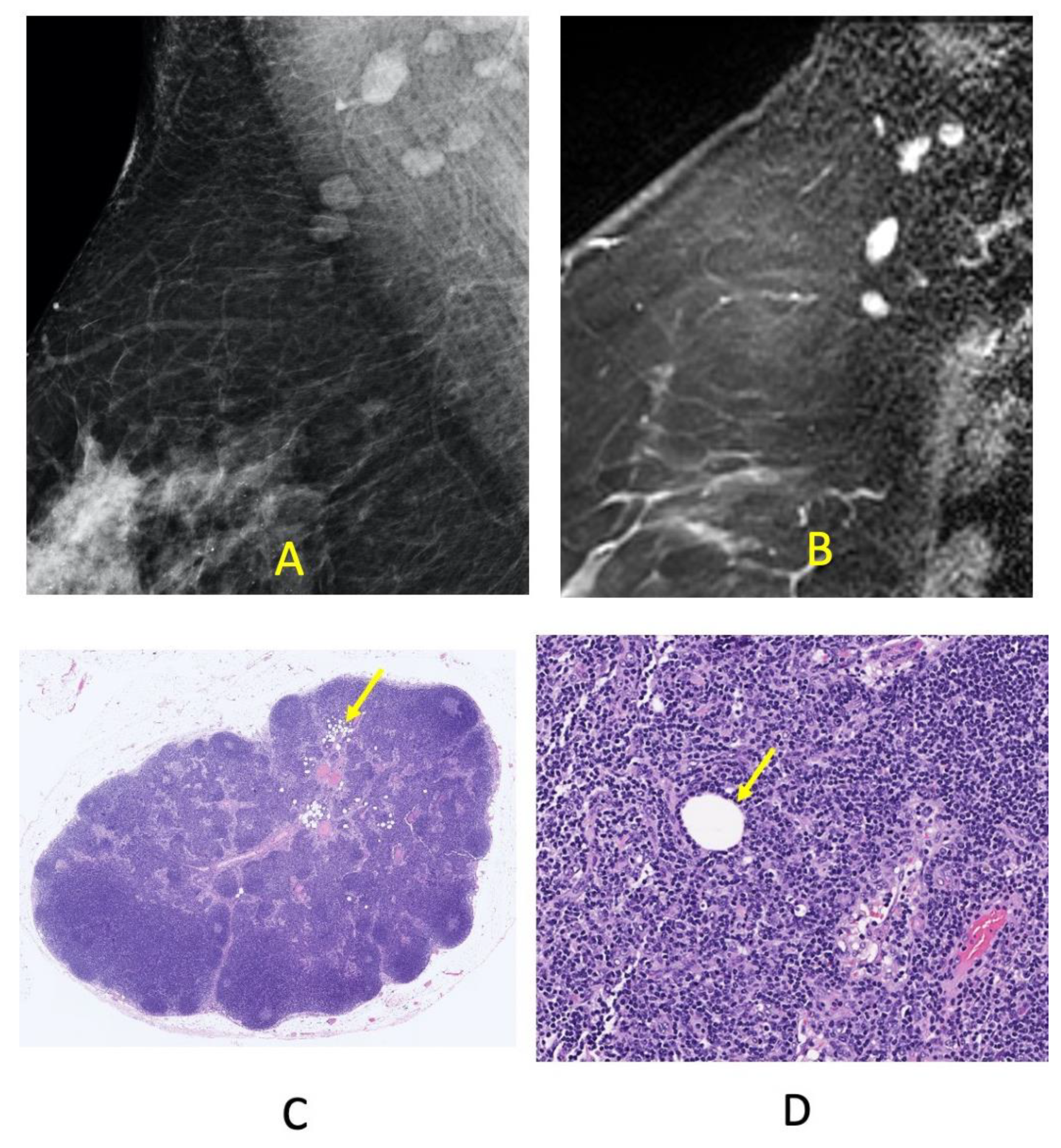
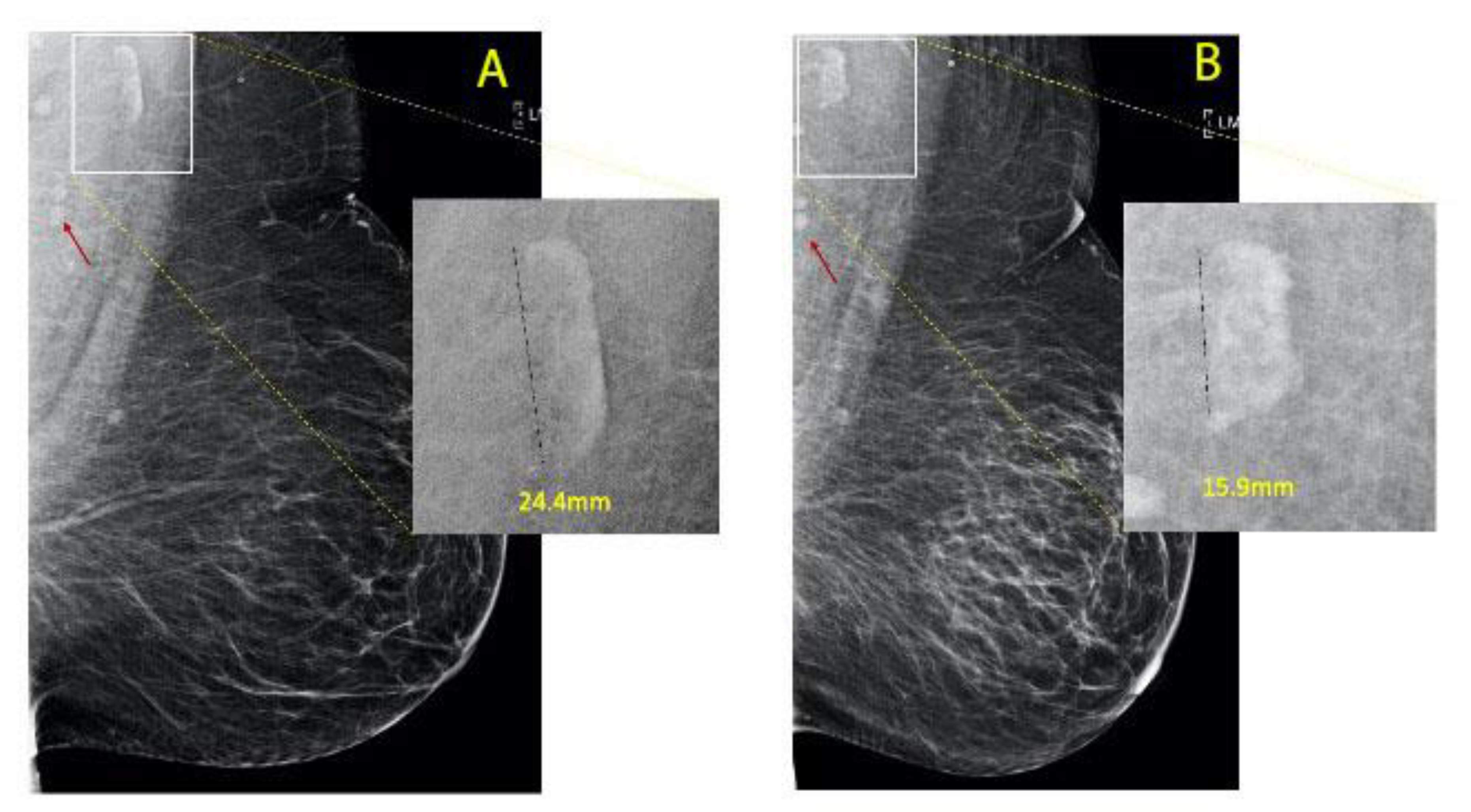
| Groups | |||
|---|---|---|---|
| No Change in FIN Size | Change in FIN Size | p-Value | |
| n | 39 | 45 | |
| Age in years (mean (SD)) | 56.26 (7.50) | 58.40 (7.10) | 0.183 |
| Pre-bypass weight (mean (SD)) | 117.0 (21.1) | 122.6 (24.0) | 0.264 |
| Post-bypass weight (mean (SD)) | 91.3 (16.9) | 89.5 (17.2) | 0.647 |
| Post-bypass change in weight (mean (SD)) | 25.71 (14.28) | 33.02 (16.94) | 0.037 |
| Percentage of patients with T2DM (%) | 28 (71.8) | 33 (78.6) | 0.654 |
| Class of Antidiabetic Drug (%) | 0.333 | ||
| None | 22 (56.4) | 26 (59.1) | |
| Biguanide | 8 (20.5) | 3 (6.8) | |
| Biguanide plus other | 4 (10.3) | 9 (20.5) | |
| Insulin | 4 (10.3) | 4 (9.1) | |
| Sulfonylurea | 1 (2.6) | 2 (4.5) | |
| Number of patients with dyslipidemia (%) | 31 (83.8) | 30 (76.9) | 0.644 |
| Class of Antilipid Drug (N (%)) | 0.979 | ||
| None | 22 (56.4) | 24 (54.5) | |
| Statin | 16 (41.0) | 19 (43.2) | |
| Statin, Fibric Acid | 1 (2.6) | 1 (2.3) | |
| Number of patients with hypertension (%) | 29 (74.4) | 30 (69.8) | 0.829 |
| Pre-bypass FIN size (mean (SD)) | 20.43 (6.17) | 26.74 (8.81) | <0.001 |
