Liraglutide Improves the Angiogenic Capability of EPC and Promotes Ischemic Angiogenesis in Mice under Diabetic Conditions through an Nrf2-Dependent Mechanism
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Hindlimb Ischemia Model Construction and Liraglutide Administration
2.3. Blood Perfusion Scanning
2.4. Immunofluorescence Staining
2.5. EPC Isolation and Identification
2.6. Cell Scratch Recovery Assay
2.7. Matrigel Capillary Formation Assay
2.8. Oxidative Stress Determination
2.9. RNA Sequencing
2.10. Detection of Nitric Oxide (NO) Production
2.11. RNA Isolation and Semi-Quantitative RT-PCR (sqRT-PCR)
2.12. Nrf2 Activity Assessment
2.13. RNA Interference
2.14. Western Blot
2.15. Statistical Analysis
3. Results
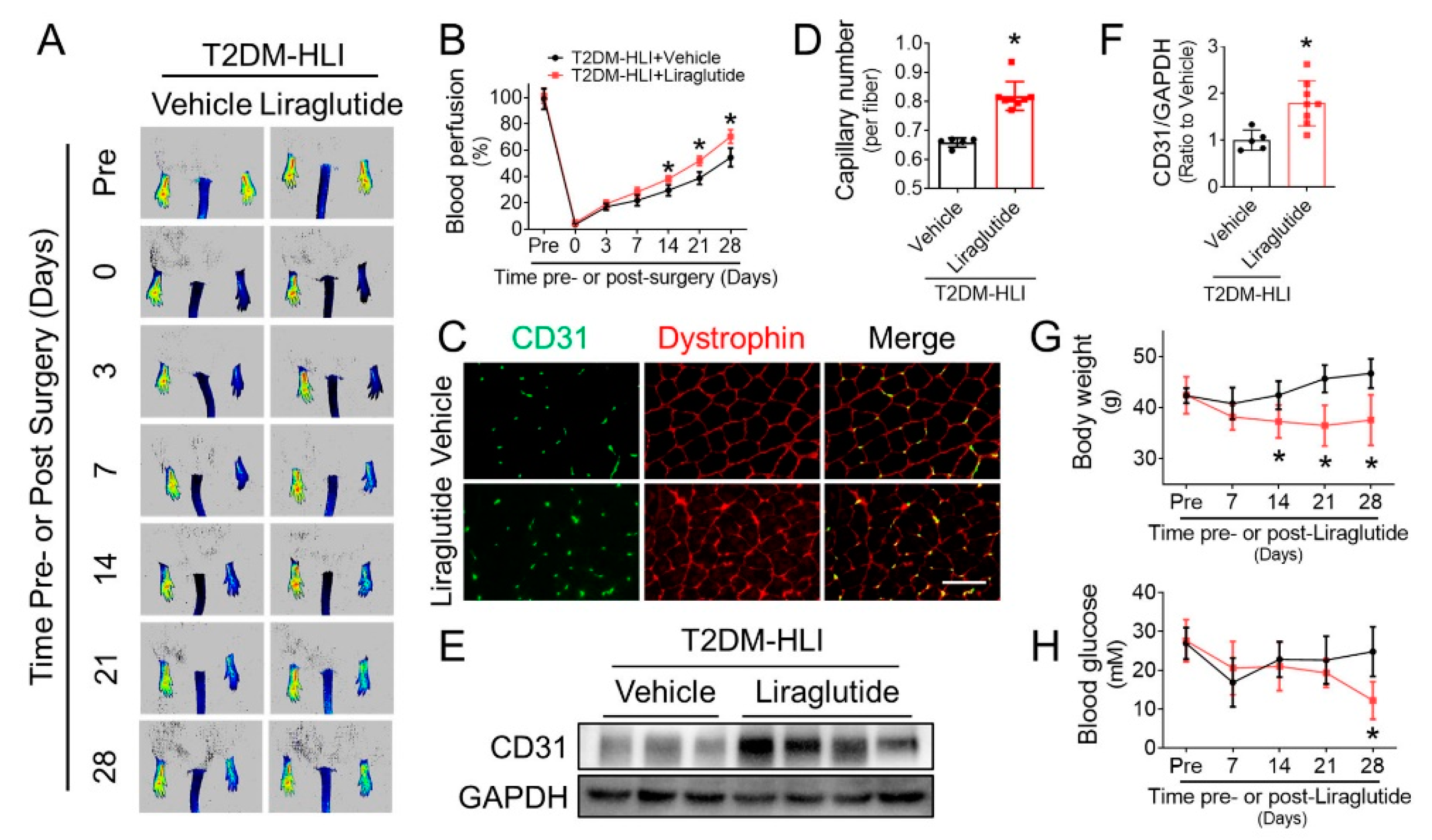
3.1. Liraglutide Improves Blood Perfusion and Angiogenesis in Ischemic Hindlimb of T2DM Mice
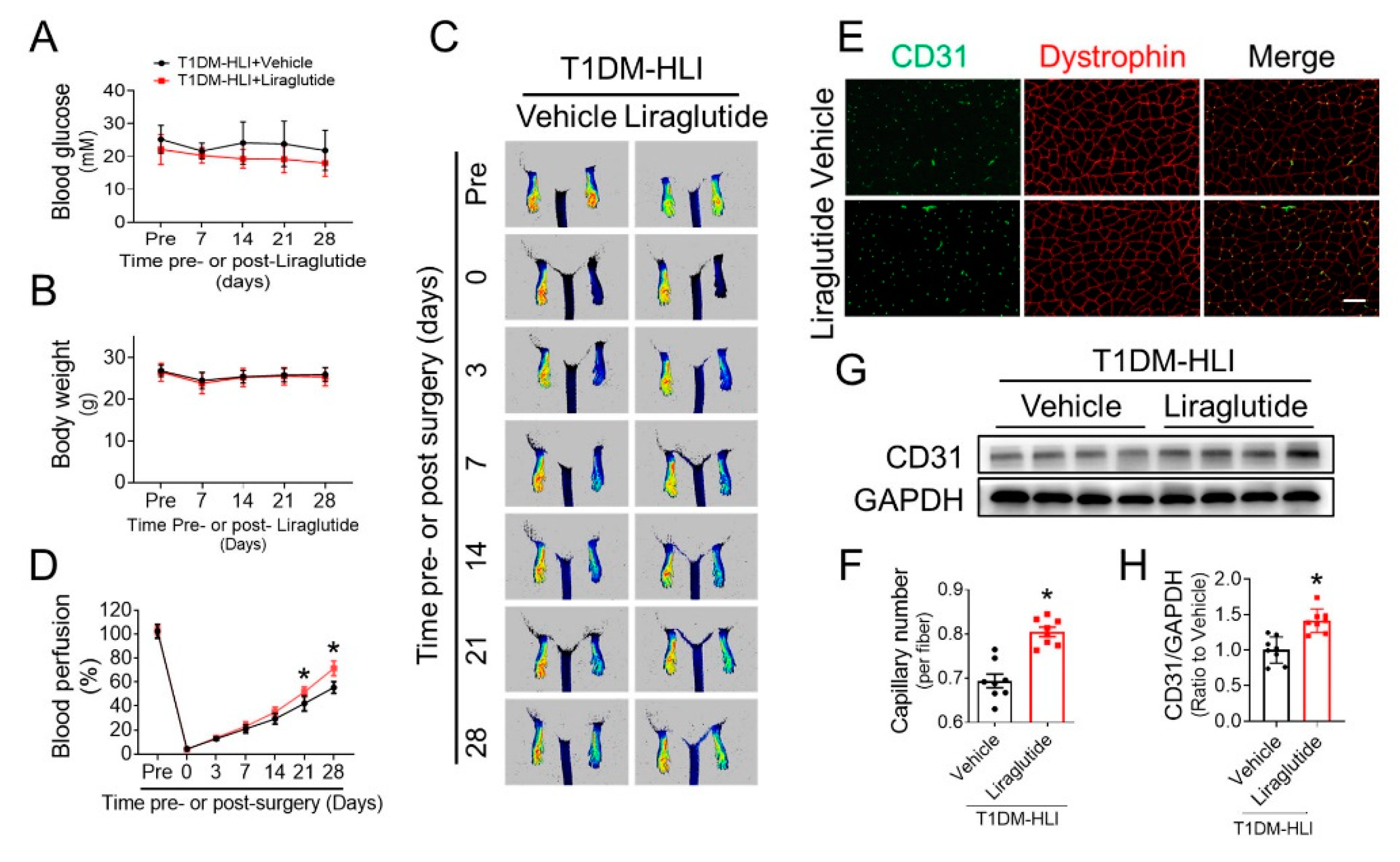
3.2. Liraglutide Improves Blood Perfusion and Angiogenesis in Ischemic Hindlimb of T1DM Mice
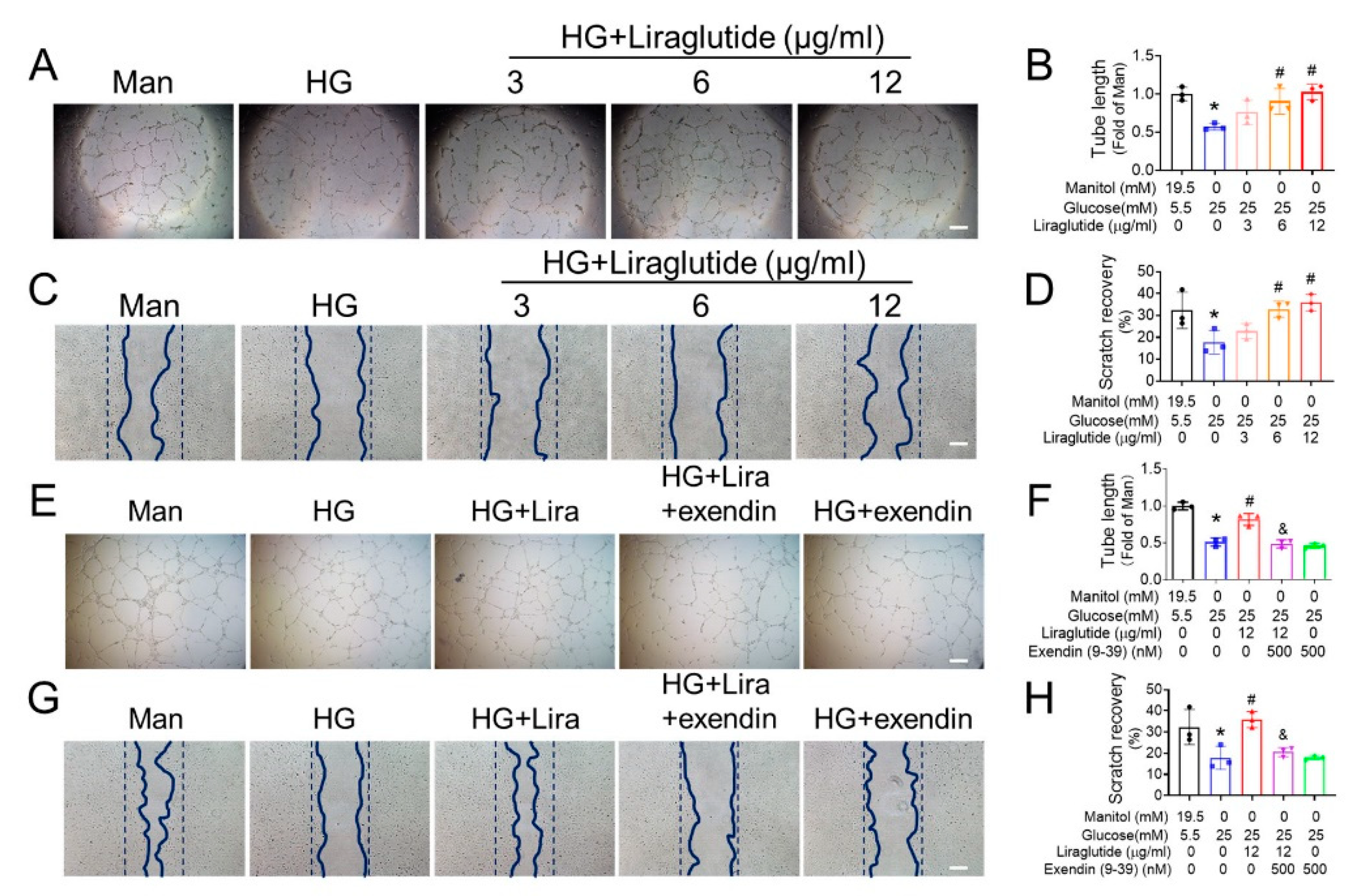
3.3. Liraglutide Ameliorates the Function of HG-Treated EPC
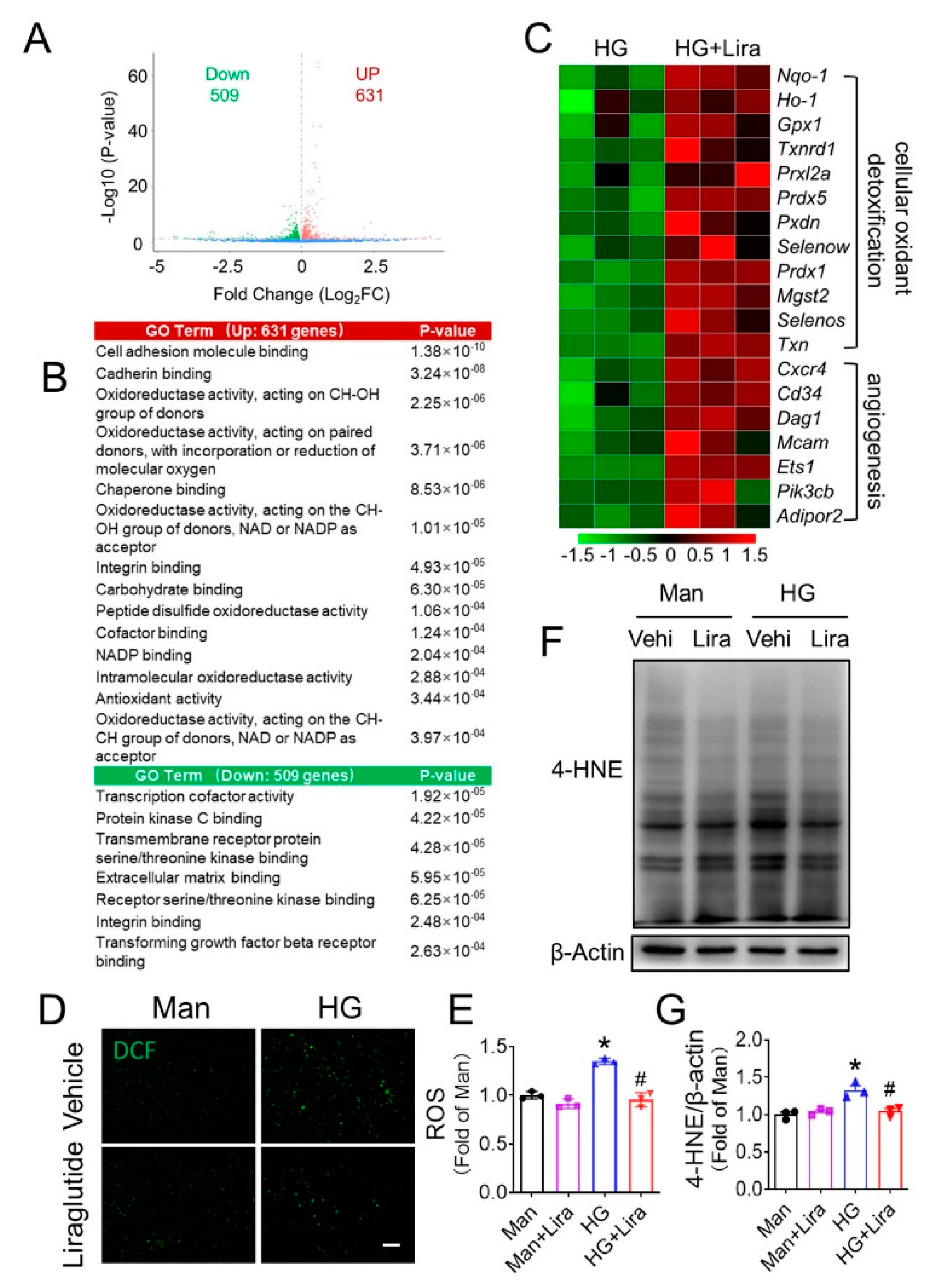
3.4. Liraglutide Attenuates HG-Induced Oxidative Stress in EPC
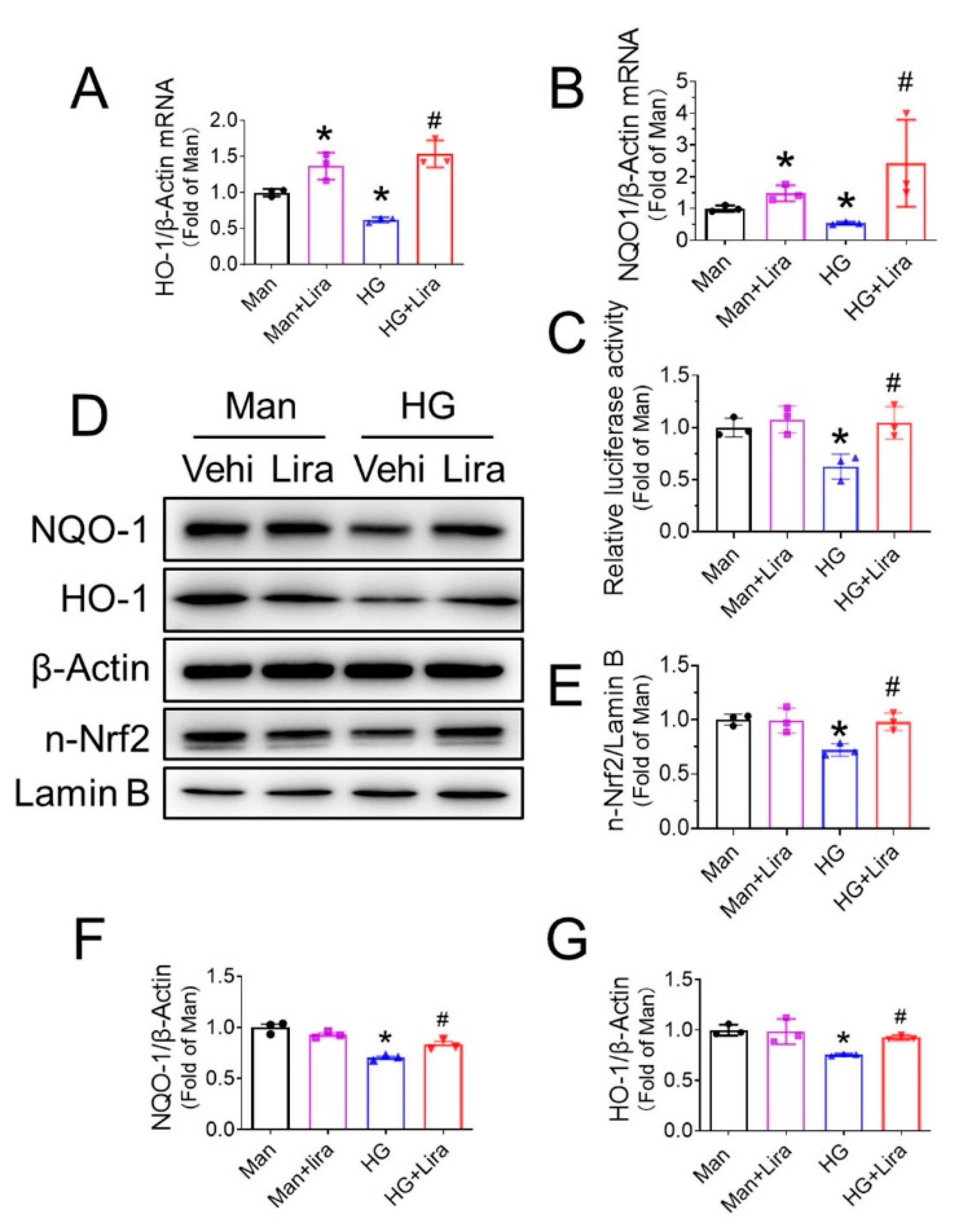
3.5. Liraglutide Enhances Nrf2 Activity in HG-Treated EPC
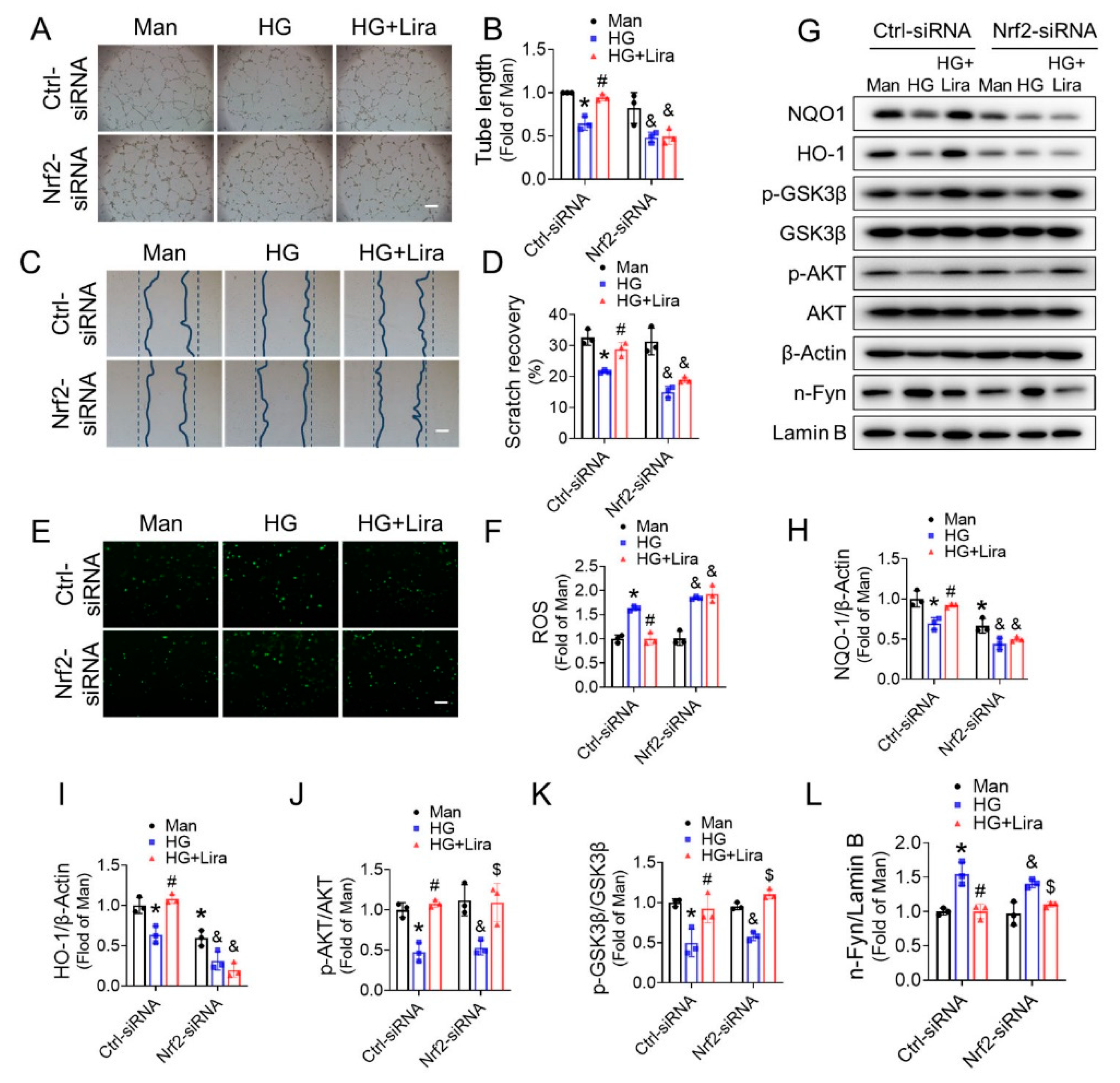
3.6. Nrf2 Mediates the Beneficial Effects of Liraglutide on HG-Treated EPC
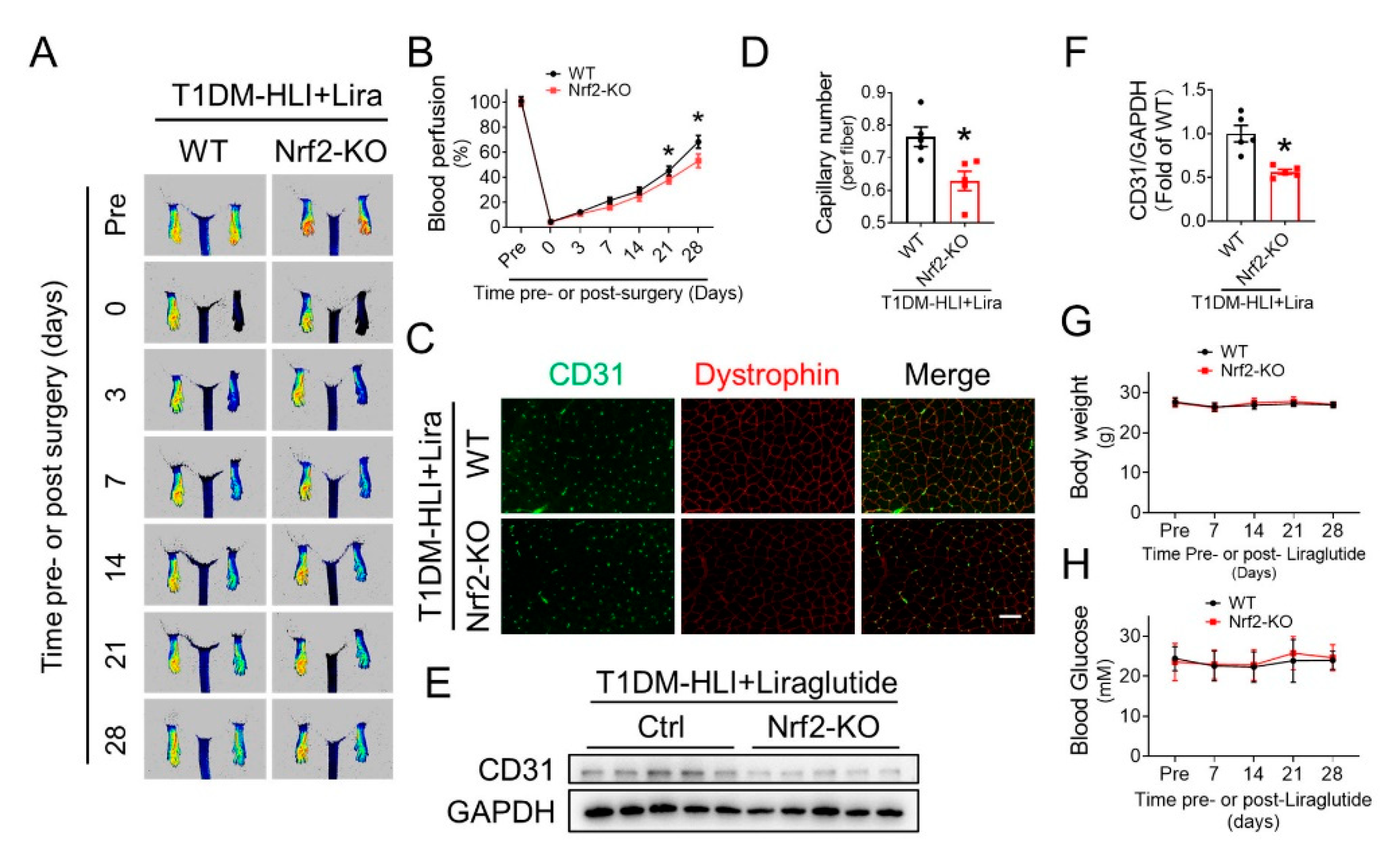
3.7. Nrf2 Deletion Attenuates the Beneficial Effect of Liraglutide in Improving Blood Perfusion and Angiogenesis in Ischemic Hindlimb of T1DM Mice
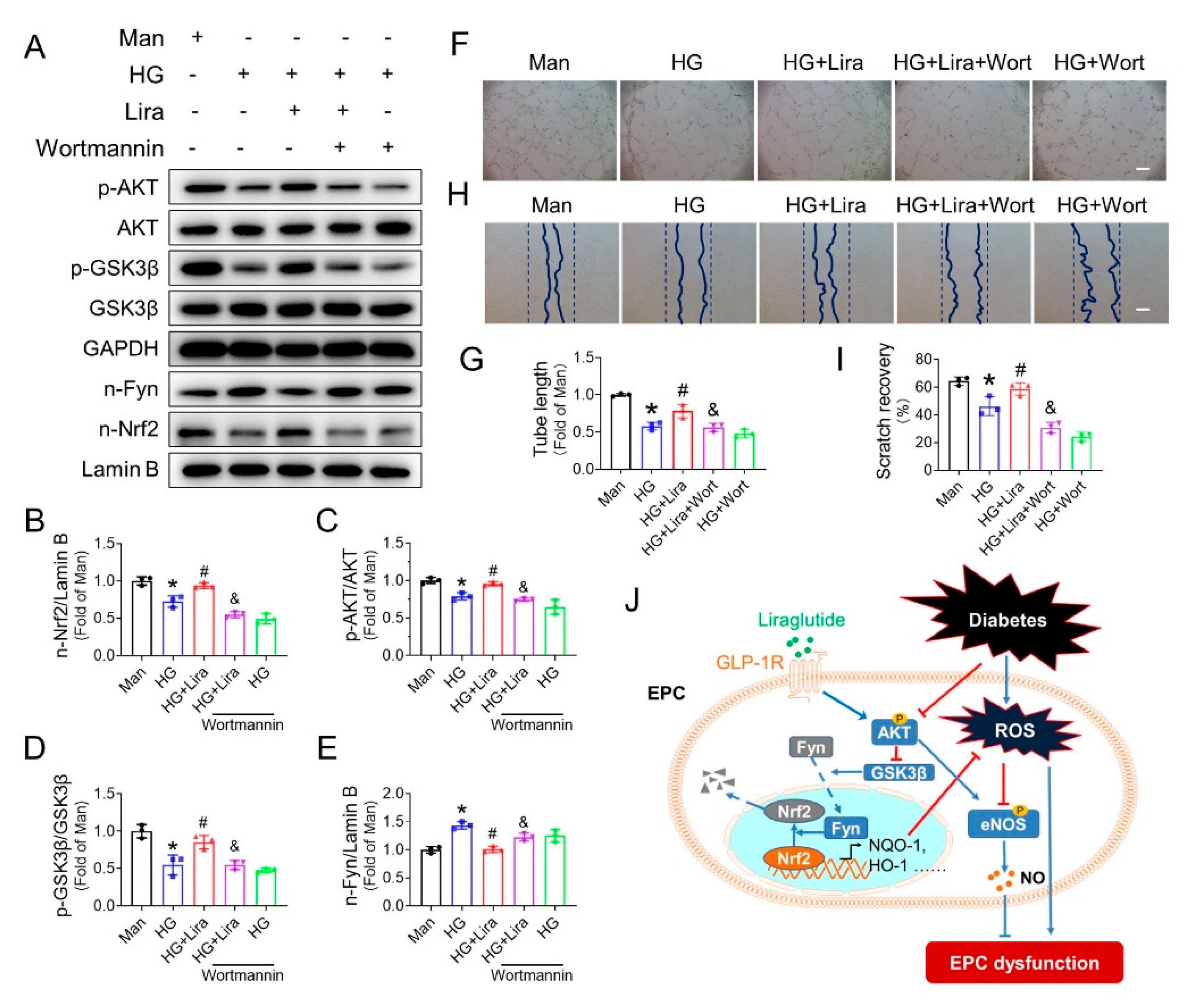
3.8. Liraglutide Activates Nrf2 through the AKT/GSK-3β/Fyn Pathway
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Petrelli, A.; Di Fenza, R.; Carvello, M.; Gatti, F.; Secchi, A.; Fiorina, P. Strategies to reverse endothelial progenitor cell dysfunction in diabetes. Exp. Diabetes Res. 2012, 2012, 471823. [Google Scholar] [CrossRef] [PubMed]
- Pompilio, G.; Capogrossi, M.; Pesce, M.; Alamanni, F.; DiCampli, C.; Achilli, F.; Germani, A.; Biglioli, P. Endothelial progenitor cells and cardiovascular homeostasis: Clinical implications. Int. J. Cardiol. 2009, 131, 156–167. [Google Scholar] [CrossRef] [PubMed]
