Targeting mGlu1 Receptors in the Treatment of Motor and Cognitive Dysfunctions in Mice Modeling Type 1 Spinocerebellar Ataxia
Abstract
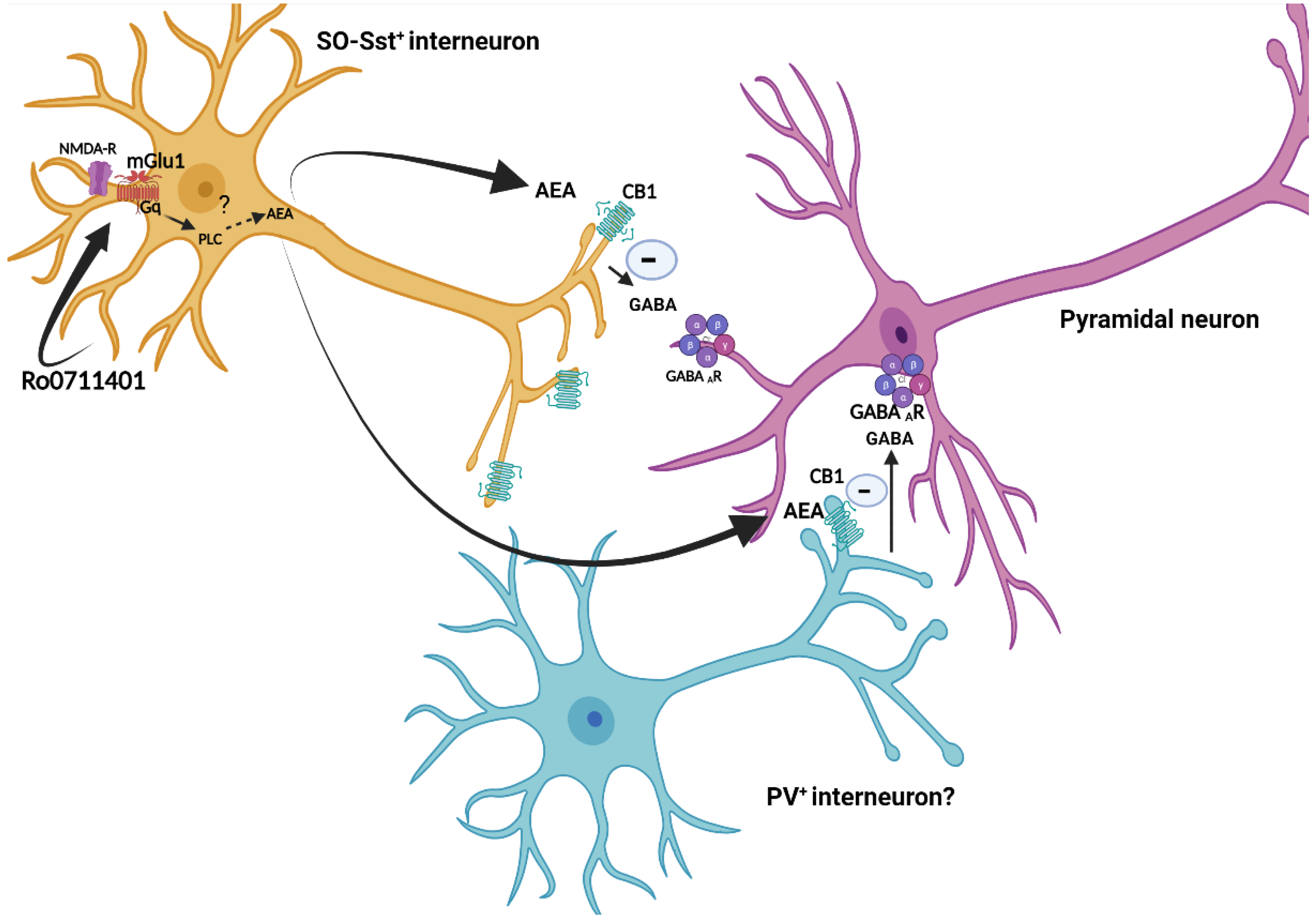
1. Introduction
1.1. mGlu1 Receptors and Cerebellar Dysfunction Associated with SCA1
1.2. Targeting mGlu1 Receptors in the Treatment of Cognitive Dysfunction Associated with SCA1
Background
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. Immunofluorescence Staining
2.4. Open-Field Test
2.5. Rotarod Test
2.6. Morris Water Maze Test
2.7. Western Blot Analysis
2.8. Measurements of Endogenous Levels of Endocannabinoids by LC-MS
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, Q.-W.; Kapfhammer, J.P. Modulation of Increased mGluR1 Signaling by RGS8 Protects Purkinje Cells From Dendritic Reduction and Could Be a Common Mechanism in Diverse Forms of Spinocerebellar Ataxia. Front. Cell Dev. Biol. 2021, 8. [Google Scholar] [CrossRef] [PubMed]
- Schöls, L.; Bauer, P.; Schmidt, T.; Schulte, T.; Riess, O. Autosomal dominant cerebellar ataxias: Clinical features, genetics, and pathogenesis. Lancet Neurol. 2004, 3, 291–304. [Google Scholar] [CrossRef]
- Tejwani, L.; Lim, J. Pathogenic mechanisms underlying spinocerebellar ataxia type 1. Exp. 2020, 77, 4015–4029. [Google Scholar] [CrossRef] [PubMed]
- Burk, K.; Globas, C.; Bosch, S.; Klockgether, T.; Zuhlke, C.; Daum, I.; Dichgans, J. Cognitive deficits in spinocerebellar ataxia type 1, 2, and 3. J. Neurol. 2003, 250, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Globas, C.; du Montcel, S.T.; Balikó, L.; Boesch, S.; Depondt, C.; DiDonato, S.; Durr, A.; Filla, A.; Klockgether, T.; Mariotti, C.; et al. Early symptoms in spinocerebellar ataxia type 1, 2, 3, and 6. Mov. Disord. 2008, 23, 2232–2238. [Google Scholar] [CrossRef] [PubMed]
- Orr, H.T. SCA1—Phosphorylation, a regulator of Ataxin-1 function and pathogenesis. Prog. Neurobiol. 2012, 99, 179–185. [Google Scholar] [CrossRef]
- Serra, H.G.; Duvick, L.; Zu, T.; Carlson, K.; Stevens, S.; Jorgensen, N.; Lysholm, A.; Burright, E.; Zoghbi, H.; Clark, H.B.; et al. RORα-Mediated Purkinje Cell Development Determines Disease Severity in Adult SCA1 Mice. Cell 2006, 127, 697–708. [Google Scholar] [CrossRef]
- Watanave, M.; Hoshino, C.; Konno, A.; Fukuzaki, Y.; Matsuzaki, Y.; Ishitani, T.; Hirai, H. Pharmacological enhancement of retinoid-related orphan receptor α function mitigates spinocerebellar ataxia type 3 pathology. Neurobiol. Dis. 2018, 121, 263–273. [Google Scholar] [CrossRef]
- Aiba, A. Reduced hippocampal long-term potentiation and context-specific deficit in associative learning in mGluR1 mutant mice. Cell 1994, 79, 365–375. [Google Scholar] [CrossRef]
- Kano, M.; Hashimoto, K.; Tabata, T. Type-1 metabotropic glutamate receptor in cerebellar Purkinje cells: A key molecule responsible for long-term depression, endocannabinoid signalling and synapse elimination. Philos. Trans. R. Soc. B: Biol. Sci. 2008, 363, 2173–2186. [Google Scholar] [CrossRef]
