Merkel Cells Are Multimodal Sensory Cells: A Review of Study Methods
Abstract
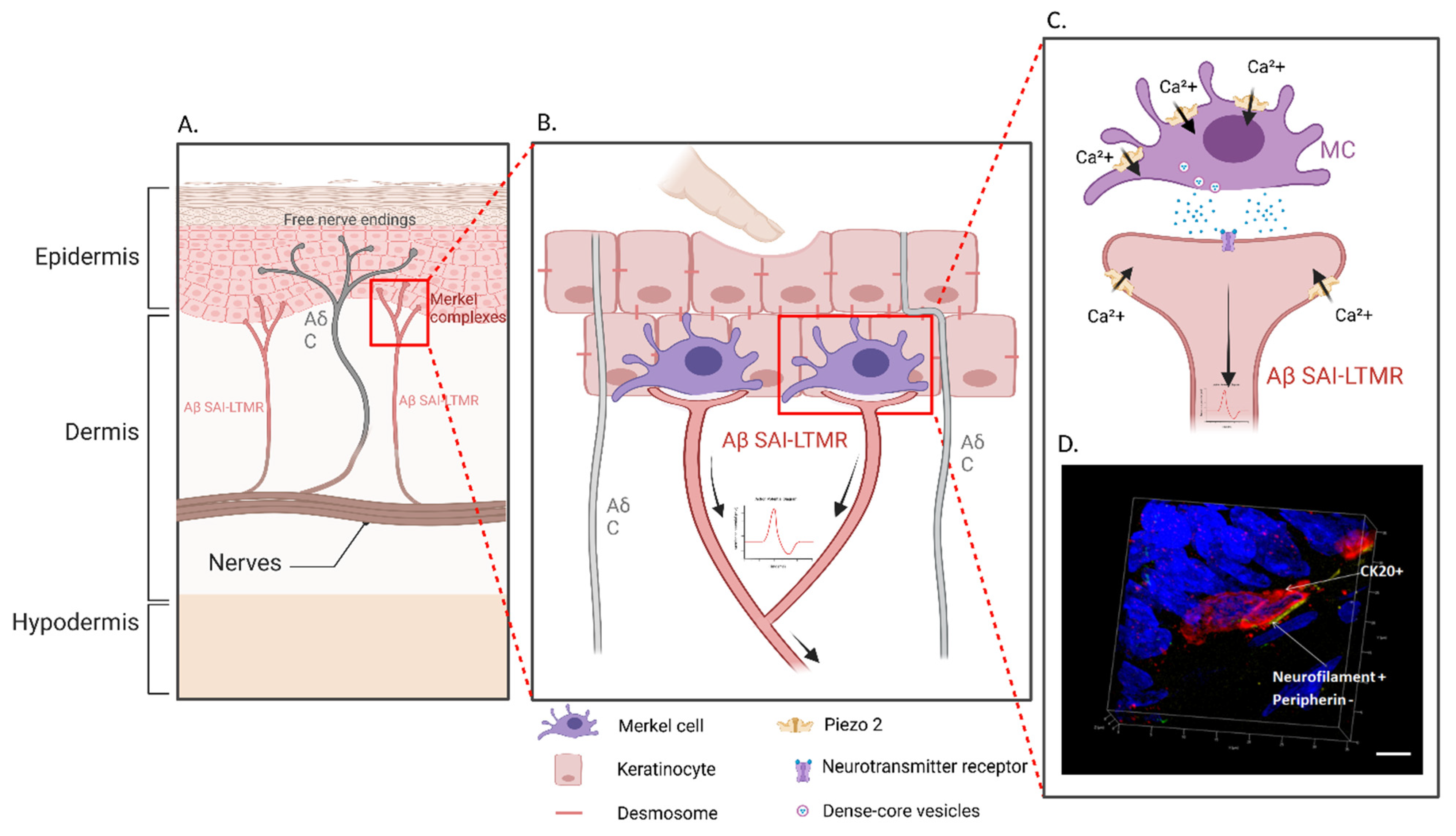
1. Introduction
2. Ex Vivo Studies (Skin Biopsies)
2.1. Histology
2.2. Functionality
3. In Vivo Studies (Mice)
3.1. Touch Perception
3.2. Mechanical Itch
3.3. Mechanical Pain
4. In Vitro Studies (Culture of Merkel Cells)
4.1. Monoculture of Merkel Cells
4.2. Co-Culture of Merkel Cells with Other Cells
5. In Silico Studies (Modeling of Merkel Cell–Neurite Complexes)
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fleming, M.S.; Luo, W. The Anatomy, Function, and Development of Mammalian Aβ Low-Threshold Mechanoreceptors. Front. Biol. 2013, 8, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Talagas, M.; Lebonvallet, N.; Misery, L. Intraepidermal Nerve Fibres Are Not the Exclusive Tranducers of Nociception. J. Neurosci. Methods 2018, 306, 92–93. [Google Scholar] [CrossRef] [PubMed]
- Boulais, N.; Misery, L. The Epidermis: A Sensory Tissue. Eur. J. Dermatol. EJD 2008, 18, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Merkel, F. Tastzellen Und Tastkörperchen Bei Den Hausthieren Und Beim Menschen. Arch. Mikrosk. Anat. 1875, 11, 636–652. [Google Scholar] [CrossRef]
- Boulais, N.; Misery, L. Merkel Cells. J. Am. Acad. Dermatol. 2007, 57, 147–165. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.O. The Roles and Functions of Cutaneous Mechanoreceptors. Curr. Opin. Neurobiol. 2001, 11, 455–461. [Google Scholar] [CrossRef]
- Zimmerman, A.; Bai, L.; Ginty, D.D. The Gentle Touch Receptors of Mammalian Skin. Science 2014, 346, 950–954. [Google Scholar] [CrossRef]
- García-Mesa, Y.; García-Piqueras, J.; García, B.; Feito, J.; Cabo, R.; Cobo, J.; Vega, J.A.; García-Suárez, O. Merkel Cells and Meissner’s Corpuscles in Human Digital Skin Display Piezo2 Immunoreactivity. J. Anat. 2017, 231, 978–989. [Google Scholar] [CrossRef]
- Maksimovic, S.; Nakatani, M.; Baba, Y.; Nelson, A.M.; Marshall, K.L.; Wellnitz, S.A.; Firozi, P.; Woo, S.-H.; Ranade, S.; Patapoutian, A.; et al. Epidermal Merkel Cells Are Mechanosensory Cells That Tune Mammalian Touch Receptors. Nature 2014, 509, 617–621. [Google Scholar] [CrossRef]
- Woo, S.-H.; Ranade, S.; Weyer, A.D.; Dubin, A.E.; Baba, Y.; Qiu, Z.; Petrus, M.; Miyamoto, T.; Reddy, K.; Lumpkin, E.A.; et al. Piezo2 Is Required for Merkel-Cell Mechanotransduction. Nature 2014, 509, 622–626. [Google Scholar] [CrossRef]
- Woo, S.-H.; Lumpkin, E.A.; Patapoutian, A. Merkel Cells and Neurons Keep in Touch. Trends Cell Biol. 2015, 25, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Ranade, S.S.; Woo, S.-H.; Dubin, A.E.; Moshourab, R.A.; Wetzel, C.; Petrus, M.; Mathur, J.; Bégay, V.; Coste, B.; Mainquist, J.; et al. Piezo2 Is the Major Transducer of Mechanical Forces for Touch Sensation in Mice. Nature 2014, 516, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, R.; Cha, M.; Ling, J.; Jia, Z.; Coyle, D.; Gu, J.G. Merkel Cells Transduce and Encode Tactile Stimuli to Drive Aβ-Afferent Impulses. Cell 2014, 157, 664–675. [Google Scholar] [CrossRef] [PubMed]
- Moll, I.; Roessler, M.; Brandner, J.M.; Eispert, A.-C.; Houdek, P.; Moll, R. Human Merkel Cells-Aspects of Cell Biology, Distribution and Functions. Eur. J. Cell Biol. 2005, 84, 259–271. [Google Scholar] [CrossRef]
- García-Caballero, T.; Gallego, R.; Rosón, E.; Basanta, D.; Morel, G.; Beiras, A. Localization of Serotonin-like Immunoreactivity in the Merkel Cells of Pig Snout Skin. Anat. Rec. 1989, 225, 267–271. [Google Scholar] [CrossRef]
- Halata, Z.; Grim, M.; Bauman, K.I. Friedrich Sigmund Merkel and His “Merkel Cell”, Morphology, Development, and Physiology: Review and New Results. Anat. Rec. A Discov. Mol. Cell Evol. Biol. 2003, 271, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Lauriano, E.R.; Pergolizzi, S.; Aragona, M.; Spanò, N.; Guerrera, M.C.; Capillo, G.; Faggio, C. Merkel Cells Immunohistochemical Study in Striped Dolphin (Stenella Coeruleoalba) Skin. Tissue Cell 2019, 56, 1–6. [Google Scholar] [CrossRef]
- Munger, B.L. The Intraepidermal Innervation of the Snout Skin of the Opossum. A Light and Electron Microscope Study, with Observations on the Nature of Merkel’s Tastzellen. J. Cell Biol. 1965, 26, 79–97. [Google Scholar] [CrossRef]
