Sézary Syndrome: Different Erythroderma Morphological Features with Proposal for a Clinical Score System
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Clinical Characteristics of Patients
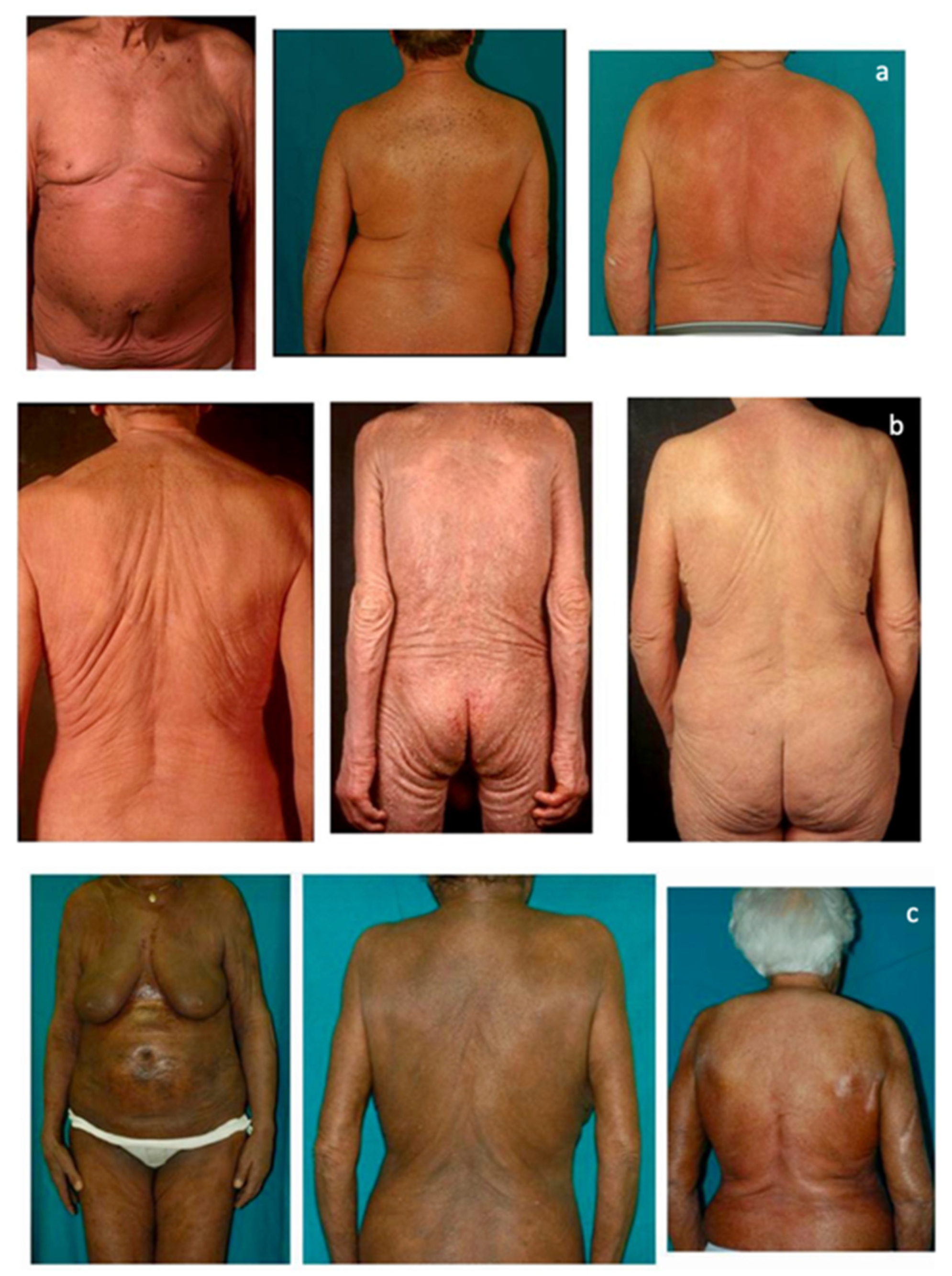
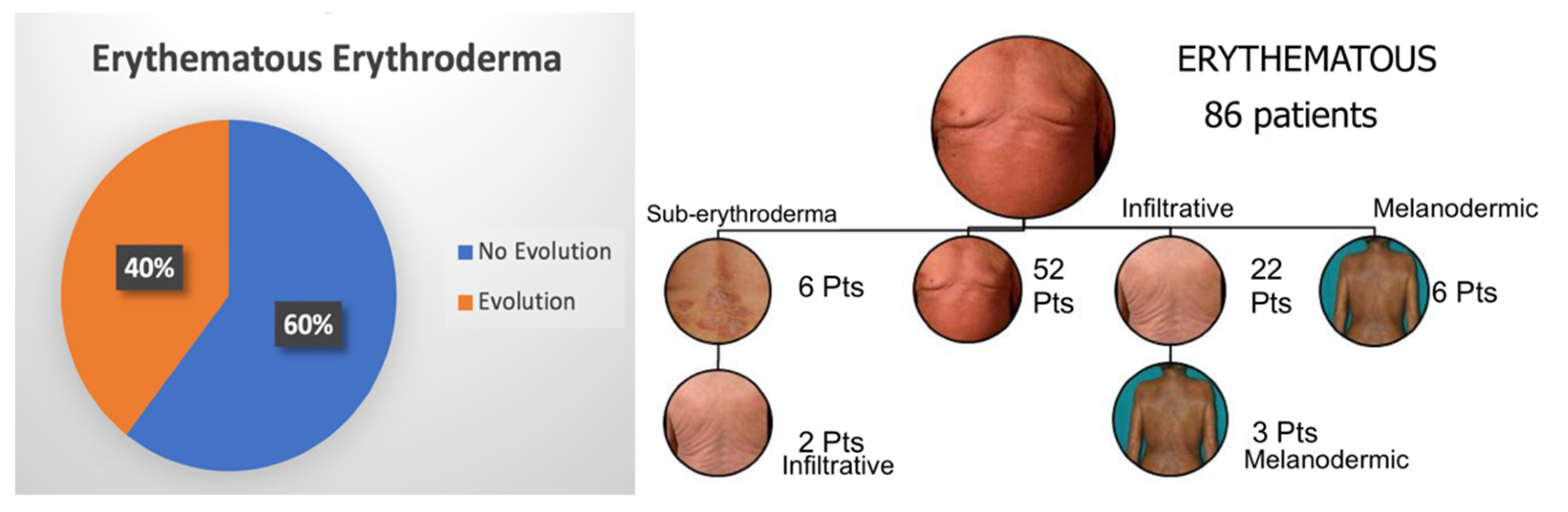
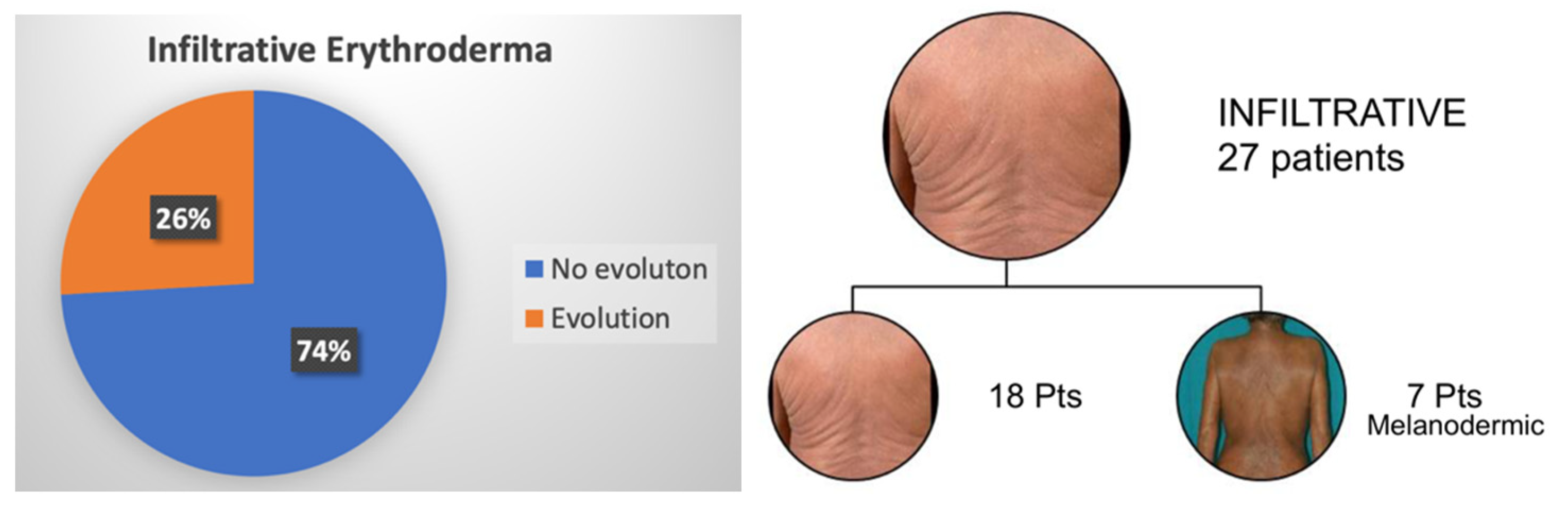
3.2. Erythroderma Morphological Features
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Willemze, R.; Jaffe, E.S.; Burg, G.; Cerroni, L.; Berti, E.; Swerdlow, S.H.; Ralfkiaer, E.; Chimenti, S.; Diaz-Perez, J.L.; Duncan, L.M.; et al. WHO-EORTC classification for cutaneous lymphomas. Blood 2005, 105, 3768–3785. [Google Scholar] [CrossRef] [Green Version]
- Agar, N.S.; Wedgeworth, E.; Crichton, S.; Mitchell, T.J.; Cox, M.; Ferreira, S.; Robson, A.; Calonje, E.; Stefanato, C.M.; Wain, E.M.; et al. Survival outcomes and prognostic factors in mycosis fungoides/Sezary syndrome: Validation of the revised International Society of Cutaneous Lymphomas/European Organisation for Research and Treatment of cancer staging proposal. J. Clin. Oncol. 2010, 28, 4730–4739. [Google Scholar] [CrossRef]
- Olsen, E.; Vonderheid, E.; Pimpinelli, N.; Willemze, R.; Kim, Y.; Knobler, R.; Zackheim, H.; Duvic, M.; Estrach, T.; Lamberg, S.; et al. Revisions to the staging and classification of mycosis fungoides and Sezary syndrome: A proposal of the International Society for Cutaneous Lymphomas (ISCL) and the Cutaneous Lymphoma Task Force of the European Organization of Research and Treatment of Cancer (EORTC). Blood 2007, 110, 1713–1722. [Google Scholar]
- Olsen, E.A.; Whittaker, S.; Willemze, R.; Pinter-Brown, L.; Foss, F.M.; Geskin, L.J.; Schwartz, L.H.; Horwitz, S.M.; Guitart, J.; Zic, J.; et al. Primary Cutaneous Lymphoma: Recommendations for Clinical Trial Design and Staging Update from the ISCL, USCLC, and EORTC. Blood 2021. [Google Scholar] [CrossRef]
