Identification and Functional Characterization of the Transcription Factors AhR/ARNT in Dendroctonus armandi
Abstract
1. Introduction
2. Materials and Methods
2.1. Insects and Reagents Preparation
2.2. RNA Extraction, cDNA Synthesis, and Reverse Transcription Quantitative PCR (qRT-qPCR)
2.3. Gene Cloning and Bioinformatic Analysis
2.4. Developmental- and Tissue-Dependent Expression Profiles of DaAhR and DaARNT
2.5. Terpenoids Exposure
2.6. Double-Strand RNA (dsRNA) Synthesis and Injection
2.7. Statistical Analysis
3. Results
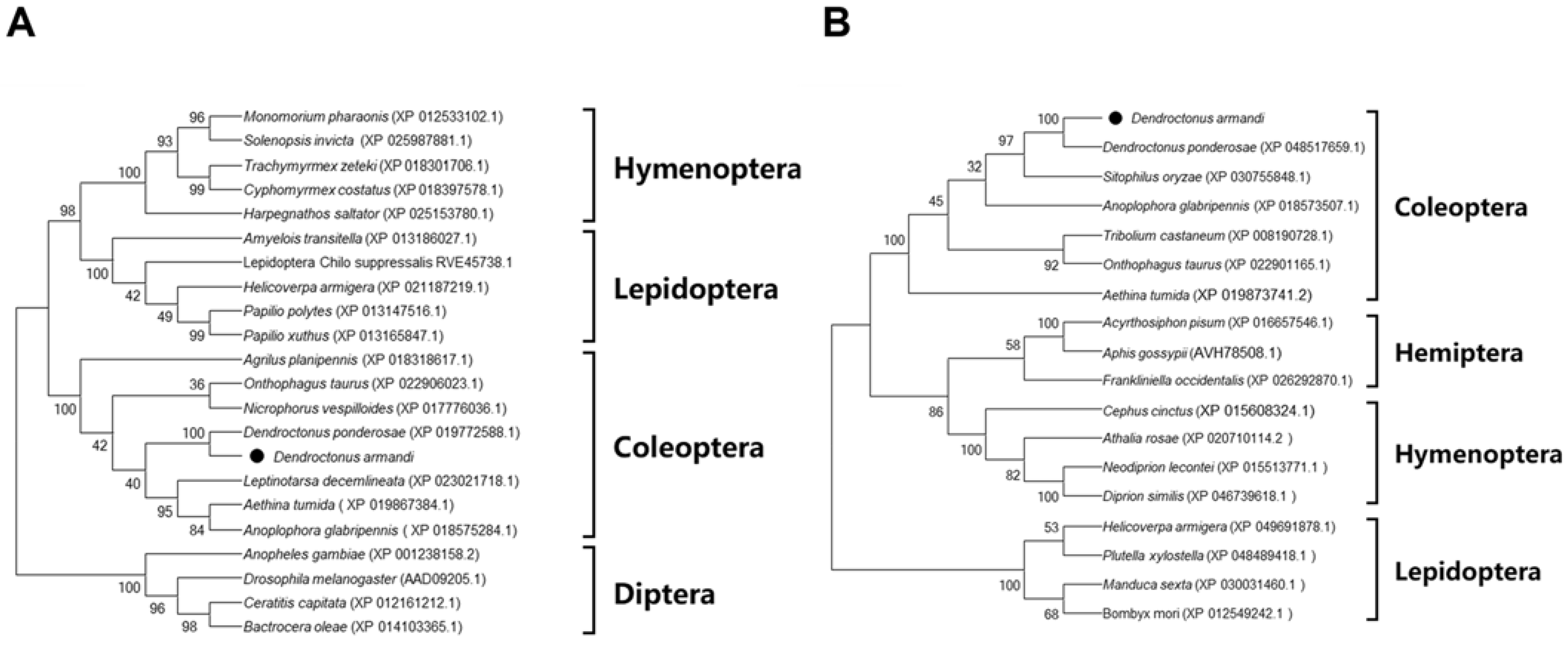
3.1. Sequencing and Bioinformatic Analysis
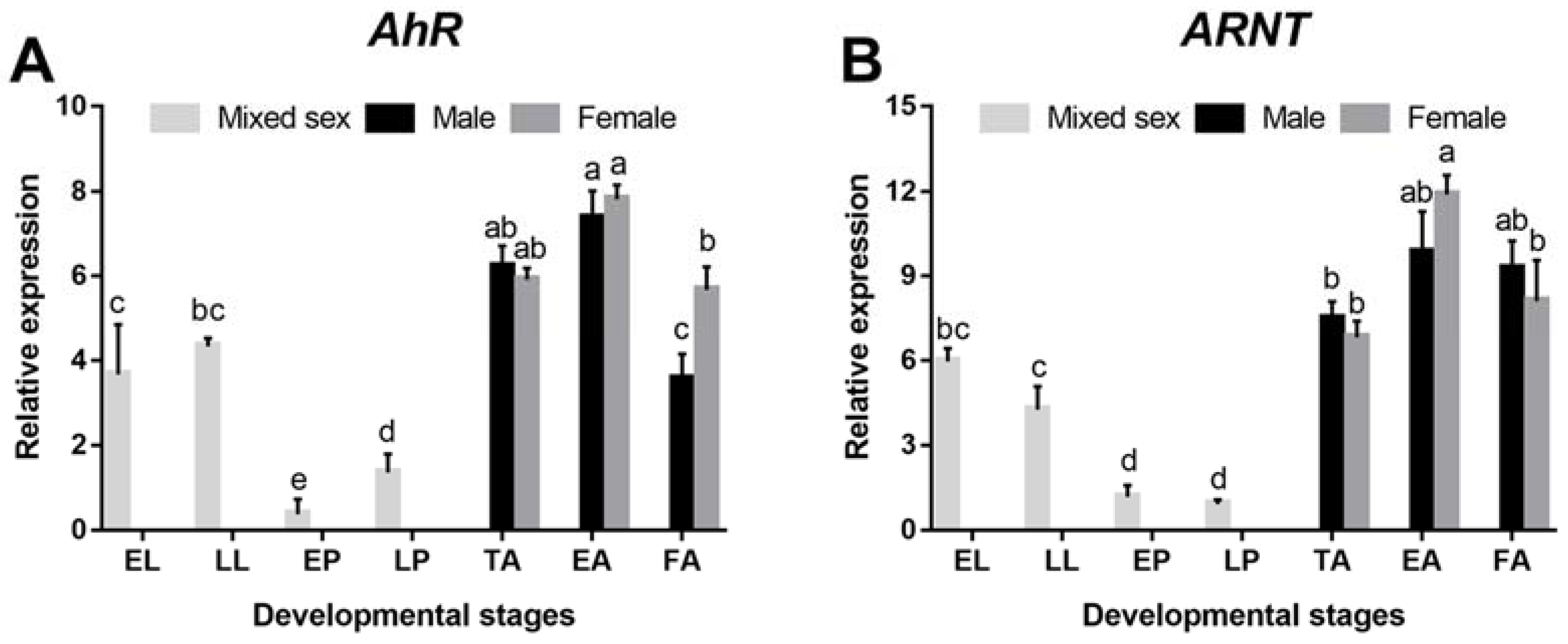
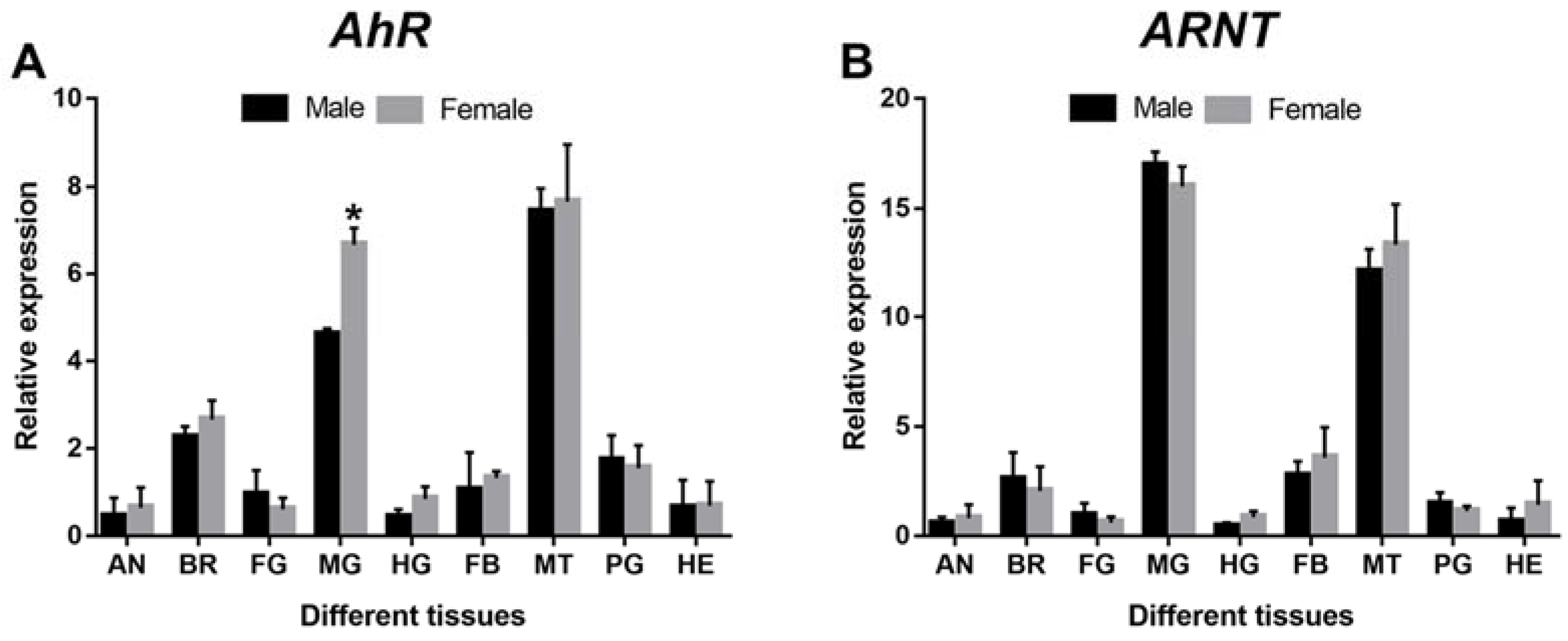
3.2. Spatiotemporal Expression Pattern of DaAhR and DaARNT
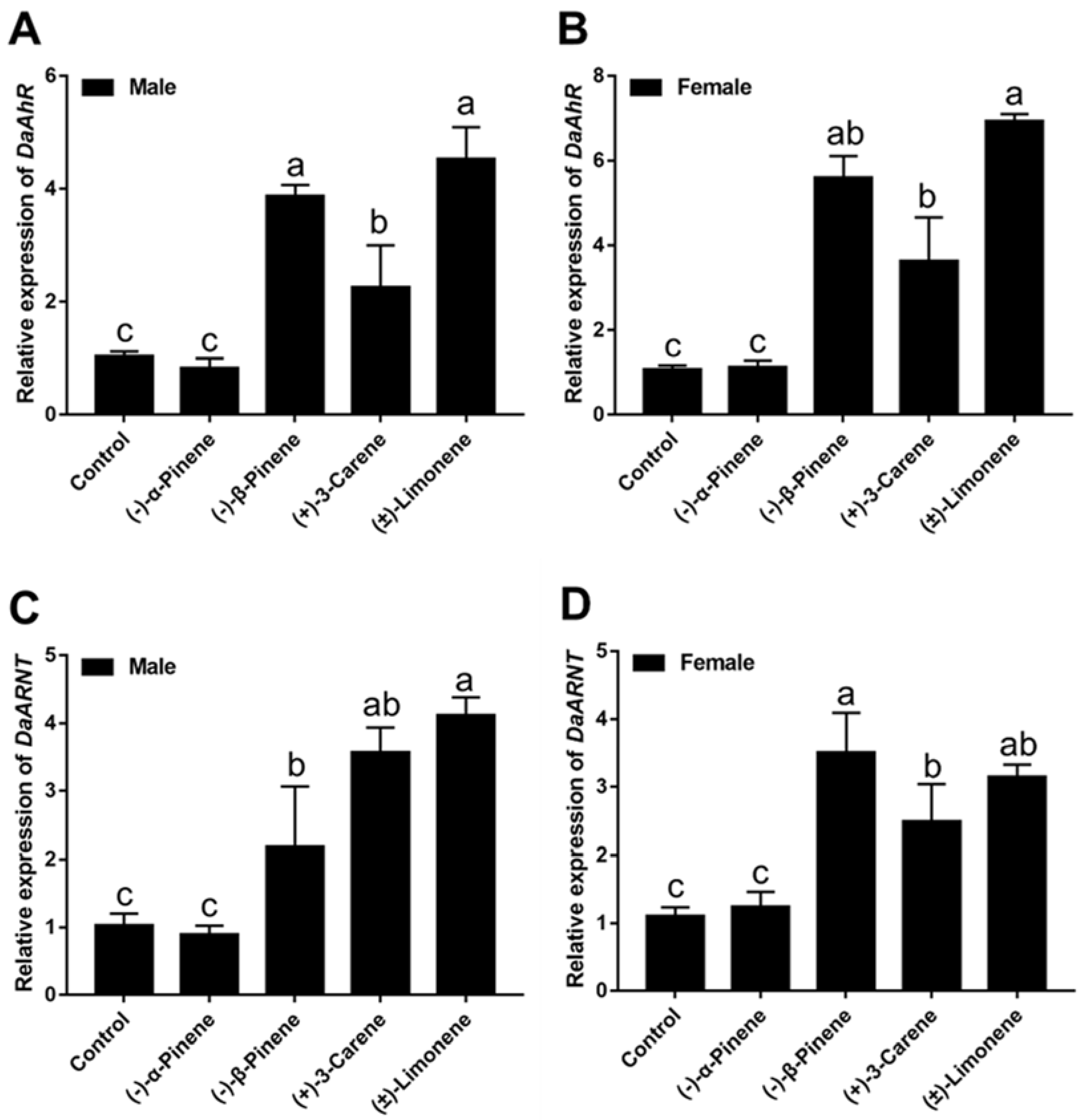
3.3. Exposure to Terpenoids
3.4. Functional Analysis of DaAhR and DaARNT by RNAi Silencing
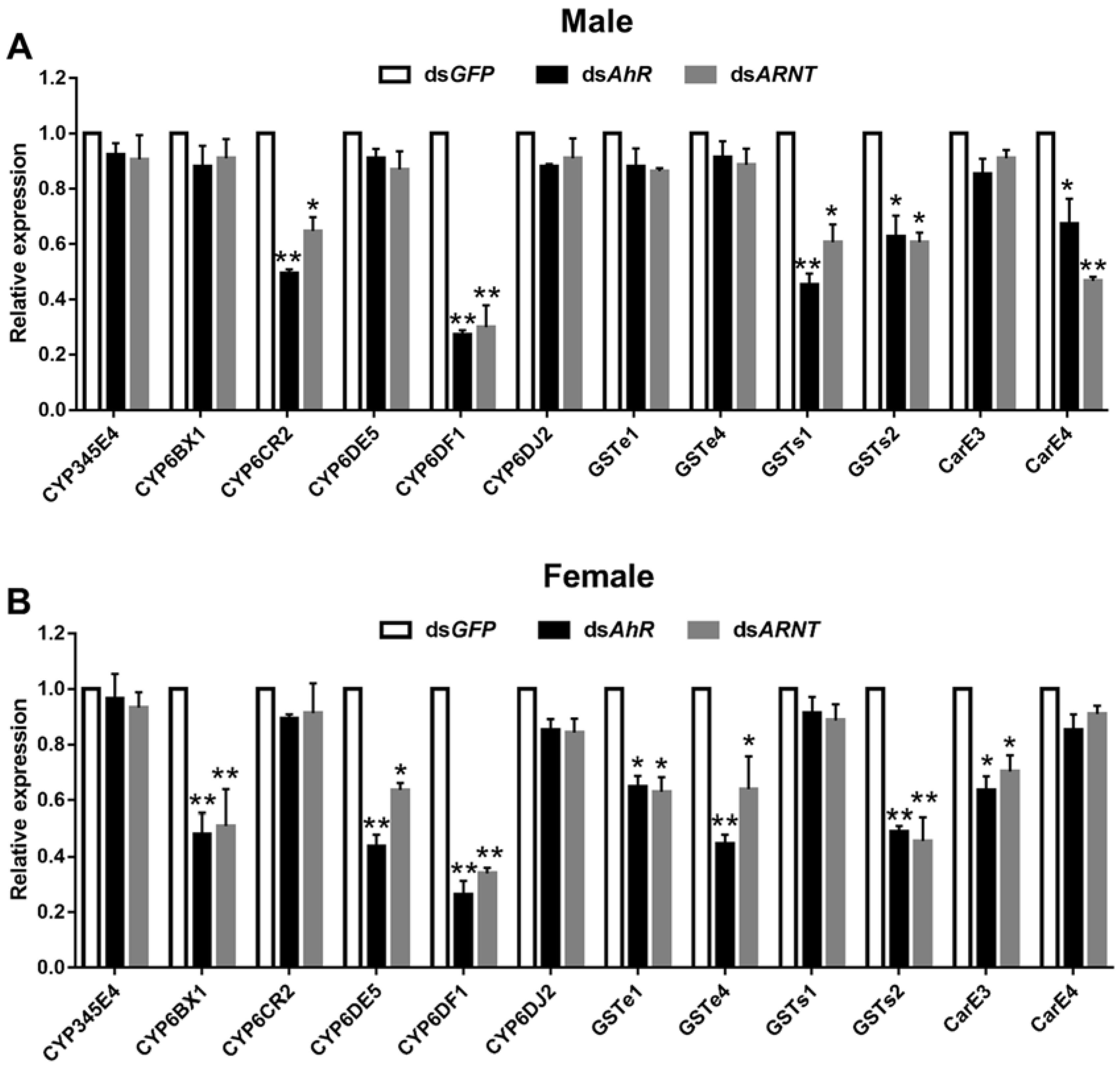
3.5. Analysis of DaAhR and DaARNT Regulation of Detoxification Genes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, H.; Li, Z.; Tang, M. Laboratory evaluation of flight activity of Dendroctonus armandi (Coleoptera: Curculionidae: Scolytinae). Can. Entomol. 2010, 142, 378–387. [Google Scholar] [CrossRef]
- Huber, D.P.W.; Robert, J.A. The proteomics and transcriptomics of early host colonization and overwintering physiology in the Mountain pine beetle, Dendroctonus ponderosae Hopkins (Coleoptera: Curculionidae). In Pine Bark Beetles; Tittiger, C., Blomquist, G.J., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 101–128. [Google Scholar]
- Chen, H.; Tang, M.; Gao, J.; Chen, X.; Li, Z. Changes in the composition of volatile monoterpenes and sesquiterpenes of Pinus armandi, P-tabulaeformis, and P-bungeana in Northwest China. Chem. Nat. Compd. 2006, 42, 534–538. [Google Scholar] [CrossRef]
