Interferon-Gamma Primed Human Clonal Mesenchymal Stromal Cell Sheets Exhibit Enhanced Immunosuppressive Function
Abstract
1. Introduction
2. Materials and Methods
2.1. hcBMSC Culture
2.2. hcBMSC Cell Sheet Fabrication
2.3. Histological Analysis
2.4. hcBMSC Sheet—Hpbmc Coculture Assays
2.5. Quantitative Real-Time PCR Analysis
2.6. Protein Secretion Assays
2.7. Statistical Analysis
3. Results
3.1. hcBMSC Sheets Respond to IFN-γ in a Dose-Dependent Manner
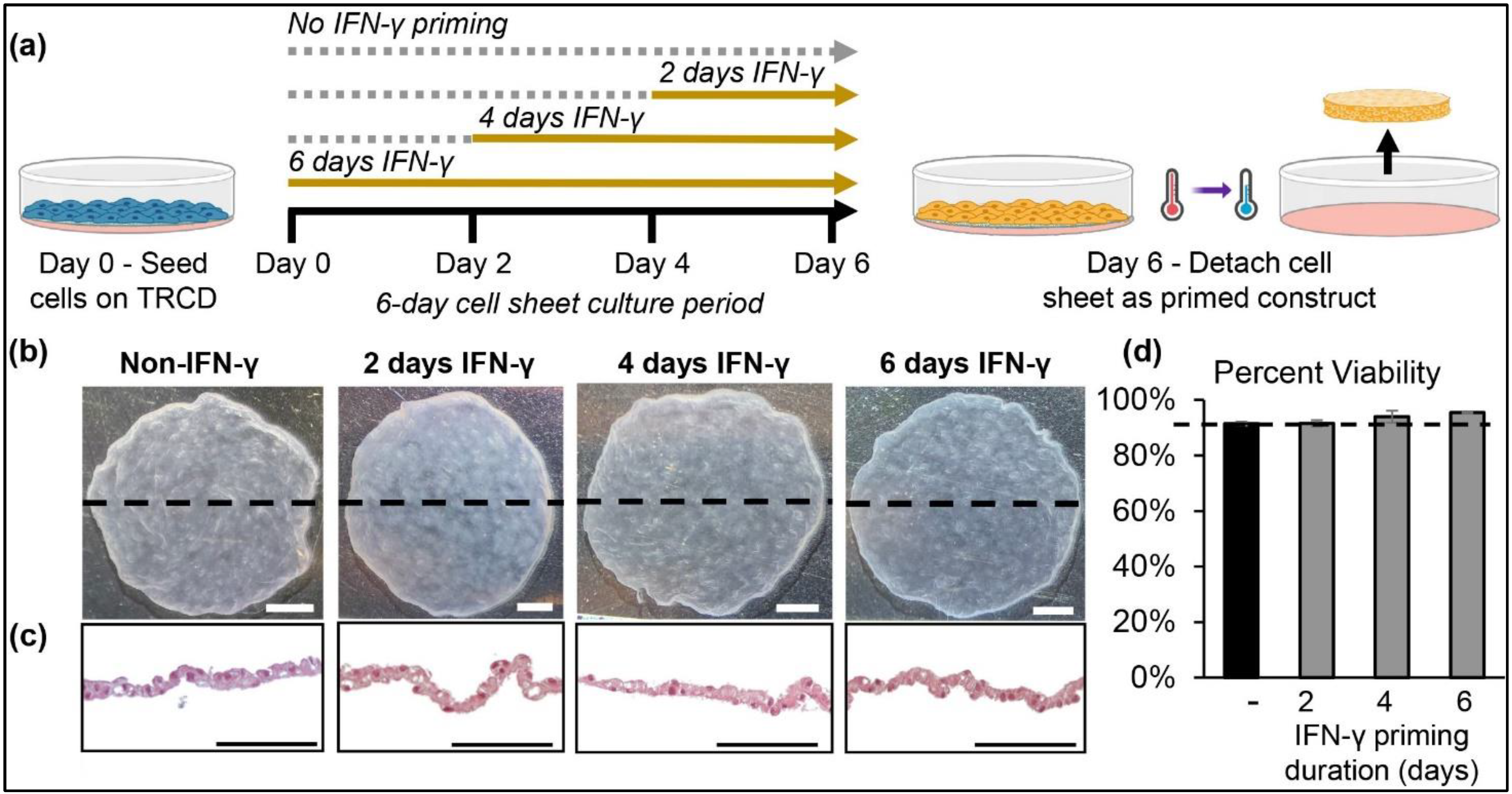
3.2. hcBMSCs Readily Detach as 3D Cell Sheets after IFN-γ Priming
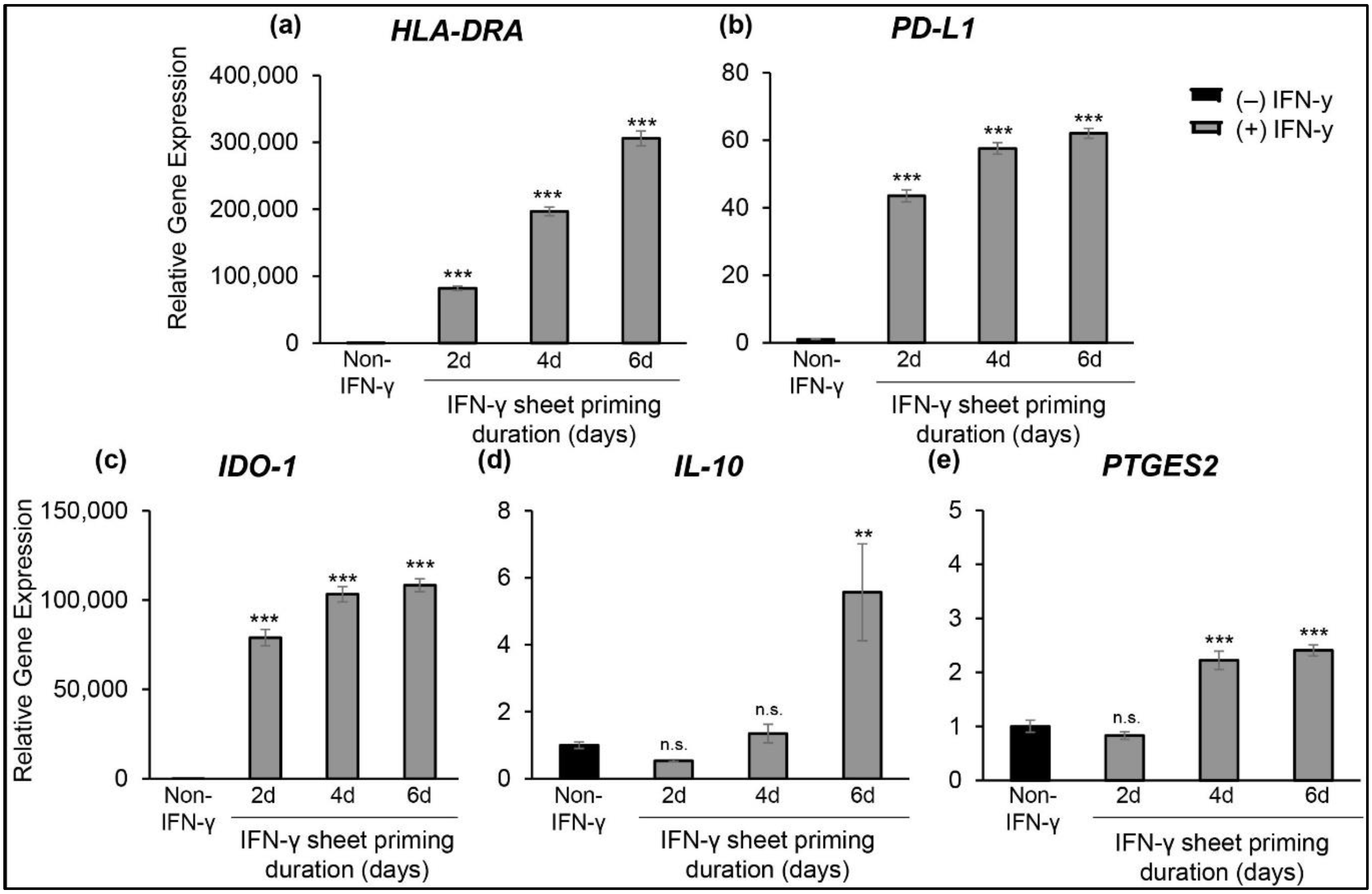
3.3. IFN-γ Priming Duration Directly Relates to hcBMSC Gene Expression
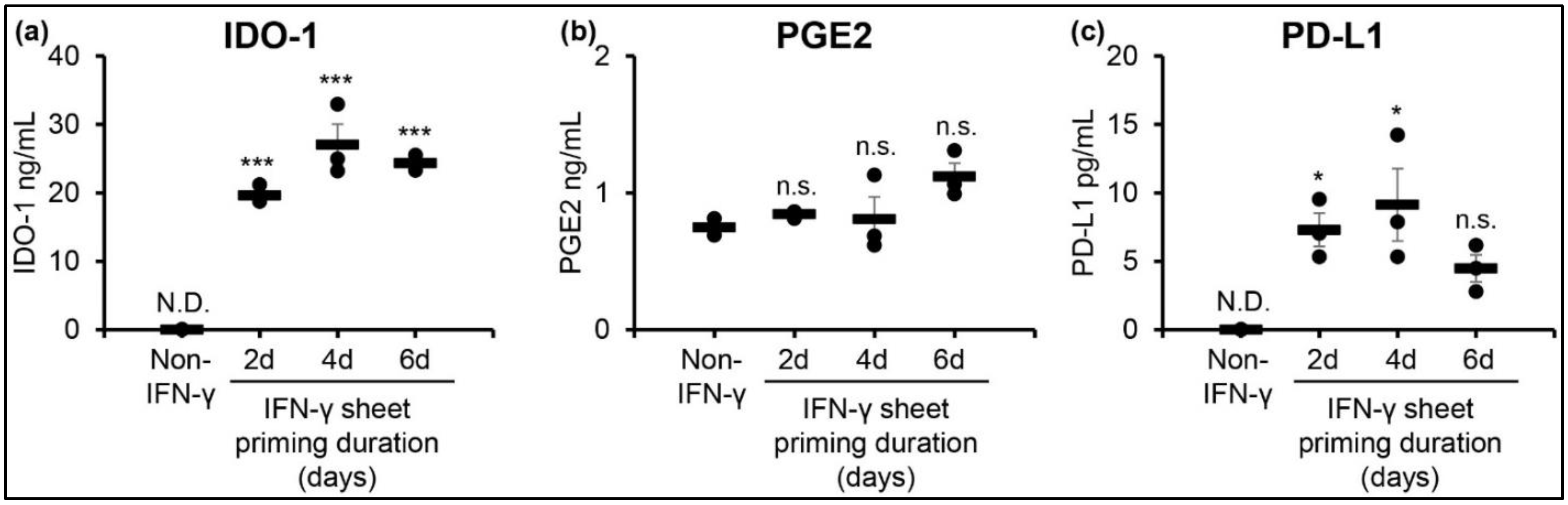
3.4. IFN-γ Primed hcBMSC Sheets Upregulate Soluble Factor Secretion for 4 Days Post-IFN-γ Removal
3.5. IFN-γ Primed hcBMSC Sheets Inhibit T Cell Proliferation in Indirect Coculture
3.6. Cell-Contact Increases Immunosuppression Activity of Primed and Non-Primed hcBMSC Sheets
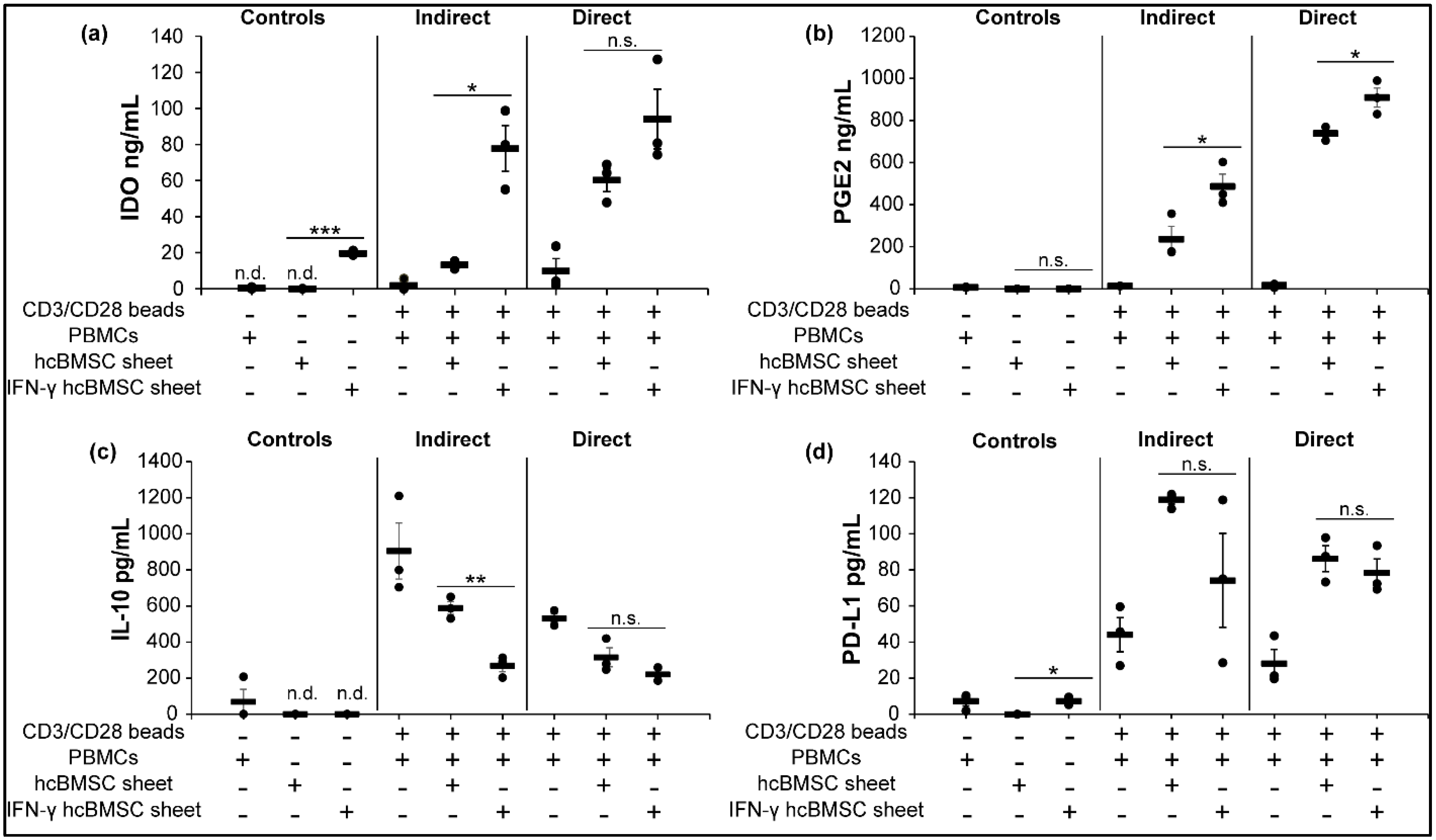
3.7. Concentration of Soluble Immunosuppressive Factors Increases in hcBMSC Sheet-hPBMC Coculture
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aggarwal, S.; Pittenger, M.F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005, 105, 1815–1822. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Scholtemeijer, M.; Shah, K. Mesenchymal stem cell immunomodulation: Mechanisms and therapeutic potential. Trends Pharmacol. Sci. 2020, 41, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Wang, Y.; Li, Q.; Liu, K.; Hou, J.; Shao, C.; Wang, Y. Immunoregulatory mechanisms of mesenchymal stem and stromal cells in inflammatory diseases. Nat. Rev. Nephrol. 2018, 14, 493–507. [Google Scholar]
- Wright, A.; Arthaud-Day, M.L.; Weiss, M.L. Therapeutic use of mesenchymal stromal cells: The need for inclusive characterization guidelines to accommodate all tissue sources and species. Front. Cell Dev. Biol. 2021, 9, 632717. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Yu, G.; Yang, K.; Xiang, W.; Li, J.; Chen, H. Efficacy and safety of mesenchymal stem cell transplantation in the treatment of autoimmune diseases (rheumatoid arthritis, systemic lupus erythematosus, inflammatory bowel disease, multiple sclerosis, and ankylosing spondylitis): A systematic review and meta-analysis of randomized controlled trial. Stem Cells Int. 2022, 2022, 9463314. [Google Scholar] [CrossRef]
