Roles of Gonadotropin Receptors in Sexual Development of Medaka
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals
2.3. TALEN Preparation
2.4. Microinjection
2.5. Genotyping
2.6. Fertility Assessment
2.7. Determination of Gonadosomatic Index (GSI)
2.8. Measurement of Steroids
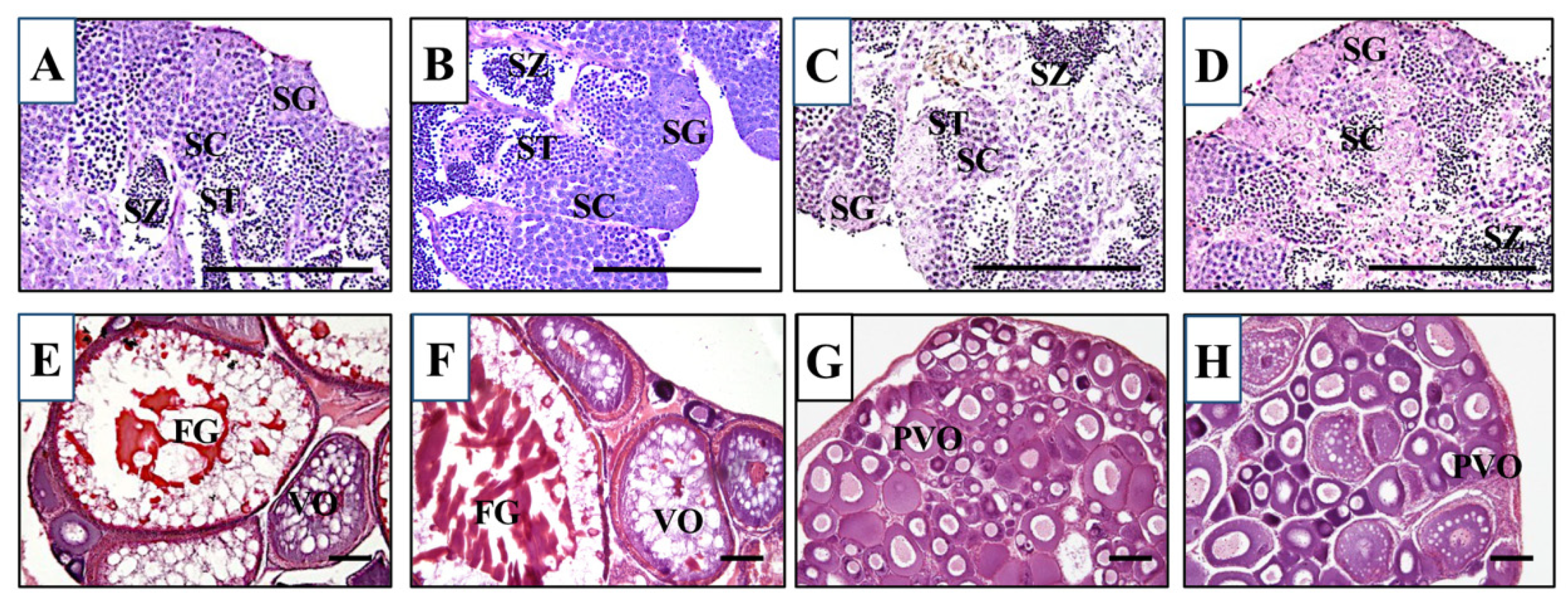
2.9. Histological Analysis of Gonads
2.10. Statistics
3. Results
3.1. Establishment of lhr-KO Lines
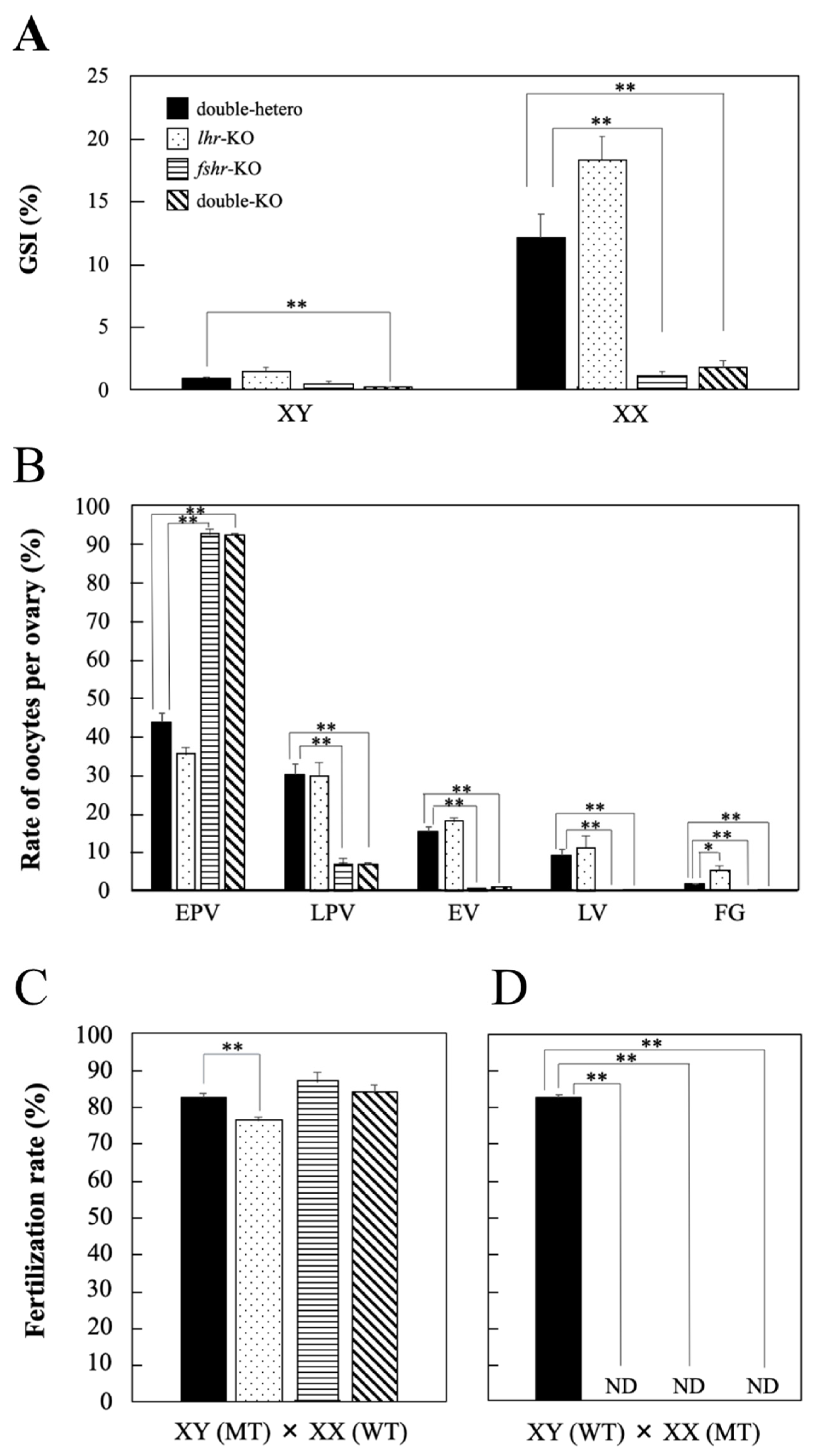
3.2. Phenotypic Analyses of lhr-KO, Double-Hetero, fshr-KO, and Double-KO Medaka
3.3. Gonad Development and Fertility in KO Medaka
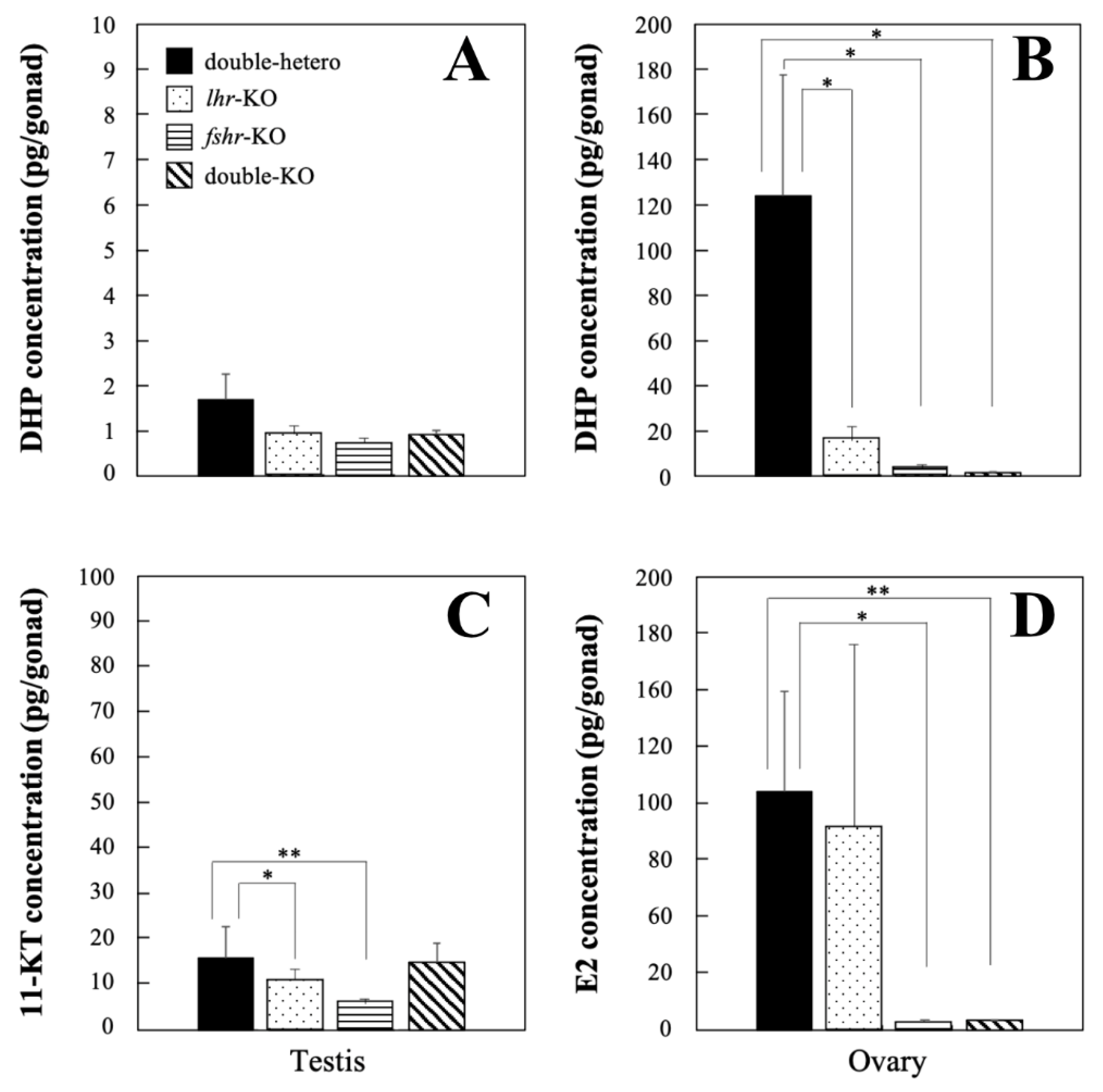
3.4. Steroid Hormone Levels in KO Medaka
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Conn, P.P.M.; Crowley, W.F. Gonadotropin-releasing hormone and its analogs. Annu. Rev. Med. 1994, 45, 391–405. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, U.B.; Conn, P.M.; Chin, W.W. Studies of gonadotropin-releasing hormone (GnRH) action using GnRH receptor-expressing pituitary cell lines. Endocr. Rev. 1997, 18, 46–70. [Google Scholar] [CrossRef] [PubMed]
- Pierce, J.G.; Parsons, T.F. Glycoprotein hormones: Structure and function. Ann. Rev. Biochem. 1981, 50, 465–495. [Google Scholar] [CrossRef] [PubMed]
- Ryan, R.J.; Charlesworth, M.C.; McCormick, D.J.; Milius, R.P.; Keutmann, H.T. The glycoprotein hormones: Recent studies of structure-function relationships. FASEB J. 1988, 2, 2661–2669. [Google Scholar] [CrossRef]
- Ulloa-Aguirre, A.; Midgley, A.R.; Beitins, I.Z.; Padmanabhan, V. Follicle-stimulating isohormones: Characterization and physiological relevance. Endocr. Rev. 1995, 16, 765–787. [Google Scholar] [CrossRef]
- Sprengel, R.; Braun, T.; Nikolics, K.; Segaloff, D.L.; Seeburg, P.H. The testicular receptor for follicle stimulating formone: Structure and functional expression of cloned cDNA. Mol. Endocrinol. 1990, 4, 525–530. [Google Scholar] [CrossRef] [Green Version]
- Kishi, H.; Kitahara, Y.; Imai, F.; Nakao, K.; Suwa, H. Expression of the gonadotropin receptors during follicular development. Reprod. Med. Biol. 2018, 17, 11–19. [Google Scholar] [CrossRef]