- Henn, A.; Michel, L.; Fite, C.; Deschamps, L.; Ortonne, N.; Ingen-Housz-Oro, S.; Marinho, E.; Beylot-Barry, M.; Bagot, M.; Laroche, L.; et al. Sézary syndrome without erythroderma. J. Am. Acad. Dermatol. 2015, 72, 1003–1009.e1. [Google Scholar] [CrossRef] [PubMed]
- Mangold, A.R.; Thompson, A.K.; Davis, M.D.; Saulite, I.; Cozzio, A.; Guenova, E.; Hodak, E.; Amitay-Laish, I.; Pujol, R.M.; Pittelkow, M.R.; et al. Early clinical manifestations of Sézary syndrome: A multicenter retrospective cohort study. J. Am. Acad. Dermatol. 2017, 77, 719–727. [Google Scholar] [CrossRef] [PubMed]
- Novelli, M.; Fava, P.; Sarda, C.; Ponti, R.; Osella-Abate, S.; Savoia, P.; Bergallo, M.; Lisa, F.; Fierro, M.T.; Quaglino, P. Blood flow cytometry in Sézary syndrome: New insights on prognostic relevance and immunophenotypic changes during follow-up. Am. J. Clin. Pathol. 2015, 143, 57–69. [Google Scholar] [CrossRef]
- Scarisbrick, J.J.; Hodak, E.; Bagot, M.; Stranzenbach, R.; Stadler, R.; Ortiz-Romero, P.L.; Papadavid, E.; Evison, F.; Knobler, R.; Quaglino, P.; et al. Blood classification and blood response criteria in mycosis fungoides and Sézary syndrome using flow cytometry: Recommendations from the EORTC cutaneous lymphoma task force. Eur. J. Cancer 2018, 93, 47–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernengo, M.G.; Novelli, M.; Quaglino, P.; Lisa, F.; De Matteis, A.; Savoia, P.; Cappello, N.; Fierro, M.T. The relevance of the CD4+ CD26—subset in the identification of circulating Sézary cells. Br. J. Dermatol. 2001, 144, 125–135. [Google Scholar] [CrossRef]
- Boonk, S.E.; Zoutman, W.H.; Marie-Cardine, A.; van der Fits, L.; Out-Luiting, J.J.; Mitchell, T.J.; Tosi, I.; Morris, S.L.; Moriarty, B.; Booken, N.; et al. Evaluation of Immunophenotypic and Molecular Biomarkers for Sézary Syndrome Using Standard Operating Procedures: A Multicenter Study of 59 Patients. J. Investig. Dermatol. 2016, 136, 1364–1372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janiga, J.; Kentley, J.; Nabhan, C.; Abdulla, F. Current Systemic Therapeutic Options for Advanced Mycosis Fungoides and Sézary Syndrome. Leuk. Lymphoma 2018, 59, 562–577. [Google Scholar] [CrossRef] [PubMed]
