Synergistic Effect of Static Magnetic Fields and 3D-Printed Iron-Oxide-Nanoparticle-Containing Calcium Silicate/Poly-ε-Caprolactone Scaffolds for Bone Tissue Engineering
Abstract
1. Introduction
2. Materials and Methods
2.1. Fabrication of Scaffolds
2.2. Physicochemical Properties of the Scaffolds
2.3. In Vitro Immersion Experiment
2.4. Magnetic Stimulation
2.5. Cell Proliferation and Morphology
2.6. Osteogenic Differentiation
2.7. Statistical Analysis
3. Results and Discussion
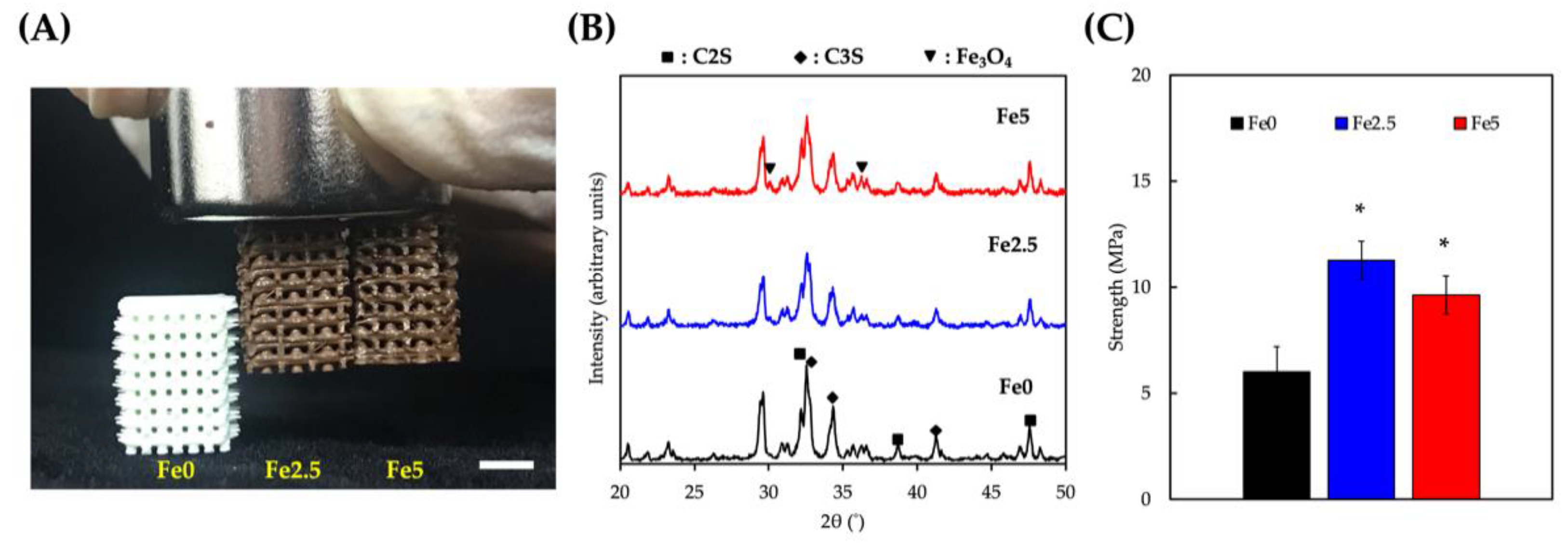
3.1. Characterization of the Physicochemical Properties of the Scaffolds
3.2. Immersed Behaviors
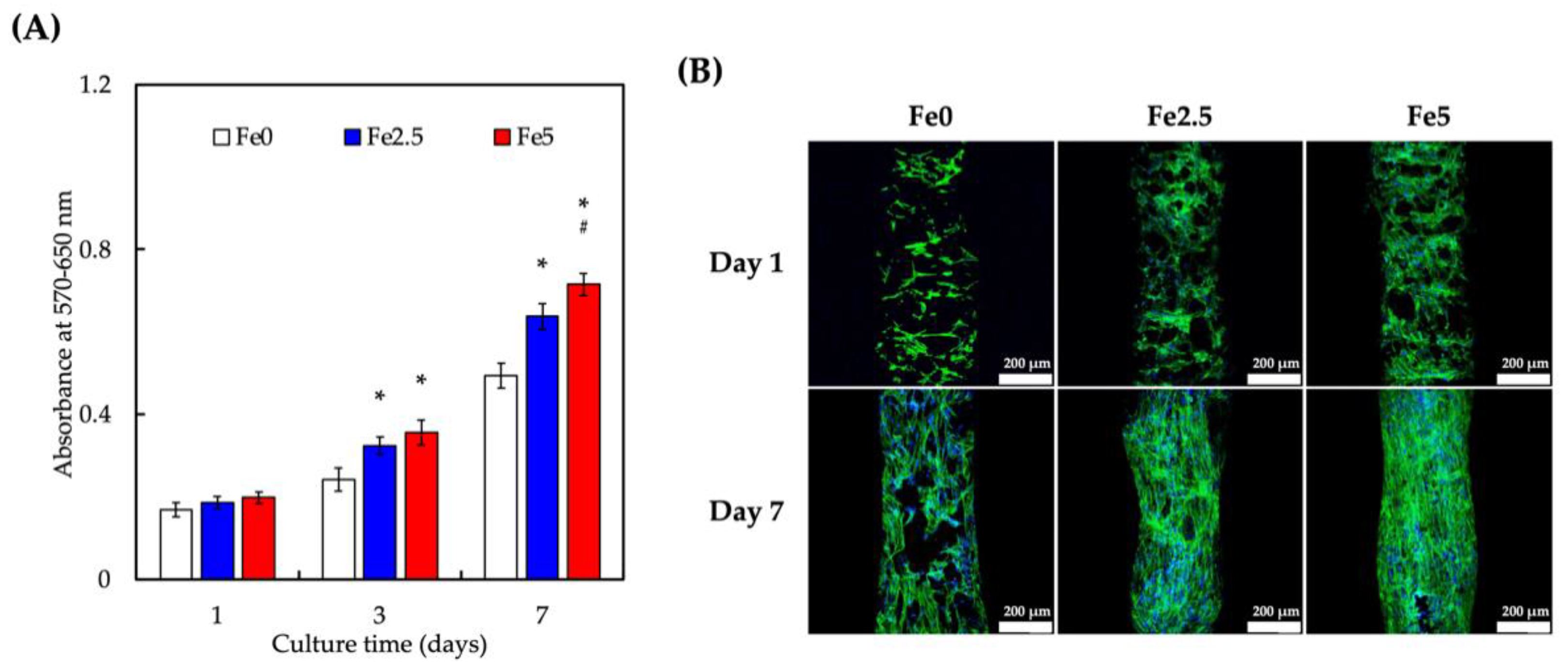
3.3. Cell Proliferation of Wharton’s Jelly Mesenchymal Stem Cells (WJMSCs) Cultured on Scaffolds
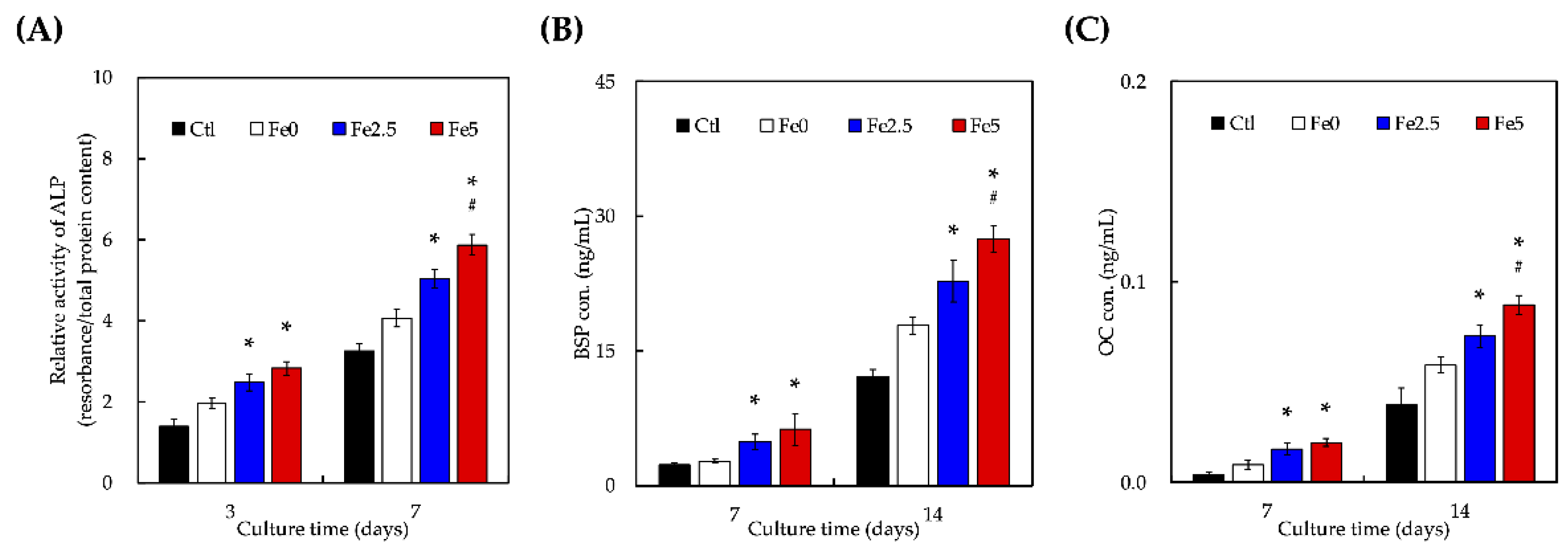
3.4. Effect of Ionic Scaffold Products on WJMSCs’ Differentiation
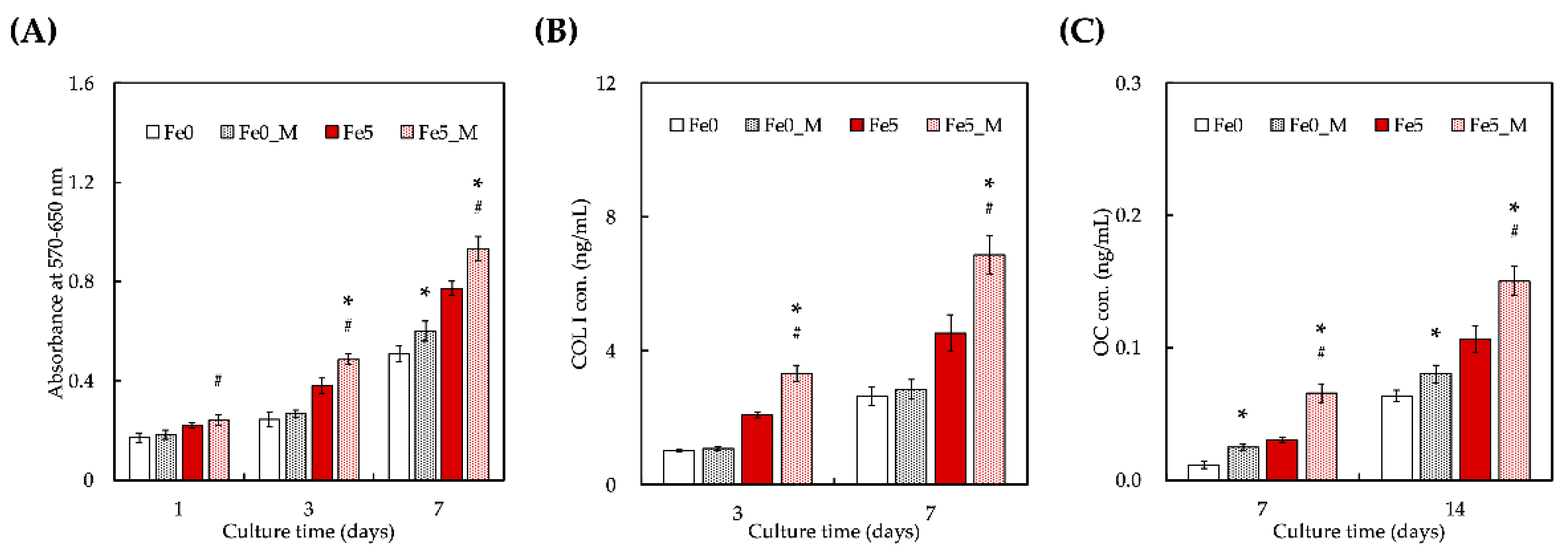
3.5. Static Magnetic Fields Enhanced Cell Proliferation and Differentiation on FeCS Scaffold
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Li, W.; Miao, W.; Liu, Y.; Wang, T.; Zhang, Y.; Wang, W.; Lu, D.; Zhou, X.; Jiao, X.; Jia, X.; et al. Bioprinted Constructs that Mimic the Ossification Center Microenvironment for Targeted Innervation in Bone Regeneration. Adv. Funct. Mater. 2021, 32, 2109871. [Google Scholar] [CrossRef]
- Jiao, C.; Xie, D.; He, Z.; Liang, H.; Shen, L.; Yang, Y.; Tian, Z.; Wu, G.; Wang, C. Additive manufacturing of Bio-inspired ceramic bone Scaffolds: Structural Design, mechanical properties and biocompatibility. Mater. Des. 2022, 217, 110610. [Google Scholar] [CrossRef]
- Ho, C.-C.; Chen, Y.-W.; Wang, K.; Lin, C.-Y.; Chen, T.-C.; Shie, M.-Y. Effect of mussel-inspired polydopamine on the reinforced properties of 3D printed β-tricalcium phosphate/polycaprolactone scaffolds for bone regeneration. J. Mater. Chem. B 2022. [Google Scholar] [CrossRef]
- Cameron, A.P.; Zeng, B.; Liu, Y.; Wang, H.; Soheilmoghaddam, F.; Cooper-White, J.; Zhao, C.-X. Biophysical properties of hydrogels for mimicking tumor extracellular matrix. Biomater. Adv. 2022, 136, 212782. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, Y.; Chen, F.; Chen, X.; Xiao, Y.; Zhang, X. Design of macropore structure and micro-nano topography to promote the early neovascularization and osteoinductivity of biphasic calcium phosphate bioceramics. Mater. Des. 2022, 216, 110581. [Google Scholar] [CrossRef]
