Phytocannabinoids Stimulate Rejuvenation and Prevent Cellular Senescence in Human Dermal Fibroblasts
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture and Maintenance
2.2. Senescence-Associated Phenotype Modelling
2.3. Cannabinoid and Nutrient Signaling Regulators Treatments
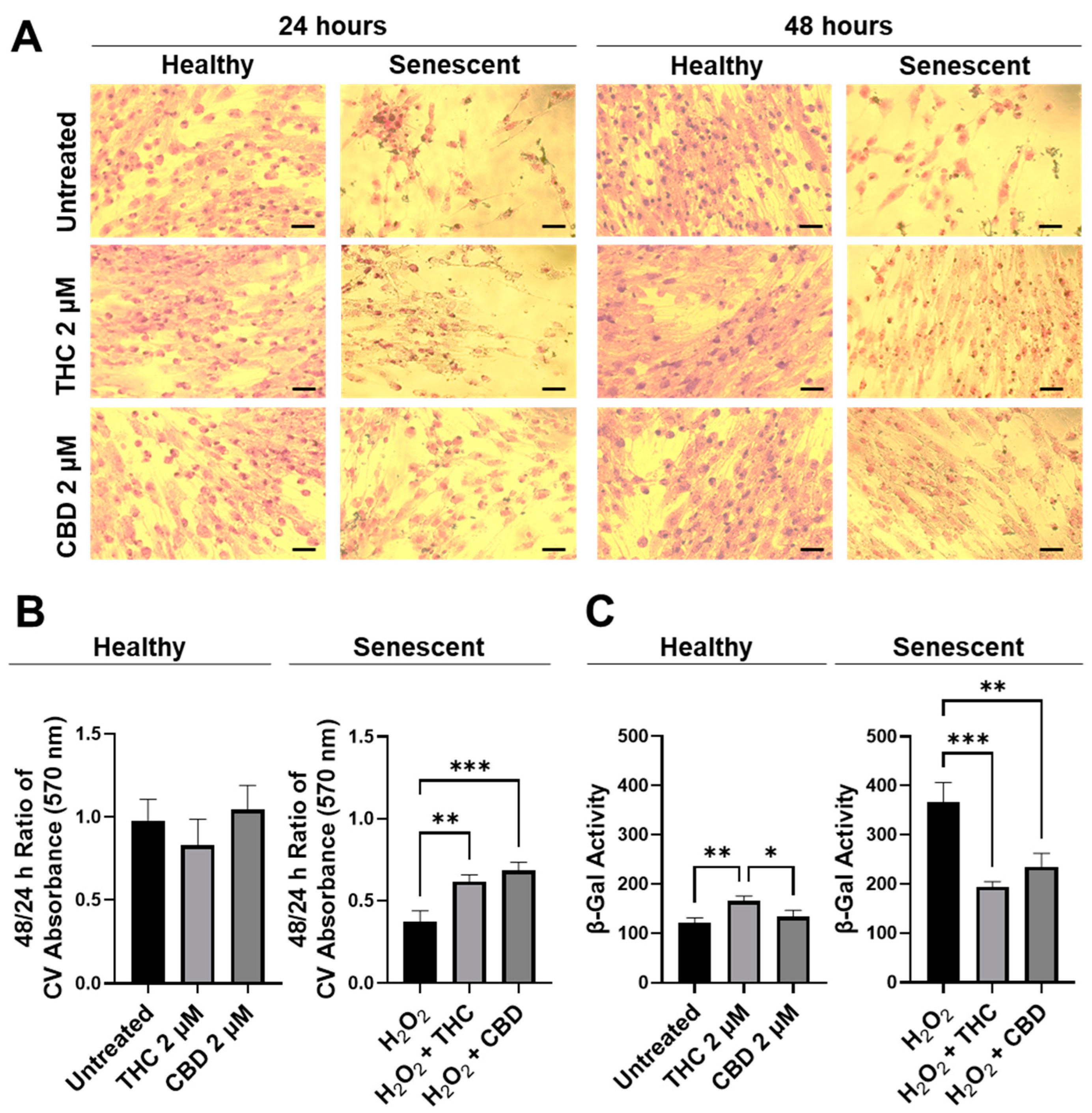
2.4. Senescence-Associated β-Galactosidase Activity
2.5. Cell Viability/Cytotoxicity Assays
