Six-Minute Walk Distance Is a Useful Outcome Measure to Detect Motor Decline in Treated Late-Onset Pompe Disease Patients
Abstract
:1. Introduction
2. Patients and Methods
2.1. Patients and Controls
2.2. Muscle Strength Assessment Using Biodex® Dynamometer
2.3. Additional Muscle Strength and Motor Function Assessments
2.4. Pulmonary Function Tests
2.5. Statistical Analysis
3. Results
3.1. Demographics and Clinical Characteristics of LOPD Patients
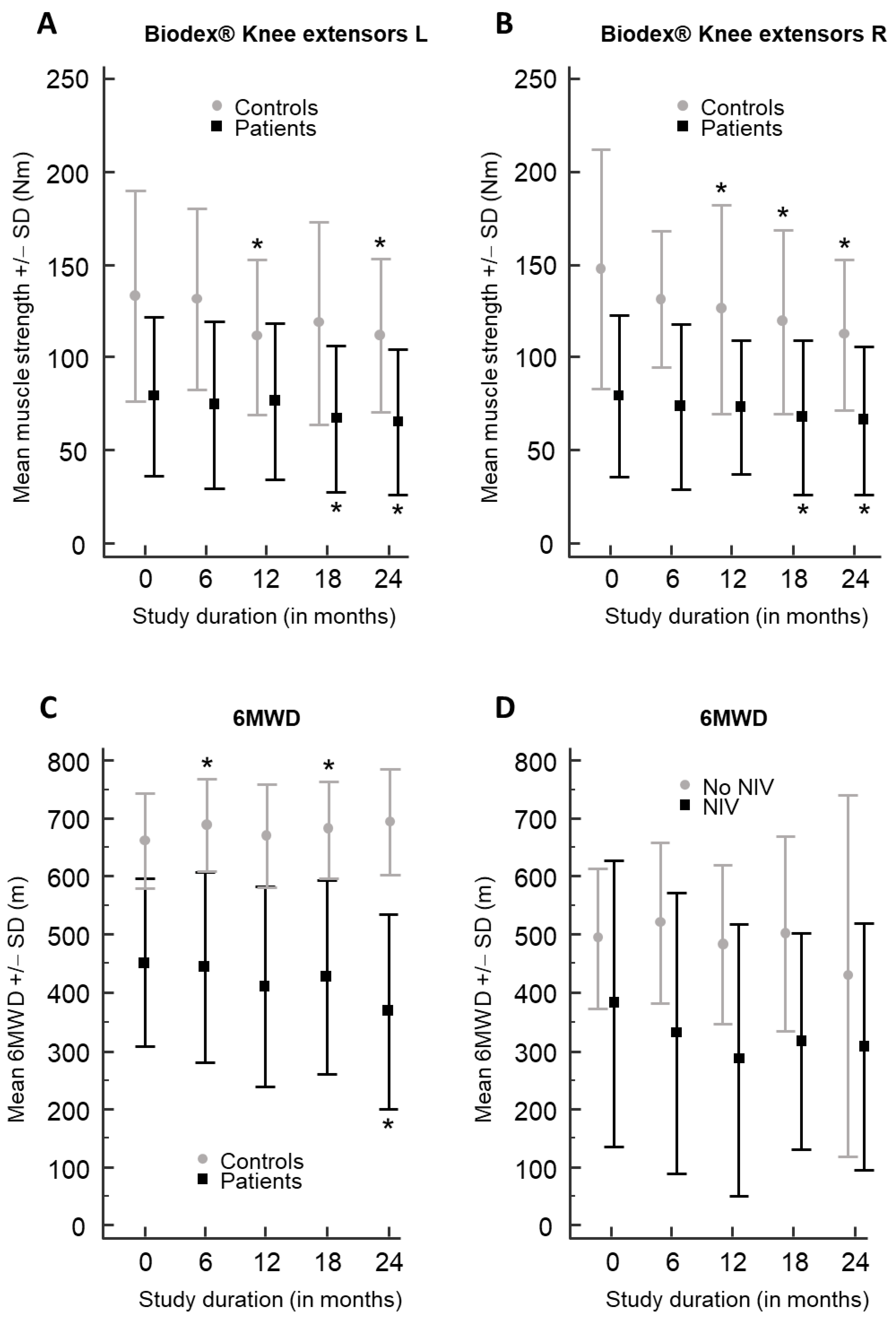
3.2. Results of Biodex® Dynamometer and Other Outcome Measures in LOPD Patients
3.3. Results of Biodex® Dynamometer and Other Outcome Measures in Controls
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schoser, B. Pompe disease: What are we missing? Ann. Transl. Med. 2019, 7, 1–7. [Google Scholar] [CrossRef]
- Toscano, A.; Rodolico, C.; Musumeci, O. Multisystem late onset Pompe disease (LOPD): An update on clinical aspects. Ann. Transl. Med. 2019, 7, 284. [Google Scholar] [CrossRef] [PubMed]
- Hahn, A.; Schänzer, A. Long-term outcome and unmet needs in infantile-onset Pompe disease. Ann. Transl. Med. 2019, 7, 1–10. [Google Scholar] [CrossRef]
- Van der Ploeg, A.T.; Kruijshaar, M.E.; Toscano, A.; Laforêt, P.; Angelini, C.; Lachmann, R.H.; Pascual, S.I.; Roberts, M.; Rösler, K.; Stulnig, T.; et al. European consensus for starting and stopping enzyme replacement therapy in adult patients with Pompe disease: A 10-year experience. Eur. J. Neurol. 2017, 24, 768.e31. [Google Scholar] [CrossRef] [PubMed]
- Angelini, C.; Semplicini, C.; Tonin, P.; Filosto, M.; Pegoraro, E.; Sorarù, G.; Fanin, M. Progress in Enzyme Replacement Therapy in Glycogen Storage Disease Type II. Ther. Adv. Neurol. Disord. 2009, 2, 143–153. [Google Scholar] [CrossRef] [Green Version]
- Merk, T.; Wibmer, T.; Schumann, C.; Krüger, S. Glycogen storage disease type II (Pompe disease)—influence of enzyme replacement therapy in adults. Eur. J. Neurol. 2009, 16, 274–277. [Google Scholar] [CrossRef]
- Bembi, B.; Pisa, F.E.; Confalonieri, M.; Ciana, G.; Fiumara, A.; Parini, R.; Rigoldi, M.; Moglia, A.; Costa, A.; Carlucci, A.; et al. Long-term observational, non-randomized study of enzyme replacement therapy in late-onset glycogenosis type II. J. Inherit. Metab. Dis. 2010, 33, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Ravaglia, S.; Pichiecchio, A.; Ponzio, M.; Danesino, C.; Saeidi Garaghani, K.; Poloni, G.U.; Toscano, A.; Moglia, A.; Carlucci, A.; Bini, P.; et al. Changes in skeletal muscle qualities during enzyme replacement therapy in late-onset type II glycogenosis: Temporal and spatial pattern of mass vs. strength response. J. Inherit. Metab. Dis. 2010, 33, 737–745. [Google Scholar] [CrossRef] [PubMed]
- Strothotte, S.; Strigl-Pill, N.; Grunert, B.; Kornblum, C.; Eger, K.; Wessig, C.; Deschauer, M.; Breunig, F.; Glocker, F.X.; Vielhaber, S.; et al. Enzyme replacement therapy with alglucosidase alfa in 44 patients with late-onset glycogen storage disease type 2: 12-month results of an observational clinical trial. J. Neurol. 2010, 257, 91–97. [Google Scholar] [CrossRef]