- Kao, C.-T.; Lin, C.-C.; Chen, Y.-W.; Yeh, C.-H.; Fang, H.-Y.; Shie, M.-Y. Poly(dopamine) coating of 3D printed poly(lactic acid) scaffolds for bone tissue engineering. Mater. Sci. Eng. C 2015, 56, 165–173. [Google Scholar] [CrossRef]
- Chen, Y.W.; Shen, Y.F.; Ho, C.C.; Yu, J.; Wu, Y.H.; Wang, K.; Shih, C.T.; Shie, M.Y. Osteogenic and Angiogenic Potentials of the Cell-Laden Hydrogel/Mussel-Inspired Calcium Silicate Complex Hierarchical Porous Scaffold Fabricated by 3D Bi-oprinting. Mater. Sci. Eng. C. 2018, 91, 679–687. [Google Scholar] [CrossRef]
- Ma, J.; Qin, C.; Wu, J.; Zhang, H.; Zhuang, H.; Zhang, M.; Zhang, Z.; Ma, L.; Wang, X.; Ma, B.; et al. 3D Printing of Strontium Silicate Microcylinder-Containing Multicellular Biomaterial Inks for Vascularized Skin Regeneration. Adv. Health Mater. 2021, 10, e2100523. [Google Scholar] [CrossRef]
- Shie, M.-Y.; Ding, S.-J.; Chang, H.-C. The role of silicon in osteoblast-like cell proliferation and apoptosis. Acta Biomater. 2011, 7, 2604–2614. [Google Scholar] [CrossRef]
- Li, J.; Deng, C.; Liang, W.; Kang, F.; Bai, Y.; Ma, B.; Wu, C.; Dong, S. Mn-containing bioceramics inhibit osteoclastogenesis and promote osteoporotic bone regeneration via scavenging ROS. Bioact. Mater. 2021, 6, 3839–3850. [Google Scholar] [CrossRef]
- Ho, C.-C.; Fang, H.-Y.; Wang, B.; Huang, T.-H.; Shie, M.-Y. The effects of Biodentine/polycaprolactone three-dimensional-scaffold with odontogenesis properties on human dental pulp cells. Int. Endod. J. 2017, 51, e291–e300. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ding, C.; Ren, L.; Zhou, Y.; Shang, P. The effects of static magnetic fields on bone. Prog. Biophys. Mol. Biol. 2014, 114, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, Y.; Zhou, N.; Feng, Y.; Yang, X. Cyclic tensile stress promotes osteogenic differentiation of adipose stem cells via ERK and p38 pathways. Stem Cell Res. 2019, 37, 101433. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, H.; He, Y.; Tang, H.; Dong, J.; Chen, X.; Lyu, F.; Dong, Y. Cyclic Pulsation Stress Promotes Bone Formation of Tissue Engineered Laminae through the F-Actin/YAP-1/β-Catenin Signaling Axis. NPJ Regen. Med. 2021, 6, 51. [Google Scholar] [CrossRef]