- Knobler, R.; Berlin, G.; Calzavara-Pinton, P.; Greinix, H.; Jaksch, P.; Laroche, L.; Ludvigsson, J.; Quaglino, P.; Reinisch, W.; Scarisbrick, J.; et al. Guidelines on the use of extracorporeal photopheresis. J. Eur. Acad Dermatol. Venereol. 2014, 28, 1–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trautinger, F.; Eder, J.; Assaf, C.; Bagot, M.; Cozzio, A.; Dummer, R.; Gniadecki, R.; Klemke, C.-D.; Ortiz-Romero, P.L.; Papadavid, E.; et al. European Organisation for Research and Treatment of Cancer consensus recommendations for the treatment of mycosis fungoides/Sézary syndrome—Update 2017. Eur. J. Cancer 2017, 77, 57–74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quaglino, P.; Fava, P.; Pileri, A.; Grandi, V.; Sanlorenzo, M.; Panasiti, V.; Guglielmo, A.; Alberti-Violetti, S.; Novelli, M.; Astrua, C.; et al. Phenotypical Markers, Molecular Mutations, and Immune Microenvironment as Targets for New Treatments in Patients with Mycosis Fungoides and/or Sézary Syndrome. J. Investig. Dermatol. 2021, 141, 484–495. [Google Scholar] [CrossRef]
- Dummer, R.; Vermeer, M.H.; Scarisbrick, J.J.; Kim, Y.H.; Stonesifer, C.; Tensen, C.P.; Geskin, L.J.; Quaglino, P.; Ramelyte, E. Cutaneous T cell lymphoma. Nat. Rev. Dis. Prim. 2021, 7, 61. [Google Scholar] [CrossRef]
- Dumont, M.; de Latour, R.P.; Ram-Wolff, C.; Bagot, M.; de Masson, A. Allogeneic Hematopoietic Stem Cell Transplantation in Cutaneous T-Cell Lymphomas. Cancers 2020, 12, 2856. [Google Scholar] [CrossRef]
- Mehta-Shah, N.; Horwitz, S.M.; Ansell, S.; Ai, W.Z.; Barnes, J.; Barta, S.K.; Clemens, M.W.; Dogan, A.; Fisher, K.; Goodman, A.M.; et al. NCCN Guidelines Insights: Primary Cutaneous Lymphomas, Version 2.2020. J. Natl. Compr. Cancer Netw. 2020, 18, 522–536. [Google Scholar] [CrossRef]
