Low WIP1 Expression Accelerates Ovarian Aging by Promoting Follicular Atresia and Primordial Follicle Activation
Abstract
1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Experimental Design
2.3. Estrous Cycle Monitoring and Mating Test
2.4. Immunohistochemistry
2.5. Serum AMH Level by ELISA
2.6. Follicle Counting
2.7. Periodic Acid-Schiff (PAS) Staining
2.8. Apoptosis Test
2.9. Western Blot
2.10. Follicle Isolation and Follicle Encapsulation
2.11. In Vitro Oocyte Maturation
2.12. Immunofluorescence
2.13. Primary Granulosa Cell Culture and siRNA Interference
2.14. Cell Proliferation Detection
2.15. Cell Apoptosis Detection
2.16. Statistical Analysis
3. Results
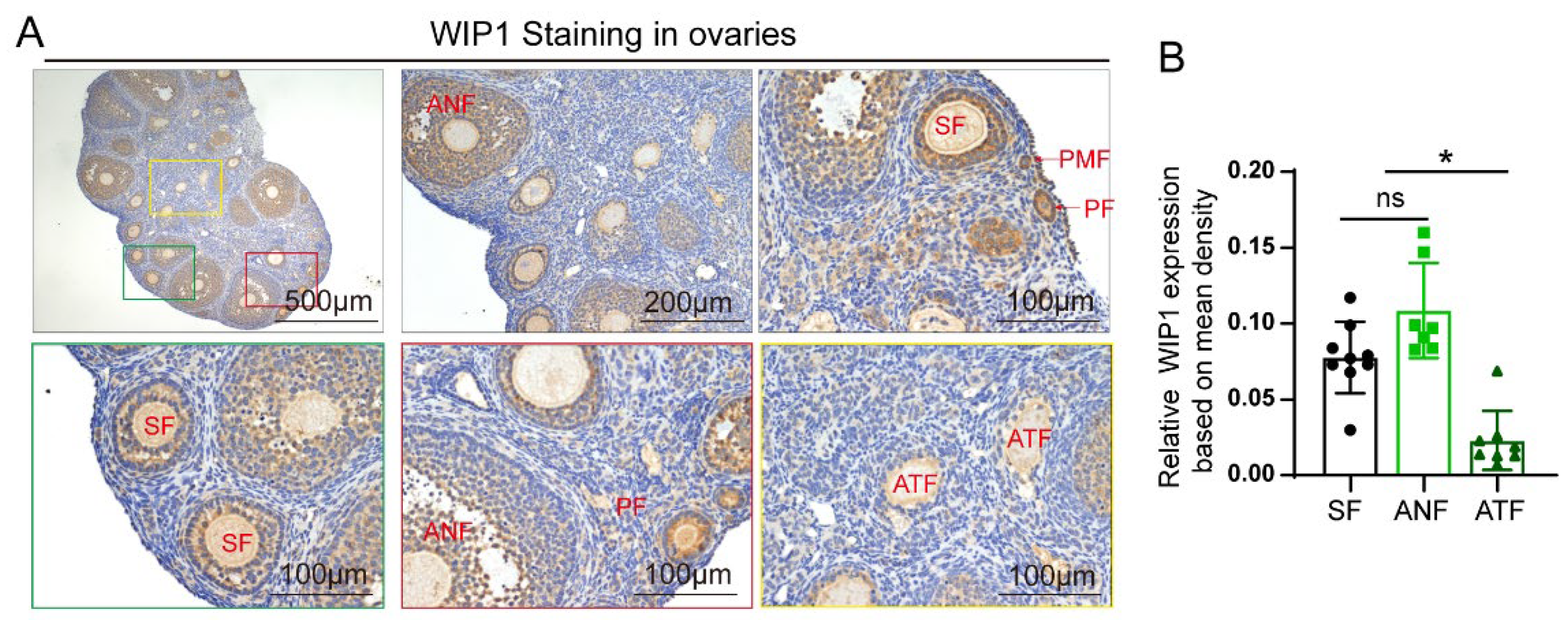
3.1. WIP1 Expression in Follicles of Different Stages in Mouse Ovaries
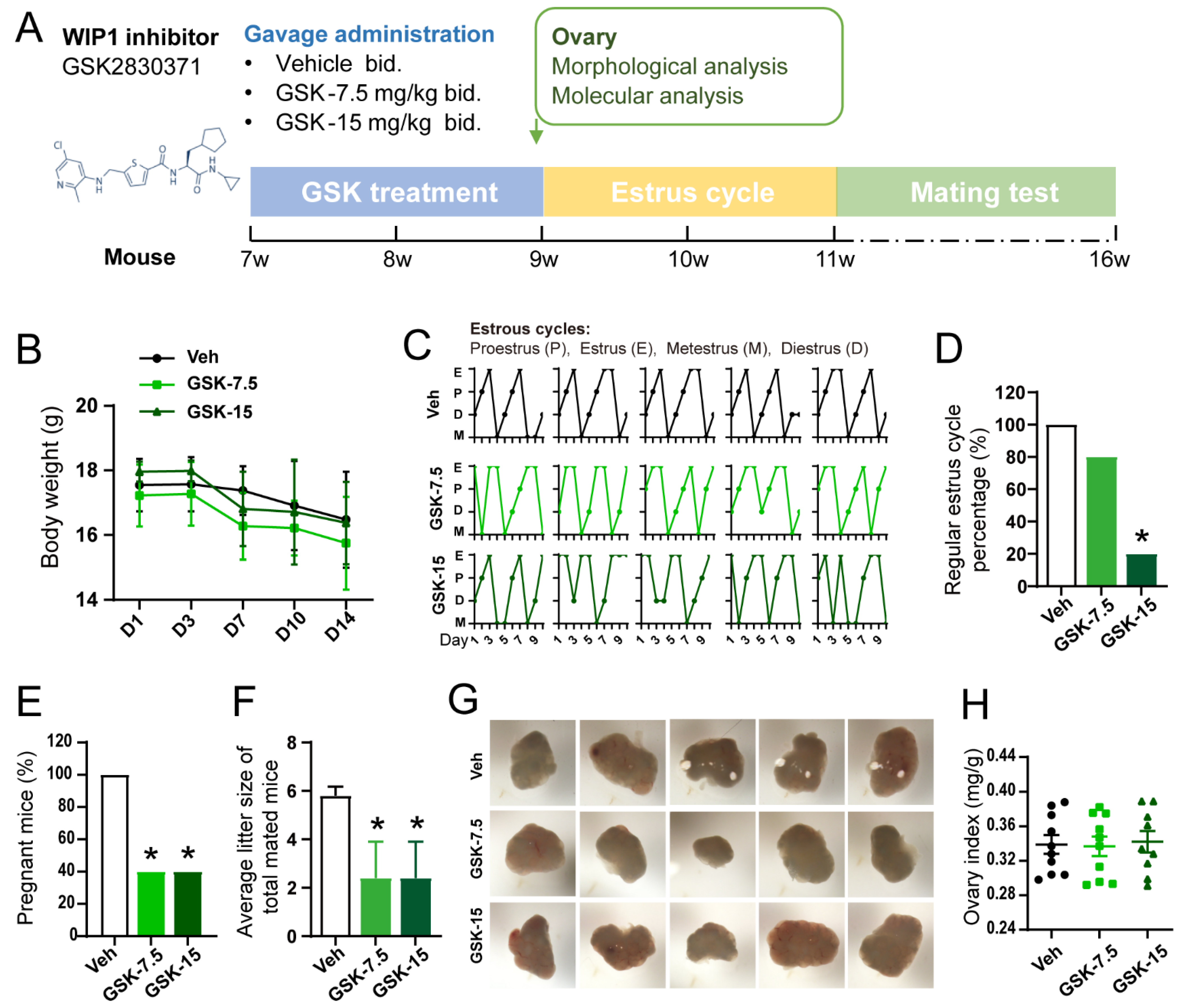
3.2. Inhibition of WIP1 Activity Induces the Irregular Estrus Cycles and Declined Reproductivity
3.3. Inhibition of WIP1 Activity Leads to Declined Ovarian Reserve
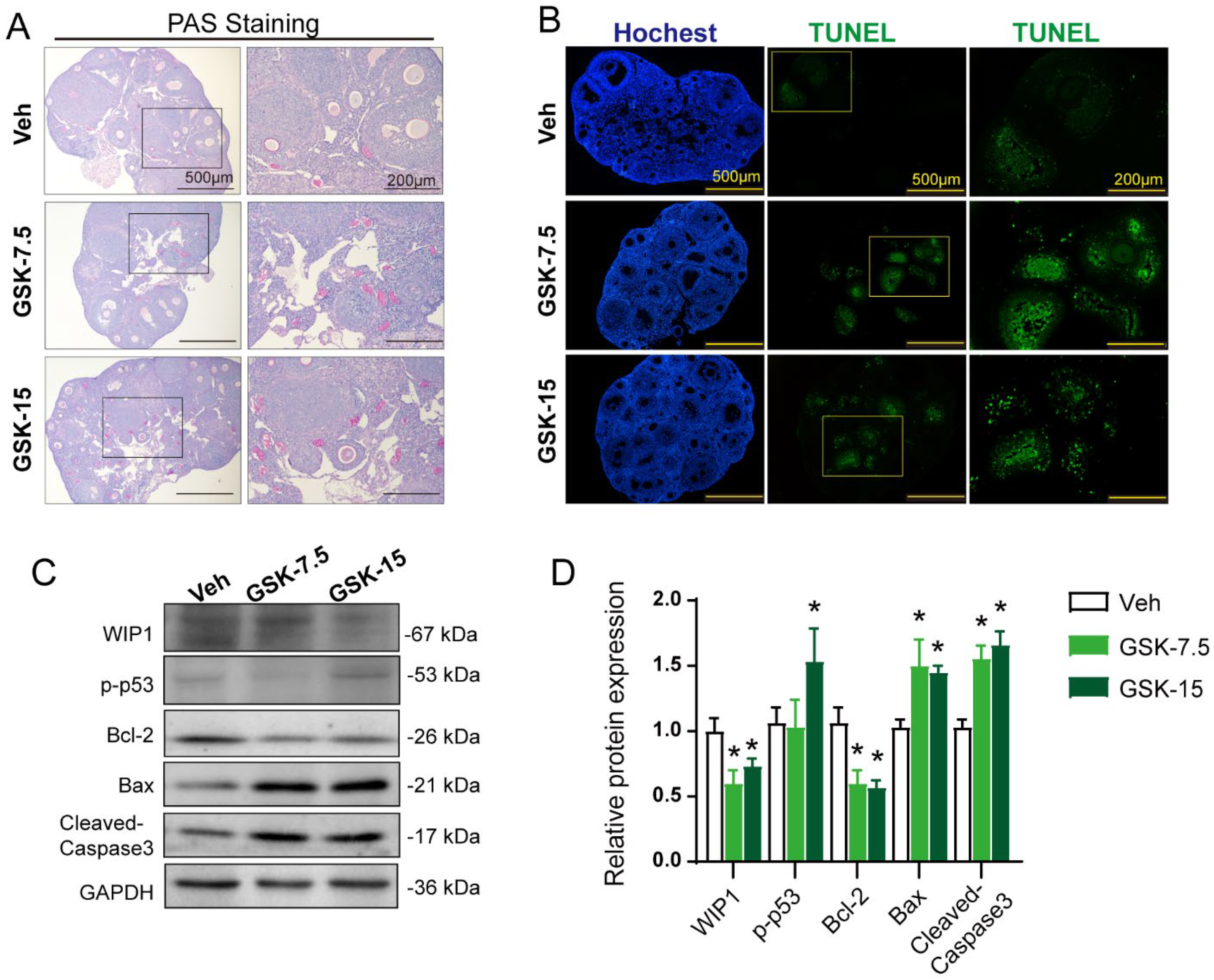
3.4. Inhibition of WIP1 Activity Promotes Follicular Atresia through Apoptosis Pathway
3.5. Inhibition of WIP1 Activity Impairs the Follicular Growth and Oocyte Quality In Vitro
3.6. Downregulating WIP1 Promotes Mouse Granulosa Cell Apoptosis
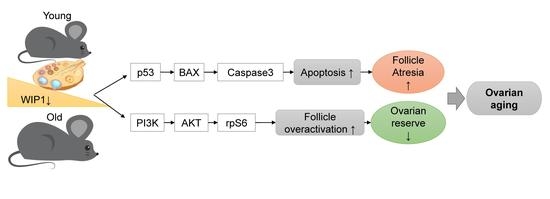
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hansen, K.R.; Knowlton, N.; Thyer, A.C.; Charleston, J.S.; Soules, M.R.; Klein, N.A. A new model of reproductive aging: The decline in ovarian non-growing follicle number from birth to menopause. Hum. Reprod. 2008, 23, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Younis, J.S. Ovarian aging: Latest thoughts on assessment and management. Curr. Opin. Obstet. Gynecol. 2011, 23, 427–434. [Google Scholar] [CrossRef] [PubMed]
- Quinn, M.M.; Cedars, M.I. Cardiovascular health and ovarian aging. Fertil. Steril. 2018, 110, 790–793. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, Z. Ovarian Aging and Osteoporosis. Adv. Exp. Med. Biol. 2018, 1086, 199–215. [Google Scholar] [CrossRef] [PubMed]
