Single-Cell Sequencing Reveals the Regulatory Role of Maresin1 on Neutrophils during Septic Lung Injury
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Animals
2.3. The Lung Histological Examination
2.4. The Neutrophil Count in Bronchoalveolar Lavage Fluid (BALF)
2.5. Immunofluorescence Assay
2.6. Single-Cell Sequenceing
2.7. Single-Cell Data Preprocessing
2.8. Pathway Enrichment Analysis
2.9. The Gene Regulatory Network Analysis
2.10. Statistical Analysis
3. Results
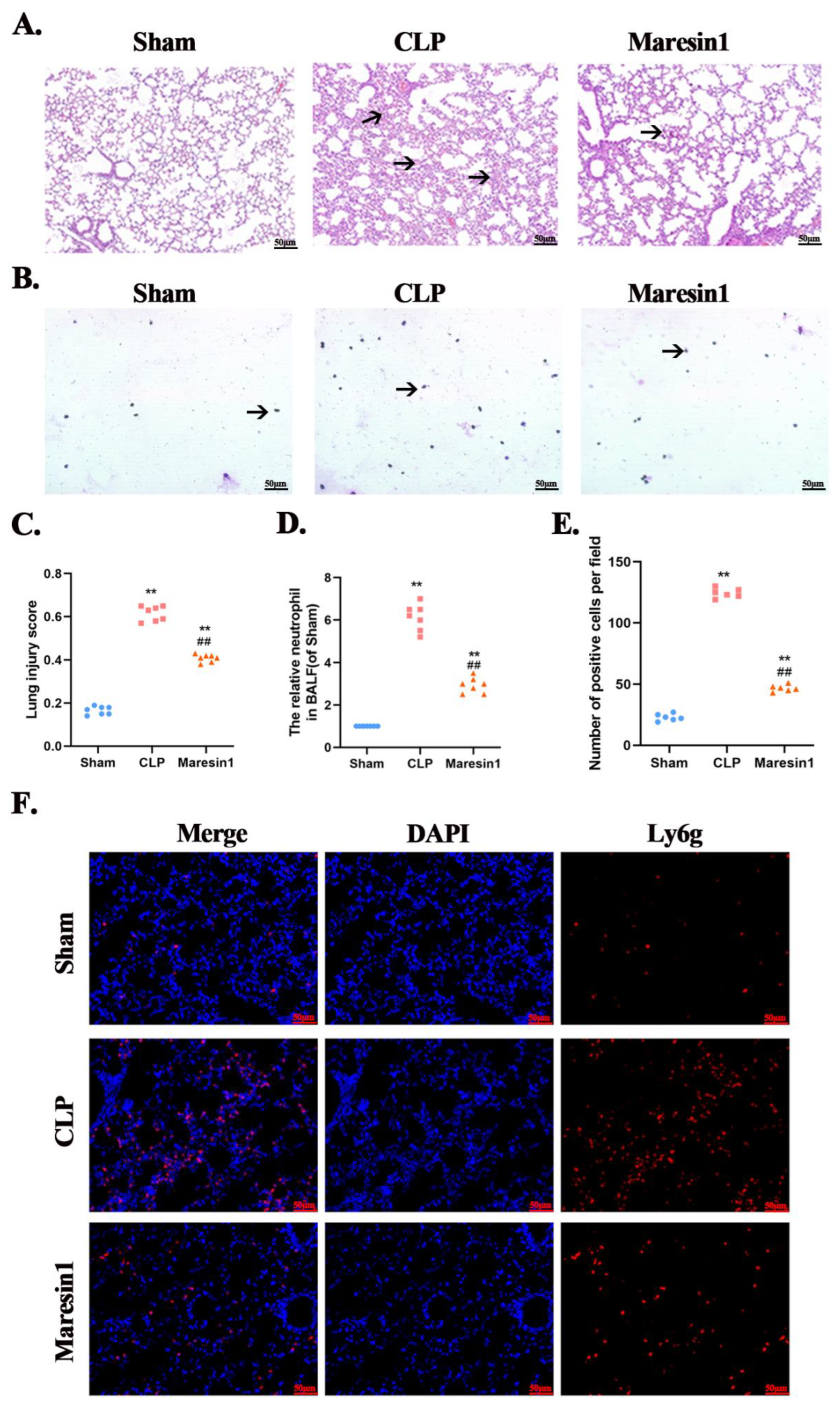
3.1. Maresin1 Attenuates the Extent of Sepsis-Induced Lung Damage
3.2. Maresin1 Reduces Neutrophil Numbers in Bronchoalveolar Lavage
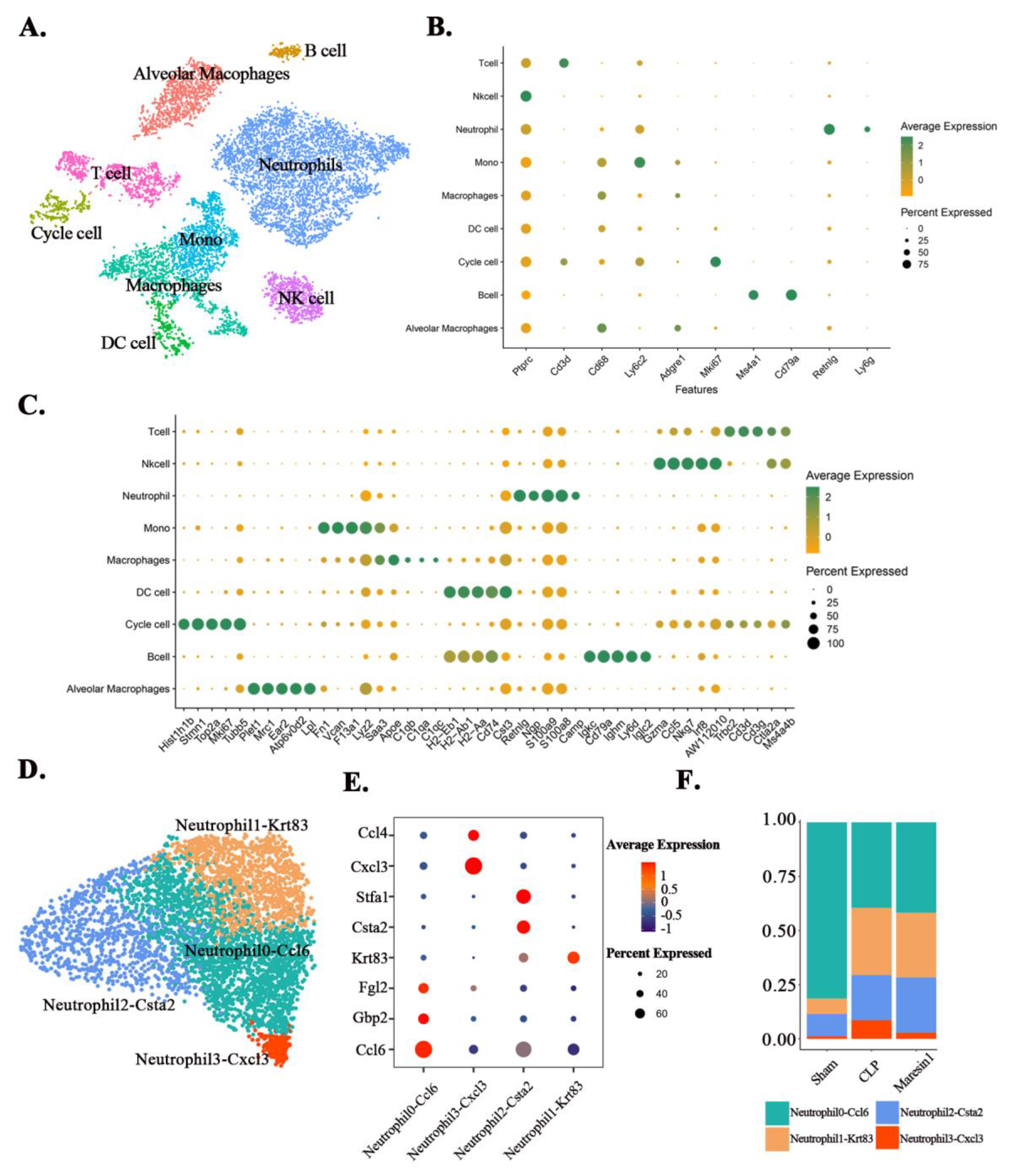
3.3. Maresin1 Alters Neutrophil Composition in Septic Lung Injury
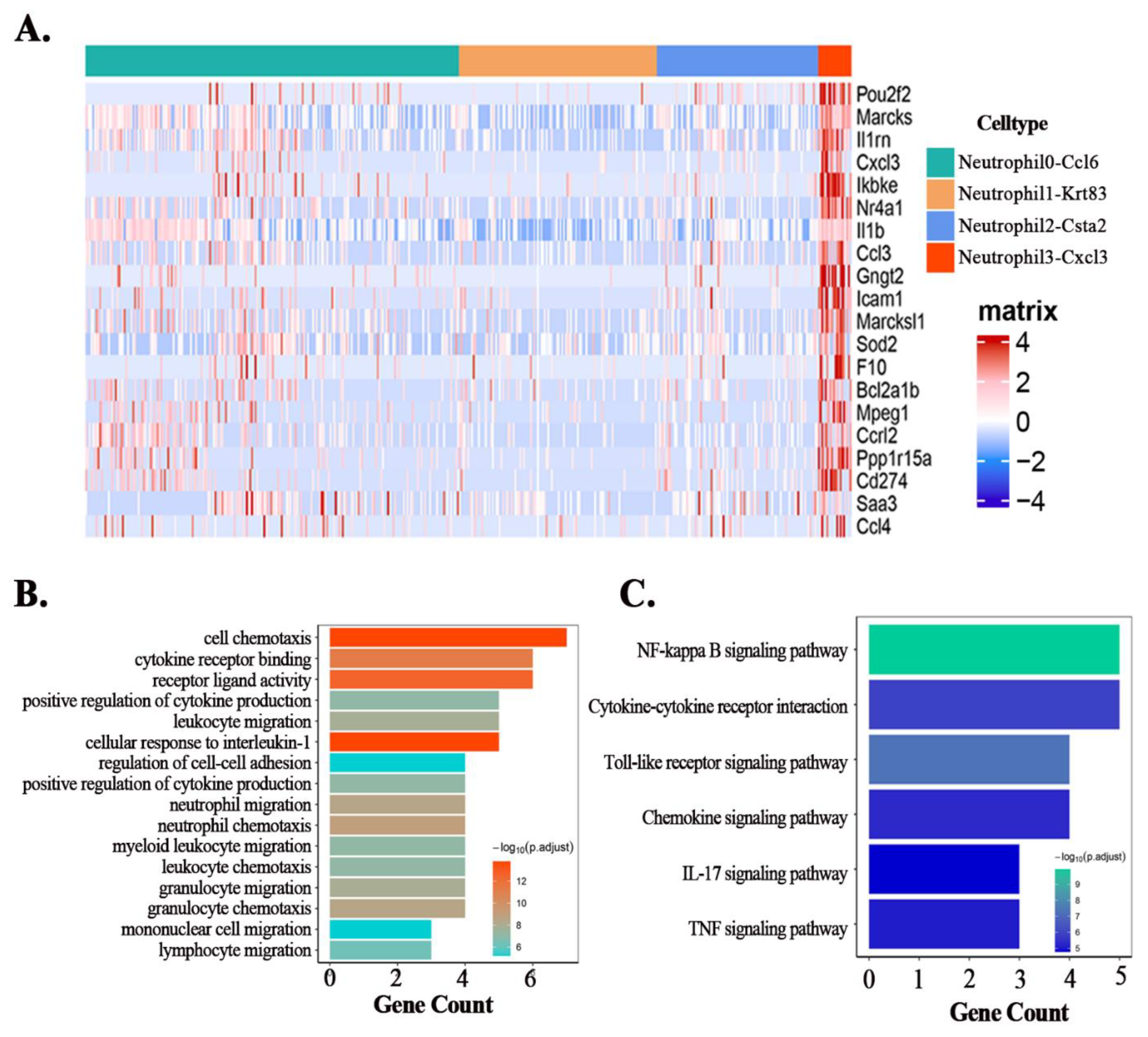
3.4. Maresin1 Inhibits the Expression of Key Genes in the Neutrophil-Cxcl3 Subpopulation
3.5. Maresin1 Represses Transcription Factors in Neutrophil-Cxcl3
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pant, A.; Mackraj, I.; Govender, T. Advances in sepsis diagnosis and management: A paradigm shift towards nanotechnology. J. Biomed. Sci. 2021, 28, 6. [Google Scholar] [CrossRef] [PubMed]
- Hasan, Z.; Palani, K.; Rahman, M.; Thorlacius, H. Targeting CD44 expressed on neutrophils inhibits lung damage in abdominal sepsis. Shock 2011, 35, 567–572. [Google Scholar] [CrossRef]
- Lagu, T.; Rothberg, M.B.; Shieh, M.S.; Pekow, P.S.; Steingrub, J.S.; Lindenauer, P.K. Hospitalizations, costs, and outcomes of severe sepsis in the United States 2003 to 2007. Crit. Care Med. 2012, 40, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Fleischmann, C.; Scherag, A.; Adhikari, N.K.; Hartog, C.S.; Tsaganos, T.; Schlattmann, P.; Angus, D.C.; Reinhart, K. Assessment of Global Incidence and Mortality of Hospital-treated Sepsis. Current Estimates and Limitations. Am. J. Respir. Crit. Care Med. 2016, 193, 259–272. [Google Scholar] [CrossRef]