- Bohlmann, J. Pine terpenoid defences in the mountain pine beetle epidemic and in other conifer pest interactions: Specialized enemies are eating holes into a diverse, dynamic and durable defence system. Tree Physiol. 2012, 32, 943–945. [Google Scholar] [CrossRef] [PubMed]
- Fernanda Lopez, M.; Cano-Ramirez, C.; Shibayama, M.; Zuniga, G. Alpha-Pinene and myrcene induce ultrastructural changes in the midgut of Dendroctonus valens (Coleoptera: Curculionidae: Scolytinae). Ann. Entomol. Soc. Am. 2011, 104, 553–561. [Google Scholar] [CrossRef]
- Chiu, C.C.; Keeling, C.; Bohlmann, J. Toxicity of pine monoterpenes to Mountain pine beetle. Sci. Rep. 2017, 7, 8858. [Google Scholar] [CrossRef]
- Lu, K.; Cheng, Y.; Li, Y.; Li, W.; Zeng, R.; Song, Y. Phytochemical flavone confers broad-spectrum tolerance to insecticides in Spodoptera litura by activating ROS/CncC-mediated xenobiotic detoxification pathways. J. Agric. Food Chem. 2021, 69, 7429–7445. [Google Scholar] [CrossRef]
- Zhang, X.; Liao, X.; Mao, K.; Yang, P.; Li, D.; Alia, E.; Wan, H.; Li, J. The role of detoxifying enzymes in field-evolved resistance to nitenpyram in the brown planthopper Nilaparvata lugens in China. Crop Prot. 2017, 94, 106–114. [Google Scholar] [CrossRef]
- Mao, K.; Zhang, X.; Ali, E.; Liao, X.; Jin, R.; Ren, Z.; Wan, H.; Li, J. Characterization of nitenpyram resistance in Nilaparvata lugens (Stal). Pestic. Biochem. Physiol. 2019, 157, 26–32. [Google Scholar] [CrossRef]
- Amezian, D.; Nauen, R.; Le Goff, G. Transcriptional regulation of xenobiotic detoxification genes in insects—An overview. Pestic. Biochem. Physiol. 2021, 174, 104822. [Google Scholar] [CrossRef]