- Wang, Y.; Bagot, M. Updates in Cutaneous Lymphoma: Evidence-Based Guidelines for the Management of Cutaneous Lymphoma 2018. Br. J. Dermatol. 2019, 180, 443–444. [Google Scholar] [CrossRef] [Green Version]
- Roccuzzo, G.; Giordano, S.; Fava, P.; Pileri, A.; Guglielmo, A.; Tonella, L.; Sanlorenzo, M.; Ribero, S.; Fierro, M.T.; Quaglino, P. Immune Check Point Inhibitors in Primary Cutaneous T-Cell Lymphomas: Biologic Rationale, Clinical Results and Future Perspectives. Front. Oncol. 2021, 11, 733770. [Google Scholar] [CrossRef]
- Hundeiker, M.; Löffler, H. Hämosiderimpigmentierung beim Sézary-Syndrom [Hemosiderin pigmentation in Sezáry's syndrome]. Hautarzt 1976, 27, 492–494. [Google Scholar]
- Sentis, H.J.; Willemze, R.; Scheffer, E. Histopathologic studies in Sezary syndrome and erythrodermic mycosis fungoides: A comparison with benign forms of erythroderma. J. Am. Acad Dermatol. 1986, 15, 1217–1226. [Google Scholar] [CrossRef]
- Trotter, M.J.; Whittaker, S.J.; Orchard, G.E.; Smith, N.P. Cutaneous histopathology of Sézary syndrome: A study of 41 cases with a proven circulating T-cell clone. J. Cutan. Pathol. 1997, 24, 286–291. [Google Scholar] [CrossRef] [PubMed]
- Klemke, C.; Booken, N.; Weiss, C.; Nicolay, J.; Goerdt, S.; Felcht, M.; Géraud, C.; Kempf, W.; Assaf, C.; Ortonne, N.; et al. Histopathological and immunophenotypical criteria for the diagnosis of Sézary syndrome in differentiation from other erythrodermic skin diseases: A European Organisation for Research and Treatment of Cancer (EORTC) Cutaneous Lymphoma Task Force Study of 97 cases. Br. J. Dermatol. 2015, 173, 93–105. [Google Scholar] [CrossRef] [PubMed]