| Post-bypass FIN size (mean (SD)) | 18.65 (6.45) | 20.37 (7.06) | 0.252 |
| Type of surgery (%) | 1 | ||
| Roux-en-y | 21 (53.8) | 24 (54.5) | |
| Sleeve gastrectomy | 18 (46.2) | 15 (38.5) | |
| Time to post-bypass mammogram (recorded in months; Mean (SD)) | 22.77 (16.58) | 21.79 (13.52) | 0.77 |
| Metabolic Disease | Mean Pre-Surgical Lymph Node Size in Patients without Cardiometabolic Disease | Mean Pre-Surgical LN Size in Patients with Cardiometabolic Disease | p-Value |
|---|---|---|---|
| Hypertension | 21.6 | 24.7 | 0.14 |
| Diabetes | 21.4 | 24.6 | 0.14 |
| Dyslipidemia | 22.5 | 24.1 | 0.5 |
| Effect Size * | Standard Error | p-Value | |
|---|---|---|---|
| Resolution of hypertension | |||
| Age | −0.07 | 0.05 | 0.18 |
| Change in weight | 0.04 | 0.03 | 0.14 |
| Change in LN size | 1.3 * | 0.67 | 0.65 |
| Surgery type | 0.36 | 0.70 | 0.15 |
| Resolution of DM | |||
| Age | −0.044 | 0.05 | 0.41 |
| Change in weight | 0.05 | 0.04 | 0.52 |
| Change in LN size | 1.28 | 0.71 | 0.73 |
| Surgery type | 1.1 | 0.75 | 0.86 |
| Resolution of dyslipidemia | |||
| Age | −0.016 | 0.04 | 0.69 |