- Huldani, H.; Margiana, R.; Ahmad, F.; Opulencia, M.J.C.; Ansari, M.J.; Bokov, D.O.; Abdullaeva, N.N.; Siahmansouri, H. Immunotherapy of inflammatory bowel disease (IBD) through mesenchymal stem cells. Int. Immunopharmacol. 2022, 107, 108698. [Google Scholar]
- Wobma, H.M.; Liu, D.; Vunjak-novakovic, G. Paracrine effects of mesenchymal stromal cells cultured in three-dimensional settings on tissue repair. ACS Biomater. Sci. Eng. 2018, 4, 1162–1175. [Google Scholar] [CrossRef]
- Ferreira, J.R.; Teixeira, G.Q.; Santos, S.G.; Barbosa, M.A.; Almeida-Porada, G.; Goncalves, R.M. Mesenchymal stromal cell secretome: Influencing therapeutic potential by cellular pre-conditioning. Front. Immunol. 2018, 9, 2837. [Google Scholar] [CrossRef]
- Galipeau, J.; Sensébé, L. Mesenchymal stromal cells: Clinical challenges and therapeutic opportunities. Cell Stem Cell 2018, 22, 824–833. [Google Scholar] [CrossRef]
- Galipeau, J.; Krampera, M.; Leblanc, K.; Nolta, J.A.; Phinney, D.G.; Shi, Y.; Tarte, K.; Viswanathan, S.; Martin, I. Mesenchymal stromal cell variables influencing clinical potency: The impact of viability, fitness, route of administration and host predisposition. Cytotherapy 2021, 23, 368–372. [Google Scholar]
- Steen, E.H.; Wang, X.; Balaji, S.; Butte, M.J.; Bollyky, P.L.; Keswani, S.G. The role of the anti-inflammatory cytokine interleukin-10 in tissue fibrosis. Adv. Wound Care 2020, 9, 184–198. [Google Scholar] [CrossRef] [PubMed]
- Qu, X.; Liu, X.; Cheng, K.; Yang, R.; Zhao, R.C.H. Mesenchymal stem cells inhibit Th17 cell differentiation by IL-10 secretion. Exp. Hematol. 2012, 40, 761–770. [Google Scholar] [PubMed]
- English, K.; Ryan, J.M.; Tobin, L.; Murphy, M.J.; Barry, F.P.; Mahon, B.P. Cell contact, prostaglandin E2 and transforming growth factor beta 1 play non-redundant roles in human mesenchymal stem cell induction of CD4+CD25 highforkhead box P3+ regulatory T cells. Clin. Exp. Immunol. 2009, 156, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.; Lin, C.; Chiang, B.; Jui, H.; Wu, K.K.; Lee, C. Prostaglandin E2 potentiates mesenchymal stem cell−induced IL-10+IFN-γ+CD4+ regulatory T cells to control transplant arteriosclerosis. J. Immunol. 2013, 190, 2372–2380. [Google Scholar] [CrossRef] [PubMed]
- Mellor, A.L.; Lemos, H.; Huang, L.; Mellor, A.L. Indoleamine 2,3-dioxygenase and tolerance: Where are we now? Front. Immunol. 2017, 8, 1–6. [Google Scholar]
- Jones, B.J.; Brooke, G.; Atkinson, K.; Mctaggart, S.J. Immunosuppression by placental indoleamine 2, 3-dioxygenase: A role for mesenchymal stem cells. Placenta 2007, 28, 1174–1181. [Google Scholar] [CrossRef]