- Dierich, A.; Sairam, M.R.; Monaco, L.; Fimia, G.M.; Gansmuller, A.; LeMeur, M.; Sassone-Corsi, P. Impairing follicle-stimulating hormone (FSH) signaling in vivo: Targeted disruption of the FSH receptor leads to aberrant gametogenesis and hormonal imbalance. Proc. Natl. Acad. Sci. USA 1998, 95, 13612–13617. [Google Scholar] [CrossRef] [Green Version]
- Kempisty, B.; Ziółkowska, A.; Ciesiolka, S.; Piotrowska, H.; Antosik, P.; Bukowska, D.; Nowicki, M.; Brüssow, K.P.; Zabel, M. Association between the expression of LHR, FSHR and CYP19 genes, cellular distribution of encoded proteins and proliferation of porcine granulosa cells in real-time. J. Boil. Regul. Homeost. Agents 2014, 28, 419–431. [Google Scholar]
- Danilovich, N.; Babu, P.S.; Xing, W.; Gerdes, M.; Krishnamurthy, H.; Sairam, M.R. Estrogen Deficiency, Obesity, and Skeletal Abnormalities in Follicle-Stimulating Hormone Receptor Knockout (FORKO) Female Mice. Endocrinology 2000, 141, 4295–4308. [Google Scholar] [CrossRef]
- Ohta, T.; Miyake, H.; Miura, C.; Kamei, H.; Aida, K.; Miura, T. Follicle-stimulating hormone induces spermatogenesis mediated by androgen production in Japanese eel, Anguilla japonica. Biol. Reprod. 2007, 77, 970–977. [Google Scholar] [CrossRef] [Green Version]
- Andersson, E.; Nijenhuis, W.; Male, R.; Swanson, P.; Bogerd, J.; Taranger, G.L.; Schulz, R.W. Pharmacological characterization, localization and quantification of expression of gonadotropin receptors in Atlantic salmon (Salmo salar L.) ovaries. Gen. Comp. Endocrinol. 2009, 163, 329–339. [Google Scholar] [CrossRef] [PubMed]
- García-López, A.; Bogerd, J.; Granneman, J.C.M.; Van Dijk, W.; Trant, J.M.; Taranger, G.L.; Schulz, R.W. Leydig cells express follicle-stimulating hormone receptors in African catfish. Endocrinology 2009, 150, 357–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-López, A.; de Jonge, H.; Nóbrega, R.H.; de Waal, P.P.; van Dijk, W.; Hemrika, W.; Taranger, G.L.; Bogerd, J.; Schulz, R.W. Studies in zebrafish reveal