| Change in weight | 0.023 | 0.023 | 0.323 |
| Change in LN size | 5.5 * | 0.63 | 0.006 |
| Surgery type | 1.25 | 0.65 | 0.73 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dwan, D.; Ramin, S.K.; Chen, Y.; Muller, K.E.; diFlorio-Alexander, R.M. Decrease in the Size of Fat-Enlarged Axillary Lymph Nodes and Serum Lipids after Bariatric Surgery. Cells 2022, 11, 482. https://doi.org/10.3390/cells11030482
Dwan D, Ramin SK, Chen Y, Muller KE, diFlorio-Alexander RM. Decrease in the Size of Fat-Enlarged Axillary Lymph Nodes and Serum Lipids after Bariatric Surgery. Cells. 2022; 11(3):482. https://doi.org/10.3390/cells11030482
Chicago/Turabian StyleDwan, Dennis, Seth K. Ramin, Youdinghuan Chen, Kristen E. Muller, and Roberta M. diFlorio-Alexander. 2022. "Decrease in the Size of Fat-Enlarged Axillary Lymph Nodes and Serum Lipids after Bariatric Surgery" Cells 11, no. 3: 482. https://doi.org/10.3390/cells11030482
APA StyleDwan, D., Ramin, S. K., Chen, Y., Muller, K. E., & diFlorio-Alexander, R. M. (2022). Decrease in the Size of Fat-Enlarged Axillary Lymph Nodes and Serum Lipids after Bariatric Surgery. Cells, 11(3), 482. https://doi.org/10.3390/cells11030482