- Van Capelle, C.I.; van der Beek, N.A.; Hagemans, M.L.; Arts, W.F.; Hop, W.C.; Lee, P.; Jaeken, J.; Frohn-Mulder, I.M.; Merkus, P.J.; Corzo, D.; et al. Effect of enzyme therapy in juvenile patients with Pompe disease: A three-year open-label study. Neuromuscul. Disord. 2010, 20, 775–782. [Google Scholar] [CrossRef]
- Van der Ploeg, A.T.; Clemens, P.R.; Corzo, D.; Escolar, D.M.; Florence, J.; Groeneveld, G.J.; Herson, S.; Kishnani, P.S.; Laforet, P.; Lake, S.L.; et al. A randomized study of alglucosidase alfa in late-onset Pompe’s disease. N. Engl. J. Med. 2010, 362, 1396–1406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Angelini, C.; Semplicini, C.; Ravaglia, S.; Bembi, B.; Servidei, S.; Pegoraro, E.; Moggio, M.; Filosto, M.; Sette, E.; Crescimanno, G.; et al. Observational clinical study in juvenile-adult glycogenosis type 2 patients undergoing enzyme replacement therapy for up to 4 years. J. Neurol. 2012, 259, 952–958. [Google Scholar] [CrossRef] [PubMed]
- Ishigaki, K.; Murakami, T.; Nakanishi, T.; Oda, E.; Sato, T.; Osawa, M. Close monitoring of initial enzyme replacement therapy in a patient with childhood-onset Pompe disease. Brain Dev. 2012, 34, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Ravaglia, S.; De Filippi, P.; Pichiecchio, A.; Ponzio, M.; Saeidi Garaghani, K.; Poloni, G.U.; Bini, P.; Danesino, C. Can genes influencing muscle function affect the therapeutic response to enzyme replacement therapy (ERT) in late-onset type II glycogenosis? Mol. Genet. Metab. 2012, 107, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Regnery, C.; Kornblum, C.; Hanisch, F.; Vielhaber, S.; Strigl-Pill, N.; Grunert, B.; Müller-Felber, W.; Glocker, F.X.; Spranger, M.; Deschauer, M.; et al. 36 months observational clinical study of 38 adult Pompe disease patients under alglucosidase alfa enzyme replacement therapy. J. Inherit. Metab. Dis. 2012, 35, 837–845. [Google Scholar] [CrossRef] [PubMed]
- Van der Ploeg, A.T.; Barohn, R.; Carlson, L.; Charrow, J.; Clemens, P.R.; Hopkin, R.J.; Kishnani, P.S.; Laforêt, P.; Morgan, C.; Nations, S.; et al. Open-label extension study following the Late-Onset Treatment Study (LOTS) of alglucosidase alfa. Mol. Genet. Metab. 2012, 107, 456–461. [Google Scholar] [CrossRef]
- Anderson, L.J.; Henley, W.; Wyatt, K.M.; Nikolaou, V.; Waldek, S.; Hughes, D.A.; Lachmann, R.H.; Logan, S. Effectiveness of enzyme replacement therapy in adults with late-onset Pompe disease: Results from the NCS-LSD cohort study. J. Inherit. Metab. Dis. 2014, 37, 945–952. [Google Scholar] [CrossRef] [PubMed]
- Andreassen, C.S.; Schlütter, J.M.; Vissing, J.; Andersen, H. Effect of enzyme replacement therapy on isokinetic strength for all major muscle groups in four patients with Pompe disease—A long-term follow-up. Mol. Genet. Metab. 2014, 112, 40–43. [Google Scholar] [CrossRef] [PubMed]
- Montagnese, F.; Barca, E.; Musumeci, O.; Mondello, S.; Migliorato, A.; Ciranni, A.; Rodolico, C.; De Filippi, P.; Danesino, C.; Toscano, A. Clinical and molecular aspects of 30 patients with late-onset Pompe disease (LOPD): Unusual features and response to treatment. J. Neurol. 2015, 262, 968–978. [Google Scholar] [CrossRef]
