Abstract
Colorectal tumorigenesis is driven by alterations in genes and proteins responsible for cancer initiation, progression, and invasion. This multistage process is based on a dense network of protein–protein interactions (PPIs) that become dysregulated as a result of changes in various cell signaling effectors. PPIs in signaling and regulatory networks are known to be mediated by short linear motifs (SLiMs), which are conserved contiguous regions of 3–10 amino acids within interacting protein domains. SLiMs are the minimum sequences required for modulating cellular PPI networks. Thus, several in silico approaches have been developed to predict and analyze SLiM-mediated PPIs. In this review, we focus on emerging evidence supporting a crucial role for SLiMs in driver pathways that are disrupted in colorectal cancer (CRC) tumorigenesis and related PPI network alterations. As a result, SLiMs, along with short peptides, are attracting the interest of researchers to devise small molecules amenable to be used as novel anti-CRC targeted therapies. Overall, the characterization of SLiMs mediating crucial PPIs in CRC may foster the development of more specific combined pharmacological approaches.
1. Introduction
Colorectal cancer (CRC) is mainly caused by abnormal proliferation of glandular epithelial cells in the colon. CRC is classified into three types: sporadic, hereditary, and colitis-associated [1]. About 65% of patients with CRC have no family history or increased risk of germline mutations and develop the disease due to acquired somatic genomic and/or epigenetic changes [2,3]. The remaining cases of CRC are linked to hereditary factors such as family history (25%), hereditary cancer syndromes (5%), low-penetration genetic variants in some known CRC genes (1%), and other unknown inherited genomic abnormalities [2,4]. Based on current cancer epidemiology, CRC is the third-most commonly diagnosed and the second-most deadly cancer in the world [3]. In 2020, 1.93 million cases of CRC were recorded worldwide, and CRC accounted for 9.4% of all cancer-related fatalities (WHO, https://www.who.int/news-room/fact-sheets/detail/cancer; accessed on 19 July 2022). Moreover, it is predicted that the global incidence of CRC will more than quadruple by 2035, with the greatest increase occurring in less developed countries due to the growing number of cases detected in the older population [1,5].
From a molecular perspective, CRC initiation and progression are complex multistage processes involving a dense network of protein–protein interactions (PPIs). These PPIs mediate cell-signaling cascades that are deregulated as a result of CRC-related alterations [6].
2. Short Linear Motifs and Their Emerging Role in Cell Biology and Cancer
It has long been believed that PPIs were mediated by large, structured domains [7,8]. Nonetheless, it is presently evident that a wide variety of protein motifs showing different levels of flexibility are involved in these interactions. Indeed, binding interfaces range from rigid globular domains to disordered regions intrinsically lacking structure [9]. Since organism complexity seems to correlate with a higher degree of disorder in major hub proteins, disordered regions are currently believed to play a functionally relevant role in cell biology [9].
PPIs involved in signaling and regulatory networks are now known to be mediated by an important subclass of disordered interfaces termed short linear motifs (SLiMs). SLiMs are typically conserved, contiguous sequences consisting of about 3–10 amino acids within interacting protein regions [10,11,12]. These motifs have been extensively investigated in the immunological field to define the microbial/host cross-talk underlying immunosurveillance against infectious diseases and some cancer-related oncovirus infections [13,14]. However, SLiMs have aroused growing interest in cancer research in the last decade because they modulate crucial PPI networks involved in the development and progression of many tumors [15]. The SLiMs identified in this field show more variable length and homology sequence levels than those previously characterized, a difference that has been the subject of controversial debate [16]. In addition to being PPI mediators, SLiMs can also function as sites for post-translational modifications, determinants of sub-cellular localization, and targets for proteolytic cleavage [12].
SLiMs are the minimum sequence determinants that can finely modulate cellular PPI networks independently of the wider sequence/structure context where they function [11]. Frequently, SLiMs correspond to consensus sequence patterns located in the disordered regions of proteins, where they mediate transient and low-affinity interactions with various interactors due to their structural flexibility [17,18]. This makes them ideal to mediate dynamic processes such as cell signaling. Consequently, SLiMs play a significant role in determining the spatio-temporal behavior of protein interaction networks [7,19]. In certain instances, a single linear motif is sufficient to mediate an interaction; nonetheless, cooperation between multiple motifs is typically necessary. Cooperation does not require physical contact between the linear motifs and usually depends on two (or more) moderate affinity contacts that synergize to produce a greater effective affinity in the interaction [11]. A significant challenge in SLiM characterization is that, despite their stability in various proteins, SLiMs can show multiple three-dimensional structures [20,21]. In native proteins, SLiMs are intertwined and overlapped to act as functional units. This variety of structural conformations for a single sequence explains why the same unit is sometimes observed in numerous unrelated non-homologous proteins. As a result, proteins may have similar functions despite showing widely different amino acid sequences and three-dimensional structures [21]. On the other hand, the majority of protein domains maintain distinct patterns of amino acid conservation, indicating that they can bind SLiMs with high intrinsic specificity, thereby modulating PPIs [22].
From a molecular point of view, cancer-causing mutations are typically thought to affect protein functionality due to the disruption of their folded globular structure. Nevertheless, 22% of human disease mutations occur in intrinsically disordered regions (IDRs) of proteins [23]. Therefore, alteration of IDR structures is implicated in numerous human diseases, including cancer. The functional role of IDRs is mainly attributable to SLiMs, but the contribution of SLiM defects to cancer pathogenesis remains unclear [11,23]. An interesting computational study including a proteome-wide comparison of the distribution of missense mutations from human disease and nondisease datasets revealed that in IDRs, aberrant mutations are more frequent within SLiM sites [23]. In addition, pathological mutations often affect functionally crucial residues within SLiMs, altering their biochemical properties and interfering with their physical interactions [23]. The analysis of these mutations yielded an exhaustive list of experimentally validated or predicted SLiMs that are disrupted in disease [23]. Compared to mutations in globular domains, the contribution of SLiM mutations to cancer disease may appear to be minor at this time. Nonetheless, emerging data on mutations in predicted SLiMs suggest that this contribution may have been underestimated [23]. In particular, growing findings indicate that a greater emphasis on SLiMs in the coming decades will improve our understanding of cancer-related networks and could be very useful for the development of targeted antitumor treatments [11,23]. Cancer is primarily caused by alterations in cell signaling pathways. Such alterations can occur in IDRs and may disrupt SLiMs, thereby contributing to carcinogenesis due to protein misrecognition or cell-signaling deregulation [23,24].
For instance, several studies have shown that SLiMs play an important role in the activity of BRCA1, a critical tumor suppressor regulating cell cycle progression and DNA-damage-induced checkpoint activation. Two regions (1648–1723; 1756–1842) of the BRCA1 C-terminus (BRCT) domain interact with the 989TSPTF993 BRIP1 (Fanconi Anemia Group J Protein) motif. Alterations in these SLiMs that mediate BRCA1–BRIP1 interaction have been associated with various cancer types, including breast cancer (BC), ovarian cancer, and pancreatic cancer [25,26,27,28,29].
Emerging evidence also suggests that specific SLiMs derived from or encompassing the alpha-fetoprotein (AFP), a well-known embryo-specific and cancer-related protein, may have antitumor effects in several types of cancer (BC, prostatic cancer, and hepatocellular carcinoma) [30,31,32]. In particular, the AFP C-terminal SLiM E489MTPVNPGV497 can inhibit mouse uterine cell proliferation and has shown anticancer effects in MCF-7 breast cancer cells [30]. In addition, local alignment analysis revealed high similarity between this AFP C-terminal SLiM and AXIN 2, a protein involved in the WNT pathway, which is deregulated in CRC and hepatocellular carcinoma [21,32]. In addition, the N-terminal heptapeptide of AFP (AFP14-20) interacts with the consensus motif (CxxGY/FxGx) of EGF family proteins, including the well-known cancer-related factor EGF [33].
Interestingly, MDMX-mediated pharmacological inhibition of the tumor suppressor P53 is also dependent on a SLiM-related mimicry effect [34]. MDMX, a p53 inhibitor, is regulated by multiple stress signaling pathways. Chen et al. identified an MDMX intramolecular interaction that mimics the interaction with p53, resulting in MDMX autoinhibition. This mechanism involves a hydrophobic peptide located in a long MDMX disordered segment, whose sequence is similar to the p53 transactivation domain [34]. Furthermore, a recent study based on NMR spectroscopy analysis demonstrated that autoinhibition of P53 binding to MDMX requires two SLiMs containing adjacent tryptophan and tryptophan–phenylalanine residues. These SLiMs directly compete for the p53 binding site on MDMX [34,35].
Overall, these data highlight the emerging biological relevance of SLiMs in modulating driver interactions in tumorigenesis and tumor progression, which is further corroborated by the number of different studies and in silico methodologies that have been developed to define a cancer-specific and SLiM-based molecular signature suitable for precision medicine approaches [23,36].
3. In Silico Methods to Study PPI Networks
A thorough understanding of PPI networks is crucial for a systems-level interpretation of the vast quantities of molecular data that are made available to researchers. Experimental detection of protein interactions is complicated by a wide variety of binding affinities, and different assays have varying strengths and weaknesses in overcoming these challenges [35].
For instance, based on the evidence that physical interaction between two proteins requires complementary three-dimensional structures, and considering that it is crucial to identify in which conformation a certain interaction can occur, Halakou et al. described a step-by-step method for incorporating the various conformations of proteins into each PPI. In particular, they showed how a PPI network can be refined by docking alternative three-dimensional conformations of each protein participating in binary interactions, thereby unifying the network view and structural perspective of the involved interactions [37].
Computational methods are a powerful tool to bridge the gaps in the experimental approaches used to investigate PPIs. In particular, they can help transfer our understanding of complex interactions from one species to another, integrate our knowledge scattered across databases covering different types of interactions, provide functional insights into the discoveries made from the analysis of omics datasets, and assemble individual interactions into higher-order functional units, i.e., protein complexes and signaling pathways [35].
In this scenario, several in silico strategies using indirect evidence or based on the ability to learn from existing interaction data gathered by experimental techniques, such as two-hybrid and affinity purification coupled with mass spectrometry, have been devised to build screening tools allowing researchers to predict and/or identify PPIs [36,37,38,39]. These tools are useful for a comprehensive interpretation of the enormous amounts of molecular data produced by high-throughput technologies. Current in silico platforms have been developed based on different biological criteria of proteins, such as similarity of amino acid sequences, evolutionary homology profiling, and comparative analysis of protein domain annotations to infer PPIs from shared functional units and similar domain composition, etc.
The above-described strategies provide a huge amount of data and information on PPIs in different species, which are annotated in specific databases. As a result, our current understanding of PPIs is fragmented across multiple sets of data that cover a variety of interaction types across multiple species. The ability to systematically query the databases of interest is crucial for the incorporation of PPI data into effective downstream analysis techniques. Various tools efficiently address this challenge.
The interpretation of high-throughput molecular profiling datasets can be supported by PPI networks. A collection of differentially expressed genes found by RNA-seq, for instance, can have their function clarified by finding enriched biological pathways or by examining their relationships in a larger network context [38,39].
Table 1 below lists various algorithms, servers, and databases that may be useful to researchers to predict in silico novel PPIs and analyze relevant networks to gain insight into their biological functions.

Table 1.
List of relevant in silico tools used to predict and analyze PPIs.
As indicated in Table 1, each tool has distinctive technical purposes that enable a deeper understanding of specific aspects of PPI analysis (e.g., identification of the motifs that mediate binary interactions, three-dimensional structures of binary complexes, evolutionary conservation of PPI motifs, tissue specificity, and particular pathological conditions). Overall, the multitude of available tools highlights the complexity of PPI analysis and the increasing efforts of the scientific community and bioinformaticians toward the development of in silico open-source resources, allowing researchers to study PPI networks in different species and varying pathophysiological contexts.
