Harnessing the Potential of Chimeric Antigen Receptor T-Cell Therapy for the Treatment of T-Cell Malignancies: A Dare or Double Dare?
Abstract
1. Background on T-Cell Malignancies
1.1. Historical Challenges
1.2. Immunotherapy in T-Cell Malignancies: About Time
1.3. Fundamentals of CAR-T Structure and Mechanisms of Action
1.4. Evolution of CARs Design
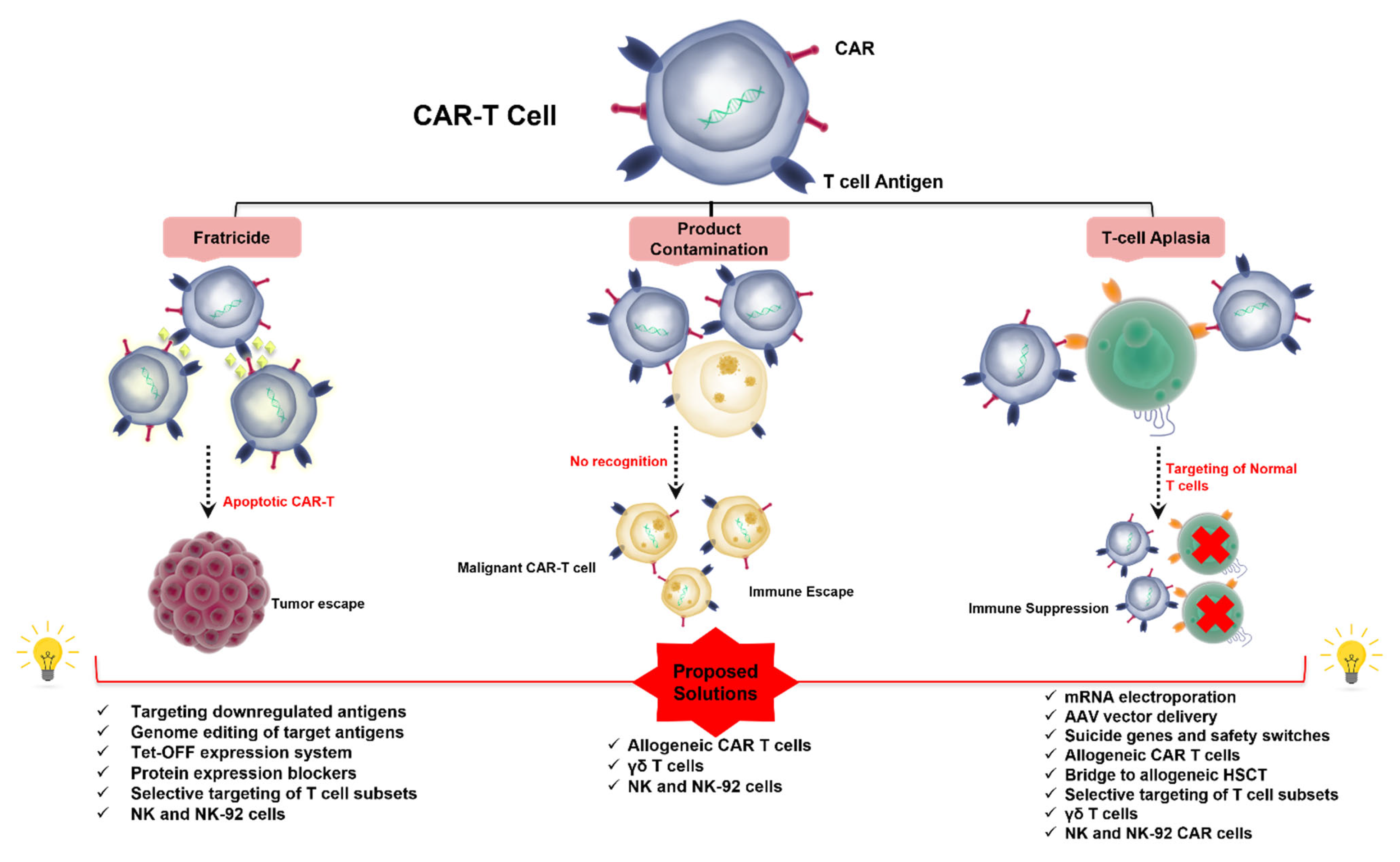
1.5. Challenges to CAR T-Cell Therapy in T-Cell Malignancies (Figure 1)
2. Addressing Fratricide
2.1. Targeting Pan-T Antigens
2.1.1. CD3
2.1.2. CD5
2.1.3. CD7
2.2. Targeting Antigens with Restricted Expression
2.2.1. CD1a
2.2.2. CD4
2.2.3. CD30
2.2.4. CCR4
2.2.5. CCR9
2.2.6. CD37
2.2.7. TRBC1 and TRBC2
3. Addressing T-Cell Aplasia
3.1. CAR-T Product Contamination with Malignant T Cells
3.2. Combining ICPIs and CAR-T Products in T-Cell Malignancies: An Ongoing Dilemma
4. Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Sehn, L.H.; Soulier, J. Introduction to the review series on T-cell malignancies. Blood J. Am. Soc. Hematol. 2017, 129, 1059–1060. [Google Scholar] [CrossRef]
- Swerdlow, S.H.; Campo, E.; Pileri, S.A.; Harris, N.L.; Stein, H.; Siebert, R.; Advani, R.; Ghielmini, M.; Salles, G.A.; Zelenetz, A.D.; et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood J. Am. Soc. Hematol. 2016, 127, 2375–2390. [Google Scholar] [CrossRef] [PubMed]
- Willemze, R.; Cerroni, L.; Kempf, W.; Berti, E.; Facchetti, F.; Swerdlow, S.H.; Jaffe, E.S. The 2018 update of the WHO-EORTC classification for primary cutaneous lymphomas. Blood J. Am. Soc. Hematol. 2019, 133, 1703–1714. [Google Scholar] [CrossRef] [PubMed]
- Tsukasaki, K.; Utsunomiya, A.; Fukuda, H.; Shibata, T.; Fukushima, T.; Takatsuka, Y.; Ikeda, S.; Masuda, M.; Nagoshi, H.; Ueda, R.; et al. VCAP-AMP-VECP Compared with Biweekly CHOP for Adult T-Cell Leukemia-Lymphoma: Japan Clinical Oncology Group Study JCOG9801. J. Clin. Oncol. 2007, 25, 5458–5464. [Google Scholar] [CrossRef]
- Maeda, Y.; Nishimori, H.; Yoshida, I.; Hiramatsu, Y.; Uno, M.; Masaki, Y.; Sunami, K.; Masunari, T.; Nawa, Y.; Yamane, H.; et al. Dose-adjusted EPOCH chemotherapy for untreated peripheral T-cell lymphomas: A multicenter phase II trial of West-JHOG PTCL0707. Haematologica 2017, 102, 2097–2103. [Google Scholar] [CrossRef]
- Wilcox, R.A. Optimising initial treatment for peripheral T-cell lymphoma: A tough nut to CHOP. Lancet Haematol. 2018, 5, e182–e183. [Google Scholar] [CrossRef]
- Horwitz, S.; O’Connor, O.A.; Pro, B.; Illidge, T.; Fanale, M.; Advani, R.; Bartlett, N.; Christensen, J.H.; Morschhauser, F.; Domenech, E.D.; et al. Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): A global, double-blind, randomised, phase 3 trial. Lancet 2018, 393, 229–240. [Google Scholar] [CrossRef]
- Virmani, P.; Hwang, S.H.; Hastings, J.G.; Haverkos, B.M.; Kohnken, R.; Gru, A.A.; Mishra, A.; Fabbro, S.K.; Horwitz, S.M.; Porcu, P. Systemic therapy for cutaneous T-cell lymphoma: Who, when, what, and why? Expert Rev. Hematol. 2016, 10, 111–121. [Google Scholar] [CrossRef]
- Mak, V.; Hamm, J.; Chhanabhai, M.; Shenkier, T.; Klasa, R.; Sehn, L.H.; Villa, D.; Gascoyne, R.D.; Connors, J.M.; Savage, K.J. Survival of Patients with Peripheral T-Cell Lymphoma after First Relapse or Progression: Spectrum of Disease and Rare Long-Term Survivors. J. Clin. Oncol. 2013, 31, 1970–1976. [Google Scholar] [CrossRef]
- Ellin, F.; Landström, J.; Jerkeman, M.; Relander, T. Real-world data on prognostic factors and treatment in peripheral T-cell lymphomas: A study from the Swedish Lymphoma Registry. Blood J. Am. Soc. Hematol. 2014, 124, 1570–1577. [Google Scholar] [CrossRef]
- Selberg, L.; Stadtherr, P.; Dietrich, S.; Tran, T.H.; Luft, T.; Hegenbart, U.; Bondong, A.; Meissner, J.; Liebers, N.; Schmitt, M.; et al. The impact of allogeneic hematopoietic cell transplantation on the mortality of poor-risk non-Hodgkin lymphoma: An intent-to-transplant analysis. Bone Marrow Transplant. 2020, 56, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Kameda, K.; Kako, S.; Kim, S.-W.; Usui, Y.; Kato, K.; Fukuda, T.; Uchida, N.; Kobayashi, H.; Wakayama, T.; Sakaida, E.; et al. Autologous or allogeneic hematopoietic cell transplantation for relapsed or refractory PTCL-NOS or AITL. Leukemia 2022, 36, 1361–1370. [Google Scholar] [CrossRef] [PubMed]
- Maus, M.V.; Grupp, S.A.; Porter, D.L.; June, C.H. Antibody-modified T cells: CARs take the front seat for hematologic malignancies. Blood J. Am. Soc. Hematol. 2014, 123, 2625–2635. [Google Scholar] [CrossRef]
- Brentjens, R.J.; Davila, M.L.; Riviere, I.; Park, J.; Wang, X.; Cowell, L.G.; Bartido, S.; Stefanski, J.; Taylor, C.; Olszewska, M.; et al. CD19-Targeted T Cells Rapidly Induce Molecular Remissions in Adults with Chemotherapy-Refractory Acute Lymphoblastic Leukemia. Sci. Transl. Med. 2013, 5, 177ra38. [Google Scholar] [CrossRef] [PubMed]
- Maude, S.L.; Frey, N.; Shaw, P.A.; Aplenc, R.; Barrett, D.M.; Bunin, N.J.; Chew, A.; Gonzalez, V.E.; Zheng, Z.; Lacey, S.F.; et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N. Engl. J. Med. 2014, 371, 1507–1517. [Google Scholar] [CrossRef] [PubMed]
- Turtle, C.J.; Hanafi, L.-A.; Berger, C.; Gooley, T.A.; Cherian, S.; Hudecek, M.; Sommermeyer, D.; Melville, K.; Pender, B.; Budiarto, T.M.; et al. CD19 CAR–T cells of defined CD4+:CD8+ composition in adult B cell ALL patients. J. Clin. Investig. 2016, 126, 2123–2138. [Google Scholar] [CrossRef]
- Mullard, A. FDA approves fourth CAR-T cell therapy. Nat. Rev. Drug Discov. 2021, 20, 166. [Google Scholar] [CrossRef]
- Voelker, R. CAR-T Therapy Is Approved for Mantle Cell Lymphoma. JAMA 2020, 324, 832. [Google Scholar] [CrossRef]
- Iqbal, J.; Wright, G.; Wang, C.; Rosenwald, A.; Gascoyne, R.D.; Weisenburger, D.D.; Greiner, T.C.; Smith, L.; Guo, S.; Wilcox, R.A.; et al. Gene expression signatures delineate biological and prognostic subgroups in peripheral T-cell lymphoma. Blood J. Am. Soc. Hematol. 2014, 123, 2915–2923. [Google Scholar] [CrossRef]