- Fadini, G.P.; Losordo, D.; Dimmeler, S. Critical reevaluation of endothelial progenitor cell phenotypes for therapeutic and diagnostic use. Circ. Res. 2012, 110, 624–637. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Yan, X.; Zeng, J.; Chen, J.; Wang, Y.; Chen, J.; Li, Y.; Barati, M.T.; Wintergerst, K.A.; Pan, K.; et al. Elevating CXCR7 Improves Angiogenic Function of EPCs via Akt/GSK-3beta/Fyn-Mediated Nrf2 Activation in Diabetic Limb Ischemia. Circ. Res. 2017, 120, e7–e23. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Cai, S.; Xiong, X.; Sun, W.; Dai, X.; Chen, S.; Ye, Q.; Song, Z.; Jiang, Q.; Xu, Z. Chemokine receptor CXCR7 mediates human endothelial progenitor cells survival, angiogenesis, but not proliferation. J. Cell. Biochem. 2012, 113, 1437–1446. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Dai, X.; He, L.; Ling, X.; Shao, M.; Zhang, C.; Wang, Y.; Xiao, J.; Cai, L.; Li, X.; et al. A Novel CXCR4 antagonist enhances angiogenesis via modifying the ischaemic tissue environment. J. Cell. Mol. Med. 2017, 21, 2298–2307. [Google Scholar] [CrossRef]
- Vasa, M.; Fichtlscherer, S.; Aicher, A.; Adler, K.; Urbich, C.; Martin, H.; Zeiher, A.M.; Dimmeler, S. Number and migratory activity of circulating endothelial progenitor cells inversely correlate with risk factors for coronary artery disease. Circ. Res. 2001, 89, e1–e7. [Google Scholar] [CrossRef] [PubMed]
- Hill, J.M.; Zalos, G.; Halcox, J.P.J.; Schenke, W.H.; Waclawiw, M.A.; Quyyumi, A.A.; Finkel, T. Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N. Engl. J. Med. 2003, 348, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Murohara, T.; Ikeda, H.; Duan, J.; Shintani, S.; Sasaki, K.; Eguchi, H.; Onitsuka, I.; Matsui, K.; Imaizumi, T. Transplanted cord blood-derived endothelial precursor cells augment postnatal neovascularization. J. Clin. Investig. 2000, 105, 1527–1536. [Google Scholar] [CrossRef]
- Kamihata, H.; Matsubara, H.; Nishiue, T.; Fujiyama, S.; Amano, K.; Iba, O.; Imada, T.; Iwasaka, T. Improvement of collateral perfusion and regional function by implantation of peripheral blood mononuclear cells into ischemic hibernating myocardium. Arterioscl. Throm. Vas. 2002, 22, 1804–1810. [Google Scholar] [CrossRef]