4. In Silico Approaches and Tools to Characterize SLiMs in PPI Networks
As indicated above, SLiMs are short, conserved stretches of amino acids that mediate PPIs. SLiMs are difficult to identify experimentally due to their extremely small size and poor folding. Based on SLiM-recognition domains, numerous computational and bioinformatics tools have been created to analyze PPI networks, especially focusing on cancer-related pathways [60]. In the last decade, several in silico algorithms able to extract SLiMs from interaction data have been developed. Surprisingly, emerging evidence suggests that these regions mediate a greater proportion of interactions than was previously anticipated. Moreover, it has been shown that SLiM-mediated interactions can be pharmacologically targeted with specific compounds, which may be used to interfere with or disrupt cancer-related PPI networks [61]. Therefore, several bioinformatics databases and tools have been developed to better characterize and predict the SLiMs involved in PPI networks. A selection of these resources is described in Table 2.

Table 2.
List of relevant in silico tools used to predict and analyze SLiMs involved in PPI networks.
The various in silico resources listed in Table 2 are valuable tools for researchers to gain insight into SLiMs involved in PPIs, but they also have limitations. Since SLiMs are very short peptides, they can be nonspecifically selected by algorithms. Moreover, their limited sequence conservation and loosely folded nature can make detection difficult. Reduced SLiM length also increases the probability of stochastic occurrence of short motifs; hence, the use of pattern matching alone generates a large number of false positive hits [8]. Therefore, methods have been developed to incorporate additional filters based on SLiM characteristics, such as sequence conservation [66,67], structural availability [9,68,69], biophysical feasibility [70], and biological keywords [71].
5. SLiMs in CRC Molecular Networks
CRC tumorigenesis is initiated by spontaneous mutations, environmental mutagens, and genetic or epigenetic alterations that trigger the transformation of normal colorectal epithelial cells into tumor cells. Mutations in crucial factors, such as APC and WNT-β catenin (CTNB1) pathway effectors, along with cytokines, chemokines, and growth factors from the tumor microenvironment, cause the hyperproliferation of initiated cells and the subsequent formation of an aberrant crypt and adenoma [6]. Next, the progression of these cells into late adenoma and colorectal adenocarcinoma is caused by mutations in other factors, such as transforming growth factor beta (TGF-β), cell division control protein 4 (CDC4), and SMAD family member 4 (SMAD4) [72]. Lastly, additional mutations targeting major tumor suppressors such as P53 and BAX, anti-apoptotic factors such as BCL-2, pro-angiogenic factors, and extracellular matrix-degrading factors promote CRC cell motility, enabling invasion and metastatization of distant organs [6,73,74].
5.1. SLiMs in CRC Signaling Pathways and Tumorigenesis
A few functionally relevant SLiMs have been identified in proteins playing a role in major signaling cascades involved in CRC onset and progression. These include the Wnt/CTNB1, the EGFR/MAPK, and the BCL-2 pathways [35,49,50,57,58].
Wnt signaling hyperactivation promotes tumor cell proliferation and is required for tumor growth [73]. After being secreted and accumulating as a result of Wnt signaling, Wnt ligands bind to Frizzled (Fz) receptors [75]. This leads to the inactivation of the multifunctional glycogen synthase kinase 3β (GSK3β) and to the stabilization, accumulation, and nuclear translocation of CTNB1, which couples with the lymphoid enhancer factor (LEF) or T-cell transcription factor (TCF) and activates specific target genes involved in proliferation and signal transmission [75,76]. Conversely, in the absence of Wnt signaling, CTNB1 is phosphorylated and targeted for ubiquitination and proteasomal degradation by casein kinase 1 (CK1), the APC core proteins, and the axin-GSK3β complex [77].
The proto-oncogene C/EBP homologous protein (CHOP) is a dominant-negative inhibitor of C/EBPs, and its disordered N-terminal region contains crucial SLiMs that are essential for CHOP oligomerization, interactions, and biological activity [78]. In particular, Singh and collaborators described a novel mechanism of CHOP-mediated inhibition of Wnt/TCF signaling and activation of the c-JUN oncogene in HT-29 and DLD-1 CRC cells. This mechanism directly involves the disordered SLiM-containing N-terminal region of CHOP [78]. Further studies will be necessary to identify the exact SLiM sequences involved in CHOP-mediated regulation of Wnt and c-JUN pathways.
Conversely, the CTNB1 SLiM D32SGIHS37 has been well characterized and is therefore reported in the ELM database (ELM ID: ELMI001302). This motif is directly involved in the aberrant proteasomal degradation of CTNB1 [15,79]. In particular, CTNB1 activates the transcription of Wnt-responsive target genes such as cytochrome C oxidase subunit 2 (COX2), matrix metalloproteinase-7 (MMP7), G1/S-specific cyclin D1 (CCND1), and others [80]. CTNB1 proteasomal degradation begins with GSK3β-mediated phosphorylation of CTNB1 residues S33, S37 (which are part of the above-cited SLiM), and T41. Upon phosphorylation, CTNB1 binds to the E3 ubiquitin ligase β-TrCP through the D32SGIHS37 SLiM and is routed to proteasomal degradation [79,81]. Point mutations of this crucial SLiM at positions D32, S33, and G34 prevent CTNB1 degradation, promoting its translocation into the nucleus where it functions as a co-activator of Wnt-responsive target genes together with TCF/LEF transcription factors [82].
The ELM database reports other SLiMs in CRC regulatory proteins that are recognized by SCF (Skp, Cullin, F-box) complexes via repeat domains of associated F-box proteins (FBPs) and are therefore routed to subsequent ubiquitin-mediated degradation [15]. For example, the F-box protein family member FBW7 is a ubiquitin ligase that ubiquitinylates various oncoproteins (including G1/S-specific cyclin-E1, MYC, c-JUN, and NOTCH), directing them to proteasomal degradation [83]. The activity of FBW7 is finely regulated by double phosphorylation at conserved TPxxS motifs, which are recognized by various kinases, including cyclin-dependent kinases (CDKs) and GSK3β. GSK3β-mediated phosphorylation links FBW7 activity to the mitogenic signaling pathway [83,84]. CRC is caused by different genomic alterations, such as microsatellite instability, altered CpG islands methylation levels, and more frequently (85% of all cases) chromosomal instability [85]. These alterations can affect the recognition sites of several GSK3β substrates, as detailed in Table 3.

Table 3.
SLiMs found in phosphorylation sites of human GSK3β substrates that can be altered in CRC 1. SLiM sequences that are subject to GSK3β phosphorylation are underlined.
The EGFR/MAPK signaling pathway is also directly related to CRC oncogenic processes and plays a critical role in tumor growth and disease progression [111]. EGFR is a transmembrane tyrosine kinase receptor (RTK) that is activated by the autophosphorylation of several tyrosine residues in its intercellular domain and dimerizes after ligand binding [112]. EGFR–ligand complexes activate the RAS protein, which in turn triggers the MAP kinase cascade and the extracellular signal-regulated kinase (ERK), both these signals being initiated through phosphorylation of specific serine and threonine residues [113,114]. As a result of its involvement in CRC tumorigenesis, this pathway and its downstream signaling cascades have been identified as potential targets for CRC therapeutic strategies [115,116].
The proliferative signal associated with the cascade activation of MAPKs depends specifically on 3 SLiM clusters: two clusters enriched in positively charged amino acids surrounding a central cluster enriched in hydrophobic amino acids. These motifs, consisting of a series of positive–hydrophobic–positive amino acids, represent the modular structure of the docking PPIs involved in MAPK signaling. Therefore, point mutations in these crucial amino acids may alter the activation of MAPK signaling cascade [117].
Apoptosis is a crucial cell-death process that is frequently dysregulated in various malignancies, including CRC [118,119]. Apoptosis is mainly controlled by the BCL-2 protein family, whose members are involved in CRC progression and chemoresistance [74]. Each member of the BCL-2 protein family exhibits homology in one or more of the four BCL-2 homology (BH) domains [74,120]. The anti-apoptotic members BCL-2, BCL-XL, MCL-1, BCL-W, and A1/BFL-1 contain four BH-domains (BH1-BH4). In their tertiary protein structure, the BH1, BH2, and BH3 domains help to create a hydrophobic pocket. The pro-apoptotic members include the BH3-only proteins and the effector proteins. BIM, BAD, BID, PUMA, NOXA, BMF, HRK, and BIK are examples of BH3-only proteins, so termed because they only exhibit homology to the BH3 domain of BCL-2. The effector proteins contain three to four BH domains and comprise BAX and BAK, which can create macropores in the mitochondrial outer membrane, thereby inducing mitoptosis [74].
The BH3 motif has been identified as a relevant SLiM in CRC tumorigenesis [16,74]. It is shared by two major BCL-2 protein subgroups, i.e., BCL-2 homologous proteins, which are evolutionarily related to BCL-2 by common ancestry, and BH3-only proteins, which do not appear to have any evolutionary or structural ties to BCL-2 homologs or to each other [16]. Recent evidence shows that the current models of apoptosis regulation ignore a third, larger group of even more varied BH3-containing proteins [16]. The ambiguous definition of the BH3 motif contributes to the lack of research on the biological effects of this third group and how it affects the BCL-2 protein network. On the other hand, the addition of this third group of proteins complicated the exact definition of the BH3 signature, though the hexameric sequence L-X(3)-G-D is frequently used to refer to this motif [121]. The alignment of the 63 reported BH3 motifs contained in the three subgroups confirms that only two crucial residues (L and D) are conserved among them [16,121,122].
Pathological variants of crucial SLiMs have also been observed in inherited CRC syndromes. In particular, familial adenomatous polyposis (FAP) is an autosomal dominant disorder characterized by a predisposition to colorectal polyposis, which in some cases will evolve into colorectal carcinoma if not surgically treated. A variant of FAP is the Gardner syndrome, in which multiple adenomas of the colon and rectum occur with desmoid tumors and osteomas [95]. This condition can be caused by the point mutation Ser171Ile in the linear motif (Q163NLTKRIDSLPLTE176) of APC nuclear export signal (NES) [96,97].
5.2. SLiMs in CRC Hallmarks: A Case Study
Recent data from our group are consistent with the crucial role played by SLiMs in the PPI network of SET and MYND domain containing 3 (SMYD3) in different cancer study models, including BC, CRC, and other gastrointestinal tumors [123,124]. In the last few years, our laboratory has focused on this methyltransferase, which is overexpressed in many types of human tumors, although its oncogenic role has not been fully understood yet [125]. In particular, we searched for novel SMYD3 interactors involved in cancer-related pathways. To this aim, based on the emerging evidence that tripeptides are the minimum determinants able to mediate PPIs [126,127,128,129], we generated a library of 19 tripeptides (termed P1 to P19) mainly composed of rare amino acids and therefore suitable to serve as valuable minimum PPI-mediating motifs. Indeed, several computational studies confirmed that amino acids occurring less commonly in proteomes (i.e., encoded by 1–3 codons) have a higher biological significance than more frequent amino acids (i.e., encoded by 4–6 codons) in PPI networks [130,131,132]. Thus, we first tested the in vitro binding affinity of tripeptides P1-P19 to SMYD3 and then used them as in silico probes to screen the human proteome in the search for novel SMYD3 interactors [123]. Enrichment of these tripeptides was observed in DNA repair pathway proteins. In particular, this analysis allowed us to identify in silico BRCA2, ATM, and CHK2 as direct SMYD3 interactors. These interactions were subsequently validated in vitro and in cellulo [123]. Furthermore, an in vitro competition assay confirmed the direct involvement of the identified tripeptides in SMYD3 binding to BRCA2 and ATM. Indeed, the purified tripeptides inhibited in a dose-dependent manner the physical interaction between SMYD3 and BRCA2/ATM fragments containing the corresponding tripeptide sequences [123].