- Gonzalez, B.R.; Zain, J.; Rosen, S.T.; Querfeld, C. Tumor microenvironment in mycosis fungoides and Sézary syndrome. Curr. Opin. Oncol. 2016, 28, 88–96. [Google Scholar] [CrossRef]
- Querfeld, C.; Leung, S.; Myskowski, P.L.; Curran, S.A.; Goldman, D.A.; Heller, G.; Wu, X.; Kil, S.H.; Sharma, S.; Finn, K.J.; et al. Primary T Cells from Cutaneous T-cell Lymphoma Skin Explants Display an Exhausted Immune Checkpoint ProfileT-cell Exhaustion in Cutaneous T-cell Lymphoma. Cancer Immunol. Res. 2018, 6, 900–909. [Google Scholar] [CrossRef] [PubMed]
- Barrett, D.M.; Grupp, S.A.; June, C.H. Chimeric Antigen Receptor– and TCR-Modified T Cells Enter Main Street and Wall Street. J. Immunol. 2015, 195, 755–761. [Google Scholar] [CrossRef] [PubMed]
- Abken, H. Building on Synthetic Immunology and T Cell Engineering: A Brief Journey Through the History of Chimeric Antigen Receptors. Hum. Gene Ther. 2021, 32, 1011–1028. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.J.; Dougan, M.; Jailkhani, N.; Ingram, J.; Fang, T.; Kummer, L.; Momin, N.; Pishesha, N.; Rickelt, S.; Hynes, R.O.; et al. Nanobody-based CAR T cells that target the tumor microenvironment inhibit the growth of solid tumors in immunocompetent mice. Proc. Natl. Acad. Sci. USA 2019, 116, 7624–7631. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Starr, R.; Chang, W.-C.; Aguilar, B.; Alizadeh, D.; Wright, S.L.; Yang, X.; Brito, A.; Sarkissian, A.; Ostberg, J.R.; et al. Chlorotoxin-directed CAR T cells for specific and effective targeting of glioblastoma. Sci. Transl. Med. 2020, 12, eaaw2672. [Google Scholar] [CrossRef] [PubMed]
- Hudecek, M.; Sommermeyer, D.; Kosasih, P.L.; Silva-Benedict, A.; Liu, L.; Rader, C.; Jensen, M.C.; Riddell, S.R. The Nonsignaling Extracellular Spacer Domain of Chimeric Antigen Receptors Is Decisive for In Vivo Antitumor Activity. Cancer Immunol. Res. 2015, 3, 125–135. [Google Scholar] [CrossRef]
- Zhao, Z.; Condomines, M.; Van Der Stegen, S.J.C.; Perna, F.; Kloss, C.C.; Gunset, G.; Plotkin, J.; Sadelain, M. Structural Design of Engineered Costimulation Determines Tumor Rejection Kinetics and Persistence of CAR T Cells. Cancer Cell 2015, 28, 415–428. [Google Scholar] [CrossRef]
- Foster, J.B.; Choudhari, N.; Perazzelli, J.; Storm, J.; Hofmann, T.J.; Jain, P.; Storm, P.B.; Pardi, N.; Weissman, D.; Waanders, A.J.; et al. Purification of mRNA Encoding Chimeric Antigen Receptor Is Critical for Generation of a Robust T-Cell Response. Hum. Gene Ther. 2019, 30, 168–178. [Google Scholar] [CrossRef]
- Lana, M.G.; Strauss, B.E. Production of Lentivirus for the Establishment of CAR-T Cells. In Chimeric Antigen Receptor T Cells; Springer: Berlin/Heidelberg, Germany, 2019; Volume 2086, pp. 61–67. [Google Scholar] [CrossRef]
- A Johnson, L.; June, C.H. Driving gene-engineered T cell immunotherapy of cancer. Cell Res. 2016, 27, 38–58. [Google Scholar] [CrossRef]
- Liadi, I.; Singh, H.; Romain, G.; Rey-Villamizar, N.; Merouane, A.; Adolacion, J.R.T.; Kebriaei, P.; Huls, H.; Qiu, P.; Roysam, B.; et al. Individual Motile CD4+ T Cells Can Participate in Efficient Multikilling through Conjugation to Multiple Tumor CellsMotile Multikiller CD4+ T Cells. Cancer Immunol. Res. 2015, 3, 473–482. [Google Scholar] [CrossRef]
- Tokarew, N.; Ogonek, J.; Endres, S.; Von Bergwelt-Baildon, M.; Kobold, S. Teaching an old dog new tricks: Next-generation CAR T cells. Br. J. Cancer 2018, 120, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Duell, J.; Lurati, S.; Dittrich, M.; Bedke, T.; Pule, M.; Einsele, H.; Rossig, C.; Topp, M.S. First Generation Chimeric Antigen Receptor Display Functional Defects In Key Signal Pathways Upon Antigen Stimulation. Blood 2010, 116, 2088. [Google Scholar] [CrossRef]
- Yeku, O.O.; Brentjens, R.J. Armored CAR T-cells: Utilizing cytokines and pro-inflammatory ligands to enhance CAR T-cell anti-tumour efficacy. Biochem. Soc. Trans. 2016, 44, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Brentjens, R.J. Are all chimeric antigen receptors created equal? J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2015, 33, 651–653. [Google Scholar] [CrossRef]
- Lock, D.; Mockel-Tenbrinck, N.; Drechsel, K.; Barth, C.; Mauer, D.; Schaser, T.; Kolbe, C.; Al Rawashdeh, W.; Brauner, J.; Hardt, O.; et al. Automated Manufacturing of Potent CD20-Directed Chimeric Antigen Receptor T Cells for Clinical Use. Hum. Gene Ther. 2017, 28, 914–925. [Google Scholar] [CrossRef]
- Chmielewski, M.; Abken, H. TRUCKs: The fourth generation of CARs. Expert Opin. Biol. Ther. 2015, 15, 1145–1154. [Google Scholar] [CrossRef]
- Kagoya, Y.; Tanaka, S.; Guo, T.; Anczurowski, M.; Wang, C.-H.; Saso, K.; Butler, M.O.; Minden, M.D.; Hirano, N. A novel chimeric antigen receptor containing a JAK–STAT signaling domain mediates superior antitumor effects. Nat. Med. 2018, 24, 352–359. [Google Scholar] [CrossRef]
- Savoldo, B.; Ramos, C.A.; Liu, E.; Mims, M.P.; Keating, M.J.; Carrum, G.; Kamble, R.T.; Bollard, C.M.; Gee, A.P.; Mei, Z.; et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor–modified T cells in lymphoma patients. J. Clin. Investig. 2011, 121, 1822–1826. [Google Scholar] [CrossRef]
- Wang, X.; Rivière, I. Clinical manufacturing of CAR T cells: Foundation of a promising therapy. Mol. Ther.-Oncolytics 2016, 3, 16015. [Google Scholar] [CrossRef]
- Berland, R.; Wortis, H.H. Origins and Functions of B-1 Cells with Notes on the Role of CD5. Annu. Rev. Immunol. 2002, 20, 253–300. [Google Scholar] [CrossRef]
- Birnbaum, M.E.; Berry, R.; Hsiao, Y.S.; Chen, Z.; Shingu-Vazquez, M.A.; Yu, X.; Waghray, D.; Fischer, S.; McCluskey, J.; Rossjohn, J.; et al. Molecular architecture of the αβ T cell receptor–CD3 complex. Proc. Natl. Acad. Sci. USA 2014, 111, 17576–17581. [Google Scholar] [CrossRef] [PubMed]
- Gomes-Silva, D.; Srinivasan, M.; Sharma, S.; Lee, C.; Wagner, D.L.; Davis, T.H.; Rouce, R.H.; Bao, G.; Brenner, M.K.; Mamonkin, M. CD7-edited T cells expressing a CD7-specific CAR for the therapy of T-cell malignancies. Blood J. Am. Soc. Hematol. 2017, 130, 285–296. [Google Scholar] [CrossRef] [PubMed]
