Tumor Cell-Intrinsic BTLA Receptor Inhibits the Proliferation of Tumor Cells via ERK1/2
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines
2.2. Flow Cytometric Analysis and Flow Cytometry Sorting
2.3. Cell Titer-Glo Luminescent Cell Viability Assay
2.4. Immunoblot
2.5. qRT-PCR
2.6. Public ChIP Sequence Data Collection
2.7. RNA-Sequence and Analysis
2.8. Expression Vectors, shRNAs, and Small Guide RNA (sgRNA)
2.9. Clone Formation Assay
2.10. Antibodies
2.11. Statistical Analysis
3. Results
3.1. BTLA Is Expressed by a Subpopulation of Tumor Cells
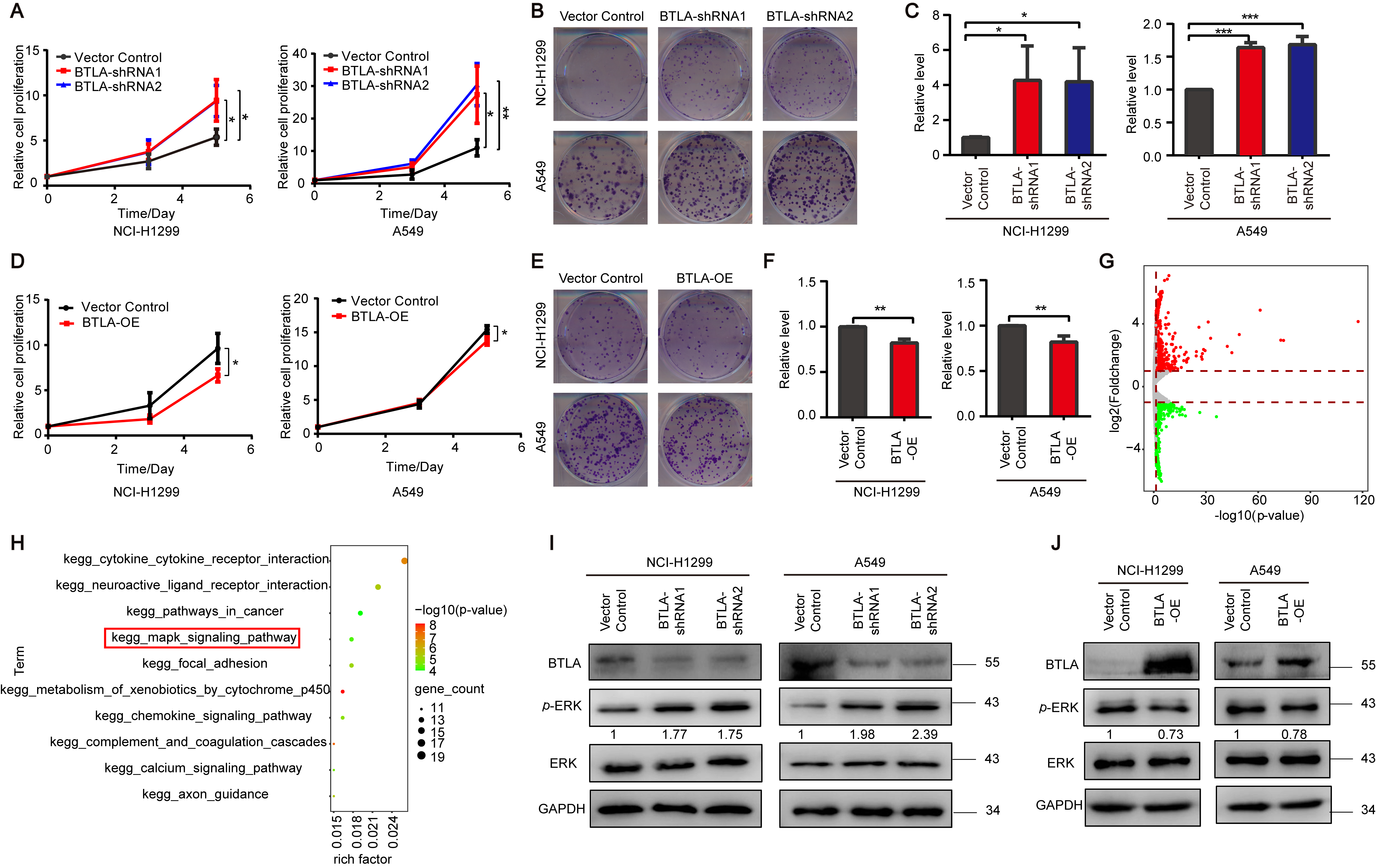
3.2. BTLA Inhibits the Growth of Tumor Cells via ERK1/2 Pathway
3.3. The Subpopulation of Low BTLA Grows Faster than High BTLA Subpopulation
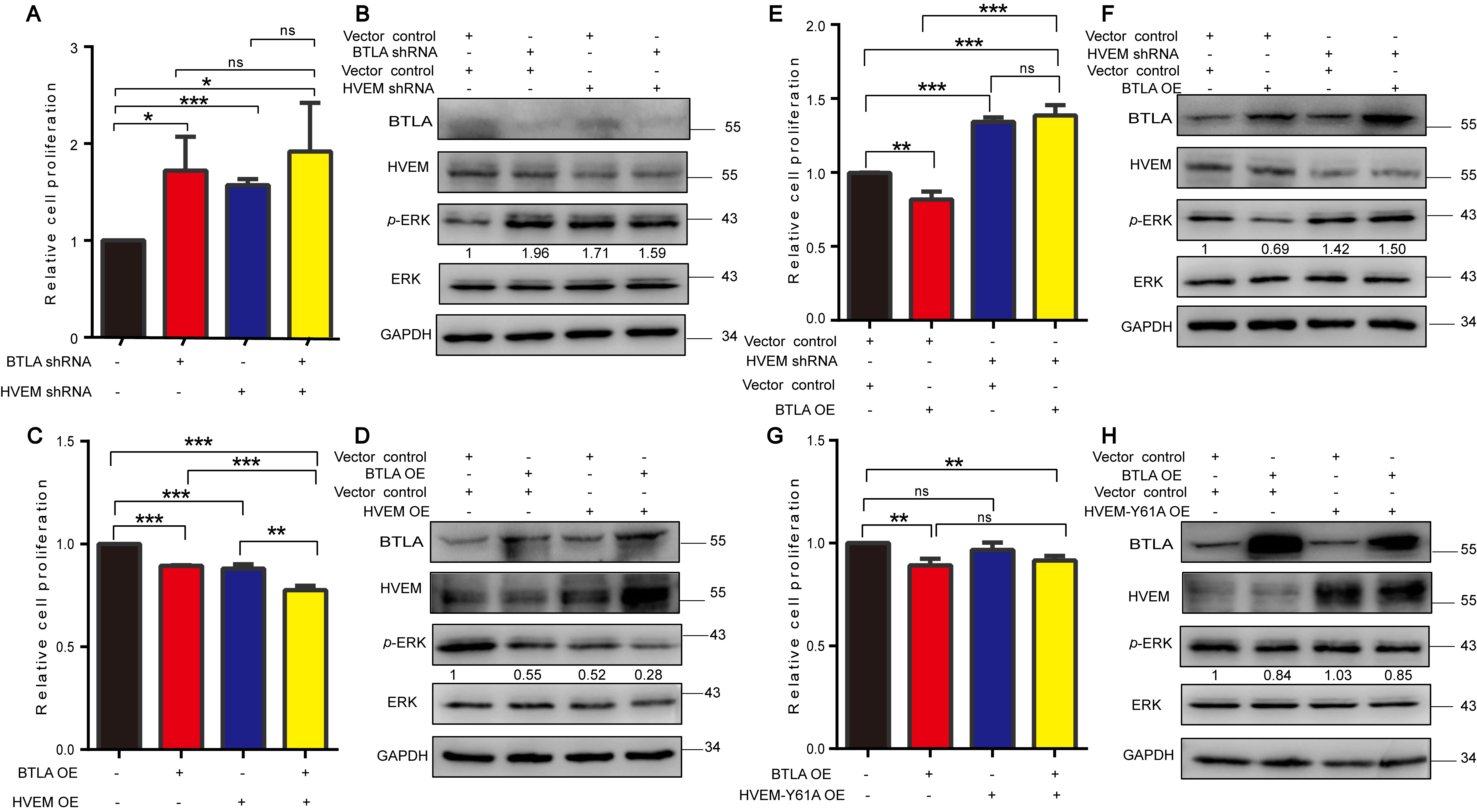
3.4. HVEM Inhibits the Growth of Tumor Cell via ERK1/2 Pathway
3.5. BTLA/HVEM Axis Functions on Growth and Signaling Pathway in Cancer Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The Next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef] [PubMed]
- Collin, M. Immune checkpoint inhibitors: A patent review (2010–2015). Expert Opin. Ther. Pat. 2016, 26, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Hoos, A. Development of immuno-oncology drugs—From CTLA4 to PD1 to the next generations. Nat. Rev. Drug Discov. 2016, 15, 235–247. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, T.; Zheng, L.; Liu, H.; Song, W.; Liu, D.; Li, Z.; Pan, C.X. Combination strategies to maximize the benefits of cancer immunotherapy. J. Hematol. Oncol. 2021, 14, 156. [Google Scholar]
- Kato, S.; Goodman, A.; Walavalkar, V.; Barkauskas, D.A.; Sharabi, A.; Kurzrock, R. Hyperprogressors after Immunotherapy: Analysis of Genomic Alterations Associated with Accelerated Growth Rate. Clin. Cancer Res. 2017, 23, 4242–4250. [Google Scholar] [CrossRef]
- Kanazu, M.; Edahiro, R.; Krebe, H.; Nishida, K.; Ishijima, M.; Uenami, T.; Akazawa, Y.; Yano, Y.; Yamaguchi, T.; Mori, M. Hyperprogressive disease in patients with non-small cell lung cancer treated with nivolumab: A case series. Thorac. Cancer 2018, 9, 1782–1787. [Google Scholar] [CrossRef]