Next, to gain insight into novel SMYD3 cancer-related activities, we focused on the whole-proteome distribution of the P1-P19 tripeptides and assessed the biological function of each putative SMYD3 interactor to identify the most important candidates associated with cancer hallmarks [124]. Surprisingly, this computational tripeptide screening of the human proteome allowed us to identify crucial cancer-related proteins such as mTOR, BLM, MET, AMPK, and RBL2 (p130) as novel SMYD3 interactors [124]. Subsequently, these interactions were validated in CRC and gastric cancer cell lines. In particular, the interaction between SMYD3 and AMPK was confirmed in multiple gastrointestinal cancer cell lines (CRC, GC, hepatocellular carcinoma, pancreatic cancer), confirming the role of SMYD3 in the metabolism of gastrointestinal cancer [124].
A significant benefit of this strategy is that our tripeptides can be used to develop pharmacological inhibitors of SMYD3 oncogenic PPIs in order to modify the composition of relevant multiprotein complexes associated with cancer driver proteins. Similar to other approaches devised in the last few years [133,134,135], our strategy provides an appropriate in silico methodology to facilitate the identification of novel interactors and generate small molecules suitable to be used as oncoprotein inhibitors.
This methodology is schematically outlined in Figure 1.
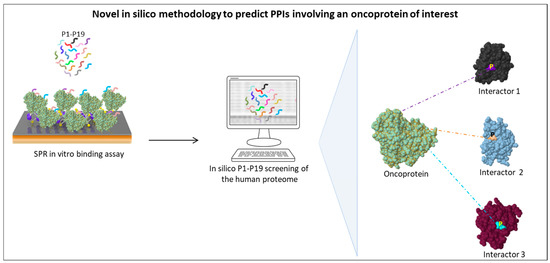
Figure 1.
Schematic representation of a novel in silico methodology developed by our group to search for new interactors of an oncoprotein of interest by taking advantage of a library of SLiMs (tripeptides P1–P19) able to bind it in vitro, as identified by surface plasmon resonance (SPR) analysis [124]. Tripeptides P1–P19 are then used as in silico probes to identify human proteins containing them, which are therefore candidate interactors of the oncoprotein of interest.
5.3. SLiMs in CRC-Related Microbiome
Growing evidence suggests that microbes represent relevant players in CRC tumorigenesis [136]. Thus, the short motifs modulating oncovirus-host PPI networks are an emerging example of SLiMs that may be involved in CRC tumorigenesis and related molecular networks [13,14,137]. Although the mechanisms underlying the interactions between colonocytes and the surrounding environment are not clear yet, the John Cunningham virus (JCV), human papillomavirus (HPV), and Epstein–Barr virus (EBV) have been linked to CRC [13]. Various viruses tend to mimic the SLiMs of host proteins to use cellular processes to their advantage. In particular, they have developed the capacity to interact with elements of the host cell via protein SLiMs that resemble those of the host, which facilitates their internalization and the manipulation of a wide range of cellular networks [137]. This mechanism, known as molecular mimicry, makes these SLiMs potential therapeutic targets [138].
Interestingly, SLiM-based molecular mimicry is investigated as a significant aspect of microbiome-host relationships, which are of crucial relevance in CRC tumorigenesis and inflammatory bowel diseases [139]. In particular, a recent computational study identified six SLiMs that are involved in F. nucleatum–human cross-talk in the context of gastrointestinal diseases [139].
Taken together, these data confirm the biological significance of SLiMs in cancer-related pathways and suggest that they may play a role in PPI networks involved in CRC. In addition, they could be used to devise pharmacological strategies to interfere with the main signaling cascades driving tumorigenesis and cancer progression.
6. Potential Small-Molecule Anticancer Drugs Based on SLiMs and Short Peptides in CRC: Where Do We Stand?
6.1. Pharmacological Suitability of SLiMs and Short Peptides as Anticancer Drugs
The biological relevance of PPIs in key cancer-related processes, including cell growth, proliferation, differentiation, and signal transduction, is corroborated by the evidence that PPIs are altered in various cancer types [140,141]. This provides alternative therapeutic prospects targeting cancer-related PPI networks to disrupt carcinogenesis and cancer progression signaling. Currently, only 1% of all human proteins that are deemed to be druggable have been targeted by small molecules based on SLiMs [142]. Targeting PPIs is more difficult than using conventional drug discovery techniques such as designing small compounds that bind to enzyme active sites.
There are different structural caveats to the pharmacological targeting of PPIs, including their large interfaces, which are frequently flat or shallow. Historically, drug design and discovery have given rise to the belief that PPIs are difficult to target because the compounds should be small enough to enter the cell and also be able to affect the large and often shallow PPI interaction sites [142]. Despite this, several studies demonstrated that protein–protein interfaces or the regions located nearby are often flexible or intrinsically disordered, allowing a small molecule to penetrate these complexes and displace the relevant protein interaction partner [143,144,145,146].
Furthermore, current advances in molecular profiling of tumor samples as a result of high throughput techniques such as NGS and mass spectrometry enable the rapid detection of alterations in genes encoding oncoproteins, providing a large number of potential therapeutic targets and offering opportunities for the design of new drugs. Notably, based on different studies carried out by pharmacological companies and academic groups on SMYD3 cancer-related activity, novel and more efficient SMYD3 inhibitors have been generated [147,148,149].
The development of novel anticancer drugs is complicated by limitations related to the specific chemical characteristics of potential therapeutic molecules and the structural requirements of druggable targets [61]. The chemical qualities required for anticancer drugs are somewhat limited and largely determined by the type of drug administration to the patient [150,151]. In particular, peptide-based drugs have several limitations, especially in the case of short peptides. These limitations range from biological stability against lytic enzymes (such as peptidases) to insufficient pharmacokinetic profiles for oral absorption or other routes of administration [151].
Most drug targets are molecules with binding sites for low-molecular-weight compounds. These compounds are then used as starting points for the development of small chemical analogs that bind to the same site and can function as competitive or irreversible inhibitors [61]. If a peptide shares the same binding characteristics as one of the physiological interaction partners of a protein of interest, it can act as a competitive inhibitor. Two types of peptides can be considered for this purpose: peptides matching one of the two interacting proteins and synthetic peptides chosen from a virtual screening of a peptide library. Usually, competitive inhibitors designed for cancer therapy can mask an interaction domain by making it inaccessible to a natural interaction partner that is required for cancer-related PPIs and phenotypes [152,153,154]. Subsequently, these peptide–ligand interactions involving essential regions of the target protein can be used in high-throughput screenings to identify low-molecular-weight molecules with functionally similar analogs [155,156,157].
As a matter of fact, SLiM-based small molecules are still in their early days. For instance, BH3-mimetics are SLiM-based engineered peptide inhibitors able to bind to the hydrophobic groove of the corresponding anti-apoptotic proteins [16,158]. In particular, in cellulo and in vivo studies demonstrated that the SMAC mimetic JP-1201 reduced HT-29 cell survival and CRC tumor growth through an additive effect on apoptosis and disruption of DNA repair mechanisms, but the underlying molecular mechanisms have not been clarified yet [159]. On the other hand, several short peptides have already shown their potential as novel antineoplastic agents.
6.2. Short Peptides as Potential Anti-CRC Drugs
One of the first examples of short peptides tested for their anticancer effects are the parasporin (PS) peptides isolated from the gram-positive bacterium Bacillus thuringiensis [160]. A total of 13 PS proteins have been identified in 11 different strains of B. thuringiensis. Eight of these proteins are included in the PS1 family (PS1Aa1, PS1Aa2, PS1Aa3, PS1Aa4, PS1Aa5, PS1Ab1, PS1Ab2, PS1Ac1), two in the PS2 family (PS2Aa1, PS2Ab1), two in the PS3 family (PS3Aa1, PS3Ab1), and one in the PS4 family (PS4Aa1) [160]. The most investigated anticancer effect is related to PS2, which functions as a cytolysin, permeabilizing the plasma membrane with target cell specificity and then inducing cell death [161]. A recent study revealed the potential of PS2 parasporin family members as anti-CRC drugs. In particular, the PS2 short peptides P264-G274, loop1-PS2Aa, and loop2-PS2Aa displayed high cytotoxicity in SW480 and SW620 CRC cells after 48-h exposure [162]. Parasporin-2Aa1 (PS2Aa1), also known as cry46Aa1, is a protoxin with known anticancer properties, which is generated by B. thuringiensis during sporulation. This 37 kDa toxin is activated by serine proteases such as proteinase K and trypsin, resulting in the production of a highly toxic 30 kDa peptide that is effective against cancer cells [162,163,164]. However, in CRC cells, the exact mechanism of PS2Aa1 activity and the receptors involved in its interaction with cells remain unclear. Another study reports the induction of apoptosis as a mechanism of cell death alongside the inhibition of various survival pathways, including AKT, E3 ubiquitin–protein ligase XIAP (XIAP), ERK1/2, and the activation of the tumor suppressor proteinase-activated receptor 4 (PAR-4) in PC-3 pancreatic and HEPG-2 hepatic cancer cells treated for 24 h with PS2Aa1 [165].
Various studies support the use of short peptide aptamers as promising pharmacological inhibitors of signal transducer and activator of transcription 3 (STAT3). STAT3 is a transcription factor that is activated by tyrosine kinase phosphorylation and has a well-defined domain structure [61,153,156]. Upon phosphorylation by tyrosine kinases activated by ligand binding to growth factor receptors, STAT3 dimerizes and translocates into the nucleus, where it binds to specific DNA response regions and regulates transcription [166]. In most cancer cells, STAT3 is phosphorylated as a result of either increased oncogenic signals or decreased tumor-suppressive pathways. It has been shown that sphingosine-1-phosphate receptor-1 (S1PR1) upregulates tyrosine–protein kinase JAK2 (JAK2) activity, increasing STAT3 phosphorylation in tumor cells, and STAT3 regulates S1PR1 and IL-6, resulting in a positive feedback loop [167,168]. STAT3 activation affects proliferation, apoptosis, differentiation, angiogenesis, immune cell recruitment, and metastasis, all of which are cancer hallmarks [167,168]. Borghouts and colleagues demonstrated that the recombinant STAT3-specific peptide aptamer rS3-PA can inhibit STAT3 and has specific anticancer activity [153]. Peptide aptamers are 12–20-amino acid-long molecules that can be selected from random, high-complexity peptide libraries in yeast two-hybrid screens [152,153]. The authors carried out an in silico virtual screening of a peptide aptamer library to identify competitive inhibitors that could interfere with cancer-related functions. They used human thioredoxin devoid of cysteines as an optimal scaffold for the display of target-interacting peptides in a restricted conformation and generated recombinant proteins for the delivery of specific peptide aptamers to cells [152]; rS3-PA was shown to rapidly enter cancer cells, decrease STAT3 phosphorylation, and increase its proteasomal degradation, promoting cell growth arrest and apoptosis [153]. Further studies will be necessary to assess the in vivo pharmacokinetic and bioavailability characteristics of rS3-PA treatment.
Anticancer peptides (ACPs) are an emerging class of naturally occurring or synthetic anticancer compounds. They show greater selectivity for cancer cells and less propensity for drug resistance [169]. In a recent study, ACP candidates were identified and selected from an in silico pepsin hydrolysate screening of Cordyceps Militaris (CM) proteome using various machine learning-based ACP prediction servers, i.e., AntiCP, iACP, and MLACP. The purpose of the authors was to select CM-derived ACPs to be used as an alternative or adjunct treatment for CRC, hence minimizing the need for chemotherapy. To confirm their anticancer effects, CM-biomimetic peptides were tested in vitro in a non-metastatic colon cancer cell line in comparison and in combination with doxorubicin, a typical chemotherapeutic agent for the treatment of colon cancer. Overall, the results demonstrated that the selected biomimetic peptide C-ori improved the efficacy of doxorubicin treatment [170].
