Impaired Autophagy Causes Severe Corneal Neovascularization
Abstract
:1. Introduction
2. Methods
2.1. Cell Culture
2.2. M1 Macrophage Polarization from THP-1 Cells and Acquisition of the Conditioned Medium
2.3. Reverse Transcription–Quantitative Polymerase Chain Reaction (RT-qPCR)
2.4. Western Blot (WB)
2.5. Scratch Wound-Healing Assay for Cell Migration
2.6. Tube Formation Assay
2.7. Cell Counting Kit-8 (CCK-8) Assay
2.8. Enzyme-Linked Immunosorbent Assay (ELISA)
2.9. Corneal Alkali Burn Model and Rapamycin Treatment
2.10. Evaluation of Corneal Morphology under Slit Lamp
2.11. Statistical Analysis
3. Results
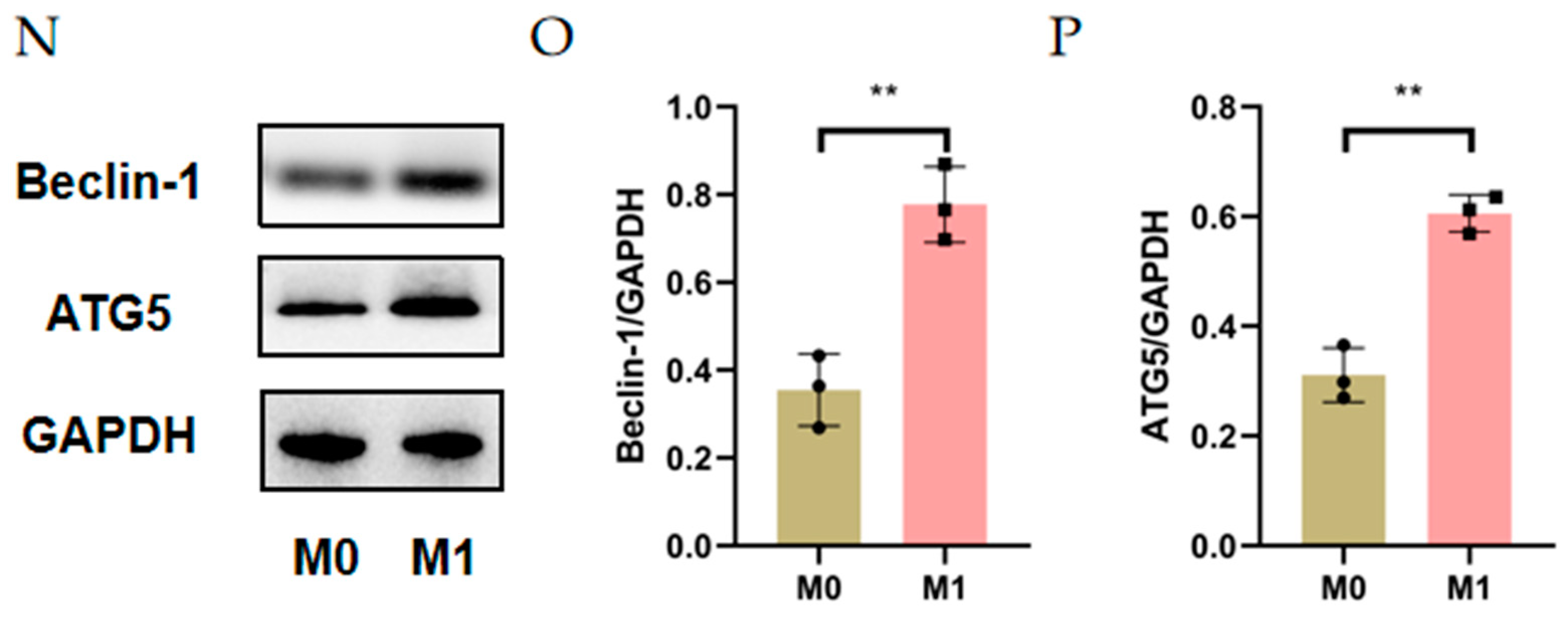
3.1. Autophagy Was Activated in Alkali Burn-Induced CNV and M1 Macrophages
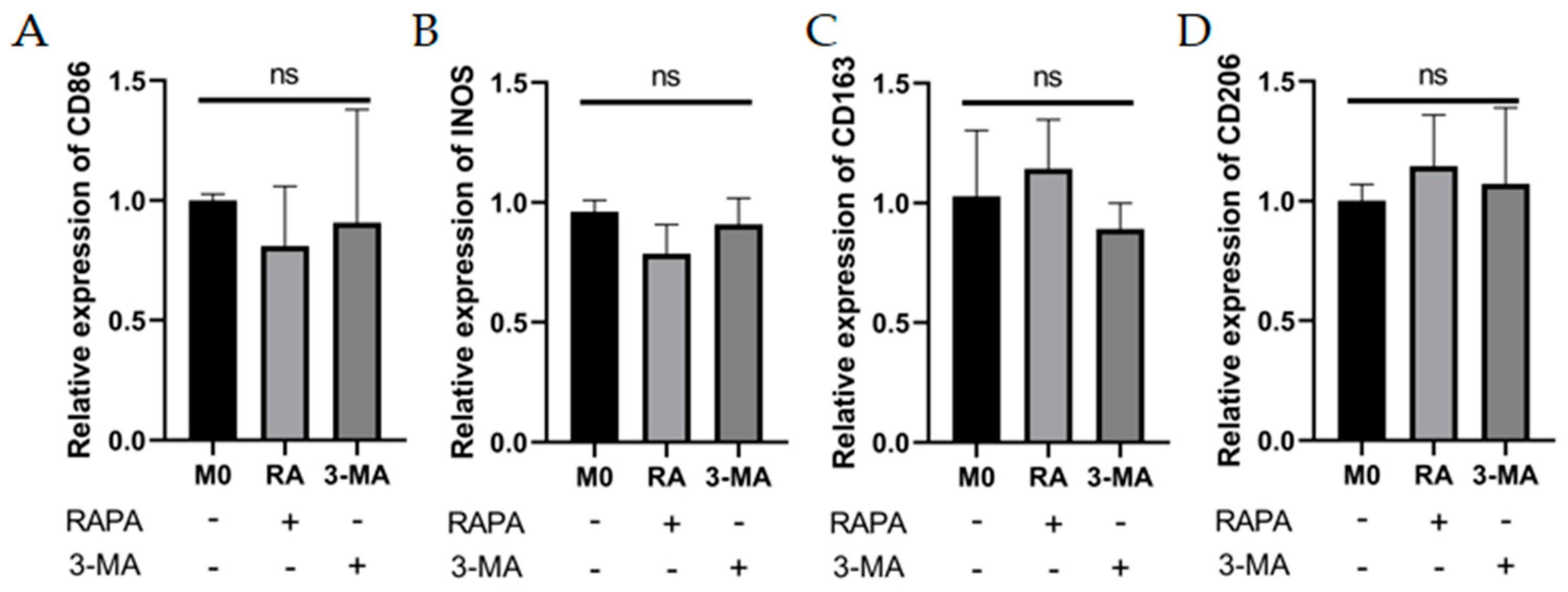
3.2. Enhanced Autophagy Suppresses M1 Macrophage Markers and Impaired Autophagy Enhances M1 Macrophage Markers
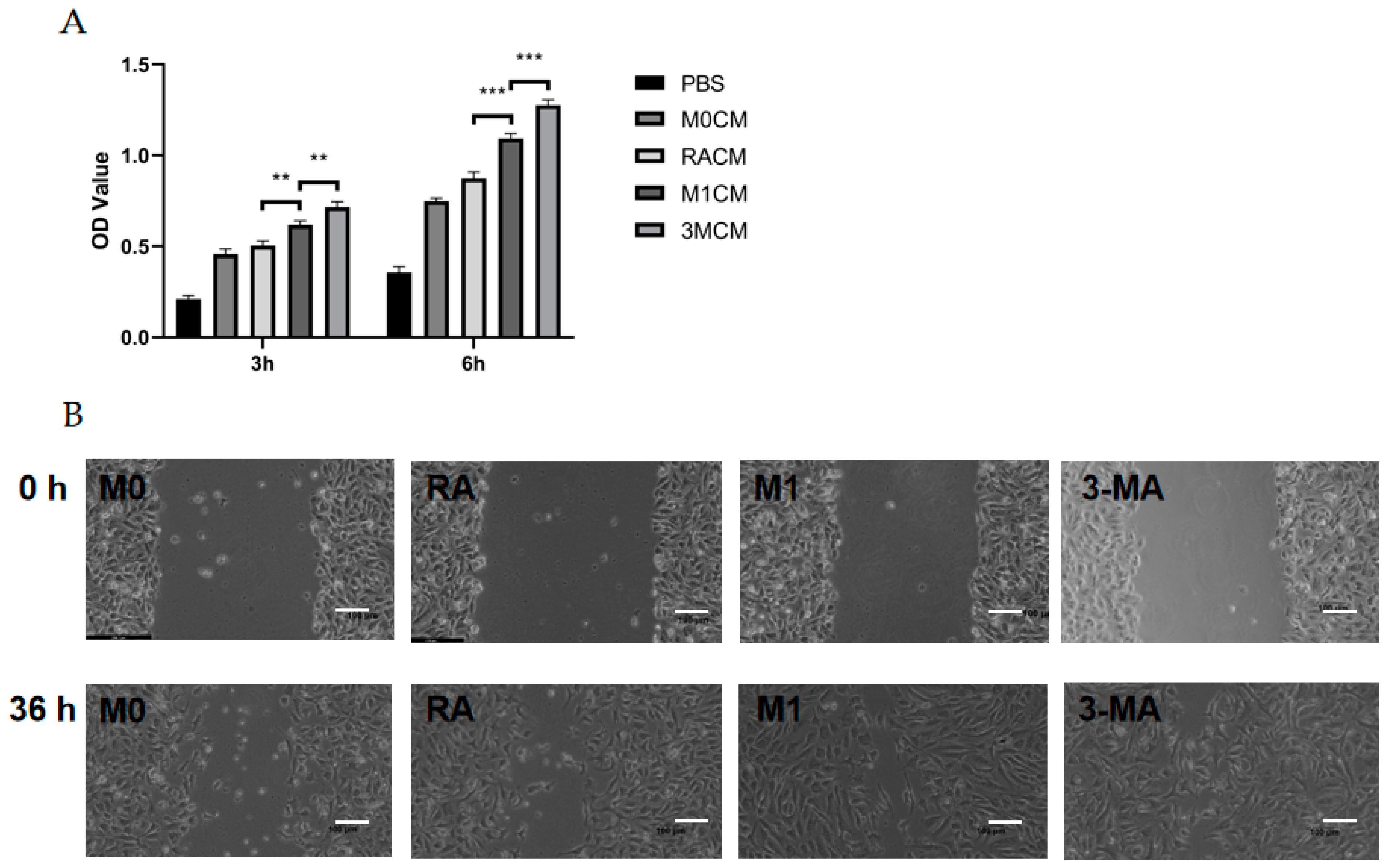
3.3. The Culture Supernatant of Impaired M1 Macrophages Enhanced the Proliferation, Migration, and Tube Formation Capacity of HUVECs
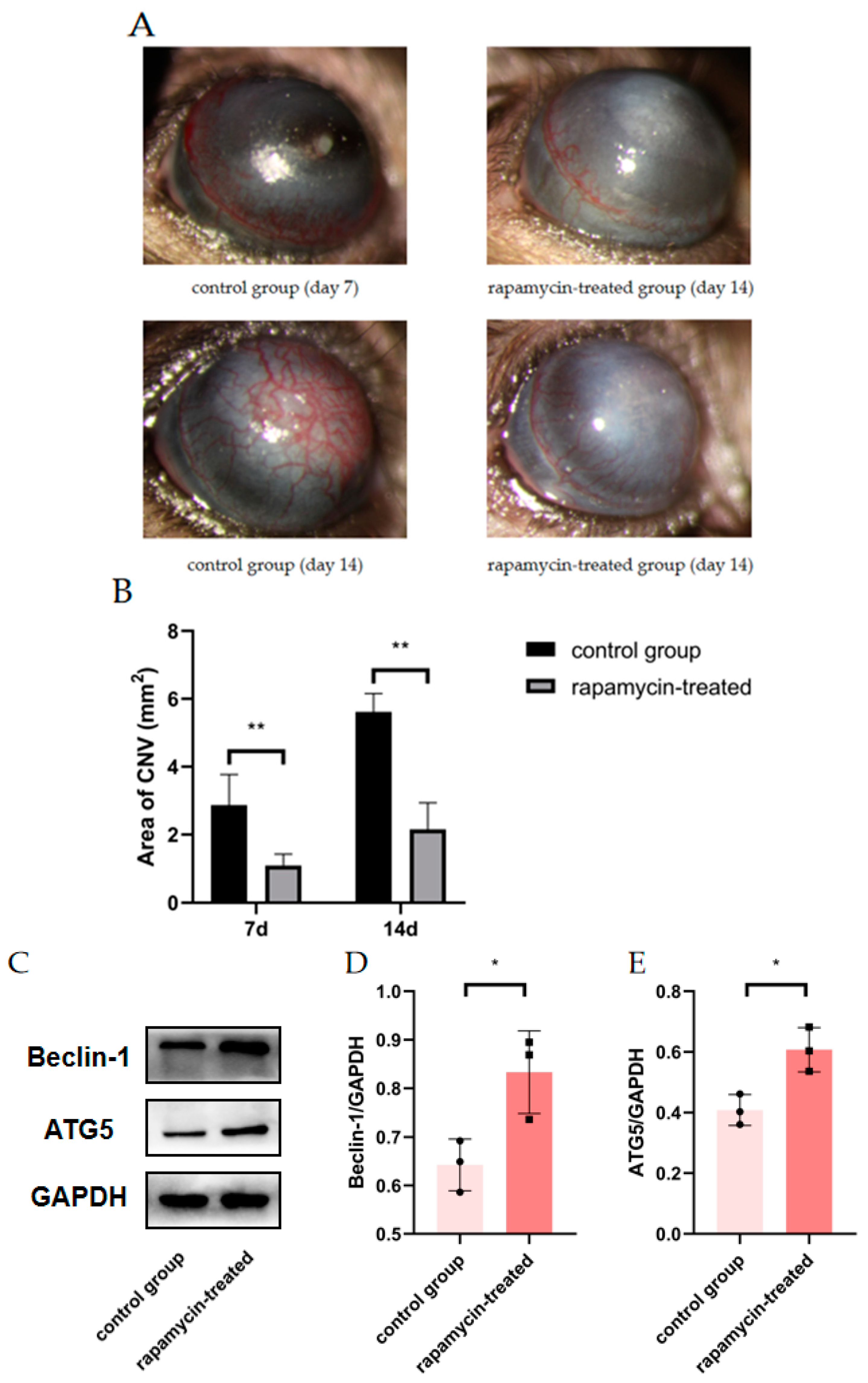
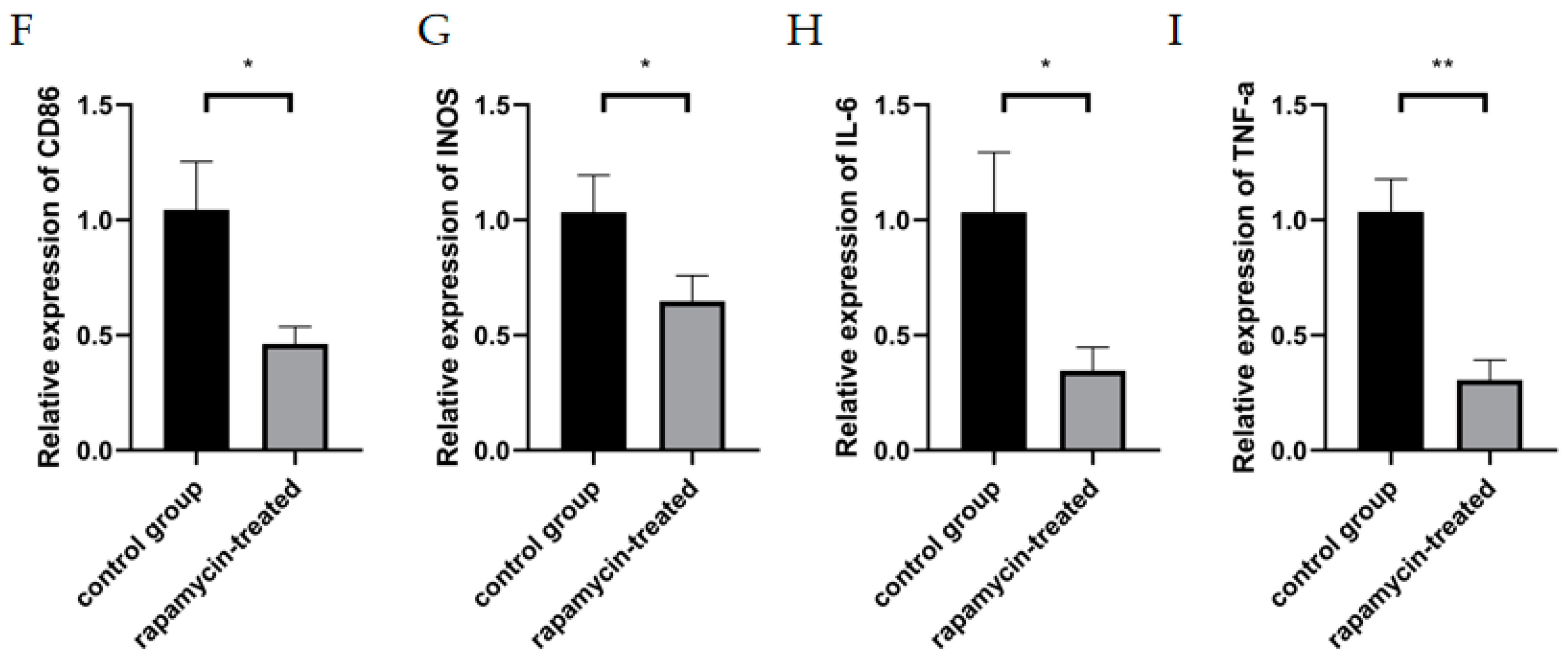
3.4. Autophagy Enhancement Improved Corneal Neovascularization in Alkali Burned Mice
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CNV | Corneal neovascularization | HUVECs | Human umbilical vein endothelial cells |
| RT-qPCR | Real-time quantitative PCR | CCK-8 | Vecsxaz Cell Counting Kit-8 |
| ELISA | Enzyme-Linked Immunosorbent Assay | ATG5 | Autophagy-related 5 |
| ATG7 | Autophagy-related 7 | INOS | Inducible nitric oxide synthase |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase | IL-6 | Interleukin-6 |