2.6. Protein Extraction and Western Blot Analysis
2.7. Reverse Transcription Polymerase Chain Reaction (RT-PCR)
2.8. Wound-Healing Assay
2.9. Immunocytochemistry, Microscopy, and QuPath Analysis
2.10. Statistical Analysis
3. Results
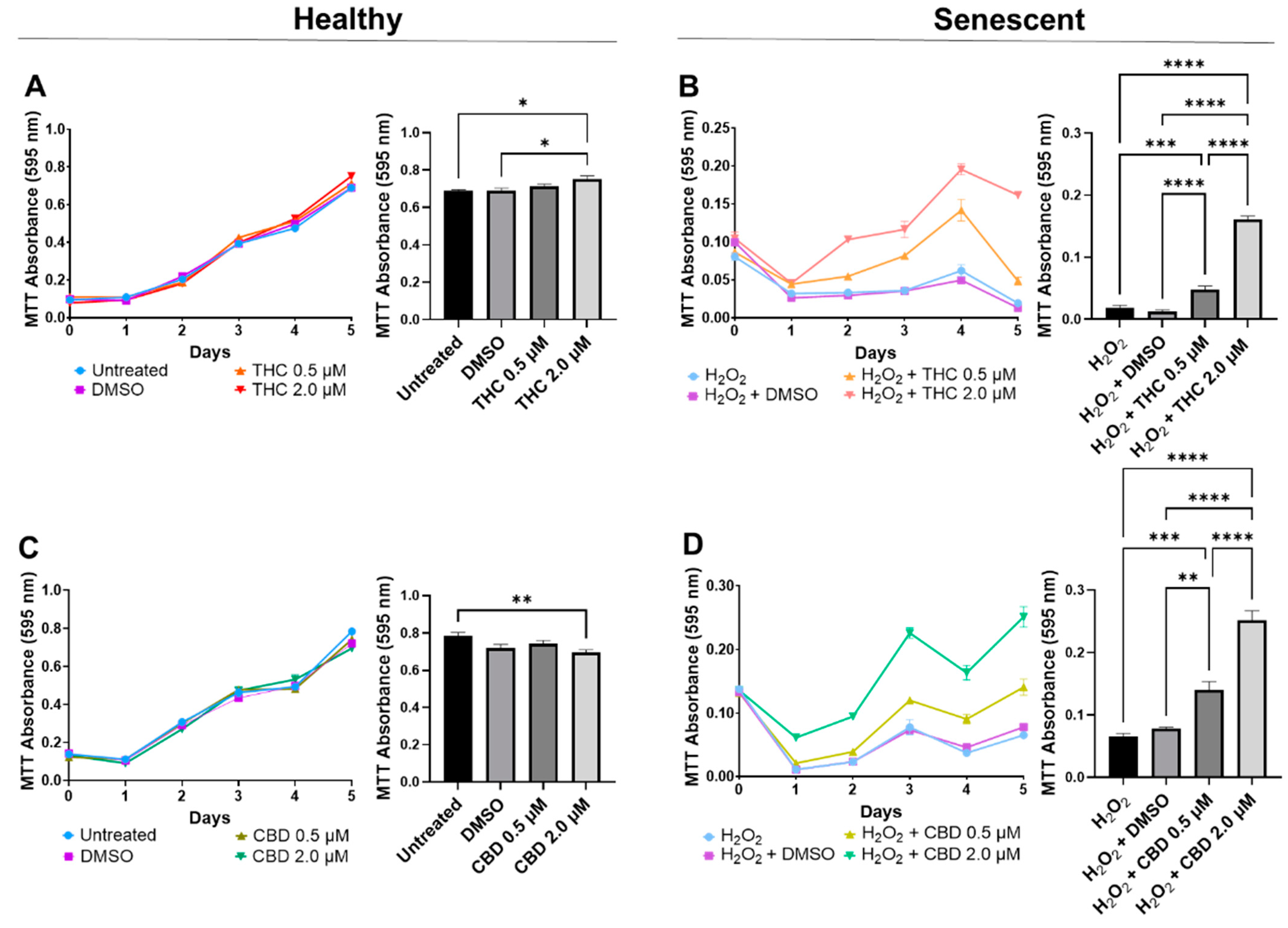
3.1. Optimizing Phytocannabinoid Dosing on Human Dermal Fibroblasts
3.2. Phytocannabinoids Enhance Cellular Viability in SIPS Fibroblasts