Another group performed an in silico analysis to generate a new library of short cationic amphiphilic α-helical ACPs with specific cytotoxicity against colorectal and cervical cancer [171]. The α-helix is the most prevalent secondary structure shown by ACPs. This study provided important findings on structural and pharmacokinetic features required for peptide-mediated anticancer activity. In particular, the authors found that peptides predominantly consisting of lysine (K) residues (e.g., CIIKKIIKKIIKK-NH2) in their hydrophilic domains exhibit more selective anticancer activity, whereas peptides containing arginine (R) (e.g., CIIRRIIRRIIRR-NH2) display strong toxicity in normal cells. Furthermore, it was shown that ACP anticancer activity depends on their helical composition and hydrophobicity [171]. Indeed, as confirmed in multicellular CRC spheroid models, the addition of two isoleucine residues at the C-terminus of selected ACPs (e.g., CIIKKIIKKIIKKII-NH2) increased their anticancer activity by boosting their hydrophobicity and helical content. The higher ACP toxicity detected in cancer cells compared to normal cells was due to better penetration into the negatively charged cancer cell membranes, resulting in greater cellular uptake, and their cytotoxic effect was primarily exerted by damaging mitochondrial membranes, resulting in apoptosis [171].
Overall, these data confirm that short peptides and SLiMs exhibit promising pharmacokinetic characteristics in vivo.
6.3. Short Peptides in Clinical Studies
Several short peptides have been recognized as PPI modulators and therefore investigated in clinical trials for CRC treatment (Table 4), which reveals an emerging interest in transferring knowledge about these compounds into clinical applications.

Table 4.
Clinical trials evaluating short peptides as a treatment for CRC. Data gathered from https://clinicaltrials.gov (accessed on 5 September 2022).
A major advantage of short peptides is their high targeting specificity; however, the number of terminated clinical trials suggests that new nanotechnologies are needed to enhance the chemical, physical, and biological stability of these compounds [151].
New therapeutic opportunities may come from highly specific small-peptide inhibitors called BH3 mimetics, which have been developed to target anti-apoptotic BCL-2 proteins by mimicking the action of BH3-only proteins [74]. By interfering with the interaction between BH3-only and prosurvival BCL-2 family proteins, these SLiM-based molecules may eventually promote cancer cell death [16,74,120]. Several BH3 mimetics-based pharmacological compounds (i.e., Venetoclax, Navitoclax, AZD5991, AMG-176, S64315, and others) are being studied in ongoing clinical trials for the treatment of leukemia and hematological malignancies (Clinical trial Ids: NCT03592576, NCT03181126, NCT03218683, NTC03797261, NTC03672695, NTC02979366; https://clinicaltrials.gov/; accessed on 5 September 2022) [172]. Notably, clinical trials on Navitoclax have been extended to solid tumors (NCT03592576 NCT03181126; https://clinicaltrials.gov/; accessed on 5 September 2022) [172]. Thus, given the involvement of BCL-2 proteins in colorectal tumorigenesis, it would be interesting to also investigate BH3 mimetics in CRC [172].
7. Conclusions
Currently available treatments for colon cancer are based on specific combinations of surgery, radiation, chemotherapy, and targeted therapy. Nevertheless, following surgical resection and intensive chemotherapy, 50 percent of CRC patients have disease recurrence. In addition, the occurrence of chemoresistance hampers the efficacy of chemotherapeutic agents [173]. Therefore, there is an urgent need for the development of safer and more selective anticancer drugs with novel modes of action.
Moreover, it is crucial to improve the clinical management of CRC patients in order to prevent relapse. This should be combined with the search for novel and more efficient approaches to obtain additional diagnostic and prognostic information.
An efficient approach to identify cancer drivers, as well as diagnostic markers for accurate tumor staging and prediction of clinical outcomes, involves in silico studies to detect differentially expressed genes across different phenotypes, followed by the analysis of the relevant pathways with specific bioinformatics tools evaluating the enrichment of altered oncoproteins [174].
In the last decade, efforts in cancer research have increasingly focused on the development of integrated strategies combining in silico, in vitro, in cellulo, and in vivo approaches to provide in-depth knowledge of cancer-related pathways, PPI networks, and the biological implications underlying a multifactorial disease such as cancer [175]. Indeed, CRC initiation and progression are complicated multistage processes depending on a dense network of PPIs organized in signaling cascades that become dysregulated as a result of abnormalities in various signaling effectors [6].
Recent advances in high-throughput biotechnology applications enable large-scale analysis of disease-associated genes and proteins involved in critical molecular processes. The biological and clinical significance of the resulting data needs careful evaluation. Characterization of the critical SLiMs that mediate PPIs between key players in CRC tumorigenesis and progression is expected to support the development of more efficient and specific pharmacological approaches based on small-molecule anticancer drugs, which may be used in combination with chemotherapeutic agents or pharmacological inhibitors (dual targeting, synthetic lethality, and co-targeting strategies). For instance, the association between pharmacological inhibitors such as ralimetinib (a MAPK14 inhibitor) and SLiM-based small molecules interfering with MAPK signaling activation may prove an effective therapeutic approach to hamper MAPK-dependent CRC development and progression [117]. Furthermore, SLiMs represent promising tools to develop novel immunotherapeutic strategies such as CRC vaccines to prevent oncovirus-dependent colorectal tumorigenesis (JCV, HPV, and EBV).
Further studies are required to gain deeper insight into the therapeutic potential of SLiM-based small molecules in CRC and to address the main limitations related to their stability, bioavailability, and pharmacokinetics.
Author Contributions
Study conceptualization, C.F. and C.S.; writing, and editing of the original draft, C.F.; revision of the manuscript for important intellectual content, V.G.; writing and curating the additional content required upon peer-review, G.F. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Italian Ministry of Health “Ricerca Corrente 2021–2023” to C.S., “Ricerca Corrente 2022–2024” to C.F., PRIN-Research Projects of National Relevance (PRIN 2017, no. 2017WNKSLr-LS4) from the Italian MUR to C.S., AIRC IG-23794 2020–2024 to C.S.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We thank Francesco Paolo Jori for his helpful discussion during manuscript preparation and editorial assistance.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Hossain, M.S.; Karuniawati, H.; Jairoun, A.A.; Urbi, Z.; Ooi, D.J.; John, A.; Lim, Y.C.; Kibria, K.M.K.; Mohiuddin, A.K.M.; Ming, L.C.; et al. Colorectal Cancer: A Review of Carcinogenesis, Global Epidemiology, Current Challenges, Risk Factors, Preventive and Treatment Strategies. Cancers 2022, 14, 1732. [Google Scholar] [CrossRef]
- Jasperson, K.W.; Tuohy, T.M.; Neklason, D.W.; Burt, R.W. Hereditary and Familial Colon Cancer. Gastroenterology 2010, 138, 2044–2058. [Google Scholar] [CrossRef]
- Keum, N.; Giovannucci, E. Global Burden of Colorectal Cancer: Emerging Trends, Risk Factors and Prevention Strategies. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 713–732. [Google Scholar] [CrossRef]
- Olkinuora, A.P.; Peltomäki, P.T.; Aaltonen, L.A.; Rajamäki, K. From APC to the Genetics of Hereditary and Familial Colon Cancer Syndromes. Hum. Mol. Genet. 2021, 30, R206–R224. [Google Scholar] [CrossRef]
- Bhandari, A.; Woodhouse, M.; Gupta, S. Colorectal Cancer Is a Leading Cause of Cancer Incidence and Mortality among Adults Younger than 50 Years in the USA: A SEER-Based Analysis with Comparison to Other Young-Onset Cancers. J. Investig. Med. 2017, 65, 311–315. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Yang, M. Molecular Network of Colorectal Cancer and Current Therapeutic Options. Front. Oncol. 2022, 12, 852927. [Google Scholar] [CrossRef]
- Perkins, J.R.; Diboun, I.; Dessailly, B.H.; Lees, J.G.; Orengo, C. Transient Protein-Protein Interactions: Structural, Functional, and Network Properties. Structure 2010, 18, 1233–1243. [Google Scholar] [CrossRef]
- Davey, N.E.; Van Roey, K.; Weatheritt, R.J.; Toedt, G.; Uyar, B.; Altenberg, B.; Budd, A.; Diella, F.; Dinkel, H.; Gibson, T.J. Attributes of Short Linear Motifs. Mol. Biosyst. 2012, 8, 268–281. [Google Scholar] [CrossRef] [PubMed]
- Mészáros, B.; Simon, I.; Dosztányi, Z. Prediction of Protein Binding Regions in Disordered Proteins. PLoS Comput. Biol. 2009, 5, e1000376. [Google Scholar] [CrossRef]
- Neduva, V.; Russell, R.B. Peptides Mediating Interaction Networks: New Leads at Last. Curr. Opin. Biotechnol. 2006, 17, 465–471. [Google Scholar] [CrossRef]
- Diella, F.; Haslam, N.; Chica, C.; Budd, A.; Michael, S.; Brown, N.P.; Trave, G.; Gibson, T.J. Understanding Eukaryotic Linear Motifs and Their Role in Cell Signaling and Regulation. Front. Biosci. 2008, 13, 6580–6603. [Google Scholar] [CrossRef]