- Kano, M.; Watanabe, T. Type-1 metabotropic glutamate receptor signaling in cerebellar Purkinje cells in health and disease. F1000Research 2017, 6, 416. [Google Scholar] [CrossRef] [PubMed]
- Kano, M.; Hashimoto, K.; Kurihara, H.; Watanabe, M.; Inoue, Y.; Aiba, A.; Tonegawa, S. Persistent Multiple Climbing Fiber Innervationof Cerebellar Purkinje Cellsin Mice Lacking mGluR1. Neuron 1997, 18, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Uesaka, N.; Uchigashima, M.; Mikuni, T.; Nakazawa, T.; Nakao, H.; Hirai, H.; Aiba, A.; Watanabe, M.; Kano, M. Retrograde semaphorin signaling regulates synapse elimination in the developing mouse brain. Science 2014, 344, 1020–1023. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, R.; Hashimoto, K.; Miyazaki, T.; Uchigashima, M.; Yamasaki, M.; Aiba, A.; Kano, M.; Watanabe, M. Territories of heterologous inputs onto Purkinje cell dendrites are segregated by mGluR1-dependent parallel fiber synapse elimination. Proc. Natl. Acad. Sci. USA 2016, 113, 2282–2287. [Google Scholar] [CrossRef] [PubMed]
- Ichise, T.; Kano, M.; Hashimoto, K.; Yanagihara, D.; Nakao, K.; Shigemoto, R.; Katsuki, M.; Aiba, A. mGluR1 in Cerebellar Purkinje Cells Essential for Long-Term Depression, Synapse Elimination, and Motor Coordination. Science 2000, 288, 1832–1835. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, Y.; Fujimichi, R.; Araishi, K.; Kawahara, S.; Kano, M.; Aiba, A.; Kirino, Y. mGluR1 in cerebellar Purkinje cells is required for normal association of temporally contiguous stimuli in classical conditioning. Eur. J. Neurosci. 2002, 16, 2416–2424. [Google Scholar] [CrossRef]
- Ohtani, Y.; Miyata, M.; Hashimoto, K.; Tabata, T.; Kishimoto, Y.; Fukaya, M.; Kase, D.; Kassai, H.; Nakao, K.; Hirata, T.; et al. The Synaptic Targeting of mGluR1 by Its Carboxyl-Terminal Domain Is Crucial for Cerebellar Function. J. Neurosci. 2014, 34, 2702–2712. [Google Scholar] [CrossRef]
- Nakao, H.; Kishimoto, Y.; Hashimoto, K.; Kitamura, K.; Yamasaki, M.; Nakao, K.; Watanabe, M.; Kano, M.; Kirino, Y.; Aiba, A. mGluR1 in cerebellar Purkinje cells is essential for the formation but not expression of associative eyeblink memory. Sci. Rep. 2019, 9, 7353. [Google Scholar] [CrossRef]
- Yamasaki, M.; Aiba, A.; Kano, M.; Watanabe, M. mGluR1 signaling in cerebellar Purkinje cells: Subcellular organization and involvement in cerebellar function and disease. Neuropharmacology 2021, 194, 108629. [Google Scholar] [CrossRef]
- Smitt, P.S.; Kinoshita, A.; De Leeuw, B.; Moll, W.; Coesmans, M.; Jaarsma, D.; Henzen-Logmans, S.; Vecht, C.; De Zeeuw, C.; Sekiyama, N.; et al. Paraneoplastic Cerebellar Ataxia Due to Autoantibodies against a Glutamate Receptor. New Engl. J. Med. 2000, 342, 21–27. [Google Scholar] [CrossRef]