- Staehlke, S.; Bielfeldt, M.; Zimmermann, J.; Gruening, M.; Barke, I.; Freitag, T.; Speller, S.; Van Rienen, U.; Nebe, B. Pulsed Electrical Stimulation Affects Osteoblast Adhesion and Calcium Ion Signaling. Cells 2022, 11, 2650. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-W.; Wang, K.; Ho, C.-C.; Kao, C.-T.; Ng, H.Y.; Shie, M.-Y. Cyclic tensile stimulation enrichment of Schwann cell-laden auxetic hydrogel scaffolds towards peripheral nerve tissue engineering. Mater. Des. 2020, 195, 108982. [Google Scholar] [CrossRef]
- Zhou, X.; Castro, N.J.; Zhu, W.; Cui, H.; Aliabouzar, M.; Sarkar, K.; Zhang, L.G. Improved Human Bone Marrow Mesen-chymal Stem Cell Osteogenesis in 3D Bioprinted Tissue Scaffolds with Low Intensity Pulsed Ultrasound Stimulation. Sci. Rep. 2016, 6, 507. [Google Scholar]
- Cai, K.; Jiao, Y.; Quan, Q.; Hao, Y.; Liu, J.; Wu, L. Improved Activity of MC3T3-E1 Cells by the Exciting Piezoelectric Ba-TiO3/TC4 Using Low-Intensity Pulsed Ultrasound. Bioact. Mater. 2021, 6, 4073–4082. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Tang, Y.; Pu, X.; Wang, M.; Chen, F.; Chen, X.; Zhu, X.; Zhang, X. The Role of Micro-Vibration Parameters in In-flammatory Responses of Macrophages Cultured on Biphasic Calcium Phosphate Ceramics and the Resultant Influence on Osteogenic Differentiation of Mesenchymal Stem Cells. J. Mater. Chem. B 2021, 9, 8003–8013. [Google Scholar] [CrossRef]
- Shi, M.; Shen, K.; Yang, B.; Zhang, P.; Lv, K.; Qi, H.; Wang, Y.; Li, M.; Yuan, Q.; Zhang, Y. An electroporation strategy to synthesize the membrane-coated nanoparticles for enhanced anti-inflammation therapy in bone infection. Theranostics 2021, 11, 2349–2363. [Google Scholar] [CrossRef]
- Yang, J.; Wu, J.; Guo, Z.; Zhang, G.; Zhang, H. Iron Oxide Nanoparticles Combined with Static Magnetic Fields in Bone Remodeling. Cells 2022, 11, 3298. [Google Scholar] [CrossRef] [PubMed]
- Byun, H.; Lee, S.; Jang, G.N.; Lee, H.; Park, S.; Shin, H. Magnetism-controlled assembly of composite stem cell spheroids for the biofabrication of contraction-modulatory 3D tissue. Biofabrication 2022, 14, 015007. [Google Scholar] [CrossRef] [PubMed]