- Bethea, C.L.; Kohama, S.G.; Reddy, A.P.; Urbanski, H.F. Ovarian steroids regulate gene expression in the dorsal raphe of old female macaques. Neurobiol. Aging 2016, 37, 179–191. [Google Scholar] [CrossRef]
- Broekmans, F.J.; Soules, M.R.; Fauser, B.C. Ovarian Aging: Mechanisms and Clinical Consequences. Endocr. Rev. 2009, 30, 465–493. [Google Scholar] [CrossRef]
- Matsuda, F.; Inoue, N.; Manabe, N.; Ohkura, S. Follicular Growth and Atresia in Mammalian Ovaries: Regulation by Survival and Death of Granulosa Cells. J. Reprod. Dev. 2012, 58, 44–50. [Google Scholar] [CrossRef]
- Turan, V.; Oktay, K. BRCA-related ATM-mediated DNA double-strand break repair and ovarian aging. Hum. Reprod. Update 2019, 26, 43–57. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, X.; Zeng, M.; Yuan, J.; Liu, M.; Yin, Y.; Wu, X.; Keefe, D.L.; Liu, L. Increased DNA damage and repair deficiency in granulosa cells are associated with ovarian aging in rhesus monkey. J. Assist. Reprod. Genet. 2015, 32, 1069–1078. [Google Scholar] [CrossRef]
- Jazayeri, A.; Falck, J.; Lukas, C.; Bartek, J.; Smith, G.C.M.; Lukas, J.; Jackson, S.P. ATM- and cell cycle-dependent regulation of ATR in response to DNA double-strand breaks. Nat. Cell Biol. 2006, 8, 37–45. [Google Scholar] [CrossRef]
- Morita, Y.; Perez, G.I.; Paris, F.; Miranda, S.R.; Ehleiter, D.; Haimovitz-Friedman, A.; Fuks, Z.; Xie, Z.; Reed, J.C.; Schuchman, E.H.; et al. Oocyte apoptosis is suppressed by disruption of the acid sphingomyelinase gene or by sphingosine -1-phosphate therapy. Nat. Med. 2000, 6, 1109–1114. [Google Scholar] [CrossRef]
- Kaipia, A.; Hsueh, A.J.W. Regulation of ovarian follicle atresia. Annu. Rev. Physiol. 1997, 59, 349–363. [Google Scholar] [CrossRef]
- Hsueh, A.J.W.; Billig, H.; Tsafriri, A. Ovarian Follicle Atresia: A Hormonally Controlled Apoptotic Process. Endocr. Rev. 1994, 15, 707–724. [Google Scholar] [CrossRef]