3.3. Phytocannabinoids Improve Wound Healing of Human Dermal Fibroblasts
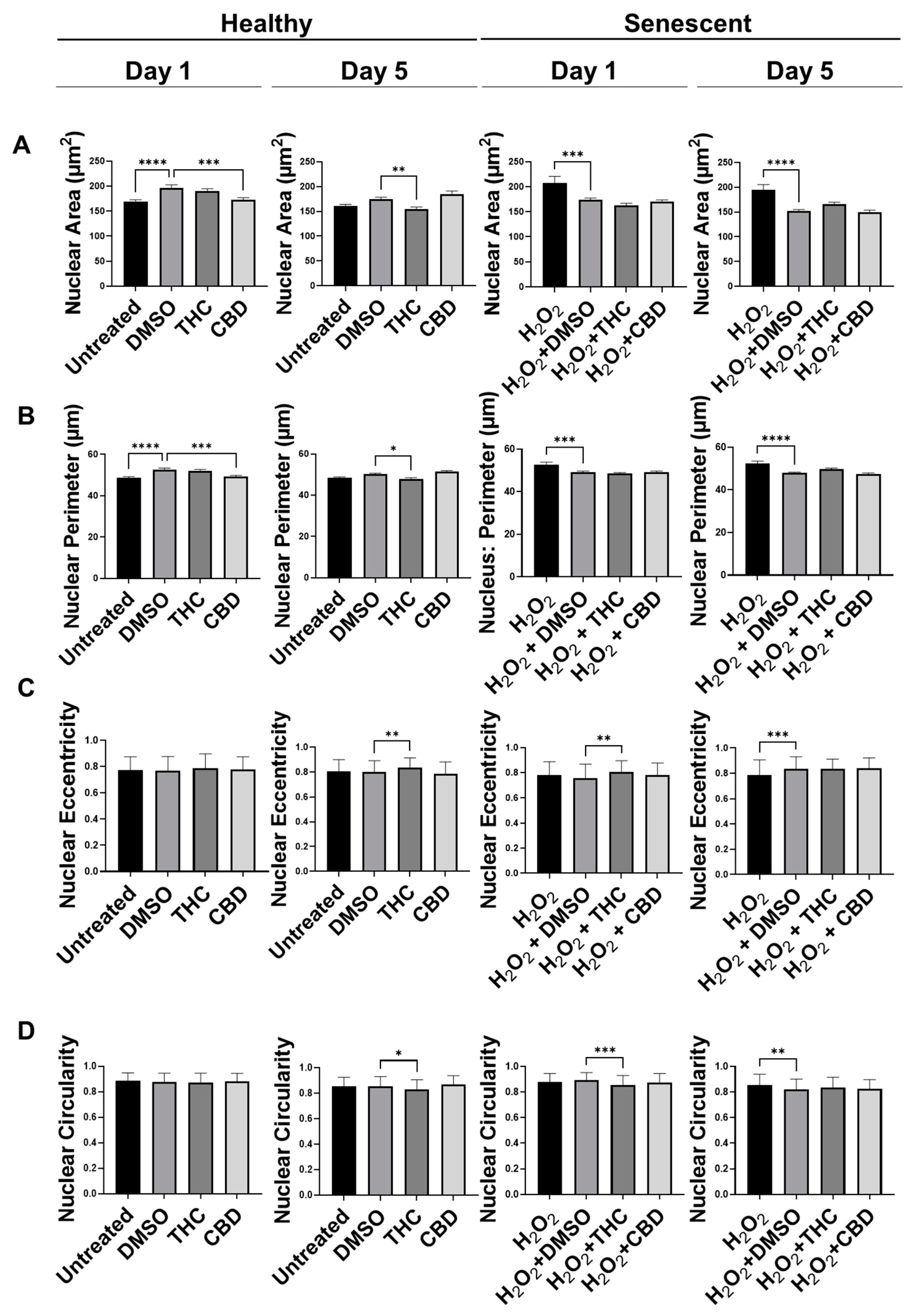
3.4. Phytocannabinoids Alter Nuclear Morphology of Human Dermal Fibroblasts
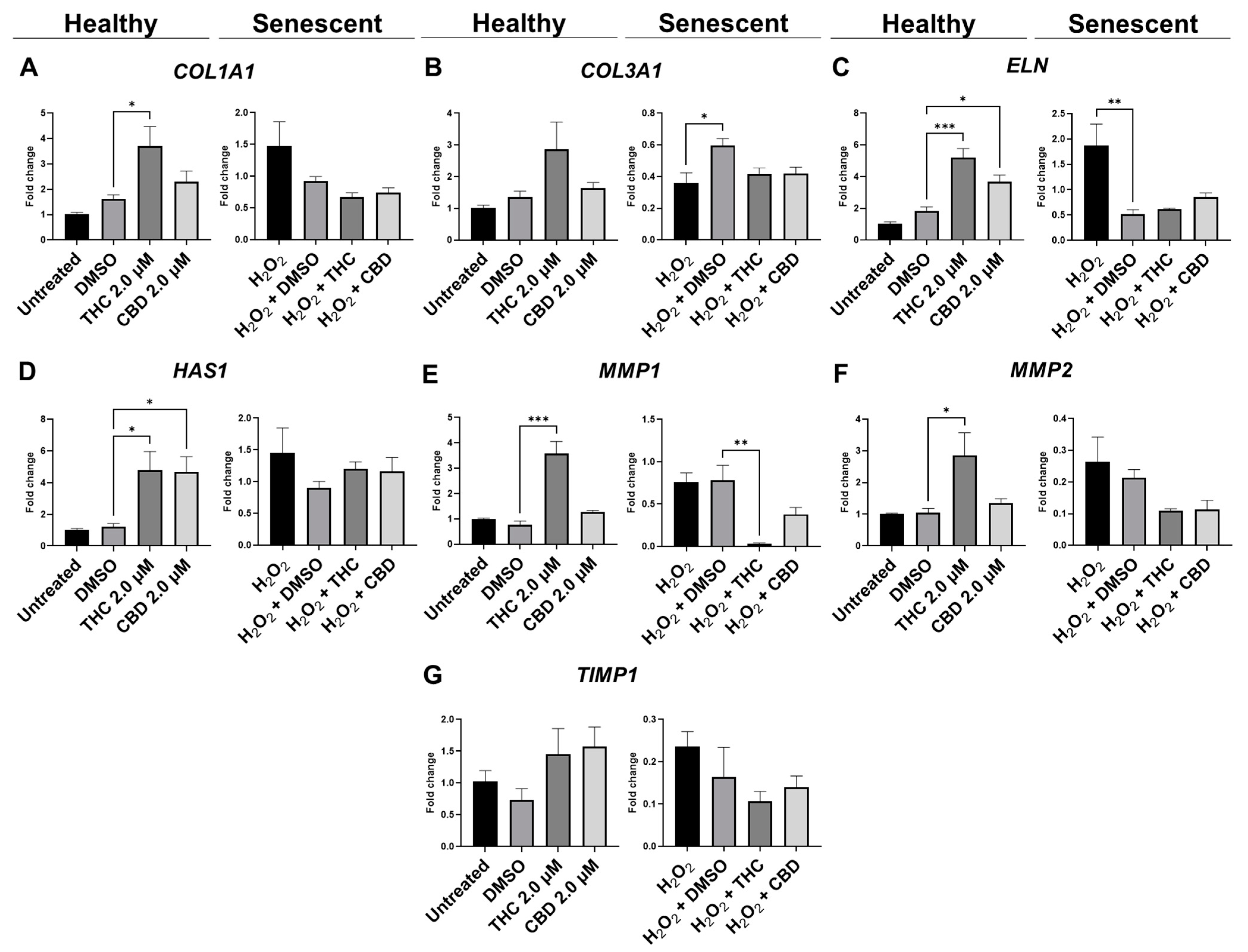
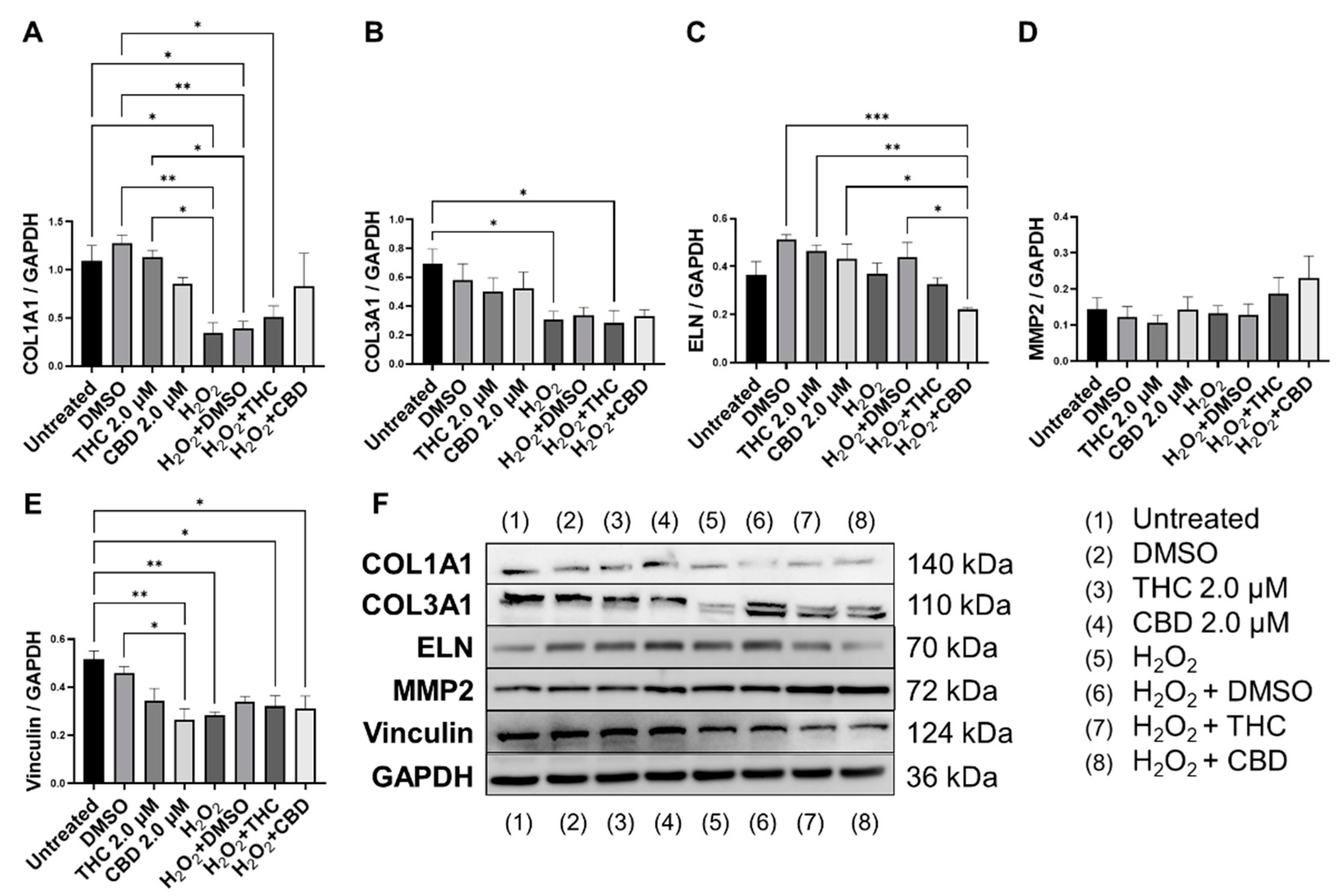
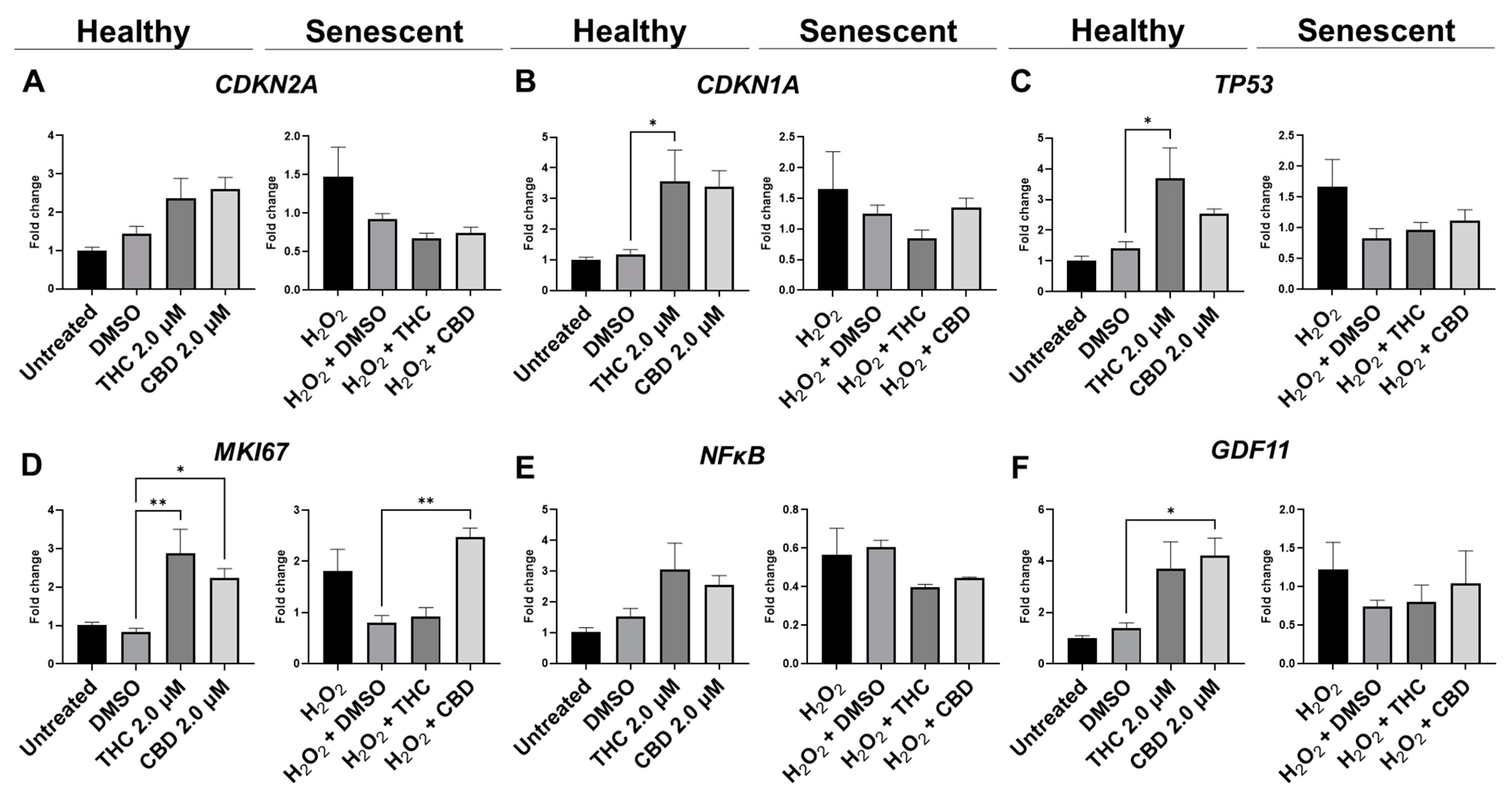