- Schoenfeld, A.J.; Hellmann, M. Acquired Resistance to Immune Checkpoint Inhibitors. Cancer Cell 2020, 37, 443–455. [Google Scholar] [CrossRef]
- Wang, X.; Yang, X.; Zhang, C.; Wang, Y.; Gao, S. Tumor cell-intrinsic PD-1 receptor is a tumor suppressor and mediates resistance to PD-1 blockade therapy. Proc. Natl. Acad. Sci. USA 2020, 117, 6640–6650. [Google Scholar]
- Kleffel, S.; Posch, C.; Barthel, S.R.; Mueller, H.; Schlapbach, C.; Guenova, E.; Elco, C.P.; Lee, N.; Juneja, V.R.; Zhan, Q.; et al. Melanoma Cell-Intrinsic PD-1 Receptor Functions Promote Tumor Growth. Cell 2015, 162, 1242–1256. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, X.; Liu, S.; Guo, L.; Zhang, B.; Zhang, J.; Ye, Q. PD-1 Checkpoint Blockade in Combination with an mTOR Inhibitor Restrains Hepatocellular Carcinoma Growth Induced by Hepatoma Cell-Intrinsic PD-1. Hepatology 2017, 66, 1920–1933. [Google Scholar] [CrossRef] [PubMed]
- Cheung, T.C.; Oborne, L.M.; Steinberg, M.W.; Macauley, M.G.; Fukuyama, S.; Sanjo, H.; D”Souza, C.; Norris, P.S.; Pfeffer, K.; Murphy, K.M. T Cell Intrinsic Heterodimeric Complexes between HVEM and BTLA Determine Receptivity to the Surrounding Microenvironment. J. Immunol. 2009, 183, 7286–7296. [Google Scholar] [CrossRef] [PubMed]
- Pasero, C.; Speiser, D.E.; Derré, L.; Olive, D. The HVEM network: New directions in targeting novel costimulatory/co-inhibitory molecules for cancer therapy. Curr. Opin. Pharmacol. 2012, 12, 478–485. [Google Scholar] [CrossRef]
- Ritthipichai, K.; Haymaker, C.L.; Martinez, M.; Aschenbrenner, A.; Yi, X.; Zhang, M.; Kale, C.; Vence, L.M.; Roszik, J.; Hailemichael, Y.; et al. Multifaceted Role of BTLA in the Control. of CD8+ T-cell Fate after Antigen Encounter. Clin. Cancer Res. 2017, 23, 6151–6164. [Google Scholar] [CrossRef]
- Cheung, T.C.; Steinberg, M.W.; Oborne, L.M.; Macauley, M.G.; Fukuyama, S.; Sanjo, H.; D’Souza, C.; Norris, P.S.; Pfeffer, K.; Murphy, K.M.; et al. Unconventional ligand activation of herpesvirus entry mediator signals cell survival. Proc. Natl. Acad. Sci. USA 2009, 106, 6244–6253. [Google Scholar] [CrossRef]
- Chen, Y.L.; Lin, H.W.; Chien, C.L.; Lai, Y.L.; Cheng, W.F. BTLA blockade enhances Cancer therapy by inhibiting IL-6/IL-10-induced CD19high B lymphocytes. J. ImmunoTher. Cancer 2019, 7, 313–326. [Google Scholar] [CrossRef]