- Fiscella, M.; Zhang, H.; Fan, S.; Sakaguchi, K.; Shen, S.; Mercer, W.E.; Vande Woude, G.F.; O’Connor, P.M.; Appella, E. Wip1, a novel human protein phosphatase that is induced in response to ionizing radiation in a p53-dependent manner. Proc. Natl. Acad. Sci. USA 1997, 94, 6048–6053. [Google Scholar] [CrossRef]
- Bulavin, D.V.; Phillips, C.; Nannenga, B.; Timofeev, O.; Donehower, L.A.; Anderson, C.W.; Appella, E.; Fornace, A.J., Jr. Inactivation of the Wip1 phosphatase inhibits mammary tumorigenesis through p38 MAPK–mediated activation of the p16Ink4a-p19Arf pathway. Nat. Genet. 2004, 36, 343–350. [Google Scholar] [CrossRef]
- Emelyanov, A.; Bulavin, D.V. Wip1 phosphatase in breast cancer. Oncogene 2014, 34, 4429–4438. [Google Scholar] [CrossRef]
- Yin, S.; Wang, P.; Yang, L.; Liu, Y.; Wang, Y.; Liu, M.; Qi, Z.; Meng, J.; Shi, T.-Y.; Yang, G.; et al. Wip1 suppresses ovarian cancer metastasis through the ATM/AKT/Snail mediated signaling. Oncotarget 2016, 7, 29359–29370. [Google Scholar] [CrossRef]
- Demidov, O.N.; Zhu, Y.; Kek, C.; Goloudina, A.R.; Motoyama, N.; Bulavin, D.V. Role of Gadd45a in Wip1-dependent regulation of intestinal tumorigenesis. Cell Death Differ. 2012, 19, 1761–1768. [Google Scholar] [CrossRef][Green Version]
- Wang, P.; Zhao, Y.; Liu, K.; Liu, X.; Liang, J.; Zhou, H.; Wang, Z.; Zhou, Z.; Xu, N. Wip1 cooperates with KPNA2 to modulate the cell proliferation and migration of colorectal cancer via a p53-dependent manner. J. Cell. Biochem. 2019, 120, 15709–15718. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, L.H.; Wan, H.; Yang, R.; Wang, Z.; Feng, J.; Yang, S.; Jones, S.; Wang, S.; Zhou, W.; et al. Exome sequencing identifies somatic gain-of-function PPM1D mutations in brainstem gliomas. Nat. Genet. 2014, 46, 726–730. [Google Scholar] [CrossRef]