- François, M.; Romieu-mourez, R.; Li, M.; Galipeau, J. Human MSC suppression correlates with cytokine induction of indoleamine 2, 3-dioxygenase and bystander M2 macrophage differentiation. Mol. Ther. 2012, 20, 187–195. [Google Scholar] [CrossRef]
- Chinnadurai, R.; Rajan, D.; Qayed, M.; Arafat, D.; Garcia, M.; Liu, Y.; Kugathasan, S.; Anderson, L.J.; Gibson, G.; Galipeau, J. Potency analysis of mesenchymal stromal cells using a combinatorial assay matrix approach. Cell Rep. 2018, 22, 2504–2517. [Google Scholar] [CrossRef]
- Davies, L.C.; Heldring, N.; Kadri, N.; le Blanc, K. Mesenchymal stromal cell secretion of programmed death-1 ligands regulates T cell mediated immunosuppression. Stem Cells 2017, 35, 766–776. [Google Scholar]
- Augello, A.; Tasso, R.; Negrini, S.M.; Amateis, A.; Indiveri, F.; Cancedda, R.; Pennesi, G. Bone marrow mesenchymal progenitor cells inhibit lymphocyte proliferation by activation of the programmed death 1 pathway. Eur. J. Immunol. 2005, 35, 1482–1490. [Google Scholar] [CrossRef]
- Selmani, Z.; Naji, A.; Zidi, I.; Favier, B.; Gaiffe, E.; Obert, L.; Borg, C.; Saas, P.; Tiberghien, P.; Rouas-Freiss, N.; et al. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells 2007, 26, 212–222. [Google Scholar] [PubMed]
- Sivanathan, K.N.; Rojas-Canales, D.M.; Hope, C.M.; Krishnan, R.; Carroll, R.P.; Gronthos, S.; Grey, S.T.; Coates, P.T. Interleukin-17A-induced human mesenchymal stem cells are superior modulators of immunological function. Stem Cells 2015, 33, 2850–2863. [Google Scholar] [PubMed]
- Melief, S.M.; Schrama, E.; Brugman, M.H.; Tiemessen, M.M.; Hoogduijn, M.J.; Fibbe, W.E.; Roelofs, H. Multipotent stromal cells induce human regulatory T cells through a novel pathway involving skewing of monocytes toward. Stem Cells Transl. Clin. Res. 2013, 31, 1980–1991. [Google Scholar]
- Corcione, A.; Benvenuto, F.; Ferretti, E.; Giunti, D.; Cappiello, V.; Cazzanti, F.; Risso, M.; Gualandi, F.; Mancardi, G.L.; Pistoia, V.; et al. Human mesenchymal stem cells modulate B-cell functions. Stem Cells Hematol. 2006, 107, 367–372. [Google Scholar]
- Asari, S.; Itakura, S.; Ferreri, K.; Liu, C.P.; Kuroda, Y.; Kandeel, F.; Mullen, Y. Mesenchymal stem cells suppress B cell terminal differentiation. Exp. Hematol. 2010, 37, 604–615. [Google Scholar]