unusual cellular expression patterns of gonadotropin receptor messenger ribonucleic acids in the testis and unexpected functional differentiation of the gonadotropins. Endocrinology 2010, 151, 2349–2360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murozumi, N.; Nakashima, R.; Hirai, T.; Kamei, Y.; Ishikawa-Fujiwara, T.; Todo, T.; Kitano, T. Loss of follicle-stimulating hormone receptor function causes masculinization and suppression of ovarian development in genetically female medaka. Endocrinology 2014, 155, 3136–3145. [Google Scholar] [CrossRef] [Green Version]
- Zheng, M.; Shi, H.; Segaloff, D.L.; Van Voorhis, B.J. Expression and localization of luteinizing hormone receptor in the female mouse reproductive tract. Biol. Reprod. 2001, 64, 179–187. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.-J.; Ma, X.; Wang, C.Q.; Ge, Y.-F.; Lian, Q.-Q.; Hardy, D.O.; Zhang, Y.-F.; Dong, Q.; Xu, Y.-F.; Ge, R.-S. Effects of luteinizing hormone and androgen on the development of rat progenitor Leydig cells in vitro and in vivo. Asian J. Androl. 2013, 15, 685–691. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.-P.; Poutanen, M.; Wilbertz, J.; Huhtaniemi, I. Normal prenatal but arrested postnatal sexual development of luteinizing hormone receptor knockout (LuRKO) mice. Mol. Endocrinol. 2001, 15, 172–183. [Google Scholar] [CrossRef]
- Lei, Z.M.; Mishra, S.; Zou, W.; Xu, B.; Foltz, M.; Li, X.; Rao, C.V. Targeted disruption of luteinizing hormone/human chorionic gonadotropin receptor gene. Mol. Endocrinol. 2001, 15, 184–200. [Google Scholar] [CrossRef]
- Ascoli, M.; Fanelli, F.; Segaloff, D.L. The lutropin/choriogonadotropin receptor, a 2002 perspective. Endocr. Rev. 2002, 23, 141–174. [Google Scholar] [CrossRef]
- Miwa, S.; Yan, L.; Swanson, P. Localization of two gonadotropin receptors in the salmon gonad by in vitro ligand autoradiography. Biol. Reprod. 1994, 50, 629–642. [Google Scholar] [CrossRef] [PubMed]