- LE Guezennec, X.; Bulavin, D.V. WIP1 phosphatase at the crossroads of cancer and aging. Trends Biochem. Sci. 2010, 35, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Sakai, H.; Fujigaki, H.; Mazur, S.; Appella, E. Wild-type p53-induced phosphatase 1 (Wip1) forestalls cellular premature senescence at physiological oxygen levels by regulating DNA damage response signaling during DNA replication. Cell Cycle 2014, 13, 1015–1029. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Kaarniranta, K. Control of p53 and NF-κB signaling by WIP1 and MIF: Role in cellular senescence and organismal aging. Cell. Signal. 2011, 23, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.S.M.; LE Guezennec, X.; Demidov, O.; Marshall, N.T.; Wang, S.T.; Krishnamurthy, J.; Sharpless, N.; Dunn, N.R.; Bulavin, D.V. p38MAPK Controls Expression of Multiple Cell Cycle Inhibitors and Islet Proliferation with Advancing Age. Dev. Cell 2009, 17, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Demidov, O.N.; Goh, A.M.; Virshup, D.M.; Lane, D.P.; Bulavin, D.V. Phosphatase WIP1 regulates adult neurogenesis and WNT signaling during aging. J. Clin. Investig. 2014, 124, 3263–3273. [Google Scholar] [CrossRef]
- Zhou, S.; Xi, Y.; Chen, Y.; Wu, T.; Yan, W.; Li, M.; Wu, M.; Luo, A.; Shen, W.; Xiang, T.; et al. The inhibition of WIP1 phosphatase accelerates the depletion of primordial follicles. Reprod. Biomed. Online 2021, 43, 161–171. [Google Scholar] [CrossRef]
- Wei, Y.; Gao, Q.; Niu, P.; Xu, K.; Qiu, Y.; Hu, Y.; Liu, S.; Zhang, X.; Yu, M.; Liu, Z.; et al. Integrative Proteomic and Phosphoproteomic Profiling of Testis from Wip1 Phosphatase-Knockout Mice: Insights into Mechanisms of Reduced Fertility. Mol. Cell. Proteom. 2019, 18, 216–230. [Google Scholar] [CrossRef]
- Niu, P.; Wei, Y.; Gao, Q.; Zhang, X.; Hu, Y.; Qiu, Y.; Mu, Y.; Li, K. Male Fertility Potential Molecular Mechanisms Revealed by iTRAQ-Based Quantitative Proteomic Analysis of the Epididymis from Wip1−/− Mice. OMICS J. Integr. Biol. 2019, 23, 54–66. [Google Scholar] [CrossRef]
