IL13 Promoter (−1055) Polymorphism Associated with Leukocyte Mitochondria DNA Copy Number in Chronic Obstructive Pulmonary Disease
Abstract
1. Introduction
2. Materials and Methods
2.1. Design
2.2. Study Participants
2.3. Genotyping of IL13 Sequencing
2.4. MtDNA-CN of Peripheral Blood Leukocyte
2.5. Ethics
2.6. Statistical analysis
3. Results
3.1. Patients’ Characteristics
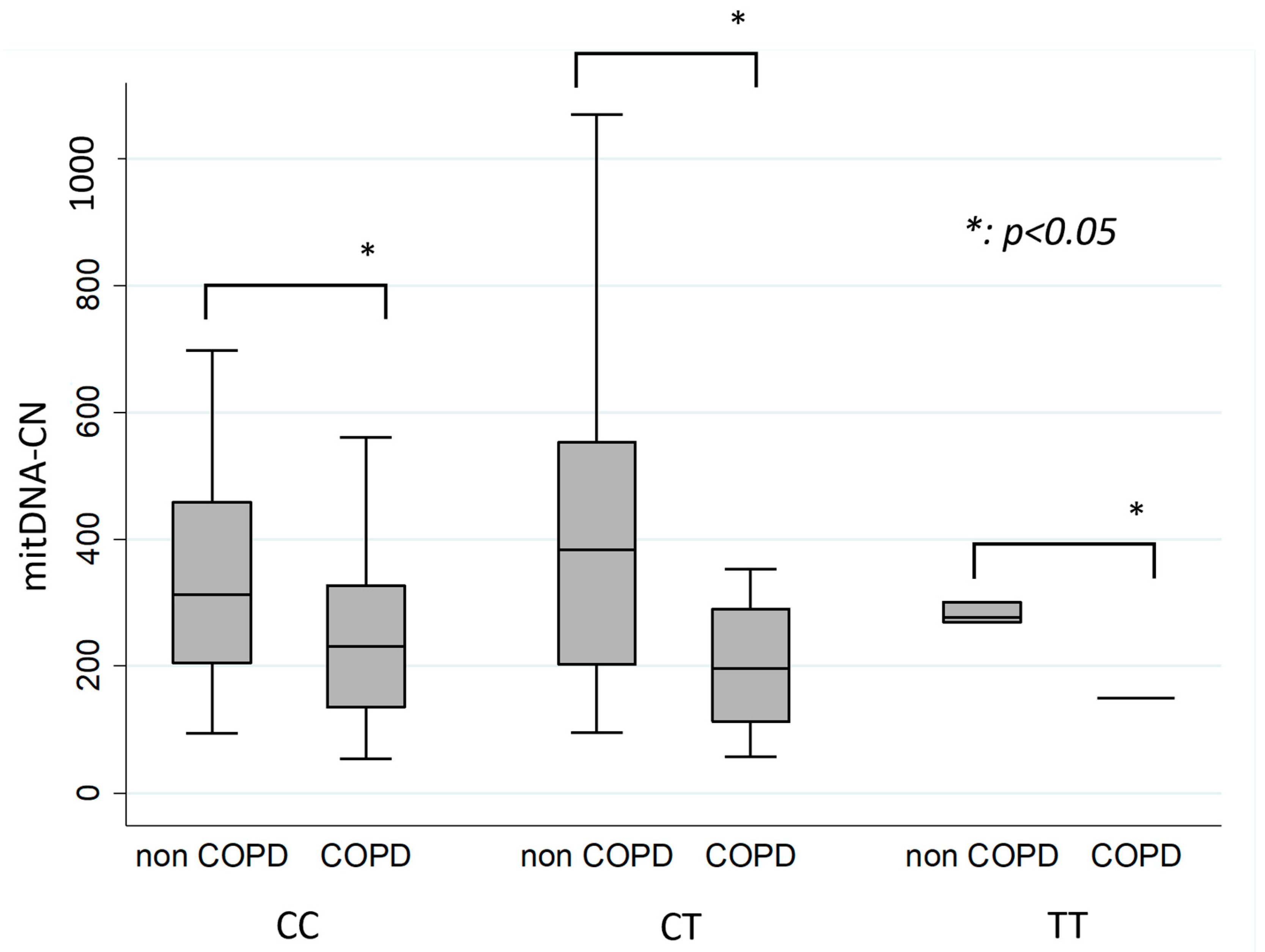
3.2. Comparison of mtDNA-CN in Individual IL 13 Genotype (CC, CT, TT) in COPD and Non-COPD Groups
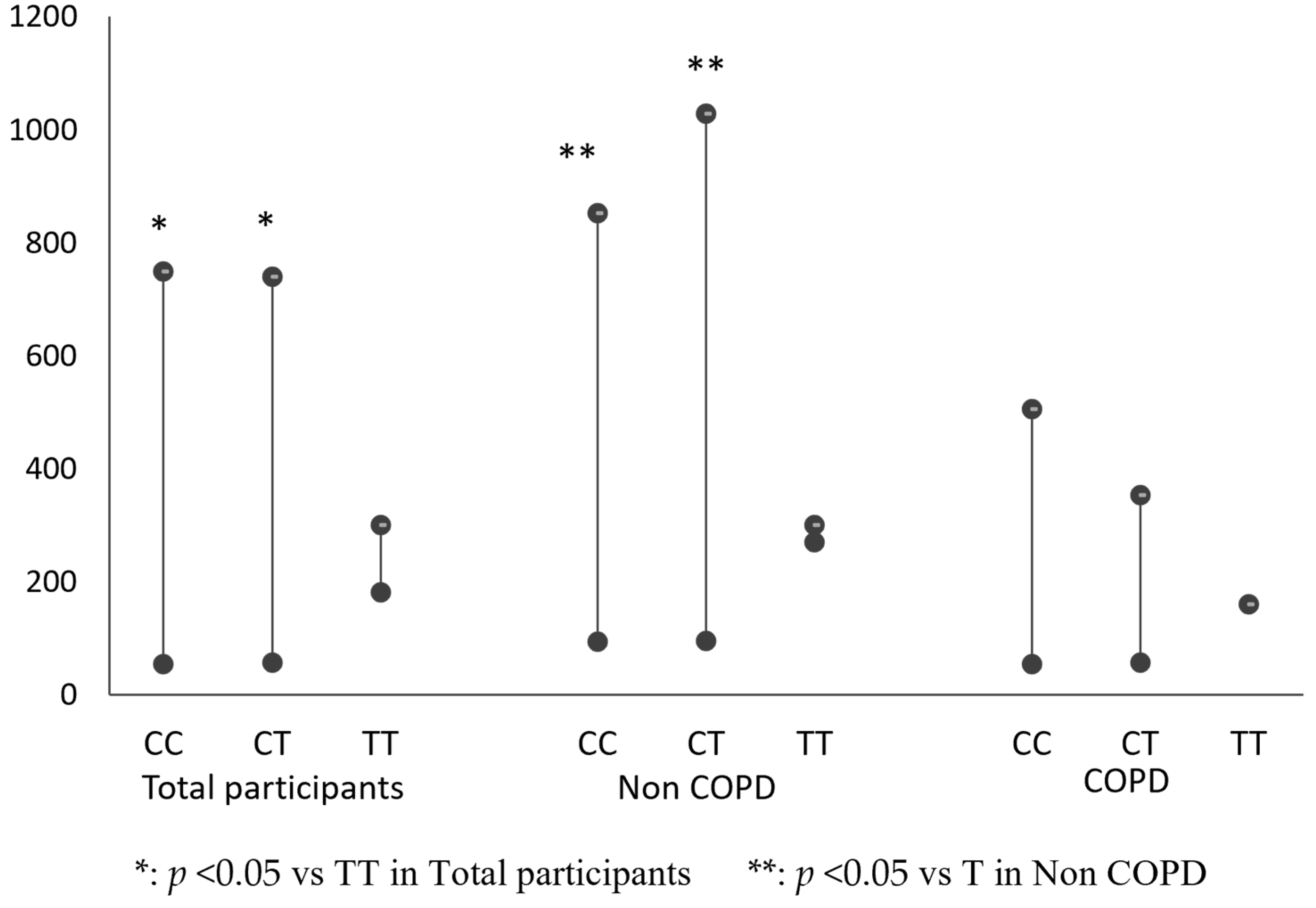
3.3. Comparison of Mitochondrial DNA Copy Number among IL 13 Genotypes (CC, CT, TT) in COPD Group, Non-COPD Group or Total Participants
4. Discussion
4.1. COPD Is Associated with Mitochondrial Dysfunction
4.2. Association between IL13 and COPD
4.3. Association between IL13 and Mitochondrial Regulation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| COPD | Chronic Obstructive Pulmonary Disease |
| GOLD | Global Initiative for Chronic Obstructive Lung Disease |
| FEV1 | Forced expiratory volume during the first second |
| FVC | Forced Vital Capacity |
References
- 2021 Global Strategy for Prevention, Diagnosis and Management of COPD. Available online: https://goldcopd.org/ (accessed on 20 July 2022).