- Oba, Y.; Hirai, T.; Yoshiura, Y.; Kobayashi, T.; Nagahama, Y. Fish gonadotropin and thyrotropin receptors: The evolution of glycoprotein hormone receptors in vertebrates. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2001, 129, 441–448. [Google Scholar] [CrossRef]
- Ogiwara, K.; Fujimori, C.; Rajapakse, S.; Takahashi, T. Characterization of luteinizing hormone and luteinizing hormone receptor and their indispensable role in the ovulatory process of the medaka. PLoS ONE 2013, 8, e54482. [Google Scholar] [CrossRef] [PubMed]
- Chu, L.; Li, J.; Liu, Y.; Hu, W.; Cheng, C.H.K. Targeted gene disruption in zebrafish reveals noncanonical functions of LH signaling in reproduction. Mol. Endocrinol. 2014, 28, 1785–1795. [Google Scholar] [CrossRef] [Green Version]
- So, W.-K.; Kwok, H.-F.; Ge, W. Zebrafish gonadotropins and their receptors: II. Cloning and characterization of zebrafish follicle-stimulating hormone and luteinizing hormone subunits—their spatial-temporal expression patterns and receptor specificity. Biol. Reprod. 2005, 72, 1382–1396. [Google Scholar] [CrossRef] [Green Version]
- Ishikawa, Y. Medakafish as a model system for vertebrate developmental genetics. Bioessays 2000, 22, 487–495. [Google Scholar] [CrossRef]
- Furukawa, F.; Hamasaki, S.; Hara, S.; Uchimura, T.; Shiraishi, E.; Osafune, N.; Takagi, H.; Yazawa, T.; Kamei, Y.; Kitano, T. Heat shock factor 1 protects germ cell proliferation during early ovarian differentiation in medaka. Sci. Rep. 2019, 9, 6927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sawamura, R.; Osafune, N.; Murakami, T.; Furukawa, F.; Kitano, T. Generation of biallelic F0 mutants in medaka using the CRISPR/Cas9 system. Genes Cells 2017, 22, 756–763. [Google Scholar] [CrossRef] [Green Version]
- Matsuda, M.; Nagahama, Y.; Shinomiya, A.; Sato, T.; Matsuda, C.; Kobayashi, T.; Morrey, C.E.; Shibata, N.; Asakawa, S.; Shimizu, N.; et al. DMY is a Y-specific DM-domain gene required for male development in the medaka fish. Nature 2002, 417, 559–563. [Google Scholar] [CrossRef]