- Polchert, D.; Sobinsky, J.; Douglas, G.W.; Kidd, M.; Moadsiri, A.; Reina, E.; Genrich, K.; Mehrotra, S.; Setty, S.; Smith, B.; et al. IFN-y activation of mesenchymal stem cells for treatment and prevention of graft versus host disease. Eur. J. Immunol. 2008, 38, 1745–1755. [Google Scholar]
- Silva-Carvalho, A.É.; Sousa, M.R.R.; Alencar-silva, T.; Carvalho, J.L.; Saldanha-araujo, F. Mesenchymal stem cells immunomodulation: The road to IFN-γ licensing and the path ahead. Cytokine Growth Factor Rev. 2019, 47, 32–42. [Google Scholar]
- Dunn, C.M.; Kameishi, S.; Grainger, D.W.; Okano, T. Strategies to address mesenchymal stem-stromal cell heterogeneity in immunomodulatory profiles to improve cell-based therapies. Acta Biomater. 2021, 133, 114–125. [Google Scholar]
- Tsuji, K.; Kitamura, S.; Wada, J. Secretomes from mesenchymal stem cells against acute kidney injury: Possible heterogeneity. Stem Cells Int. 2018, 2018, 8693137. [Google Scholar] [CrossRef] [PubMed]
- Szabó, E.; Fajka-Boja, R.; Kriston-Pál, É.; Hornung, Á.; Makra, I.; Kudlik, G.; Uher, F.; Katona, R.L.; Monostori, É.; Czibula, A. Licensing by inflammatory cytokines abolishes heterogeneity of immunosuppressive function of mesenchymal stem cell population. Stem Cells Dev. 2015, 24, 2171–2180. [Google Scholar] [CrossRef]
- Krampera, M.; Cosmi, L.; Angeli, R.; Pasini, A.; Liotta, F.; Andreini, A.; Santarlasci, V.; Mazzinghi, B.; Pizzolo, G.; Vinante, F.; et al. Role for interferon-y in the immunomodulatory activity of human bone marrow mesenchymal stem cells. Stem Cells Transl. Clin. Res. 2006, 24, 386–398. [Google Scholar]
- Kim, D.S.; Jang, I.K.; Lee, M.W.; Ko, Y.J.; Lee, D.H.; Lee, J.W.; Sung, K.W.; Koo, H.H.; Yoo, K.H. Enhanced immunosuppressive properties of human mesenchymal stem cells primed by interferon-γ. EBioMedicine 2018, 28, 261–273. [Google Scholar] [PubMed]
- Duijvestein, M.; Wildenberg, M.E.; Welling, M.M.; Hennink, S.; Molendijk, I.; van Zuylen, V.L.; Bosse, T.; Vos, A.C.W.; de Jonge-Muller, E.S.; Roelofs, H.; et al. Pretreatment with interferon-y enhances the therapeutic activity of mesenchymal stromal cells in animal models of colitis. Stem Cells Regen. Med. 2011, 29, 1549–1558. [Google Scholar]
- Kanai, R.; Nakashima, A.; Doi, S.; Kimura, T.; Yoshida, K. Interferon-γ enhances the therapeutic effect of mesenchymal stem cells on experimental renal fibrosis. Sci. Rep. 2021, 11, 850. [Google Scholar] [CrossRef] [PubMed]