- Centers for Disease Control and Prevention. Health Effects of Cigarette Smoking. Available online: https://www.cdc.gov/tobacco/data_statistics/fact_sheets/health_effects/effects_cig_smoking/index.htm (accessed on 20 July 2022).
- Chen, L.; Shen, Y.; Liu, L.; Li, X.; Wang, T.; Wen, F. Interleukin-13 -1112 C/T promoter polymorphism confers risk for COPD: A meta-analysis. PLoS ONE 2013, 8, e68222. [Google Scholar] [CrossRef] [PubMed][Green Version]
- van der Pouw Kraan, T.C.; Küçükaycan, M.; Bakker, A.M.; Baggen, J.M.; van der Zee, J.S.; Dentener, M.A.; Wouters, E.F.; Verweij, C.L. Chronic obstructive pulmonary disease is associated with the -1055 IL-13 promoter polymorphism. Genes Immun. 2002, 3, 436–439. [Google Scholar] [CrossRef] [PubMed]
- Meyers, D.A.; Larj, M.J.; Lange, L. Genetics of asthma and COPD. Similar results for different phenotypes. Chest 2014, 126, 105S–110S, 159S–161S. [Google Scholar] [CrossRef] [PubMed]
- Liao, N.; Zhao, H.; Chen, M.L.; Xie, Z.F. Association of the IL-13 polymorphisms rs1800925 and rs20541 with chronic obstructive pulmonary disease risk: An updated meta-analysis. Medicine 2017, 96, e8556. [Google Scholar] [CrossRef]
- Ahmadi, A.; Ghaedi, H.; Salimian, J.; Azimzadeh Jamalkandi, S.; Ghanei, M. Association between chronic obstructive pulmonary disease and interleukins gene variants: A systematic review and meta-analysis. Cytokine 2019, 117, 65–71. [Google Scholar] [CrossRef]
- Ohar, J.A.; Bleecker, E.R.; Howard, T.D. IL-13 polymorphism: Predisposition towards COPD and asbestos induced diseases. Eur. Respir. J. 2001, 18, P3588. [Google Scholar]
- Hegab, A.E.; Sakamoto, T.; Saitoh, W.; Massoud, H.H.; Massoud, H.M.; Hassanein, K.M.; Sekizawa, K. Polymorphisms of IL4, IL13, and ADRB2 genes in COPD. Chest 2004, 126, 1832–1839. [Google Scholar] [CrossRef]
- Liu, S.F.; Chen, Y.C.; Wang, C.C.; Fang, W.F.; Chin, C.H.; Su, M.C.; Lin, M.C. Il13 promoter (-1055) polymorphisms associated with chronic obstructive pulmonary disease in Taiwanese. Exp. Lung Res. 2009, 35, 807–816. [Google Scholar] [CrossRef]
- Barnes, P.J.; Baker, J.; Donnelly, L.E. Autophagy in asthma and chronic obstructive pulmonary disease. Clin. Sci. 2022, 136, 733–746. [Google Scholar] [CrossRef]
- Liu, S.F.; Kuo, H.C.; Tseng, C.W.; Huang, H.T.; Chen, Y.C.; Tseng, C.C.; Lin, M.C. Leukocyte Mitochondrial DNA Copy Number Is Associated with Chronic Obstructive Pulmonary Disease. PLoS ONE 2015, 10, e0138716. [Google Scholar] [CrossRef]
- Guo, X.; Hong, T.; Zhang, S.; Wei, Y.; Jin, H.; Miao, Q.; Wang, K.; Zhou, M.; Wang, C.; He, B. IL-13 Alleviates Cardiomyocyte Apoptosis by Improving Fatty Acid Oxidation in Mitochondria. Front. Cell Dev. Biol. 2021, 9, 736603. [Google Scholar] [CrossRef]