- Winkelmann, R.K.; Breathnach, A.S. The Merkel Cell. J. Investig. Dermatol. 1973, 60, 2–15. [Google Scholar] [CrossRef]
- Fradette, J.; Larouche, D.; Fugère, C.; Guignard, R.; Beauparlant, A.; Couture, V.; Caouette-Laberge, L.; Roy, A.; Germain, L. Normal Human Merkel Cells Are Present in Epidermal Cell Populations Isolated and Cultured from Glabrous and Hairy Skin Sites. J. Investig. Dermatol. 2003, 120, 313–317. [Google Scholar] [CrossRef]
- Boulais, N.; Pennec, J.-P.; Lebonvallet, N.; Pereira, U.; Rougier, N.; Dorange, G.; Chesné, C.; Misery, L. Rat Merkel Cells Are Mechanoreceptors and Osmoreceptors. PLoS ONE 2009, 4, e7759. [Google Scholar] [CrossRef] [PubMed]
- Eispert, A.-C.; Fuchs, F.; Brandner, J.M.; Houdek, P.; Wladykowski, E.; Moll, I. Evidence for Distinct Populations of Human Merkel Cells. Histochem. Cell Biol. 2009, 132, 83–93. [Google Scholar] [CrossRef]
- Morrison, K.M.; Miesegaes, G.R.; Lumpkin, E.A.; Maricich, S.M. Mammalian Merkel Cells Are Descended from the Epidermal Lineage. Dev. Biol. 2009, 336, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, G.A.; Rodríguez, F.; Herráez, P.; Suárez-Bonnet, A.; Andrada, M.; Espinosa-de-Los-Monteros, A. Morphologic and Immunohistochemical Features of Merkel Cells in the Dog. Res. Vet. Sci. 2014, 97, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Maksimovic, S.; Baba, Y.; Lumpkin, E.A. Neurotransmitters and Synaptic Components in the Merkel Cell-Neurite Complex, a Gentle-Touch Receptor. Ann. N. Y. Acad. Sci. 2013, 1279, 13–21. [Google Scholar] [CrossRef]
- Lacour, J.P.; Dubois, D.; Pisani, A.; Ortonne, J.P. Anatomical Mapping of Merkel Cells in Normal Human Adult Epidermis. Br. J. Dermatol. 1991, 125, 535–542. [Google Scholar] [CrossRef]
- Szeder, V.; Grim, M.; Halata, Z.; Sieber-Blum, M. Neural Crest Origin of Mammalian Merkel Cells. Dev. Biol. 2003, 253, 258–263. [Google Scholar] [CrossRef]
- Van Keymeulen, A.; Mascre, G.; Youseff, K.K.; Harel, I.; Michaux, C.; De Geest, N.; Szpalski, C.; Achouri, Y.; Bloch, W.; Hassan, B.A.; et al. Epidermal Progenitors Give Rise to Merkel Cells during Embryonic Development and Adult Homeostasis. J. Cell Biol. 2009, 187, 91–100. [Google Scholar] [CrossRef]
- Moll, R.; Moll, I.; Franke, W.W. Identification of Merkel Cells in Human Skin by Specific Cytokeratin Antibodies: Changes of Cell Density and Distribution in Fetal and Adult Plantar Epidermis. Differ. Res. Biol. Divers. 1984, 28, 136–154. [Google Scholar] [CrossRef]
- Doucet, Y.S.; Woo, S.-H.; Ruiz, M.E.; Owens, D.M. The Touch Dome Defines an Epidermal Niche Specialized for Mechanosensory Signaling. Cell Rep. 2013, 3, 1759–1765. [Google Scholar] [CrossRef]
- Woo, S.-H.; Stumpfova, M.; Jensen, U.B.; Lumpkin, E.A.; Owens, D.M. Identification of Epidermal Progenitors for the Merkel Cell Lineage. Dev. Camb. Engl. 2010, 137, 3965–3971. [Google Scholar] [CrossRef] [PubMed]
- Wright, M.C.; Reed-Geaghan, E.G.; Bolock, A.M.; Fujiyama, T.; Hoshino, M.; Maricich, S.M. Unipotent, Atoh1+ Progenitors Maintain the Merkel Cell Population in Embryonic and Adult Mice. J. Cell Biol. 2015, 208, 367–379. [Google Scholar] [CrossRef] [PubMed]
- Maricich, S.M.; Wellnitz, S.A.; Nelson, A.M.; Lesniak, D.R.; Gerling, G.J.; Lumpkin, E.A.; Zoghbi, H.Y. Merkel Cells Are Essential for Light-Touch Responses. Science 2009, 324, 1580–1582. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, S.M.; Wright, M.C.; Bolock, A.M.; Geng, X.; Maricich, S.M. Ectopic Atoh1 Expression Drives Merkel Cell Production in Embryonic, Postnatal and Adult Mouse Epidermis. Dev. Camb. Engl. 2015, 142, 2533–2544. [Google Scholar] [CrossRef]
- Ben-Arie, N.; Hassan, B.A.; Bermingham, N.A.; Malicki, D.M.; Armstrong, D.; Matzuk, M.; Bellen, H.J.; Zoghbi, H.Y. Functional Conservation of Atonal and Math1 in the CNS and PNS. Dev. Camb. Engl. 2000, 127, 1039–1048. [Google Scholar] [CrossRef]
- Moll, I.; Troyanovsky, S.M.; Moll, R. Special Program of Differentiation Expressed in Keratinocytes of Human Haarscheiben: An Analysis of Individual Cytokeratin Polypeptides. J. Investig. Dermatol. 1993, 100, 69–76. [Google Scholar] [CrossRef]
- Lesko, M.H.; Driskell, R.R.; Kretzschmar, K.; Goldie, S.J.; Watt, F.M. Sox2 Modulates the Function of Two Distinct Cell Lineages in Mouse Skin. Dev. Biol. 2013, 382, 15–26. [Google Scholar] [CrossRef]