- Cho, H.J.; Lee, N.; Lee, J.Y.; Choi, Y.J.; Ii, M.; Wecker, A.; Jeong, J.O.; Curry, C.; Qin, G.; Yoon, Y.S.; et al. Role of host tissues for sustained humoral effects after endothelial progenitor cell transplantation into the ischemic heart. J. Exp. Med. 2007, 204, 3257–3269. [Google Scholar] [CrossRef] [PubMed]
- Loomans, C.J.M.; de Koning, E.J.P.; Staal, F.J.T.; Rookmaaker, M.B.; Verseyden, C.; de Boer, H.C.; Verhaar, M.C.; Braam, B.; Rabelink, T.J.; van Zonneveld, A.J. Endothelial progenitor cell dysfunction—A novel concept in the pathogenesis of vascular complications of type 1 diabetes. Diabetes 2004, 53, 195–199. [Google Scholar] [CrossRef]
- Fadini, G.P.; Boscaro, E.; Albiero, M.; Menegazzo, L.; Frison, V.; de Kreutzenberg, S.; Agostini, C.; Tiengo, A.; Avogaro, A. The Oral Dipeptidyl Peptidase-4 Inhibitor Sitagliptin Increases Circulating Endothelial Progenitor Cells in Patients With Type 2 Diabetes Possible role of stromal-derived factor-1 alpha. Diabetes Care 2010, 33, 1607–1609. [Google Scholar] [CrossRef]
- Meier, J. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2012, 8, 728–742. [Google Scholar] [CrossRef] [PubMed]
- Drucker, D.J. The Cardiovascular Biology of Glucagon-like Peptide-1. Cell Metab. 2016, 24, 15–30. [Google Scholar] [CrossRef] [PubMed]
- Kieffer, T.J.; Habener, J.F. The glucagon-like peptides. Endocr. Rev. 1999, 20, 876–913. [Google Scholar] [CrossRef] [PubMed]
- Marso, S.P.; Daniels, G.H.; Brown-Frandsen, K.; Kristensen, P.; Mann, J.F.; Nauck, M.A.; Nissen, S.E.; Pocock, S.; Poulter, N.R.; Ravn, L.S.; et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Mann, J.F.; Fonseca, V.; Mosenzon, O.; Raz, I.; Goldman, B.; Idorn, T.; von Scholten, B.J.; Poulter, N.R. Effects of Liraglutide Versus Placebo on Cardiovascular Events in Patients With Type 2 Diabetes Mellitus and Chronic Kidney Disease. Circulation 2018, 138, 2908–2918. [Google Scholar] [CrossRef]