- Chiang, M.-Y.; Cheng, I.-Y.; Chou, S.-H.; Tsai, J.-H.; Chen, Y.-J.; Lu, H.-E.; Yang, S.-W.; Chang, S.-J.; Chen, S.-Y. A smart injectable composite hydrogel with magnetic navigation and controlled glutathione release for promoting in situ chondrocyte array and self-healing in damaged cartilage tissue. J. Mater. Chem. B 2021, 9, 9370–9382. [Google Scholar] [CrossRef] [PubMed]
- Shuai, C.; Yang, W.; He, C.; Peng, S.; Gao, C.; Yang, Y.; Qi, F.; Feng, P. A magnetic micro-environment in scaffolds for stimulating bone regeneration. Mater. Des. 2019, 185, 108275. [Google Scholar] [CrossRef]
- Shuai, C.; Cheng, Y.; Yang, W.; Feng, P.; Yang, Y.; He, C.; Qi, F.; Peng, S. Magnetically Actuated Bone Scaffold: Micro-structure, Cell Response and Osteogenesis. Compos. Part B Eng. 2020, 192, 107986. [Google Scholar] [CrossRef]
- Jia, Y.; Zhang, P.; Sun, Y.; Kang, Q.; Xu, J.; Zhang, C.; Chai, Y. Regeneration of large bone defects using mesoporous silica coated magnetic nanoparticles during distraction osteogenesis. Nanomed. Nanotechnol. Biol. Med. 2019, 21, 102040. [Google Scholar] [CrossRef]
- Xia, Y.; Chen, H.; Zhao, Y.; Zhang, F.; Li, X.; Wang, L.; Weir, M.D.; Ma, J.; Reynolds, M.A.; Gu, N.; et al. Novel Magnetic Calcium Phosphate-Stem Cell Construct with Magnetic Field Enhances Osteogenic Differentiation and Bone Tissue Engi-neering. Mater. Sci. Eng. C 2019, 98, 30–41. [Google Scholar] [CrossRef]
- De Santis, R.; Gloria, A.; Russo, T.; D’Amora, U.; Zeppetelli, S.; Tampieri, A.; Herrmannsdörfer, T.; Ambrosio, L. A route toward the development of 3D magnetic scaffolds with tailored mechanical and morphological properties for hard tissue regeneration: Preliminary study. Virtual Phys. Prototyp. 2011, 6, 189–195. [Google Scholar] [CrossRef]
- Vieira, J.; Maurmann, N.; Venturini, J.; Pranke, P.; Bergmann, C.P. PCL-coated magnetic Fe3O4 nanoparticles: Production, characterization and viability on stem cells. Mater. Today Commun. 2022, 31, 103416. [Google Scholar] [CrossRef]
- Feng, P.; Wang, K.; Shuai, Y.; Peng, S.; Hu, Y.; Shuai, C. Hydroxyapatite nanoparticles in situ grown on carbon nanotube as a reinforcement for poly (ε-caprolactone) bone scaffold. Mater. Today Adv. 2022, 15, 100272. [Google Scholar] [CrossRef]
- Jiao, Z.; Luo, B.; Xiang, S.; Ma, H.; Yu, Y.; Yang, W. 3D Printing of HA / PCL Composite Tissue Engineering Scaffolds. Adv. Industrial. Eng. Polym. Res. 2019, 2, 196–202. [Google Scholar] [CrossRef]