- Zinzani, P.L.; Quaglino, P.; Violetti, S.A.; Cantonetti, M.; Goteri, G.; Onida, F.; Paulli, M.; Rupoli, S.; Barosi, G.; Pimpinelli, N. Critical concepts and management recommendations for cutaneous T-cell lymphoma: A consensus-based position paper from the Italian Group of Cutaneous Lymphoma. Hematol. Oncol. 2021, 39, 275–283. [Google Scholar] [CrossRef] [PubMed]
- Olsen, E.A.; Whittaker, S.; Kim, Y.H.; Duvic, M.; Prince, H.M.; Lessin, S.R.; Wood, G.S.; Willemze, R.; Demierre, M.-F.; Pimpinelli, N.; et al. Clinical end points and response criteria in mycosis fungoides and Sézary syndrome: A consensus statement of the International Society for Cutaneous Lymphomas, the United States Cutaneous Lymphoma Consortium, and the Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer. J. Clin. Oncol. 2011, 29, 2598–2607. [Google Scholar] [CrossRef]
- Nagler, A.R.; Samimi, S.; Schaffer, A.; Vittorio, C.C.; Kim, E.J.; Rook, A.H. Peripheral blood findings in erythrodermic patients: Importance for the differential diagnosis of Sézary syndrome. J. Am. Acad. Dermatol. 2012, 66, 503–508. [Google Scholar] [CrossRef]

| Parameters at Diagnosis | N° Patients | Erythematous (E1) | Infiltrative (E2) | Melanoderma (E3) | Confluent Patches/Plaques/ Sub-Erythroderma |
|---|---|---|---|---|---|
| N° patients | 144 | 86 | 27 | 9 | 22 |
| Gender M-F (% Male patients) | 88–56 (61.1%) | 51–35 (59.3%) | 22–5 (81.5%) | 5–4 (55.5%) | 10–12 (45.5%) |
| Age (years) (median; range) | 70 (25–97) | 70 (25–97) | 69 (50–84) | 73 (55–86) | 67 (49–93) |
| Previous MF | 40 (27.8%) | 23/86 (26.7%) | 8/27 (29.6%) | 1/9 (11.1%) | 8/22 (36.4%) |
| Circulating SC (median; range)(/mm3) | 2691 (147–52,419) | 3228 (147–44,153) | 1698 (212–13,349) | 4398 (420–52,419) | 3160 (261–50,730) |
| SS Subtype | Proposed Staging | Clinical Presentation | Features |
|---|---|---|---|
| Patches, Plaques, Sub-erythroderma | E0 | 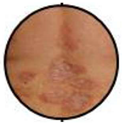 | No frank erythroderma. It may characterize early as well as atypical forms of SS. |