- Davey, N.E.; Edwards, R.J.; Shields, D.C. Computational Identification and Analysis of Protein Short Linear Motifs. Front. Biosci. (Landmark Ed) 2010, 15, 801–825. [Google Scholar] [CrossRef]
- Marongiu, L.; Allgayer, H. Viruses in Colorectal Cancer. Mol. Oncol. 2022, 16, 1423–1450. [Google Scholar] [CrossRef] [PubMed]
- Sai Krishna, A.V.S.; Sinha, S.; Donakonda, S. Virus-Host Interaction Analysis in Colorectal Cancer Identifies Core Virus Network Signature and Small Molecules. Comput. Struct. Biotechnol. J. 2022, 20, 4025–4039. [Google Scholar] [CrossRef]
- Kumar, M.; Michael, S.; Alvarado-Valverde, J.; Mészáros, B.; Sámano-Sánchez, H.; Zeke, A.; Dobson, L.; Lazar, T.; Örd, M.; Nagpal, A.; et al. The Eukaryotic Linear Motif Resource: 2022 Release. Nucleic Acids Res. 2022, 50, D497–D508. [Google Scholar] [CrossRef] [PubMed]
- Aouacheria, A.; Combet, C.; Tompa, P.; Hardwick, J.M. Redefining the BH3 Death Domain as a “Short Linear Motif”. Trends Biochem. Sci. 2015, 40, 736–748. [Google Scholar] [CrossRef]
- Petsalaki, E.; Russell, R.B. Peptide-Mediated Interactions in Biological Systems: New Discoveries and Applications. Curr. Opin. Biotechnol. 2008, 19, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Clerc, I.; Sagar, A.; Barducci, A.; Sibille, N.; Bernadó, P.; Cortés, J. The Diversity of Molecular Interactions Involving Intrinsically Disordered Proteins: A Molecular Modeling Perspective. Comput. Struct. Biotechnol. J. 2021, 19, 3817–3828. [Google Scholar] [CrossRef]
- Kim, I.; Lee, H.; Han, S.K.; Kim, S. Linear Motif-Mediated Interactions Have Contributed to the Evolution of Modularity in Complex Protein Interaction Networks. PLoS Comput. Biol. 2014, 10, e1003881. [Google Scholar] [CrossRef]
- Verschueren, E.; Vanhee, P.; van der Sloot, A.M.; Serrano, L.; Rousseau, F.; Schymkowitz, J. Protein Design with Fragment Databases. Curr. Opin. Struct. Biol. 2011, 21, 452–459. [Google Scholar] [CrossRef] [PubMed]
- Sologova, S.S.; Zavadskiy, S.P.; Mokhosoev, I.M.; Moldogazieva, N.T. Short Linear Motifs Orchestrate Functioning of Human Proteins during Embryonic Development, Redox Regulation, and Cancer. Metabolites 2022, 12, 464. [Google Scholar] [CrossRef] [PubMed]
- Hartooni, N.; Sung, J.; Jain, A.; Morgan, D.O. Single-Molecule Analysis of Specificity and Multivalency in Binding of Short Linear Substrate Motifs to the APC/C. Nat. Commun. 2022, 13, 341. [Google Scholar] [CrossRef]
- Uyar, B.; Weatheritt, R.J.; Dinkel, H.; Davey, N.E.; Gibson, T.J. Proteome-Wide Analysis of Human Disease Mutations in Short Linear Motifs: Neglected Players in Cancer? Mol. Biosyst. 2014, 10, 2626–2642. [Google Scholar] [CrossRef] [PubMed]
- Tompa, P. Unstructural Biology Coming of Age. Curr. Opin. Struct. Biol. 2011, 21, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Chini, C.C.S.; He, M.; Mer, G.; Chen, J. The BRCT Domain Is a Phospho-Protein Binding Domain. Science 2003, 302, 639–642. [Google Scholar] [CrossRef] [PubMed]
- Clapperton, J.A.; Manke, I.A.; Lowery, D.M.; Ho, T.; Haire, L.F.; Yaffe, M.B.; Smerdon, S.J. Structure and Mechanism of BRCA1 BRCT Domain Recognition of Phosphorylated BACH1 with Implications for Cancer. Nat. Struct. Mol. Biol. 2004, 11, 512–518. [Google Scholar] [CrossRef] [PubMed]
- Al-Sukhni, W.; Rothenmund, H.; Borgida, A.E.; Zogopoulos, G.; O’Shea, A.-M.; Pollett, A.; Gallinger, S. Germline BRCA1 Mutations Predispose to Pancreatic Adenocarcinoma. Hum. Genet. 2008, 124, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Jubb, H.; Blundell, T.L. Phosphopeptide Interactions with BRCA1 BRCT Domains: More than Just a Motif. Prog. Biophys. Mol. Biol. 2015, 117, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Cantor, S.; Drapkin, R.; Zhang, F.; Lin, Y.; Han, J.; Pamidi, S.; Livingston, D.M. The BRCA1-Associated Protein BACH1 Is a DNA Helicase Targeted by Clinically Relevant Inactivating Mutations. Proc. Natl. Acad. Sci. USA 2004, 101, 2357–2362. [Google Scholar] [CrossRef]
- Moldogazieva, N.T.; Terent’ev, A.A.; Shaĭtan, K.V. Relationship between structure and function of alpha-fetoprotein: Conformational status and biological activity. Biomed. Khim. 2005, 51, 127–151. [Google Scholar]
- Zhu, Z.; West, G.R.; Wang, D.C.; Collins, A.B.; Xiao, H.; Bai, Q.; Mesfin, F.B.; Wakefield, M.R.; Fang, Y. AFP Peptide (AFPep) as a Potential Growth Factor for Prostate Cancer. Med. Oncol. 2021, 39, 2. [Google Scholar] [CrossRef] [PubMed]
- Moldogazieva, N.T.; Zavadskiy, S.P.; Sologova, S.S.; Mokhosoev, I.M.; Terentiev, A.A. Predictive Biomarkers for Systemic Therapy of Hepatocellular Carcinoma. Expert Rev. Mol. Diagn. 2021, 21, 1147–1164. [Google Scholar] [CrossRef] [PubMed]
- Moldogazieva, N.T.; Shaitan, K.V.; Antonov, M.Y.; Mokhosoev, I.M.; Levtsova, O.V.; Terentiev, A.A. Human EGF-Derived Direct and Reverse Short Linear Motifs: Conformational Dynamics Insight into the Receptor-Binding Residues. J. Biomol. Struct. Dyn. 2018, 36, 1286–1305. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Borcherds, W.; Wu, S.; Becker, A.; Schonbrunn, E.; Daughdrill, G.W.; Chen, J. Autoinhibition of MDMX by Intramolecular P53 Mimicry. Proc. Natl. Acad. Sci. USA 2015, 112, 4624–4629. [Google Scholar] [CrossRef] [PubMed]
- Fenton, M.; Borcherds, W.; Chen, L.; Anbanandam, A.; Levy, R.; Chen, J.; Daughdrill, G. The MDMX Acidic Domain Uses Allovalency to Bind Both P53 and MDMX. J. Mol. Biol. 2022, 434, 167844. [Google Scholar] [CrossRef] [PubMed]
- Edwards, R.J.; Davey, N.E.; O’Brien, K.; Shields, D.C. Interactome-Wide Prediction of Short, Disordered Protein Interaction Motifs in Humans. Mol. Biosyst. 2012, 8, 282–295. [Google Scholar] [CrossRef] [PubMed]
- Halakou, F.; Gursoy, A.; Keskin, O. Embedding Alternative Conformations of Proteins in Protein-Protein Interaction Networks. Methods Mol. Biol. 2020, 2074, 113–124. [Google Scholar] [CrossRef]
- Ivanov, A.A.; Revennaugh, B.; Rusnak, L.; Gonzalez-Pecchi, V.; Mo, X.; Johns, M.A.; Du, Y.; Cooper, L.A.D.; Moreno, C.S.; Khuri, F.R.; et al. The OncoPPi Portal: An Integrative Resource to Explore and Prioritize Protein-Protein Interactions for Cancer Target Discovery. Bioinformatics 2018, 34, 1183–1191. [Google Scholar] [CrossRef]
- Ivanov, A.A. Explore Protein–Protein Interactions for Cancer Target Discovery Using the OncoPPi Portal. In Protein-Protein Interaction Networks: Methods and Protocols; Canzar, S., Ringeling, F.R., Eds.; Methods in Molecular Biology; Springer US: New York, NY, USA, 2020; pp. 145–164. ISBN 978-1-4939-9873-9. [Google Scholar]
- Du, Y.; Cai, M.; Xing, X.; Ji, J.; Yang, E.; Wu, J. PINA 3.0: Mining Cancer Interactome. Nucleic Acids Res. 2021, 49, D1351–D1357. [Google Scholar] [CrossRef]
- Wu, J.; Vallenius, T.; Ovaska, K.; Westermarck, J.; Mäkelä, T.P.; Hautaniemi, S. Integrated Network Analysis Platform for Protein-Protein Interactions. Nat. Methods 2009, 6, 75–77. [Google Scholar] [CrossRef]
- Li, Y.; Ilie, L. SPRINT: Ultrafast Protein-Protein Interaction Prediction of the Entire Human Interactome. BMC Bioinform. 2017, 18, 485. [Google Scholar] [CrossRef]
- Philipp, O.; Osiewacz, H.D.; Koch, I. Path2PPI: An R Package to Predict Protein-Protein Interaction Networks for a Set of Proteins. Bioinformatics 2016, 32, 1427–1429. [Google Scholar] [CrossRef]
- Gueudré, T.; Baldassi, C.; Pagnani, A.; Weigt, M. Predicting Interacting Protein Pairs by Coevolutionary Paralog Matching. Methods Mol. Biol. 2020, 2074, 57–65. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Zeng, H.; Wang, S.; Xu, J. A Web-Based Protocol for Interprotein Contact Prediction by Deep Learning. Methods Mol. Biol. 2020, 2074, 67–80. [Google Scholar] [CrossRef] [PubMed]
- Furmanová, K.; Byška, J.; Gröller, E.M.; Viola, I.; Paleček, J.J.; Kozlíková, B. COZOID: Contact Zone Identifier for Visual Analysis of Protein-Protein Interactions. BMC Bioinform. 2018, 19, 125. [Google Scholar] [CrossRef]
- Peterson, L.X.; Togawa, Y.; Esquivel-Rodriguez, J.; Terashi, G.; Christoffer, C.; Roy, A.; Shin, W.-H.; Kihara, D. Modeling the Assembly Order of Multimeric Heteroprotein Complexes. PLoS Comput. Biol. 2018, 14, e1005937. [Google Scholar] [CrossRef]
- Loney, F.; Wu, G. Automation of ReactomeFIViz via CyREST API. F1000Research 2018, 7, 531. [Google Scholar] [CrossRef]
- Alcaraz, N.; Pauling, J.; Batra, R.; Barbosa, E.; Junge, A.; Christensen, A.G.L.; Azevedo, V.; Ditzel, H.J.; Baumbach, J. KeyPathwayMiner 4.0: Condition-Specific Pathway Analysis by Combining Multiple Omics Studies and Networks with Cytoscape. BMC Syst. Biol. 2014, 8, 99. [Google Scholar] [CrossRef]
- Biedermann, S.; Henzinger, M.; Schulz, C.; Schuster, B. Vienna Graph Clustering. In Protein-Protein Interaction Networks: Methods and Protocols; Canzar, S., Ringeling, F.R., Eds.; Methods in Molecular Biology; Springer US: New York, NY, USA, 2020; pp. 215–231. ISBN 978-1-4939-9873-9. [Google Scholar]
- Re, A.; Lecca, P. On TD-WGcluster: Theoretical Foundations and Guidelines for the User. In Protein-Protein Interaction Networks: Methods and Protocols; Canzar, S., Ringeling, F.R., Eds.; Methods in Molecular Biology; Springer US: New York, NY, USA, 2020; pp. 233–262. ISBN 978-1-4939-9873-9. [Google Scholar]
- Mamano, N.; Hayes, W.B. SANA: Simulated Annealing Far Outperforms Many Other Search Algorithms for Biological Network Alignment. Bioinformatics 2017, 33, 2156–2164. [Google Scholar] [CrossRef]
- Zhou, A.; Zhang, F.; Chen, J.Y. PEPPI: A Peptidomic Database of Human Protein Isoforms for Proteomics Experiments. BMC Bioinform. 2010, 11 (Suppl. 6), S7. [Google Scholar] [CrossRef]