- Leonard, W.J. Cytokines and immunodeficiency diseases. Nat. Rev. Immunol. 2001, 1, 200–208. [Google Scholar] [CrossRef] [PubMed]
- Mamonkin, M.; Rouce, R.H.; Tashiro, H.; Brenner, M.K. A T-cell–directed chimeric antigen receptor for the selective treatment of T-cell malignancies. Blood J. Am. Soc. Hematol. 2015, 126, 983–992. [Google Scholar] [CrossRef]
- Cooper, M.L.; Choi, J.; Staser, K.; Ritchey, J.K.; Devenport, J.M.; Eckardt, K.; Rettig, M.P.; Wang, B.; Eissenberg, L.G.; Ghobadi, A.; et al. An “off-the-shelf” fratricide-resistant CAR-T for the treatment of T cell hematologic malignancies. Leukemia 2018, 32, 1970–1983. [Google Scholar] [CrossRef]
- Fathi, E.; Farahzadi, R.; Sheervalilou, R.; Sanaat, Z.; Vietor, I. A general view of CD33+ leukemic stem cells and CAR-T cells as interesting targets in acute myeloblatsic leukemia therapy. Blood Res. 2020, 55, 10–16. [Google Scholar] [CrossRef]
- Frankel, A.E.; Zuckero, S.L.; A Mankin, A.; Grable, M.; Mitchell, K.; Lee, Y.J.; Neville, D.M.; Woo, J.H. Anti-CD3 Recombinant Diphtheria Immunotoxin Therapy of Cutaneous T Cell Lymphoma. Curr. Drug Targets 2009, 10, 104–109. [Google Scholar] [CrossRef]
- Frankel, A.E.; Woo, J.H.; Ahn, C.; Foss, F.M.; Duvic, M.; Neville, P.H.; Neville, D.M. Resimmune, an anti-CD3ε recombinant immunotoxin, induces durable remissions in patients with cutaneous T-cell lymphoma. Haematologica 2015, 100, 794–800. [Google Scholar] [CrossRef]
- Chen, K.H.; Wada, M.; Firor, A.E.; Pinz, K.G.; Jares, A.; Liu, H.; Salman, H.; Golightly, M.; Lan, F.; Jiang, X.; et al. Novel anti-CD3 chimeric antigen receptor targeting of aggressive T cell malignancies. Oncotarget 2016, 7, 56219–56232. [Google Scholar] [CrossRef]
- Rasaiyaah, J.; Georgiadis, C.; Preece, R.; Mock, U.; Qasim, W. TCRαβ/CD3 disruption enables CD3-specific antileukemic T cell immunotherapy. JCI Insight 2018, 3, e99442. [Google Scholar] [CrossRef]
- Suck, G.; Odendahl, M.; Nowakowska, P.; Seidl, C.; Wels, W.S.; Klingemann, H.G.; Tonn, T. NK-92: An ‘off-the-shelf therapeutic’ for adoptive natural killer cell-based cancer immunotherapy. Cancer Immunol. Immunother. 2016, 65, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Klingemann, H. Are natural killer cells superior CAR drivers? Oncoimmunology 2014, 3, e28147. [Google Scholar] [CrossRef] [PubMed]
- Glienke, W.; Esser, R.; Priesner, C.; Suerth, J.D.; Schambach, A.; Wels, W.S.; Grez, M.; Kloess, S.; Arseniev, L.; Koehl, U. Advantages and applications of CAR-expressing natural killer cells. Front. Pharmacol. 2015, 6, 21. [Google Scholar] [CrossRef] [PubMed]
- Campana, D.; van Dongen, J.J.; Mehta, A.; Coustan-Smith, E.; Wolvers-Tettero, I.L.; Ganeshaguru, K.; Janossy, G. Stages of T-cell receptor protein expression in T-cell acute lymphoblastic leukemia. Blood 1991, 77, 1546–1554. [Google Scholar] [CrossRef]
- Patel, J.L.; Smith, L.M.; Anderson, J.; Abromowitch, M.; Campana, D.; Jacobsen, J.; Lones, M.A.; Gross, T.G.; Cairo, M.S.; Perkins, S.L. The immunophenotype of T-lymphoblastic lymphoma in children and adolescents: A C hildren’s O ncology G roup report. Br. J. Haematol. 2012, 159, 454–461. [Google Scholar] [CrossRef]
- Perez-Villar, J.J.; Whitney, G.S.; Bowen, M.A.; Hewgill, D.H.; Aruffo, A.A.; Kanner, S.B. CD5 Negatively Regulates the T-Cell Antigen Receptor Signal Transduction Pathway: Involvement of SH2-Containing Phosphotyrosine Phosphatase SHP-1. Mol. Cell. Biol. 1999, 19, 2903–2912. [Google Scholar] [CrossRef]
- Gary, H.; Sainz-Perez, A.; Marteau, J.-B.; Marfaing-Koka, A.; Delic, J.; Merle-Béral, H.; Galanaud, P.; Dalloul, A. Natural Phosphorylation of CD5 in Chronic Lymphocytic Leukemia B Cells and Analysis of CD5-Regulated Genes in a B Cell Line Suggest a Role for CD5 in Malignant Phenotype. J. Immunol. 2007, 179, 4335–4344. [Google Scholar] [CrossRef]
- LeMaistre, C.F.; Rosen, S.; Frankel, A.; Kornfeld, S.; Saria, E.; Meneghetti, C.; Drajesk, J.; Fishwild, D.; Scannon, P.; Byers, V. Phase I trial of H65-RTA immunoconjugate in patients with cutaneous T-cell lymphoma. Blood 1991, 78, 1173–1182. [Google Scholar] [CrossRef]
- Chen, K.H.; Wada, M.; Pinz, K.G.; Liu, H.; Lin, K.-W.; Jares, A.; Firor, A.E.; Shuai, X.; Salman, H.; Golightly, M.; et al. Preclinical targeting of aggressive T-cell malignancies using anti-CD5 chimeric antigen receptor. Leukemia 2017, 31, 2151–2160. [Google Scholar] [CrossRef]
- Raikar, S.S.; Fleischer, L.C.; Moot, R.; Fedanov, A.; Paik, N.Y.; Knight, K.A.; Doering, C.B.; Spencer, H.T. Development of chimeric antigen receptors targeting T-cell malignancies using two structurally different anti-CD5 antigen binding domains in NK and CRISPR-edited T cell lines. Oncoimmunology 2017, 7, e1407898. [Google Scholar] [CrossRef]
- Mamonkin, M.; Mukherjee, M.; Srinivasan, M.; Sharma, S.; Gomes-Silva, D.; Mo, F.; Krenciute, G.; Orange, J.S.; Brenner, M.K. Reversible Transgene Expression Reduces Fratricide and Permits 4-1BB Costimulation of CAR T Cells Directed to T-cell MalignanciesRegulated CAR Expression Minimizes Tonic Signaling. Cancer Immunol. Res. 2018, 6, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Liu, Q.; Zhong, M.; Wang, Z.; Chen, Z.; Zhang, Y.; Xing, H.; Tian, Z.; Tang, K.; Liao, X.; et al. 2B4 costimulatory domain enhancing cytotoxic ability of anti-CD5 chimeric antigen receptor engineered natural killer cells against T cell malignancies. J. Hematol. Oncol. 2019, 12, 1–13. [Google Scholar] [CrossRef]
- Rouce, R.H.; Hill, L.C.; Smith, T.S.; Yang, L.; Boriskie, B.; Srinivasan, M.; Zhang, H.; Perconti, S.; Mehta, B.; Dakhova, O.; et al. Early Signals of Anti-Tumor Efficacy and Safety with Autologous CD5.CAR T-Cells in Patients with Refractory/Relapsed T-Cell Lymphoma. Blood 2021, 138, 654. [Google Scholar] [CrossRef]
- Kaufman, R.E.; Haynes, B.F.; Sempowski, G.D.; Lee, D.M. Structure and function of the CD7 molecule. Crit. Rev. Immunol. 1999, 19, 18. [Google Scholar] [CrossRef]