- Van der Ploeg, A.; Carlier, P.G.; Carlier, R.Y.; Kissel, J.T.; Schoser, B.; Wenninger, S.; Pestronk, A.; Barohn, R.J.; Dimachkie, M.M.; Goker-Alpan, O.; et al. Prospective exploratory muscle biopsy, imaging, and functional assessment in patients with late-onset Pompe disease treated with alglucosidase alfa: The EMBASSY Study. Mol. Genet. Metab. 2016, 119, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Kuperus, E.; Kruijshaar, M.E.; Wens, S.C.A.; de Vries, J.M.; Favejee, M.M.; van der Meijden, J.C.; Rizopoulos, D.; Brusse, E.; van Doorn, P.A.; van der Ploeg, A.T.; et al. Long-term benefit of enzyme replacement therapy in Pompe disease: A 5-year prospective study. Neurology 2017, 89, 2365–2373. [Google Scholar] [CrossRef] [PubMed]
- Witkowski, G.; Konopko, M.; Rola, R.; Ługowska, A.; Ryglewicz, D.; Sienkiewicz-Jarosz, H. Enzymatic replacement therapy in patients with late-onset Pompe disease—6-Year follow up. Neurol. Neurochir. Pol. 2018, 52, 465–469. [Google Scholar] [CrossRef]
- Semplicini, C.; De Antonio, M.; Taouagh, N.; Béhin, A.; Bouhour, F.; Echaniz-Laguna, A.; Magot, A.; Nadaj-Pakleza, A.; Orlikowski, D.; Sacconi, S.; et al. Long-term benefit of enzyme replacement therapy with alglucosidase alfa in adults with Pompe disease: Prospective analysis from the French Pompe Registry. J. Inherit. Metab. Dis. 2020, 43, 1219–1231. [Google Scholar] [CrossRef]
- Vanherpe, P.; Fieuws, S.; D’Hondt, A.; Bleyenheuft, C.; Demaerel, P.; De Bleecker, J.; Van den Bergh, P.; Baets, J.; Remiche, G.; Verhoeven, K.; et al. Late-onset Pompe disease (LOPD) in Belgium: Clinical characteristics and outcome measures. Orphanet J. Rare Dis. 2020, 15, 83. [Google Scholar] [CrossRef]
- Buckon, C.; Sienko, S.; Bagley, A.; Sison-Williamson, M.; Fowler, E.; Staudt, L.; Heberer, K.; McDonald, C.M.; Sussman, M. Can Quantitative Muscle Strength and Functional Motor Ability Differentiate the Influence of Age and Corticosteroids in Ambulatory Boys with Duchenne Muscular Dystrophy? PLoS Curr. 2016, 8. [Google Scholar] [CrossRef]
- Plewa, J.; Surampalli, A.; Wencel, M.; Milad, M.; Donkervoort, S.; Caiozzo, V.J.; Goyal, N.; Mozaffar, T.; Kimonis, V. A cross-sectional analysis of clinical evaluation in 35 individuals with mutations of the valosin-containing protein gene. Neuromuscul. Disord. 2018, 28, 778–786. [Google Scholar] [CrossRef]
- Schoonjans, F.; Zalata, A.; Depuydt, C.E.; Comhaire, F.H. MedCalc: A new computer program for medical statistics. Comput. Methods Programs Biomed. 1995, 48, 257–262. [Google Scholar] [CrossRef]
- ATS Committee on Proficiency Standards for Clinical Pulmonary Function Laboratories. ATS statement: Guidelines for the 6-min walk test. Am. J. Respir. Crit. Care Med. 2002, 166, 111–117. [Google Scholar] [CrossRef]
- Goemans, N.M.; Tulinius, M.; van den Akker, J.T.; Burm, B.E.; Ekhart, P.F.; Heuvelmans, N.; Holling, T.; Janson, A.A.; Platenburg, G.J.; Sipkens, J.A.; et al. Systemic administration of PRO051 in Duchenne’s muscular dystrophy. N. Engl. J. Med. 2011, 364, 1513–1522. [Google Scholar] [CrossRef] [PubMed]