- Coesmans, M.; Sillevis Smitt, P.A.; Linden, D.J.; Shigemoto, R.; Hirano, T.; Yamakawa, Y.; van Alphen, A.M.; Luo, C.; van der Geest, J.N.; Kros, J.M.; et al. Mechanisms underlying cerebellar motor deficits due to mGluR1-autoantibodies. Ann. Neurol. 2003, 53, 325–336. [Google Scholar] [CrossRef]
- Marignier, R.; Chenevier, F.; Rogemond, V.; Smitt, P.S.; Renoux, C.; Cavillon, G.; Androdias, G.; Vukusic, S.; Graus, F.; Honnorat, J.; et al. Metabotropic Glutamate Receptor Type 1 Autoantibody–Associated Cerebellitis. Arch. Neurol. 2010, 67, 627–630. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ferraguti, F.; Crepaldi, L.; Nicoletti, F. Metabotropic Glutamate 1 Receptor: Current Concepts and Perspectives. Pharmacol. Rev. 2008, 60, 536–581. [Google Scholar] [CrossRef] [PubMed]
- Shuvaev, A.N.; Horiuchi, H.; Seki, T.; Goenawan, H.; Irie, T.; Iizuka, A.; Sakai, N.; Hirai, H. Mutant PKC in Spinocerebellar Ataxia Type 14 Disrupts Synapse Elimination and Long-Term Depression in Purkinje Cells In Vivo. J. Neurosci. 2011, 31, 14324–14334. [Google Scholar] [CrossRef] [PubMed]
- Armbrust, K.R.; Wang, X.; Hathorn, T.J.; Cramer, S.W.; Chen, G.; Zu, T.; Kangas, T.; Zink, A.N.; Öz, G.; Ebner, T.J.; et al. Mutant -III Spectrin Causes mGluR1 Mislocalization and Functional Deficits in a Mouse Model of Spinocerebellar Ataxia Type 5. J. Neurosci. 2014, 34, 9891–9904. [Google Scholar] [CrossRef] [PubMed]
- Hamilton, B.A.; Frankel, W.N.; Kerrebrock, A.W.; Hawkins, T.L.; FitzHugh, W.; Kusumi, K.; Russell, L.B.; Mueller, K.L.; Van Berkel, V.; Birren, B.W.; et al. Disruption of the nuclear hormone receptor RORα in staggerer mice. Nature 1996, 379, 736–739. [Google Scholar] [CrossRef]
- Mitsumura, K.; Hosoi, N.; Furuya, N.; Hirai, H. Disruption of metabotropic glutamate receptor signalling is a major defect at cerebellar parallel fibre-Purkinje cell synapses in staggerer mutant mice. J. Physiol. 2011, 589, 3191–3209. [Google Scholar] [CrossRef]
- Steinmayr, M.; André, E.; Conquet, F.; Rondi-Reig, L.; Delhaye-Bouchaud, N.; Auclair, N.; Daniel, H.; Crépel, F.; Mariani, J.; Sotelo, C.; et al. staggerer phenotype in retinoid-related orphan receptor α-deficient mice. Proc. Natl. Acad. Sci. USA 1998, 95, 3960–3965. [Google Scholar] [CrossRef]
- Doulazmi, M.; Frédéric, F.; Capone, F.; Becker-André, M.; Delhaye-Bouchaud, N.; Mariani, J. A comparative study of Purkinje cells in two RORα gene mutant mice: Staggerer and RORα−/−. Dev. Brain Res. 2001, 127, 165–174. [Google Scholar] [CrossRef]
- Shuvaev, A.N.; Hosoi, N.; Sato, Y.; Yanagihara, D.; Hirai, H. Progressive impairment of cerebellar mGluR signalling and its therapeutic potential for cerebellar ataxia in spinocerebellar ataxia type 1 model mice. J. Physiol. 2016, 595, 141–164. [Google Scholar] [CrossRef]
- De Vries, L.; Zheng, B.; Fischer, T.; Elenko, E.; Farquhar, M.G. The Regulator of G Protein Signaling Family. Annu. Rev. Pharmacol. Toxicol. 2000, 40, 235–271. [Google Scholar] [CrossRef] [PubMed]