3.5. Protective Effects of Phytocannabinoids on the Expression of Age-Related Genes Involved in Extracellular Matrix Maintenance
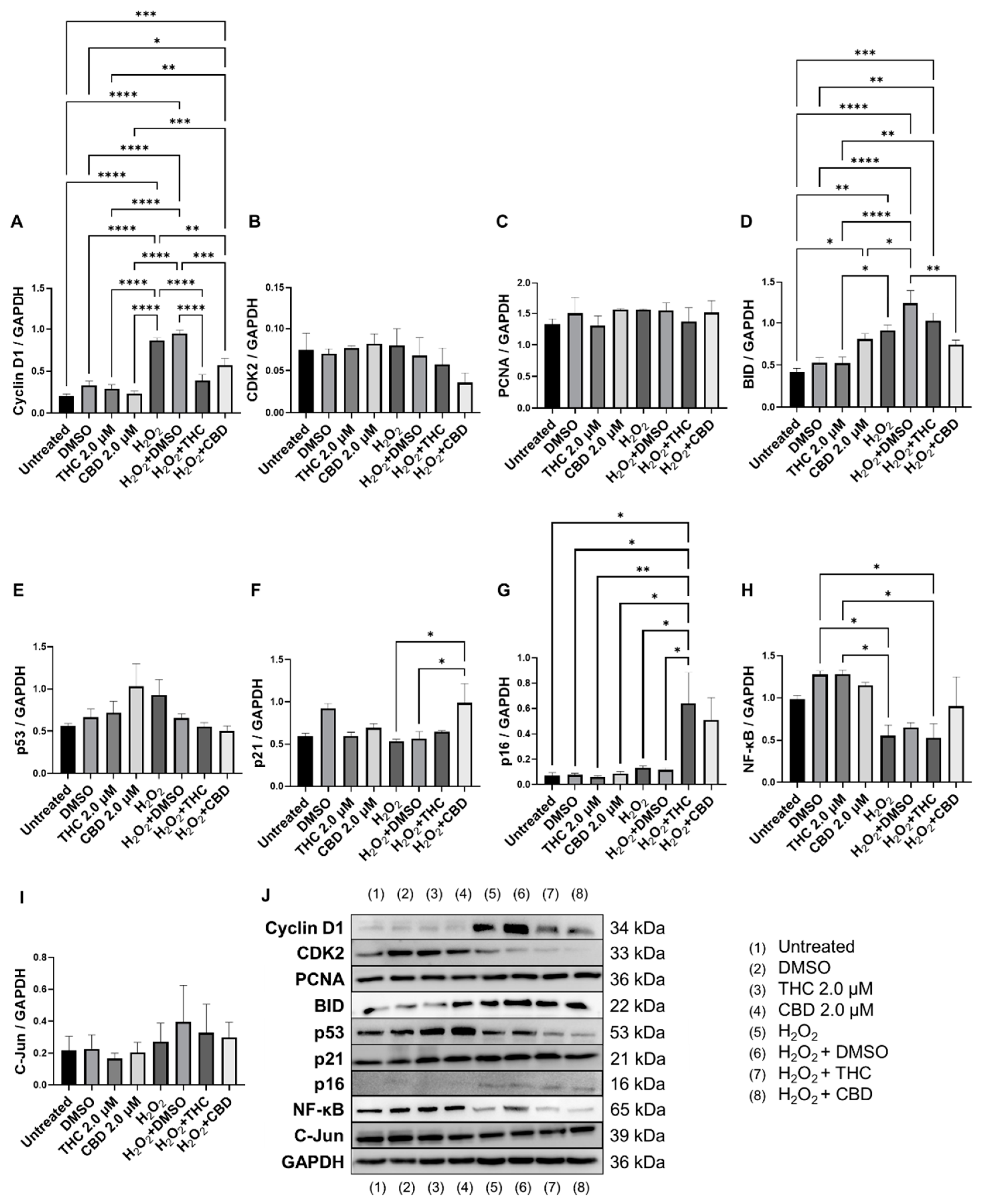
3.6. Effects of Phytocannabinoids on Cell Cycle Regulators
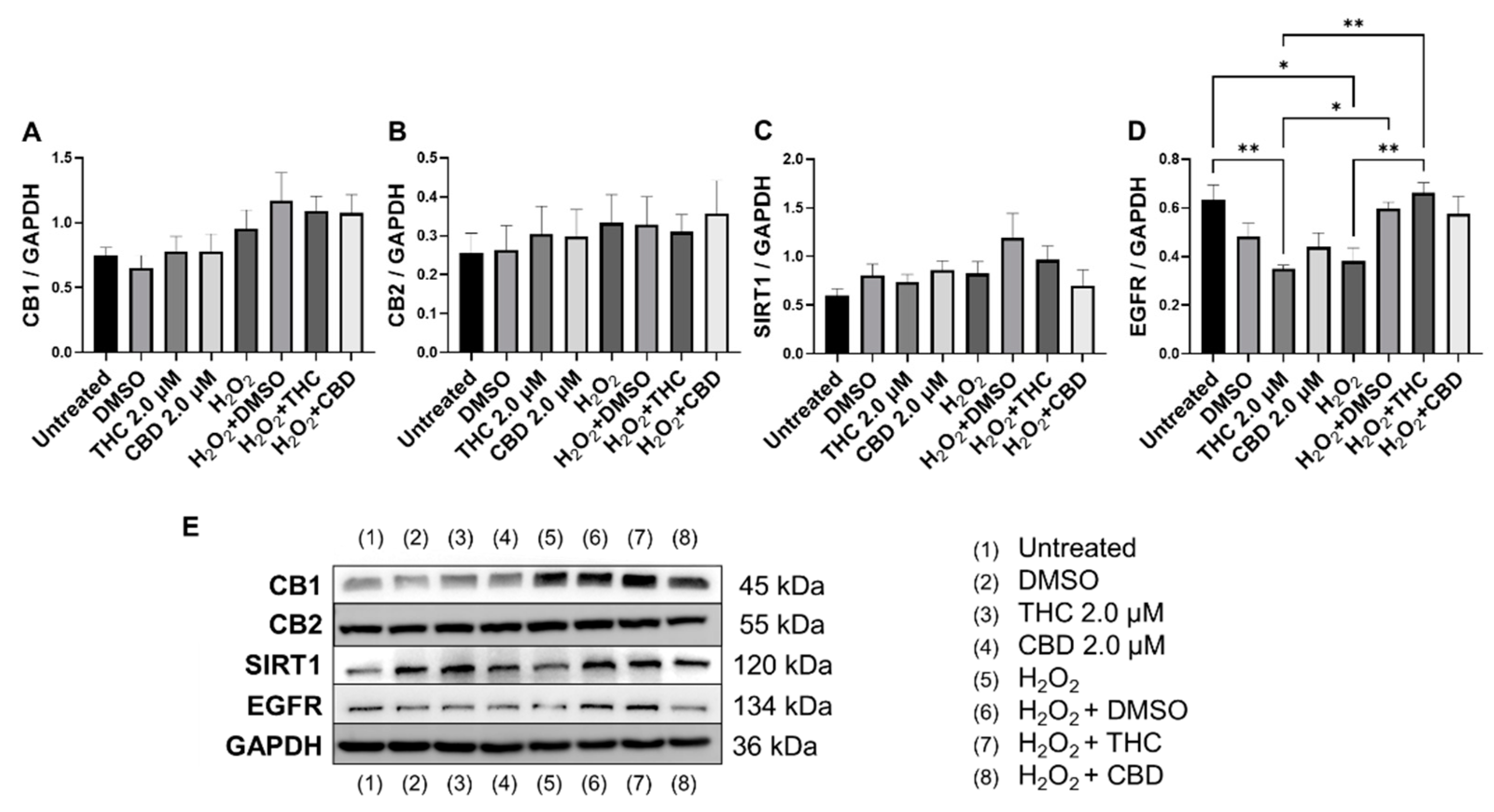
3.7. Effects of Phytocannabinoids on Metabolic Regulators and Cannabinoid Receptors
4. Discussion
5. Conclusions
- (i)
- pCBs have a therapeutic range of 0.5–2 µM in dermal fibroblasts,
- (ii)
- THC and CBD stimulated fibroblasts’ ability to close damaged wounds, while THC induced wound healing better than common nutrient signaling regulators,
- (iii)
- THC reduced morphological alterations in skin cells, potentiated cellular viability via preserving cell-cycle regulators, and boosted ECM production,
- (iv)
- CBD provided few beneficial effects on dermal fibroblasts.