- Schrepfer, S.; Deuse, T.; Reichenspurner, H.; Fischbein, M.P.; Robbins, R.C.; Pelletier, M.P. Stem cell transplantation: The lung barrier. Transplant. Proc. 2007, 39, 573–576. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Bou-ghannam, S.; Kameishi, S.; Oka, M.; Grainger, D.W.; Okano, T. Allogeneic mesenchymal stem cell sheet therapy: A new frontier in drug delivery systems. J. Control. Release 2021, 330, 696–704. [Google Scholar] [PubMed]
- Okano, T.; Yamada, N.; Okuhara, M.; Sakai, H. Mechanism of cell detachment from hydrophobic polymer surfaces. Biomaterials 1995, 16, 297–303. [Google Scholar]
- Yamato, M.; Okano, T. Cell sheet engineering. Mater. Today 2004, 7, 42–47. [Google Scholar]
- Imafuku, A.; Oka, M.; Miyabe, Y.; Sekiya, S.; Nitta, K.; Shimizu, T. Rat mesenchymal stromalcell sheets suppress renal fibrosis via microvascular protection. Stem Cells Transl. Med. 2019, 8, 1330–1341. [Google Scholar] [PubMed]
- Sekine, H.; Shimizu, T.; Dobashi, I.; Matsuura, K.; Hagiwara, N.; Takahashi, M.; Kobayashi, E.; Yamato, M.; Okano, T. Cardiac cell sheet transplantation improves damaged heart function via superior cell survival in comparison with dissociated cell injection. Tissue Eng. Part A 2011, 17, 2973–2980. [Google Scholar]
- Narita, T.; Shintani, Y.; Ikebe, C.; Kaneko, M.; Campbell, N.G.; Coppen, S.R.; Uppal, R.; Sawa, Y.; Yashiro, K.; Suzuki, K. The use of scaffold-free cell sheet technique to refine mesenchymal stromal cell-based therapy for heart failure. Mol. Ther. 2013, 21, 860–867. [Google Scholar] [CrossRef] [PubMed]
- Yi, T.; Kim, S.; Lee, H.; Kim, J.; Cho, Y. Manufacture of clinical-grade human clonal colony forming unit-derived colonies based on the subfractionation culturing method. Tissue Eng. Part C 2015, 21, 1251–1262. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Peinado, P.; Pascual-García, S.; Roche, E.; Sempere-Ortells, J.M. Differences of clonogenic mesenchymal stem cells on immunomodulation of lymphocyte subsets. J. Immunol. Res. 2018, 2018, 7232717. [Google Scholar] [CrossRef] [PubMed]
- Phinney, D.G. Functional heterogeneity of mesenchymal stem cells: Implications for cell therapy. J. Cell. Biochem. 2012, 113, 2806–2812. [Google Scholar] [CrossRef]
- Marklein, R.A.; Klinker, M.W.; Drake, K.A.; Polikowsky, H.G.; Lessey-morillon, E.C.; Bauer, S.R. Morphological profiling using machine learning reveals emergent subpopulations of interferon-y-stimulated mesenchymal stromal cells that predict immunosuppression. Cytotherapy 2019, 21, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.X.; Han, Z.B.; Ji, Y.R.; Wang, Y.W.; Liang, L.; Chi, Y.; Yang, S.G.; Li, L.N.; Luo, W.F.; Li, J.P.; et al. CD106 identifies a subpopulation of mesenchymal stem cells with unique immunomodulatory properties. PLoS ONE 2013, 8, e59354. [Google Scholar] [CrossRef] [PubMed]