- Verma, S.; Bhatt, D.L.; Bain, S.C.; Buse, J.B.; Mann, J.F.; Marso, S.P.; Nauck, M.A.; Poulter, N.R.; Pratley, R.E.; Zinman, B.; et al. Effect of Liraglutide on Cardiovascular Events in Patients With Type 2 Diabetes Mellitus and Polyvascular Disease: Results of the LEADER Trial. Circulation 2018, 137, 2179–2183. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Poulter, N.R.; Bhatt, D.L.; Bain, S.C.; Buse, J.B.; Leiter, L.A.; Nauck, M.A.; Pratley, R.E.; Zinman, B.; Ørsted, D.D.; et al. Effects of Liraglutide on Cardiovascular Outcomes in Patients With Type 2 Diabetes Mellitus With or Without History of Myocardial Infarction or Stroke. Circulation 2018, 138, 2884–2894. [Google Scholar] [CrossRef]
- Svanström, H.; Ueda, P.; Melbye, M.; Eliasson, B.; Svensson, A.M.; Franzén, S.; Gudbjörnsdottir, S.; Hveem, K.; Jonasson, C.; Pasternak, B.; et al. Use of liraglutide and risk of major cardiovascular events: A register-based cohort study in Denmark and Sweden. Lancet Diabetes Endocrinol. 2019, 7, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Niiyama, H.; Huang, N.F.; Rollins, M.D.; Cooke, J.P. Murine model of hindlimb ischemia. J. Vis. Exp. 2009, 23, e1035. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.; Fan, X.; Meng, X.; Sun, S.; Su, Y.; Ling, X.; Chen, X.; Wang, K.; Dai, X.; Zhang, C.; et al. FGF21 promotes ischaemic angiogenesis and endothelial progenitor cells function under diabetic conditions in an AMPK/NAD+-dependent manner. J. Cell. Mol. Med. 2021, 25, 3091–3102. [Google Scholar] [CrossRef]
- Dai, X.; Cai, S.; Xiong, X.; Wang, L.; Ye, Q.; Yan, X.; Ma, K.; Cai, L.; Tan, Y. The Role of CXCR7 on the Adhesion, Proliferation and Angiogenesis of Endothelial Progenitor Cells. J. Cell. Mol. Med. 2011, 15, 1299–1309. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Tan, Y.; Eton, D.; Yang, Z.; Uberti, Z.; Li, S.; Schulick, A.; Yu, H. Statin and Stromal Cell-Derived Factor-1 Additively Promote Angiogenesis by Enhancement of Progenitor Cells Incorporation into New Vessels. Stem Cells 2008, 26, 1376–1384. [Google Scholar] [CrossRef] [PubMed]
- Murohara, T.; Asahara, T.; Silver, M.; Bauters, C.; Masuda, H.; Kalka, C.; Kearney, M.; Chen, D.; Symes, J.F.; Fishman, M.C.; et al. Nitric oxide synthase modulates angiogenesis in response to tissue ischemia. J. Clin. Investig. 1998, 101, 2567–2578. [Google Scholar] [CrossRef] [PubMed]