- Choi, J.; Nannenga, B.; Demidov, O.N.; Bulavin, D.V.; Cooney, A.; Brayton, C.; Zhang, Y.; Mbawuike, I.N.; Bradley, A.; Appella, E.; et al. Mice Deficient for the Wild-Type p53-Induced Phosphatase Gene (Wip1) Exhibit Defects in Reproductive Organs, Immune Function, and Cell Cycle Control. Mol. Cell. Biol. 2002, 22, 1094–1105. [Google Scholar] [CrossRef]
- Gilmartin, A.G.; Faitg, T.H.; Richter, M.; Groy, A.; Seefeld, M.A.; Darcy, M.G.; Peng, X.; Federowicz, K.; Yang, J.; Zhang, S.-Y.; et al. Allosteric Wip1 phosphatase inhibition through flap-subdomain interaction. Nat. Chem. Biol. 2014, 10, 181–187. [Google Scholar] [CrossRef]
- Zhou, S.; Xi, Y.; Chen, Y.; Zhang, Z.; Wu, C.; Yan, W.; Luo, A.; Wu, T.; Zhang, J.; Wu, M.; et al. Ovarian Dysfunction Induced by Chronic Whole-Body PM2.5 Exposure. Small 2020, 16, e2000845. [Google Scholar] [CrossRef]
- Fu, D.A.; Campbell-Thompson, M. Periodic Acid-Schiff Staining with Diastase. Methods Mol. Biol. 2017, 1639, 145–149. [Google Scholar] [CrossRef]
- Shen, L.; Chen, Y.; Cheng, J.; Yuan, S.; Zhou, S.; Yan, W.; Liu, J.; Luo, A.; Wang, S. CCL5 secreted by senescent theca-interstitial cells inhibits preantral follicular development via granulosa cellular apoptosis. J. Cell. Physiol. 2019, 234, 22554–22564. [Google Scholar] [CrossRef]
- Shikanov, A.; Xu, M.; Woodruff, T.K.; Shea, L.D. A Method for Ovarian Follicle Encapsulation and Culture in a Proteolytically Degradable 3 Dimensional System. J. Vis. Exp. 2011, 49, e2695. [Google Scholar] [CrossRef]
- Tian, Y.; Shen, W.; Lai, Z.; Shi, L.; Yang, S.; Ding, T.; Wang, S.; Luo, A. Isolation and identification of ovarian theca-interstitial cells and granulose cells of immature female mice. Cell Biol. Int. 2015, 39, 584–590. [Google Scholar] [CrossRef]
- Akhtari, K.; Razi, M.; Malekinejad, H. Uterine artery interruption: Evidence for follicular growth and histochemical and biochemical changes. J. Reprod. Infertil. 2012, 13, 193–203. [Google Scholar]