- Qasim, W.; Zhan, H.; Samarasinghe, S.; Adams, S.; Amrolia, P.; Stafford, S.; Butler, K.; Rivat, C.; Wright, G.; Somana, K.; et al. Molecular remission of infant B-ALL after infusion of universal TALEN gene-edited CAR T cells. Sci. Transl. Med. 2017, 9, eaaj2013. [Google Scholar] [CrossRef]
- Jacobson, C.A.; Herrera, A.F.; E Budde, L.; DeAngelo, D.J.; Heery, C.; Stein, A.; Jain, M.D.; Shah, B. Initial Findings of the Phase 1 Trial of PBCAR0191, a CD19 Targeted Allogeneic CAR-T Cell Therapy. Blood 2019, 134, 4107. [Google Scholar] [CrossRef]
- Png, Y.T.; Vinanica, N.; Kamiya, T.; Shimasaki, N.; Coustan-Smith, E.; Campana, D. Blockade of CD7 expression in T cells for effective chimeric antigen receptor targeting of T-cell malignancies. Blood Adv. 2017, 1, 2348–2360. [Google Scholar] [CrossRef]
- You, F.; Wang, Y.; Jiang, L.; Zhu, X.; Chen, D.; Yuan, L.; An, G.; Meng, H.; Yang, L. A novel CD7 chimeric antigen receptor-modified NK-92MI cell line targeting T-cell acute lymphoblastic leukemia. Am. J. Cancer Res. 2019, 9, 64–78. [Google Scholar]
- Pan, J.; Tan, Y.; Wang, G.; Deng, B.; Ling, Z.; Song, W.; Seery, S.; Zhang, Y.; Peng, S.; Xu, J.; et al. Donor-Derived CD7 Chimeric Antigen Receptor T Cells for T-Cell Acute Lymphoblastic Leukemia: First-in-Human, Phase I Trial. J. Clin. Oncol. 2021, 39, 3340–3351. [Google Scholar] [CrossRef]
- Burger, R.; E Hansen-Hagge, T.; Drexler, H.G.; Gramatzki, M. Heterogeneity of T-acute lymphoblastic leukemia (T-ALL) cell lines: Suggestion for classification by immunophenotype and T-cell receptor studies. Leuk. Res. 1998, 23, 19–27. [Google Scholar] [CrossRef]
- Niehues, T.; Kapaun, P.; Harms, D.; Burdach, S.; Kramm, C.; Körholz, D.; Janka-Schaub, G.; Göbel, U. A classification based on T cell selection-related phenotypes identifies a subgroup of childhood T-ALL with favorable outcome in the COALL studies. Leukemia 1999, 13, 614–617. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, D.; Baroni, M.L.; Gutiérrez-Agüera, F.; Roca-Ho, H.; Blanch-Lombarte, O.; González-García, S.; Torrebadell, M.; Junca, J.; Ramírez-Orellana, M.; Velasco-Hernández, T.; et al. Fratricide-resistant CD1a-specific CAR T cells for the treatment of cortical T-cell acute lymphoblastic leukemia. Blood J. Am. Soc. Hematol. 2019, 133, 2291–2304. [Google Scholar] [CrossRef]
- Knox, S.; Hoppe, R.; Maloney, D.; Gibbs, I.; Fowler, S.; Marquez, C.; Cornbleet, P.; Levy, R. Treatment of cutaneous T-cell lymphoma with chimeric anti-CD4 monoclonal antibody. Blood 1996, 87, 893–899. [Google Scholar] [CrossRef]
- Hagberg, H.; Pettersson, M.; Bjerner, T.; Enblad, G. Treatment of a patient with a nodal peripheral T-cell lymphoma (angioimmunoblastic T-Cell lymphoma) with a human monoclonal antibody against the CD4 antigen (HuMax-CD4). Med. Oncol. 2005, 22, 191–194. [Google Scholar] [CrossRef]
- Kim, Y.H.; Duvic, M.; Obitz, E.; Gniadecki, R.; Iversen, L.; Österborg, A.; Whittaker, S.; Illidge, T.M.; Schwarz, T.; Kaufmann, R.; et al. Clinical efficacy of zanolimumab (HuMax-CD4): Two phase 2 studies in refractory cutaneous T-cell lymphoma. Blood J. Am. Soc. Hematol. 2007, 109, 4655–4662. [Google Scholar] [CrossRef]
- D’Amore, F.; Radford, J.; Relander, T.; Jerkeman, M.; Tilly, H.; Österborg, A.; Morschhauser, F.; Gramatzki, M.; Dreyling, M.; Bang, B.; et al. Phase II trial of zanolimumab (HuMax-CD4) in relapsed or refractory non-cutaneous peripheral T cell lymphoma. Br. J. Haematol. 2010, 150, 565–573. [Google Scholar] [CrossRef]
- Pinz, K.; Liu, H.; Golightly, M.; Jares, A.; Lan, F.; Zieve, G.W.; Hagag, N.; Schuster, M.; Firor, A.; Jiang, X.; et al. Preclinical targeting of human T-cell malignancies using CD4-specific chimeric antigen receptor (CAR)-engineered T cells. Leukemia 2015, 30, 701–707. [Google Scholar] [CrossRef]
- Pinz, K.G.; Yakaboski, E.; Jares, A.; Liu, H.; Firor, A.E.; Chen, K.H.; Wada, M.; Salman, H.; Tse, W.; Hagag, N.; et al. Targeting T-cell malignancies using anti-CD4 CAR NK-92 cells. Oncotarget 2017, 8, 112783–112796. [Google Scholar] [CrossRef]
- Alcantara, M.; Tesio, M.; June, C.H.; Houot, R. CAR T-cells for T-cell malignancies: Challenges in distinguishing between therapeutic, normal, and neoplastic T-cells. Leukemia 2018, 32, 2307–2315. [Google Scholar] [CrossRef]
- Ma, G.; Shen, J.; Pinz, K.; Wada, M.; Park, J.; Kim, S.; Togano, T.; Tse, W. Targeting T Cell Malignancies Using CD4CAR T-Cells and Implementing a Natural Safety Switch. Stem Cell Rev. Rep. 2019, 15, 443–447. [Google Scholar] [CrossRef]
- Falini, B.; Pileri, S.; Pizzolo, G.; Durkop, H.; Flenghi, L.; Stirpe, F.; Martelli, M.; Stein, H. CD30 (Ki-1) molecule: A new cytokine receptor of the tumor necrosis factor receptor superfamily as a tool for diagnosis and immunotherapy. Blood 1995, 85, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Bossard, C.; Dobay, M.P.; Parrens, M.; Lamant, L.; Missiaglia, E.; Haioun, C.; Martin, A.; Fabiani, B.; Delarue, R.; Tournilhac, O.; et al. Immunohistochemistry as a valuable tool to assess CD30 expression in peripheral T-cell lymphomas: High correlation with mRNA levels. Blood J. Am. Soc. Hematol. 2014, 124, 2983–2986. [Google Scholar] [CrossRef]
- Sabattini, E.; Pizzi, M.; Tabanelli, V.; Baldin, P.; Sacchetti, C.S.; Agostinelli, C.; Zinzani, P.L.; Pileri, S.A. CD30 expression in peripheral T-cell lymphomas. Haematologica 2013, 98, e81–e82. [Google Scholar] [CrossRef] [PubMed]
- Zheng, W.; Medeiros, L.J.; Young, K.H.; Goswami, M.; Powers, L.; Kantarjian, H.H.; Thomas, D.A.; Cortes, J.; Wang, S.A. CD30 expression in acute lymphoblastic leukemia as assessed by flow cytometry analysis. Leuk. Lymphoma 2013, 55, 624–627. [Google Scholar] [CrossRef][Green Version]
- Bonthapally, V.; Wu, E.; Macalalad, A.; Yang, H.; Shonukan, O.; Liu, Y.; Chi, A.; Huebner, D. Brentuximab vedotin in relapsed/refractory Hodgkin lymphoma post-autologous transplant: Meta-analysis versus historical data. Curr. Med Res. Opin. 2015, 31, 993–1001. [Google Scholar] [CrossRef] [PubMed]