- (v)
- Our data helps fill the gap regarding the potential role of THC/CBD in anti-aging research and demonstrates THC has therapeutic potential for cosmetic applications.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lim, M.; Kirchhof, M.G. Dermatology-Related Uses of Medical Cannabis Promoted by Dispensaries in Canada, Europe, and the United States. J. Cutan. Med. Surg. 2019, 23, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Dhadwal, G.; Kirchhof, M.G. The Risks and Benefits of Cannabis in the Dermatology Clinic. J. Cutan. Med. Surg. 2018, 22, 194–199. [Google Scholar] [CrossRef] [PubMed]
- MacCallum, C.A.; Russo, E.B. Practical considerations in medical cannabis administration and dosing. Eur. J. Intern. Med. 2018, 49, 12–19. [Google Scholar] [CrossRef]
- Ramot, Y.; Sugawara, K.; Zákány, N.; Tóth, B.I.; Bíró, T.; Paus, R. A novel control of human keratin expression: Cannabinoid receptor 1-mediated signaling down-regulates the expression of keratins K6 and K16 in human keratinocytes in vitro and in situ. PeerJ 2013, 1, e40. [Google Scholar] [CrossRef] [PubMed]
- Oláh, A.; Szabó-Papp, J.; Soeberdt, M.; Knie, U.; Dähnhardt-Pfeiffer, S.; Abels, C.; Bíró, T. Echinacea purpurea-derived alkylamides exhibit potent anti-inflammatory effects and alleviate clinical symptoms of atopic eczema. J. Dermatol. Sci. 2017, 88, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Ikeda, S.R. Endocannabinoids modulate N-type calcium channels and G-protein-coupled inwardly rectifying potassium channels via CB1 cannabinoid receptors heterologously expressed in mammalian neurons. Mol. Pharmacol. 2004, 65, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Moreno, F.A.; Wiegand, C.B.; Taitano, E.K.; Delgado, P.L. Safety, tolerability, and efficacy of psilocybin in 9 patients with obsessive-compulsive disorder. J. Clin. Psychiatry 2006, 67, 1735–1740. [Google Scholar] [CrossRef] [PubMed]
- Toutfaire, M.; Bauwens, E.; Debacq-Chainiaux, F. The impact of cellular senescence in skin ageing: A notion of mosaic and therapeutic strategies. Biochem. Pharmacol. 2017, 142, 1–12. [Google Scholar] [CrossRef]
- Gerasymchuk, M.; Cherkasova, V.; Kovalchuk, O.; Kovalchuk, I. The Role of microRNAs in Organismal and Skin Aging. Int. J. Mol. Sci. 2020, 21, 5281. [Google Scholar] [CrossRef] [PubMed]
- Liszewski, W.; Farah, R.S. Response to: “The role of cannabinoids in dermatology”. J. Am. Acad. Dermatol. 2017, 77, e87–e88. [Google Scholar] [CrossRef] [PubMed]
- Maida, V.; Shi, R.B.; Fazzari, F.G.T.; Zomparelli, L. Promoting wound healing of uremic calciphylaxis leg ulcers using topical cannabis-based medicines. Dermatol. Ther. 2020, 33, e14419. [Google Scholar] [CrossRef]
- Chelliah, M.P.; Zinn, Z.; Khuu, P.; Teng, J.M.C. Self-initiated use of topical cannabidiol oil for epidermolysis bullosa. Pediatr. Dermatol. 2018, 35, e224–e227. [Google Scholar] [CrossRef]
- Eberlein, B.; Eicke, C.; Reinhardt, H.W.; Ring, J. Adjuvant treatment of atopic eczema: Assessment of an emollient containing N-palmitoylethanolamine (ATOPA study). J. Eur. Acad. Dermatol. Venereol. 2008, 22, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Hampson, A.J.; Grimaldi, M.; Axelrod, J.; Wink, D. Cannabidiol and (-)Delta9-tetrahydrocannabinol are neuroprotective antioxidants. Proc. Natl. Acad. Sci. USA 1998, 95, 8268–8273. [Google Scholar] [CrossRef] [PubMed]
- Oláh, A.; Tóth, B.I.; Borbíró, I.; Sugawara, K.; Szöllõsi, A.G.; Czifra, G.; Pál, B.; Ambrus, L.; Kloepper, J.; Camera, E.; et al. Cannabidiol exerts sebostatic and antiinflammatory effects on human sebocytes. J. Clin. Invest. 2014, 124, 3713–3724. [Google Scholar] [CrossRef] [PubMed]
- Kozela, E.; Juknat, A.; Kaushansky, N.; Rimmerman, N.; Ben-Nun, A.; Vogel, Z. Cannabinoids Decrease the Th17 Inflammatory Autoimmune Phenotype. J. Neuroimmune Pharmacol. 2013, 8, 1265–1276. [Google Scholar] [CrossRef]
- Gerasymchuk, M.; Robinson, G.I.; Kovalchuk, O.; Kovalchuk, I. Modeling of the Senescence-Associated Phenotype in Human Skin Fibroblasts. Int. J. Mol. Sci. 2022, 23, 7124. [Google Scholar] [CrossRef] [PubMed]
- Gerasymchuk, M.; Robinson, G.I.; Kovalchuk, O.; Kovalchuk, I. The Effects of Nutrient Signaling Regulators in Combination with Phytocannabinoids on the Senescence-Associated Phenotype in Human Dermal Fibroblasts. Int. J. Mol. Sci. 2022, 23, 8804. [Google Scholar] [CrossRef] [PubMed]
- Di Giacomo, V.; Recinella, L.; Chiavaroli, A.; Orlando, G.; Cataldi, A.; Rapino, M.; Di Valerio, V.; Politi, M.; Antolini, M.; Acquaviva, A.; et al. Metabolomic Profile and Antioxidant/Anti-Inflammatory Effects of Industrial Hemp Water Extract in Fibroblasts, Keratinocytes and Isolated Mouse Skin Specimens. Antioxidants 2021, 10, E44. [Google Scholar] [CrossRef]
- Styrczewska, M.; Kostyn, A.; Kulma, A.; Majkowska-Skrobek, G.; Augustyniak, D.; Prescha, A.; Czuj, T.; Szopa, J. Flax Fiber Hydrophobic Extract Inhibits Human Skin Cells Inflammation and Causes Remodeling of Extracellular Matrix and Wound Closure Activation. Biomed. Res. Int. 2015, 2015, 862391. [Google Scholar] [CrossRef]
- Martinelli, G.; Magnavacca, A.; Fumagalli, M.; Dell’Agli, M.; Piazza, S.; Sangiovanni, E. Cannabis sativa and Skin Health: Dissecting the Role of Phytocannabinoids. Planta Med. 2022, 88, 492–506. [Google Scholar] [CrossRef] [PubMed]
- Szachowicz-Petelska, B.; Łuczaj, W.; Wroński, A.; Jastrząb, A.; Dobrzyńska, I. The Differential Effect of Cannabidiol on the Composition and Physicochemical Properties of Keratinocyte and Fibroblast Membranes from Psoriatic Patients and Healthy People. Membranes 2021, 11, 111. [Google Scholar] [CrossRef] [PubMed]