- Kamburov, A.; Wierling, C.; Lehrach, H.; Herwig, R. ConsensusPathDB--a Database for Integrating Human Functional Interaction Networks. Nucleic Acids Res. 2009, 37, D623–D628. [Google Scholar] [CrossRef]
- Kotlyar, M.; Pastrello, C.; Sheahan, N.; Jurisica, I. Integrated Interactions Database: Tissue-Specific View of the Human and Model Organism Interactomes. Nucleic Acids Res. 2016, 44, D536–D541. [Google Scholar] [CrossRef] [PubMed]
- Alanis-Lobato, G.; Andrade-Navarro, M.A.; Schaefer, M.H. HIPPIE v2.0: Enhancing Meaningfulness and Reliability of Protein–Protein Interaction Networks. Nucleic Acids Res. 2017, 45, D408–D414. [Google Scholar] [CrossRef] [PubMed]
- Pagel, P.; Kovac, S.; Oesterheld, M.; Brauner, B.; Dunger-Kaltenbach, I.; Frishman, G.; Montrone, C.; Mark, P.; Stümpflen, V.; Mewes, H.-W.; et al. The MIPS Mammalian Protein-Protein Interaction Database. Bioinformatics 2005, 21, 832–834. [Google Scholar] [CrossRef] [PubMed]
- Oughtred, R.; Rust, J.; Chang, C.; Breitkreutz, B.-J.; Stark, C.; Willems, A.; Boucher, L.; Leung, G.; Kolas, N.; Zhang, F.; et al. The BioGRID Database: A Comprehensive Biomedical Resource of Curated Protein, Genetic, and Chemical Interactions. Protein Sci. 2021, 30, 187–200. [Google Scholar] [CrossRef]
- Orchard, S.; Ammari, M.; Aranda, B.; Breuza, L.; Briganti, L.; Broackes-Carter, F.; Campbell, N.H.; Chavali, G.; Chen, C.; del-Toro, N.; et al. The MIntAct Project--IntAct as a Common Curation Platform for 11 Molecular Interaction Databases. Nucleic Acids Res. 2014, 42, D358–D363. [Google Scholar] [CrossRef]
- Reys, V.; Labesse, G. SLiMAn: An Integrative Web Server for Exploring Short Linear Motif-Mediated Interactions in Interactomes. J. Proteome Res. 2022, 21, 1654–1663. [Google Scholar] [CrossRef]
- Groner, B.; Weber, A.; Mack, L. Increasing the Range of Drug Targets: Interacting Peptides Provide Leads for the Development of Oncoprotein Inhibitors. Bioengineered 2012, 3, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Amos-Binks, A.; Patulea, C.; Pitre, S.; Schoenrock, A.; Gui, Y.; Green, J.R.; Golshani, A.; Dehne, F. Binding Site Prediction for Protein-Protein Interactions and Novel Motif Discovery Using Re-Occurring Polypeptide Sequences. BMC Bioinform. 2011, 12, 225. [Google Scholar] [CrossRef]
- Mi, T.; Merlin, J.C.; Deverasetty, S.; Gryk, M.R.; Bill, T.J.; Brooks, A.W.; Lee, L.Y.; Rathnayake, V.; Ross, C.A.; Sargeant, D.P.; et al. Minimotif Miner 3.0: Database Expansion and Significantly Improved Reduction of False-Positive Predictions from Consensus Sequences. Nucleic Acids Res. 2012, 40, D252–D260. [Google Scholar] [CrossRef]
- Sarkar, D.; Jana, T.; Saha, S. LMPID: A Manually Curated Database of Linear Motifs Mediating Protein-Protein Interactions. Database (Oxford) 2015, 2015, bav014. [Google Scholar] [CrossRef]
- Encinar, J.A.; Fernandez-Ballester, G.; Sánchez, I.E.; Hurtado-Gomez, E.; Stricher, F.; Beltrao, P.; Serrano, L. ADAN: A Database for Prediction of Protein-Protein Interaction of Modular Domains Mediated by Linear Motifs. Bioinformatics 2009, 25, 2418–2424. [Google Scholar] [CrossRef]
- Dinkel, H.; Sticht, H. A Computational Strategy for the Prediction of Functional Linear Peptide Motifs in Proteins. Bioinformatics 2007, 23, 3297–3303. [Google Scholar] [CrossRef]
- Davey, N.E.; Haslam, N.J.; Shields, D.C.; Edwards, R.J. SLiMSearch 2.0: Biological Context for Short Linear Motifs in Proteins. Nucleic Acids Res. 2011, 39, W56–W60. [Google Scholar] [CrossRef]
- Dosztányi, Z.; Csizmok, V.; Tompa, P.; Simon, I. IUPred: Web Server for the Prediction of Intrinsically Unstructured Regions of Proteins Based on Estimated Energy Content. Bioinformatics 2005, 21, 3433–3434. [Google Scholar] [CrossRef] [PubMed]
- Via, A.; Gould, C.M.; Gemünd, C.; Gibson, T.J.; Helmer-Citterich, M. A Structure Filter for the Eukaryotic Linear Motif Resource. BMC Bioinform. 2009, 10, 351. [Google Scholar] [CrossRef]
- Petsalaki, E.; Stark, A.; García-Urdiales, E.; Russell, R.B. Accurate Prediction of Peptide Binding Sites on Protein Surfaces. PLoS Comput. Biol. 2009, 5, e1000335. [Google Scholar] [CrossRef]
- Ramu, C. SIRW: A Web Server for the Simple Indexing and Retrieval System That Combines Sequence Motif Searches with Keyword Searches. Nucleic Acids Res. 2003, 31, 3771–3774. [Google Scholar] [CrossRef]
- Wang, G.; Matsuura, I.; He, D.; Liu, F. Transforming Growth Factor-{beta}-Inducible Phosphorylation of Smad3. J. Biol. Chem. 2009, 284, 9663–9673. [Google Scholar] [CrossRef]
- Koveitypour, Z.; Panahi, F.; Vakilian, M.; Peymani, M.; Seyed Forootan, F.; Nasr Esfahani, M.H.; Ghaedi, K. Signaling Pathways Involved in Colorectal Cancer Progression. Cell Biosci. 2019, 9, 97. [Google Scholar] [CrossRef]
- Ramesh, P.; Medema, J.P. BCL-2 Family Deregulation in Colorectal Cancer: Potential for BH3 Mimetics in Therapy. Apoptosis 2020, 25, 305–320. [Google Scholar] [CrossRef] [PubMed]
- Bienz, M.; Clevers, H. Linking Colorectal Cancer to Wnt Signaling. Cell 2000, 103, 311–320. [Google Scholar] [CrossRef] [PubMed]
- Bian, J.; Dannappel, M.; Wan, C.; Firestein, R. Transcriptional Regulation of Wnt/β-Catenin Pathway in Colorectal Cancer. Cells 2020, 9, 2125. [Google Scholar] [CrossRef]
- Wnt Signaling in Colorectal Cancer: Pathogenic Role and Therapeutic Target-PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/35836256/ (accessed on 3 August 2022).
- Singh, V.K.; Pacheco, I.; Uversky, V.N.; Smith, S.P.; MacLeod, R.J.; Jia, Z. Intrinsically Disordered Human C/EBP Homologous Protein Regulates Biological Activity of Colon Cancer Cells during Calcium Stress. J. Mol. Biol. 2008, 380, 313–326. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wu, G.; Xu, G.; Schulman, B.A.; Jeffrey, P.D.; Harper, J.W.; Pavletich, N.P. Structure of a Beta-TrCP1-Skp1-Beta-Catenin Complex: Destruction Motif Binding and Lysine Specificity of the SCF(Beta-TrCP1) Ubiquitin Ligase. Mol. Cell 2003, 11, 1445–1456. [Google Scholar] [CrossRef]
- Lustig, B.; Behrens, J. The Wnt Signaling Pathway and Its Role in Tumor Development. J. Cancer Res. Clin. Oncol. 2003, 129, 199–221. [Google Scholar] [CrossRef] [PubMed]
- Sadot, E.; Conacci-Sorrell, M.; Zhurinsky, J.; Shnizer, D.; Lando, Z.; Zharhary, D.; Kam, Z.; Ben-Ze’ev, A.; Geiger, B. Regulation of S33/S37 Phosphorylated Beta-Catenin in Normal and Transformed Cells. J. Cell Sci. 2002, 115, 2771–2780. [Google Scholar] [CrossRef] [PubMed]
- Provost, E.; McCabe, A.; Stern, J.; Lizardi, I.; D’Aquila, T.G.; Rimm, D.L. Functional Correlates of Mutation of the Asp32 and Gly34 Residues of Beta-Catenin. Oncogene 2005, 24, 2667–2676. [Google Scholar] [CrossRef]
- Welcker, M.; Clurman, B.E. FBW7 Ubiquitin Ligase: A Tumour Suppressor at the Crossroads of Cell Division, Growth and Differentiation. Nat. Rev. Cancer 2008, 8, 83–93. [Google Scholar] [CrossRef]
- Hao, B.; Oehlmann, S.; Sowa, M.E.; Harper, J.W.; Pavletich, N.P. Structure of a Fbw7-Skp1-Cyclin E Complex: Multisite-Phosphorylated Substrate Recognition by SCF Ubiquitin Ligases. Mol. Cell 2007, 26, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Sameer, A.S. Colorectal Cancer: Molecular Mutations and Polymorphisms. Front. Oncol. 2013, 3, 114. [Google Scholar] [CrossRef]
- Byeon, I.-J.L.; Li, H.; Song, H.; Gronenborn, A.M.; Tsai, M.-D. Sequential Phosphorylation and Multisite Interactions Characterize Specific Target Recognition by the FHA Domain of Ki67. Nat. Struct. Mol. Biol. 2005, 12, 987–993. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Hu, X.; Guo, Q.; Shi, Y.; Liu, L.; Ying, G. Discovery and Validation of a Novel Metastasis-Related LncRNA Prognostic Signature for Colorectal Cancer. Front. Genet. 2022, 13, 704988. [Google Scholar] [CrossRef] [PubMed]
- Picard, N.; Caron, V.; Bilodeau, S.; Sanchez, M.; Mascle, X.; Aubry, M.; Tremblay, A. Identification of Estrogen Receptor β as a SUMO-1 Target Reveals a Novel Phosphorylated Sumoylation Motif and Regulation by Glycogen Synthase Kinase 3β. Mol. Cell. Biol. 2012, 32, 2709–2721. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Li, R.; Shi, W.; Huang, Z. Discovery of the Anti-Tumor Mechanism of Calycosin Against Colorectal Cancer by Using System Pharmacology Approach. Med. Sci. Monit. 2019, 25, 5589–5593. [Google Scholar] [CrossRef]
- Hayakawa, M.; Matsushima, M.; Hagiwara, H.; Oshima, T.; Fujino, T.; Ando, K.; Kikugawa, K.; Tanaka, H.; Miyazawa, K.; Kitagawa, M. Novel Insights into FGD3, a Putative GEF for Cdc42, That Undergoes SCF(FWD1/Beta-TrCP)-Mediated Proteasomal Degradation Analogous to That of Its Homologue FGD1 but Regulates Cell Morphology and Motility Differently from FGD1. Genes Cells 2008, 13, 329–342. [Google Scholar] [CrossRef] [PubMed]
- El-Masry, O.S.; Goja, A.; Rateb, M.; Owaidah, A.Y.; Alsamman, K. RNA Sequencing Identified Novel Target Genes for Adansonia Digitata in Breast and Colon Cancer Cells. Sci. Prog. 2021, 104, 368504211032084. [Google Scholar] [CrossRef] [PubMed]
- Sapkota, G.; Alarcón, C.; Spagnoli, F.M.; Brivanlou, A.H.; Massagué, J. Balancing BMP Signaling through Integrated Inputs into the Smad1 Linker. Mol. Cell 2007, 25, 441–454. [Google Scholar] [CrossRef]
- Chu, B.; Soncin, F.; Price, B.D.; Stevenson, M.A.; Calderwood, S.K. Sequential Phosphorylation by Mitogen-Activated Protein Kinase and Glycogen Synthase Kinase 3 Represses Transcriptional Activation by Heat Shock Factor-1. J. Biol. Chem. 1996, 271, 30847–30857. [Google Scholar] [CrossRef]