- McDonald, C.M.; Campbell, C.; Torricelli, R.E.; Finkel, R.S.; Flanigan, K.M.; Goemans, N.; Heydemann, P.; Kaminska, A.; Kirschner, J.; Muntoni, F.; et al. Ataluren in patients with nonsense mutation Duchenne muscular dystrophy (ACT DMD): A multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2017, 390, 1489–1498. [Google Scholar] [CrossRef]
- De Wel, B.; Goosens, V.; Sobota, A.; Van Camp, E.; Geukens, E.; Van Kerschaver, G.; Jagut, M.; Claes, K.; Claeys, K.G. Nusinersen treatment significantly improves hand grip strength, hand motor function and MRC sum scores in adult patients with spinal muscular atrophy types 3 and 4. J. Neurol. 2021, 268, 923–935. [Google Scholar] [CrossRef] [PubMed]
- Wokke, J.H.; Escolar, D.M.; Pestronk, A.; Jaffe, K.M.; Carter, G.T.; van den Berg, L.H.; Florence, J.M.; Mayhew, J.; Skrinar, A.; Corzo, D.; et al. Clinical features of late-onset Pompe disease: A prospective cohort study. Muscle Nerve 2008, 38, 1236–1245. [Google Scholar] [CrossRef]
- Casanova, C.; Celli, B.R.; Barria, P.; Casas, A.; Cote, C.; de Torres, J.P.; Jardim, J.; Lopez, M.V.; Marin, J.M.; de Oca, M.; et al. Six Minute Walk Distance Project (ALAT). The 6-min walk distance in healthy subjects: Reference standards from seven countries. Eur. Respir. J. 2011, 37, 150–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harlaar, L.; Hogrel, J.Y.; Perniconi, B.; Kruijshaar, M.E.; Rizopoulos, D.; Taouagh, N.; Canal, A.; Brusse, E.; van Doorn, P.A.; van der Ploeg, A.T.; et al. Large variation in effects during 10 years of enzyme therapy in adults with Pompe disease. Neurology 2019, 93, e1756–e1767. [Google Scholar] [CrossRef] [Green Version]
- Bohannon, R.W.; Crouch, R. Minimal clinically important difference for change in 6-min walk test distance of adults with pathology: A systematic review. J. Eval. Clin. Pract. 2017, 23, 377–381. [Google Scholar] [CrossRef] [PubMed]
- Van der Beek, N.A.; Hagemans, M.L.; van der Ploeg, A.T.; van Doorn, P.A.; Merkies, I.S. The Rasch-built Pompe-specific activity (R-PAct) scale. Neuromuscul. Disord. 2013, 23, 256–264. [Google Scholar] [CrossRef] [Green Version]
- Yuan, M.; Andrinopoulou, E.R.; Kruijshaar, M.E.; Lika, A.; Harlaar, L.; van der Ploeg, A.T.; Rizopoulos, D.; van der Beek, N.A.M.E. Positive association between physical outcomes and patient-reported outcomes in late-onset Pompe disease: A cross sectional study. Orphanet J. Rare Dis. 2020, 15, 232. [Google Scholar] [CrossRef]
- Van der Beek, N.A.; de Vries, J.M.; Hagemans, M.L.; Hop, W.C.; Kroos, M.A.; Wokke, J.H.; de Visser, M.; van Engelen, B.G.; Kuks, J.B.; van der Kooi, A.J.; et al. Clinical features and predictors for disease natural progression in adults with Pompe disease: A nationwide prospective observational study. Orphanet J. Rare Dis. 2012, 7, 88. [Google Scholar] [CrossRef] [Green Version]
- Diaz-Manera, J.; Kishnani, P.S.; Kushlaf, H.; Ladha, S.; Mozaffar, T.; Straub, V.; Toscano, A.; van der Ploeg, A.T.; Berger, K.I.; Clemens, P.R.; et al. Safety and efficacy of avalglucosidase alfa versus alglucosidase alfa in patients with late-onset Pompe disease (COMET): A phase 3, randomised, multicentre trial. Lancet Neurol. 2021, 20, 1012–1026. [Google Scholar] [CrossRef]