- Celis-Gutierrez, J.; Blattmann, P.; Zhai, Y.; Jarmuzynski, N.; Ruminski, K. Quantitative Interactomics in Primary T Cells Provides a Rationale for Concomitant PD-1 and BTLA Coinhibitor Blockade in Cancer Immunotherapy—ScienceDirect. Cell Rep. 2019, 27, 3315–3330. [Google Scholar] [CrossRef]
- Zhang, R.R.; Wang, L.M.; Shen, J.J. Overexpression of miR-32 inhibits the proliferation and metastasis of ovarian cancer cells by targeting BTLA. Eur. Rev. Med. Pharmacol. 2020, 24, 4671–4678. [Google Scholar]
- Feng, X.Y.; Wen, X.Z.; Tan, X.J.; Hou, J.H.; Ding, Y.; Wang, K.F.; Dong, J.; Zhou, Z.W.; Chen, Y.B.; Zhang, X.S. Ectopic expression of B and T lymphocyte attenuator in gastric cancer: A potential independent prognostic factor in patients with gastric cancer. Mol. Med. Rep. 2015, 11, 658–664. [Google Scholar] [CrossRef]
- Lan, X.; Li, S.; Gao, H.; Nanding, A.; Quan, L.; Yang, C.; Ding, S.; Xue, Y. Increased BTLA and HVEM in gastric cancer are associated with progression and poor prognosis. OncoTargets Ther. 2017, 10, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, Z.; Cui, G.; Yu, L.; Zhang, X. BTLA Expression in Stage I–III Non–Small-Cell Lung Cancer and Its Correlation with PD-1/PD-L1 and Clinical Outcomes. OncoTargets Ther. 2020, 13, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Ren, S.; Tian, Q.; Amar, N.; Yu, H.; Rivard, C.J.; Caldwell, C.; Ng, T.L.; Tu, M.; Liu, Y.; Gao, D. The immune checkpoint, HVEM may contribute to immune escape in non-small cell lung cancer lacking PD-L1 expression. Lung Cancer 2018, 125, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Boice, M.; Salloum, D.; Mourcin, F.; Sanghvi, V.; Wendel, H.G. Loss of the HVEM Tumor Suppressor in Lymphoma and Restoration by Modified CAR-T Cells. Cell 2016, 167, 405–418. [Google Scholar] [CrossRef] [PubMed]
- Mintz, M.A.; Felce, J.H.; Chou, M.Y.; Mayya, V.; Cyster, J.G. The HVEM-BTLA Axis Restrains T Cell Help to Germinal Center B Cells and Functions as a Cell-Extrinsic Suppressor in Lymphomagenesis. Immunity 2019, 51, 310–323.e7. [Google Scholar] [CrossRef]