- Nanda, I.; Kondo, M.; Hornung, U.; Asakawa, S.; Winkler, C.; Shimizu, A.; Shan, Z.; Haaf, T.; Shimizu, N.; Shima, A.; et al. A duplicated copy of DMRT1 in the sex-determining region of the Y chromosome of the medaka, Oryzias latipes. Proc. Natl. Acad. Sci. USA 2002, 99, 11778–11783. [Google Scholar] [CrossRef] [Green Version]
- Matsuda, M.; Shinomiya, A.; Kinoshita, M.; Suzuki, A.; Kobayashi, T.; Paul-Prasanth, B.; Lau, E.-L.; Hamaguchi, S.; Sakaizumi, M.; Nagahama, Y. DMY gene induces male development in genetically female (XX) medaka fish. Proc. Natl. Acad. Sci. USA 2007, 104, 3865–3870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cermak, T.; Doyle, E.L.; Christian, M.; Wang, L.; Zhang, Y.; Schmidt, C.; Baller, J.A.; Somia, N.V.; Bogdanove, A.J.; Voytas, D.F. Efficient design and assembly of custom TALEN and other TAL effector-based constructs for DNA targeting. Nucleic Acids Res. 2011, 39, e82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayashi, Y.; Kobira, H.; Yamaguchi, T.; Shiraishi, E.; Yazawa, T.; Hirai, T.; Kamei, Y.; Kitano, T. High temperature causes masculinization of genetically female medaka by elevation of cortisol. Mol. Reprod. Dev. 2010, 77, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Kitano, T.; Takamune, K.; Kobayashi, T.; Nagahama, Y.; Abe, S.-I. Suppression of P450 aromatase gene expression in sex-reversed males produced by rearing genetically female larvae at a high water temperature during a period of sex differentiation in the Japanese flounder (Paralichthys olivaceus). J. Mol. Endocrinol. 1999, 23, 167–176. [Google Scholar] [CrossRef]
- Yamada, H.; Satoh, R.-I.; Ogoh, M.; Takaji, K.; Fujimoto, Y.; Hakuba, T.; Chiba, H.; Kambegawa, A.; Iwata, M. Circadian changes in serum concentrations of steroids in Japanese char Salvelinus leucomaenis at the stage of final maturation. Zool. Sci. 2002, 19, 891–898. [Google Scholar] [CrossRef]
- Iwamatsu, T. Stages of normal development in the medaka Oryzias latipes. Mech. Dev. 2004, 121, 605–618. [Google Scholar] [CrossRef]
- Kawai, T.; Yoshimura, A.; Oka, Y. Neurones in the preoptic area of the male goldfish are activated by a sex pheromone 17α,20β-dihydroxy-4-pregnen-3-one. J. Neuroendocrinol. 2015, 27, 123–130. [Google Scholar] [CrossRef]