| ID | Gender | Age at Symptom Onset (y) | Symptoms at Onset | GAA Mutations | Age at Study Inclusion (y) | Disease Duration at Inclusion (y) | Duration of ERT (y) | NIV at Night (Y/N), Age at Start NIV (y) | FVC Sitting Visit 1 (0 Months) (%) | FVC Supine Visit 1 (0 Months) (%) | FVC Sitting Visit 5 (24 Months) (%) | FVC Supine Visit 5 (24 Months) (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | F | 42 | LW | c.-32-13T>G; c.482_483delCC | 48 | 6 | 6 | N | 101 | 97 | 96 | 94 |
| 2 | F | 1 | LW | c.-32-13T>G; c.923A>C | 22 | 21 | 13 | N | 106 | 91 | 106 | 107 |
| 3 | M | 39 | R | c.-32-13T>G; c.258dupC | 61 | 22 | 11 | Y (40) | 33 | ND | 30 | ND |
| 4 | F | 35 | LW | c.-32-13T>G; c.1548G>A | 49 | 14 | 12 | Y (36) | 48 | 33 | 51 | 38 |
| 5 | M | 17 | LW | c.-32-13T>G; c.482_483del | 43 | 26 | 8 | N | 73 | 67 | 76 | 62 |
| 6 | F | 27 | hyperCK, F | c.-32-13T>G; c.525delT | 40 | 13 | 8 | N | 114 | 93 | 121 | 105 |
| 7 | M | 42 | LW | c.-32-13T>G; c.2608C>T | 54 | 12 | 0.5 | Y (53) | 76 | 45 | 84 | 45 |
| 8 | M | 45 | LW | c.-32-13T>G; c.1681_1699dup19 | 67 | 22 | 11 | Y (60) | 101 | 49 | 105 | 59 |
| 9 | F | 44 | LW | c.-32-13T>G; del exon 18 | 62 | 18 | 10 | N | 70 | 43 | 64 | 32 |
| 10 | F | 52 | LW | c.-32-13T>G; c.258dupC | 63 | 11 | 8 | N | 108 | 78 | 73 | 56 |
| 11 | F | 25 | LW | c.-32-13T>G; c.186dup11 | 61 | 36 | 9 | N | 84 | 64 | 79 | 62 |
| 12 | M | 25 | LW | c.-32-13T>G; c.1075G>A | 45 | 20 | 9 | N | 80 | 62 | 79 | 56 |
| 32.8 (1–52) | 51.3 (22–67) | 18.4 (0.5–36) | 8.8 (0.5–13) |
| Outcome | Visit 1 (0 Months) | Visit 2 (6 Months) | Visit 3 (12 Months) | Visit 4 (18 Months) | Visit 5 (24 Months) | ANOVA | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Measures | Mean | ±SD | Mean | ±SD | V2 vs. V1 | 95%CI | p | Mean | ±SD | V3 vs. V1 | 95%CI | p | Mean | ±SD | V4 vs. V1 | 95%CI | p | Mean | ±SD | V5 vs. V1 | 95%CI | p | p |
| Hip ext. R | 54.1 | 19.8 | 62.0 | 29.6 | 7.7 | −0.4 to 15.8 | 0.061 | 59.6 | 24.7 | 6.0 | −3.6 to 15.7 | 0.191 | 59.4 | 27.1 | 5.3 | −2.5 to 13.2 | 0.162 | 61.5 | 28.5 | 7.4 | −2.5 to 17.3 | 0.126 | 0.959 |
| Hip ext. L | 55.5 | 26.4 | 62.3 | 31.1 | 6.7 | −2.1 to 15.5 | 0.119 | 59.1 | 30.3 | 5.6 | −4.8 to 16.0 | 0.253 | 60.1 | 28.4 | 4.6 | −4.3 to 13.5 | 0.274 | 57.8 | 24.3 | 2.3 | −9.5 to 14.1 | 0.677 | 0.986 |
| Hip flex. R | 19.2 | 14.8 | 22.5 | 14.2 | 3.7 | −1.8 to 9.2 | 0.162 | 23.9 | 16.6 | 4.2 | −1.9 to 10.4 | 0.156 | 24.9 | 11.5 | 5.8 | 0.6 to 10.9 | 0.031 | 26.4 | 15.6 | 7.3 | −1.0 to 15.5 | 0.079 | 0.813 |
| Hip flex. L | 17.9 | 12.4 | 20.9 | 13.8 | 3.3 | −1.5 to 8.2 | 0.153 | 22.0 | 15.4 | 3.5 | −2.7 to 9.7 | 0.233 | 23.1 | 14.2 | 5.2 | −1.6 to 11.9 | 0.119 | 22.1 | 12.8 | 4.2 | −2.1 to 10.5 | 0.170 | 0.921 |
| Knee ext. R | 79.5 | 43.4 | 73.5 | 44.5 | −3.3 | −8.5 to 2.0 | 0.194 | 73.2 | 35.9 | −6.4 | −18.1 to 5.3 | 0.256 | 67.7 | 41.3 | −11.8 | −20.2 to −3.5 | 0.010 | 66.2 | 39.6 | −13.3 | −20.5 to −6.2 | 0.002 | 0.937 |