- Zu, T.; Duvick, L.A.; Kaytor, M.D.; Berlinger, M.S.; Zoghbi, H.; Clark, H.B.; Orr, H. Recovery from Polyglutamine-Induced Neurodegeneration in Conditional SCA1 Transgenic Mice. J. Neurosci. 2004, 24, 8853–8861. [Google Scholar] [CrossRef] [PubMed]
- Notartomaso, S.; Zappulla, C.; Biagioni, F.; Cannella, M.; Bucci, D.; Mascio, G.; Scarselli, P.; Fazio, F.; Weisz, F.; Lionetto, L.; et al. Pharmacological enhancement of mGlu1 metabotropic glutamate receptors causes a prolonged symptomatic benefit in a mouse model of spinocerebellar ataxia type 1. Mol. Brain 2013, 6, 48. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.J.; Steinberg, J.P.; Huganir, R.L.; Linden, D.J. Requirement of AMPA Receptor GluR2 Phosphorylation for Cerebellar Long-Term Depression. Science 2003, 300, 1751–1755. [Google Scholar] [CrossRef]
- Power, E.; Morales, A.; Empson, R.M. Prolonged Type 1 Metabotropic Glutamate Receptor Dependent Synaptic Signaling Contributes to Spino-Cerebellar Ataxia Type 1. J. Neurosci. 2016, 36, 4910–4916. [Google Scholar] [CrossRef]
- Notartomaso, S.; Nakao, H.; Mascio, G.; Scarselli, P.; Cannella, M.; Zappulla, C.; Madonna, M.; Motolese, M.; Gradini, R.; Liberatore, F.; et al. mGlu1 Receptors Monopolize the Synaptic Control of Cerebellar Purkinje Cells by Epigenetically Down-Regulating mGlu5 Receptors. Sci. Rep. 2018, 8, 13361. [Google Scholar] [CrossRef]
- Harbers, M.; Nakao, H.; Watanabe, T.; Matsuyama, K.; Tohyama, S.; Nakao, K.; Kishimoto, Y.; Kano, M.; Aiba, A. mGluR5 Is Substitutable for mGluR1 in Cerebellar Purkinje Cells for Motor Coordination, Developmental Synapse Elimination, and Motor Learning. Cells 2022, 11, 2004. [Google Scholar] [CrossRef]
- Illarioshkin, S.N.; Slominsky, P.A.; Ovchinnikov, I.V.; Markova, E.D.; Miklina, N.I.; Klyushnikov, S.A.; Shadrina, M.; Vereshchagin, N.V.; Limborskaya, S.A.; Ivanova-Smolenskaya, I.A. Spinocerebellar ataxia type 1 in Russia. J. Neurol. 1996, 243, 506–510. [Google Scholar] [CrossRef]
- Manto, M.; Lorivel, T. Cognitive repercussions of hereditary cerebellar disorders. Cortex 2011, 47, 81–100. [Google Scholar] [CrossRef]
- Fancellu, R.; Paridi, D.; Tomasello, C.; Panzeri, M.; Castaldo, A.; Genitrini, S.; Soliveri, P.; Girotti, F. Longitudinal study of cognitive and psychiatric functions in spinocerebellar ataxia types 1 and 2. J. Neurol. 2013, 260, 3134–3143. [Google Scholar] [CrossRef]
- Jacobi, H.; Reetz, K.; du Montcel, S.T.; Bauer, P.; Mariotti, C.; Nanetti, L.; Rakowicz, M.; Sulek, A.; Durr, A.; Charles, P.; et al. Biological and clinical characteristics of individuals at risk for spinocerebellar ataxia types 1, 2, 3, and 6 in the longitudinal RISCA study: Analysis of baseline data. Lancet Neurol. 2013, 12, 650–658. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wu, C.; Lei, J.; Zhang, X. Cognitive impairments in patients with spinocerebellar ataxia types 1, 2 and 3 are positively correlated to the clinical severity of ataxia symptoms. Int. J. Clin. Exp. Med. 2014, 7, 5765–5771. [Google Scholar] [PubMed]