- Selich, A.; Daudert, J.; Hass, R.; Philipp, F.; von Kaisenberg, C.; Paul, G.; Cornils, K.; Fehse, B.; Rittinghausen, S.; Schambach, A.; et al. Massive clonal selection and transiently contributing clones during expansion of mesenchymal stem cell cultures revealed by lentiviral RGB-barcode technology. Stem Cells Transl. Med. 2016, 5, 591–601. [Google Scholar] [CrossRef]
- Marrazzo, P.; Pizzuti, V.; Zia, S.; Sargenti, A.; Gazzola, D.; Roda, B.; Bonsi, L.; Alviano, F. Microfluidic Tools for Enhanced Characterization of Therapeutic Stem Cells and Prediction of Their Potential Antimicrobial Secretome. Antibiotics 2021, 10, 750. [Google Scholar] [CrossRef]
- Jayaraman, P.; Lim, R.; Ng, J.; Vemuri, M.C. Acceleration of Translational Mesenchymal Stromal Cell Therapy through Consistent Quality GMP Manufacturing. Front. Cell Dev. Biol. 2021, 9, 648472. [Google Scholar] [CrossRef] [PubMed]
- Roda, B.; Lanzoni, G.; Alviano, F.; Zattoni, A.; Costa, R.; Di Carlo, A.; Marchionni, C.; Franchina, M.; Ricci, F.; Tazzari, P.L.; et al. A Novel Stem Cell Tag-Less Sorting Method. Stem Cell Rev. Rep. 2009, 5, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Wilson, S.; Fitzpatrick, I.; Barabadi, M.; Chan, S.T.; Krause, M.; Kusuma, G.D.; James, D.; Lim, R. Automated Counterflow Centrifugal System for Small-Scale Cell Processing. J. Vis. Exp. 2019, 12, e60423. [Google Scholar] [CrossRef] [PubMed]
- Dargitz, C.T.; Daoudi, S.; Dunn, S.; Jeu, X.d.d.; Ravinder, N. Rotea: A closed and automated instrument for efficient cell isolation, washing and concentration in cell therapy workflows. Cytotherapy 2020, 22, S200. [Google Scholar]
- Kim, M.; Kim, K.H.; Song, S.U.; Yi, T.G.; Yoon, S.H.; Park, S.R.; Choi, B.H. Transplantation of human bone marrow-derived clonal mesenchymal stem cells reduces fibrotic scar formation in a rat spinal cord injury model. J. Tissue Eng. Regen. Med. 2017, 12, 1034–1045. [Google Scholar] [CrossRef] [PubMed]
- Yi, H.G.; Yahng, S.A.; Kim, I.; Lee, J.H.; Min, C.K.; Kim, J.H.; Kim, C.S.; Song, S.U. Allogeneic clonal mesenchymal stem cell therapy for refractory graft-versus-host disease to standard treatment: A phase I study. Korean J. Physiol. Pharm. 2016, 20, 63–67. [Google Scholar] [CrossRef]