- Kim, D.K.; Holbrook, K.A. The Appearance, Density, and Distribution of Merkel Cells in Human Embryonic and Fetal Skin: Their Relation to Sweat Gland and Hair Follicle Development. J. Investig. Dermatol. 1995, 104, 411–416. [Google Scholar] [CrossRef]
- Fradette, J.; Godbout, M.J.; Michel, M.; Germain, L. Localization of Merkel Cells at Hairless and Hairy Human Skin Sites Using Keratin 18. Biochem. Cell Biol. Biochim. Biol. Cell. 1995, 73, 635–639. [Google Scholar] [CrossRef]
- Barrett, A.W.; Cort, E.M.; Patel, P.; Berkovitz, B.K. An Immunohistological Study of Cytokeratin 20 in Human and Mammalian Oral Epithelium. Arch. Oral Biol. 2000, 45, 879–887. [Google Scholar] [CrossRef]
- Boulais, N.; Pereira, U.; Lebonvallet, N.; Gobin, E.; Dorange, G.; Rougier, N.; Chesne, C.; Misery, L. Merkel Cells as Putative Regulatory Cells in Skin Disorders: An in Vitro Study. PLoS ONE 2009, 4, e6528. [Google Scholar] [CrossRef] [PubMed]
- Moll, I.; Kuhn, C.; Moll, R. Cytokeratin 20 Is a General Marker of Cutaneous Merkel Cells While Certain Neuronal Proteins Are Absent. J. Investig. Dermatol. 1995, 104, 910–915. [Google Scholar] [CrossRef] [PubMed]
- Polakovičová, S.; Csöbönyeiová, M.; Filova, B.; Borovský, M.; Maršík, L.; Kvasilová, A.; Polák, Š. Merkel-like Cell Distribution in the Epithelium of the Human Vagina. An Immunohistochemical and TEM Study. Eur. J. Histochem. EJH 2018, 62, 2836. [Google Scholar] [CrossRef] [PubMed]
- Iggo, A.; Muir, A.R. The Structure and Function of a Slowly Adapting Touch Corpuscle in Hairy Skin. J. Physiol. 1969, 200, 763–796. [Google Scholar] [CrossRef] [PubMed]
- Hartschuh, W.; Weihe, E. Fine Structural Analysis of the Synaptic Junction of Merkel Cell-Axon-Complexes. J. Investig. Dermatol. 1980, 75, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Lucarz, A.; Brand, G. Current Considerations about Merkel Cells. Eur. J. Cell Biol. 2007, 86, 243–251. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, T. The Merkel Cell: Recent Findings and Unresolved Problems. Arch. Histol. Cytol. 1995, 58, 379–396. [Google Scholar] [CrossRef][Green Version]
- Tachibana, T.; Nawa, T. Recent Progress in Studies on Merkel Cell Biology. Anat. Sci. Int. 2002, 77, 26–33. [Google Scholar] [CrossRef]
- Cheng Chew, S.B.; Leung, P.Y. Species Variability in the Expression of Met- and Leu-Enkephalin-like Immunoreactivity in Mammalian Merkel Cell Dense-Core Granules. A Light- and Electron-Microscopic Immunohistochemical Study. Cell Tissue Res. 1992, 269, 347–351. [Google Scholar] [CrossRef]
- Gauweiler, B.; Weihe, E.; Hartschuh, W.; Yanaihara, N. Presence and Coexistence of Chromogranin A and Multiple Neuropeptides in Merkel Cells of Mammalian Oral Mucosa. Neurosci. Lett. 1988, 89, 121–126. [Google Scholar] [CrossRef]
- Hartschuh, W.; Weihe, E.; Yanaihara, N.; Reinecke, M. Immunohistochemical Localization of Vasoactive Intestinal Polypeptide (VIP) in Merkel Cells of Various Mammals: Evidence for a Neuromodulator Function of the Merkel Cell. J. Investig. Dermatol. 1983, 81, 361–364. [Google Scholar] [CrossRef]
- Tachibana, T.; Nawa, T. Immunohistochemical Reactions of Receptors to Met-Enkephalin, VIP, Substance P, and CGRP Located on Merkel Cells in the Rat Sinus Hair Follicle. Arch. Histol. Cytol. 2005, 68, 383–391. [Google Scholar] [CrossRef]
- Hartschuh, W.; Weihe, E.; Büchler, M.; Helmstaedter, V.; Feurle, G.E.; Forssmann, W.G. Met Enkephalin-like Immunoreactivity in Merkel Cells. Cell Tissue Res. 1979, 201, 343–348. [Google Scholar] [CrossRef]
- Gallego, R.; García-Caballero, T.; Fraga, M.; Beiras, A.; Forteza, J. Neural Cell Adhesion Molecule Immunoreactivity in Merkel Cells and Merkel Cell Tumours. Virchows Arch. Int. J. Pathol. 1995, 426, 317–321. [Google Scholar] [CrossRef]
- McNiff, J.M.; Cowper, S.E.; Lazova, R.; Subtil, A.; Glusac, E.J. CD56 Staining in Merkel Cell Carcinoma and Natural Killer-Cell Lymphoma: Magic Bullet, Diagnostic Pitfall, or Both? J. Cutan. Pathol. 2005, 32, 541–545. [Google Scholar] [CrossRef]
- Zaccone, G. Neuron-Specific Enolase and Serotonin in the Merkel Cells of Conger-Eel (Conger Conger) Epidermis. An Immunohistochemical Study. Histochemistry 1986, 85, 29–34. [Google Scholar] [CrossRef]