- Moriarty, A.; Cook, A.; Hunt, H.; Adams, M.E.; Cipolotti, L.; Giunti, P. A longitudinal investigation into cognition and disease progression in spinocerebellar ataxia types 1, 2, 3, 6, and 7. Orphanet, J. Rare Dis. 2016, 11, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zoghbi, H.Y.; Pollack, M.S.; Lyons, L.A.; Ferrell, R.E.; Daiger, S.P.; Beaudet, A.L. Spinocerebellar ataxia: Variable age of onset and linkage to human leukocyte antigen in a large kindred. Ann Neurol 1988, 23, 580–584. [Google Scholar] [CrossRef] [PubMed]
- Watase, K.; Weeber, E.J.; Xu, B.; Antalffy, B.; Yuva-Paylor, L.; Hashimoto, K.; Kano, M.; Atkinson, R.; Sun, Y.; Armstrong, D.L.; et al. A Long CAG Repeat in the Mouse Sca1 Locus Replicates SCA1 Features and Reveals the Impact of Protein Solubility on Selective Neurodegeneration. Neuron 2002, 34, 905–919. [Google Scholar] [CrossRef]
- Asher, M.; Rosa, J.-G.; Rainwater, O.; Duvick, L.; Bennyworth, M.; Lai, R.-Y.; Kuo, S.-H.; Cvetanovic, M.; Sca, C. Cerebellar contribution to the cognitive alterations in SCA1: Evidence from mouse models. Hum. Mol. Genet. 2019, 29, 117–131. [Google Scholar] [CrossRef]
- Shigemoto, R.; Nakanishi, S.; Mizuno, N. Distribution of the mRNA for a metabotropic glutamate receptor (mGluR1) in the central nervous system: An in situ hybridization study in adult and developing rat. J. Comp. Neurol. 1992, 322, 121–135. [Google Scholar] [CrossRef]
- Berthele, A.; Laurie, D.; Platzer, S.; Zieglgänsberger, W.; Tölle, T.; Sommer, B. Differential expression of rat and human type 1 metabotropic glutamate receptor splice variant messenger RNAs. Neuroscience 1998, 85, 733–749. [Google Scholar] [CrossRef]
- Conquet, F.; Bashir, Z.I.; Davies, C.H.; Daniel, H.; Ferraguti, F.; Bordi, F.; Franz-Bacon, K.; Reggiani, A.; Matarese, V.; Condé, F.; et al. Motor deficit and impairment of synaptic plasticity in mice lacking mGluR1. Nature 1994, 372, 237–243. [Google Scholar] [CrossRef]
- Perez, Y.; Morin, F.; Lacaille, J.-C. A hebbian form of long-term potentiation dependent on mGluR1a in hippocampal inhibitory interneurons. Proc. Natl. Acad. Sci. USA 2001, 98, 9401–9406. [Google Scholar] [CrossRef]
- Lapointe, V.; Morin, F.; Ratté, S.; Croce, A.; Conquet, F.; Lacaille, J.-C. Synapse-specific mGluR1-dependent long-term potentiation in interneurones regulates mouse hippocampal inhibition. J. Physiol. 2004, 555, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Naie, K.; Manahan-Vaughan, D. Pharmacological antagonism of metabotropic glutamate receptor 1 regulates long-term potentiation and spatial reference memory in the dentate gyrus of freely moving rats viaN-methyl-d-aspartate and metabotropic glutamate receptor-dependent mechanisms. Eur. J. Neurosci. 