- Serhan, C.N. Pro-resolving lipid mediators are leads for resolution physiology. Nature 2014, 510, 92–101. [Google Scholar] [CrossRef]
- Schwab, J.M.; Chiang, N.; Arita, M.; Serhan, C.N. Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature 2007, 447, 869–874. [Google Scholar] [CrossRef]
- Buckley, C.D.; Gilroy, D.W.; Serhan, C.N. Proresolving lipid mediators and mechanisms in the resolution of acute inflammation. Immunity 2014, 40, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Che, C.; Lin, J.; He, H.; Zhao, W.; Lv, L.; Zhao, G. Maresin1 regulates neutrophil recruitment and IL-10 expression in Aspergillus fumigatus keratitis. Int. Immunopharmacol. 2019, 69, 103–108. [Google Scholar] [CrossRef]
- Qiao, N.; Lin, Y.; Wang, Z.; Chen, J.Y.; Ge, Y.Y.; Yao, S.L.; Gong, J. Maresin1 Promotes M2 Macrophage Polarization Through Peroxisome Proliferator-Activated Receptor-γ Activation to Expedite Resolution of Acute Lung Injury. J. Surg. Res. 2020, 256, 584–594. [Google Scholar] [CrossRef]
- Gong, J.; Liu, H.; Wu, J.; Qi, H.; Wu, Z.Y.; Shu, H.Q.; Li, H.B.; Chen, L.; Wang, Y.X.; Li, B.; et al. Maresin 1 prevents lipopolysaccharide-induced neutrophil survival and accelerates resolution of acute lung injurY. Shock 2015, 44, 371–380. [Google Scholar] [CrossRef]
- Wang, F.; Wang, M.; Wang, J.; Chen, M.; Sun, S.; Yao, S.; Xia, H. Maresin1 ameliorates sepsis-associated lung injury by inhibiting the activation of the JAK2/STAT3 and MAPK/ NF-κB signaling pathways. Microb. Pathog. 2020, 148, 104468. [Google Scholar] [CrossRef] [PubMed]
- Grommes, J.; Soehnlein, O. Contribution of neutrophils to acute lung injury. Mol. Med. 2011, 17, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Rahman, M.; Zhang, S.; Qi, Z.; Thorlacius, H. Simvastatin antagonizes CD40L secretion, CXC chemokine formation, and pulmonary infiltration of neutrophils in abdominal sepsis. J. Leukoc. Biol. 2011, 89, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Nicolás-Ávila, J.; Adrover, J.M.; Hidalgo, A. Neutrophils in Homeostasis, Immunity, and Cancer. Immunity 2017, 46, 15–28. [Google Scholar] [CrossRef]