- Hamed, S.; Brenner, B.; Roguin, A. Nitric oxide: A key factor behind the dysfunctionality of endothelial progenitor cells in diabetes mellitus type-2. Cardiovasc. Res. 2011, 91, 9–15. [Google Scholar] [CrossRef]
- Dai, X.; Yan, X.; Wintergerst, K.; Cai, L.; Keller, L.; Tan, Y. Nrf2: Redox and Metabolic Regulator of Stem Cell State and Function. Trends Mol. Med. 2020, 26, 185–200. [Google Scholar] [CrossRef] [PubMed]
- Xin, Y.; Bai, Y.; Jiang, X.; Zhou, S.; Wang, Y.; Wintergerst, K.A.; Cui, T.; Ji, H.; Tan, Y.; Cai, L. Sulforaphane prevents angiotensin II-induced cardiomyopathy by activation of Nrf2 via stimulating the Akt/GSK-3ß/Fyn pathway. Redox Biol. 2018, 15, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Tepper, O.M.; Galiano, R.D.; Capla, J.M.; Kalka, C.; Gagne, P.J.; Jacobowitz, G.R.; Levine, J.P.; Gurtner, G.C. Human endothelial progenitor exhibit impaired proliferation, cells from type II diabetics adhesion, and incorporation into vascular structures. Circulation 2002, 106, 2781–2786. [Google Scholar] [CrossRef] [PubMed]
- Wils, J.; Favre, J.; Bellien, J. Modulating putative endothelial progenitor cells for the treatment of endothelial dysfunction and cardiovascular complications in diabetes. Pharmacol. Ther. 2017, 170, 98–115. [Google Scholar] [CrossRef] [PubMed]
- Sivertsen, J.; Rosenmeier, J.; Holst, J.J.; Vilsbøll, T. The effect of glucagon-like peptide 1 on cardiovascular risk. Nat. Rev. Cardiol. 2012, 9, 209–222. [Google Scholar] [CrossRef] [PubMed]
- Wegeberg, A.; Hansen, C.; Farmer, A.; Karmisholt, J.; Drewes, A.; Jakobsen, P.; Brock, B.; Brock, C. Liraglutide accelerates colonic transit in people with type 1 diabetes and polyneuropathy: A randomised, double-blind, placebo-controlled trial. United Eur. Gastroenterol. 2020, 8, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Romaní-Pérez, M.; Outeiriño-Iglesias, V.; Moya, C.; Santisteban, P.; González-Matías, L.; Vigo, E.; Mallo, F. Activation of the GLP-1 Receptor by Liraglutide Increases ACE2 Expression, Reversing Right Ventricle Hypertrophy, and Improving the Production of SP-A and SP-B in the Lungs of Type 1 Diabetes Rats. Endocrinology 2015, 156, 3559–3569. [Google Scholar] [CrossRef] [PubMed]