- Nagahama, Y.; Yamashita, M. Regulation of oocyte maturation in fish. Dev. Growth Differ. 2008, 50, S195–S219. [Google Scholar] [CrossRef]
- Li, J.; Chu, L.; Sun, X.; Liu, Y.; Cheng, C.H. IGFs mediate the action of LH on oocyte maturation in zebrafish. Mol. Endocrinol. 2015, 29, 373–383. [Google Scholar] [CrossRef] [Green Version]
- Silva, J.R.V.; Figueiredo, J.R.; van den Hurk, R. Involvement of growth hormone (GH) and insulin-like growth factor (IGF) system in ovarian folliculogenesis. Theriogenology 2009, 71, 1193–1208. [Google Scholar] [CrossRef]
- Zhang, Z.; Lau, S.-W.; Zhang, L.; Ge, W. Disruption of zebrafish follicle-stimulating hormone receptor (fshr) but not luteinizing hormone receptor (lhcgr) gene by TALEN leads to failed follicle activation in females followed by sexual reversal to males. Endocrinology 2015, 156, 3747–3762. [Google Scholar] [CrossRef] [PubMed]
- Hara, A.; Hiramatsu, N.; Fujita, T. Vitellogenesis and choriogenesis in fishes. Fish. Sci. 2016, 82, 187–202. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Zhu, B.; Ge, W. Genetic analysis of zebrafish gonadotropin (FSH and LH) functions by TALEN-mediated gene disruption. Mol. Endocrinol. 2015, 29, 76–98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levavi-Sivan, B.; Bogerd, J.; Mañanós, E.; Gómez, A.; Lareyre, J. Perspectives on fish gonadotropins and their receptors. Gen. Comp. Endocrinol. 2010, 165, 412–437. [Google Scholar] [CrossRef] [PubMed]
- Aizen, J.; Kowalsman, N.; Kobayashi, M.; Hollander, L.; Sohn, Y.C.; Yoshizaki, G.; Niv, M.Y.; Levavi-Sivan, B. Experimental and computational study of inter- and intra- species specificity of gonadotropins for various gonadotropin receptors. Mol. Cell. Endocrinol. 2012, 364, 89–100. [Google Scholar] [CrossRef]
- Schulz, R.W.; de França, L.R.; Lareyre, J.J.; Le Gac, F.; Chiarini-Garcia, H.; Nobrega, R.H.; Miura, T. Spermatogenesis in fish. Gen. Comp. Endocrinol. 2010, 165, 390–411. [Google Scholar] [CrossRef]