- Nauseef, W.M.; Borregaard, N. Neutrophils at work. Nat. Immunol. 2014, 15, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Silvestre-Roig, C.; Hidalgo, A.; Soehnlein, O. Neutrophil heterogeneity: Implications for homeostasis and pathogenesis. Blood 2016, 127, 2173–2181. [Google Scholar] [CrossRef]
- Xie, X.; Shi, Q.; Wu, P.; Zhang, X.; Kambara, H.; Su, J.; Yu, H.; Park, S.Y.; Guo, R.; Ren, Q.; et al. Single-cell transcriptome profiling reveals neutrophil heterogeneity in homeostasis and infection. Nat. Immunol. 2020, 21, 1119–1133. [Google Scholar] [CrossRef]
- Gong, J.; Wu, Z.Y.; Qi, H.; Chen, L.; Li, H.B.; Li, B.; Yao, C.Y.; Wang, Y.X.; Wu, J.; Yuan, S.Y.; et al. Maresin 1 mitigates LPS-induced acute lung injury in mice. Br. J. Pharmacol. 2014, 171, 3539–3550. [Google Scholar] [CrossRef]
- Stubbington, M.J.T.; Rozenblatt-Rosen, O.; Regev, A.; Teichmann, S.A. Single-cell transcriptomics to explore the immune system in health and disease. Science 2017, 358, 58–63. [Google Scholar] [CrossRef]
- Sun, S.; Wang, J.; Wang, J.; Wang, F.; Yao, S.; Xia, H. Maresin 1 Mitigates Sepsis-Associated Acute Kidney Injury in Mice via Inhibition of the NF-κB/STAT3/MAPK Pathways. Front. Pharmacol. 2019, 10, 1323. [Google Scholar] [CrossRef]
- Matute-Bello, G.; Downey, G.; Moore, B.B.; Groshong, S.D.; Matthay, M.A.; Slutsky, A.S.; Kuebler, W.M. An official American Thoracic Society workshop report: Features and measurements of experimental acute lung injury in animals. Am. J. Respir. Cell Mol. Biol. 2011, 44, 725–738. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhou, L.; Liu, L.; Hou, Y.; Xiong, M.; Yang, Y.; Hu, J.; Chen, K. Single-cell RNA sequencing highlights the role of inflammatory cancer-associated fibroblasts in bladder urothelial carcinoma. Nat. Commun. 2020, 11, 5077. [Google Scholar] [CrossRef] [PubMed]