| Knee ext. L | 79.3 | 42.6 | 74.8 | 44.8 | −2.4 | −7.7 to 2.9 | 0.332 | 76.7 | 42.0 | −2.6 | −13.3 to 8.2 | 0.609 | 67.1 | 39.6 | −12.1 | −20.3 to −4.0 | 0.007 | 65.1 | 39.0 | −14.1 | −25.2 to −3.1 | 0.017 | 0.901 |
| Knee flex. R | 52.6 | 24.7 | 51.9 | 25.7 | −1.3 | −4.6 to 1.9 | 0.378 | 44.3 | 21.6 | −8.2 | −16.6 to 0.2 | 0.055 | 48.1 | 24.6 | −4.4 | −9.7 to 0.9 | 0.095 | 47.6 | 23.9 | −5.0 | −11.0 to 1.0 | 0.093 | 0.919 |
| Knee flex. L | 47.4 | 21.3 | 48.2 | 24.8 | 0.4 | −3.2 to 4.0 | 0.807 | 42.3 | 20.4 | −5.1 | −11.0 to 0.7 | 0.081 | 46.6 | 22.0 | −0.9 | −4.2 to 2.5 | 0.577 | 44.9 | 22.2 | −2.5 | −6.2 to 1.2 | 0.167 | 0.970 |
| Shoulder abd. R | 18.8 | 5.0 | 18.6 | 6.1 | 0.4 | −3.4 to 4.2 | 0.821 | 18.3 | 8.7 | 0.4 | −4.2 to 5.1 | 0.839 | 17.7 | 6.7 | −0.4 | −2.9 to 2.1 | 0.726 | 16.8 | 6.1 | −1.2 | −3.4 to 0.9 | 0.223 | 0.953 |
| Shoulder abd. L | 16.8 | 6.8 | 16.9 | 7.8 | 1.1 | −2.4 to 4.5 | 0.498 | 17.5 | 6.9 | 0.8 | −1.5 to 3.2 | 0.437 | 16.2 | 7.0 | −0.7 | −3.6 to 2.2 | 0.596 | 17.3 | 7.1 | 0.6 | −2.1 to 3.2 | 0.646 | 0.993 |
| Shoulder add. R | 42.3 | 14.4 | 40.6 | 14.5 | −1.2 | −3.5 to 1.2 | 0.291 | 40.1 | 14.8 | −2.1 | −6.2 to 1.9 | 0.269 | 41.2 | 15.0 | −0.6 | −3.9 to 2.7 | 0.696 | 41.6 | 15.3 | −0.3 | −3.4 to 2.8 | 0.853 | 0.997 |
| Shoulder add. L | 47.3 | 14.9 | 44.7 | 13.9 | −2.2 | −6.0 to 1.7 | 0.236 | 46.5 | 13.7 | −1.0 | −5.2 to 3.3 | 0.627 | 45.5 | 14.0 | −1.7 | −6.3 to 3.0 | 0.443 | 47.3 | 14.5 | −0.1 | −5.1 to 4.8 | 0.952 | 0.991 |
| Elbow ext. R | 40.8 | 13.8 | 40.9 | 13.8 | 1.8 | −4.4 to 8.0 | 0.534 | 42.1 | 14.9 | 1.2 | −4.0 to 6.4 | 0.614 | 43.3 | 14.3 | 2.5 | −3.4 to 8.4 | 0.378 | 40.0 | 13.0 | −0.9 | −7.0 to 5.3 | 0.765 | 0.982 |
| Elbow ext. L | 44.8 | 16.9 | 45.7 | 13.1 | 3.2 | −1.5 to 8.0 | 0.159 | 43.2 | 16.3 | −1.6 | −6.0 to 2.8 | 0.446 | 44.8 | 15.5 | 0.0 | −4.7 to 4.7 | 0.988 | 43.1 | 13.7 | −1.7 | −7.0 to 3.7 | 0.506 | 0.993 |
| Elbow flex. R | 22.6 | 10.9 | 21.0 | 10.2 | −1.4 | −6.0 to 3.1 | 0.506 | 22.0 | 12.2 | −0.6 | −6.0 to 4.8 | 0.810 | 22.2 | 11.2 | −0.5 | −4.7 to 3.7 | 0.805 | 22.7 | 11.7 | 0.0 | −5.2 to 5.2 | 0.995 | 0.997 |
| Elbow flex. L | 22.2 | 14.1 | 22.3 | 12.3 | 2.1 | −1.1 to 5.4 | 0.178 | 22.7 | 12.1 | 0.5 | −4.0 to 4.9 | 0.816 | 22.9 | 12.0 | 0.7 | −2.3 to 3.7 | 0.626 | 23.1 | 11.2 | 0.9 | −3.2 to 4.9 | 0.646 | 1.000 |
| Hand grip R(kg) | 37.3 | 10.7 | 39.3 | 8.9 | 1.1 | −1.3 to 3.5 | 0.323 | 37.4 | 7.9 | −0.8 | −5.1 to 3.5 | 0.680 | 37.5 | 9.3 | −0.5 | −4.0 to 3.0 | 0.743 | 36.8 | 7.7 | −2.2 | −7.1 to 2.7 | 0.328 | 0.974 |
| MRC-SS (/80) | 67.2 | 8.2 | 70.4 | 7.7 | 1.0 | −5.5 to 7.5 | 0.733 | 71.1 | 8.3 | 1.8 | −4.9 to 8.5 | 0.556 | 70.4 | 8.3 | 1.2 | −5.8 to 8.1 | 0.709 | 71.3 | 8.1 | 2.3 | −5.3 to 9.8 | 0.506 | 0.785 |
| 6MWD (m) | 451.9 | 143.3 | 443.9 | 163.6 | −19.1 | −58.8 to 20.6 | 0.305 | 410.6 | 172.2 | −41.3 | −86.9 to 4.3 | 0.071 | 427.6 | 167.0 | −28.5 | −63.9 to 6.9 | 0.102 | 368.1 | 167.6 | −60.6 | −92.0 to −29.1 | 0.003 | 0.826 |
| 10MWT (s) | 8.4 | 2.8 | 8.6 | 2.8 | 0.2 | −0.7 to 1.2 | 0.626 | 9.4 | 3.7 | 1.0 | −0.3 to 2.4 | 0.127 | 9.0 | 3.8 | 0.7 | −0.4 to 1.7 | 0.214 | 9.1 | 3.5 | 0.8 | −1.3 to 2.9 | 0.414 | 0.960 |