- Song, S.U.; Kim, C.S.; Yoon, S.P.; Kim, S.K.; Lee, M.H.; Kang, J.S.; Choi, G.S.; Moon, S.H.; Choi, M.S.; Cho, Y.K.; et al. Variations of clonal marrow stem cell lines established from human bone marrow in surface epitopes, differentation potential, gene expression, and cytokine secretion. Stem Cells Dev. 2008, 461, 451–461. [Google Scholar]
- Jung, K.H.; Song, S.U.; Yi, T.; Jeon, M.S.; Hong, S.W.; Zheng, H.M.; Lee, H.S.; Choi, M.J.; Lee, D.H.; Hong, S.S. Human bone marrow–derived clonal mesenchymal stem cells inhibit inflammation and reduce acute pancreatitis in rats. Gastroenterology 2011, 140, 998–1008. [Google Scholar] [PubMed]
- Thorp, H.; Kim, K.; Kondo, M.; Grainger, D.W.; Okano, T. Fabrication of hyaline-like cartilage constructs using mesenchymal stem cell sheets. Sci. Rep. 2020, 10, 20869. [Google Scholar] [CrossRef]
- Boyt, D.T.; Boland, L.K.; Jr, A.J.B.; Brown, A.J.; Ankrum, J.A. Dose and duration of interferonγ pre-licensing interact with donor characteristics to influence the expression and function of indoleamine-2,3-dioxygenase in mesenchymal stromal cells. Interface 2020, 17, 20190815. [Google Scholar] [CrossRef]
- Burand, A.J., Jr.; Di, L.; Boland, L.K.; Boyt, D.T.; Schrodt, M.V.; Santillan, D.A.; Ankrum, J.A. Aggregation of human mesenchymal stromal cells eliminates their ability to suppress human T cells. Front. Immunol. 2020, 11, 143. [Google Scholar] [CrossRef]
- Lee, H.; Kim, S.; Jeon, M.; Yi, T.; Song, S.U. ICOSL expression in human bone marrow-derived mesenchymal stem cells promotes induction of regulatory T cells. Sci. Rep. 2017, 7, 44486. [Google Scholar] [CrossRef]
- Piekarska, K.; Urban-Wójciuk, Z.; Kurkowiak, M.; Pelikant-Małecka, I.; Schumacher, A.; Sakowska, J.; Spodnik, J.H.; Arcimowicz, Ł.; Zielińska, H.; Tymoniuk, B.; et al. Mesenchymal stem cells transfer mitochondria to allogeneic Tregs in an HLA-dependent manner improving their immunosuppressive activity. Nat. Commun. 2022, 13, 856. [Google Scholar] [CrossRef] [PubMed]
- English, K.; Barry, F.P.; Field-corbett, C.P.; Mahon, B.P. IFN-y and TNF-a differentially regulate immunomodulation by murine mesenchymal stem cells. Immunol. Lett. 2007, 110, 91–100. [Google Scholar] [PubMed]
- Puccetti, P.; Grohmann, U. IDO and regulatory T cells: A role for reverse signalling and non-canonical NF-κB activation. Nat. Rev. Immunol. 2007, 7, 817–823. [Google Scholar] [PubMed]