- Van Der Weyden, C.; Dickinson, M.; Whisstock, J.; Prince, H.M. Brentuximab vedotin in T-cell lymphoma. Expert Rev. Hematol. 2019, 12, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.H.; Prince, H.M.; Whittaker, S.; Horwitz, S.M.; Duvic, M.; Bechter, O.; Sanches, J.A.; Stadler, R.; Scarisbrick, J.; Quaglino, P.; et al. Response to brentuximab vedotin versus physician’s choice by CD30 expression and large cell transformation status in patients with mycosis fungoides: An ALCANZA sub-analysis. Eur. J. Cancer 2021, 148, 411–421. [Google Scholar] [CrossRef]
- Thurber, G.M.; Schmidt, M.M.; Wittrup, K.D. Antibody tumor penetration: Transport opposed by systemic and antigen-mediated clearance. Adv. Drug Deliv. Rev. 2008, 60, 1421–1434. [Google Scholar] [CrossRef]
- Hombach, A.; Heuser, C.; Sircar, R.; Tillmann, T.; Diehl, V.; Pohl, C.; Abken, H. An anti-CD30 chimeric receptor that mediates CD3-ζ-independent T-cell activation against Hodgkin’s lymphoma cells in the presence of soluble CD30. Cancer Res. 1998, 58, 1116–1119. [Google Scholar]
- Hombach, A.; Heuser, C.; Sircar, R.; Tillmann, T.; Diehl, V.; Pohl, C.; Abken, H. Characterization of a Chimeric T-Cell Receptor with Specificity for the Hodgkin??s Lymphoma-Associated CD30 Antigen. J. Immunother. 1999, 22, 473–480. [Google Scholar] [CrossRef]
- Ramos, C.A.; Ballard, B.; Zhang, H.; Dakhova, O.; Gee, A.P.; Mei, Z.; Bilgi, M.; Wu, M.-F.; Liu, H.; Grilley, B.; et al. Clinical and immunological responses after CD30-specific chimeric antigen receptor–redirected lymphocytes. J. Clin. Investig. 2017, 127, 3462–3471. [Google Scholar] [CrossRef]
- Park, S.I.; Serody, J.S.; Shea, T.C.; Grover, N.S.; Ivanova, A.; Morrison, K.; Eldridge, P.; McKay, K.; Cheng, C.J.; Covington, D.; et al. A phase 1b/2 study of CD30-specific chimeric antigen receptor T-cell (CAR-T) therapy in combination with bendamustine in patients with CD30+ Hodgkin and non-Hodgkin lymphoma. J. Clin. Oncol. 2017, 35, TPS3095. [Google Scholar] [CrossRef]
- Ramos, C.A.; Grover, N.S.; Beaven, A.W.; Lulla, P.D.; Wu, M.-F.; Ivanova, A.; Wang, T.; Shea, T.C.; Rooney, C.M.; Dittus, C.; et al. Anti-CD30 CAR-T Cell Therapy in Relapsed and Refractory Hodgkin Lymphoma. J. Clin. Oncol. 2020, 38, 3794–3804. [Google Scholar] [CrossRef] [PubMed]
- Di Stasi, A.; De Angelis, B.; Rooney, C.M.; Zhang, L.; Mahendravada, A.; Foster, A.E.; Heslop, H.E.; Brenner, M.K.; Dotti, G.; Savoldo, B. T lymphocytes coexpressing CCR4 and a chimeric antigen receptor targeting CD30 have improved homing and antitumor activity in a Hodgkin tumor model. Blood J. Am. Soc. Hematol. 2009, 113, 6392–6402. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.M.; Wu, Z.Q.; Wang, Y.; Guo, Y.L.; Dai, H.R.; Wang, X.H.; Li, X.; Zhang, Y.J.; Zhang, W.Y.; Chen, M.X.; et al. Autologous T Cells Expressing CD30 Chimeric Antigen Receptors for Relapsed or Refractory Hodgkin Lymphoma: An Open-Label Phase I TrialCART-30 Cell Therapy for Relapsed or Refractory Hodgkin Lymphoma. Clin. Cancer Res. 2017, 23, 1156–1166. [Google Scholar] [CrossRef]
- Guercio, M.; Orlando, D.; Di Cecca, S.; Sinibaldi, M.; Boffa, I.; Caruso, S.; Abbaszadeh, Z.; Camera, A.; Cembrola, B.; Bovetti, K.; et al. CD28.OX40 co-stimulatory combination is associated with long in vivo persistence and high activity of CAR.CD30 T-cells. Haematologica 2020, 106, 987–999. [Google Scholar] [CrossRef]
- Tobinai, K.; Takahashi, T.; Akinaga, S. Targeting Chemokine Receptor CCR4 in Adult T-Cell Leukemia-Lymphoma and Other T-Cell Lymphomas. Curr. Hematol. Malign-Rep. 2012, 7, 235–240. [Google Scholar] [CrossRef][Green Version]
- Sugaya, M.; Morimura, S.; Suga, H.; Kawaguchi, M.; Miyagaki, T.; Ohmatsu, H.; Fujita, H.; Sato, S. CCR4 is expressed on infiltrating cells in lesional skin of early mycosis fungoides and atopic dermatitis. J. Dermatol. 2015, 42, 613–615. [Google Scholar] [CrossRef]
- Moore, D.C.; Elmes, J.B.; Shibu, P.A.; Larck, C.; Park, S.I. Mogamulizumab: An Anti-CC Chemokine Receptor 4 Antibody for T-Cell Lymphomas. Ann. Pharmacother. 2019, 54, 371–379. [Google Scholar] [CrossRef]
- Perera, L.P.; Zhang, M.; Nakagawa, M.; Petrus, M.N.; Maeda, M.; Kadin, M.E.; Waldmann, T.A.; Perera, P.-Y. Chimeric antigen receptor modified T cells that target chemokine receptor CCR4 as a therapeutic modality for T-cell malignancies. Am. J. Hematol. 2017, 92, 892–901. [Google Scholar] [CrossRef]
- Ishida, T.; Joh, T.; Uike, N.; Yamamoto, K.; Utsunomiya, A.; Yoshida, S.; Saburi, Y.; Miyamoto, T.; Takemoto, S.; Suzushima, H.; et al. Defucosylated Anti-CCR4 Monoclonal Antibody (KW-0761) for Relapsed Adult T-Cell Leukemia-Lymphoma: A Multicenter Phase II Study. J. Clin. Oncol. 2012, 30, 837–842. [Google Scholar] [CrossRef] [PubMed]
- Ogura, M.; Ishida, T.; Hatake, K.; Taniwaki, M.; Ando, K.; Tobinai, K.; Fujimoto, K.; Yamamoto, K.; Miyamoto, T.; Uike, N.; et al. Multicenter Phase II Study of Mogamulizumab (KW-0761), a Defucosylated Anti-CC Chemokine Receptor 4 Antibody, in Patients With Relapsed Peripheral T-Cell Lymphoma and Cutaneous T-Cell Lymphoma. J. Clin. Oncol. 2014, 32, 1157–1163. [Google Scholar] [CrossRef] [PubMed]
- Grover, N.S.; Ivanova, A.; Moore, D.T.; Cheng, C.J.A.; Babinec, C.; West, J.; Cavallo, T.; Morrison, J.K.; Buchanan, F.B.; Bowers, E.; et al. CD30-Directed CAR-T Cells Co-Expressing CCR4 in Relapsed/Refractory Hodgkin Lymphoma and CD30+ Cutaneous T Cell Lymphoma. Blood 2021, 138, 742. [Google Scholar] [CrossRef]
- Uehara, S.; Grinberg, A.; Farber, J.M.; Love, P.E. A Role for CCR9 in T Lymphocyte Development and Migration. J. Immunol. 2002, 168, 2811–2819. [Google Scholar] [CrossRef] [PubMed]
- Wurbel, M.-A.; Malissen, M.; Guy-Grand, D.; Meffre, E.; Nussenzweig, M.C.; Richelme, M.; Carrier, A.; Malissen, B. Mice lacking the CCR9 CC-chemokine receptor show a mild impairment of early T- and B-cell development and a reduction in T-cell receptor γδ+ gut intraepithelial lymphocytes. Blood J. Am. Soc. Hematol. 2001, 98, 2626–2632. [Google Scholar] [CrossRef]
- Maciocia, P.M.; Wawrzyniecka, P.A.; Maciocia, N.C.; Burley, A.; Karpanasamy, T.; Devereaux, S.; Hoekx, M.; O’Connor, D.; Leon, T.E.; Rapoz-D’Silva, T.; et al. Anti-CCR9 chimeric antigen receptor T cells for T-cell acute lymphoblastic leukemia. Blood 2022, 140, 25–37. [Google Scholar] [CrossRef]