- Zhao, Y.Z.; Chen, R.; Xue, P.P.; Luo, L.Z.; Zhong, B.; Tong, M.Q.; Chen, B.; Yao, Q.; Yuan, J.D.; Xu, H.L. Magnetic PLGA Microspheres Loaded with SPIONs Promoted the Reconstruction of Bone Defects through Regulating the Bone Mesen-chymal Stem Cells under an External Magnetic Field. Mater. Sci. Eng. C 2021, 122, 111877. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-W.; Yeh, C.-H.; Shie, M.-Y. Stimulatory effects of the fast setting and suitable degrading Ca–Si–Mg cement on both cementogenesis and angiogenesis differentiation of human periodontal ligament cells. J. Mater. Chem. B 2015, 3, 7099–7108. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-Y.; Lin, Y.-H.; Lee, A.K.-X.; Kuo, T.-Y.; Tsai, C.-H.; Shie, M.-Y. Combined Effects of Polydopamine-Assisted Copper Immobilization on 3D-Printed Porous Ti6Al4V Scaffold for Angiogenic and Osteogenic Bone Regeneration. Cells 2022, 11, 2824. [Google Scholar] [CrossRef] [PubMed]
- Tien, N.; Lee, J.-J.; Lee, A.K.-X.; Lin, Y.-H.; Chen, J.-X.; Kuo, T.-Y.; Shie, M.-Y. Additive Manufacturing of Caffeic Acid-Inspired Mineral Trioxide Aggregate/Poly-ε-Caprolactone Scaffold for Regulating Vascular Induction and Osteogenic Regeneration of Dental Pulp Stem Cells. Cells 2021, 10, 2911. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Chiu, Y.-C.; Shen, Y.-F.; Wu, Y.-H.A.; Shie, M.-Y. Bioactive calcium silicate/poly-ε-caprolactone composite scaffolds 3D printed under mild conditions for bone tissue engineering. J. Mater. Sci. Mater. Med. 2017, 29, 11. [Google Scholar] [CrossRef]
- Kao, C.-T.; Chiu, Y.-C.; Lee, A.K.-X.; Lin, Y.-H.; Huang, T.-H.; Liu, Y.-C.; Shie, M.-Y. The synergistic effects of Xu Duan combined Sr-containing calcium silicate/poly-ε-caprolactone scaffolds for the promotion of osteogenesis marker expression and the induction of bone regeneration in osteoporosis. Mater. Sci. Eng. C 2020, 119, 111629. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhai, D.; Xu, M.; Yao, Q.; Chang, J.; Wu, C. 3D-Printed Bioceramic Scaffolds with a Fe3O4/Graphene Oxide Nanocomposite Interface for Hyperthermia Therapy of Bone Tumor Cells. J. Mater. Chem. B 2016, 4, 2874–2886. [Google Scholar] [CrossRef]