- Korsunsky, I.; Millard, N.; Fan, J.; Slowikowski, K.; Zhang, F.; Wei, K.; Baglaenko, Y.; Brenner, M.; Loh, P.R.; Raychaudhuri, S. Fast, sensitive and accurate integration of single-cell data with Harmony. Nat. Methods 2019, 16, 1289–1296. [Google Scholar] [CrossRef] [PubMed]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene ontology: Tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. ClusterProfiler: An R package for comparing biological themes among gene clusters. Omics A J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Aibar, S.; González-Blas, C.B.; Moerman, T.; Huynh-Thu, V.A.; Imrichova, H.; Hulselmans, G.; Rambow, F.; Marine, J.C.; Geurts, P.; Aerts, J.; et al. SCENIC: Single-cell regulatory network inference and clustering. Nat. Methods 2017, 14, 1083–1086. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef]
- Hurskainen, M.; Mižíková, I.; Cook, D.P.; Andersson, N.; Cyr-Depauw, C.; Lesage, F.; Helle, E.; Renesme, L.; Jankov, R.P.; Heikinheimo, M.; et al. Single cell transcriptomic analysis of murine lung development on hyperoxia-induced damage. Nat. Commun. 2021, 12, 1565. [Google Scholar] [CrossRef]
- Chen, L.; Chou, C.L.; Knepper, M.A. Targeted Single-Cell RNA-seq Identifies Minority Cell Types of Kidney Distal Nephron. J. Am. Soc. Nephrol. JASN 2021, 32, 886–896. [Google Scholar] [CrossRef]
- Farooq, M.; Ito, M.; Naito, M.; Shimomura, Y. A case of monilethrix caused by novel compound heterozygous mutations in the desmoglein 4 (DSG4) gene. Br. J. Dermatol. 2011, 165, 425–431. [Google Scholar] [CrossRef]
- Sagy, M.; Al-Qaqaa, Y.; Kim, P. Definitions and pathophysiology of sepsis. Curr. Probl. Pediatr. Adolesc. Health Care 2013, 43, 260–263. [Google Scholar] [CrossRef]
- Wang, J.F.; Li, J.B.; Zhao, Y.J.; Yi, W.J.; Bian, J.J.; Wan, X.J.; Zhu, K.M.; Deng, X.M. Up-regulation of programmed cell death 1 ligand 1 on neutrophils may be involved in sepsis-induced immunosuppression: An animal study and a prospective case-control study. Anesthesiology 2015, 122, 852–863. [Google Scholar] [CrossRef]