- Kushima, H.; Mori, Y.; Koshibu, M.; Hiromura, M.; Kohashi, K.; Terasaki, M.; Fukui, T.; Hirano, T. The role of endothelial nitric oxide in the anti-restenotic effects of liraglutide in a mouse model of restenosis. Cardiovasc. Diabetol. 2017, 16, 122. [Google Scholar] [CrossRef] [PubMed]
- Helmstädter, J.; Frenis, K.; Filippou, K.; Grill, A.; Dib, M.; Kalinovic, S.; Pawelke, F.; Kus, K.; Kröller-Schön, S.; Oelze, M.; et al. Endothelial GLP-1 (Glucagon-Like Peptide-1) Receptor Mediates Cardiovascular Protection by Liraglutide In Mice With Experimental Arterial Hypertension. Arterioscl. Throm. Vas. 2020, 40, 145–158. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Duan, X.; Yuan, M.; Yu, J.; Hu, X.; Han, X.; Lan, L.; Liu, B.; Wang, B.; Qin, J. Glucagon-like peptide-1 receptor activation by liraglutide promotes breast cancer through NOX4/ROS/VEGF pathway. Life Sci. 2022, 294, 120370. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.W.; Deng, Y.P.; Han, X.; Ren, G.F.; Cai, J.; Jiang, G.J. Metformin improves the angiogenic functions of endothelial progenitor cells via activating AMPK/eNOS pathway in diabetic mice. Cardiovasc. Diabetol. 2016, 15, 88. [Google Scholar] [CrossRef] [PubMed]
- Sorrentino, S.A.; Bahlmann, F.H.; Besler, C.; Mueller, M.; Schulz, S.; Kirchhoff, N.; Doerries, C.; Horvath, T.; Limbourg, A.; Limbourg, F.; et al. Oxidant stress impairs in vivo reendothelialization capacity of endothelial progenitor cells from patients with type 2 diabetes mellitus—Restoration by the peroxisome proliferator-activated receptor-gamma agonist rosiglitazone. Circulation 2007, 116, 163–173. [Google Scholar] [CrossRef] [PubMed]
- Walter, D.H.; Rittig, K.; Bahlmann, F.H.; Kirchmair, R.; Silver, M.; Murayama, T.; Nishimura, H.; Losordo, D.W.; Asahara, T.; Isner, J.M. Statin therapy accelerates reendothelialization—A novel effect involving mobilization and incorporation of bone marrow-derived endothelial progenitor cells. Circulation 2002, 105, 3017–3024. [Google Scholar] [CrossRef] [PubMed]