- Kalsi, M.; Palli, S.R. Transcription factors, CncC and Maf, regulate expression of CYP6BQ genes responsible for deltamethrin resistance in Tribolium castaneum. Insect Biochem. Mol. Biol. 2015, 65, 47–56. [Google Scholar] [CrossRef]
- Kalsi, M.; Palli, S.R. Transcription factor cap n collar C regulates multiple cytochrome P450 genes conferring adaptation to potato plant allelochemicals and resistance to imidacloprid in Leptinotarsa decemlineata (Say). Insect Biochem. Mol. Biol. 2017, 83, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Misra, J.R.; Lam, G.; Thummel, C.S. Constitutive activation of the Nrf2/Keap1 pathway in insecticide-resistant strains of Drosophila. Insect Biochem. Mol. Biol. 2013, 43, 1116–1124. [Google Scholar] [CrossRef]
- Palli, S.R. CncC/Maf-mediated xenobiotic response pathway in insects. Arch. Insect Biochem. Physiol. 2020, 104, e21674. [Google Scholar] [CrossRef]
- Li, X.; Shan, C.; Li, F.; Liang, P.; Smagghe, G.; Gao, X. Transcription factor FTZ-F1 and cis -acting elements mediate expression of CYP6BG1 conferring resistance to chlorantraniliprole in Plutella xylostella. Pest Manag. Sci. 2018, 75, 1172–1180. [Google Scholar] [CrossRef]
- Misra, J.R.; Horner, M.A.; Lam, G.; Thummel, C.S. Transcriptional regulation of xenobiotic detoxification in Drosophila. Genes Dev. 2011, 25, 1796–1806. [Google Scholar] [CrossRef] [PubMed]
- Saxlund, M.A.; Sadler-Riggleman, I.; Skinner, M.K. Role of basic helix-loop-helix (bHLH) and CREB transcription factors in the regulation of sertoli cell androgen-binding protein expression. Mol. Reprod. Dev. 2004, 68, 269–278. [Google Scholar] [CrossRef]