- Kuramoto, H. An Immunohistochemical Study of Cellular and Nervous Elements in the Taste Organ of the Bullfrog, Rana Catesbeiana. Arch. Histol. Cytol. 1988, 51, 205–221. [Google Scholar] [CrossRef]
- English, K.B.; Wang, Z.Z.; Stayner, N.; Stensaas, L.J.; Martin, H.; Tuckett, R.P. Serotonin-like Immunoreactivity in Merkel Cells and Their Afferent Neurons in Touch Domes from the Hairy Skin of Rats. Anat. Rec. 1992, 232, 112–120. [Google Scholar] [CrossRef]
- Nakatani, M.; Maksimovic, S.; Baba, Y.; Lumpkin, E.A. Mechanotransduction in Epidermal Merkel Cells. Pflug. Arch. 2015, 467, 101–108. [Google Scholar] [CrossRef]
- Toyoshima, K.; Shimamura, A. Uranaffin Reaction of Merkel Corpuscles in the Lingual Mucosa of the Finch, Lonchula Striata Var. Domestica. J. Anat. 1991, 179, 197–201. [Google Scholar]
- Nunzi, M.-G.; Pisarek, A.; Mugnaini, E. Merkel Cells, Corpuscular Nerve Endings and Free Nerve Endings in the Mouse Palatine Mucosa Express Three Subtypes of Vesicular Glutamate Transporters. J. Neurocytol. 2004, 33, 359–376. [Google Scholar] [CrossRef] [PubMed]
- Hitchcock, I.S.; Genever, P.G.; Cahusac, P.M.B. Essential Components for a Glutamatergic Synapse between Merkel Cell and Nerve Terminal in Rats. Neurosci. Lett. 2004, 362, 196–199. [Google Scholar] [CrossRef]
- Hartschuh, W.; Weihe, E.; Egner, U. Chromogranin A in the Mammalian Merkel Cell: Cellular and Subcellular Distribution. J. Investig. Dermatol. 1989, 93, 641–648. [Google Scholar] [CrossRef]
- García-Mesa, Y.; Feito, J.; Cuendias, P.; García-Piqueras, J.; Germanà, A.; García-Suárez, O.; Martín-Biedma, B.; Vega, J.A. The Acquisition of Mechanoreceptive Competence by Human Digital Merkel Cells and Sensory Corpuscles during Development: An Immunohistochemical Study of PIEZO2. Ann. Anat. Anat. Anz. Off. Organ Anat. Ges. 2022, 243, 151953. [Google Scholar] [CrossRef]
- Fukuda, J.; Ishimine, H.; Masaki, Y. Long-Term Staining of Live Merkel Cells with FM Dyes. Cell Tissue Res. 2003, 311, 325–332. [Google Scholar] [CrossRef]
- Marasco, P.D.; Tsuruda, P.R.; Bautista, D.M.; Julius, D.; Catania, K.C. Neuroanatomical Evidence for Segregation of Nerve Fibers Conveying Light Touch and Pain Sensation in Eimer’s Organ of the Mole. Proc. Natl. Acad. Sci. USA 2006, 103, 9339–9344. [Google Scholar] [CrossRef]
- Nishikawa, S. Fluorescent AM1-43 and FM1-43 Probes for Dental Sensory Nerves and Cells: Their Labeling Mechanisms and Applications. Jpn. Dent. Sci. Rev. 2011, 47, 150–156. [Google Scholar] [CrossRef]
- Rizzoli, S.O.; Richards, D.A.; Betz, W.J. Monitoring Synaptic Vesicle Recycling in Frog Motor Nerve Terminals with FM Dyes. J. Neurocytol. 2003, 32, 539–549. [Google Scholar] [CrossRef]
- Nurse, C.A.; Mearow, K.M.; Holmes, M.; Visheau, B.; Diamond, J. Merkel Cell Distribution in the Epidermis as Determined by Quinacrine Fluorescence. Cell Tissue Res. 1983, 228, 511–524. [Google Scholar] [CrossRef]
- Vos, P.; Stark, F.; Pittman, R.N. Merkel Cells in Vitro: Production of Nerve Growth Factor and Selective Interactions with Sensory Neurons. Dev. Biol. 1991, 144, 281–300. [Google Scholar] [CrossRef]
- Crowe, R.; Whitear, M. Quinacrine Fluorescence of Merkel Cells in Xenopus Laevis. Cell Tissue Res. 1978, 190, 273–283. [Google Scholar] [CrossRef] [PubMed]
- Brumback, A.C.; Lieber, J.L.; Angleson, J.K.; Betz, W.J. Using FM1-43 to Study Neuropeptide Granule Dynamics and Exocytosis. Methods 2004, 33, 287–294. [Google Scholar] [CrossRef] [PubMed]
- Drew, L.J.; Wood, J.N. FM1-43 Is a Permeant Blocker of Mechanosensitive Ion Channels in Sensory Neurons and Inhibits Behavioural Responses to Mechanical Stimuli. Mol. Pain 2007, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Gale, J.E.; Marcotti, W.; Kennedy, H.J.; Kros, C.J.; Richardson, G.P. FM1-43 Dye Behaves as a Permeant Blocker of the Hair-Cell Mechanotransducer Channel. J. Neurosci. Off. J. Soc. Neurosci. 2001, 21, 7013–7025. [Google Scholar] [CrossRef]
- Nagano, N.; Imaizumi, Y.; Watanabe, M. Novel Blockade of Ca2+ Current by Quinacrine in Smooth Muscle Cells of the Guinea Pig. Jpn. J. Pharmacol. 1996, 71, 51–60. [Google Scholar] [CrossRef][Green Version]
- Diamond, J.; Holmes, M.; Nurse, C.A. Are Merkel Cell-Neurite Reciprocal Synapses Involved in the Initiation of Tactile Responses in Salamander Skin? J. Physiol. 1986, 376, 101–120. [Google Scholar] [CrossRef]