- Planas, J. Maturation-associated changes in the response of the salmon testis to the steroidogenic actions of gonadotropins (GTH I and GTH II) in vitro. Biol. Reprod. 1995, 52, 697–704. [Google Scholar] [CrossRef] [Green Version]
- Kamei, H.; Kaneko, T.; Aida, K. In vivo gonadotropic effects of recombinant Japanese eel follicle-stimulating hormone. Aquaculture 2006, 261, 771–775. [Google Scholar] [CrossRef]
- Kazeto, Y.; Kohara, M.; Miura, T.; Miura, C.; Yamaguchi, S.; Trant, J.M.; Adachi, S.; Yamauchi, K. Japanese eel follicle-stimulating hormone (Fsh) and luteinizing hormone (Lh): Production of biologically active recombinant Fsh and Lh by Drosophila S2 cells and their differential actions on the reproductive biology. Biol. Reprod. 2008, 79, 938–9466. [Google Scholar] [CrossRef] [Green Version]
- Burow, S.; Mizrahi, N.; Maugars, G.; von Krogh, K.; Nourizadeh-Lillabadi, R.; Hollander-Cohen, L.; Shpilman, M.; Atre, I.; Weltzien, F.-A.; Levavi-Sivan, B. Characterization of gonadotropin receptors Fshr and Lhr in Japanese medaka, Oryzias latipes. Gen. Comp. Endocrinol. 2020, 285, 113276. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kitano, T.; Takenaka, T.; Takagi, H.; Yoshiura, Y.; Kazeto, Y.; Hirai, T.; Mukai, K.; Nozu, R. Roles of Gonadotropin Receptors in Sexual Development of Medaka. Cells 2022, 11, 387. https://doi.org/10.3390/cells11030387
Kitano T, Takenaka T, Takagi H, Yoshiura Y, Kazeto Y, Hirai T, Mukai K, Nozu R. Roles of Gonadotropin Receptors in Sexual Development of Medaka. Cells. 2022; 11(3):387. https://doi.org/10.3390/cells11030387
Chicago/Turabian StyleKitano, Takeshi, Tomoaki Takenaka, Hisanori Takagi, Yasutoshi Yoshiura, Yukinori Kazeto, Toshiaki Hirai, Koki Mukai, and Ryo Nozu. 2022. "Roles of Gonadotropin Receptors in Sexual Development of Medaka" Cells 11, no. 3: 387. https://doi.org/10.3390/cells11030387
APA StyleKitano, T., Takenaka, T., Takagi, H., Yoshiura, Y., Kazeto, Y., Hirai, T., Mukai, K., & Nozu, R. (2022). Roles of Gonadotropin Receptors in Sexual Development of Medaka. Cells, 11(3), 387. https://doi.org/10.3390/cells11030387