- Zafeiris, K.; Brasinika, D.; Karatza, A.; Koumoulos, E.; Karoussis, I.K.; Kyriakidou, K.; Charitidis, C.A. Additive Manufac-turing of Hydroxyapatite–Chitosan–Genipin Composite Scaffolds for Bone Tissue Engineering Applications. Mater. Sci. Eng. C 2021, 119, 111639. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, S.; Zhou, J.; Zhu, K.; Cui, X.; Huang, W.; Rahaman, M.N.; Zhang, C.; Wang, D. Biocompatibility and Os-teogenic Capacity of Borosilicate Bioactive Glass Scaffolds Loaded with Fe3O4 Magnetic Nanoparticles. J. Mater. Chem. B 2015, 3, 4377–4387. [Google Scholar] [CrossRef]
- Yang, C.; Huan, Z.; Wang, X.; Wu, C.; Chang, J. 3D Printed Fe Scaffolds with HA Nanocoating for Bone Regeneration. ACS Biomater. Sci. Eng. 2018, 4, 608–616. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, H.; Qin, C.; Zhang, M.; Ma, J.; Zhai, D.; Ma, B.; Ma, N.; Huan, Z.; Wu, C. 3D-printed bioceramic scaffolds with Fe3S4 microflowers for magnetothermal and chemodynamic therapy of bone tumor and regeneration of bone defects. Biofabrication 2021, 13, 045010. [Google Scholar] [CrossRef] [PubMed]
- Yue, C.; Li, M.; Liu, Y.; Fang, Y.; Song, Y.; Xu, M.; Li, J. Three-dimensional printing of cellulose nanofibers reinforced PHB/PCL/Fe3O4 magneto-responsive shape memory polymer composites with excellent mechanical properties. Addit. Manuf. 2021, 46, 102146. [Google Scholar] [CrossRef]
- Wang, X.; Zhai, D.; Yao, X.; Wang, Y.; Ma, H.; Yu, X.; Du, L.; Lin, H.; Wu, C. 3D printing of pink bioceramic scaffolds for bone tumor tissue therapy. Appl. Mater. Today 2022, 27, 101443. [Google Scholar] [CrossRef]
- Lin, Y.H.; Lee, K.X.; Ho, C.C.; Fang, M.J.; Kuo, T.Y.; Shie, M.Y. The Effects of a 3D-Printed Magnesium-/Strontium-Doped Calcium Silicate Scaffold on Regulation of Bone Regeneration via Dual-Stimulation of the AKT and WNT Signaling Pathways. Biomater. Adv. 2022, 135, 112660. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ling, C.; Chen, J.; Liu, H.; Mo, Q.; Zhang, W.; Yao, Q. 3D-printed composite scaffold with gradient structure and programmed biomolecule delivery to guide stem cell behavior for osteochondral regeneration. Biomater. Adv. 2022, 140, 213067. [Google Scholar] [CrossRef]
- Lee, J.-J.; Ng, H.Y.; Lin, Y.-H.; Liu, E.-W.; Lin, T.-J.; Chiu, H.-T.; Ho, X.-R.; Yang, H.-A.; Shie, M.-Y. The 3D printed conductive grooved topography hydrogel combined with electrical stimulation for synergistically enhancing wound healing of dermal fibroblast cells. Biomater. Adv. 2022, 142, 213132. [Google Scholar] [CrossRef]