- He, L.; Tuckett, R.P.; English, K.B. 5-HT2 and 3 Receptor Antagonists Suppress the Response of Rat Type I Slowly Adapting Mechanoreceptor: An in Vitro Study. Brain Res. 2003, 969, 230–236. [Google Scholar] [CrossRef]
- Hoffman, B.U.; Baba, Y.; Griffith, T.N.; Mosharov, E.V.; Woo, S.-H.; Roybal, D.D.; Karsenty, G.; Patapoutian, A.; Sulzer, D.; Lumpkin, E.A. Merkel Cells Activate Sensory Neural Pathways through Adrenergic Synapses. Neuron 2018, 100, 1401–1413.e6. [Google Scholar] [CrossRef] [PubMed]
- Koltzenburg, M.; Stucky, C.L.; Lewin, G.R. Receptive Properties of Mouse Sensory Neurons Innervating Hairy Skin. J. Neurophysiol. 1997, 78, 1841–1850. [Google Scholar] [CrossRef]
- Takeda, M.; Nishikawa, T.; Sato, S.; Aiyama, S.; Matsumoto, S. Effects of Gadolinium and Tetrodotoxin on the Response of Slowly Adapting Type I Mechanoreceptors to Mechanical Stimulation in Frog Dorsal Skin. J. Peripher. Nerv. Syst. JPNS 2003, 8, 271–281. [Google Scholar] [CrossRef]
- Toda, K.; Ishii, N.; Nakamura, Y. An in Vitro Jaw-Nerve Preparation for Oral Sensory Study in the Rat. J. Neurosci. Methods 1995, 61, 85–90. [Google Scholar] [CrossRef]
- Wellnitz, S.A.; Lesniak, D.R.; Gerling, G.J.; Lumpkin, E.A. The Regularity of Sustained Firing Reveals Two Populations of Slowly Adapting Touch Receptors in Mouse Hairy Skin. J. Neurophysiol. 2010, 103, 3378–3388. [Google Scholar] [CrossRef] [PubMed]
- Adrian, E.D. The Impulses Produced by Sensory Nerve Endings: Part I. J. Physiol. 1926, 61, 49–72. [Google Scholar] [CrossRef]
- Adrian, E.D.; Zotterman, Y. The Impulses Produced by Sensory Nerve-Endings: Part II. The Response of a Single End-Organ. J. Physiol. 1926, 61, 151–171. [Google Scholar] [CrossRef] [PubMed]
- Iggo, A. New Specific Sensory Structures in Hairy Skin. Acta Neuroveg. 1962, 24, 175–180. [Google Scholar] [CrossRef]
- Reed-Geaghan, E.G.; Wright, M.C.; See, L.A.; Adelman, P.C.; Lee, K.H.; Koerber, H.R.; Maricich, S.M. Merkel Cell-Driven BDNF Signaling Specifies SAI Neuron Molecular and Electrophysiological Phenotypes. J. Neurosci. Off. J. Soc. Neurosci. 2016, 36, 4362–4376. [Google Scholar] [CrossRef]
- Chambers, M.R.; Andres, K.H.; von Duering, M.; Iggo, A. The Structure and Function of the Slowly Adapting Type II Mechanoreceptor in Hairy Skin. Q. J. Exp. Physiol. Cogn. Med. Sci. 1972, 57, 417–445. [Google Scholar] [CrossRef]
- Feng, J.; Luo, J.; Yang, P.; Du, J.; Kim, B.S.; Hu, H. Piezo2 Channel-Merkel Cell Signaling Modulates the Conversion of Touch to Itch. Science 2018, 360, 530–533. [Google Scholar] [CrossRef]
- Fagan, B.M.; Cahusac, P.M. Evidence for Glutamate Receptor Mediated Transmission at Mechanoreceptors in the Skin. Neuroreport 2001, 12, 341–347. [Google Scholar] [CrossRef]
- Haeberle, H.; Fujiwara, M.; Chuang, J.; Medina, M.M.; Panditrao, M.V.; Bechstedt, S.; Howard, J.; Lumpkin, E.A. Molecular Profiling Reveals Synaptic Release Machinery in Merkel Cells. Proc. Natl. Acad. Sci. USA 2004, 101, 14503–14508. [Google Scholar] [CrossRef]
- Higashikawa, A.; Kimura, M.; Shimada, M.; Ohyama, S.; Ofusa, W.; Tazaki, M.; Shibukawa, Y. Merkel Cells Release Glutamate Following Mechanical Stimulation: Implication of Glutamate in the Merkel Cell-Neurite Complex. Front. Cell. Neurosci. 2019, 13, 255. [Google Scholar] [CrossRef] [PubMed]
- Piskorowski, R.; Haeberle, H.; Panditrao, M.V.; Lumpkin, E.A. Voltage-Activated Ion Channels and Ca(2+)-Induced Ca (2+) Release Shape Ca (2+) Signaling in Merkel Cells. Pflug. Arch. 2008, 457, 197–209. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moll, I.; Zieger, W.; Schmelz, M. Proliferative Merkel Cells Were Not Detected in Human Skin. Arch. Dermatol. Res. 1996, 288, 184–187. [Google Scholar] [CrossRef] [PubMed]
- Nakafusa, J.; Narisawa, Y.; Shinogi, T.; Taira, K.; Tanaka, T.; Inoue, T.; Misago, N. Changes in the Number of Merkel Cells with the Hair Cycle in Hair Discs on Rat Back Skin. Br. J. Dermatol. 2006, 155, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, K. The Ultrastructure of the Skin of Human Embryos. X. Merkel Tactile Cells in the Finger and Nail. J. Anat. 1972, 111, 99–120. [Google Scholar]