2005, 21, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Neyman, S.; Manahan-Vaughan, D. Metabotropic glutamate receptor 1 (mGluR1) and 5 (mGluR5) regulate late phases of LTP and LTD in the hippocampal CA1 region in vitro. Eur. J. Neurosci. 2008, 27, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Ran, I.; Laplante, I.; Bourgeois, C.; Pépin, J.; Lacaille, P.; Costa-Mattioli, M.; Pelletier, J.; Sonenberg, N.; Lacaille, J.-C. Persistent Transcription- and Translation-Dependent Long-Term Potentiation Induced by mGluR1 in Hippocampal Interneurons. J. Neurosci. 2009, 29, 5605–5615. [Google Scholar] [CrossRef]
- Tigaret, C.M.; Chamberlain, S.E.; Sadowski, J.H.; Hall, J.; Ashby, M.C.; Mellor, J.R. Convergent Metabotropic Signaling Pathways Inhibit SK Channels to Promote Synaptic Plasticity in the Hippocampus. J. Neurosci. 2018, 38, 9252–9262. [Google Scholar] [CrossRef]
- Friend, L.N.; Williamson, R.C.; Merrill, C.B.; Newton, S.T.; Christensen, M.T.; Petersen, J.; Wu, B.; Ostlund, I.; Edwards, J.G. Hippocampal Stratum Oriens Somatostatin-Positive Cells Undergo CB1-Dependent Long-Term Potentiation and Express Endocannabinoid Biosynthetic Enzymes. Molecules 2019, 24, 1306. [Google Scholar] [CrossRef]
- Racine, A.-S.; Michon, F.-X.; Laplante, I.; Lacaille, J.-C. Somatostatin contributes to long-term potentiation at excitatory synapses onto hippocampal somatostatinergic interneurons. Mol. Brain 2021, 14, 1–20. [Google Scholar] [CrossRef]
- Morris, R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 1984, 11, 47–60. [Google Scholar] [CrossRef]
- Landucci, E.; Boscia, F.; Gerace, E.; Scartabelli, T.; Cozzi, A.; Moroni, F.; Mannaioni, G.; Pellegrini-Giampietro, D.E. Chapter 23 Involvement of Endocannabinoid Signaling in the Neuroprotective Effects of Subtype 1 Metabotropic Glutamate Receptor Antagonists in Models of Cerebral Ischemia. Int. Rev. Neurobiol. 2009, 85, 337–350. [Google Scholar] [CrossRef]
- Inada, H.; Maejima, T.; Nakahata, Y.; Yamaguchi, J.; Nabekura, J.; Ishibashi, H. Endocannabinoids contribute to metabotropic glutamate receptor-mediated inhibition of GABA release onto hippocampal CA3 pyramidal neurons in an isolated neuron/bouton preparation. Neuroscience 2010, 165, 1377–1389. [Google Scholar] [CrossRef]
- Huang, G.Z.; Woolley, C.S. Estradiol Acutely Suppresses Inhibition in the Hippocampus through a Sex-Specific Endocannabinoid and mGluR-Dependent Mechanism. Neuron 2012, 74, 801–808. [Google Scholar] [CrossRef] [PubMed]
- Landucci, E.; Berlinguer-Palmini, R.; Baccini, G.; Boscia, F.; Gerace, E.; Mannaioni, G.; Pellegrini-Giampietro, D.E. The Neuroprotective Effects of mGlu1 Receptor Antagonists Are Mediated by an Enhancement of GABAergic Synaptic Transmission via a Presynaptic CB1 Receptor Mechanism. Cells 2022, 11, 3015. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V.; Fontana, A.; Cadas, H.; Schinelli, S.; Cimino, G.; Schwartz, J.-C.; Piomelli, D. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature 1994, 372, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Simon, G.M.; Cravatt, B.F. Endocannabinoid Biosynthesis Proceeding through Glycerophospho-N-acyl Ethanolamine and a Role for α/β-Hydrolase 4 in This Pathway. J. Biol. Chem. 2006, 281, 26465–26472. [Google Scholar] [CrossRef]