- Qi, D.; Su, J.; Li, S.; Zhu, H.; Cheng, L.; Hua, S.; Yuan, X.; Jiang, J.; Shu, Z.; Shi, Y.; et al. 3D printed magnesium-doped β-TCP gyroid scaffold with osteogenesis, angiogenesis, immunomodulation properties and bone regeneration capability in vivo. Biomater. Adv. 2022, 136, 212759. [Google Scholar] [CrossRef]
- Dang, W.; Li, T.; Li, B.; Ma, H.; Zhai, D.; Wang, X.; Chang, J.; Xiao, Y.; Wang, J.; Wu, C. A bifunctional scaffold with CuFeSe2 nanocrystals for tumor therapy and bone reconstruction. Biomaterials 2018, 160, 92–106. [Google Scholar] [CrossRef]
- Aghajanian, A.H.; Bigham, A.; Sanati, A.; Kefayat, A.; Salamat, M.R.; Sattary, M.; Rafienia, M. A 3D macroporous and magnetic Mg2SiO4-CuFe2O4 scaffold for bone tissue regeneration: Surface modification, in vitro and in vivo studies. Biomater. Adv. 2022, 137, 212809. [Google Scholar] [CrossRef]
- Xia, Y.; Guo, Y.; Yang, Z.; Chen, H.; Ren, K.; Weir, M.D.; Chow, L.C.; Reynolds, M.A.; Zhang, F.; Gu, N.; et al. Iron Oxide Nanoparticle-Calcium Phosphate Cement Enhanced the Osteogenic Activities of Stem Cells through WNT/β-Catenin Sig-naling. Mater. Sci. Eng. C 2019, 104, 109955. [Google Scholar] [CrossRef]
- Lew, W.-Z.; Feng, S.-W.; Lee, S.-Y.; Huang, H.-M. The Review of Bioeffects of Static Magnetic Fields on the Oral Tissue-Derived Cells and Its Application in Regenerative Medicine. Cells 2021, 10, 2662. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Li, C.; Masood, M.; Zhang, Z.; González-Almela, E.; Castells-Garcia, A.; Zou, G.; Xu, X.; Wang, L.; Zhao, G.; et al. Static Magnetic Fields Regulate T-Type Calcium Ion Channels and Mediate Mesenchymal Stem Cells Proliferation. Cells 2022, 11, 2460. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; LV, C.; Zhou, X.; Wu, Z.; Wang, Z.; Wang, Y.; Wang, L.; Zhu, Y.; Guo, M.; Zhang, P. Tannin-Bridged Magnetic Responsive Multifunctional Hydrogel for Enhanced Wound Healing by Mechanical Stimulation-Induced Early Vasculariza-tion. J. Mater. Chem. B 2022, 10, 7808–7826. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, X.; Fu, K.; Wei, G.; Su, Z. Stimulus-responsive nanomaterials under physical regulation for biomedical applications. J. Mater. Chem. B 2021, 9, 9642–9657. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Chen, H.; Zhou, F.; Liu, J.; Qian, Y.; Hu, K.; Yan, J.; Gu, Z.; Guo, Z.; Zhang, F.; et al. Superparamagnetic core–shell electrospun scaffolds with sustained release of IONPs facilitating in vitro and in vivo bone regeneration. J. Mater. Chem. B 2021, 9, 8980–8993. [Google Scholar] [CrossRef]
- Hao, S.; Meng, J.; Zhang, Y.; Liu, J.; Nie, X.; Wu, F.; Yang, Y.; Wang, C.; Gu, N.; Xu, H. Macrophage Phenotypic Mecha-nomodulation of Enhancing Bone Regeneration by Superparamagnetic Scaffold upon Magnetization. Biomaterials 2017, 140, 16–25. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, Q.; Yang, M.; Zhan, X.; Lan, F.; He, J.; Gu, Z.; Wu, Y. Protein Corona of Magnetic Hydroxyapatite Scaffold Improves Cell Proliferation via Activation of Mitogen-Activated Protein Kinase Signaling Pathway. ACS Nano 2017, 11, 3690–3704. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kao, C.-Y.; Lin, T.-L.; Lin, Y.-H.; Lee, A.K.-X.; Ng, S.Y.; Huang, T.-H.; Hsu, T.-T. Synergistic Effect of Static Magnetic Fields and 3D-Printed Iron-Oxide-Nanoparticle-Containing Calcium Silicate/Poly-ε-Caprolactone Scaffolds for Bone Tissue Engineering. Cells 2022, 11, 3967. https://doi.org/10.3390/cells11243967
Kao C-Y, Lin T-L, Lin Y-H, Lee AK-X, Ng SY, Huang T-H, Hsu T-T. Synergistic Effect of Static Magnetic Fields and 3D-Printed Iron-Oxide-Nanoparticle-Containing Calcium Silicate/Poly-ε-Caprolactone Scaffolds for Bone Tissue Engineering. Cells. 2022; 11(24):3967. https://doi.org/10.3390/cells11243967
Chicago/Turabian StyleKao, Chuan-Yi, Tsung-Li Lin, Yen-Hong Lin, Alvin Kai-Xing Lee, Sing Yee Ng, Tsui-Hsien Huang, and Tuan-Ti Hsu. 2022. "Synergistic Effect of Static Magnetic Fields and 3D-Printed Iron-Oxide-Nanoparticle-Containing Calcium Silicate/Poly-ε-Caprolactone Scaffolds for Bone Tissue Engineering" Cells 11, no. 24: 3967. https://doi.org/10.3390/cells11243967
APA StyleKao, C.-Y., Lin, T.-L., Lin, Y.-H., Lee, A. K.-X., Ng, S. Y., Huang, T.-H., & Hsu, T.-T. (2022). Synergistic Effect of Static Magnetic Fields and 3D-Printed Iron-Oxide-Nanoparticle-Containing Calcium Silicate/Poly-ε-Caprolactone Scaffolds for Bone Tissue Engineering. Cells, 11(24), 3967. https://doi.org/10.3390/cells11243967