- Yang, Y.-Q.; Zheng, Y.-H.; Zhang, C.-T.; Liang, W.-W.; Wang, S.-Y.; Wang, X.-D.; Wang, Y.; Wang, T.-H.; Jiang, H.-Q.; Feng, H.-L. Wild-type p53-induced phosphatase 1 down-regulation promotes apoptosis by activating the DNA damage-response pathway in amyotrophic lateral sclerosis. Neurobiol. Dis. 2020, 134, 104648. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, L.; Yao, D.; Yang, T.; Cao, W.-M.; Dou, J.; Pang, J.; Guan, S.; Zhang, H.; Yu, Y.; et al. Wip1 inhibitor GSK2830371 inhibits neuroblastoma growth by inducing Chk2/p53-mediated apoptosis. Sci. Rep. 2016, 6, 38011. [Google Scholar] [CrossRef]
- Zhu, Y.-H.; Bulavin, D.V. Wip1-Dependent Signaling Pathways in Health and Diseases. Prog. Mol. Biol. Transl. Sci. 2012, 106, 307–325. [Google Scholar] [CrossRef]
- Reddy, P.; Liu, L.; Adhikari, D.; Jagarlamudi, K.; Rajareddy, S.; Shen, Y.; Du, C.; Tang, W.; Huhtaniemi, T.; Peng, S.L.; et al. Oocyte-Specific Deletion of Pten Causes Premature Activation of the Primordial Follicle Pool. Science 2008, 319, 611–613. [Google Scholar] [CrossRef]
- Xia, Y.; Ongusaha, P.; Lee, S.W.; Liou, Y.-C. Loss of Wip1 Sensitizes Cells to Stress- and DNA Damage-induced Apoptosis. J. Biol. Chem. 2009, 284, 17428–17437. [Google Scholar] [CrossRef] [PubMed]
- Lowe, J.; Cha, H.; Lee, M.-O.; Mazur, S.J.; Appella, E.; Fornace, A.J., Jr. Regulation of the Wip1 phosphatase and its effects on the stress response. Front. Biosci. 2012, 17, 1480–1498. [Google Scholar] [CrossRef] [PubMed]
- Uyanik, B.; Grigorash, B.B.; Goloudina, A.R.; Demidov, O.N. DNA damage-induced phosphatase Wip1 in regulation of hematopoiesis, immune system and inflammation. Cell Death Discov. 2017, 3, 17018. [Google Scholar] [CrossRef] [PubMed]
- Moskalev, A.A.; Shaposhnikov, M.V.; Plyusnina, E.N.; Zhavoronkov, A.; Budovsky, A.; Yanai, H.; Fraifeld, V.E. The role of DNA damage and repair in aging through the prism of Koch-like criteria. Ageing Res. Rev. 2013, 12, 661–684. [Google Scholar] [CrossRef] [PubMed]
- Hoeijmakers, J.H.J. DNA Damage, Aging, and Cancer. New Engl. J. Med. 2009, 361, 1475–1485. [Google Scholar] [CrossRef]