- Wang, H.; Wang, X.; Zhang, H.; Deng, T.; Liu, R.; Liu, Y.; Li, H.; Bai, M.; Ning, T.; Wang, J.; et al. The HSF1/MiR-135b-5p Axis Induces Protective Autophagy to Promote Oxaliplatin Resistance through the MUL1/ULK1 Pathway in Colorectal Cancer. Oncogene 2021, 40, 4695–4708. [Google Scholar] [CrossRef]
- Hayakawa, M.; Kitagawa, H.; Miyazawa, K.; Kitagawa, M.; Kikugawa, K. The FWD1/Beta-TrCP-Mediated Degradation Pathway Establishes a “turning off Switch” of a Cdc42 Guanine Nucleotide Exchange Factor, FGD1. Genes Cells 2005, 10, 241–251. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Zhao, J.; Yin, F.; Sharen, G.; Wu, Q.; Chen, Q.; Sun, X.; Yang, J.; Wang, H.; Zhang, D. A Methylation-Driven Gene Panel Predicts Survival in Patients with Colon Cancer. FEBS Open. Biol. 2021, 11, 2490–2506. [Google Scholar] [CrossRef]
- Ershov, P.; Poyarkov, S.; Konstantinova, Y.; Veselovsky, E.; Makarova, A. Transcriptomic Signatures in Colorectal Cancer Progression. Curr. Mol. Med. 2022, 23, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Fei, E.; Jia, N.; Zhang, T.; Ma, X.; Wang, H.; Liu, C.; Zhang, W.; Ding, L.; Nukina, N.; Wang, G. Phosphorylation of Ataxin-3 by Glycogen Synthase Kinase 3beta at Serine 256 Regulates the Aggregation of Ataxin-3. Biochem. Biophys. Res. Commun. 2007, 357, 487–492. [Google Scholar] [CrossRef]
- Li, D.; Zhang, T.; Lai, J.; Zhang, J.; Wang, T.; Ling, Y.; He, S.; Hu, Z. MicroRNA-25/ATXN3 Interaction Regulates Human Colon Cancer Cell Growth and Migration. Mol. Med. Rep. 2019, 19, 4213–4221. [Google Scholar] [CrossRef]
- Hart, M.; Concordet, J.P.; Lassot, I.; Albert, I.; del los Santos, R.; Durand, H.; Perret, C.; Rubinfeld, B.; Margottin, F.; Benarous, R.; et al. The F-Box Protein Beta-TrCP Associates with Phosphorylated Beta-Catenin and Regulates Its Activity in the Cell. Curr. Biol. 1999, 9, 207–210. [Google Scholar] [CrossRef]
- Liu, C.; Li, Y.; Semenov, M.; Han, C.; Baeg, G.H.; Tan, Y.; Zhang, Z.; Lin, X.; He, X. Control of Beta-Catenin Phosphorylation/Degradation by a Dual-Kinase Mechanism. Cell 2002, 108, 837–847. [Google Scholar] [CrossRef]
- Su, Y.; Fu, C.; Ishikawa, S.; Stella, A.; Kojima, M.; Shitoh, K.; Schreiber, E.M.; Day, B.W.; Liu, B. APC Is Essential for Targeting Phosphorylated Beta-Catenin to the SCFbeta-TrCP Ubiquitin Ligase. Mol. Cell 2008, 32, 652–661. [Google Scholar] [CrossRef]
- Boyle, W.J.; Smeal, T.; Defize, L.H.; Angel, P.; Woodgett, J.R.; Karin, M.; Hunter, T. Activation of Protein Kinase C Decreases Phosphorylation of C-Jun at Sites That Negatively Regulate Its DNA-Binding Activity. Cell 1991, 64, 573–584. [Google Scholar] [CrossRef]
- Turenne, G.A.; Price, B.D. Glycogen Synthase Kinase3 Beta Phosphorylates Serine 33 of P53 and Activates P53’s Transcriptional Activity. BMC Cell Biol. 2001, 2, 12. [Google Scholar] [CrossRef]
- Gregory, M.A.; Qi, Y.; Hann, S.R. Phosphorylation by Glycogen Synthase Kinase-3 Controls c-Myc Proteolysis and Subnuclear Localization. J. Biol. Chem. 2003, 278, 51606–51612. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.; Peng, D.; Cheng, Y. Significant Position of C-Myc in Colorectal Cancer: A Promising Therapeutic Target. Clin. Transl. Oncol. 2022, 24, 2295–2304. [Google Scholar] [CrossRef] [PubMed]
- Zhou, B.P.; Deng, J.; Xia, W.; Xu, J.; Li, Y.M.; Gunduz, M.; Hung, M.-C. Dual Regulation of Snail by GSK-3beta-Mediated Phosphorylation in Control of Epithelial-Mesenchymal Transition. Nat. Cell Biol. 2004, 6, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Galindo-Pumariño, C.; Collado, M.; Castillo, M.E.; Barquín, J.; Romio, E.; Larriba, M.J.; Muñoz de Mier, G.J.; Carrato, A.; de la Pinta, C.; Pena, C. SNAI1-Expressing Fibroblasts and Derived-Extracellular Matrix as Mediators of Drug Resistance in Colorectal Cancer Patients. Toxicol. Appl. Pharmacol. 2022, 450, 116171. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, C.M.; Heist, E.K.; Beals, C.R.; Crabtree, G.R.; Gardner, P. Protein Kinase A Negatively Modulates the Nuclear Accumulation of NF-ATc1 by Priming for Subsequent Phosphorylation by Glycogen Synthase Kinase-3. J. Biol. Chem. 2002, 277, 48664–48676. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Yue, C.; Wang, X.; Wang, Z.; Wu, Y.; Zhao, C.; Chang, P.; Sun, X.; Wang, W. NFATc1 Promotes Epithelial-Mesenchymal Transition and Facilitates Colorectal Cancer Metastasis by Targeting SNAI1. Exp. Cell Res. 2021, 408, 112854. [Google Scholar] [CrossRef]
- Jeong, W.-J.; Ro, E.J.; Choi, K.-Y. Interaction between Wnt/β-Catenin and RAS-ERK Pathways and an Anti-Cancer Strategy via Degradations of β-Catenin and RAS by Targeting the Wnt/β-Catenin Pathway. NPJ Precis. Oncol. 2018, 2, 5. [Google Scholar] [CrossRef]
- Nicholson, R.I.; Gee, J.M.; Harper, M.E. EGFR and Cancer Prognosis. Eur J. Cancer 2001, 37 (Suppl. 4), S9–S15. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.Y.; Richardson, B.C. The MAPK Signalling Pathways and Colorectal Cancer. Lancet Oncol. 2005, 6, 322–327. [Google Scholar] [CrossRef]
- Ahmad, R.; Singh, J.K.; Wunnava, A.; Al-Obeed, O.; Abdulla, M.; Srivastava, S.K. Emerging Trends in Colorectal Cancer: Dysregulated Signaling Pathways (Review). Int. J. Mol. Med. 2021, 47, 14. [Google Scholar] [CrossRef]
- Mizukami, T.; Izawa, N.; Nakajima, T.E.; Sunakawa, Y. Targeting EGFR and RAS/RAF Signaling in the Treatment of Metastatic Colorectal Cancer: From Current Treatment Strategies to Future Perspectives. Drugs 2019, 79, 633–645. [Google Scholar] [CrossRef]
- Kong, L.; Zhang, Q.; Mao, J.; Cheng, L.; Shi, X.; Yu, L.; Hu, J.; Yang, M.; Li, L.; Liu, B.; et al. A Dual-Targeted Molecular Therapy of PP242 and Cetuximab Plays an Anti-Tumor Effect through EGFR Downstream Signaling Pathways in Colorectal Cancer. J. Gastrointestig. Oncol. 2021, 12, 1625–1642. [Google Scholar] [CrossRef]
- Tanoue, T.; Yamamoto, T.; Nishida, E. Modular Structure of a Docking Surface on MAPK Phosphatases. J. Biol. Chem. 2002, 277, 22942–22949. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, Y.; Steller, H. Live to Die Another Way: Modes of Programmed Cell Death and the Signals Emanating from Dying Cells. Nat. Rev. Mol. Cell Biol. 2015, 16, 329–344. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yu, J. Role of Apoptosis in Colon Cancer Biology, Therapy, and Prevention. Curr. Colorectal Cancer Rep. 2013, 9, 331–340. [Google Scholar] [CrossRef]
- Montero, J.; Letai, A. Why Do BCL-2 Inhibitors Work and Where Should We Use Them in the Clinic? Cell Death Differ. 2018, 25, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Aouacheria, A.; Rech de Laval, V.; Combet, C.; Hardwick, J.M. Evolution of Bcl-2 Homology Motifs: Homology versus Homoplasy. Trends Cell Biol. 2013, 23, 103–111. [Google Scholar] [CrossRef]
- Letai, A.; Bassik, M.C.; Walensky, L.D.; Sorcinelli, M.D.; Weiler, S.; Korsmeyer, S.J. Distinct BH3 Domains Either Sensitize or Activate Mitochondrial Apoptosis, Serving as Prototype Cancer Therapeutics. Cancer Cell 2002, 2, 183–192. [Google Scholar] [CrossRef]
- Sanese, P.; Fasano, C.; Buscemi, G.; Bottino, C.; Corbetta, S.; Fabini, E.; Silvestri, V.; Valentini, V.; Disciglio, V.; Forte, G.; et al. Targeting SMYD3 to Sensitize Homologous Recombination-Proficient Tumors to PARP-Mediated Synthetic Lethality. iScience 2020, 23, 101604. [Google Scholar] [CrossRef]
- Fasano, C.; Lepore Signorile, M.; De Marco, K.; Forte, G.; Sanese, P.; Grossi, V.; Simone, C. Identifying Novel SMYD3 Interactors on the Trail of Cancer Hallmarks. Comput. Struct. Biotechnol. J. 2022, 20, 1860–1875. [Google Scholar] [CrossRef]
- Bottino, C.; Peserico, A.; Simone, C.; Caretti, G. SMYD3: An Oncogenic Driver Targeting Epigenetic Regulation and Signaling Pathways. Cancers 2020, 12, 142. [Google Scholar] [CrossRef] [PubMed]
- Stefanucci, A.; Luisi, G.; Zengin, G.; Macedonio, G.; Dimmito, M.P.; Novellino, E.; Mollica, A. Discovery of Arginine-Containing Tripeptides as a New Class of Pancreatic Lipase Inhibitors. Future Med. Chem. 2019, 11, 5–19. [Google Scholar] [CrossRef] [PubMed]
- McInnes, C.; Estes, K.; Baxter, M.; Yang, Z.; Farag, D.B.; Johnston, P.; Lazo, J.S.; Wang, J.; Wyatt, M.D. Targeting Subcellular Localization through the Polo-Box Domain: Non-ATP Competitive Inhibitors Recapitulate a PLK1 Phenotype. Mol. Cancer Ther. 2012, 11, 1683–1692. [Google Scholar] [CrossRef] [PubMed]
- Milorey, B.; Schweitzer-Stenner, R.; Andrews, B.; Schwalbe, H.; Urbanc, B. Short Peptides as Predictors for the Structure of Polyarginine Sequences in Disordered Proteins. Biophys. J. 2021, 120, 662–676. [Google Scholar] [CrossRef] [PubMed]
- Su, A.; Tabata, Y.; Aoki, K.; Sada, A.; Ohki, R.; Nagatoishi, S.; Tsumoto, K.; Wang, S.; Otani, Y.; Ohwada, T. Elaboration of Non-Naturally Occurring Helical Tripeptides as P53-MDM2/MDMX Interaction Inhibitors. Chem. Pharm. Bull. (Tokyo) 2021, 69, 681–692. [Google Scholar] [CrossRef] [PubMed]
- Trost, B.; Kanduc, D.; Kusalik, A. Rare Peptide Segments Are Found Significantly More Often in Proto-Oncoproteins than Control Proteins: Implications for Immunology and Oncology. J. R. Soc. Interface 2009, 6, 123–127. [Google Scholar] [CrossRef][Green Version]
- Kusalik, A.; Trost, B.; Bickis, M.; Fasano, C.; Capone, G.; Kanduc, D. Codon Number Shapes Peptide Redundancy in the Universal Proteome Composition. Peptides 2009, 30, 1940–1944. [Google Scholar] [CrossRef]
- Kanduc, D. Protein Information Content Resides in Rare Peptide Segments. Peptides 2010, 31, 983–988. [Google Scholar] [CrossRef]
- Ahn, Y.-H.; Chang, Y.-T. Tagged Small Molecule Library Approach for Facilitated Chemical Genetics. Acc. Chem. Res. 2007, 40, 1025–1033. [Google Scholar] [CrossRef]