| TNF-α | Tumor necrosis factor-alpha | RAPA | Rapamycin |
| 3-MA | 3-methyladenine | LPS | Lipopolysaccharide |
| INF-γ | Interferon-gamma | ATCC | American Type Culture Collection |
| FBS | Fetal bovine serum | PMA | Phorbol 12-myristate 13-acetate |
| LSCD | Limbal stem cell deficiency | DMEM | Dulbecco’s modified eagle medium |
| M0CM | M0 macrophage-conditioned medium | MCM | Macrophage-conditioned medium |
| M1CM | M1 macrophage-conditioned medium with no pretreatment | RACM | M1 macrophage-conditioned Medium with rapamycin pretreatment |
| 3MCM | M1 macrophage-conditioned medium with 3-MA pretreatment | CG | Control group |
References
- Nicholas, M.P.; Mysore, N. Corneal neovascularization. Exp. Eye Res. 2021, 202, 108363. [Google Scholar] [CrossRef] [PubMed]
- Austin, A.; Lietman, T.; Rose-Nussbaumer, J. Update on the Management of Infectious Keratitis. Ophthalmology 2017, 124, 1678–1689. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Jurkunas, U. Limbal Stem Cell Transplantation and Complications. Semin. Ophthalmol. 2018, 33, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Kaur, M.; Agarwal, T.; Sangwan, V.S.; Vajpayee, R.B. Treatment of acute ocular chemical burns. Surv. Ophthalmol. 2018, 63, 214–235. [Google Scholar] [CrossRef]
- Mamikonyan, V.R.; Pivin, E.A.; Krakhmaleva, D.A. Mechanisms of corneal neovascularization and modern options for its suppression. Vestn. Oftalmol. 2016, 132, 81–87. [Google Scholar] [CrossRef]
- Su, W.; Sun, S.; Tian, B.; Tai, P.W.; Luo, Y.; Ko, J.; Zhan, W.; Ke, X.; Zheng, Q.; Li, X.; et al. Efficacious, safe, and stable inhibition of corneal neovascularization by AAV-vectored anti-VEGF therapeutics. Mol. Ther. Methods Clin. Dev. 2021, 22, 107–121. [Google Scholar] [CrossRef]