- Zhu, M.; Min, S.; Mao, X.; Zhou, Y.; Zhang, Y.; Li, W.; Li, L.; Wu, L.; Cong, X.; Yu, G. Interleukin-13 promotes cellular senescence through inducing mitochondrial dysfunction in IgG4-related sialadenitis. Int. J. Oral Sci. 2020, 14, 29. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, N.H.; Stanya, K.J.; Hyde, A.L.; Chalom, M.M.; Alexander, R.K.; Liou, Y.H.; Starost, K.A.; Gangl, M.R.; Jacobi, D.; Liu, S.; et al. Interleukin-13 drives metabolic conditioning of muscle to endurance exercise. Science 2020, 368, eaat3987. [Google Scholar] [CrossRef]
- Kuo, C.L.; Chou, H.Y.; Chiu, Y.C.; Cheng, A.N.; Fan, C.C.; Chang, Y.N.; Chen, C.H.; Jiang, S.S.; Chen, N.J.; Lee, A.Y. Mitochondrial oxidative stress by Lon-PYCR1 maintains an immunosuppressive tumor microenvironment that promotes cancer progression and metastasis. Cancer Lett. 2020, 474, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Dong, M.; Teng, F.; Cui, J.; Zhu, X.; Wang, W.; Wuniqiemu, T.; Qin, J.; Yi, L.; Wang, S.; et al. Environmental allergens house dust mite-induced asthma is associated with ferroptosis in the lungs. Exp. Ther. Med. 2021, 22, 1483. [Google Scholar] [CrossRef]
- Zhan, D.; Tanavalee, A.; Tantavisut, S.; Ngarmukos, S.; Edwards, S.W.; Honsawek, S. Relationships between blood leukocyte mitochondrial DNA copy number and inflammatory cytokines in knee osteoarthritis. J. Zhejiang Univ. Sci. B 2020, 21, 42–52. [Google Scholar] [CrossRef]
- Doyle, A.D.; Mukherjee, M.; LeSuer, W.E.; Bittner, T.B.; Pasha, S.M.; Frere, J.J.; Neely, J.L.; Kloeber, J.A.; Shim, K.P.; Ochkur, S.I.; et al. Eosinophil-derived IL-13 promotes emphysema. Eur. Respir. J. 2019, 53, 1801291. [Google Scholar] [CrossRef] [PubMed]
- Obling, N.; Backer, V.; Hurst, J.R.; Bodtger, U. Nasal and systemic inflammation in Chronic Obstructive Pulmonary Disease (COPD). Respir. Med. 2022, 195, 106774. [Google Scholar] [CrossRef] [PubMed]
- Balaban, R.S.; Nemoto, S.; Finkel, T. Mitochondria, oxidants, and aging. Cell 2005, 120, 483–495. [Google Scholar] [CrossRef]
- Sena, L.A.; Chandel, N.S. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell 2012, 48, 158–167. [Google Scholar] [CrossRef]
- Cross, C.E.; Halliwell, B.; Borish, E.T.; Pryor, W.A.; Ames, B.N.; Saul, R.L.; McCord, J.M.; Harman, D. Oxygen radicals and human disease. Ann. Intern. Med. 1987, 107, 526–545. [Google Scholar] [CrossRef] [PubMed]
- Barnes, P.J. Oxidative Stress in Chronic Obstructive Pulmonary Disease. Antioxidants 2022, 11, 965. [Google Scholar] [CrossRef] [PubMed]
- Park, K.W.; Baik, H.H.; Jin, B.K. IL-13-induced oxidative stress via microglial NADPH oxidase contributes to death of hippocampal neurons in vivo. J. Immunol. 2009, l183, 4666–4674. [Google Scholar] [CrossRef] [PubMed]