- Gęgotek, A.; Atalay, S.; Domingues, P.; Skrzydlewska, E. The Differences in the Proteome Profile of Cannabidiol-Treated Skin Fibroblasts following UVA or UVB Irradiation in 2D and 3D Cell Cultures. Cells 2019, 8, 995. [Google Scholar] [CrossRef] [PubMed]
- Brand, R.M.; Charron, A.R.; Sandler, V.L.; Jendrzejewski, J.L. Moisturizing Lotions can Increase Transdermal Absorption of the Herbicide 2,4-Dichlorophenoxacetic Acid Across Hairless Mouse Skin. Cutan. Ocul. Toxicol. 2007, 26, 15–23. [Google Scholar] [CrossRef]
- Gęgotek, A.; Bielawska, K.; Biernacki, M.; Dobrzyńska, I.; Skrzydlewska, E. Time-dependent effect of rutin on skin fibroblasts membrane disruption following UV radiation. Redox Biol. 2017, 12, 733–744. [Google Scholar] [CrossRef]
- Feoktistova, M.; Geserick, P.; Leverkus, M. Crystal Violet Assay for Determining Viability of Cultured Cells. Cold Spring Harb. Protoc. 2016, 2016, pdb.prot087379. [Google Scholar] [CrossRef]
- Repetto, G.; del Peso, A.; Zurita, J.L. Neutral red uptake assay for the estimation of cell viability/cytotoxicity. Nat. Protoc. 2008, 3, 1125–1131. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing real-time PCR data by the comparative CT method. Nat. Protoc. 2008, 3, 1101–1108. [Google Scholar] [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef]
- Dimri, G.P.; Lee, X.; Basile, G.; Acosta, M.; Scott, G.; Roskelley, C.; Medrano, E.E.; Linskens, M.; Rubelj, I.; Pereira-Smith, O.; et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc. Natl. Acad. Sci. USA 1995, 92, 9363–9367. [Google Scholar] [CrossRef]
- Baldin, V.; Lukas, J.; Marcote, M.J.; Pagano, M.; Draetta, G. Cyclin D1 is a nuclear protein required for cell cycle progression in G1. Genes Dev. 1993, 7, 812–821. [Google Scholar] [CrossRef] [PubMed]
- Fu, M.; Wang, C.; Li, Z.; Sakamaki, T.; Pestell, R.G. Minireview: Cyclin D1: Normal and abnormal functions. Endocrinology 2004, 145, 5439–5447. [Google Scholar] [CrossRef] [PubMed]
- Saab, R.; Rodriguez-Galindo, C.; Matmati, K.; Rehg, J.E.; Baumer, S.H.; Khoury, J.D.; Billups, C.; Neale, G.; Helton, K.J.; Skapek, S.X. p18Ink4c and p53 Act as tumor suppressors in cyclin D1-driven primitive neuroectodermal tumor. Cancer Res. 2009, 69, 440–448. [Google Scholar] [CrossRef]
- Demidenko, Z.N.; Blagosklonny, M.V. Growth stimulation leads to cellular senescence when the cell cycle is blocked. Cell Cycle 2008, 7, 3355–3361. [Google Scholar] [CrossRef]
- Chia, C.W.; Sherman-Baust, C.A.; Larson, S.A.; Pandey, R.; Withers, R.; Karikkineth, A.C.; Zukley, L.M.; Campisi, J.; Egan, J.M.; Sen, R.; et al. Age-associated expression of p21and p53 during human wound healing. Aging Cell 2021, 20, e13354. [Google Scholar] [CrossRef] [PubMed]
- Wisdom, R.; Johnson, R.S.; Moore, C. c-Jun regulates cell cycle progression and apoptosis by distinct mechanisms. EMBO J. 1999, 18, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Tito, A.; Barbulova, A.; Zappelli, C.; Leone, M.; Ruvo, M.; Mercurio, F.A.; Chambery, A.; Russo, R.; Colucci, M.G.; Apone, F. The Growth Differentiation Factor 11 is Involved in Skin Fibroblast Ageing and is Induced by a Preparation of Peptides and Sugars Derived from Plant Cell Cultures. Mol. Biotechnol. 2019, 61, 209–220. [Google Scholar] [CrossRef]
- Shiraha, H.; Gupta, K.; Drabik, K.; Wells, A. Aging fibroblasts present reduced epidermal growth factor (EGF) responsiveness due to preferential loss of EGF receptors. J. Biol. Chem. 2000, 275, 19343–19351. [Google Scholar] [CrossRef]
- Ketcherside, A.; Noble, L.J.; McIntyre, C.K.; Filbey, F.M. Cannabinoid Receptor 1 Gene by Cannabis Use Interaction on CB1 Receptor Density. Cannabis Cannabinoid Res. 2017, 2, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Zuardi, A.W. History of cannabis as a medicine: A review. Braz. J. Psychiatry 2006, 28, 153–157. [Google Scholar] [CrossRef]
- Merve, A.O.; Sobiecka, P.; Remeškevičius, V.; Taylor, L.; Saskoy, L.; Lawton, S.; Jones, B.P.; Elwakeel, A.; Mackenzie, F.E.; Polycarpou, E.; et al. Metabolites of Cannabis Induce Cardiac Toxicity and Morphological Alterations in Cardiac Myocytes. Int. J. Mol. Sci. 2022, 23, 1401. [Google Scholar] [CrossRef] [PubMed]
- Hohmann, T.; Feese, K.; Ghadban, C.; Dehghani, F.; Grabiec, U. On the influence of cannabinoids on cell morphology and motility of glioblastoma cells. PLoS ONE 2019, 14, e0212037. [Google Scholar] [CrossRef]
- McClean, D.K.; Zimmerman, A.M. Action of delta 9-tetrahydrocannabinol on cell division and macromolecular synthesis in division-synchronized protozoa. Pharmacology 1976, 14, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Ngoi, N.Y.; Liew, A.Q.; Chong, S.J.F.; Davids, M.S.; Clement, M.V.; Pervaiz, S. The redox-senescence axis and its therapeutic targeting. Redox Biol. 2021, 45, 102032. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, M.; Kolbus, A.; Piu, F.; Szabowski, A.; Möhle-Steinlein, U.; Tian, J.; Karin, M.; Angel, P.; Wagner, E.F. Control of cell cycle progression by c-Jun is p53 dependent. Genes Dev. 1999, 13, 607–619. [Google Scholar] [CrossRef]
- Birch, J.; Gil, J. Senescence and the SASP: Many therapeutic avenues. Genes Dev. 2020, 34, 1565–1576. [Google Scholar] [CrossRef]
- Guarente, L. Franklin, H. Epstein Lecture: Sirtuins, aging, and medicine. N. Engl. J. Med. 2011, 364, 2235–2244. [Google Scholar] [CrossRef]
- Turan, V.; Oktay, K. BRCA-related ATM-mediated DNA double-strand break repair and ovarian aging. Hum. Reprod. Update 2020, 26, 43–57. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef]