- Malissen, N.; Macagno, N.; Granjeaud, S.; Granier, C.; Moutardier, V.; Gaudy-Marqueste, C.; Habel, N.; Mandavit, M.; Guillot, B.; Pasero, C.; et al. HVEM has a broader expression than PD-L1 and constitutes a negative prognostic marker and potential treatment target for melanoma. Oncoimmunology 2019, 8, e1665976. [Google Scholar] [CrossRef]
- Tang, M.; Cao, X.; Li, Y.; Li, G.Q.; He, Q.H.; Li, S.J.; Chen, J.; Xu, G.L.; Zhang, K.Q. High expression of herpes virus entry mediator is associated with poor prognosis in clear cell renal cell carcinoma. Am. J. Cancer Res. 2019, 9, 975–987. [Google Scholar]
- Fang, Y.; Ye, L.; Zhang, T.; He, Q.Z.; Zhu, J.L. High expression of herpesvirus entry mediator (HVEM) in ovarian serous adenocarcinoma tissue. J. BUON 2017, 22, 80–86. [Google Scholar]
- Kaur, J.; Bachhawat, A.K. A modified Western blot protocol. for enhanced sensitivity in the detection of a membrane protein. Anal. Biochem. 2009, 384, 348–349. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–410. [Google Scholar] [CrossRef]
- Gu, Y.; Niu, S.; Wang, Y.; Duan, L.; Pan, Y.; Tong, Z.; Zhang, X.; Yang, Z.; Peng, B.; Wang, X.; et al. DMDRMR-Mediated Regulation of m(6)A-Modified CDK4 by m(6)A Reader IGF2BP3 Drives ccRCC Progression. Cancer Res. 2021, 81, 923–934. [Google Scholar] [CrossRef] [PubMed]
- Consortium, E.P. An integrated encyclopedia of DNA elements in the human genome. Nature 2012, 489, 57–74. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wen, Y.; Liu, S.; Duan, L.; Liu, T.; Tong, Z.; Wang, Z.; Gu, Y.; Gao, S. LCDR regulates the integrity of lysosomal membrane by hnRNP K-stabilized LAPTM5 transcript and promotes cell survival. Proc. Natl. Acad. Sci. USA 2022, 119, e2110428119. [Google Scholar] [CrossRef] [PubMed]
- Shalem, O.; Sanjana, N.E.; Hartenian, E.; Shi, X.; Scott, D.A.; Mikkelsen, T.S.; Heckl, D.; Ebert, B.L.; Root, D.E.; Doench, J.G. Genome-Scale CRISPR-Cas9 Knockout Screening in Human Cells. Science 2014, 343, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Barretina, J.; Caponigro, G.; Stransky, N.; Venkatesan, K.; Al, E. The Cancer Cell Line Encyclopedia enables predictive modelling of anticancer drug sensitivity. Nature 2012, 483, 603–610. [Google Scholar] [CrossRef] [PubMed]