- Wolf, M.; Clay, S.M.; Zheng, S.; Pan, P.; Chan, M.F. MMP12 Inhibits Corneal Neovascularization and Inflammation through Regulation of CCL2. Sci. Rep. 2019, 9, 11579. [Google Scholar] [CrossRef] [Green Version]
- Roshandel, D.; Eslani, M.; Baradaran-Rafii, A.; Cheung, A.Y.; Kurji, K.; Jabbehdari, S.; Maiz, A.; Jalali, S.; Djalilian, A.R.; Holland, E.J. Current and emerging therapies for corneal neovascularization. Ocul. Surf. 2018, 16, 398–414. [Google Scholar] [CrossRef]
- Abdelfattah, N.S.; Amgad, M.; Zayed, A.A. Host immune cellular reactions in corneal neovascularization. Int. J. Ophthalmol. 2016, 9, 625–633. [Google Scholar]
- Usui, T.; Yamagami, S.; Kishimoto, S.; Seiich, Y.; Nakayama, T.; Amano, S. Role of macrophage migration inhibitory factor in corneal neovascularization. Investig. Ophthalmol. Vis. Sci. 2007, 48, 3545–3550. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Wu, H.; Lu, P.; Zhang, X. Interleukin (IL)-17A Promotes Angiogenesis in an Experimental Corneal Neovascularization Model. Curr. Eye Res. 2017, 42, 368–379. [Google Scholar] [CrossRef]
- Tobita, Y.; Arima, T.; Nakano, Y.; Uchiyama, M.; Shimizu, A.; Takahashi, H. Peroxisome Proliferator-Activated Receptor Beta/Delta Agonist Suppresses Inflammation and Promotes Neovascularization. Int. J. Mol. Sci. 2020, 21, 5296. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Li, L.; Liu, G.; Baba, T.; Ishida, Y.; Nosaka, M.; Kondo, T.; Zhang, X.; Mukaida, N. Critical role of TNF-alpha-induced macrophage VEGF and iNOS production in the experimental corneal neovascularization. Investig. Ophthalmol. Vis. Sci. 2012, 53, 3516–3526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, Y.H.; Lv, Y.; Wang, W.Q.; Sun, G.L.; Zhang, H.H. LncRNA NEAT1 promotes inflammatory response and induces corneal neovascularization. J. Mol. Endocrinol. 2018, 61, 231–239. [Google Scholar] [CrossRef]