- Yo, K.; Rünger, T.M. The long non-coding RNA FLJ46906 binds to the transcription factors NF-κB and AP-1 and regulates expression of aging-associated genes. Aging 2018, 10, 2037–2050. [Google Scholar] [CrossRef]
- Ruland, J. Return to homeostasis: Downregulation of NF-κB responses. Nat. Immunol. 2011, 12, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Lee, J.H.; Lee, H.Y.; Min, K.J. Sirtuin signaling in cellular senescence and aging. BMB Rep. 2019, 52, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Bonkowski, M.S.; Sinclair, D.A. Slowing ageing by design: The rise of NAD+ and sirtuin-activating compounds. Nat. Rev. Mol. Cell Biol. 2016, 17, 679–690. [Google Scholar] [CrossRef]
- Song, J.; Yang, B.; Jia, X.; Li, M.; Tan, W.; Ma, S.; Shi, X.; Feng, L. Distinctive Roles of Sirtuins on Diabetes, Protective or Detrimental? Front. Endocrinol. 2018, 9, 724. [Google Scholar] [CrossRef]
- Baur, J.A. Biochemical effects of SIRT1 activators. Biochim. Biophys. Acta 2010, 1804, 1626–1634. [Google Scholar] [CrossRef] [PubMed]
- Yessenkyzy, A.; Saliev, T.; Zhanaliyeva, M.; Masoud, A.-R.; Umbayev, B.; Sergazy, S.; Krivykh, E.; Gulyayev, A.; Nurgozhin, T. Polyphenols as Caloric-Restriction Mimetics and Autophagy Inducers in Aging Research. Nutrients 2020, 12, 1344. [Google Scholar] [CrossRef] [PubMed]
- Betsinger, C.N.; Cristea, I.M. Mitochondrial Function, Metabolic Regulation, and Human Disease Viewed through the Prism of Sirtuin 4 (SIRT4) Functions. J. Proteome Res. 2019, 18, 1929–1938. [Google Scholar] [CrossRef]
- Kofman, A.E.; Huszar, J.M.; Payne, C.J. Transcriptional analysis of histone deacetylase family members reveal similarities between differentiating and aging spermatogonial stem cells. Stem Cell Rev. Rep. 2013, 9, 59–64. [Google Scholar] [CrossRef] [PubMed]
- Dong, K.; Pelle, E.; Yarosh, D.B.; Pernodet, N. Sirtuin 4 identification in normal human epidermal keratinocytes and its relation to sirtuin 3 and energy metabolism under normal conditions and UVB-induced stress. Exp. Dermatol. 2012, 21, 231–233. [Google Scholar] [CrossRef]
- Lang, A.; Grether-Beck, S.; Singh, M.; Kuck, F.; Jakob, S.; Kefalas, A.; Altinoluk-Hambüchen, S.; Graffmann, N.; Schneider, M.; Lindecke, A.; et al. MicroRNA-15b regulates mitochondrial ROS production and the senescence-associated secretory phenotype through sirtuin 4/SIRT4. Aging 2016, 8, 484–505. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.-X.; Tang, X.; An, X.-Z.; Xie, X.-M.; Chen, X.-F.; Zhao, X.; Hao, D.-L.; Chen, H.-Z.; Liu, D.-P. SIRT4 accelerates Ang II-induced pathological cardiac hypertrophy by inhibiting manganese superoxide dismutase activity. Eur. Heart J. 2017, 38, 1389–1398. [Google Scholar] [CrossRef]
- Lago, J.C.; Puzzi, M.B. The effect of aging in primary human dermal fibroblasts. PLoS ONE 2019, 14, e0219165. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Qi, X.; Alhabeil, J.; Lu, H.; Zhou, Z. Activation of cannabinoid receptors promote periodontal cell adhesion and migration. J. Clin. Periodontol. 2019, 46, 1264–1272. [Google Scholar] [CrossRef] [PubMed]



| Characteristic | Young Fibroblasts | Senescent Fibroblasts | ||
|---|---|---|---|---|
| THC | CBD | H2O2 + THC | H2O2 + CBD | |
| Shape and size of the cell | Elongated, small | Elongated, small | Enlarged, flattened | Enlarged, flattened |
| Nuclear Area | ↓ | ↔ | ↔ | ↔ |
| Nuclear Perimeter | ↓ | ↔ | ↔ | ↔ |
| Nuclear Eccentricity | ↑ | ↔ | ↔ | ↔ |
| Nuclear circularity (roundness) | ↓ | ↔ | ↔ | ↔ |
| β-Gal level | ↑ | ↔ | ↓ | ↓ |
| BID | ↔ | ↔↑ | ↔↓ | ↓ |
| CB1 | ↔↑ | ↔↑ | ↔ | ↔ |
| CB2 | ↔↑ | ↔↑ | ↔ | ↔↑ |
| CDK2 | ↔↑ | ↔↑ | ↔↓ | ↔↓ |
| c-Jun | ↔↓ | ↔ | ↔ | ↔↓ |
| Collagen (type I) | ↔ | ↔↓ | ↔↑ | ↔↑ |
| Collagen (type III) | ↔ | ↔ | ↔ | ↔ |
| Cyclin D1 | ↔ | ↔↓ | ↓ | ↓ |
| EGFR | ↓ | ↔ | ↑ | ↔ |
| Elastin | ↔ | ↔ | ↔↓ | ↓ |
| GDF11 | ↔↑ | ↑ | ↔ | ↔↑ |
| Hyaluronan synthase | ↑ | ↑ | ↔↑ | ↔↑ |
| MMP 1 | ↑ | ↔ | ↓ | ↔↓ |
| MMP 2 | ↔ | ↔ | ↔↑ | ↔↑ |
| MKI67 | ↑ | ↑ | ↔ | ↑ |
| NF-κB | ↔↑ | ↔↑ | ↔↓ | ↔↑ |
| P16 | ↔ | ↔↑ | ↑ | ↔↑ |
| P21 | ↔↓ | ↔↓ | ↔ | ↑ |
| P53 | ↔↑ | ↔↑ | ↔ | ↔↓ |
| PCNA | ↔ | ↔ | ↔ | ↔ |
| SIRT 1 | ↔↑ | ↔↑ | ↔ | ↔ |
| SIRT 3, 4 | ↔↑ | ↔↑ | ↔↓ | ↔ |
| SIRT 6 | ↑ | ↔↑ | ↔↓ | ↔ |
| TIMP 1 | ↔↑ | ↔↑ | ↔↓ | ↔↓ |
| Vinculin | ↔↓ | ↓ | ↔ | ↔ |
| Cell viability | ↑ | ↔ | ↑ | ↑ |
| Wound healing | ↑ | ↑ | ↑ | ↑ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerasymchuk, M.; Robinson, G.I.; Groves, A.; Haselhorst, L.; Nandakumar, S.; Stahl, C.; Kovalchuk, O.; Kovalchuk, I. Phytocannabinoids Stimulate Rejuvenation and Prevent Cellular Senescence in Human Dermal Fibroblasts. Cells 2022, 11, 3939. https://doi.org/10.3390/cells11233939
Gerasymchuk M, Robinson GI, Groves A, Haselhorst L, Nandakumar S, Stahl C, Kovalchuk O, Kovalchuk I. Phytocannabinoids Stimulate Rejuvenation and Prevent Cellular Senescence in Human Dermal Fibroblasts. Cells. 2022; 11(23):3939. https://doi.org/10.3390/cells11233939
Chicago/Turabian StyleGerasymchuk, Marta, Gregory Ian Robinson, Alyssa Groves, Lucie Haselhorst, Sanjana Nandakumar, Cora Stahl, Olga Kovalchuk, and Igor Kovalchuk. 2022. "Phytocannabinoids Stimulate Rejuvenation and Prevent Cellular Senescence in Human Dermal Fibroblasts" Cells 11, no. 23: 3939. https://doi.org/10.3390/cells11233939
APA StyleGerasymchuk, M., Robinson, G. I., Groves, A., Haselhorst, L., Nandakumar, S., Stahl, C., Kovalchuk, O., & Kovalchuk, I. (2022). Phytocannabinoids Stimulate Rejuvenation and Prevent Cellular Senescence in Human Dermal Fibroblasts. Cells, 11(23), 3939. https://doi.org/10.3390/cells11233939