- Vorrink, S.U.; Domann, F.E. Regulatory crosstalk and interference between the xenobiotic and hypoxia sensing pathways at the AhR-ARNT-HIF1 alpha signaling node. Chem. Biol. Interact. 2014, 218, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Ji, C.; Ren, F.; Aniagu, S.; Tong, J.; Jiang, Y.; Chen, T. AHR-mediated oxidative stress contributes to the cardiac developmental toxicity of trichloroethylene in zebrafish embryos. J. Hazard. Mater. 2019, 385, 121521. [Google Scholar] [CrossRef]
- Berg, P.; Pongratz, I. Differential usage of nuclear export sequences regulates intracellular localization of the dioxin (aryl hydrocarbon) receptor. J. Biol. Chem. 2001, 276, 43231–43238. [Google Scholar] [CrossRef]
- Kikuchi, Y.; Ohsawa, S.; Mimura, J.; Ema, M.; Takasaki, C.; Sogawa, K.; Fujii-Kuriyama, Y. Heterodimers of bHLH-PAS protein fragments derived from AhR, AhRR, and Arnt prepared by co-expression in Escherichia coli: Characterization of their DNA binding activity and preparation of a DNA complex. J. Biochem. 2003, 134, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Kudo, I.; Hosaka, M.; Haga, A.; Tsuji, N.; Nagata, Y.; Okada, H.; Fukuda, K.; Kakizaki, Y.; Okamoto, T.; Grave, E.; et al. The regulation mechanisms of AhR by molecular chaperone complex. J. Biochem. 2017, 163, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Peng, T.; Xu, P.; Zeng, X.; Tian, F.; Song, J.; Shang, Q. Transcription factors AhR/ARNT regulate the expression of CYP6CY3 and CYP6CY4 switch conferring nicotine adaptation. Int. J. Mol. Sci. 2019, 20, 4521. [Google Scholar] [CrossRef] [PubMed]
- Peng, T.; Chen, X.; Pan, Y.; Zheng, Z.; Wei, X.; Xi, J.; Zhang, J.; Gao, X.; Shang, Q. Transcription factor aryl hydrocarbon receptor/aryl hydrocarbon receptor nuclear translocator is involved in regulation of the xenobiotic tolerance-related cytochrome P450 CYP6DA2 in Aphis gossypii Glover. Insect Mol. Biol. 2017, 26, 485–495. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Jie, D.; Liu, J.; Zhang, J.; Zhang, T.; Zhang, J.; Ma, E. Aryl hydrocarbon receptor regulates the expression of LmGSTd7 and is associated with chlorpyrifos susceptibility in Locusta migratoria. Pest Manag. Sci. 2019, 75, 2916–2924. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Fu, D.; Gao, H.; Ning, H.; Sun, Y.; Chen, H.; Tang, M. Cloning and expression of the neuropeptide F and neuropeptide F receptor genes and their regulation of food intake in the Chinese white pine beetle Dendroctonus armandi. Front. Physiol. 2021, 12, 662651. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Fu, D.; Ning, H.; Tang, M.; Chen, H. Identification of the short neuropeptide F and short neuropeptide F receptor genes and their roles of food intake in Dendroctonus armandi. Insects 2021, 12, 844. [Google Scholar] [CrossRef]
- Liu, B.; Fu, D.; Ning, H.; Tang, M.; Chen, H. Identification and functional characterization of the sulfakinin and sulfakinin receptor in the Chinese white pine beetle Dendroctonus armandi. Front. Physiol. 2022, 13, 927890. [Google Scholar] [CrossRef]
- Dai, L.; Ma, M.; Wang, C.; Shi, Q.; Zhang, R.; Chen, H. Cytochrome P450s from the Chinese white pine beetle, Dendroctonus armandi (Curculionidae: Scolytinae): Expression profiles of different stages and responses to host allelochemicals. Insect Biochem. Mol. Biol. 2015, 65, 35–46. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, research0034.1. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2–ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis Version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Kang, X.; An, H.; Liu, B.; Chen, H. Two key volatiles of Chinese white pine (Pinus armandii) (Pinales: Pinaceae: Pinoideae) phloem resist invasion by Chinese white pine beetle (Dendroctonus armandii) (Coleoptera: Curculionidae: Scolytinae). Appl. Ecol. Environ. Res. 2022, 20, 587–600. [Google Scholar] [CrossRef]