| Erythematous Erythroderma | E1 | 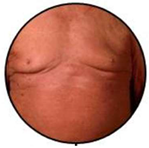 | Widespread reddening, with or without exfoliation, affecting at least 80% of body surface area. No appreciable signs of increased skin fold thickness. |
| Infiltrative Erythroderma | E2 | 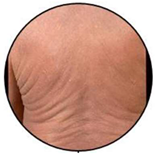 | Widespread reddening, with or without exfoliation, affecting at least 80% of body surface area and clear features of increased skin fold thickness. |
| Melanoderma | E3 |  | Widespread darkening affecting at least 80% body surface area. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roccuzzo, G.; Giordano, S.; Avallone, G.; Rubatto, M.; Canonico, S.; Funaro, A.; Ortolan, E.; Senetta, R.; Fava, P.; Fierro, M.T.; et al. Sézary Syndrome: Different Erythroderma Morphological Features with Proposal for a Clinical Score System. Cells 2022, 11, 333. https://doi.org/10.3390/cells11030333
Roccuzzo G, Giordano S, Avallone G, Rubatto M, Canonico S, Funaro A, Ortolan E, Senetta R, Fava P, Fierro MT, et al. Sézary Syndrome: Different Erythroderma Morphological Features with Proposal for a Clinical Score System. Cells. 2022; 11(3):333. https://doi.org/10.3390/cells11030333
Chicago/Turabian StyleRoccuzzo, Gabriele, Silvia Giordano, Gianluca Avallone, Marco Rubatto, Silvia Canonico, Ada Funaro, Erika Ortolan, Rebecca Senetta, Paolo Fava, Maria Teresa Fierro, and et al. 2022. "Sézary Syndrome: Different Erythroderma Morphological Features with Proposal for a Clinical Score System" Cells 11, no. 3: 333. https://doi.org/10.3390/cells11030333
APA StyleRoccuzzo, G., Giordano, S., Avallone, G., Rubatto, M., Canonico, S., Funaro, A., Ortolan, E., Senetta, R., Fava, P., Fierro, M. T., Ribero, S., & Quaglino, P. (2022). Sézary Syndrome: Different Erythroderma Morphological Features with Proposal for a Clinical Score System. Cells, 11(3), 333. https://doi.org/10.3390/cells11030333