- García-Piqueras, J.; García-Mesa, Y.; Cárcaba, L.; Feito, J.; Torres-Parejo, I.; Martín-Biedma, B.; Cobo, J.; García-Suárez, O.; Vega, J.A. Ageing of the Somatosensory System at the Periphery: Age-Related Changes in Cutaneous Mechanoreceptors. J. Anat. 2019, 234, 839–852. [Google Scholar] [CrossRef]
- Cha, M.; Ling, J.; Xu, G.-Y.; Gu, J.G. Shear Mechanical Force Induces an Increase of Intracellular Ca2+ in Cultured Merkel Cells Prepared from Rat Vibrissal Hair Follicles. J. Neurophysiol. 2011, 106, 460–469. [Google Scholar] [CrossRef]
- Ikeda, R.; Ling, J.; Cha, M.; Gu, J.G. In Situ Patch-Clamp Recordings from Merkel Cells in Rat Whisker Hair Follicles, an Experimental Protocol for Studying Tactile Transduction in Tactile-End Organs. Mol. Pain 2015, 11, 23. [Google Scholar] [CrossRef]
- Yamashita, Y.; Akaike, N.; Wakamori, M.; Ikeda, I.; Ogawa, H. Voltage-Dependent Currents in Isolated Single Merkel Cells of Rats. J. Physiol. 1992, 450, 143–162. [Google Scholar] [CrossRef]
- Coste, B.; Mathur, J.; Schmidt, M.; Earley, T.J.; Ranade, S.; Petrus, M.J.; Dubin, A.E.; Patapoutian, A. Piezo1 and Piezo2 Are Essential Components of Distinct Mechanically Activated Cation Channels. Science 2010, 330, 55–60. [Google Scholar] [CrossRef]
- Severson, K.S.; Xu, D.; Van de Loo, M.; Bai, L.; Ginty, D.D.; O’Connor, D.H. Active Touch and Self-Motion Encoding by Merkel Cell-Associated Afferents. Neuron 2017, 94, 666–676.e9. [Google Scholar] [CrossRef] [PubMed]
- Senok, S.S.; Baumann, K.I. Functional Evidence for Calcium-Induced Calcium Release in Isolated Rat Vibrissal Merkel Cell Mechanoreceptors. J. Physiol. 1997, 500, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Maricich, S.M.; Morrison, K.M.; Mathes, E.L.; Brewer, B.M. Rodents Rely on Merkel Cells for Texture Discrimination Tasks. J. Neurosci. Off. J. Soc. Neurosci. 2012, 32, 3296–3300. [Google Scholar] [CrossRef]
- Chang, W.; Kanda, H.; Ikeda, R.; Ling, J.; DeBerry, J.J.; Gu, J.G. Merkel Disc Is a Serotonergic Synapse in the Epidermis for Transmitting Tactile Signals in Mammals. Proc. Natl. Acad. Sci. USA 2016, 113, E5491–E5500. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Kanda, H.; Ikeda, R.; Ling, J.; Gu, J.G. Serotonergic Transmission at Merkel Discs: Modulation by Exogenously Applied Chemical Messengers and Involvement of Ih Currents. J. Neurochem. 2017, 141, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Chang, W.; Gu, J.G. Effects on Tactile Transmission by Serotonin Transporter Inhibitors at Merkel Discs of Mouse Whisker Hair Follicles. Mol. Pain 2020, 16, 1744806920938237. [Google Scholar] [CrossRef] [PubMed]
- Sonekatsu, M.; Gu, S.L.; Kanda, H.; Gu, J.G. Effects of Norepinephrine and Β2 Receptor Antagonist ICI 118,551 on Whisker Hair Follicle Mechanoreceptors Dissatisfy Merkel Discs Being Adrenergic Synapses. Mol. Brain 2019, 12, 31. [Google Scholar] [CrossRef]
- Bouvier, V.; Roudaut, Y.; Osorio, N.; Aimonetti, J.-M.; Ribot-Ciscar, E.; Penalba, V.; Merrot, T.; Lebonvallet, N.; Le Gall-Ianotto, C.; Misery, L.; et al. Merkel Cells Sense Cooling with TRPM8 Channels. J. Investig. Dermatol. 2018, 138, 946–956. [Google Scholar] [CrossRef]
- Misery, L.; Brenaut, E.; Pierre, O.; Le Garrec, R.; Gouin, O.; Lebonvallet, N.; Abasq-Thomas, C.; Talagas, M.; Le Gall-Ianotto, C.; Besner-Morin, C.; et al. Chronic Itch: Emerging Treatments Following New Research Concepts. Br. J. Pharmacol. 2021, 178, 4775–4791. [Google Scholar] [CrossRef]
- Sakai, K.; Akiyama, T. Disinhibition of Touch-Evoked Itch in a Mouse Model of Psoriasis. J. Investig. Dermatol. 2019, 139, 1407–1410. [Google Scholar] [CrossRef]
- Xu, Z.-Z.; Kim, Y.H.; Bang, S.; Zhang, Y.; Berta, T.; Wang, F.; Oh, S.B.; Ji, R.-R. Inhibition of Mechanical Allodynia in Neuropathic Pain by TLR5-Mediated A-Fiber Blockade. Nat. Med. 2015, 21, 1326–1331. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, Y.; Geng, J.; Zhou, S.; Xiao, B. Mechanically Activated Piezo Channels Mediate Touch and Suppress Acute Mechanical Pain Response in Mice. Cell Rep. 2019, 26, 1419–1431.e4. [Google Scholar] [CrossRef] [PubMed]
- Fukuoka, M.; Miyachi, Y.; Ikoma, A. Mechanically Evoked Itch in Humans. Pain 2013, 154, 897–904. [Google Scholar] [CrossRef] [PubMed]
- Koch, S.C.; Acton, D.; Goulding, M. Spinal Circuits for Touch, Pain, and Itch. Annu. Rev. Physiol. 2018, 80, 189–217. [Google Scholar] [CrossRef] [PubMed]