| TUG (s) | 7.4 | 4.7 | 7.8 | 5.8 | 0.2 | −1.0 to 1.3 | 0.779 | 8.2 | 5.4 | 0.6 | −0.5 to 1.7 | 0.265 | 7.8 | 5.4 | 1.1 | −0.3 to 2.5 | 0.112 | 9.0 | 6.2 | 1.3 | −1.4 to 4.0 | 0.289 | 0.981 |
| Outcome | Visit 1 (0 Months) | Visit 2 (6 Months) | Visit 3 (12 Months) | Visit 4 (18 Months) | Visit 5 (24 Months) | ANOVA | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Measures | Mean | ±SD | Mean | ±SD | V2 vs. V1 | 95%CI | p | Mean | ±SD | V3 vs. V1 | 95%CI | p | Mean | ±SD | V4 vs. V1 | 95%CI | p | Mean | ±SD | V5 vs. V1 | 95%CI | p | p |
| Hip ext. R | 107.7 | 31.8 | 115.6 | 41.2 | 3.1 | −14.7 to 20.9 | 0.705 | 110.4 | 47.5 | 2.7 | −24.4 to 29.7 | 0.833 | 108.3 | 42.8 | −2.1 | −23.4 to 19.3 | 0.833 | 109.5 | 36.3 | −7.1 | −25.9 to 11.6 | 0.407 | 0.992 |
| Hip ext. L | 100.1 | 33.3 | 115.4 | 38.1 | 12.1 | −8.9 to 33.0 | 0.229 | 102.3 | 35.1 | 2.2 | −21.3 to 25.6 | 0.843 | 105.9 | 39.9 | 3.6 | −21.3 to 28.5 | 0.755 | 107.2 | 32.1 | −1.8 | −24.6 to 21.1 | 0.864 | 0.872 |
| Hip flex. R | 52.1 | 22.6 | 51.7 | 19.3 | −3.9 | −10.4 to 2.6 | 0.207 | 45.5 | 16.0 | −6.6 | −15.9 to 2.6 | 0.142 | 42.7 | 19.5 | −8.8 | −19.6 to 2.1 | 0.102 | 46.0 | 17.8 | −5.2 | −12.6 to 2.2 | 0.141 | 0.723 |
| Hip flex. L | 47.1 | 22.5 | 48.8 | 22.6 | −1.2 | −5.6 to 3.3 | 0.570 | 45.1 | 21.7 | −2.1 | −10.8 to 6.7 | 0.610 | 46.6 | 24.6 | −0.8 | −13.8 to 12.3 | 0.896 | 42.7 | 19.8 | −5.6 | −20.7 to 9.6 | 0.422 | 0.980 |
| Knee ext. R | 147.7 | 64.5 | 131.4 | 36.4 | −23.1 | −49.7 to 3.5 | 0.082 | 125.9 | 56.5 | −21.8 | −38.3 to −5.3 | 0.014 | 119.1 | 49.4 | −29.7 | −55.8 to −3.6 | 0.030 | 112.2 | 40.2 | −32.4 | −53.2 to −11.7 | 0.007 | 0.557 |
| Knee ext. L | 133.0 | 56.7 | 131.7 | 48.8 | −8.1 | −24.2 to 8.0 | 0.289 | 111.0 | 41.7 | −22.0 | −39.0 to −5.0 | 0.016 | 118.4 | 54.5 | −16.4 | −35.5 to 2.8 | 0.086 | 111.8 | 41.3 | −21.6 | −41.3 to −2.0 | 0.035 | 0.725 |
| Knee flex. R | 98.4 | 34.9 | 100.8 | 40.4 | −1.1 | −11.1 to 9.0 | 0.819 | 89.2 | 35.3 | −9.2 | −17.5 to −0.8 | 0.034 | 92.2 | 43.7 | −4.1 | −15.4 to 7.1 | 0.431 | 86.1 | 27.4 | −9.5 | −17.1 to −2.0 | 0.020 | 0.880 |
| Knee flex. L | 94.4 | 39.1 | 98.0 | 45.0 | −1.5 | −11.8 to 8.8 | 0.754 | 86.7 | 38.5 | −7.7 | −16.9 to 1.6 | 0.095 | 89.6 | 42.7 | −4.0 | −15.9 to 7.9 | 0.474 | 86.6 | 28.8 | −8.9 | −22.0 to 4.1 | 0.153 | 0.951 |
| Shoulder abd. R | 32.4 | 21.1 | 32.0 | 21.2 | −0.4 | −3.9 to 3.2 | 0.827 | 29.6 | 21.4 | −2.8 | −7.0 to 1.4 | 0.167 | 31.0 | 22.7 | −1.1 | −5.5 to 3.3 | 0.593 | 27.3 | 12.5 | −1.8 | −8.3 to 4.7 | 0.535 | 0.981 |
| Shoulder abd. L | 29.8 | 16.8 | 30.1 | 16.7 | 0.3 | −2.6 to 3.1 | 0.843 | 28.2 | 18.0 | −1.6 | −5.3 to 2.0 | 0.345 | 27.8 | 16.7 | −1.3 | −4.1 to 1.4 | 0.297 | 24.9 | 12.7 | −1.5 | −4.4 to 1.4 | 0.265 | 0.958 |
| Shoulder add. R | 78.2 | 37.5 | 74.2 | 31.6 | −4.0 | −12.6 to 4.6 | 0.333 | 70.6 | 25.5 | −7.6 | −18.0 to 2.9 | 0.140 | 73.2 | 31.9 | −4.3 | −16.2 to 7.7 | 0.444 | 67.9 | 24.5 | −7.7 | −22.3 to 6.8 | 0.256 | 0.954 |