- Dai, L.; Gao, H.; Chen, H. Expression levels of detoxification enzyme genes from Dendroctonus armandi (Coleoptera: Curculionidae) fed on a solid diet containing pine phloem and terpenoids. Insects 2021, 12, 926. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Fu, D.; Ning, H.; Tang, M.; Chen, H. Knockdown of CYP6CR2 and CYP6DE5 reduces tolerance to host plant allelochemicals in the Chinese white pine beetle Dendroctonus armandi. Pestic. Biochem. Physiol. 2022, 187, 105180. [Google Scholar] [CrossRef] [PubMed]
- Liao, X.; Xu, P.; Gong, P.; Wan, H.; Li, J. Current susceptibilities of brown planthopper Nilaparvata lugens to triflumezopyrim and other frequently used insecticides in China. Insect Sci. 2020, 28, 115–126. [Google Scholar] [CrossRef]
- Zhang, X.; Dong, J.; Wu, H.; Zhang, H.; Zhang, J.; Ma, E. Knockdown of cytochrome P450 CYP6 family genes increases susceptibility to carbamates and pyrethroids in the migratory locust, Locusta migratoria. Chemosphere 2019, 223, 48–57. [Google Scholar] [CrossRef]
- Dow, J.A.T.; Davies, S.A. The Malpighian tubule: Rapid insights from post-genomic biology. J. Insect Physiol. 2006, 52, 365–378. [Google Scholar] [CrossRef]
- Huang, Y.; Shen, G.-M.; Jiang, H.-B.; Jiang, X.-Z.; Dou, W.; Wang, J.-J. Multiple P450 genes: Identification, tissue-specific expression and their responses to insecticide treatments in the oriental fruit fly, Bactrocera dorsalis (Hendel) (Diptera: Tephritidea). Pestic. Biochem. Physiol. 2013, 106, 1–7. [Google Scholar] [CrossRef]