- Dai, X.; Zeng, J.; Yan, X.; Lin, Q.; Wang, K.; Chen, J.; Shen, F.; Gu, X.; Wang, Y.; Chen, J.; et al. Sitagliptin-mediated preservation of endothelial progenitor cell function via augmenting autophagy enhances ischaemic angiogenesis in diabetes. J. Cell. Mol. Med. 2018, 22, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Nandula, S.; Kundu, N.; Awal, H.; Brichacek, B.; Fakhri, M.; Aimalla, N.; Elzarki, A.; Amdur, R.; Sen, S. Role of Canagliflozin on function of CD34+ve endothelial progenitor cells (EPC) in patients with type 2 diabetes. Cardiovasc. Diabetol. 2021, 20, 44. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wang, S.; Chen, X.; Wang, Z.; Wang, X.; Zhou, Q.; Fang, W.; Zheng, C. Liraglutide prevents high glucose induced HUVECs dysfunction via inhibition of PINK1/Parkin-dependent mitophagy. Mol. Cell. Endocrinol. 2022, 545, 111560. [Google Scholar] [CrossRef] [PubMed]
- Callaghan, M.J.; Ceradini, D.J.; Gurtner, G.C. Hyperglycemia-induced reactive oxygen species and impaired endothelial progenitor cell function. Antioxid. Redox Signal. 2005, 7, 1476–1482. [Google Scholar] [CrossRef]
- Kim, K.A.; Shin, Y.J.; Kim, Y.J.; Lee, H.; Noh, S.Y.; Jang, S.H.; Bae, O.N. Dysfunction of Endothelial Progenitor Cells under Diabetic Conditions and its Underlying Mechanisms. Arch. Pharm. Res. 2012, 35, 223–234. [Google Scholar] [CrossRef] [PubMed]
- Florczyk, U.; Jazwa, A.; Maleszewska, M.; Mendel, M.; Szade, K.; Kozakowska, M.; Grochot-Przeczek, A.; Viscardi, M.; Czauderna, S.; Bukowska-Strakova, K.; et al. Nrf2 regulates angiogenesis: Effect on endothelial cells, bone marrow-derived proangiogenic cells and hind limb ischemia. Antioxid. Redox Sign. 2014, 20, 1693–1708. [Google Scholar] [CrossRef] [PubMed]
- Dai, G.; Vaughn, S.; Zhang, Y.; Wang, E.; Garcia-Cardena, G.; Gimbrone, M. Biomechanical forces in atherosclerosis-resistant vascular regions regulate endothelial redox balance via phosphoinositol 3-kinase/Akt-dependent activation of Nrf2. Circ. Res. 2007, 101, 723–733. [Google Scholar] [CrossRef] [PubMed]
- Farhat, F.; Nofal, S.; Raafat, E.; Eissa Ahmed, A. Akt/GSK3β/Nrf2/HO-1 pathway activation by flurbiprofen protects the hippocampal neurons in a rat model of glutamate excitotoxicity. Neuropharmacology 2021, 196, 108654. [Google Scholar] [CrossRef] [PubMed]