- Rebetz, J.; Semple, J.W.; Kapur, R. The Pathogenic Involvement of Neutrophils in Acute Respiratory Distress Syndrome and Transfusion-Related Acute Lung Injury. Transfus. Med. Hemother. 2018, 45, 290–298. [Google Scholar] [CrossRef]
- Wang, J.F.; Wang, Y.P.; Xie, J.; Zhao, Z.Z.; Gupta, S.; Guo, Y.; Jia, S.H.; Parodo, J.; Marshall, J.C.; Deng, X.M. Upregulated PD-L1 delays human neutrophil apoptosis and promotes lung injury in an experimental mouse model of sepsis. Blood 2021, 138, 806–810. [Google Scholar] [CrossRef]
- Liu, L.; Sun, B. Neutrophil pyroptosis: New perspectives on sepsis. Cell. Mol. Life Sci. CMLS 2019, 76, 2031–2042. [Google Scholar] [CrossRef]
- Park, I.; Kim, M.; Choe, K.; Song, E.; Seo, H.; Hwang, Y.; Ahn, J.; Lee, S.H.; Lee, J.H.; Jo, Y.H.; et al. Neutrophils disturb pulmonary microcirculation in sepsis-induced acute lung injury. Eur. Respir. J. 2019, 53, 1800786. [Google Scholar] [CrossRef]
- Burnett, A.; Gomez, I.; De Leon, D.D.; Ariaans, M.; Progias, P.; Kammerer, R.A.; Velasco, G.; Marron, M.; Hellewell, P.; Ridger, V. Angiopoietin-1 enhances neutrophil chemotaxis in vitro and migration in vivo through interaction with CD18 and release of CCL4. Sci. Rep. 2017, 7, 2332. [Google Scholar] [CrossRef]
- Pelisch, N.; Rosas Almanza, J.; Stehlik, K.E.; Aperi, B.V.; Kroner, A. CCL3 contributes to secondary damage after spinal cord injury. J. Neuroinflamm. 2020, 17, 362. [Google Scholar] [CrossRef]
- Lyck, R.; Enzmann, G. The physiological roles of ICAM-1 and ICAM-2 in neutrophil migration into tissues. Curr. Opin. Hematol. 2015, 22, 53–59. [Google Scholar] [CrossRef]
- Sokulsky, L.A.; Garcia-Netto, K.; Nguyen, T.H.; Girkin, J.L.N.; Collison, A.; Mattes, J.; Kaiko, G.; Liu, C.; Bartlett, N.W.; Yang, M.; et al. A Critical Role for the CXCL3/CXCL5/CXCR2 Neutrophilic Chemotactic Axis in the Regulation of Type 2 Responses in a Model of Rhinoviral-Induced Asthma Exacerbation. J. Immunol. 2020, 205, 2468–2478. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, Z.; Tang, D. Aerobic exercise improves LPS-induced sepsis via regulating the Warburg effect in mice. Sci. Rep. 2021, 11, 17772. [Google Scholar] [CrossRef]