- Li, X.; Schuler, M.A.; Berenbaum, M.R. Molecular mechanisms of metabolic resistance to synthetic and natural xenobiotics. Annu. Rev. Èntomol. 2007, 52, 231–253. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, Y.; Li, W.; Song, Y.; Zeng, R.; Lu, K. Inhibition of hepatocyte nuclear factor 4 confers imidacloprid resistance in Nilaparvata lugens via the activation of cytochrome P450 and UDP-glycosyltransferase genes. Chemosphere 2020, 263, 128269. [Google Scholar] [CrossRef]
- Wittkopp, P.J.; Kalay, G. Cis-regulatory elements: Molecular mechanisms and evolutionary processes underlying divergence. Nat. Rev. Genet. 2012, 13, 59–69. [Google Scholar] [CrossRef]
- Lenhard, B.; Sandelin, A.; Carninci, P. Metazoan promoters: Emerging characteristics and insights into transcriptional regulation. Nat. Rev. Genet. 2012, 13, 233–245. [Google Scholar] [CrossRef]
- Zhang, C.; Luo, X.; Ni, X.; Zhang, Y.; Li, X. Functional characterization of cis-acting elements mediating flavone-inducible expression of CYP321A1. Insect Biochem. Mol. Biol. 2010, 40, 898–908. [Google Scholar] [CrossRef]
- Chen, S.; Lu, M.; Zhang, N.; Zou, X.; Mo, M.; Zheng, S. Nuclear factor erythroid-derived 2-related factor 2 activates glutathione S-transferase expression in the midgut of Spodoptera litura (Lepidoptera: Noctuidae) in response to phytochemicals and insecticides. Insect Mol. Biol. 2018, 27, 522–532. [Google Scholar] [CrossRef]
- Billiard, S.M.; Timme-Laragy, A.; Wassenberg, D.M.; Cockman, C.; Di Giulio, R.T. The role of the aryl hydrocarbon receptor pathway in mediating synergistic developmental toxicity of polycyclic aromatic hydrocarbons to zebrafish. Toxicol. Sci. 2006, 92, 526–536. [Google Scholar] [CrossRef]