- Kaspar, J.; Jaiswal, A. Tyrosine phosphorylation controls nuclear export of Fyn, allowing Nrf2 activation of cytoprotective gene expression. FASEB J. 2011, 25, 1076–1087. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, H.; Wu, W.; Shi, W.; Hu, S.; Yin, T.; Ma, Q.; Han, T.; Zhang, Y.; Tian, F.; et al. Liraglutide protects cardiac microvascular endothelial cells against hypoxia/reoxygenation injury through the suppression of the SR-Ca(2+)-XO-ROS axis via activation of the GLP-1R/PI3K/Akt/survivin pathways. Free Radical Bio. Med. 2016, 95, 278–292. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Ji, Y.; Jiang, Y.; Zhou, Y.; Xu, Y.; Li, Y.; Jiang, W.; Meng, P.; Liu, X. Liraglutide attenuates high glucose-induced abnormal cell migration, proliferation, and apoptosis of vascular smooth muscle cells by activating the GLP-1 receptor, and inhibiting ERK1/2 and PI3K/Akt signaling pathways. Cardiovasc. Diabetol. 2015, 14, 18. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, X.; Su, Y.; Fan, X.; Chen, H.; Lu, Z.; Liu, Z.; Li, Y.; Yi, M.; Zhang, G.; Gu, C.; et al. Liraglutide Improves the Angiogenic Capability of EPC and Promotes Ischemic Angiogenesis in Mice under Diabetic Conditions through an Nrf2-Dependent Mechanism. Cells 2022, 11, 3821. https://doi.org/10.3390/cells11233821
Yan X, Su Y, Fan X, Chen H, Lu Z, Liu Z, Li Y, Yi M, Zhang G, Gu C, et al. Liraglutide Improves the Angiogenic Capability of EPC and Promotes Ischemic Angiogenesis in Mice under Diabetic Conditions through an Nrf2-Dependent Mechanism. Cells. 2022; 11(23):3821. https://doi.org/10.3390/cells11233821
Chicago/Turabian StyleYan, Xiaoqing, Yue Su, Xia Fan, Hui Chen, Zixian Lu, Zijuan Liu, Yingjian Li, Mei Yi, Guigui Zhang, Chunjie Gu, and et al. 2022. "Liraglutide Improves the Angiogenic Capability of EPC and Promotes Ischemic Angiogenesis in Mice under Diabetic Conditions through an Nrf2-Dependent Mechanism" Cells 11, no. 23: 3821. https://doi.org/10.3390/cells11233821
APA StyleYan, X., Su, Y., Fan, X., Chen, H., Lu, Z., Liu, Z., Li, Y., Yi, M., Zhang, G., Gu, C., Wang, K., Wu, J., Sun, D., Zhang, Y., Zhang, C., Dai, X., & Zheng, C. (2022). Liraglutide Improves the Angiogenic Capability of EPC and Promotes Ischemic Angiogenesis in Mice under Diabetic Conditions through an Nrf2-Dependent Mechanism. Cells, 11(23), 3821. https://doi.org/10.3390/cells11233821