- Birney, E.; Stamatoyannopoulos, J.A.; Dutta, A.; Guigo, T.R.; Margulies, E.H.; Weng, Z.; Snyder, M.; Dermitzakis, E.T. Identification and analysis of functional elements in 1% of the human genome by the ENCODE pilot project. Nature 2007, 447, 799–816. [Google Scholar] [PubMed]
- Ye, R.; Cao, C.; Xue, Y. Enhancer RNA: Biogenesis, function, and regulation. Essays Biochem. 2020, 64, 883–894. [Google Scholar]
- Gavrieli, M.; Murphy, K.M. Association of Grb-2 and PI3K p85 with phosphotyrosile peptides derived from BTLA. Biochem. Biophys. Res. Commun. 2006, 345, 1440–1445. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Nielsen, F.M.L.; Riis, S.E.; Andersen, J.I.; Lesage, R.; Fink, T.; Pennisi, C.P.; Zachar, V. Discrete adipose-derived stem cell subpopulations may display differential functionality after in vitro expansion despite convergence to a common phenotype distribution. Stem Cell Res. Ther. 2016, 7, 177. [Google Scholar] [CrossRef]
- Cai, G.; Freeman, G.J. The CD160, BTLA, LIGHT/HVEM pathway: A bidirectional switch regulating T-cell activation. Immunol. Rev. 2010, 229, 244–258. [Google Scholar] [CrossRef] [PubMed]
- Compaan, D.M.; Gonzalez, L.C.; Tom, I.; Loyet, K.M.; Eaton, D.; Hymowitz, S.G. Attenuating lymphocyte activity: The crystal structure of the BTLA-HVEM complex. J. Biol. Chem. 2005, 280, 39553–39561. [Google Scholar] [CrossRef]
- Cheung, T.; Humphreys, I.; Potter, K.; Norris, P.; Shumway, H.; Tran, B.; Patterson, G.; Jean-Jacques, R.; Yoon, M.; Spear, P. Evolutionarily divergent herpesviruses modulate T cell activation by targeting the herpesvirus entry mediator cosignaling pathway. Proc. Natl. Acad. Sci. USA 2005, 102, 13218–13223. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, N.; Gavrieli, M.; Sedy, J.R.; Yang, J.; Fallarino, F.; Loftin, S.K.; Hurchla, M.A.; Zimmerman, N.; Sim, J.; Zang, X. BTLA is a lymphocyte inhibitory receptor with similarities to CTLA-4 and PD-1. Nat. Immunol. 2003, 4, 670–679. [Google Scholar] [CrossRef] [PubMed]
- Gavrieli, M.; Sedy, J.; Nelson, C.; Murphy, K.M. BTLA and HVEM cross talk regulates inhibition and costimulation. Adv. Immunol. 2006, 92, 157–185. [Google Scholar]