- Zahir-Jouzdani, F.; Atyabi, F.; Mojtabavi, N. Interleukin-6 participation in pathology of ocular diseases. Pathophysiology 2017, 24, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Zhang, Y. Autophagy and Inflammatory Diseases. Adv. Exp. Med. Biol. 2020, 1207, 391–400. [Google Scholar]
- Yao, R.Q.; Ren, C.; Xia, Z.F.; Yao, Y.M. Organelle-specific autophagy in inflammatory diseases: A potential therapeutic target underlying the quality control of multiple organelles. Autophagy 2021, 17, 385–401. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.Y.; Lu, J.H. Autophagy and Macrophage Functions: Inflammatory Response and Phagocytosis. Cells 2019, 9, 70. [Google Scholar] [CrossRef] [Green Version]
- Vergadi, E.; Ieronymaki, E.; Lyroni, K.; Vaporidi, K.; Tsatsanis, C. Akt Signaling Pathway in Macrophage Activation and M1/M2 Polarization. J. Immunol. 2017, 198, 1006–1014. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Zhu, S.; Li, J.; Jiang, T.; Feng, L.; Pei, J.; Wang, G.; Ouyang, L.; Liu, B. Targeting autophagy using small-molecule compounds to improve potential therapy of Parkinson’s disease. Acta Pharm. Sin. B 2021, 11, 3015–3034. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H. Regulation of Autophagy by mTOR Signaling Pathway. Adv. Exp. Med. Biol. 2019, 1206, 67–83. [Google Scholar] [PubMed]