- Ng Kee Kwong, F.; Nicholson, A.G.; Harrison, C.L.; Hansbro, P.M.; Adcock, I.M.; Chung, K.F. Is mitochondrial dysfunction a driving mechanism linking COPD to nonsmall cell lung carcinoma? Eur. Respir. Rev. 2017, 26, 170040. [Google Scholar] [CrossRef]

| Non COPD (n = 117) | COPD (n = 99) | p Value | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| Age | 55.50 (9.67) | 69.34 (11.043) | <0.001 |
| Gender (male, %) | 87 | 96 | 0.023 |
| Pack-years | 36.75 (30.59) | 50.94 (31.19) | 0.014 |
| BMI | 24.43 (3.33) | 23.67 (4.01) | 0.140 |
| FVC | 89.95 (11.63) | 80.80 (12.97) | <0.001 |
| FEV1 | 93.91 (1.35) | 56.82 (19.93) | <0.001 |
| FEV1/FVC | 83.82 (7.78) | 53.17 (11.29) | <0.001 |
| mMRC | 1.38 | 2.14 | 0.003 |
| 6MWD | 520.17 (134.14) | 437.28 (109.91) | 0.018 |
| mtDNA-CN | 444.03 (433.08) | 250.34 (199.75) | <0.001 |
| Non COPD (n = 117) | COPD (n = 99) | ||||
|---|---|---|---|---|---|
| IL13 Genotypes | N | mitDNA Copy Number Mean ± SD | N. | mitDNA Copy Number Mean ± SD | p Value |
| Total | 117 | 444.03 ± 433.08 | 99 | 250.34 ± 199.75 | 0.000 |
| CC | 92 | 451.40 ± 455.35 | 71 | 260.10 ± 208.32 | 0.0038 |
| CT | 21 | 533.57 ± 494.72 | 27 | 230.48 ± 174.37 | 0.0111 |
| TT | 4 | 282.79 ± 16.29 | 1 | 149.85 ± 0 | 0.0194 |
| CC N(%) | mtDNA-CN Mean ± SD | CT N(%) | mtDNA-CN Mean ± SD | TT N(%) | mtDNA-CN Mean ± SD | p Value | |
|---|---|---|---|---|---|---|---|
| COPD | 71(71.6%) | 260.10 ± 208.32 | 27(27.2%) | 230.48 ± 174.37 | 1 (1.2%) | 149.85 ± 0.00 | 0.343 |
| Non-COPD | 92(78.7%) | 448.37 ± 457.69 | 21(18.1%) | 533.57 ± 494.72 | 4(3.2%) | 282.79 ± 16.29 | 0.005 |
| Total participants | 163(75.6%) | 368.80 ± 380.42 | 48(22.2%) | 362.59 ± 378.20 | 5(2.3%) | 249.56 ± 67.79 | 0.030 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.-F.; Chang, H.-C.; Chang, Y.-P.; Kuo, H.-C.; Tsai, Y.-C. IL13 Promoter (−1055) Polymorphism Associated with Leukocyte Mitochondria DNA Copy Number in Chronic Obstructive Pulmonary Disease. Cells 2022, 11, 3787. https://doi.org/10.3390/cells11233787
Liu S-F, Chang H-C, Chang Y-P, Kuo H-C, Tsai Y-C. IL13 Promoter (−1055) Polymorphism Associated with Leukocyte Mitochondria DNA Copy Number in Chronic Obstructive Pulmonary Disease. Cells. 2022; 11(23):3787. https://doi.org/10.3390/cells11233787
Chicago/Turabian StyleLiu, Shih-Feng, Hui-Chuan Chang, Yu-Ping Chang, Ho-Chang Kuo, and Yuh-Chyn Tsai. 2022. "IL13 Promoter (−1055) Polymorphism Associated with Leukocyte Mitochondria DNA Copy Number in Chronic Obstructive Pulmonary Disease" Cells 11, no. 23: 3787. https://doi.org/10.3390/cells11233787
APA StyleLiu, S.-F., Chang, H.-C., Chang, Y.-P., Kuo, H.-C., & Tsai, Y.-C. (2022). IL13 Promoter (−1055) Polymorphism Associated with Leukocyte Mitochondria DNA Copy Number in Chronic Obstructive Pulmonary Disease. Cells, 11(23), 3787. https://doi.org/10.3390/cells11233787