- Contardi, E.; Palmisano, G.L.; Tazzari, P.L.; Martelli, A.M.; Fala, F.; Fabbi, M.; Kato, T.; Lucarelli, E.; Donati, D.; Polito, L.; et al. CTLA-4 is constitutively expressed on tumor cells and can trigger apoptosis upon ligand interaction. Int. J. Cancer 2005, 117, 538–550. [Google Scholar] [CrossRef]
- Zhang, H.; Dutta, P.; Liu, J.; Sabri, N.; Song, Y.; Li, W.X.; Li, J. Tumour cell-intrinsic CTLA4 regulates PD-L1 expression in non-small cell lung cancer. J. Cell. Mol. Med. 2019, 23, 535–542. [Google Scholar] [CrossRef]
- Haymaker, C.L.; Wu, R.C.; Ritthipichai, K.; Bernatchez, C.; Forget, M.A.; Chen, J.Q.; Liu, H.; Wang, E.; Marincola, F.; Hwu, P.; et al. BTLA marks a less-differentiated tumor-infiltrating lymphocyte subset in melanoma with enhanced survival properties. OncoImmunology 2015, 4, e1014246. [Google Scholar] [CrossRef]
- Demerle, C.; Gorvel, L.; Olive, D. BTLA-HVEM Couple in Health and Diseases: Insights for Immunotherapy in Lung Cancer. Front. Oncol. 2021, 11, 682007. [Google Scholar] [CrossRef]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, T.-Y.; Liu, Y.-J.; Yan, H.; Xi, Y.-B.; Duan, L.-Q.; Wang, Y.; Zhang, T.-T.; Gu, Y.-M.; Wang, X.-D.; Wu, C.-X.; et al. Tumor Cell-Intrinsic BTLA Receptor Inhibits the Proliferation of Tumor Cells via ERK1/2. Cells 2022, 11, 4021. https://doi.org/10.3390/cells11244021
Cheng T-Y, Liu Y-J, Yan H, Xi Y-B, Duan L-Q, Wang Y, Zhang T-T, Gu Y-M, Wang X-D, Wu C-X, et al. Tumor Cell-Intrinsic BTLA Receptor Inhibits the Proliferation of Tumor Cells via ERK1/2. Cells. 2022; 11(24):4021. https://doi.org/10.3390/cells11244021
Chicago/Turabian StyleCheng, Tian-You, Ya-Juan Liu, Hong Yan, Yi-Bo Xi, Li-Qiang Duan, Yang Wang, Tian-Tian Zhang, Yin-Min Gu, Xiao-Dong Wang, Chang-Xin Wu, and et al. 2022. "Tumor Cell-Intrinsic BTLA Receptor Inhibits the Proliferation of Tumor Cells via ERK1/2" Cells 11, no. 24: 4021. https://doi.org/10.3390/cells11244021
APA StyleCheng, T.-Y., Liu, Y.-J., Yan, H., Xi, Y.-B., Duan, L.-Q., Wang, Y., Zhang, T.-T., Gu, Y.-M., Wang, X.-D., Wu, C.-X., & Gao, S. (2022). Tumor Cell-Intrinsic BTLA Receptor Inhibits the Proliferation of Tumor Cells via ERK1/2. Cells, 11(24), 4021. https://doi.org/10.3390/cells11244021