- Liu, J.; Wang, L.; Harvey-White, J.; Osei-Hyiaman, D.; Razdan, R.; Gong, Q.; Chan, A.C.; Zhou, Z.; Huang, B.X.; Kim, H.-Y.; et al. A biosynthetic pathway for anandamide. Proc. Natl. Acad. Sci. USA 2006, 103, 13345–13350. [Google Scholar] [CrossRef]
- Liu, J.; Wang, L.; Harvey-White, J.; Huang, B.X.; Kim, H.-Y.; Luquet, S.; Palmiter, R.D.; Krystal, G.; Rai, R.; Mahadevan, A.; et al. Multiple pathways involved in the biosynthesis of anandamide. Neuropharmacology 2008, 54, 1–7. [Google Scholar] [CrossRef]
- Pollock, P.M.; Cohen-Solal, K.; Sood, R.; Namkoong, J.; Martino, J.J.; Koganti, A.; Zhu, H.; Robbins, C.; Makalowska, I.; Shin, S.-S.; et al. Melanoma mouse model implicates metabotropic glutamate signaling in melanocytic neoplasia. Nat. Genet. 2003, 34, 108–112. [Google Scholar] [CrossRef]
- Eddy, K.; Eddin, M.N.; Fateeva, A.; Pompili, S.V.B.; Shah, R.; Doshi, S.; Chen, S. Implications of a Neuronal Receptor Family, Metabotropic Glutamate Receptors, in Cancer Development and Progression. Cells 2022, 11, 2857. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liberatore, F.; Antenucci, N.; Tortolani, D.; Mascio, G.; Fanti, F.; Sergi, M.; Battaglia, G.; Bruno, V.; Nicoletti, F.; Maccarrone, M.; et al. Targeting mGlu1 Receptors in the Treatment of Motor and Cognitive Dysfunctions in Mice Modeling Type 1 Spinocerebellar Ataxia. Cells 2022, 11, 3916. https://doi.org/10.3390/cells11233916
Liberatore F, Antenucci N, Tortolani D, Mascio G, Fanti F, Sergi M, Battaglia G, Bruno V, Nicoletti F, Maccarrone M, et al. Targeting mGlu1 Receptors in the Treatment of Motor and Cognitive Dysfunctions in Mice Modeling Type 1 Spinocerebellar Ataxia. Cells. 2022; 11(23):3916. https://doi.org/10.3390/cells11233916
Chicago/Turabian StyleLiberatore, Francesca, Nico Antenucci, Daniel Tortolani, Giada Mascio, Federico Fanti, Manuel Sergi, Giuseppe Battaglia, Valeria Bruno, Ferdinando Nicoletti, Mauro Maccarrone, and et al. 2022. "Targeting mGlu1 Receptors in the Treatment of Motor and Cognitive Dysfunctions in Mice Modeling Type 1 Spinocerebellar Ataxia" Cells 11, no. 23: 3916. https://doi.org/10.3390/cells11233916
APA StyleLiberatore, F., Antenucci, N., Tortolani, D., Mascio, G., Fanti, F., Sergi, M., Battaglia, G., Bruno, V., Nicoletti, F., Maccarrone, M., & Notartomaso, S. (2022). Targeting mGlu1 Receptors in the Treatment of Motor and Cognitive Dysfunctions in Mice Modeling Type 1 Spinocerebellar Ataxia. Cells, 11(23), 3916. https://doi.org/10.3390/cells11233916