- Bhat, P.; Kriel, J.; Shubha Priya, B.; Basappa Shivananju, N.S.; Loos, B. Modulating autophagy in cancer therapy: Advancements and challenges for cancer cell death sensitization. Biochem. Pharmacol. 2018, 147, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Torres, P.F.; Kijlstra, A. The role of cytokines in corneal immunopathology. Ocul. Immunol. Inflamm. 2001, 9, 9–24. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, A. Immunological aspects of corneal transplant. Immunol. Investig. 2014, 43, 888–901. [Google Scholar] [CrossRef] [PubMed]
- De Palma, M.; Biziato, D.; Petrova, T.V. Microenvironmental regulation of tumour angiogenesis. Nat. Rev. Cancer 2017, 17, 457–474. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.Q.; Du, W.L.; Cai, M.H.; Yao, J.Y.; Zhao, Y.Y.; Mou, X.Z. The roles of tumor-associated macrophages in tumor angiogenesis and metastasis. Cell. Immunol. 2020, 353, 104119. [Google Scholar] [CrossRef] [PubMed]
- Glick, D.; Barth, S.; Macleod, K.F. Autophagy: Cellular and molecular mechanisms. J. Pathol. 2010, 221, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Parzych, K.R.; Klionsky, D.J. An overview of autophagy: Morphology, mechanism, and regulation. Antioxid. Redox Signal. 2014, 20, 460–473. [Google Scholar] [CrossRef] [Green Version]
- Levine, B.; Kroemer, G. Biological Functions of Autophagy Genes: A Disease Perspective. Cell 2019, 176, 11–42. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Li, Y.; Fu, M.; Xin, H.B. Polarizing Macrophages In Vitro. Methods Mol. Biol. 2018, 1784, 119–126. [Google Scholar]