- Shi, L.; Shi, Y.; Liu, M.F.; Zhang, Y.; Liao, X.L. Transcription factor CncC potentially regulates the expression of multiple detoxification genes that mediate indoxacarb resistance in Spodoptera litura. Insect Sci. 2020, 28, 1426–1438. [Google Scholar] [CrossRef]
- Wang, Y.; Jin, R.; Liu, C.; Gao, Y.; Deng, X.; Wan, H.; Li, J. Functional characterization of the transcription factors AhR and ARNT in Nilaparvata lugens. Pestic. Biochem. Physiol. 2021, 176, 104875. [Google Scholar] [CrossRef]
- Hu, B.; Huang, H.; Wei, Q.; Ren, M.; Mburu, D.K.; Tian, X.; Su, J. Transcription factors CncC/Maf and AhR/ARNT coordinately regulate the expression of multiple GSTs conferring resistance to chlorpyrifos and cypermethrin in Spodoptera exigua. Pest Manag. Sci. 2019, 75, 2009–2019. [Google Scholar] [CrossRef]


| Gene Name | Accession No | ORF (bp) a | Amino Acid Residues a | MW (KDa) a | IP a |
|---|---|---|---|---|---|
| AhR | OP378567 | 2412 | 803 | 90.63 | 7.29 |
| ARNT | ON378568 | 2106 | 701 | 77.29 | 6.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, B.; Chen, H. Identification and Functional Characterization of the Transcription Factors AhR/ARNT in Dendroctonus armandi. Cells 2022, 11, 3856. https://doi.org/10.3390/cells11233856
Liu B, Chen H. Identification and Functional Characterization of the Transcription Factors AhR/ARNT in Dendroctonus armandi. Cells. 2022; 11(23):3856. https://doi.org/10.3390/cells11233856
Chicago/Turabian StyleLiu, Bin, and Hui Chen. 2022. "Identification and Functional Characterization of the Transcription Factors AhR/ARNT in Dendroctonus armandi" Cells 11, no. 23: 3856. https://doi.org/10.3390/cells11233856
APA StyleLiu, B., & Chen, H. (2022). Identification and Functional Characterization of the Transcription Factors AhR/ARNT in Dendroctonus armandi. Cells, 11(23), 3856. https://doi.org/10.3390/cells11233856