- Philip, B.; Kokalaki, E.; Mekkaoui, L.; Thomas, S.; Straathof, K.; Flutter, B.; Marin, V.; Marafioti, T.; Chakraverty, R.; Linch, D.; et al. A highly compact epitope-based marker/suicide gene for easier and safer T-cell therapy. Blood J. Am. Soc. Hematol. 2014, 124, 1277–1287. [Google Scholar] [CrossRef]
- Hamilton, B.K.; Rybicki, L.; Abounader, D.; Adekola, K.; Advani, A.; Aldoss, I.; Bachanova, V.; Bashey, A.; Brown, S.; DeLima, M.; et al. Allogeneic Hematopoietic Cell Transplantation for Adult T Cell Acute Lymphoblastic Leukemia. Biol. Blood Marrow Transplant. 2017, 23, 1117–1121. [Google Scholar] [CrossRef]
- Van Spriel, A.B.; Puls, K.L.; Sofi, M.; Pouniotis, D.; Hochrein, H.; Orinska, Z.; Knobeloch, K.P.; Plebanski, M.; Wright, M.D. A regulatory role for CD37 in T cell proliferation. J. Immunol. 2004, 172, 2953–2961. [Google Scholar] [CrossRef]
- Barrena, S.; Almeida, J.; Yunta, M.; López, A.; Fernández-Mosteirín, N.; Giralt, M.; Romero, M.; Perdiguer, L.; Delgado, M.; Orfao, A.; et al. Aberrant expression of tetraspanin molecules in B-cell chronic lymphoproliferative disorders and its correlation with normal B-cell maturation. Leukemia 2005, 19, 1376–1383. [Google Scholar] [CrossRef]
- Lapalombella, R.; Yeh, Y.-Y.; Wang, L.; Ramanunni, A.; Rafiq, S.; Jha, S.; Staubli, J.; Lucas, D.M.; Mani, R.; Herman, S.E.; et al. Tetraspanin CD37 Directly Mediates Transduction of Survival and Apoptotic Signals. Cancer Cell 2012, 21, 694–708. [Google Scholar] [CrossRef] [PubMed]
- Sawas, A.; Savage, K.J.; Perez, R.; Advani, R.H.; Butturini, A.; Lackey, J.; Trave, F.; Anand, B.; Huang, Y.; Reyno, L.; et al. A phase 1 study of the anti-CD37 antibody-drug conjugate AGS67E in advanced lymphoid malignancies. Interim results. Hematol. Oncol. 2017, 35, 49. [Google Scholar] [CrossRef]
- Scarfò, I.; Ormhøj, M.; Frigault, M.J.; Castano, A.P.; Lorrey, S.; Bouffard, A.A.; Van Scoyk, A.; Rodig, S.J.; Shay, A.J.; Aster, J.C.; et al. Anti-CD37 chimeric antigen receptor T cells are active against B- and T-cell lymphomas. Blood J. Am. Soc. Hematol. 2018, 132, 1495–1506. [Google Scholar] [CrossRef] [PubMed]
- Tunnacliffe, A.; Kefford, R.; Milstein, C.; Forster, A.; Rabbitts, T.H. Sequence and evolution of the human T-cell antigen receptor beta-chain genes. Proc. Natl. Acad. Sci. USA 1985, 82, 5068–5072. [Google Scholar] [CrossRef] [PubMed]
- Jamal, S.; Picker, L.J.; Aquino, D.B.; McKenna, R.W.; Dawson, D.B.; Kroft, S.H. Immunophenotypic analysis of peripheral T-cell neoplasms: A multiparameter flow cytometric approach. Am. J. Clin. Pathol. 2001, 116, 512–526. [Google Scholar] [CrossRef] [PubMed]
- Maciocia, P.; A Wawrzyniecka, P.; Philip, B.; Ricciardelli, I.; Akarca, A.U.; Onuoha, S.C.; Legut, M.; Cole, D.; Sewell, A.K.; Gritti, G.; et al. Targeting the T cell receptor β-chain constant region for immunotherapy of T cell malignancies. Nat. Med. 2017, 23, 1416–1423. [Google Scholar] [CrossRef]
- Morton, L.T.; Reijmers, R.M.; Wouters, A.K.; Kweekel, C.; Remst, D.F.G.; Pothast, C.R.; Falkenburg, J.H.F.; Heemskerk, M.H.M. Simultaneous Deletion of Endogenous TCRαβ for TCR Gene Therapy Creates an Improved and Safe Cellular Therapeutic. Mol. Ther. 2020, 28, 64–74. [Google Scholar] [CrossRef]
- Sun, S.; Hao, H.; Yang, G.; Zhang, Y.; Fu, Y. Immunotherapy with CAR-Modified T Cells: Toxicities and Overcoming Strategies. J. Immunol. Res. 2018, 2018, 1–10. [Google Scholar] [CrossRef]
- Svoboda, J.; Rheingold, S.R.; Gill, S.I.; Grupp, S.A.; Lacey, S.F.; Kulikovskaya, I.; Suhoski, M.M.; Melenhorst, J.J.; Loudon, B.; Mato, A.R.; et al. Nonviral RNA chimeric antigen receptor–modified T cells in patients with Hodgkin lymphoma. Blood J. Am. Soc. Hematol. 2018, 132, 1022–1026. [Google Scholar] [CrossRef]
- Birkholz, K.; A Hombach, A.; A Krug, C.; Reuter, S.; Kershaw, M.; Kampgen, E.; Schuler, G.; Abken, H.; Schaft, N.; Dörrie, J. Transfer of mRNA encoding recombinant immunoreceptors reprograms CD4+ and CD8+ T cells for use in the adoptive immunotherapy of cancer. Gene Ther. 2009, 16, 596–604. [Google Scholar] [CrossRef]
- Barrett, D.M.; Zhao, Y.; Liu, X.; Jiang, S.; Carpenito, C.; Kalos, M.; Carroll, R.G.; June, C.H.; Grupp, S.A. Treatment of Advanced Leukemia in Mice with mRNA Engineered T Cells. Hum. Gene Ther. 2011, 22, 1575–1586. [Google Scholar] [CrossRef] [PubMed]
- Di Stasi, A.; Tey, S.K.; Dotti, G.; Fujita, Y.; Kennedy-Nasser, A.; Martinez, C.; Straathof, K.; Liu, E.; Durett, A.G.; Grilley, B.; et al. Inducible apoptosis as a safety switch for adoptive cell therapy. N. Engl. J. Med. 2011, 365, 1673–1683. [Google Scholar] [CrossRef] [PubMed]
- Traversari, C.; Marktel, S.; Magnani, Z.I.; Mangia, P.; Russo, V.; Ciceri, F.; Bonini, M.C.; Bordignon, C. The potential immunogenicity of the TK suicide gene does not prevent full clinical benefit associated with the use of TK-transduced donor lymphocytes in HSCT for hematologic malignancies. Blood J. Am. Soc. Hematol. 2007, 109, 4708–4715. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Yi, M.; Qin, S.; Wu, K. Next generation chimeric antigen receptor T cells: Safety strategies to overcome toxicity. Mol. Cancer 2019, 18, 125. [Google Scholar] [CrossRef] [PubMed]
- Safarzadeh Kozani, P.; Safarzadeh Kozani, P.; Rahbarizadeh, F.; Khoshtinat Nikkhoi, S. Strategies for dodging the obstacles in CAR T cell therapy. Front. Oncol. 2021, 11, 627549. [Google Scholar] [CrossRef]
- Ruella, M.; Xu, J.; Barrett, D.M.; Fraietta, J.A.; Reich, T.J.; Ambrose, D.E.; Klichinsky, M.; Shestova, O.; Patel, P.R.; Kulikovskaya, I.; et al. Induction of resistance to chimeric antigen receptor T cell therapy by transduction of a single leukemic B cell. Nat. Med. 2018, 24, 1499–1503. [Google Scholar] [CrossRef]
- Depil, S.; Duchateau, P.; Grupp, S.; Mufti, G.; Poirot, L. ‘Off-the-shelf’allogeneic CAR T cells: Development and challenges. Nat. Rev. Drug Discov. 2020, 19, 185–199. [Google Scholar] [CrossRef]