- Cao, W.; Li, J.; Yang, K.; Cao, D. An overview of autophagy: Mechanism, regulation and research progress. Bull. Cancer 2021, 108, 304–322. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Gu, J.; Liu, R.; Wei, S.; Wang, Q.; Shen, H.; Dai, Y.; Zhou, H.; Zhang, F.; Lu, L. Spermine Alleviates Acute Liver Injury by Inhibiting Liver-Resident Macrophage Pro-Inflammatory Response Through ATG5-Dependent Autophagy. Front. Immunol. 2018, 9, 948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, K.; Zhao, E.; Ilyas, G.; Lalazar, G.; Lin, Y.; Haseeb, M.; Tanaka, K.E.; Czaja, M.J. Impaired macrophage autophagy increases the immune response in obese mice by promoting proinflammatory macrophage polarization. Autophagy 2015, 11, 271–284. [Google Scholar] [CrossRef] [PubMed]
- Keating, A.M.; Jacobs, D.S. Anti-VEGF Treatment of Corneal Neovascularization. Ocul. Surf. 2011, 9, 227–237. [Google Scholar] [CrossRef]
- Chang, J.H.; Gabison, E.E.; Kato, T.; Azar, D.T. Corneal neovascularization. Curr. Opin. Ophthalmol. 2001, 12, 242–249. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.; Zhang, J.; Wang, Y.; Chen, J.; Li, X.; Liu, X.; Kong, E.; Su, S.B.; Zhang, Z. The Effect of Interleukin 38 on Inflammation-induced Corneal Neovascularization. Curr. Mol. Med. 2019, 19, 589–596. [Google Scholar] [CrossRef]
- Ferrari, G.; Bignami, F.; Rama, P. Tumor necrosis factor-alpha inhibitors as a treatment of corneal hemangiogenesis and lymphangiogenesis. Eye Contact Lens 2015, 41, 72–76. [Google Scholar] [CrossRef]
- Harris, J. Autophagy and cytokines. Cytokine 2011, 56, 140–144. [Google Scholar] [CrossRef]
- Ge, Y.; Huang, M.; Yao, Y.M. Autophagy and proinflammatory cytokines: Interactions and clinical implications. Cytokine Growth Factor Rev. 2018, 43, 38–46. [Google Scholar] [CrossRef]