- Cha, H.; Lowe, J.M.; Li, H.; Lee, J.-S.; Belova, G.I.; Bulavin, D.V.; Fornace, A.J. Wip1 Directly Dephosphorylates γ-H2AX and Attenuates the DNA Damage Response. Cancer Res. 2010, 70, 4112–4122. [Google Scholar] [CrossRef]
- Lu, X.; Nguyen, T.-A.; Moon, S.-H.; Darlington, Y.; Sommer, M.; Donehower, L.A. The type 2C phosphatase Wip1: An oncogenic regulator of tumor suppressor and DNA damage response pathways. Cancer Metastasis Rev. 2008, 27, 123–135. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, L.; Guo, H.; Yang, J.; Jones, S.N.; Jochemsen, A.; Lu, X. Phosphorylation and Degradation of MdmX Is Inhibited by Wip1 Phosphatase in the DNA Damage Response. Cancer Res. 2009, 69, 7960–7968. [Google Scholar] [CrossRef]
- Yoshida, K.; Miki, Y. Role of BRCA1 and BRCA2 as regulators of DNA repair, transcription, and cell cycle in response to DNA damage. Cancer Sci. 2004, 95, 866–871. [Google Scholar] [CrossRef]
- Titus, S.; Li, F.; Stobezki, R.; Akula, K.; Unsal, E.; Jeong, K.; Dickler, M.; Robson, M.; Moy, F.; Goswami, S.; et al. Impairment of BRCA1-Related DNA Double-Strand Break Repair Leads to Ovarian Aging in Mice and Humans. Sci. Transl. Med. 2013, 5, 172ra21. [Google Scholar] [CrossRef]
- Miao, Y.; Wang, P.; Xie, B.; Yang, M.; Li, S.; Cui, Z.; Fan, Y.; Li, M.; Xiong, B. BRCA2 deficiency is a potential driver for human primary ovarian insufficiency. Cell Death Dis. 2019, 10, 474. [Google Scholar] [CrossRef]
- Moolhuijsen, L.M.E.; Visser, J.A. Anti-Müllerian Hormone and Ovarian Reserve: Update on Assessing Ovarian Function. J. Clin. Endocrinol. Metab. 2020, 105, 3361–3373. [Google Scholar] [CrossRef]
- Shreeram, S.; Demidov, O.; Hee, W.K.; Yamaguchi, H.; Onishi, N.; Kek, C.; Timofeev, O.N.; Dudgeon, C.; Fornace, A.; Anderson, C.W.; et al. Wip1 Phosphatase Modulates ATM-Dependent Signaling Pathways. Mol. Cell 2006, 23, 757–764. [Google Scholar] [CrossRef]