- Simonetta, F.; Alvarez, M.; Negrin, R.S. Natural Killer Cells in Graft-versus-Host-Disease after Allogeneic Hematopoietic Cell Transplantation. Front. Immunol. 2017, 8, 465. [Google Scholar] [CrossRef]
- Zhang, C.; Oberoi, P.; Oelsner, S.; Waldmann, A.; Lindner, A.; Tonn, T.; Wels, W.S. Chimeric Antigen Receptor-Engineered NK-92 Cells: An Off-the-Shelf Cellular Therapeutic for Targeted Elimination of Cancer Cells and Induction of Protective Antitumor Immunity. Front. Immunol. 2017, 8, 533. [Google Scholar] [CrossRef]
- Liu, Q.; Xu, Y.; Mou, J.; Tang, K.; Fu, X.; Li, Y.; Xing, Y.; Rao, Q.; Xing, H.; Tian, Z.; et al. Irradiated chimeric antigen receptor engineered NK-92MI cells show effective cytotoxicity against CD19+ malignancy in a mouse model. Cytotherapy 2020, 22, 552–562. [Google Scholar] [CrossRef]
- Allan, D.S.; Chakraborty, M.; Waller, G.C.; Hochman, M.J.; Poolcharoen, A.; Reger, R.N.; Childs, R.W. Systematic improvements in lentiviral transduction of primary human natural killer cells undergoing ex vivo expansion. Mol. Ther.-Methods Clin. Dev. 2021, 20, 559–571. [Google Scholar] [CrossRef] [PubMed]
- Morgan, M.A.; Büning, H.; Sauer, M.; Schambach, A. Use of Cell and Genome Modification Technologies to Generate Improved “Off-the-Shelf” CAR T and CAR NK Cells. Front. Immunol. 2020, 11, 1965. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, A.; Gerdemann, U.; Katari, U.L.; Tzannou, I.; Liu, H.; Martinez, C.; Leung, K.; Carrum, G.; Gee, A.P.; Vera, J.F.; et al. Activity of Broad-Spectrum T Cells as Treatment for AdV, EBV, CMV, BKV, and HHV6 Infections after HSCT. Sci. Transl. Med. 2014, 6, 242ra83. [Google Scholar] [CrossRef]
- Houghtelin, A.; Bollard, C.M. Virus-Specific T Cells for the Immunocompromised Patient. Front. Immunol. 2017, 8, 1272. [Google Scholar] [CrossRef] [PubMed]
- Melenhorst, J.J.; Leen, A.M.; Bollard, C.M.; Quigley, M.F.; Price, D.A.; Rooney, C.M.; Brenner, M.K.; Barrett, A.J.; Heslop, H.E. Allogeneic virus-specific T cells with HLA alloreactivity do not produce GVHD in human subjects. Blood J. Am. Soc. Hematol. 2010, 116, 4700–4702. [Google Scholar] [CrossRef]
- Lança, T.; Correia, D.V.; Moita, C.F.; Raquel, H.; Neves-Costa, A.; Ferreira, C.; Ramalho, J.S.; Barata, J.T.; Moita, L.F.; Gomes, A.Q.; et al. The MHC class Ib protein ULBP1 is a nonredundant determinant of leukemia/lymphoma susceptibility to γδ T-cell cytotoxicity. Blood J. Am. Soc. Hematol. 2010, 115, 2407–2411. [Google Scholar] [CrossRef]
- Nielsen, M.M.; Witherden, D.A.; Havran, W.L. γδ T cells in homeostasis and host defence of epithelial barrier tissues. Nat. Rev. Immunol. 2017, 17, 733–745. [Google Scholar] [CrossRef]
- McCarthy, N.E.; Eberl, M. Human γδ T-cell control of mucosal immunity and inflammation. Front. Immunol. 2018, 9, 985. [Google Scholar] [CrossRef]
- Capsomidis, A.; Benthall, G.; Van Acker, H.H.; Fisher, J.; Kramer, A.M.; Abeln, Z.; Majani, Y.; Gileadi, T.; Wallace, R.; Gustafsson, K.; et al. Chimeric Antigen Receptor-Engineered Human Gamma Delta T Cells: Enhanced Cytotoxicity with Retention of Cross Presentation. Mol. Ther. 2018, 26, 354–365. [Google Scholar] [CrossRef]
- Uchida, R.; Ashihara, E.; Sato, K.; Kimura, S.; Kuroda, J.; Takeuchi, M.; Kawata, E.; Taniguchi, K.; Okamoto, M.; Shimura, K.; et al. γδT cells kill myeloma cells by sensing mevalonate metabolites and ICAM-1 molecules on cell surface. Biochem. Biophys. Res. Commun. 2007, 354, 613–618. [Google Scholar] [CrossRef]
- Li, J.; Herold, M.; Kimmel, B.; Müller, I.; Rincon-Orozco, B.; Kunzmann, V.; Herrmann, T. Reduced Expression of the Mevalonate Pathway Enzyme Farnesyl Pyrophosphate Synthase Unveils Recognition of Tumor Cells by Vγ9Vδ2 T Cells. J. Immunol. 2009, 182, 8118–8124. [Google Scholar] [CrossRef] [PubMed]
- Rischer, M.; Pscherer, S.; Duwe, S.; Vormoor, J.; Jürgens, H.; Rossig, C. Human γδ T cells as mediators of chimaeric-receptor redirected anti-tumour immunity. Br. J. Haematol. 2004, 126, 583–592. [Google Scholar] [CrossRef] [PubMed]
- Harrer, D.C.; Simon, B.; Fujii, S.I.; Shimizu, K.; Uslu, U.; Schuler, G.; Gerer, K.F.; Hoyer, S.; Dörrie, J.; Schaft, N. RNA-transfection of γ/δ T cells with a chimeric antigen receptor or an α/β T-cell receptor: A safer alternative to genetically engineered α/β T cells for the immunotherapy of melanoma. BMC Cancer 2017, 17, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Ratner, L.; Waldmann, T.A.; Janakiram, M.; Brammer, J.E. Rapid Progression of Adult T-Cell Leukemia–Lymphoma after PD-1 Inhibitor Therapy. N. Engl. J. Med. 2018, 378, 1947–1948. [Google Scholar] [CrossRef] [PubMed]
- Ghione, P.; Moskowitz, A.J.; De Paola, N.E.K.; Horwitz, S.M.; Ruella, M. Novel Immunotherapies for T Cell Lymphoma and Leukemia. Curr. Hematol. Malign-Rep. 2018, 13, 494–506. [Google Scholar] [CrossRef] [PubMed]
- Hill, B.T.; Roberts, Z.J.; Xue, A.; Rossi, J.M.; Smith, M.R. Rapid tumor regression from PD-1 inhibition after anti-CD19 chimeric antigen receptor T-cell therapy in refractory diffuse large B-cell lymphoma. Bone Marrow Transplant. 2019, 55, 1184–1187. [Google Scholar] [CrossRef] [PubMed]
| Target Receptor | Notes on CAR Construct | Target Disease | Main Eligibility Criteria | Phase | Target Accrual | Outcomes | Age Group (Years) | Status | ClinicalTrials.gov Identifier |
|---|---|---|---|---|---|---|---|---|---|
| CD4 | |||||||||
| Anti-CD4 CAR transduced with a lentiviral vector | R/R CD4+ T-NHL | After failure of standard therapy | I | 12 | Toxicity DFS; PFS; OS | ≥18 | Recruiting | NCT04162340 | |
| Autologous LCAR-T2C | T-ALL T-NHL | PD or SD after ≥1 prior line of therapy | I | 33 | DLT, AE, RP2D, PK | 18–75 | Recruiting | NCT04973527 | |
| Autologous LCAR-T2C | R/R CD4+ T-NHL | PD or SD after ≥1 prior line of therapy | I | 32 | DLT, AE, RP2D, PK | 18–75 | Recruiting | NCT04219319 | |
| Autologous T cells transduced with a lentiviral vector | R/R CD4+ T-NHL | R/R including ASCT | I | 20 | Safety, feasibility In vivo survival, clinical response | ≥18 | Active, not recruiting | NCT03829540 | |