- Phinney, D.G.; Di Giuseppe, M.; Njah, J.; Sala, E.; Shiva, S.; St Croix, C.M.; Stolz, D.B.; Watkins, S.C.; Di, Y.P.; Leikauf, G.D.; et al. Mesenchymal stem cells use extracellular vesicles to outsource mitophagy and shuttle microRNAs. Nat. Commun. 2015, 6, 8472. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Liu, F.; Zhou, P.; Wang, Q.; Xu, C.; Li, Y.; Bian, L.; Liu, Y.; Zhou, J.; Wang, F.; et al. The MTOR signaling pathway regulates macrophage differentiation from mouse myeloid progenitors by inhibiting autophagy. Autophagy 2019, 15, 1150–1162. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.J.; Chen, Y.Y.; Hsiao, C.M.; Pan, M.H.; Wang, B.J.; Chen, Y.C.; Ho, C.T.; Huang, K.C.; Chen, R.J. Induction of Autophagy by Pterostilbene Contributes to the Prevention of Renal Fibrosis via Attenuating NLRP3 Inflammasome Activation and Epithelial-Mesenchymal Transition. Front. Cell Dev. Biol. 2020, 8, 436. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Ma, Y.; Huang, W.; Gao, J.; Guo, M.; Li, J.; Kong, L.; Liang, G.; Du, R.; Xu, Q.; et al. Typically inhibiting USP14 promotes autophagy in M1-like macrophages and alleviates CLP-induced sepsis. Cell Death Dis. 2020, 11, 666. [Google Scholar] [CrossRef] [PubMed]
- Mills, C.D. M1 and M2 Macrophages: Oracles of Health and Disease. Crit. Rev. Immunol. 2012, 32, 463–488. [Google Scholar] [CrossRef] [PubMed]




| Primer Name | 5′->3′ Forward | 5′->3′ Reverse |
|---|---|---|
| mice GAPDH | GAGAGTGTTTCCTCGTCCC | ATTTGCCGTGAGTGGAGTC |
| mice Beclin-1 | GGGGGTTTGCGGTTTTTCTG | CTGCCTCCAGTGTCTTCAATCT |
| mice ATG5 | TATCAGACCACGACGGAGC | CAGAGGGGTTTCCAGCATTG |
| mice ATG7 | CCAACTCCACACTGCTTTCCT | CTACTGTTCTTACCAGCCTCACT |
| mice CD86 | TCAGTATCTCCAACAGCCTCTC | TCCAGAACACACACAACGGT |
| mice INOS | GCCACCTCTACATTTGCGGA | CTGCTCCTCGCTCAAGTTCA |