- Cisar, J.S.; Cravatt, B.F. Fully Functionalized Small-Molecule Probes for Integrated Phenotypic Screening and Target Identification. J. Am. Chem. Soc. 2012, 134, 10385–10388. [Google Scholar] [CrossRef]
- Kambe, T.; Correia, B.E.; Niphakis, M.J.; Cravatt, B.F. Mapping the Protein Interaction Landscape for Fully Functionalized Small-Molecule Probes in Human Cells. J. Am. Chem. Soc. 2014, 136, 10777–10782. [Google Scholar] [CrossRef]
- Cheng, Y.; Ling, Z.; Li, L. The Intestinal Microbiota and Colorectal Cancer. Front. Immunol. 2020, 11, 615056. [Google Scholar] [CrossRef]
- Via, A.; Uyar, B.; Brun, C.; Zanzoni, A. How Pathogens Use Linear Motifs to Perturb Host Cell Networks. Trends Biochem. Sci. 2015, 40, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Sámano-Sánchez, H.; Gibson, T.J. Mimicry of Short Linear Motifs by Bacterial Pathogens: A Drugging Opportunity. Trends Biochem. Sci. 2020, 45, 526–544. [Google Scholar] [CrossRef] [PubMed]
- Zanzoni, A.; Spinelli, L.; Braham, S.; Brun, C. Perturbed Human Sub-Networks by Fusobacterium Nucleatum Candidate Virulence Proteins. Microbiome 2017, 5, 89. [Google Scholar] [CrossRef] [PubMed]
- Schuster-Böckler, B.; Bateman, A. Protein Interactions in Human Genetic Diseases. Genome Biol. 2008, 9, R9. [Google Scholar] [CrossRef] [PubMed]
- Teng, S.; Madej, T.; Panchenko, A.; Alexov, E. Modeling Effects of Human Single Nucleotide Polymorphisms on Protein-Protein Interactions. Biophys. J. 2009, 96, 2178–2188. [Google Scholar] [CrossRef]
- Whitty, A.; Kumaravel, G. Between a Rock and a Hard Place? Nat. Chem. Biol. 2006, 2, 112–118. [Google Scholar] [CrossRef]
- Arkin, M.R.; Wells, J.A. Small-Molecule Inhibitors of Protein-Protein Interactions: Progressing towards the Dream. Nat. Rev. Drug Discov. 2004, 3, 301–317. [Google Scholar] [CrossRef] [PubMed]
- Higueruelo, A.P.; Schreyer, A.; Bickerton, G.R.J.; Pitt, W.R.; Groom, C.R.; Blundell, T.L. Atomic Interactions and Profile of Small Molecules Disrupting Protein-Protein Interfaces: The TIMBAL Database. Chem. Biol. Drug Des. 2009, 74, 457–467. [Google Scholar] [CrossRef] [PubMed]
- Morell, M.; Avilés, F.X.; Ventura, S. Detecting and Interfering Protein Interactions: Towards the Control of Biochemical Pathways. Curr. Med. Chem. 2009, 16, 362–379. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.J. Inhibition of Protein-Protein Interactions Using Designed Molecules. Chem. Soc. Rev. 2009, 38, 3289–3300. [Google Scholar] [CrossRef] [PubMed]
- Peserico, A.; Germani, A.; Sanese, P.; Barbosa, A.J.; Di Virgilio, V.; Fittipaldi, R.; Fabini, E.; Bertucci, C.; Varchi, G.; Moyer, M.P.; et al. A SMYD3 Small-Molecule Inhibitor Impairing Cancer Cell Growth. J. Cell. Physiol. 2015, 230, 2447–2460. [Google Scholar] [CrossRef] [PubMed]
- Van Aller, G.S.; Graves, A.P.; Elkins, P.A.; Bonnette, W.G.; McDevitt, P.J.; Zappacosta, F.; Annan, R.S.; Dean, T.W.; Su, D.-S.; Carpenter, C.L.; et al. Structure-Based Design of a Novel SMYD3 Inhibitor That Bridges the SAM-and MEKK2-Binding Pockets. Structure 2016, 24, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, L.H.; Boriack-Sjodin, P.A.; Smith, S.; Thomenius, M.; Rioux, N.; Munchhof, M.; Mills, J.E.; Klaus, C.; Totman, J.; Riera, T.V.; et al. Novel Oxindole Sulfonamides and Sulfamides: EPZ031686, the First Orally Bioavailable Small Molecule SMYD3 Inhibitor. ACS Med. Chem. Lett. 2016, 7, 134–138. [Google Scholar] [CrossRef]
- Jain, A.K.; Jain, S. Advances in Oral Delivery of Anti-Cancer Prodrugs. Expert Opin. Drug Deliv. 2016, 13, 1759–1775. [Google Scholar] [CrossRef] [PubMed]
- Santos, G.B.; Ganesan, A.; Emery, F.S. Oral Administration of Peptide-Based Drugs: Beyond Lipinski’s Rule. ChemMedChem 2016, 11, 2245–2251. [Google Scholar] [CrossRef]
- Borghouts, C.; Kunz, C.; Delis, N.; Groner, B. Monomeric Recombinant Peptide Aptamers Are Required for Efficient Intracellular Uptake and Target Inhibition. Mol. Cancer Res. 2008, 6, 267–281. [Google Scholar] [CrossRef]
- Borghouts, C.; Tittmann, H.; Delis, N.; Kirchenbauer, M.; Brill, B.; Groner, B. The Intracellular Delivery of a Recombinant Peptide Derived from the Acidic Domain of PIAS3 Inhibits STAT3 Transactivation and Induces Tumor Cell Death. Mol. Cancer Res. 2010, 8, 539–553. [Google Scholar] [CrossRef] [PubMed]
- Weiss, A.; Brill, B.; Borghouts, C.; Delis, N.; Mack, L.; Groner, B. Survivin Inhibition by an Interacting Recombinant Peptide, Derived from the Human Ferritin Heavy Chain, Impedes Tumor Cell Growth. J. Cancer Res. Clin. Oncol. 2012, 138, 1205–1220. [Google Scholar] [CrossRef]
- Kadkhodayan, S.; Elliott, L.O.; Mausisa, G.; Wallweber, H.A.; Deshayes, K.; Feng, B.; Fairbrother, W.J. Evaluation of Assay Technologies for the Identification of Protein-Peptide Interaction Antagonists. Assay Drug Dev. Technol. 2007, 5, 501–513. [Google Scholar] [CrossRef] [PubMed]
- Uehara, Y.; Mochizuki, M.; Matsuno, K.; Haino, T.; Asai, A. Novel High-Throughput Screening System for Identifying STAT3-SH2 Antagonists. Biochem. Biophys. Res. Commun. 2009, 380, 627–631. [Google Scholar] [CrossRef]
- Murray, E.J.; Leaman, D.P.; Pawa, N.; Perkins, H.; Pickford, C.; Perros, M.; Zwick, M.B.; Butler, S.L. A Low-Molecular-Weight Entry Inhibitor of Both CCR5- and CXCR4-Tropic Strains of Human Immunodeficiency Virus Type 1 Targets a Novel Site on Gp41. J. Virol. 2010, 84, 7288–7299. [Google Scholar] [CrossRef] [PubMed]
- Townsend, P.A.; Kozhevnikova, M.V.; Cexus, O.N.F.; Zamyatnin, A.A.; Soond, S.M. BH3-Mimetics: Recent Developments in Cancer Therapy. J. Exp. Clin. Cancer Res. 2021, 40, 355. [Google Scholar] [CrossRef] [PubMed]
- Huerta, S.; Gao, X.; Livingston, E.H.; Kapur, P.; Sun, H.; Anthony, T. In Vitro and in Vivo Radiosensitization of Colorectal Cancer HT-29 Cells by the Smac Mimetic JP-1201. Surgery 2010, 148, 346–353. [Google Scholar] [CrossRef]
- Ohba, M.; Mizuki, E.; Uemori, A. Parasporin, a New Anticancer Protein Group from Bacillus Thuringiensis. Anticancer Res. 2009, 29, 427–433. [Google Scholar]
- Kitada, S.; Abe, Y.; Shimada, H.; Kusaka, Y.; Matsuo, Y.; Katayama, H.; Okumura, S.; Akao, T.; Mizuki, E.; Kuge, O.; et al. Cytocidal Actions of Parasporin-2, an Anti-Tumor Crystal Toxin from Bacillus Thuringiensis. J. Biol. Chem. 2006, 281, 26350–26360. [Google Scholar] [CrossRef]
- Cruz, J.; Suárez-Barrera, M.O.; Rondón-Villarreal, P.; Olarte-Diaz, A.; Guzmán, F.; Visser, L.; Rueda-Forero, N.J. Computational Study, Synthesis and Evaluation of Active Peptides Derived from Parasporin-2 and Spike Protein from Alphacoronavirus against Colorectal Cancer Cells. Biosci. Rep. 2021, 41, BSR20211964. [Google Scholar] [CrossRef] [PubMed]
- Akiba, T.; Okumura, S. Parasporins 1 and 2: Their Structure and Activity. J. Invertebr. Pathol. 2017, 142, 44–49. [Google Scholar] [CrossRef]
- Mizuki, E.; Park, Y.S.; Saitoh, H.; Yamashita, S.; Akao, T.; Higuchi, K.; Ohba, M. Parasporin, a Human Leukemic Cell-Recognizing Parasporal Protein of Bacillus Thuringiensis. Clin. Diagn. Lab. Immunol. 2000, 7, 625–634. [Google Scholar] [CrossRef]
- Brasseur, K.; Auger, P.; Asselin, E.; Parent, S.; Côté, J.-C.; Sirois, M. Parasporin-2 from a New Bacillus Thuringiensis 4R2 Strain Induces Caspases Activation and Apoptosis in Human Cancer Cells. PLoS ONE 2015, 10, e0135106. [Google Scholar] [CrossRef]
- Reich, N.C.; Liu, L. Tracking STAT Nuclear Traffic. Nat. Rev. Immunol. 2006, 6, 602–612. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Deng, J.; Kujawski, M.; Yang, C.; Liu, Y.; Herrmann, A.; Kortylewski, M.; Horne, D.; Somlo, G.; Forman, S.; et al. STAT3-Induced S1PR1 Expression Is Crucial for Persistent STAT3 Activation in Tumors. Nat. Med. 2010, 16, 1421–1428. [Google Scholar] [CrossRef] [PubMed]
- Li, G.-H.; Wei, H.; Lv, S.-Q.; Ji, H.; Wang, D.-L. Knockdown of STAT3 Expression by RNAi Suppresses Growth and Induces Apoptosis and Differentiation in Glioblastoma Stem Cells. Int. J. Oncol. 2010, 37, 103–110. [Google Scholar] [PubMed]
- Norouzi, P.; Mirmohammadi, M.; Houshdar Tehrani, M.H. Anticancer Peptides Mechanisms, Simple and Complex. Chem. Biol. Interact 2022, 368, 110194. [Google Scholar] [CrossRef] [PubMed]
- Chantawannakul, J.; Chatpattanasiri, P.; Wattayagorn, V.; Kongsema, M.; Noikaew, T.; Chumnanpuen, P. Virtual Screening for Biomimetic Anti-Cancer Peptides from Cordyceps Militaris Putative Pepsinized Peptidome and Validation on Colon Cancer Cell Line. Molecules 2021, 26, 5767. [Google Scholar] [CrossRef] [PubMed]
- Hadianamrei, R.; Tomeh, M.A.; Brown, S.; Wang, J.; Zhao, X. Rationally Designed Short Cationic α-Helical Peptides with Selective Anticancer Activity. J. Colloid Interface Sci. 2022, 607, 488–501. [Google Scholar] [CrossRef] [PubMed]
- Cerella, C.; Dicato, M.; Diederich, M. BH3 Mimetics in AML Therapy: Death and Beyond? Trends Pharmacol. Sci. 2020, 41, 793–814. [Google Scholar] [CrossRef]
- Hao, Y.; Chung, C.K.; Gu, Z.; Schomann, T.; Dong, X.; Veld, R.V.H.; Camps, M.G.M.; Ten Dijke, P.; Ossendorp, F.A.; Cruz, L.J. Combinatorial Therapeutic Approaches of Photodynamic Therapy and Immune Checkpoint Blockade for Colon Cancer Treatment. Mol. Biomed. 2022, 3, 26. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Bioinformatics Enrichment Tools: Paths toward the Comprehensive Functional Analysis of Large Gene Lists. Nucleic Acids Res. 2009, 37, 1–13. [Google Scholar] [CrossRef]
- Verhagen, A.M.; Lock, P. Revealing the Intricacies of Cancer. Genome Biol. 2002, 3, reports4015.1. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).