- Tipnis, S.; Viswanathan, C.; Majumdar, A.S. Immunosuppressive properties of human umbilical cord-dervied mesenchymal stem cells: Role of B7-H1 and IDO. Immunol. Cell Biol. 2010, 88, 795–806. [Google Scholar] [CrossRef]
- Romieu-mourez, R.; François, M.; Boivin, M.-N.; Stagg, J.; Galipeau, J. Regulation of MHC class II expression and antigen processing in murine and human mesenchymal stromal cells by IFN-γ, TGF-β, and Cell Density. J. Immunol. 2007, 179, 1549–1558. [Google Scholar] [CrossRef] [PubMed]
- Le Blanc, K.; Frassoni, F.; Ball, L.; Locatelli, F.; Roelofs, H.; Lewis, I.; Lanino, E.; Sundberg, B.; Bernardo, M.E.; Remberger, M.; et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: A phase II study. Lancet 2008, 371, 1579–1586. [Google Scholar]
- Barrachina, L.; Remacha, A.R.; Romero, A.; Vitoria, A.; Albareda, J.; Prades, M.; Roca, M.; Zaragoza, P.; Vázquez, F.J.; Rodellar, C. Assessment of effectiveness and safety of repeat administration of proinflammatory primed allogeneic mesenchymal stem cells in an equine model of chemically induced osteoarthritis. BMC Vet. Res. 2018, 14, 241. [Google Scholar] [CrossRef]
- Lohan, P.; Treacy, O.; Morcos, M.; Donohoe, E.; O’donoghue, Y.; Ryan, A.E.; Elliman, S.J.; Ritter, T.; Griffin, M.D. Interspecies incompatibilities limit the immunomodulatory effect of human mesenchymal stromal cells in the rat. Stem Cells Regen. Med. 2018, 36, 1210–1215. [Google Scholar]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dunn, C.M.; Kameishi, S.; Cho, Y.-K.; Song, S.U.; Grainger, D.W.; Okano, T. Interferon-Gamma Primed Human Clonal Mesenchymal Stromal Cell Sheets Exhibit Enhanced Immunosuppressive Function. Cells 2022, 11, 3738. https://doi.org/10.3390/cells11233738
Dunn CM, Kameishi S, Cho Y-K, Song SU, Grainger DW, Okano T. Interferon-Gamma Primed Human Clonal Mesenchymal Stromal Cell Sheets Exhibit Enhanced Immunosuppressive Function. Cells. 2022; 11(23):3738. https://doi.org/10.3390/cells11233738
Chicago/Turabian StyleDunn, Celia M., Sumako Kameishi, Yun-Kyoung Cho, Sun U. Song, David W. Grainger, and Teruo Okano. 2022. "Interferon-Gamma Primed Human Clonal Mesenchymal Stromal Cell Sheets Exhibit Enhanced Immunosuppressive Function" Cells 11, no. 23: 3738. https://doi.org/10.3390/cells11233738
APA StyleDunn, C. M., Kameishi, S., Cho, Y.-K., Song, S. U., Grainger, D. W., & Okano, T. (2022). Interferon-Gamma Primed Human Clonal Mesenchymal Stromal Cell Sheets Exhibit Enhanced Immunosuppressive Function. Cells, 11(23), 3738. https://doi.org/10.3390/cells11233738