| LB1901- autologous CD4-targeted CAR-T | T-NHL | Failed ≥2 prior lines of therapy | I (FIH) | 50 | RP2D ORR, TTR | ≥18 | Active, not recruiting | NCT04712864 | |
| CD5 | |||||||||
| Anti-CD5 CAR transduced with a lentiviral vector | R/R T-ALL and T-NHL | After failure of standard therapy | I | 20 | Toxicity DFS; PFS; OS | ≥8 | Recruiting | NCT04594135 | |
| Donor-derived CD5 CAR T cells | R/R T-ALL | After failure of standard therapy | I (FIH) | 18 | DLT, AEs ORR, BOR | 1–70 | Recruiting | NCT05032599 | |
| Autologous CD5.CAR/28zeta CAR T cells Allogeneic CD5.CAR/28zeta CAR T cells | R/R T-ALL and T-NHL | Suitable for ASCT R/R post ASCT | I | 42 | DLT, ORR | Up to 75 | Active Not recruiting | NCT03081910 MAGENTA | |
| Endogenous CD5 in CT125A cells knocked out by CRISPR/Cas9 genome editing | PTCL CD5+ R/R | Failed at ≥1 prior therapy | I | 18 | DLT, AE ORR, CR | 18–70 | Not yet recruiting | NCT04767308 | |
| CD7 | |||||||||
| BT-007, transduced with 4-1BB/CD3ζ lentiviral vector, expanded in vitro | CD7+ R/R T-NHL | Failed ≥1 line of therapy | I | 15 | ORR, retention time and amount of CAR-T cells remaining in vivo | 18–70 | Recruiting | NCT05554575 | |
| CD30 | |||||||||
| Carmelizumab + CD30 CAR-T cells | CD30+ R/R T-NHL | Failed ≥2 lines of systemic treatment | II | 30 | ORR, OS, DOR, PFS, AEs | 18–70 | Recruiting | NCT05320081 | |
| Allogeneic CD30.CAR-EBVSTs | CD30+ R/R T-NHL | ALK+/ALK- ALCL PTCL | I | 18 | DLT, ORR, DOR, SD, PFS | 12–75 | Recruiting | NCT04288726 | |
| CD30.CAR-EBVST cells | CD30+ R/RT-NHL | ALK+/ALK- ALCL PTCL | I | 18 | DLT, ORR, DOR, SD, PFS | 12–75 | Not yet recruiting | NCT04952584 | |
| Autologous 3rd generation anti-CD30 CAR T cells. | CD30+ R/R T-NHL | ATLL, ALCL, AITL, NK/TCL, PTCL | I | 50 | AEs, OS, EFS, RFS, retention amount | 18–70 | Recruiting | NCT04008394 | |
| Autologous, uses retroviral vector | CD30+ R/R T-NHL | PTCL, AITL, NK/TCL, ALCL, etc. | I | 66 | DLT, ORR | 12–75 | Recruiting | NCT02917083 RELY-30 | |
| Autologous CAR.CD30 T CELLS | CD30+ R/R T-NHL | PTCL, AITL, NK/TCL, ALCL, etc. | I | 9 | MTD, AE, ORR, DCR, DOR, PFS, OS | 18–70 | Not yet recruiting | NCT05208853 | |
| CD30-Directed Genetically Modified Autologous T-Cells | CD30+ R/R T-NHL | PTCL, ALCL, NK/TCL | I | 21 | DLT, ORR, DOR, PFS | 18–75 | Active, not recruiting | NCT04526834 | |
| HSP-CAR30; Autologous second generation (4-1BBz) | CD30+ R/R T-NHL | ALK+/ALK- ALCL, PTCL | I/II | 30 | Safety, toxicity, MTD, CR | 18–70 | Recruiting | NCT04653649 | |
| Autologous CAR.CD30 T CELLS | CD30+ T-NHL | Relapsing after ASCT, or refractory to 2 multidrug regimens and/or anti-CD30 antibody treatment. Newly diagnosed patients unable to receive or complete standard chemotherapy | I/II | 30 | AE, anti-tumor responses, in vivo existence | 16–80 | Recruiting | NCT02259556 | |
| Autologous activated T lymphocytes (ATLs) expressing CD30 CAR | CD30+ R/R T-NHL | PTCL | II | 20 | PFS, BOR, ORR, DLT, OS | 18–99 | Recruiting | NCT04083495 | |
| Autologous CAR.CD30 T CELLS | CD30+ R/R T-NHL | Failed >2 prior regimens. Subjects relapsed after ASCT or APSCT also eligible | Ib/II | 40 | 2-y OS, 2-y PFS, ORR, DOR, AEs | ≥3 | Recruiting | NCT02690545 | |
| Transduced with lentivirus bearing anti-CD30 antibody scFV and the activation signals of second-generation CART designation. | CD30+ R/R T-NHL | ALK+/ALK- ALCL, PTCL | I | 20 | Safety, anti-tumor efficacy | 2–80 | Recruiting | NCT03383965 | |
| Autologous CAR.CD30 EBV specific-CTLs | Newly diagnosed and R/R T-NHL | CD30+; include failure post APSCT | I | 18 | Survival and anti-tumor effects in vivo | All ages | Active, not recruiting | NCT01192464 CARCD30 | |
| Autologous CAR.CD30 T CELLS | CD30+ T-NHL | CD30+; include failure post APSCT | I | 10 | Safety; Survival and anti-tumor effects in vivo | All ages | Active, not recruiting | NCT01316146 CART CD30 | |
| Autologous CAR.CD30 T CELLS with CCR4 (ATLCAR.CD30.CCR4) | CD30+ CTCL | All cutaneous CD30+ T-NHL | I | 59 | AE, PFS, BOR, ORR, OS | ≥18 | Recruiting | NCT03602157 | |
| Autologous CAR.CD30 T CELLS with CCR4 (ATLCAR.CD30.CCR4) | CD30+ T-NHL | Relapse after high dose therapy and APSCT (ATLAS) | I | 18 | AEs, PFS, OS, survival of CAR in vivo | ≥3 | Active, not recruiting | NCT02663297 | |
| CD37 | |||||||||
| CAR-37 T cells | CD37+ R/R T-NHL | Mature T cell neoplasms R/R after 2 or more prior lines of therapy or Relapse after APSCT | I (FIH) | 18 | DLT, AE, OS, PFS, RR | ≥18 | Recruiting | NCT04136275 | |
| CCR4 | |||||||||
| Autologous CAR.CD30 T CELLS with CCR4 (ATLCAR.CD30.CCR4) | CD30+ CTCL | All cutaneous CD30+ T-NHL | I | 59 | AE, PFS, BOR, ORR, OS | ≥18 | Recruiting | NCT03602157 | |
| TRBC1/2 | |||||||||
| Autologous anti-TRBC1 CAR | R/R TRBC1+ T-NHL | PTCL, AITL, ALCL, T-ALL | I | 9 | CAR-T cell expansion and persistence ORR, DOR, OS, PFS | 18–70 | Recruiting | NCT04828174 | |
| AUTO4; RQR8/aTRBC1 CAR T cells | R/R TRBC1+ T-NHL | PTCL, AITL, ALCL | I/II | 200 | Safety, CR+PR, AE, time to response | ≥18 | Recruiting | NCT03590574 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Assi, R.; Salman, H. Harnessing the Potential of Chimeric Antigen Receptor T-Cell Therapy for the Treatment of T-Cell Malignancies: A Dare or Double Dare? Cells 2022, 11, 3971. https://doi.org/10.3390/cells11243971
Assi R, Salman H. Harnessing the Potential of Chimeric Antigen Receptor T-Cell Therapy for the Treatment of T-Cell Malignancies: A Dare or Double Dare? Cells. 2022; 11(24):3971. https://doi.org/10.3390/cells11243971
Chicago/Turabian StyleAssi, Rita, and Huda Salman. 2022. "Harnessing the Potential of Chimeric Antigen Receptor T-Cell Therapy for the Treatment of T-Cell Malignancies: A Dare or Double Dare?" Cells 11, no. 24: 3971. https://doi.org/10.3390/cells11243971
APA StyleAssi, R., & Salman, H. (2022). Harnessing the Potential of Chimeric Antigen Receptor T-Cell Therapy for the Treatment of T-Cell Malignancies: A Dare or Double Dare? Cells, 11(24), 3971. https://doi.org/10.3390/cells11243971