- Gupta, S.; Silveira, D.A.; Mombach, J.C.M. Towards DNA-damage induced autophagy: A Boolean model of p53-induced cell fate mechanisms. DNA Repair 2020, 96, 102971. [Google Scholar] [CrossRef]
- Leem, J.; Kim, J.-S.; Oh, J.S. WIP1 phosphatase suppresses the DNA damage response during G2/prophase arrest in mouse oocytes†. Biol. Reprod. 2018, 99, 798–805. [Google Scholar] [CrossRef]
- Chen, Z.; Yi, W.; Morita, Y.; Wang, H.; Cong, Y.; Liu, J.-P.; Xiao, Z.; Rudolph, K.L.; Cheng, T.; Ju, Z. Wip1 deficiency impairs haematopoietic stem cell function via p53 and mTORC1 pathways. Nat. Commun. 2015, 6, 6808. [Google Scholar] [CrossRef]
- Yi, W.; Hu, X.; Chen, Z.; Liu, L.; Tian, Y.; Chen, H.; Cong, Y.-S.; Yang, F.; Zhang, L.; Rudolph, K.L.; et al. Phosphatase Wip1 controls antigen-independent B-cell development in a p53-dependent manner. Blood 2015, 126, 620–628. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, S.; Xi, Y.; Chen, Y.; Fu, F.; Yan, W.; Li, M.; Wu, Y.; Luo, A.; Li, Y.; Wang, S. Low WIP1 Expression Accelerates Ovarian Aging by Promoting Follicular Atresia and Primordial Follicle Activation. Cells 2022, 11, 3920. https://doi.org/10.3390/cells11233920
Zhou S, Xi Y, Chen Y, Fu F, Yan W, Li M, Wu Y, Luo A, Li Y, Wang S. Low WIP1 Expression Accelerates Ovarian Aging by Promoting Follicular Atresia and Primordial Follicle Activation. Cells. 2022; 11(23):3920. https://doi.org/10.3390/cells11233920
Chicago/Turabian StyleZhou, Su, Yueyue Xi, Yingying Chen, Fangfang Fu, Wei Yan, Milu Li, Yaling Wu, Aiyue Luo, Ya Li, and Shixuan Wang. 2022. "Low WIP1 Expression Accelerates Ovarian Aging by Promoting Follicular Atresia and Primordial Follicle Activation" Cells 11, no. 23: 3920. https://doi.org/10.3390/cells11233920
APA StyleZhou, S., Xi, Y., Chen, Y., Fu, F., Yan, W., Li, M., Wu, Y., Luo, A., Li, Y., & Wang, S. (2022). Low WIP1 Expression Accelerates Ovarian Aging by Promoting Follicular Atresia and Primordial Follicle Activation. Cells, 11(23), 3920. https://doi.org/10.3390/cells11233920