- Khoyratty, T.E.; Ai, Z.; Ballesteros, I.; Eames, H.L.; Mathie, S.; Martín-Salamanca, S.; Wang, L.; Hemmings, A.; Willemsen, N.; von Werz, V.; et al. Distinct transcription factor networks control neutrophil-driven inflammation. Nat. Immunol. 2021, 22, 1093–1106. [Google Scholar] [CrossRef]
- Schwartz, J.T.; Bandyopadhyay, S.; Kobayashi, S.D.; McCracken, J.; Whitney, A.R.; Deleo, F.R.; Allen, L.A. Francisella tularensis alters human neutrophil gene expression: Insights into the molecular basis of delayed neutrophil apoptosis. J. Innate Immun. 2013, 5, 124–136. [Google Scholar] [CrossRef]
- Ward, C.; Chilvers, E.R.; Lawson, M.F.; Pryde, J.G.; Fujihara, S.; Farrow, S.N.; Haslett, C.; Rossi, A.G. NF-kappaB activation is a critical regulator of human granulocyte apoptosis in vitro. J. Biol. Chem. 1999, 274, 4309–4318. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, F.; Chen, M.; Wang, C.; Xia, H.; Zhang, D.; Yao, S. Single-Cell Sequencing Reveals the Regulatory Role of Maresin1 on Neutrophils during Septic Lung Injury. Cells 2022, 11, 3733. https://doi.org/10.3390/cells11233733
Wang F, Chen M, Wang C, Xia H, Zhang D, Yao S. Single-Cell Sequencing Reveals the Regulatory Role of Maresin1 on Neutrophils during Septic Lung Injury. Cells. 2022; 11(23):3733. https://doi.org/10.3390/cells11233733
Chicago/Turabian StyleWang, Fuquan, Ming Chen, Chenchen Wang, Haifa Xia, Dingyu Zhang, and Shanglong Yao. 2022. "Single-Cell Sequencing Reveals the Regulatory Role of Maresin1 on Neutrophils during Septic Lung Injury" Cells 11, no. 23: 3733. https://doi.org/10.3390/cells11233733
APA StyleWang, F., Chen, M., Wang, C., Xia, H., Zhang, D., & Yao, S. (2022). Single-Cell Sequencing Reveals the Regulatory Role of Maresin1 on Neutrophils during Septic Lung Injury. Cells, 11(23), 3733. https://doi.org/10.3390/cells11233733