- McGlone, F.; Wessberg, J.; Olausson, H. Discriminative and Affective Touch: Sensing and Feeling. Neuron 2014, 82, 737–755. [Google Scholar] [CrossRef]
- Olausson, H.; Lamarre, Y.; Backlund, H.; Morin, C.; Wallin, B.G.; Starck, G.; Ekholm, S.; Strigo, I.; Worsley, K.; Vallbo, A.B.; et al. Unmyelinated Tactile Afferents Signal Touch and Project to Insular Cortex. Nat. Neurosci. 2002, 5, 900–904. [Google Scholar] [CrossRef]
- Abraira, V.E.; Ginty, D.D. The Sensory Neurons of Touch. Neuron 2013, 79, 618–639. [Google Scholar] [CrossRef]
- Reinisch, C.M.; Tschachler, E. The Touch Dome in Human Skin Is Supplied by Different Types of Nerve Fibers. Ann. Neurol. 2005, 58, 88–95. [Google Scholar] [CrossRef]
- Ebara, S.; Kumamoto, K.; Baumann, K.I.; Halata, Z. Three-Dimensional Analyses of Touch Domes in the Hairy Skin of the Cat Paw Reveal Morphological Substrates for Complex Sensory Processing. Neurosci. Res. 2008, 61, 159–171. [Google Scholar] [CrossRef]
- Zhang, J.; Huang, W.; Tuckett, R.P. C-Fiber Modulation of the Rat Type I Slowly Adapting Mechanoreceptor. Neuroscience 2002, 115, 797–804. [Google Scholar] [CrossRef]
- Bataille-Savattier, A.; Le Gall-Ianotto, C.; Lebonvallet, N.; Misery, L.; Talagas, M. Do Merkel Complexes Initiate Mechanical Itch? Exp. Dermatol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Szczot, M.; Liljencrantz, J.; Ghitani, N.; Barik, A.; Lam, R.; Thompson, J.H.; Bharucha-Goebel, D.; Saade, D.; Necaise, A.; Donkervoort, S.; et al. PIEZO2 Mediates Injury-Induced Tactile Pain in Mice and Humans. Sci. Transl. Med. 2018, 10, eaat9892. [Google Scholar] [CrossRef] [PubMed]
- Murthy, S.E.; Loud, M.C.; Daou, I.; Marshall, K.L.; Schwaller, F.; Kühnemund, J.; Francisco, A.G.; Keenan, W.T.; Dubin, A.E.; Lewin, G.R.; et al. The Mechanosensitive Ion Channel Piezo2 Mediates Sensitivity to Mechanical Pain in Mice. Sci. Transl. Med. 2018, 10, eaat9897. [Google Scholar] [CrossRef]
- Gregory, N.S.; Harris, A.L.; Robinson, C.R.; Dougherty, P.M.; Fuchs, P.N.; Sluka, K.A. An Overview of Animal Models of Pain: Disease Models and Outcome Measures. J. Pain 2013, 14, 1255–1269. [Google Scholar] [CrossRef] [PubMed]
- Tominaga, M.; Caterina, M.J.; Malmberg, A.B.; Rosen, T.A.; Gilbert, H.; Skinner, K.; Raumann, B.E.; Basbaum, A.I.; Julius, D. The Cloned Capsaicin Receptor Integrates Multiple Pain-Producing Stimuli. Neuron 1998, 21, 531–543. [Google Scholar] [CrossRef] [PubMed]
- Hill, R.Z.; Bautista, D.M. Getting in Touch with Mechanical Pain Mechanisms. Trends Neurosci. 2020, 43, 311–325. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.M.; Moehring, F.; Itson-Zoske, B.; Fan, F.; Stucky, C.L.; Hogan, Q.H.; Yu, H. Piezo2 Mechanosensitive Ion Channel Is Located to Sensory Neurons and Nonneuronal Cells in Rat Peripheral Sensory Pathway: Implications in Pain. Pain 2021, 162, 2750–2768. [Google Scholar] [CrossRef] [PubMed]
- Jeon, S.-M.; Chang, D.; Geske, A.; Ginty, D.D.; Caterina, M.J. Sex-Dependent Reduction in Mechanical Allodynia in the Sural-Sparing Nerve Injury Model in Mice Lacking Merkel Cells. J. Neurosci. 2021, 41, 5595–5619. [Google Scholar] [CrossRef]
- Moll, I. Merkel Cell Distribution in Human Hair Follicles of the Fetal and Adult Scalp. Cell Tissue Res. 1994, 277, 131–138. [Google Scholar] [CrossRef]
- Narisawa, Y.; Hashimoto, K.; Kohda, H. Merkel Cells of the Terminal Hair Follicle of the Adult Human Scalp. J. Investig. Dermatol. 1994, 102, 506–510. [Google Scholar] [CrossRef] [PubMed]
- Lumpkin, E.A.; Collisson, T.; Parab, P.; Omer-Abdalla, A.; Haeberle, H.; Chen, P.; Doetzlhofer, A.; White, P.; Groves, A.; Segil, N.; et al. Math1-Driven GFP Expression in the Developing Nervous System of Transgenic Mice. Gene Expr. Patterns GEP 2003, 3, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, J. A Pure, Monolayer Culture of Merkel Cells from Sinus Hair Follicles of the Rat. Neurosci. Lett. 1996, 216, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Shimohira-Yamasaki, M.; Toda, S.; Narisawa, Y.; Sugihara, H. Merkel Cell-Nerve Cell Interaction Undergoes Formation of a Synapse-like Structure in a Primary Culture. Cell Struct. Funct. 2006, 31, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Parisi, I.; Collinson, J.M. Regulation of Merkel Cell Development by Pax6. Int. J. Dev. Biol. 2012, 56, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Soya, M.; Sato, M.; Sobhan, U.; Tsumura, M.; Ichinohe, T.; Tazaki, M.; Shibukawa, Y. Plasma Membrane Stretch Activates Transient Receptor Potential Vanilloid and Ankyrin Channels in Merkel Cells from Hamster Buccal Mucosa. Cell Calcium 2014, 55, 208–218. [Google Scholar] [CrossRef]