| Shoulder add. L | 73.3 | 31.9 | 69.9 | 27.8 | −3.4 | −8.6 to 1.8 | 0.180 | 69.3 | 22.6 | −4.0 | −13.0 to 5.0 | 0.348 | 69.5 | 28.3 | −3.3 | −13.7 to 7.0 | 0.490 | 64.1 | 18.6 | −5.4 | −21.1 to 10.2 | 0.446 | 0.959 |
| Elbow ext. R | 44.6 | 19.4 | 44.3 | 19.6 | −0.3 | −3.6 to 3.1 | 0.864 | 43.8 | 17.2 | −0.7 | −4.7 to 3.2 | 0.693 | 43.6 | 18.8 | 0.2 | −3.5 to 4.0 | 0.892 | 40.3 | 13.0 | −2.4 | −10.2 to 5.4 | 0.500 | 0.986 |
| Elbow ext. L | 46.3 | 19.2 | 45.5 | 21.1 | −0.8 | −3.1 to 1.5 | 0.470 | 44.9 | 13.9 | −1.4 | −6.3 to 3.6 | 0.552 | 44.5 | 18.2 | −0.5 | −5.8 to 4.9 | 0.853 | 43.5 | 14.5 | 0.2 | −5.1 to 5.5 | 0.937 | 0.998 |
| Elbow flex. R | 37.2 | 20.8 | 36.2 | 20.1 | −1.0 | −2.7 to 0.8 | 0.249 | 36.0 | 17.0 | −1.1 | −4.5 to 2.2 | 0.470 | 32.8 | 18.3 | −4.1 | −11.3 to 3.1 | 0.230 | 32.8 | 16.2 | −2.1 | −6.6 to 2.3 | 0.298 | 0.971 |
| Elbow flex. L | 32.4 | 18.6 | 34.6 | 18.2 | 2.2 | −1.0 to 5.4 | 0.159 | 31.8 | 15.2 | −0.6 | −4.6 to 3.4 | 0.742 | 31.0 | 16.5 | −0.9 | −4.9 to 3.1 | 0.623 | 29.3 | 11.4 | 0.7 | −4.3 to 5.7 | 0.752 | 0.964 |
| Hand grip R(kg) | 36.5 | 9.6 | 34.9 | 9.5 | −1.6 | −4.5 to 1.2 | 0.233 | 34.7 | 12.2 | −1.8 | −5.8 to 2.1 | 0.331 | 36.2 | 12.7 | −0.3 | −3.5 to 2.8 | 0.814 | 35.4 | 11.5 | −0.9 | −3.2 to 1.4 | 0.401 | 0.993 |
| MRC−SS (/80) | 80.0 | 0.0 | 80.0 | 0.0 | 0.0 | 0.0 to 0.0 | 1.000 | 80.0 | 0.0 | 0.0 | 0.0 to 0.0 | 1.000 | 80.0 | 0.0 | 0.0 | 0.0 to 0.0 | 1.000 | 80.0 | 0.0 | 0.0 | 0.0 to 0.0 | 1.000 | 1.000 |
| 6MWD (m) | 661.3 | 81.8 | 688.2 | 78.7 | 16.0 | 0.5 to 31.5 | 0.045 | 670.4 | 88.8 | 9.2 | −9.2 to 27.5 | 0.295 | 680.2 | 82.9 | 17.7 | 3.5 to 32.0 | 0.020 | 694.6 | 91.0 | 23.3 | −0.4 to 47.1 | 0.054 | 0.896 |
| 10MWT (s) | 4.2 | 1.4 | 4.7 | 1.4 | 0.5 | −0.4 to 1.3 | 0.242 | 4.4 | 1.2 | 0.2 | −0.5 to 0.9 | 0.486 | 4.5 | 1.1 | 0.3 | −0.6 to 1.1 | 0.502 | 4.2 | 0.9 | 0.2 | −0.7 to 1.1 | 0.702 | 0.867 |
| TUG (s) | 1.8 | 0.3 | 1.8 | 0.5 | 0.0 | −0.2 to 0.2 | 0.927 | 1.9 | 0.9 | 0.2 | −0.4 to 0.7 | 0.540 | 2.1 | 1.0 | 0.2 | −0.3 to 0.8 | 0.357 | 1.8 | 0.5 | 0.0 | −0.3 to 0.3 | 1.000 | 0.799 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Claeys, K.G.; D’Hondt, A.; Fache, L.; Peers, K.; Depuydt, C.E. Six-Minute Walk Distance Is a Useful Outcome Measure to Detect Motor Decline in Treated Late-Onset Pompe Disease Patients. Cells 2022, 11, 334. https://doi.org/10.3390/cells11030334
Claeys KG, D’Hondt A, Fache L, Peers K, Depuydt CE. Six-Minute Walk Distance Is a Useful Outcome Measure to Detect Motor Decline in Treated Late-Onset Pompe Disease Patients. Cells. 2022; 11(3):334. https://doi.org/10.3390/cells11030334
Chicago/Turabian StyleClaeys, Kristl G., Ann D’Hondt, Lucas Fache, Koen Peers, and Christophe E. Depuydt. 2022. "Six-Minute Walk Distance Is a Useful Outcome Measure to Detect Motor Decline in Treated Late-Onset Pompe Disease Patients" Cells 11, no. 3: 334. https://doi.org/10.3390/cells11030334
APA StyleClaeys, K. G., D’Hondt, A., Fache, L., Peers, K., & Depuydt, C. E. (2022). Six-Minute Walk Distance Is a Useful Outcome Measure to Detect Motor Decline in Treated Late-Onset Pompe Disease Patients. Cells, 11(3), 334. https://doi.org/10.3390/cells11030334