| mice IL-6 | GCCTTCTTGGGACTGATGCT | GGTCTGTTGGGAGTGGTATCC |
| mice TNF-α | GAGAAGGGGGACCAACTCAG | CTCCAAAGTAGACCTGCCCG |
| human GAPDH | ATCCCATCACCATCTTCCAGG | AAATGAGCCCCAGCCTTCTC |
| human Beclin-1 | GAGGTTGAGAAAGGCGAGACA | GAGGACACCCAAGCAAGACC |
| human ATG5 | CGAGATGTGTGGTTTGGACGA | TGGTTCTGCTTCCCTTTCAGTT |
| human ATG7 | AACCTCTCTTGGGCTTGTGC | GGCTGACGGGAAGGACATT |
| human CD11B | TATGACTGGGCTGGTGGAGT | TCTGCCTGAACATCGCTACC |
| human CD68 | ACTGAACCCCAACAAAACCA | GGAATGAGAGAAGCAGGTGG |
| human INOS | GATGGGAGAAGGGGATGAGC | GAATGTGCTGTTTGCCTCGG |
| human CD86 | CCCCAGACCACATTCCTTGG | TGTTCACTCTCTTCCCTCTCCA |
| human CD163 | GCTCAGGAAACCAGTCCCAA | TACCAGGCGAAGTTGACCAC |
| human CD206 | TACTGAACCCCCACAACTGC | ACCAGAGAGGAACCCATTCG |
| human IL-6 | CTTCGGTCCAGTTGCCTTCT | GGTGAGTGGCTGTCTGTGTG |
| human TNF-α | ACCTCCTCTCTGCCATCAAGA | TCCCAAAGTAGACCTGCCCA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, K.; Yang, Y.; Yuan, Y.; Xiang, Y.; Zhou, S. Impaired Autophagy Causes Severe Corneal Neovascularization. Cells 2022, 11, 3895. https://doi.org/10.3390/cells11233895
Yi K, Yang Y, Yuan Y, Xiang Y, Zhou S. Impaired Autophagy Causes Severe Corneal Neovascularization. Cells. 2022; 11(23):3895. https://doi.org/10.3390/cells11233895
Chicago/Turabian StyleYi, Kun, Yuping Yang, Ye Yuan, Yingqian Xiang, and Shanbi Zhou. 2022. "Impaired Autophagy Causes Severe Corneal Neovascularization" Cells 11, no. 23: 3895. https://doi.org/10.3390/cells11233895
APA StyleYi, K., Yang, Y., Yuan, Y., Xiang, Y., & Zhou, S. (2022). Impaired Autophagy Causes Severe Corneal Neovascularization. Cells, 11(23), 3895. https://doi.org/10.3390/cells11233895