- Château, Y.; Dorange, G.; Clément, J.-F.; Pennec, J.-P.; Gobin, E.; Griscom, L.; Baudrimont, M.; Rougier, N.; Chesné, C.; Misery, L. In Vitro Reconstruction of Neuro-Epidermal Connections. J. Investig. Dermatol. 2007, 127, 979–981. [Google Scholar] [CrossRef]
- Ishida, K.; Saito, T.; Mitsui, T. In Vitro Formation of the Merkel Cell-Neurite Complex in Embryonic Mouse Whiskers Using Organotypic Co-Cultures. Dev. Growth Differ. 2018, 60, 291–299. [Google Scholar] [CrossRef]
- Hahn, J.M.; Combs, K.A.; Lloyd, C.M.; McFarland, K.L.; Boyce, S.T.; Supp, D.M. Identification of Merkel Cells Associated with Neurons in Engineered Skin Substitutes after Grafting to Full Thickness Wounds. PLoS ONE 2019, 14, e0213325. [Google Scholar] [CrossRef]
- Stella, M.; Calcagni, M.; Teich-Alasia, S.; Ramieri, G.; Cellino, G.; Panzica, G. Sensory Endings in Skin Grafts and Scars after Extensive Burns. Burn. J. Int. Soc. Burn INJ 1994, 20, 491–495. [Google Scholar] [CrossRef]
- Wright, M.C.; Logan, G.J.; Bolock, A.M.; Kubicki, A.C.; Hemphill, J.A.; Sanders, T.A.; Maricich, S.M. Merkel Cells Are Long-Lived Cells Whose Production Is Stimulated by Skin Injury. Dev. Biol. 2017, 422, 4–13. [Google Scholar] [CrossRef]
- Lee, J.; Rabbani, C.C.; Gao, H.; Steinhart, M.R.; Woodruff, B.M.; Pflum, Z.E.; Kim, A.; Heller, S.; Liu, Y.; Shipchandler, T.Z.; et al. Hair-bearing human skin generated entirely from pluripotent stem cells. Nature 2020, 582, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Dong, Y.; Mihalas, S.; Kim, S.S.; Yoshioka, T.; Bensmaia, S.; Niebur, E. A Simple Model of Mechanotransduction in Primate Glabrous Skin. J. Neurophysiol. 2013, 109, 1350–1359. [Google Scholar] [CrossRef]
- Kim, S.S.; Sripati, A.P.; Bensmaia, S.J. Predicting the Timing of Spikes Evoked by Tactile Stimulation of the Hand. J. Neurophysiol. 2010, 104, 1484–1496. [Google Scholar] [CrossRef] [PubMed]
- Wheat, H.E.; Goodwin, A.W. Tactile Discrimination of Edge Shape: Limits on Spatial Resolution Imposed by Parameters of the Peripheral Neural Population. J. Neurosci. Off. J. Soc. Neurosci. 2001, 21, 7751–7763. [Google Scholar] [CrossRef] [PubMed]
- Gerling, G.J.; Rivest, I.I.; Lesniak, D.R.; Scanlon, J.R.; Wan, L. Validating a Population Model of Tactile Mechanotransduction of Slowly Adapting Type I Afferents at Levels of Skin Mechanics, Single-Unit Response and Psychophysics. IEEE Trans. Haptics 2014, 7, 216–228. [Google Scholar] [CrossRef] [PubMed]
- Lesniak, D.R.; Marshall, K.L.; Wellnitz, S.A.; Jenkins, B.A.; Baba, Y.; Rasband, M.N.; Gerling, G.J.; Lumpkin, E.A. Computation Identifies Structural Features That Govern Neuronal Firing Properties in Slowly Adapting Touch Receptors. eLife 2014, 3, e01488. [Google Scholar] [CrossRef] [PubMed]
- Chalfie, M. Neurosensory Mechanotransduction. Nat. Rev. Mol. Cell Biol. 2009, 10, 44–52. [Google Scholar] [CrossRef]
- Gerling, G.J.; Wan, L.; Hoffman, B.U.; Wang, Y.; Lumpkin, E.A. Computation Predicts Rapidly Adapting Mechanotransduction Currents Cannot Account for Tactile Encoding in Merkel Cell-Neurite Complexes. PLoS Comput. Biol. 2018, 14, e1006264. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bataille, A.; Le Gall, C.; Misery, L.; Talagas, M. Merkel Cells Are Multimodal Sensory Cells: A Review of Study Methods. Cells 2022, 11, 3827. https://doi.org/10.3390/cells11233827
Bataille A, Le Gall C, Misery L, Talagas M. Merkel Cells Are Multimodal Sensory Cells: A Review of Study Methods. Cells. 2022; 11(23):3827. https://doi.org/10.3390/cells11233827
Chicago/Turabian StyleBataille, Adeline, Christelle Le Gall, Laurent Misery, and Matthieu Talagas. 2022. "Merkel Cells Are Multimodal Sensory Cells: A Review of Study Methods" Cells 11, no. 23: 3827. https://doi.org/10.3390/cells11233827
APA StyleBataille, A., Le Gall, C., Misery, L., & Talagas, M. (2022). Merkel Cells Are Multimodal Sensory Cells: A Review of Study Methods. Cells, 11(23), 3827. https://doi.org/10.3390/cells11233827







